Introduction
Ultraviolet (UV) radiation is generally divided into
two spectral components consisting of UVB (290–320 nm) and UVA
(320–400 nm). It is the main causative factor of skin cancer and
also causes chronic damage to the eye, eventually leading to eye
cancer (1). Cellular metabolic
pathways generate molecules involved in causing photochemical
damage to areas of skin exposed to UVB and UVA (2). For instance, UV radiation generates
free radicals, including oxygen-derived radical species, which are
known to cause lipid peroxidation in cellular membranes (3). In addition, it has been demonstrated
that UV light directly damages DNA (4,5),
decreases mitochondrial function, induces apoptosis (4) and affects ocular structures, including
the cornea, lens and retina (6).
Histatins (Hsts) are proteins found in saliva and
are ubiquitously present in all living organisms. Hsts display
anti-microbial and anti-fungal properties and are known to have a
role in wound closure (7). Three
major types of Hsts (Hst1, 3 and 5) have been identified, and Hst1
is encoded by the gene HTN1 (8). In
humans, Hsts are secreted into saliva by the parotid and
submandibular glands (9). Oudhoff
et al (10) reported that
Hst1 not only promotes cell-substrate adhesion but also cell-cell
adhesion, which facilitates the creation of epithelial barrier
junctions. Hsts are ubiquitously present in all living organisms,
and in addition to their anti-microbial functions, also appear to
have growth-stimulating properties (10). While Hsts exert wound-healing effects
in cultured epithelial cells, it has not been determined whether
they protect human corneal epithelial cells (HCECs) from damage
induced by exposure to UV radiation. Insulin-like growth factor
(IGF)-1 expressed in corneal tissues is known to promote corneal
epithelial cell proliferation, migration and differentiation, and
to have an important role in maintaining normal corneal growth.
Individuals with diabetic retinopathy display an increased
expression of IGF-1 (11), which
enhances the ability of their corneal epithelial cells to heal
after being damaged.
The present study investigated the role of Hst1 in
HCECs damaged by exposure to UV radiation. The results suggested
that Hst1 may be a potential therapeutic agent for protecting HCECs
against damage induced by UV radiation.
Materials and methods
Cell culture and treatment
HCECs from cultures of <30 passages were provided
by the Department of Plastic, Shandong Provincial Qianfoshan
Hospital affiliated Shandong University (Shandong, China); the
cells were purchased from the American Type Culture Collection
(Manassas, VA, USA). The HCECs were cultured in a growth medium
consisting of 10% fetal bovine serum (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), 100 IU/ml penicillin
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), 100 IU/ml
streptomycin (Sigma-Aldrich; Merck KGaA) and Dulbecco's modified
Eagle's medium/F12 (Invitrogen; Thermo Fisher Scientific, Inc.).
The cells were maintained in a humidified atmosphere with 5%
CO2 at 37°C. When the cells had formed a sheath on the
bottom of the culture vessel, they were re-suspended by incubation
with 0.125% trypsin, collected and seeded at a ratio of 1:2 in a
fresh 25 cm2 culture flask. They were then sub-cultured
in 5 ml complete medium.
Exposure of cells to UV light
When the HCECs had grown to ~80% confluency, the
residual growth medium was discarded and replaced with 1 ml
pre-heated PBS. The cells were then gently agitated and washed with
PBS. Next, the culture dish lids were removed and the cells were
placed 12 cm below an UVB lamp (wavelength range, 250–350 nm; peak
wavelength, 297 nm) for direct irradiation. The intensity of UV
radiation directly measured at a 12-cm vertical distance below the
UVB lamp was 20 µw/cm2, and was calculated using the
following formula: H=TxE; where H was the radiant exposure
(J/cm2), t was the exposure duration (seconds) and E was
the measured irradiance (W/cm2). Radiation dosages
corresponding to UVB irradiation times of 1, 2 and 3 h were 0.072,
0.144 and 0.216 J/cm2, respectively. Following
irradiation, the cells were once again cultured in fresh
medium.
Hst1 treatment
Hst1 was obtained from the Chinese Peptide Company
(Hangzhou, China). The sequence was DSH EKR HHG YRR KFH EKH HSH REF
PFY GDY GSN YLY DN. Four doses of Hst1 (0, 10, 50 and 100 µg/ml)
were added to the cells for 12 h prior to UV radiation treatment or
other assays.
AlamarBlue® assay
The viability of HCECs was measured by examining
their general health and proliferation using the
alamarBlue® assay. HCECs (~105) were seeded
into the wells of 24-well plates and then incubated at 37°C with 5%
CO2 for 24 h. The cells were exposed to UV light when
the cultures were ~75% confluent. Following UV exposure, the
cultures were again incubated for 24 h. Subsequently, the medium
was aspirated from each well and the cells were rinsed with 1 ml
culture medium containing serum. After aspirating the residual
medium, 1 ml 10% alamarBlue® (Thermo Fisher Scientific,
Inc.) plus growth medium without serum was added to each well, and
the cells were incubated at 37°C for 3 h. Following incubation, the
fluorescence in each well was measured at 530/590 nm using a Thermo
Plate microplate reader (Rayto Life and Analytical Science Co.
Ltd., Shenzhen, China).
Proliferation of cultured cells
The proliferation of cultured HCECs was evaluated
using the MTT colorimetric assay, which is based on the chemical
reduction of MTT by living cells to form formazan crystals. In
brief, HCECs were seeded into 96-well tissue culture plates
(2×104 cells/well) and incubated at 37°C under 5%
CO2 for 0, 12, 24 or 48 h. Next, the cells were
incubated in 100 µl MTT solution (5 mg/ml; cat. no. M6494;
Invitrogen; Thermo Fisher Scientific, Inc.) for 3 h, and then
washed with PBS. The purple formazan crystals formed in living
cells were then solubilized with dimethylsulfoxide (Invitrogen;
Thermo Fisher Scientific, Inc.). The absorbance of the formazan
solution was measured at 450 nm using a Thermo Plate microplate
reader (Rayto Life and Analytical Science Co. Ltd.).
Cell apoptosis analysis
Apoptosis assays were performed using the
Annexin-V-FITC Apoptosis Detection Kit (Nanjing KeyGen Biotech Co.,
Ltd., Nanjing, China) according to the manufacturer's instructions.
Early apoptotic cells were defined as annexin-V-positive or
propidium iodide-negative cells. Analyses were performed using a
Beckman Gallios Flow Cytometer (Beckman Coulter, Brea, CA,
USA).
Semi-quantitative polymerase chain
reaction (qPCR)
Total cellular RNA was isolated using TRIzol reagent
(Takara Biotechnology Co., Ltd., Dalian, China). The first strand
cDNA was synthesized using the PrimeScript™ 1st strand cDNA
Synthesis kit (cat. no. 6110A; Takara Biotechnology Co., Ltd.).
Next, RNA samples were subjected to qPCR analysis to measure their
levels of IGF-1, B-cell lymphoma 2 (Bcl-2) and Bcl-2-associated X
(Bax) mRNA. The primers used for IGF-1, Bcl-2, Bax and GAPDH are
listed in Table I. qPCR reactions
were conducted using the SYBR® Fast qPCR mix (cat. no.
RR430A; Takara Biotechnology Co., Ltd.). The PCR amplification
conditions were as followings, pre-denaturation at 95°C for 30 sec,
denaturation at 95°C for 5 sec and annealing at 60°C for 10 sec (40
cycles). The PCR products in 4 µl of the product mixture were
separated by electrophoresis on a 1.25% agarose gel, the gel was
stained with ethidium bromide solution for 15 min at <50°C. The
densities of the various complementary DNA bands were analyzed by
scanning their absorbance areas with an AlphaImager gel imaging and
analysis system (ProteinSimple, San Jose, CA, USA).
Alphalmager® Ger Documentation (version 1.0;
ProteinSimple) was used to assess the density the bands. Band
intensity values for IGF-1, Bcl-2 and Bax were normalized to those
of GAPDH and the relative expression was calculated using the
2−ΔΔCq method (12).
 | Table I.Primer pairs used for polymerase chain
reaction. |
Table I.
Primer pairs used for polymerase chain
reaction.
|
| Primers (5′-3′) |
|---|
|
|
|
|---|
| Gene | Forward | Reverse |
|---|
| GAPDH |
GGCCTCCAAGGAGTAAGAAA |
GCCCCTCCTGTTATTATGG |
| IGF-1 |
TAAGGAGGCTGGAGATGTATTGC |
GGCTGATACTTCTGGGTCTTGG |
| Bcl-2 |
GGGAGGATTGTGGCCTTCTTTG |
TGTGCAGGTGCCGGTTCAG |
| Bax |
TTTGCTTCAGGGTTTCATCCA |
TGAGACACTCGCTCAGCTTCTTG |
ELISA
Cellular levels of malondialdehyde (MDA; cat. no.
A003-4) and superoxide dismutase (SOD; cat. no. A001-1) were
detected using ELISA kits (both Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) according to the manufacturer's
instructions. In brief, 100 µl of a prepared standard solution or
sample was added to each well of a sample plate and incubated
overnight at 4°C. The plate was then washed four times with buffer,
and subsequently, 100 µl biotinylated primary antibody was added to
each well, followed by incubation at room temperature (RT) for 1 h.
The plates were then washed four times with buffer and incubated
with peroxidase-conjugated secondary antibody for 45 min. Next, the
plates were washed three times with buffer, and 100 µl
tetramethylbenzidine was added to each well to induce a color
reaction at RT, which was stopped after 30 min. The optical density
of each well at 450 nm was determined using an automated microplate
reader.
Western blot analysis
Cells were harvested in radioimmunoprecipitation
assay lysis buffer and centrifuged at 13,200 × g for 15 min at 4°C.
The supernatant fractions were collected and the protein
concentration was determined by the Bio-Rad Protein Assay kit II
(cat. no. 5000002; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Protein samples (40 µg/lane of total protein) were separated by 12%
SDS-PAGE and electro-transferred to polyvinylidene difluoride
membranes (cat. no. IPVH00010; EMD Millipore, Billerica, MA, USA).
The membranes were then incubated with 5% non-fat skimmed milk in
Tris-buffered saline containing 0.1% Tween 20 (TBST) for 1 h. Next,
the membranes were incubated overnight with anti-IGF-1 (1:1,000;
cat. no. ab9572; Abcam, Cambridge, UK), anti-Bcl-2 (1:1,000; cat.
no. 2872), anti-Bax (1:1,000; cat. no. 5023) and anti-GAPDH
(1:2,000; cat. no. 97166) (all Cell Signaling Technology, Inc.,
Danvers, MA, USA) primary antibodies at 4°C, followed by a
subsequent incubation with horseradish peroxidase-conjugated goat
anti-mouse (cat. no. A4416) and anti-rabbit (cat. no. A6154)
secondary antibodies (both 1:10,000; Sigma-Aldrich; Merck KGaA) for
1 h at room temperature. The membranes were then rinsed with TBST,
blots were visualized with enhanced chemiluminescence western blot
detection reagents (cat. no. 321096; Thermo Fisher Scientific,
Inc.) and then exposed to an X-ray film (Kodak, Rochester, NY,
USA). The resultant protein bands were scanned using a Gel-doc2000
imaging system (Bio-Rad Laboratories, Inc.) and analyzed using
Quantity One 1-D Analysis software (version 4.6.9; Bio-Rad
Laboratories, Inc.).
Statistical analysis
All data were analyzed using Predictive Analytics
Software for Windows (version 18.0; IBM Corp., Armonk, NY, USA).
Statistical significance was assessed by Student's t-test and
one-way analysis of variance followed by Tukey's post-hoc test.
Statistical values are expressed as the mean ± standard error of
the mean. P<0.05 was considered to indicate a statistically
significant difference between groups. All experiments were
performed at least three times.
Results
Cell model of UV irradiation-induced
injury
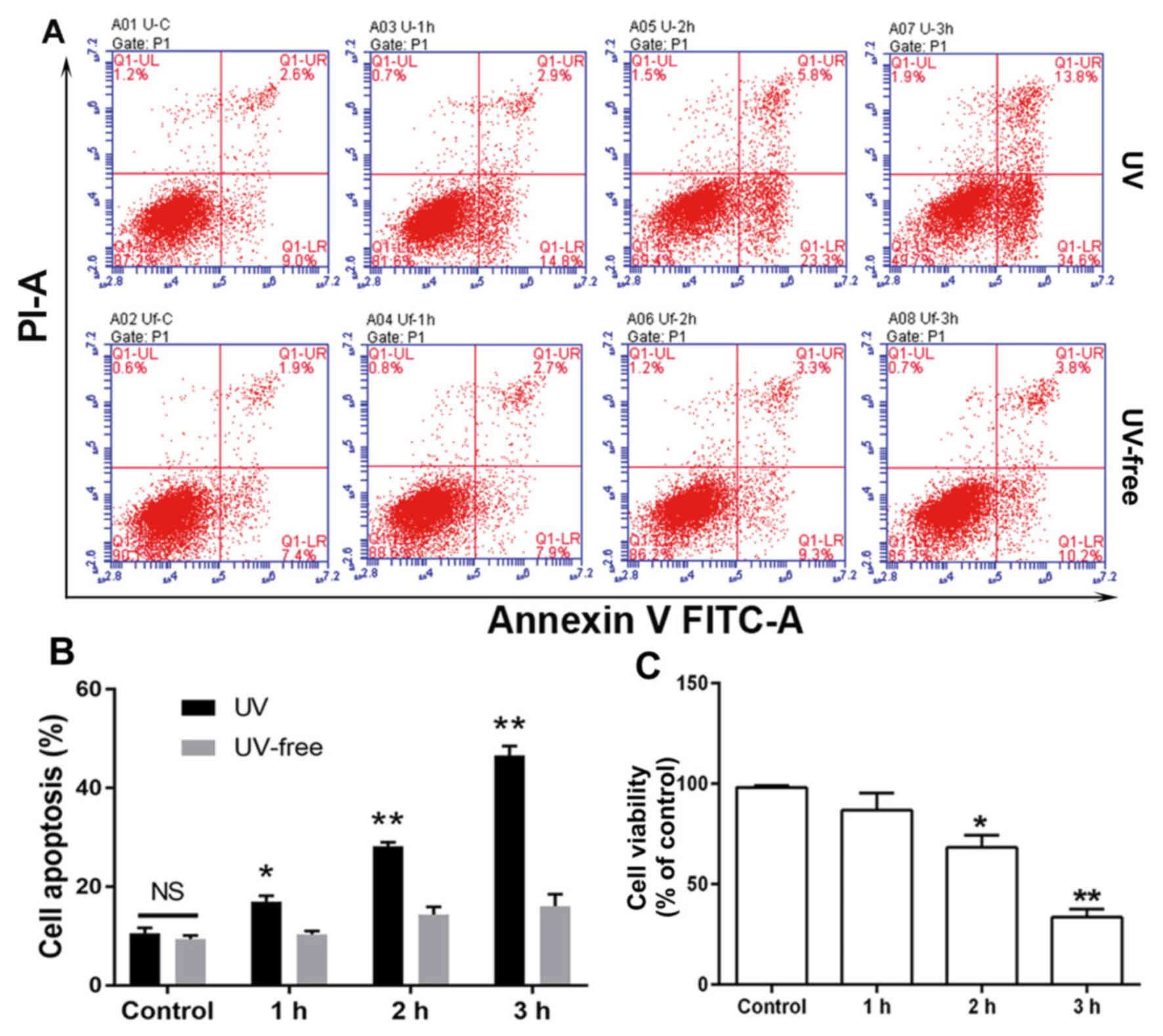
The percentage of HCECs undergoing apoptosis
increased with increasing exposure to UV light (Fig. 1A). While 29.1% of cells exposed to UV
radiation for 2 h had entered apoptosis (early and late apoptosis),
this percentage increased to 48.4% after 3 h of exposure,
indicating that UV radiation can greatly damage HCECs (Fig. 1B). Furthermore, cells exposed to UV
radiation for 3 h demonstrated a significant loss of viability as
measured by alamarBlue® assay, while a 1 h exposure had
little or no effect (Fig. 1C).
Simultaneously, a UV-free group was set as a control, and cells in
this group were treated under the above conditions apart from being
irradiated with UV light. Cell apoptosis slightly increased in the
UV-free group with time; however, compared with this group at 0 h,
no significant differences were demonstrated. Based on these
results, a 2 h UV radiation exposure period (total radiation
dosage, 0.144 J/cm2) was selected for use in the
subsequent UV-induced cell damage model.
Hst1 enhances the proliferation and
reduces oxidative stress in HCECs
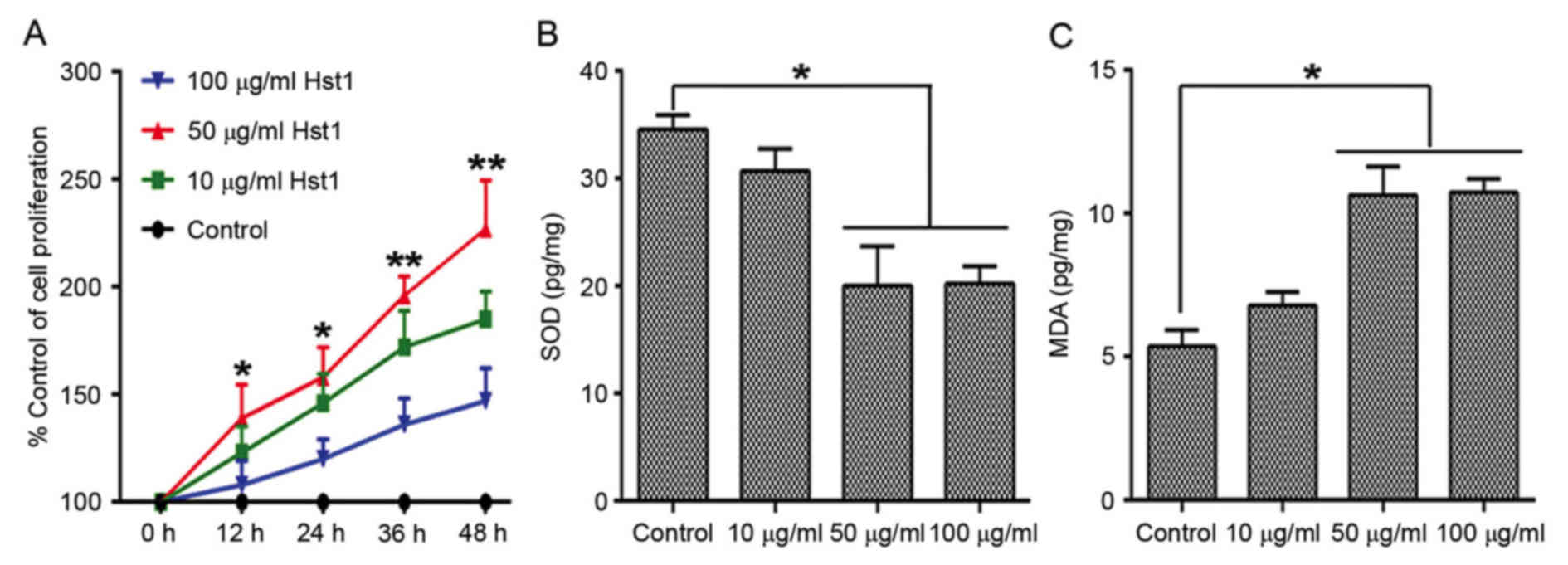
Three concentrations of Hst1 (10, 50 and 100 µg/ml)
were used to treat the HCECs prior to UV radiation. Cell
proliferation, which was assessed by the MTT assay was increased in
the 10, 50 and 100 µg/ml Hst1 treatment groups. Cell proliferation
was significantly induced in the 50 µg/ml Hst1 treatment group
compared with the control group (Fig.
2A). However, cell proliferation in the 100 µg/ml Hst1
treatment group was markedly lower compared with the 10 µg/ml Hst1
treatment group, but it remained higher than that in the control
group. Furthermore, by performing an MTT assay the proliferation of
irradiated HCECs pre-treated with Hst1 was demonstrated to be
increased compared with that of untreated irradiated cells. The
same cells were also subjected to analysis of cellular SOD and MDA
levels. The results revealed that the Hst1 treatment groups had
lower SOD levels (Fig. 2B), but
higher MDA levels (Fig. 2C) than the
control group (0 µg/ml and UV irradiated). When pre-treated with 50
µg/ml Hst1, the intracellular content of SOD was significantly
decreased, while the MDA concentration was increased when compared
with that in the control group. Therefore, 50 µg/ml was selected as
the concentration of Hst1 to be used in the subsequent
experiments.
Hst1 protects HCECs against UV-induced
damage
It was then examined how Hst1 protected HCECs
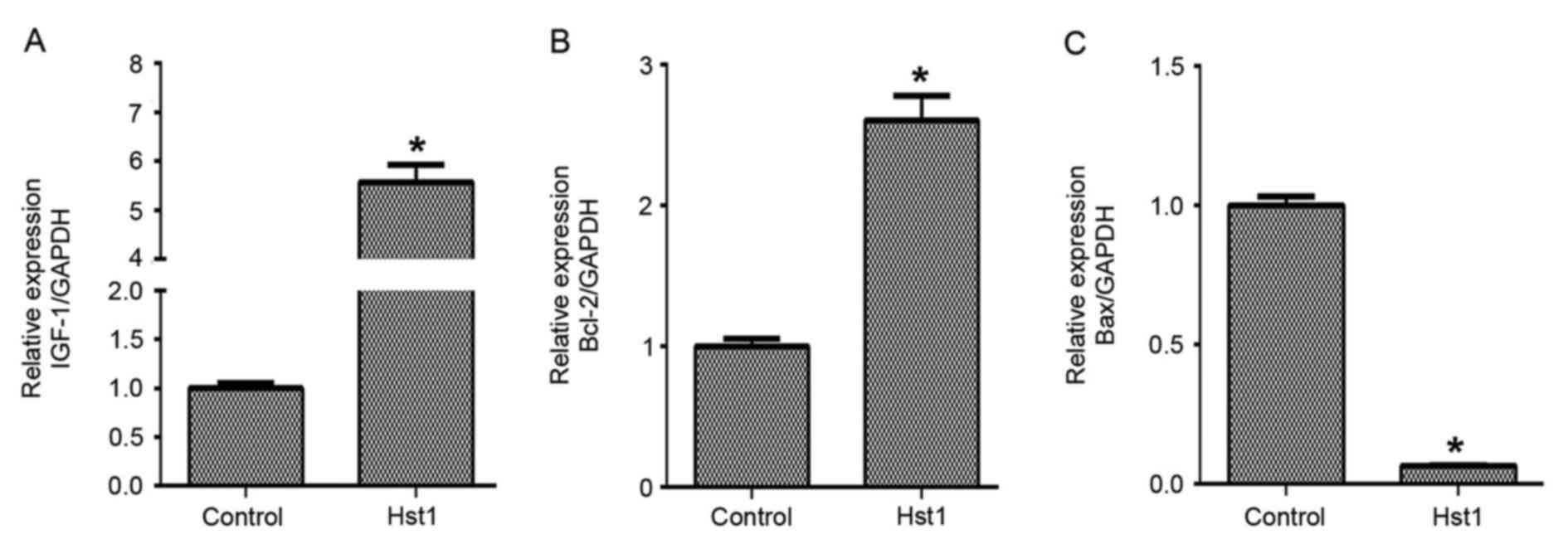
against UV-induced damage. qPCR analysis demonstrated that the mRNA
expression levels of IGF-1 (Fig. 3A)
and Bcl-2 (Fig. 3B) were upregulated
after pre-treatment of HCECs with 50 µg/ml Hst1 prior to
irradiation, while and Bax was downregulated (Fig. 3C). Furthermore, Hst1 inhibited
irradiation-induced apoptosis in HCECs (Fig. 4A and B). In addition, Hst1 was found
to affect IGF-1, Bcl-2 and Bax protein expression in a similar way
to the corresponding mRNA levels: The expression of IGF-1 and Bcl-2
in HCECs treated with Hst1 was significantly upregulated when
compared with that in non-treated control HCECs. By contrast, Bax
expression was significantly downregulated in Hst1-treated cells
after irradiation (Fig. 4C-E). These
results suggested that Hst1 may be a potential prophylactic
therapeutic agent to protect against UV damage. Further studies may
demonstrate that Hst1 improves corneal epithelial wound healing in
UV-damaged eyes.
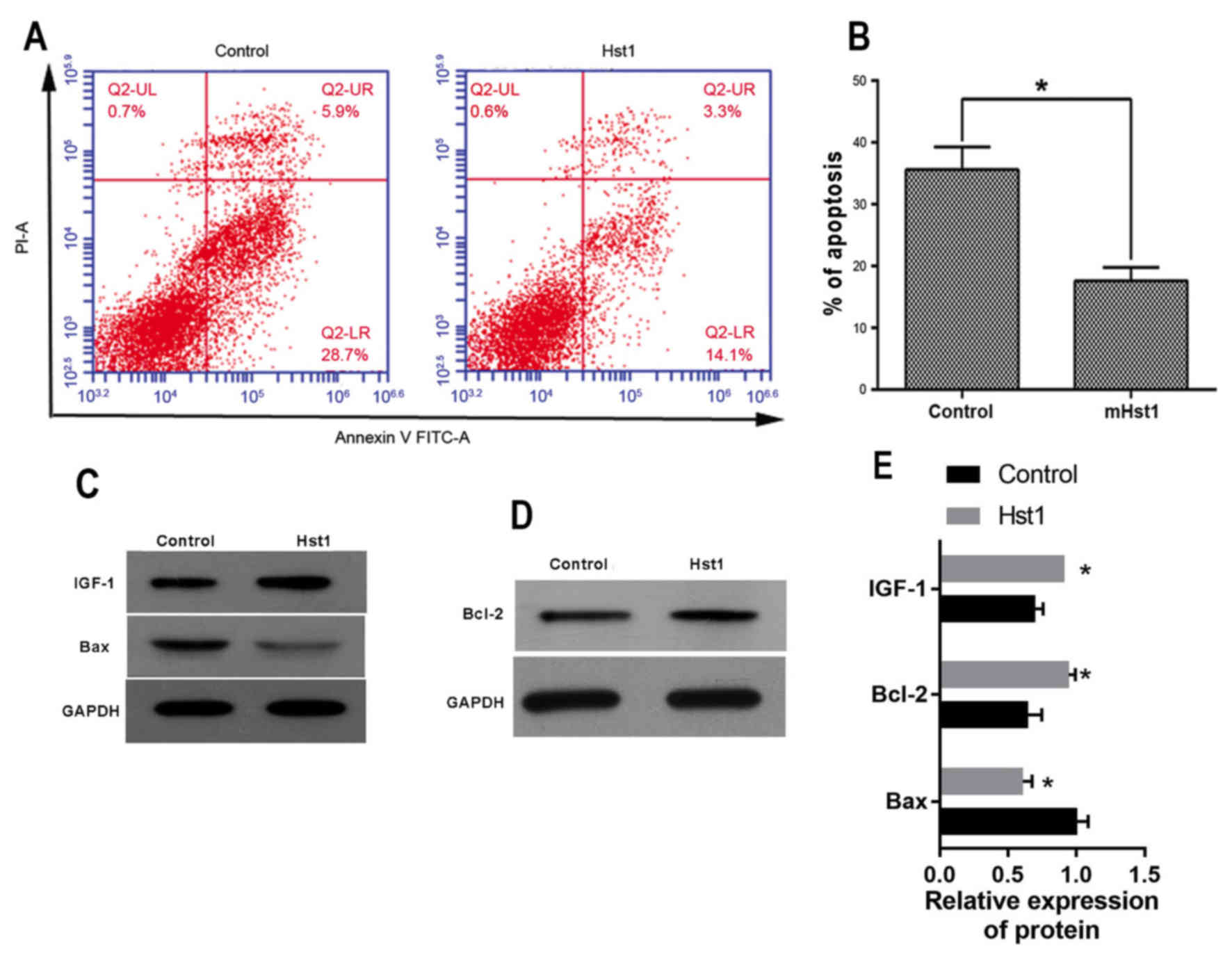 | Figure 4.Protective effect of Hst1 against
ultraviolet irradiation-induced damage to HCECs. (A) Cell apoptosis
assay. (B) The percentage of HCECs undergoing apoptosis. Expression
of (C) IGF-1, Bax and (D) Bcl-2, as detected by western blotting
and (E) western blot analysis. *P<0.05 vs. control. Hst,
histatin; IGF, insulin-like growth factor; Bcl-2, B-cell lymphoma
2; Bax, Bcl-2-associated X protein; HCEC, human corneal epithelial
cell; FITC, fluorescein isothiocyanate; PI, propidium iodide; UL,
upper left; LR, lower right. |
Discussion
Irradiation of cells with UV light triggers a
variety of signaling cascades that promote apoptosis. UV light is
absorbed by the cornea, resulting in the generation of large
amounts of free radicals, which may damage biological tissues if
the supply of free radical scavengers in the irradiated tissue is
insufficient (13).
It is well known that UV radiation causes cell
damage and even the death of cells located on the ocular surface
(13,14), and this damage to corneal epithelial
cells contributes to ocular pathologies such as photokeratitis. The
present study was performed to determine whether Hst1 protects
HCECs against UV radiation. The alamarBlue® assay was
performed to evaluate differences between the viability of HCECs
treated with Hst1 and control HCECs treated with PBS prior to
irradiation with UV light for time periods of 1, 2 and 3 h,
respectively. The results demonstrated that relevant doses (0.072,
0.144 and 0.216 J/cm2) of UV radiation decreased the
viability of HCECs. However, HCECs pre-treated with Hst1 were less
susceptible to damage by UV radiation, suggesting a protective
effect of Hst1.
Histatins are anti-microbial and anti-fungal
proteins found in human saliva, and have been demonstrated to have
a role in wound closure (10).
Certain mammalian anti-microbial peptides were found to induce the
expression of proteoglycans that promote cell proliferation,
migration, angiogenesis and collagen synthesis, all of which are
involved in the wound healing process (15). Due to their specific distribution in
the human body, studies on Hsts have mostly concentrated on their
role in the oral environment (16).
The present study revealed that Hst1 protected HCECs and decreased
their entry into apoptosis. Free radicals may inactivate
Na+, K+ and adenosine triphosphate-dependent
enzymes in the corneal epithelium, resulting in disorders of cell
metabolism. At the same time, they consume large amounts of
anti-oxidants, which decreases the anti-oxidant capacity of
biological tissue as indicated by increased MDA and decreased SOD
levels, resulting in tissue damage (17). The results of the present study
demonstrated that pre-treatment with Hst1 decreases SOD activity
and increases MDA levels compared with those in untreated
UV-irradiated HCECs, indicating that free radical oxidation
reactions are involved in the corneal damage produced by UV
light.
IGF-1 is the most important growth factor known to
regulate cell proliferation, migration and apoptosis, and its
expression is elevated in the vitreous of diabetic retinopathy
patients. Bcl-2 and Bax mRNA have been detected in the primary
cultures of three types of corneal cells, and are known to be
involved in regulating apoptosis in corneal cells (18). In the present study, IGF-1 and Bcl-2
expression were increased in the Hst1 group, while Bax expression
was decreased compared with that in untreated UV-irradiated
HCECs.
Our group will continue to study the underlying
mechanisms involved in UV-induced damage to biological tissue, with
the goal of gaining novel information that may be used to help
prevent or treat certain diseases caused by exposure to UV light.
These results of the present suggested that Hst1 may be a potential
prophylactic therapeutic agent to protect against UV damage.
Further studies may demonstrate that Hst1 also improves corneal
epithelial wound healing in UV-damaged eyes. Studies on corneal
damage produced by exposure to UV light may provide a factual or
theoretical basis for the clinical use of Hst1, and a better
understanding of its pharmacological effects.
References
|
1
|
Ng J, Coroneo MT, Wakefield D and Di
Girolamo N: Ultraviolet radiation and the role of matrix
metalloproteinases in the pathogenesis of ocular surface squamous
neoplasia. Investigative Ophthalmol Visual Sci. 49:5295–5306. 2008.
View Article : Google Scholar
|
|
2
|
Youn HY, McCanna DJ, Sivak JG and Jones
LW: In vitro ultraviolet-induced damage in human corneal, lens, and
retinal pigment epithelial cells. Mol Vis. 17:237–246.
2011.PubMed/NCBI
|
|
3
|
Halliwell B: Antioxidant defence
mechanisms: From the beginning to the end (of the beginning). Free
Radic Res. 31:261–272. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cadet J, Berger M, Douki T, Morin B, Raoul
S, Ravanat JL and Spinelli S: Effects of UV and visible radiation
on DNA-final base damage. Biol Chem. 378:1275–1286. 1997.PubMed/NCBI
|
|
5
|
Jou MJ, Peng TI, Hsu LF, Jou SB, Reiter
RJ, Yang CM, Chiao CC, Lin YF and Chen CC: Visualization of
melatonin's multiple mitochondrial levels of protection against
mitochondrial Ca(2+)-mediated permeability transition and beyond in
rat brain astrocytes. J Pineal Res. 48:20–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Clydesdale GJ, Dandie GW and Muller HK:
Ultraviolet light induced injury: Immunological and inflammatory
effects. Immunol Cell Biol. 79:547–568. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
vanderSpek JC, Offner GD, Troxler RF and
Oppenheim FG: Molecular cloning of human submandibular histatins.
Arch Oral Biol. 35:137–143. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sabatini LM and Azen EA: Histatins, a
family of salivary histidine-rich proteins, are encoded by at least
two loci (HIS1 and HIS2). Biochem Biophys Res Commun. 160:495–502.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
vanderSpek JC, Wyandt HE, Skare JC,
Milunsky A, Oppenheim FG and Troxler RF: Localization of the genes
for histatins to human chromosome 4q13 and tissue distribution of
the mRNAs. Am J Hum Genet. 45:381–387. 1989.PubMed/NCBI
|
|
10
|
Oudhoff MJ, Bolscher JG, Nazmi K, Kalay H,
van't Hof W, Amerongen AV and Veerman EC: Histatins are the major
wound-closure stimulating factors in human saliva as identified in
a cell culture assay. FASEB J. 22:3805–3812. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chiarelli F, Santilli F and Mohn A: Role
of growth factors in the development of diabetic complications.
Horm Res. 53:53–67. 2000.PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pauloin T, Dutot M, Joly F, Warnet JM and
Rat P: High molecular weight hyaluronan decreases UVB-induced
apoptosis and inflammation in human epithelial corneal cells. Mol
Vis. 15:577–583. 2009.PubMed/NCBI
|
|
14
|
Lu L, Wang L and Shell B: UV-induced
signaling pathways associated with corneal epithelial cell
apoptosis. Invest Ophthalmol Vis Sci. 44:5102–5109. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirsch T, Spielmann M, Zuhaili B, Fossum
M, Metzig M, Koehler T, Steinau HU, Yao F, Onderdonk AB,
Steinstraesser L and Eriksson E: Human beta-defensin-3 promotes
wound healing in infected diabetic wounds. J Gene Med. 11:220–228.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kavanagh K and Dowd S: Histatins:
Antimicrobial peptides with therapeutic potential. J Pharm
Pharmacol. 56:285–289. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rivera-Pastrana DM, Gardea AA, Yahia EM,
Martinez-Téllez MA and González-Aguilar GA: Effect of UV-C
irradiation and low temperature storage on bioactive compounds,
antioxidant enzymes and radical scavenging activity of papaya
fruit. J Food Sci Technol. 51:3821–3829. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li Q, Weng J, Mohan RR, Bennett GL,
Schwall R, Wang ZF, Tabor K, Kim J, Hargrave S, Cuevas KH and
Wilson SE: Hepatocyte growth factor and hepatocyte growth factor
receptor in the lacrimal gland, tears, and cornea. Invest
Ophthalmol Vis Sci. 37:727–739. 1996.PubMed/NCBI
|