Introduction
Osteoporosis is a metabolic disease that is mainly
characterized by low bone mineral density. Osteoporosis has become
one of the most serious public health problems worldwide, and is
considered by the World Health Organization (WHO) to be an
important global health issue (1,2). The
most frequent and severe clinical complication of osteoporosis is
bone fracture, and the morbidity or mortality associated with
osteoporosis is one of the most important causes of the millions of
fractures that occur annually (1,3,4). It is estimated that the number of
people suffering from osteoporosis is >90 million in China
(2), and ~200 million worldwide
(5). The burden of osteoporosis,
which results in a cost of 10 billion dollars annually, is
reflected significantly by the ever increasing hospital and medical
expenditures for fracture-associated problems worldwide (6–8). In
addition, >40% of postmenopausal women are affected by
osteoporosis, and this number is expected to steadily increase with
the aging population in the near future (9). Therefore, the economic burden of
osteoporosis is expected to markedly increase with the expansion of
the aging world population (10).
MicroRNAs (miRNAs or miRs) are a type of
small-molecule endogenous RNAs that regulate thousands of genes
(11–14). It is believed that 30% of the human
genome is manipulated by miRNAs through binding to the
3′-untranslated region (UTR) of their target mRNAs, resulting in
mRNA degradation or translational repression (15–17).
miRNAs have been demonstrated to function as regulators in numerous
diseases; however, few studies discussing the miRNAs associated
with osteoblastogenesis have been reported (13,18).
Although the biological functions of the majority of miRNAs are not
yet fully understood, they may serve an important role in the
regulation of various biological processes, including apoptosis,
cell proliferation and cell differentiation. Among these processes,
osteoblast differentiation and bone formation regulated by miRNAs
are currently widely researched. Studies have indicated that miRNAs
positively regulate osteoblast differentiation and bone formation
by targeting negative regulators of osteogenesis, or negatively
regulate it by targeting important osteogenic factors (19–22). In
particular, miR-153 has been proven to regulate cell proliferation
and differentiation in osteosarcoma, prostate cancer, gastric
cancer and venous smooth muscle cell lines (23–26).
Therefore, miR-153 is one of the most representative miRNAs that
can impact proliferation and osteoblastic differentiation. However,
the association among miR-153, MC3T3-E1 cells and osteoporosis
remains unclear.
Icariin, a prenylated flavonol glycoside isolated
from the Epimedium herb, has been observed to have
bone-strengthening activities in Chinese herbal medicine (27). It has been demonstrated that icariin
improved osteoblast differentiation and mineralization in rat
osteoblasts in vitro (28).
Furthermore, it was reported that icariin prevented bone loss
induced by ovariectomization in rats (29). In addition, icariin was found to
increase osteoblast differentiation in MC3T3-E1 cells through the
Runt-related transcription factor 2 (Runx2) and bone morphogenetic
protein (BMP) signaling pathways (30). These results suggested that icariin
may prevent bone loss by stimulating bone formation. However, the
understanding of the mechanism underlying the icariin function with
regard to the microRNA and bone association remains unclear.
In the present study, a discrepancy in the
expression levels of miR-153 in MC3T3-E1 cells was observed between
the icariin-treated and the control groups. To reveal the potential
mechanism of icariin, a functional study was performed, which
identified that miR-153 promoted the osteoblast differentiation and
cell proliferation of MC3T3-E1 cells through directly targeting and
upregulating Runx2 following icariin treatment. Therefore, the
findings of the present study suggested that icariin may serve as a
potential drug for the prevention and treatment of
osteoporosis.
Materials and methods
Materials
The clonal mouse MC3T3-E1 preosteoblastic cell line
was obtained from the Central Laboratory of Wuhan University
(Wuhan, China). Icariin (purity, >98%) was purchased from Abcam
(Cambridge, MA, USA), dissolved in dimethyl sulfoxide (DMSO) and
stored in the dark at −20°C. Throughout the experiments, the final
concentration of DMSO in the medium was kept at ≤0.1%, and a DMSO
control was compared with the icariin-treated cells. The 6-, 24-
and 96-well plates, as well as the 25 mm culture bottles, were
purchased from Corning, Inc. (Corning, NY, USA). Fetal bovine serum
(FBS) was purchased from Thermo Fisher Scientific, Inc. (Waltham,
MA, USA). Phosphate-buffered saline (PBS), dH2O,
paraformaldehyde, cetylpyridinium chloride, α-minimal essential
medium (α-MEM), b-glycerophosphate, ascorbic acid 2-phosphate
(ASAP) and DMSO were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). The alizarin red S staining kit, alkaline
phosphatase (ALP) assay kit and Cell Counting kit-8 (CCK-8) for
cell viability assay were obtained from Nanjing Jiancheng
Bioengineering Institute (Nanjing, China). The RNAiso kit was
purchased from Takara Biotechnology Co., Ltd. (Dalian, China).
Antibodies against GAPDH (ab22555), Runx2 (ab54868), ALP
(ab108337), BMP2 (ab82511), BMP4 (ab39973) and osteopontin (OPN;
ab8448) were all purchased from Abcam. Polyvinylidene fluoride
(PVDF) membranes (0.22 mm) were purchased from EMD Millipore
(Billerica, MA, USA). All experiments were repeated three times.
All control groups consisted of MC3T3-E1 cells without treatment or
transfection.
Cell culture and proliferation
assay
MC3T3-E1 cells were cultured in α-MEM supplemented
with 10% FBS, 10 mmol/l b-glycerophosphate and 50 µg/ml ASAP in a
humidified atmosphere containing 5% CO2 at 37°C. The
medium was replaced every 3 days. When 80–90% confluence was
reached in the 25 mm culture bottle, cells were seeded in 96-well
plates at a density of 3,000 cells/well, in 24-well plates at a
density of 5,000 cells/well or in 6-well plates at a density of
1×105 cells/well for different assays.
The effect of icariin on cell proliferation was
evaluated using the CCK-8 assay. Briefly, MC3T3-E1 cells were
plated in 96-well plates (3,000 cells/well) and treated with
different concentrations of icariin (0.001–100 µM) for 48 h.
Subsequently, the cell numbers were assessed using CCK-8 according
to the manufacturer's instructions. The results were presented as
the absorbance at 450 nm recorded by a multifunctional microplate
reader (ELx800; BioTek Instruments, Inc., Winooski, VT, USA).
Mineral content and ALP activity
assay
The mineral content of cells was quantitatively
assessed using an alizarin red S assay (31). Briefly, cells were plated in 24-well
plates at 5,000 cells/well. According to the results of the CCK-8
assay, the doses of 0.1, 1 and 10 µM icariin were selected as the
effective concentrations. After 14 days of icariin treatment, cells
were fixed in 4% paraformaldehyde for 24 h at 4°C. Next,
paraformaldehyde was removed from the wells and cells were washed
with PBS and dH2O for three times. Subsequently, 0.04 M
alizarin red S was used to stain the cells for 30 min at room
temperature while shaking. Cells were then washed with
dH2O for three times, and precipitated alizarin red S
was extracted by the addition of 10% cetylpyridinium chloride for 1
h at room temperature. The mineral content was quantified by
measuring the optical density at 550 nm.
MC3T3-E1 cells were seeded into 24-well plates
(three replicate wells per group) at a density of 5,000 cells/well
and the doses of 0.1, 1 and 10 µM icariin were selected as the
effective concentrations. ALP activity in the various groups was
assayed at 3, 10 and 14 days of treatment at a wavelength of 520
nm, according to the manufacturer's protocol of the kit.
Transfection, miRNA target site
prediction and luciferase reporter assays
In order to promote or inhibit the miR-153 activity,
the synthetic miR-153 mimics (sense, 5′-UUGCAUAGUCACAAAAGUGAUC-3′
and antisense, 5′-GAUCACUUUUGUGACUAUGCAA-3′) and miR-153 inhibitors
(5′-GAUCACUUUUGUGACUAUGCAA-3′; both Biomics Biotechnologies Co.,
Ltd., Nantong, China) were transfected into 1 µM icariin treated
MC3T3-E1 cultures using Lipofectamine 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.). Negative controls [transfected with
negative mimics (sense, 5′-UCACAACCUCCUAGAAAGAGUAGA-3′ and
antisense, 5′-UCUACUCUUUCUAGGAGGUUGUGA-3′) or negative inhibitors
(5′-UCUACUCUUUCUAGGAGGUUGUGA-3′) in 1 µM icariin treated MC3T3-E1
cultures] were used in all reactions. The final concentration of
the mimics and inhibitors was 100 nM. Subsequent experiments were
performed 3 days after transfection.
The online software TargetScan (release 6.2;
http://www.targetscan.org) and miRanda
(http://www.microrna.org/microrna/home.do) were used to
predict the miR-153 targets. The sequence was inserted into the
psi-CHECK2 vector (Promega Corp., Madison, WI, USA) within
XhoI and NotI restriction sites. Mutation of the
miR-153 binding sites was introduced by a fast mutation kit (New
England Biolabs, Ipswich, MA, USA). MC3T3-E1 cells were
co-transfected with psi-CHECK2-Runx2 3′UTR Wt or psi-CHECK2-Runx2
3′UTR mutant (Mut), and the miR-153 or negative control mimics at a
final concentration of 100 nM. After 48 h of transfection, the
luciferase activity was determined according to the manufacturer's
recommended protocols of the Dual-Luciferase Reporter Assay System
(Promega Corp.).
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
According to the manufacturer's instructions, total
RNA was extracted with TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and was quantitatively measured by
Quant-iT™ RNA HS reagent (Thermo Fisher Scientific,
Inc.). Complementary (c)DNA was synthesized from total RNA using
PrimeScript RT reagent kit (Perfect Real Time; Takara Bio, Inc.,
Tokyo, Japan). The mRNA expression in the cells was quantified by
qPCR using the SYBR-Green/ROX qPCRMaster Mix (Takara Bio, Inc.) and
the StepOnePlus Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The following primers were used: Runx2
forward, 5′-AGCGGACGAGGCAAGAGTTT-3′, and reverse,
5′-AGGCGGGACACCTACTCTCATA-3′; ALP forward,
5′-AGCGGACGAGGCAAGAGTTT-3′ and reverse,
5′-AGGCGGGACACCTACTCTCATA-3′; BMP2 forward,
5′-AACGAGAAAAGCGTCAAGCC-3′ and reverse, 5′-AGGTGCCACGATCCAGTCAT-3′;
BMP4 forward, 5′-AACTGCCGTCGCCATTCACT-3′ and reverse,
5′-TCAACACCACCTTGTCATACTCAT-3′; OPN forward,
5′-CCCTCCCGAGTAAGTCCAAT-3′ and reverse, 5′-ACACTATCACCTCGGCCATC-3′;
U6 forward, 5′-CGCTTCACGAATTTGCGTGTCAT-3′ and reverse,
5′-GCTTCGGCAGCACATATACTAAAAT-3′; GAPDH forward,
5′-ACCACAGTCCATGCCATCAC-3′ and reverse, 5′-TCCACCCTGTTGCTGTA-3′.
Subsequent to denaturing the DNA templates at 95°C for 10 min, the
reactions were followed by 40 cycles at 95°C for 15 sec, 60°C for
20 sec and 75°C for 10 sec. The expression of Runx2 was normalized
to GAPDH, and the miR-153 level was normalized to U6. Relative
expression levels were determined using the 2−ΔΔCq
method (32).
Western blot analysis
Cells were lysed in RIPA buffer (Sigma Aldrich;
Merck KGaA) to extract the protein, the protein concentration was
quantitative measured by Pierce™BCA Protein Assay kit
(Thermo Fisher Scientific, Inc.). A total of 20–30 µg/lane of
protein was loaded onto 10% SDS-PAGE gels, and then transferred
onto PVDF membranes. After blocking in Tris-buffered
saline/Tween-20 containing 5% fat-free milk for 1 h at room
temperature, the membranes were probed overnight at 4°C with the
following monoclonal primary antibodies: Anti-GAPDH (1:10,000),
anti-Runx2 (1:10,000), anti-ALP (1:1,000), anti-BMP2 (1:1,000),
anti-BMP4 (1:1,000) and anti-OPN (1:10,000) antibodies. After
washing by TBST three times, the membranes were incubated with
horseradish peroxide-conjugated secondary antibodies (1:10,000;
ab97023; Abcam) for 1 h at room temperature. GAPDH was used as a
loading control. The protein bands were visualized and detected
using an ECL system (Pierce; Thermo Fisher Scientific, Inc.).
Statistical analysis
Data was presented as the mean ± standard deviation.
Comparisons between groups were analyzed by paired-sample t-test or
analysis of variance using SPSS version 17.0 software (SPSS, Inc.,
Chicago, IL, USA). A P<0.05 value was considered to indicate a
difference that was statistically significant.
Results
Effect of icariin on proliferation,
ALP activity and mineral deposition in MC3T3-E1 cells
The present study first examined the individual
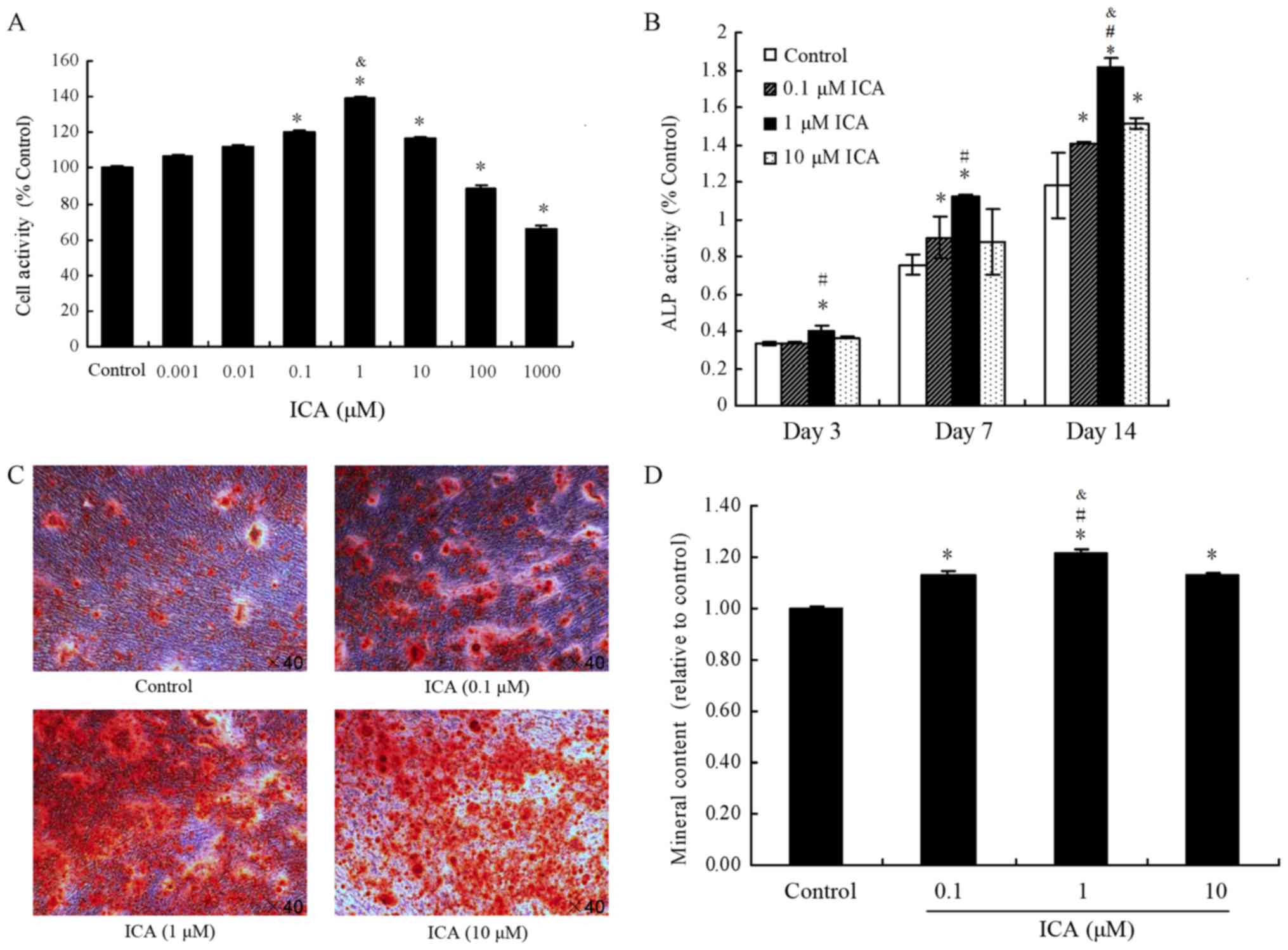
effects of 0.001, 0.01, 0.1, 1, 10, 100 and 1,000 µM icariin on the
proliferation activity of MC3T3-E1 cells by CCK-8 assay (Fig. 1A). Icariin concentrations of 100 and
1,000 µM were found to significantly decrease cell proliferation
compared with the control group (P<0.05). In contrast to the
control group, icariin at concentrations of 0.1, 1 and 10 µM
significantly increased cell proliferation (P<0.05). Finally,
icariin at 0.01 and 0.001 µM increased cell proliferation, but the
difference with not statistically significant (P>0.05). These
results indicated that icariin promoted proliferation at 0.1, 1 and
10 µM in MC3T3-E1 cells, particularly when used at a concentration
of 1 µM. Thus, these concentrations were used in subsequent
experiments.
ALP activity was used as an osteoblast
differentiation marker in MC3T3-E1 cells. As shown in Fig. 1B, following treatment of MC3T3-E1
cells with 0.1, 1 and 10 µM icariin, ALP activity was increased by
0.12%, 19.93% (P<0.05) and 8.3%, respectively, at day 3 in
comparison with the control group. At day 10, the ALP activity was
increased by 19.52% (P<0.05), 48.57% (P<0.05) and 16.1%,
respectively, while at day 14, it was enhanced by 19.39%, 53.94%
and 28.25% (all P<0.05), respectively. These results indicated
that icariin promoted ALP activity at concentrations of 1 and 10 µM
in MC3T3-E1 cells, particularly upon treatment with 1 µM icariin
(Fig. 1B).
According to the cell proliferation activity tests,
0.1, 1 and 10 µM icariin concentrations were selected for
examination of mineralization in MC3T3-E1 cells by alizarin red S
staining (Fig. 1C and D). In
contrast to the control group, icariin (0.1, 1 and 10 µM) resulted
in significantly higher mineral content (P<0.05). These results
indicated that icariin enhanced the deposition of calcium on
nodules formed in MC3T3-E1 cells.
Effect of icariin on the expression
levels of osteoblast differentiation-associated genes in MC3T3-E1
cells
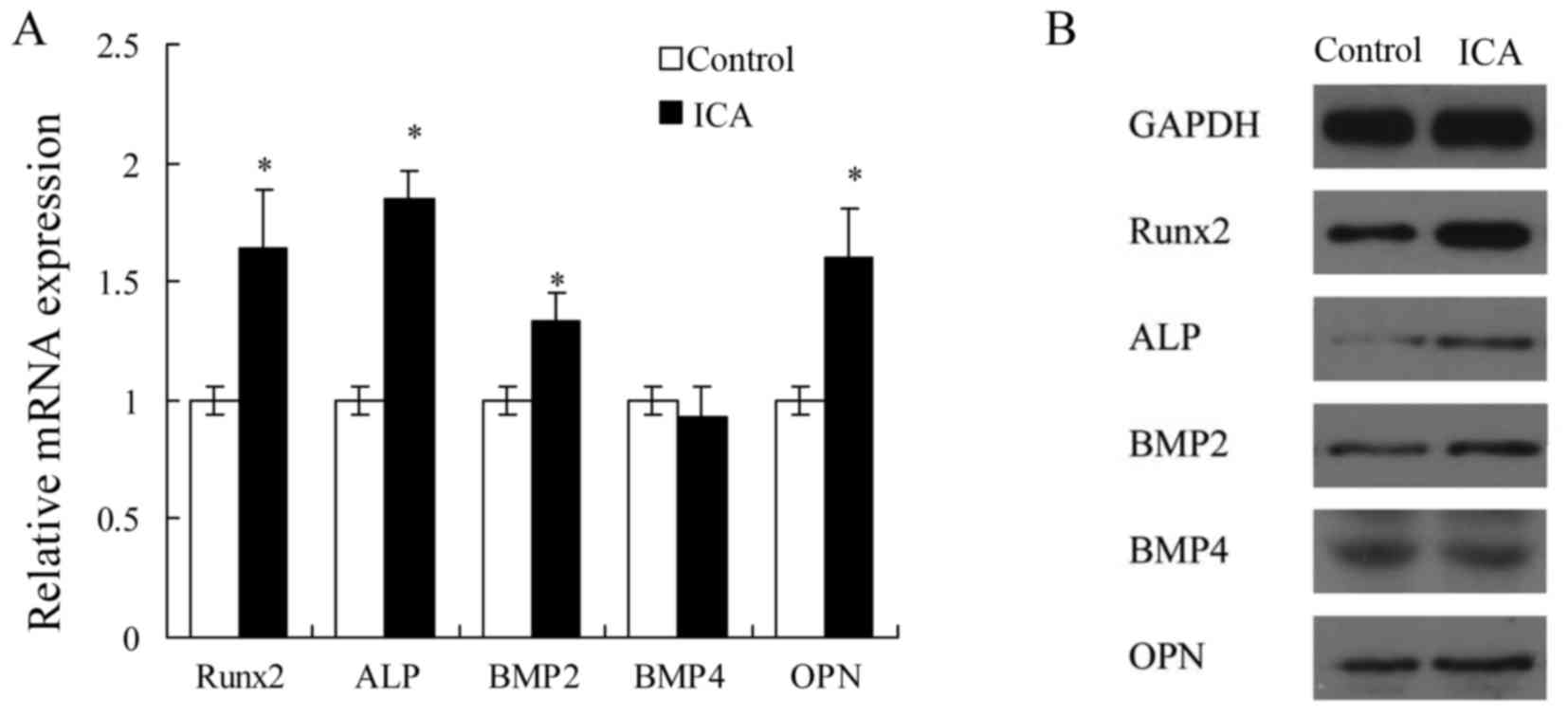
According to the results of cell proliferation,
alizarin red S staining and ALP activity assays, icariin at 1 µM
was selected to examine the effect of treatment on the mRNA and
protein expression levels of osteoblast differentiation-associated
genes in MC3T3-E1 cells. Treatment with 1 µM icariin evidently
increased the mRNA expression levels of Runx2, ALP, BMP2 and OPN
(P<0.05; Fig. 2A), and the
protein expression levels of Runx2 and ALP (Fig. 2B). These results suggested that
icariin may promote the early stage of osteoblast differentiation
in MC3T3-E1 cells.
Icariin increases miR-153 expression
in MC3T3-E1 cells, and Runx2 is positively targeted by miR-153
To determine whether the expression of miR-153 was
correlated with icariin treatment, the expression level of miR-153
in MC3T3-E1 cells was evaluated. As shown in Fig. 3A, the relative expression of miR-153
in MC3T3-E1 cells was significantly increased following treatment
with icariin at concentrations of 0.1, 1 and 10 µM for 14 days, as
compared with the control group. However, there was no
significantly difference among the three icariin treatment groups.
Next, miR-153 mimics (100 nM) or miR-153 inhibitor (100 nM) was
transfected into the MC3T3-E1 cells with Lipofectamine 2000. As
observed earlier, a significant increase in miR-153 expression was
observed in the icariin (1 µM) treatment group. Notably, icariin
enhanced miR-153 expression following transfection with an miR-153
mimics (Fig. 3B), however, it did
not increase the expression of miR-153 following transfection with
an miR-153 inhibitor (Fig. 3C).
These results indicated that icariin promoted miR-153 expression in
MC3T3-E1 cells.
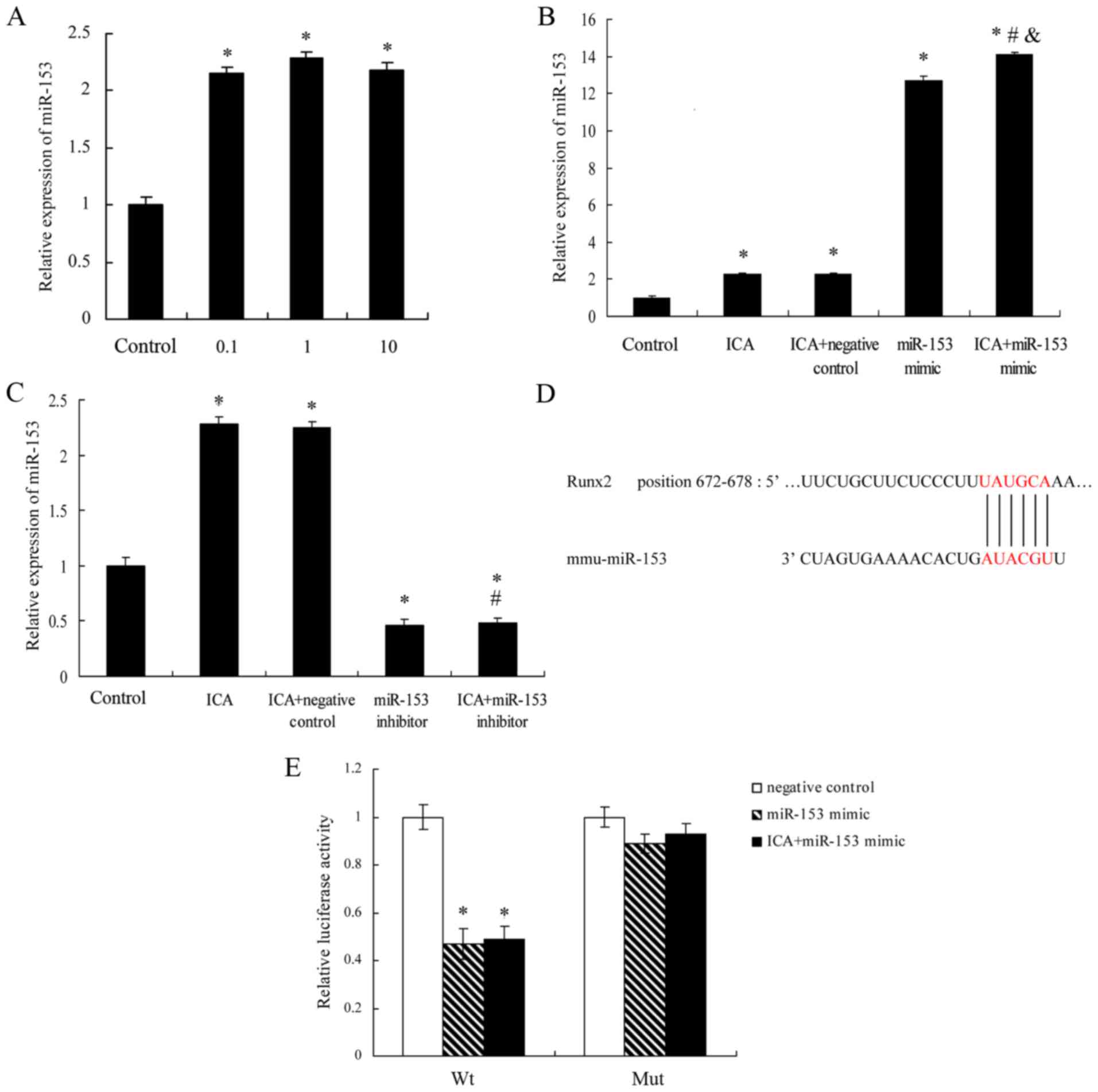 | Figure 3.ICA treatment increased miR-153
expression in MC3T3-E1 cells. The effect of ICA on the expression
of miR-153 was measured by reverse transcription-quantitative
polymerase chain reaction analysis in various groups of MC3T3-E1
cells (A) treated with ICA (0.1, 1 and 10 µM), (B) treated with 1
µM ICA and transfected with miR-153 mimics (100 nM) or negative
control, and (C) treated with 1 µMICA and transfected with miR-153
inhibitor (100 nM) or negative controlfor 14 days. Results were
obtained from three independent experiments and are expressed as
the mean ± standard error. *P<0.05 vs. control group;
#P<0.05 vs. ICA + negative control group;
&P<0.05 vs. miR-153 mimic group. (D) Schematic
diagram of miR-153 target site in the 3′-UTR of Runx2 mRNA. (E)
psiCHECK2-Runx2 3′-UTR was co-transfected with miR-153 mimic
negative control, miR-153 mimic or miR-153 mimic with ICA (1 µM)
treatment in MC3T3-E1 cells. After 48 h of transfection, luciferase
activities were measured. *P<0.05 vs. negative control group.
ICA, icariin; Runx2, Runt-related transcription factor 2; miR,
microRNA; 3′-UTR, 3′-untranslated region; Wt, wild-type; Mut,
mutant. |
To define the possible mechanisms by which miR-153
affects proliferation and osteoblast differentiation in MC3T3-E1
cells, the miRNA target analysis tools TargetScan (release 6.2) and
miRanda were used to determine the possible target genes of
miR-153. Based on the results using these two software programs,
Runx2 was predicted to be a target of miR-153 and selected for
further investigation. Subsequently, 3′UTR reporter plasmids
containing the 3′UTR of Runx2 with the predicted miR-153 3′UTR
sites of Runx2 were generated (Fig.
3D). The results of luciferase assay suggested that miR-153
significantly upregulated the luciferase activity of Wt, but of not
Mut, Runx2 3′UTR in MC3T3-E1 cells compared with the negative
control (Fig. 3E).
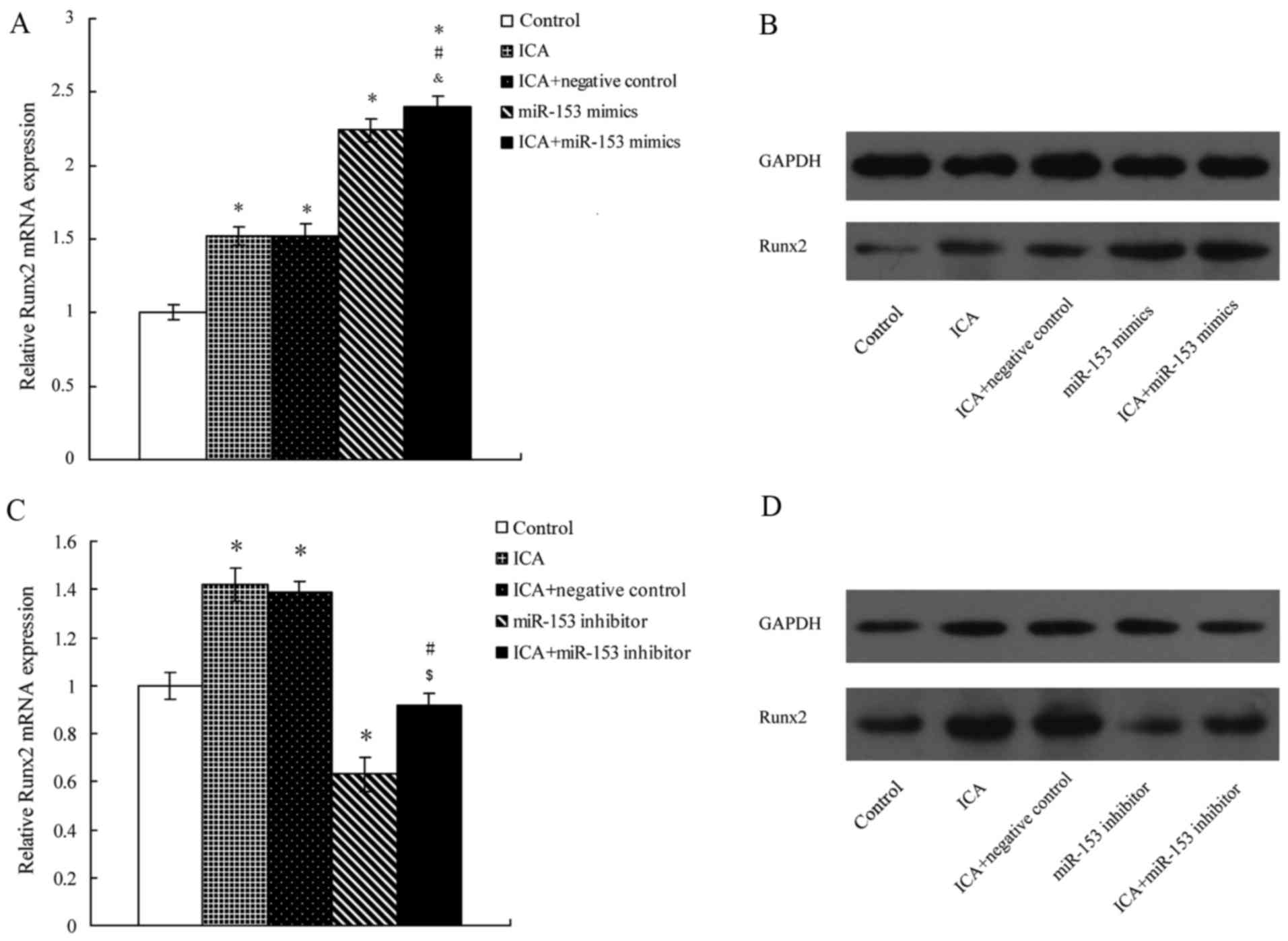
Transfection of miR-153 mimic markedly
increases Runx2 expression in icariin-treated MC3T3-E1 cells
The potential target protein of miR-153, namely
Runx2, was selected to study the effect of icariin on MC3T3-E1
cells. The miR-153 mimic was transfected into the MC3T3-E1 cells
using Lipofectamine 2000, and the results demonstrated that icariin
treatment or miR-153 mimic transfection significantly increased the
expression level of Runx2 in the MC3T3-E1 cells (Fig. 4A). Furthermore, the combination of
miR-153 mimic and icariin group had a significantly higher
expression of Runx2 as compared with the miR-153 mimic alone group.
The western blot analysis presented similar results (Fig. 4B).
Transfection with miR-153 inhibitor
affects the expression of Runx2 protein in icariin-treated MC3T3-E1
cells
To further investigate the potential connection
between knockdown of miR-153 and the potential effect of icariin
treatment on MC3T3-E1 cells, an miR-153 inhibitor was transfected
into the MC3T3-E1 cells using Lipofectamine 2000. The results
indicated that transfection with the miR-153 inhibitor
significantly decreased Runx2 expression in MC3T3-E1 cells when
compared with the control group (Fig.
4C). However, icariin treatment reversed the potential effect
of the miR-153 inhibitor on MC3T3-E1 cells, as the expression level
of Runx2 in the miR-153 inhibitor with icariin treatment group was
significantly higher in comparison with the miR-153 inhibitor alone
group. Similar results were observed by western blot analysis
(Fig. 4D).
Discussion
There is increasing interest in investigating the
efficacy of icariin as a potential alternative therapy for
improving bone formation, preventing bone loss and treating
osteoporosis. In the present study, it was observed that icariin
improved osteoblast differentiation and cell proliferation of
MC3T3-E1 cells, as demonstrated by CCK-8, mineral deposition and
ALP activity assays, as well as by stimulating the gene expression
of Runx2. In addition, the current study demonstrated that icariin
regulated Runx2 expression through miR-153 in MC3T3-E1 cells.
The present study demonstrated that treatment of
MC3T3-E1 cells with icariin at concentrations of 0.1, 1 and 10 µM
significantly increased cell proliferation and differentiation.
However, higher concentrations of icariin may have a negative
effect on cell proliferation, while low concentrations had a
positive effect. Numerous studies have indicated that icariin at
different concentrations enhanced cell proliferation and osteoblast
differentiation. For instance, Mok et al (33) observed that icariin significantly
enhanced the proliferation at concentrations ranging between
10−14 and 10−6 M in UMR-106 cells. A
dose-dependent study by Ma et al (34) on osteoblast differentiation measuring
ALP activity revealed that the optimal concentration of icariin for
stimulating osteogenesis was 10−5 M in ROB cells. In
addition, a recently study suggested that icariin stimulated the
differentiation of primary osteoblasts at final concentrations of
0.1–10 µM (35). These findings were
generally in agreement with the results of the present study.
According to the observed results of the cell proliferation
activity test, alizarin red S staining and ALP activity assay, 1 µM
icariin was selected as the final concentration to further examine
the effect on mRNA and protein expression levels of osteoblast
differentiation-associated genes and to investigate the underlying
mechanism of action in MC3T3-E1 cells.
Several studies have reported that icariin increases
mineral deposition and ALP activity in multiple cells and animal
studies. For instance, Mok et al (33) observed that icariin stimulated ALP
activity in a dose-dependent manner in rat osteoblast-like UMR-106
cells. Furthermore, Ma et al (34) identified that icariin had a stronger
ability in improving ALP activity, calcium deposition and the
number of mineralized bone nodules in rat calvarial osteoblasts
(ROB cells). Li et al (36)
further reported that the level of serum ALP was higher in
ovariectomized rats treated with icariin in comparison with the
controls. The present study results were consistent with these
aforementioned studies. In addition, the present data revealed that
icariin increased miR-153, as well as Runx2 mRNA expression, in
MC3T3-E1 cells. These results suggested that icariin may stimulate
the process of osteogenesis by modulating the expression of Runx2
in MC3T3-E1 cells. However, the signaling pathway involved in the
osteoblast differentiation effects of icariin remains unclear.
miRNAs contribute to osteogenesis in the embryonic
bone development, while they are also involved in the maintenance
of adult bone tissue by regulating the growth, differentiation and
functional activity of cells (37).
miR-153 has been reported to facilitate the cell proliferation in
multiple cancer cells by suppressing important cancer-relevant
genes, such as AKT (38), CACNA1C
(39) and WWOX (40). Similarly, the present study indicated
that miR-153 improved osteoblast differentiation and cell
proliferation in MC3T3-E1 cells, while icariin was demonstrated to
increase the miR-153 expression level in these cells. To the best
of our knowledge, the present study is the first to investigate the
role of miR-153 in the osteogenesis effects of icariin on MC3T3-E1
cells. However, the role of miR-153 in regulating the osteoblast
differentiation remains unclear. In the current study, a new role
of miR-153 in the regulation of osteoblast differentiation in
MC3T3-E1 cells was detected. Luciferase activity assay combined
with RT-qPCR and western blot analysis demonstrated that Runx2 was
a direct target of miR-153. These results indicated that miR-153
upregulated the mRNA expression and protein levels of Runx2 during
the osteoblast differentiation in MC3T3-E1 cells. However, the role
of miRNA-Runx2 regulation in osteoporosis requires further
clarification.
Recent studies have shown that icariin stimulates
the expression of Runx2 by modulating different signal transduction
pathways, including the estrogen receptor-mediated pathway
(35), BMP-2/Smad4 signal
transduction pathway (41) and BMP
signaling pathway (30). These
studies suggested that icariin stimulated osteoblast
differentiation and maturation through multiple pathways. The
present study demonstrated that icariin regulated Runx2 expression
through miR-153 in MC3T3-E1 cells. Notably, the expression of Runx2
was stimulated by icariin following transfection with miR-153
inhibitor in MC3T3-E1 cells (Fig. 4C and
D), which indicated that icariin may also regulate the
osteoblast differentiation and cell proliferation of MC3T3-E1 cells
through other mechanisms. However, the particular mechanisms of how
icariin influenced miR-153 expression remain unclear, and further
studies are required.
In conclusion, icariin is an active constituent of
the herb Epimedium, a Chinese herb commonly used for the
prevention and treatment of osteoporosis in traditional Chinese
medicine formulas. The present study clearly demonstrated that
icariin had a strong osteoblast differentiation effect in MC3T3-E1
cells through the miR-153/Runx2 pathway. Therefore, the current
study provided evidence that icariin should be consider as an
effective candidate for the management of osteoporosis. Further
studies are needed in order to investigate the underlying molecular
mechanisms for bone mass preservation and bone loss prevention
in vivo.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. NSFC-81201413 and
NSFC-81371973).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
ZH, SY and XW designed the study. CC, JW, XL, HW and
YH conducted the experiments. ZH and CC analyzed the data. ZH, SY
and XW contributed to the writing of the manuscript. All authors
reviewed the manuscript.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhao LJ, Liu XG, Liu YZ, Liu YJ, Papasian
CJ, Sha BY, Pan F, Guo YF, Wang L, Yan H, et al: Genome-wide
association study for femoral neck bone geometry. J Bone Miner Res.
25:320–329. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
An J, Yang H, Zhang Q, Liu C, Zhao J,
Zhang L and Chen B: Natural products for treatment of osteoporosis:
The effects and mechanisms on promoting osteoblast-mediated bone
formation. Life Sci. 147:46–58. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu YJ, Shen H, Xiao P, Xiong DH, Li LH,
Recker RR and Deng HW: Molecular genetic studies of gene
identification for osteoporosis: A 2004 update. J Bone Miner Res.
21:1511–1535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Piscitelli P, Iolascon G, Gimigliano F,
Muratore M, Camboa P, Borgia O, Forcina B, Fitto F, Robaud V,
Termini G, et al: Incidence and costs of hip fractures compared to
acute myocardial infarction in the Italian population: A 4-year
survey. Osteoporos Int. 18:211–219. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Reginster JY and Burlet N: Osteoporosis: A
still increasing prevalence. Bone. 38 2 Suppl 1:4–9. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Budhia S, Mikyas Y, Tang M and Badamgarav
E: Osteoporotic fractures: A systematic review of U.S. healthcare
costs and resource utilization. Pharmacoeconomics. 30:147–170.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Du F, Ye W, Chen Y, Li J, Zhang J,
Nicely H and Burge R: Inpatient cost of treating osteoporotic
fractures in mainland China: A descriptive analysis. Clinicoecon
Outcomes Res. 7:205–212. 2015.PubMed/NCBI
|
|
8
|
Chan DC, Lee YS, Wu YJ, Tsou HH, Chen CT,
Hwang JS, Tsai KS and Yang RS: A 12-year ecological study of hip
fracture rates among older Taiwanese adults. Calcif Tissue Int.
93:397–404. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Melton LJ 3rd, Chrischilles EA, Cooper C,
Lane AW and Riggs BL: Perspective. How many women have
osteoporosis? J Bone Miner Res. 7:1005–1010. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burge R, Dawson-Hughes B, Solomon DH, Wong
JB, King A and Tosteson A: Incidence and economic burden of
osteoporosis-related fractures in the United States, 2005–2025. J
Bone Miner Res. 22:465–475. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ahmed FE, Ahmed NC, Vos PW, Bonnerup C,
Atkins JN, Casey M, Nuovo GJ, Naziri W, Wiley JE, Mota H, et al:
Diagnostic microRNA markers to screen for sporadic human colon
cancer in stool: I. Proof of principle. Cancer Genomics Proteomics.
10:93–113. 2013.PubMed/NCBI
|
|
12
|
van Wijnen AJ, van de Peppel J, van
Leeuwen JP, Lian JB, Stein GS, Westendorf JJ, Oursler MJ, Im HJ,
Taipaleenmäki H, Hesse E, Riester S, et al: MicroRNA functions in
osteogenesis and dysfunctions in osteoporosis. Curr Osteoporos Rep.
11:72–82. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamasaki K, Nakasa T, Miyaki S, Yamasaki
T, Yasunaga Y and Ochi M: Angiogenic microRNA-210 is present in
cells surrounding osteonecrosis. J Orthop Res. 30:1263–1270. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ukai T, Sato M, Akutsu H, Umezawa A and
Mochida J: MicroRNA-199a-3p, microRNA-193b, and microRNA-320c are
correlated to aging and regulate human cartilage metabolism. J
Orthop Res. 30:1915–1922. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lagos-Quintana M, Rauhut R, Lendeckel W
and Tuschl T: Identification of novel genes coding for small
expressed RNAs. Science. 294:853–858. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lau NC, Lim LP, Weinstein EG and Bartel
DP: An abundant class of tiny RNAs with probable regulatory roles
in Caenorhabditis elegans. Science. 294:858–862. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee RC and Ambros V: An extensive class of
small RNAs in Caenorhabditis elegans. Science. 294:862–864.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duan Z, Choy E, Nielsen GP, Rosenberg A,
Iafrate J, Yang C, Schwab J, Mankin H, Xavier R and Hornicek FJ:
Differential expression of microRNA (miRNA) in chordoma reveals a
role for miRNA-1 in Met expression. J Orthop Res. 28:746–752.
2010.PubMed/NCBI
|
|
19
|
Dong S, Yang B, Guo H and Kang F:
MicroRNAs regulate osteogenesis and chondrogenesis. Biochem Biophys
Res Commun. 418:587–591. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li H, Xie H, Liu W, Hu R, Huang B, Tan YF,
Xu K, Sheng ZF, Zhou HD, Wu XP, et al: A novel microRNA targeting
HDAC5 regulates osteoblast differentiation in mice and contributes
to primary osteoporosis in humans. J Clin Invest. 119:3666–3677.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guo J, Ren F, Wang Y, Li S, Gao Z, Wang X,
Ning H, Wu J, Li Y, Wang Z, et al: miR-764-5p promotes osteoblast
differentiation through inhibition of CHIP/STUB1 expression. J Bone
Miner Res. 27:1607–1618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gao J, Yang T, Han J, Yan K, Qiu X, Zhou
Y, Fan Q and Ma B: MicroRNA expression during osteogenic
differentiation of human multipotent mesenchymal stromal cells from
bone marrow. J Cell Biochem. 112:1844–1856. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Niu G, Li B, Sun L and An C: MicroRNA-153
inhibits osteosarcoma cells proliferation and invasion by targeting
TGF-β2. PLoS One. 10:e01192252015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu Z, He B, He J and Mao X: Upregulation
of miR-153 promotes cell proliferation via downregulation of the
PTEN tumor suppressor gene in human prostate cancer. Prostate.
73:596–604. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xie L, Zhang Z, Tan Z, He R, Zeng X, Xie
Y, Li S, Tang G, Tang H and He X: MicroRNA-124 inhibits
proliferation and induces apoptosis by directly repressing EZH2 in
gastric cancer. Mol Cell Biochem. 392:153–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Song L, Duan P, Guo P, Li D, Li S, Xu Y
and Zhou Q: Downregulation of miR-223 and miR-153 mediates
mechanical stretch-stimulated proliferation of venous smooth muscle
cells via activation of the insulin-like growth factor-1 receptor.
Arch Biochem Biophys. 528:204–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ming LG, Chen KM and Xian CJ: Functions
and action mechanisms of flavonoids genistein and icariin in
regulating bone remodeling. J Cell Physiol. 228:513–521. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang J, Yuan L, Wang X, Zhang TL and Wang
K: Icaritin and its glycosides enhance osteoblastic, but suppress
osteoclastic, differentiation and activity in vitro. Life
Sci. 81:832–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Nian H, Ma MH, Nian SS and Xu LL:
Antiosteoporotic activity of icariin in ovariectomized rats.
Phytomedicine. 16:320–326. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhao J, Ohba S, Shinkai M, Chung UI and
Nagamune T: Icariin induces osteogenic differentiation in
vitro in a BMP- and Runx2-dependent manner. Biochem Biophys Res
Commun. 369:444–448. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nash LA, Peters SJ, Sullivan PJ and Ward
WE: Supraphysiological levels of quercetin glycosides are required
to alter mineralization in Saos2 cells. Int J Environ Res Public
Health. 13:2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mok SK, Chen WF, Lai WP, Leung PC, Wang
XL, Yao XS and Wong MS: Icariin protects against bone loss induced
by oestrogen deficiency and activates oestrogen receptor-dependent
osteoblastic functions in UMR 106 cells. Br J Pharmacol.
159:939–949. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma HP, Ming LG, Ge BF, Zhai YK, Song P,
Xian CJ and Chen KM: Icariin is more potent than genistein in
promoting osteoblast differentiation and mineralization in
vitro. J Cell Biochem. 112:916–923. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang D, Fong C, Jia Z, Cui L, Yao X and
Yang M: Icariin stimulates differentiation and suppresses
adipocytic transdifferentiation of primary osteoblasts through
estrogen receptor-mediated pathway. Calcif Tissue Int. 187–198.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li GW, Xu Z, Chang SX, Nian H, Wang XY and
Qin LD: Icariin prevents ovariectomy-induced bone loss and lowers
marrow adipogenesis. Menopause. 21:1007–1016. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Meng YB, Li X, Li ZY, Zhao J, Yuan XB, Ren
Y, Cui ZD, Liu YD and Yang XJ: microRNA-21 promotes osteogenic
differentiation of mesenchymal stem cells by the PI3K/β-catenin
pathway. J Orthop Res. 33:957–964. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yuan Y, Du W, Wang Y, Xu C, Wang J, Zhang
Y, Wang H, Ju J, Zhao L, Wang Z, et al: Suppression of AKT
expression by miR-153 produced anti-tumor activity in lung cancer.
Int J Cancer. 136:1333–1340. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu H, Abuhatzira L, Carmona GN, Vadrevu S,
Satin LS and Notkins AL: The Ia-2β intronic miRNA, miR-153, is a
negative regulator of insulin and dopamine secretion through its
effect on the Cacna1c gene in mice. Diabetologia. 58:2298–2306.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hua HW, Jiang F, Huang Q, Liao Z and Ding
G: MicroRNA-153 promotes Wnt/β-catenin activation in hepatocellular
carcinoma through suppression of WWOX. Oncotarget. 6:3840–3847.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Liang W, Lin M, Li X, Li C, Gao B, Gan H,
Yang Z, Lin X, Liao L and Yang M: Icariin promotes bone formation
via the BMP-2/Smad4 signal transduction pathway in the hFOB 1.19
human osteoblastic cell line. Int J Mol Med. 30:889–895. 2012.
View Article : Google Scholar : PubMed/NCBI
|