Introduction
Preeclampsia is a hypertensive disorder of pregnancy
that is characterized by systolic blood pressure (SBP) ≥140 mmHg or
diastolic BP ≥90 mmHg, as well as proteinuria (≥0.3 g/24 h) after
the 20th week of pregnancy (1–4). It is a
multisystem syndrome, and the maternal and neonatal morbidity in
the setting of preeclampsia is significantly greater than that
associated with normal pregnancies (5,6).
Animal models are effective tools for studying the
pathogenesis, diagnostic criteria and treatment methods of
preeclampsia. It is well known that nitric oxide (NO) in vascular
endothelial cells is critical for regulating vascular tension.
During normal pregnancy, the cardiac output, blood volume and heart
rate are increased; however, the BP typically remains normal or is
slightly lower than that prior to pregnancy (7). NO has an important role in the
regulation of the cardiovascular system during pregnancy (7). N-nitro-L-arginine methyl ester (L-NAME)
is an inhibitor of NO synthase (NOS), and inhibition of NOS has
been demonstrated to alleviate hypertension in pregnant rats as
well as maintain normal BP levels until delivery (8). Since preeclampsia is associated with
vascular endothelial disorders and excessive inflammation (9), it is important to construct a
preeclampsia-like animal model that mimics the vascular pathology
of preeclampsia to better understand its onset and progression.
It has been proposed that the development of
preeclampsia is likely associated with placenta formation (10), particularly with placental
dysfunction in early pregnancy (6,11,12).
Although the exact mechanisms of preeclampsia remain elusive,
vascular dysfunction, oxidative stress and metabolic abnormalities
are possible causes of maternal preeclampsia syndrome. In addition,
vasoactive substances are considered to be another pathogenic
factor for the development of preeclampsia (13). Hypoxia caused by the lack of blood
flow in the placenta may induce the release of various vasoactive
substances, including anti-angiogenic factors (14). Furthermore, a previous study
indicated that abnormalities in circulating angiogenic factors have
an important role in the pathogenesis of preeclampsia (15). To date, a large number of clinical
studies and experimental studies have provided strong evidence that
the soluble fms-like tyrosine kinase-1 (sFlt-1)/placental growth
factor (PLGF) ratio may be used as a predictor of preeclampsia,
particularly early-onset preeclampsia (16). Therefore, the present study aimed to
establish an L-NAME-induced rat model of preeclampsia and evaluate
vascular injury, oxidative stress and the circulating vasoactive
substances in the rats in order to confirm the feasibility of their
use as a model of preeclampsia.
Materials and methods
Reagents
N-nitro-L-arginine methyl ester (L-NAME) was
purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). The
bicinchoninic acid (BCA) protein assay kit was acquired from Thermo
Fisher Scientific (Waltham, MA, USA). PLGF, sFlt-1,
8-iso-prostaglandin F2α (8-iso-PGF2α) and MDA activity was measured
using a commercial kit from Jiancheng Institute (cat. no. A003-1;
Nanjing, China). Levels of common inflammatory markers were
measured using a cytometric beads assay (LEGENDplex™ Rat
Panel; BioLegend, San Diego, CA, USA).
Animals
A total of 70 female SD rats (weight, 200–220 g;
age, 6–7 weeks) were bred at the Laboratory Animal Center of the
Academy of Military Medical Sciences of Beijing People's Liberation
Army (Beijing, China). Respective parental rats were not subjected
to any experiments or treatments prior to breeding. The animals
were individually housed at 24°C with a humidity of 55±10% under a
12 h light/dark cycle and were provided regular chow and water at
libitum. At the age of 8 weeks, the female rats were pair-housed
with a male rat. On the morning after mating, clean and wet cotton
swabs (rinsed with physiological saline) were gently inserted into
the vagina of female rats rotated twice to obtain the vaginal
secretions, which were smeared on clean and dry slides and observed
under an optical microscope. The presence of sperm in the vaginal
smears was indicative of gestational day 0 (GD 0). Rats were
randomly assigned to 7 groups, with dosages selected according to a
previous study (17): Control rats;
rats treated with low-dose L-NAME (40 mg/kg body weight/day)
starting from GD 9 (9D LL); rats treated with medium-dose L-NAME
(75 mg/kg body weight/day; selected according to a pervious study)
(18) starting from GD 9 (9D ML);
rats treated with high-dose L-NAME (125 mg/kg body weight/day)
starting from GD 9 (9D HL); rats treated with low-dose L-NAME (40
mg/kg body weight/day) starting from GD 10 (10D LL); rats treated
with medium-dose L-NAME (75 mg/kg body weight/day) starting from GD
10 (10D ML); and rats treated with high-dose L-NAME (125 mg/kg body
weight/day) starting from GD 10 (10D HL). Pregnant rats were
administered different doses of L-NAME starting on the ninth or
tenth day of gestation via subcutaneous injection on the neck back.
All treatments were maintained until GD 20. The combination of
different dosages and time-points is the core of the present
experiment. Multiple injections were applied to maintain the drug
effect of L-NAME, resulting in a persistent high BP of pregnant
rats and other physiological changes, including abnormal urine
protein and endothelial dysfunction (17,19). All
experimental animals were euthanized on GD 20.
Ethics statements
The experiments were approved by the ethics
committee of the Investigation of Logistics University of the
Chinese People's Armed Police Force (Tianjin, China), and the
treatment of all 70 animals was compliant with the Public Health
Service Policy on the Humane Care and Use of Laboratory Animals of
the National Institutes of Health.
Measurement of BP
BP was non-invasively measured during pregnancy on
GD 0, 6, 12 and 18 by determining the tail blood volume with a
volume pressure-recording sensor and an occlusion tail cuff (CODA
System; Kent Scientific, Torrington, CT, USA) (19).
Urine protein quantitation
Rats were placed in metabolic cages for 24 h for
urine collection at baseline (prior to mating) and on GD19. The
24-h urine protein in each group was measured with a BCA protein
assay kit (Thermo Fisher Scientific, Inc.).
Tissue collection
Maternal blood was collected on GD 20 by abdominal
aorta puncture after the rats were anesthetized with 3%
intraperitoneal pentobarbital sodium (50 mg/kg). Animals were then
sacrificed via exsanguination, which was confirmed following
following a complete non-autonomous response to external stimuli.
Plasma was prepared by centrifugation at 1,006.2 × g for 20 min at
4°C. The fetuses, placentas and kidneys were removed, weighed and
then fixed with 4% paraformaldehyde for histological evaluation.
The uterus was cut and the placenta and fetuses were removed
quickly. Fetal processing was conducted in accordance with CCAC
Guidelines (20). The plasma samples
and the remaining placentas and kidneys were stored at −80°C until
analysis.
Histological assay
Placenta and kidney specimens were cut into 5-µm
paraffin sections using a microtome and were stained with
hematoxylin and eosin for conventional morphological evaluation
under a light microscope (Olympus BX51; Olympus, Tokyo, Japan).
LEGENDplex™
The LEGENDplex™ Rat Panel is a bead-based
multiplex assay panel using fluorescence-encoded beads that are
suitable for use on various flow cytometers. Tumor necrosis
factor-α (TNF-α), interleukin-1β (IL-1β), IL-6, IL-17A and monocyte
chemoattractant protein 1 (MCP-1) levels were examined according to
methods described in a previous study (21–23). In
brief, the standard was prepared by dissolving in 250 µl Assay
Buffer and 4-fold dilution; Assay Buffer was used as the C0
standard (0 pg/ml). Matrix B was added to the samples, and
subsequently, Assay Buffer and beads were added to reach a final
volume of 100 µl per tube. The samples were then mixed and
incubated in the dark at room temperature. Subsequently,
Streptavidin and R-Phycoerythrin conjugate was added and the sample
was mixed. Following centrifugation at a speed of 1,000 × g for 5
min at 22°C, the supernatant was discarded, and the beads were
vortexed and analyzed with a flow cytometer. The signal intensity
of the microbeads was detected using a BD LSRFortessa instrument
(BD Biosciences, Franklin Lakes, NJ, USA), and the data were
analyzed using LEGENDplex software, version 8.0 (BioLegend).
ELISA
Plasma was separated by centrifuging the blood at
1,006.2 × g for 20 min at 4°C. sFlt-1, PLGF and 8-iso-PFG2α were
measured by quantitative sandwich enzyme immunoassays using
commercial ELISA kits.
Thiobarbituric acid reactive
substances (TBARS) assay
The supernatant of the tissue homogenate (with PBS)
was decanted into glass tubes and mixed with 500 µl 2% phosphoric
acid. Subsequently, 7% H3PO4 (200 µl),
TBA/butylated hydroxytoluene (400 µl) and 1 M HCl (200 µl) were
added. The tubes were heated at 100°C for 15 min and then cooled to
room temperature. Butanol (1,500 µl) was then added to each tube,
followed by vortexing. The upper phase of each vial was transferred
to triplicate wells of a 96-well plate, and the absorbance (Abs.)
was measured at 532 and 600 nm using a Spectrostarnano plate reader
(BMG Labtech, Ortenberg, Germany). The concentration was determined
using the following formula: Concentration (nmol/mgprot)=[(Abs.532
nm-Abs.600 nm)/156] ×1,000.
Measurement of serum NO levels
The serum concentration of NO was measured with an
NO assay kit (nitrate reductase method; cat. no. A012; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) in accordance
with the manufacturer's protocol.
Statistical analysis
All values are expressed as the mean ± standard
deviation. Statistical analysis was performed using STATA version
14.2 (StataCorp LP, College Station, TX, USA). Statistical analyses
were performed by one-way analysis of variance (ANOVA) followed by
an S-N-K post-hoc test. BP during pregnancy was analyzed by
repeated-measures ANOVA. P<0.05 was considered to indicate a
statistically significant difference.
Results
Systolic and mean arterial pressure
changes in the pregnant rat
The variation of SBP and MAP during pregnancy in
each group is displayed in Fig. 1A and
B. Prior to L-NAME administration, no significant differences
in SBP and mean arterial pressure (MAP) were present among the 7
groups of rats (the 9D LL, 9D ML, 9D HL, 10D LL, 10D ML, 10D HL and
normal pregnancy groups). The SBP of the L-NAME groups steadily
increased with L-NAME treatment, and the SBP of the 9D ML, 9D HL
and 10D ML groups was slightly increased during late gestation
(Fig. 1A). Of note, the SBP of the
L-NAME-treated pregnant rats in the 9D ML group was significantly
higher than that of rats in the control group (P<0.01) and the
other L-NAME-treated groups (P<0.05). The MAP in the
L-NAME-treated groups was higher than that in the control group
(P<0.05), and no significant difference in MAP was present among
the groups treated with L-NAME (Fig.
1B).
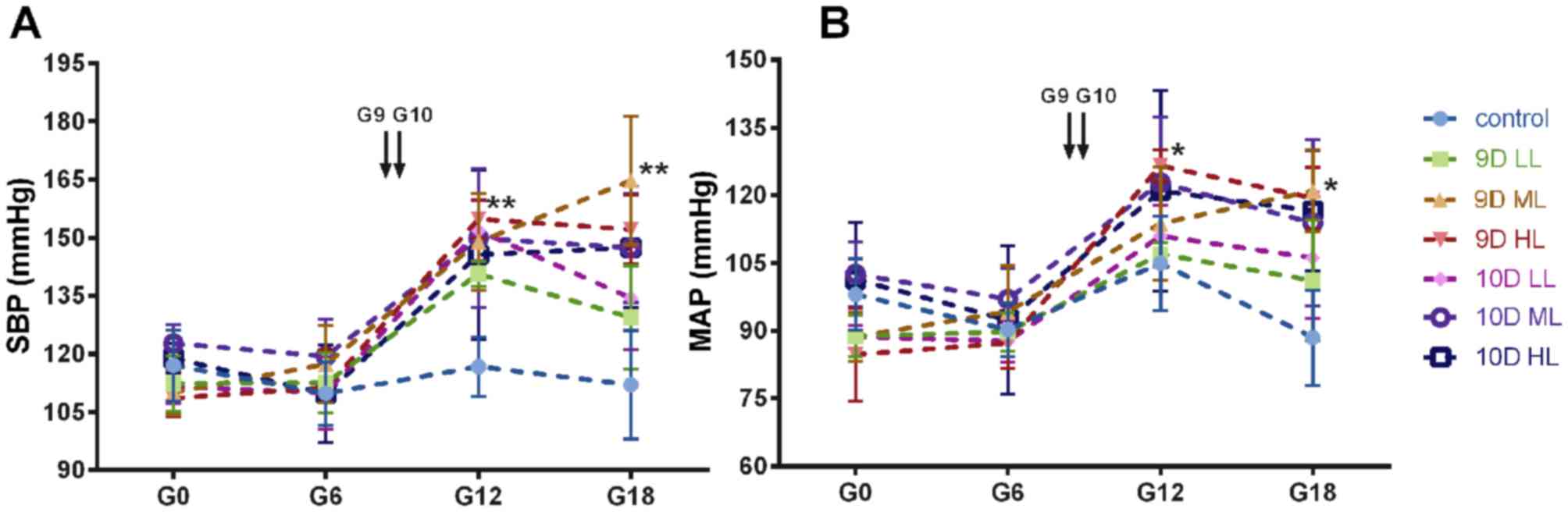 | Figure 1.Blood pressure of pregnant rats was
non-invasively measured with the CODA system (Kent Scientific,
Torrington, CT, USA). The variation tendency of (A) SBP and (B) MAP
during pregnancy in each group. *P<0.05; **P<0.01 vs. the
control group. Values are expressed as the mean ± 95% confidence
interval. Groups: 9D LL, rats treated with low-dose L-NAME (40
mg/kg body weight/day) starting from GD 9; 9D ML, rats treated with
medium-L-NAME (75 mg/kg body weight/day) starting from GD 9; 9D HL,
rats treated with high-dose L-NAME (125 mg/kg body weight/day)
starting from GD 9; 10D LL, rats treated with low-dose L-NAME (40
mg/kg body weight/day) starting from GD 10; 10D ML, rats treated
with medium-L-NAME (75 mg/kg body weight/day) starting from GD 10;
10D HL, rats treated with high-dose L-NAME (125 mg/kg body
weight/day) starting from GD 10; control, normal pregnancy group.
GD, gestational day; L-NAME, N-nitro-L-arginine methyl ester; SBP,
systolic blood pressure; MAP, mean arterial pressure. |
The rats in all groups except the 9D ML group
exhibited patterns that are normal during pregnancy, with a
decrease in SBP and MAP during the late stage. However, the rats in
the 9D ML group did not exhibit this drop in BP, as their BP
steadily increased during pregnancy.
Changes in kidney structure and
function in the pregnant rats injected with L-NAME
Renal histology of the pregnant rats in the
L-NAME-treated groups exhibited significant changes that are
consistent with those observed in human preeclampsia patients. The
kidneys of the pregnant L-NAME-treated rats exhibited an increased
glomerular area, capillary structure disorder and abnormal protein
cast. Representative images for the control, 9D ML and 10D ML group
are presented in Fig. 2A, B and C,
respectively.
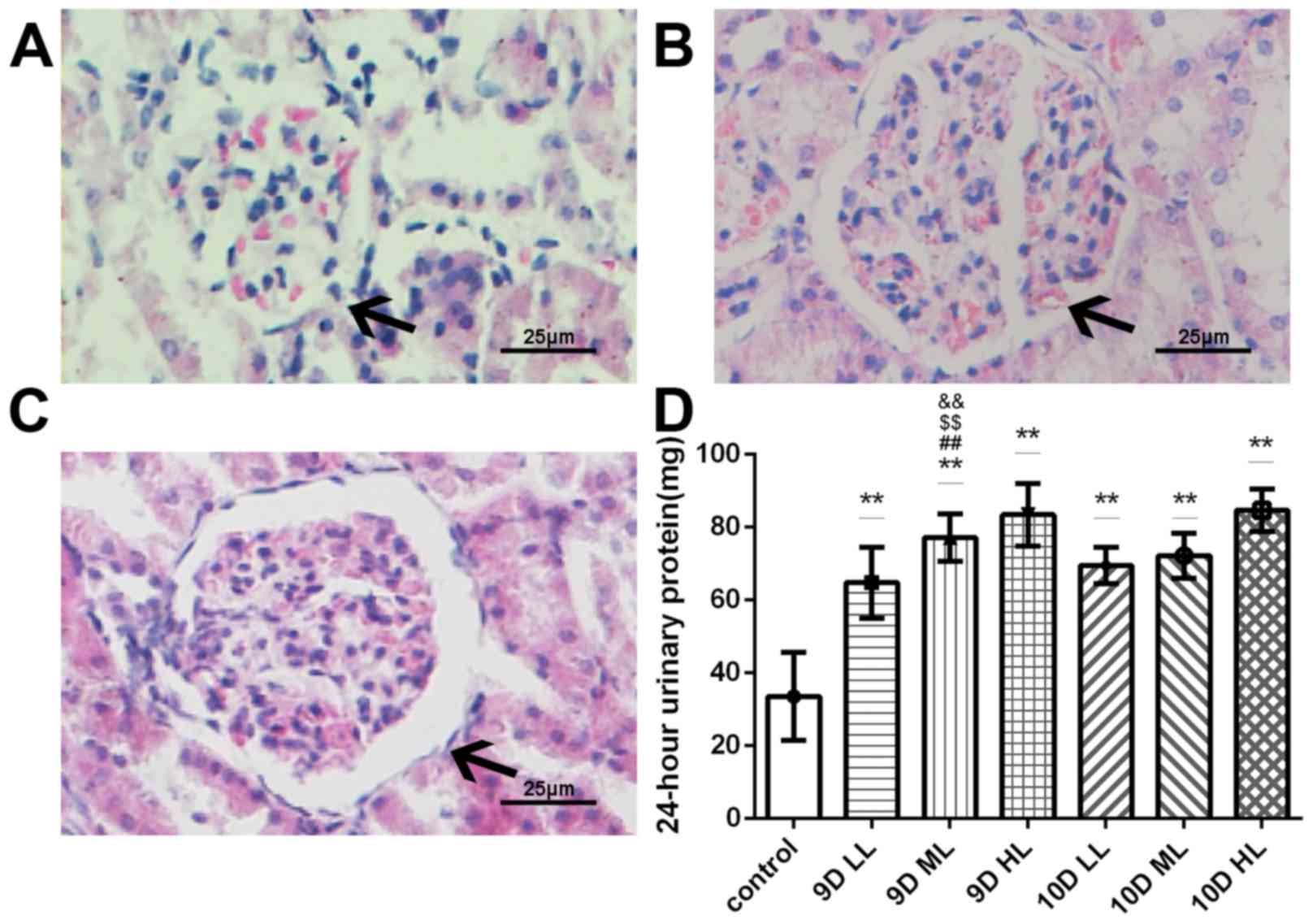 | Figure 2.Changes of kidney structure and
function in pregnant rats (GD20). (A-C) Histopathological images of
glomeruli in pregnant rats of (A) the control group, (B) the 9D ML
group and (C) the 10D ML group (H&E; magnification, ×400; scale
bar, 25 µm). Black arrows indicate glomeruli. (D) The 24-h urine
protein of the pregnant rats in GD20. **P<0.05 vs. control
group; ##P<0.05 9D ML group vs. 9D LL group;
&&P<0.05 9D ML group vs. 10D LL group;
&&P<0.05 9D ML group vs. 10D ML group. Values
are expressed as the mean ± standard deviation. Groups: 9D LL, rats
treated with low-dose L-NAME (40 mg/kg body weight/day) starting
from at GD 9; 9D ML, rats treated with medium-L-NAME (75 mg/kg body
weight/day) starting from GD 9; 9D HL, rats treated with high-dose
L-NAME (125 mg/kg body weight/day) starting from GD 9; 10D LL, rats
treated with low-dose L-NAME (40 mg/kg body weight/day) starting
from GD 10; 10D ML, rats treated with medium-L-NAME (75 mg/kg body
weight/day) starting from GD 10; 10D HL, rats treated with
high-dose L-NAME (125 mg/kg body weight/day) starting from GD 10;
control, normal pregnancy group. GD, gestational day; L-NAME,
N-nitro-L-arginine methyl ester. |
The results of the 24-h urine protein quantitation
indicated that the urinary protein levels were similar among the 7
groups prior to L-NAME treatment. At the end of pregnancy, an
increase in urinary protein was observed in animals that received
injections of L-NAME (P<0.05); furthermore, the urinary protein
content in the L-NAME-treated pregnant rats was significantly
higher than that of the rats in the normal pregnancy group
(P<0.05; Fig. 2D). No difference
in the amount of excreted urinary protein among the 9D LL, 10D LL
and 10D ML groups was detected. At the same time, there were
significant differences between 9D ML and 9D LL, and 10D LL and 10D
ML. (P<0.05).
Adverse pregnancy outcomes in the
L-NAME-treated groups
Adverse pregnancy outcomes were observed in the
experimental groups, which may have resulted from high SBP or MAP.
The pups of rats in the L-NAME-treated groups were significantly
smaller than those of the rats in the control group, with a shorter
crown-rump length and lower fetal weight (P<0.01). The placentas
of the L-NAME-treated groups were smaller but heavier (P<0.01)
and exhibited spherical edema compared with the placentas of the
normal pregnancy rats. The placental diameter, placental weight,
fetal weight and crown-rump length in the 9D ML and 10D ML groups
were the most significantly different compared with those in the
control group (Fig. 3A-D). However,
the 10D ML group exhibited a more severe fetal absorption
percentage than the 9D ML group (fetal malformations in 25% of
fetuses).
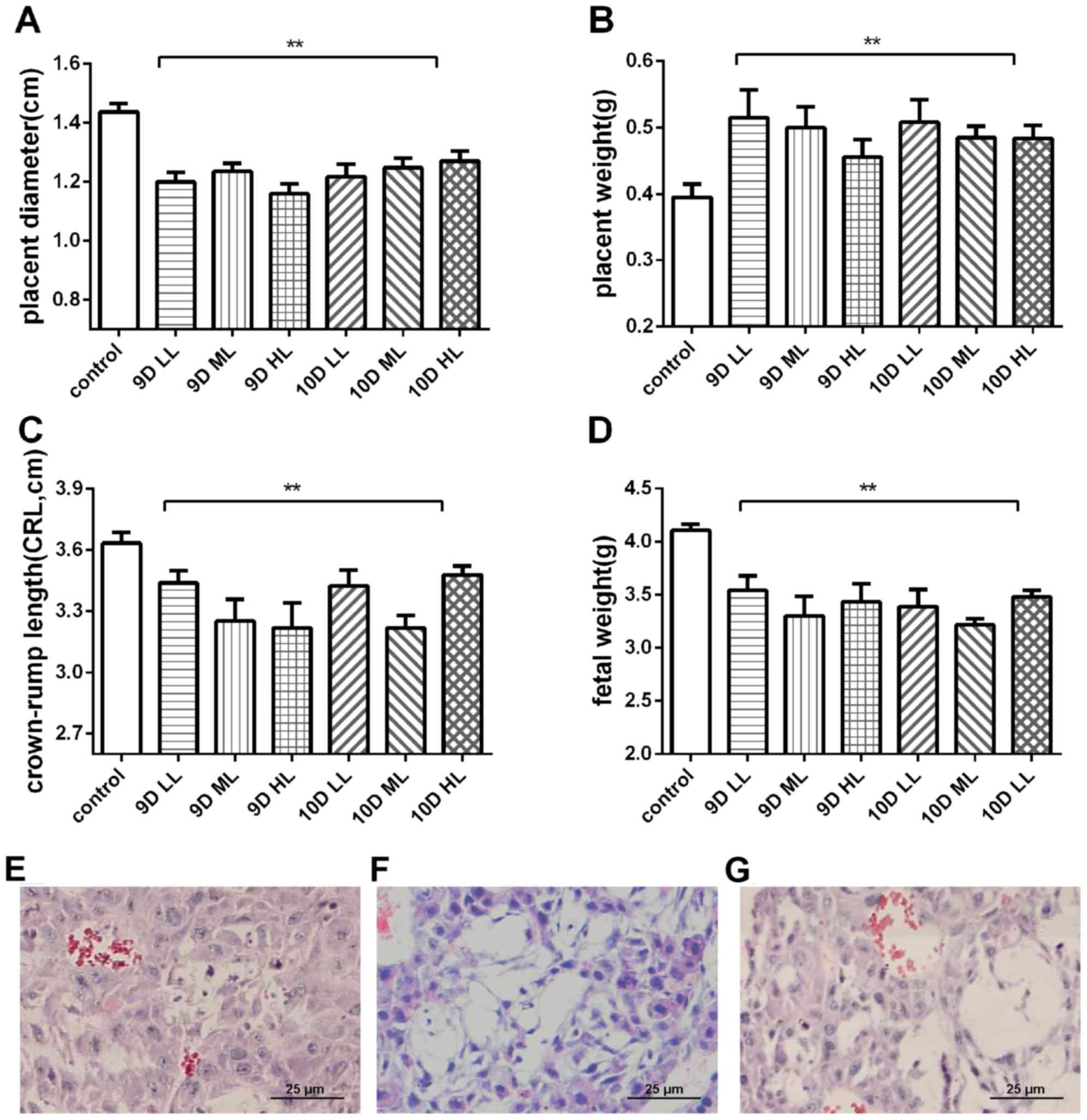 | Figure 3.Pregnancy outcome and changes in
placental trophoblast structure in the pregnant rats at the end of
pregnancy (GD20). (A) Diameter of the placenta in each group. (B)
Weight of the placenta in each group. (C) Crown-rump length in each
group. (D) Fetal weight in each group. (E-G) Histopathological
images of placental trophoblasts in pregnant rats of (E) the
control group, (F) 9D ML group and (G) 10D ML group (H&E
staining; magnification, ×400; scale bar, 25 µm). **P<0.05 vs.
control group. Values are expressed as the mean ± standard
deviation. Groups: 9D LL, rats treated with low-dose L-NAME (40
mg/kg body weight/day) starting from GD 9; 9D ML, rats treated with
medium-L-NAME (75 mg/kg body weight/day) starting from GD 9; 9D HL,
rats treated with high-dose L-NAME (125 mg/kg body weight/day)
starting from GD 9; 10D LL, rats treated with low-dose L-NAME (40
mg/kg body weight/day) starting from GD 10; 10D ML, rats treated
with medium-L-NAME (75 mg/kg body weight/day) starting from GD 10;
10D HL, rats treated with high-dose L-NAME (125 mg/kg body
weight/day) starting from GD 10; control, normal pregnancy group.
GD, gestational day; L-NAME, N-nitro-L-arginine methyl ester. |
The pregnant rats in the L-NAME-treated groups also
exhibited significant changes in placental histology that are
consistent with those reported in human preeclampsia patients. The
placental trophoblast cells of the L-NAME-treated pregnant rats
exhibited vacuolar aggregation (Fig.
3E-G).
Circulating sFlt-1 levels and the
sFlt-1/PLGF ratio are significantly elevated in the pregnant rats
injected with L-NAME
The plasma levels of sFlt-1 and PLGF in the pregnant
rats of each group were measured by ELISA (Fig. 4). The concentration of sFlt-1 in the
plasma of rats treated with L-NAME was increased, but no
dose-dependent effect was observed. The plasma concentration of
sFlt-1 in rats of the 9D ML group (373.85±4.67 ng/ml) and the 9D HL
group (346.30±8.23 ng/ml) was significantly increased compared with
that in rats of the control group (281.02±5.77 ng/ml; P<0.01;
Fig. 4A). The plasma concentration
of PLGF in rats of the 9D ML group (74.54±4.75 ng/ml) and the 10D
ML group (80.69±5.14 ng/ml) was significantly decreased compared
with that in rats of the control group (95.39±3.80 ng/ml;
P<0.01; Fig. 4B).
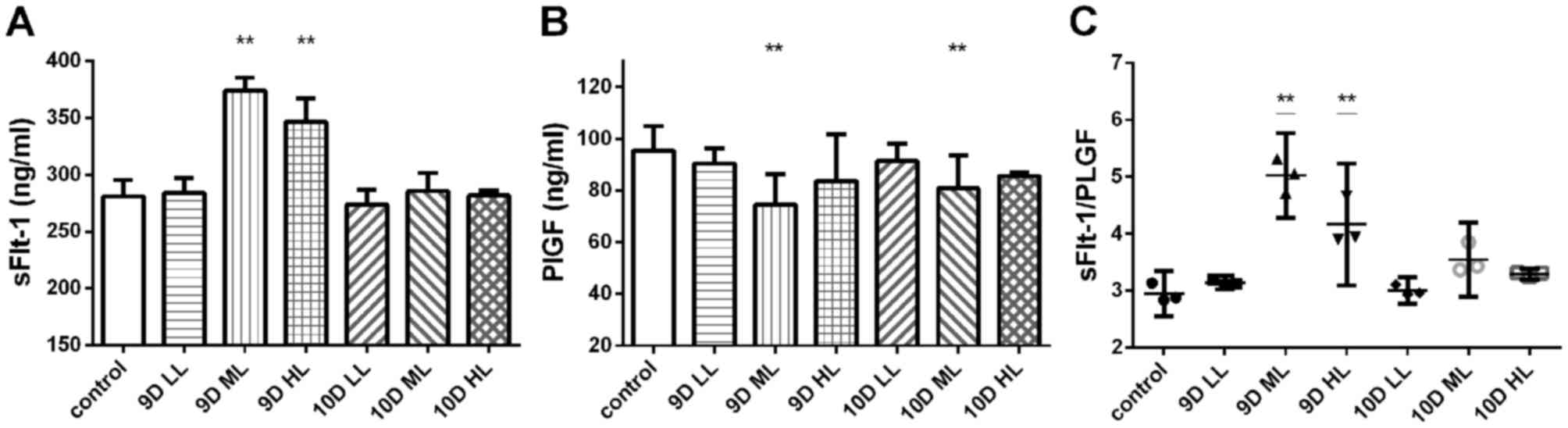 | Figure 4.Plasma levels of sFlt-1 and PLGF in
the pregnant rats in each group at the end of pregnancy (GD20) were
determined by ELISA. (A) Circulating sFlt-1 levels. (B) Circulating
PLGF levels. (C) Circulating sFlt-1/PLGF ratio in the pregnant
rats. **P<0.05 vs. control group. Values are expressed as the
mean ± standard deviation. Groups: 9D LL, rats treated with
low-dose L-NAME (40 mg/kg body weight/day) starting from GD 9; 9D
ML, rats treated with medium-L-NAME (75 mg/kg body weight/day)
starting from GD 9; 9D HL, rats treated with high-dose L-NAME (125
mg/kg body weight/day) starting from GD 9; 10D LL, rats treated
with low-dose L-NAME (40 mg/kg body weight/day) starting from GD
10; 10D ML, rats treated with medium-L-NAME (75 mg/kg body
weight/day) starting from GD 10; 10D HL, rats treated with
high-dose L-NAME (125 mg/kg body weight/day) starting from GD 10;
control, normal pregnancy group. GD, gestational day; L-NAME,
N-nitro-L-arginine methyl ester; sFlt-1, soluble fms-like tyrosine
kinase-1; PLGF, placental growth factor. |
The sFlt-1/PLGF ratio in the 9D ML group (5.03±0.30
ng/ml) and the 9D HL group (4.17±0.43 ng/ml) was significantly
higher than that in the control group (2.95±0.16 ng/ml; P<0.01;
Fig. 4C). However, no statistically
significant differences were detected for the other groups.
Circulating oxidative stress levels
are increased in rats after L-NAME treatment
The plasma levels of 8-iso-PFG2α and malondialdehyde
were measured in pregnant rats (Fig.
5). The levels of 8-iso-PFG2α in the L-NAME-treated groups were
higher than those in the control group (96.71±1.12 ng/ml), and a
significant difference was present among the 9D ML (115.29±7.67
ng/ml), 10D ML (118.26±22.69 ng/ml) and 10D HL groups (121.44±9.82
ng/ml; P<0.01). However, the levels of 8-iso-PFG2α in the other
three experimental groups were not significantly different from
those in the control group (Fig.
5A).
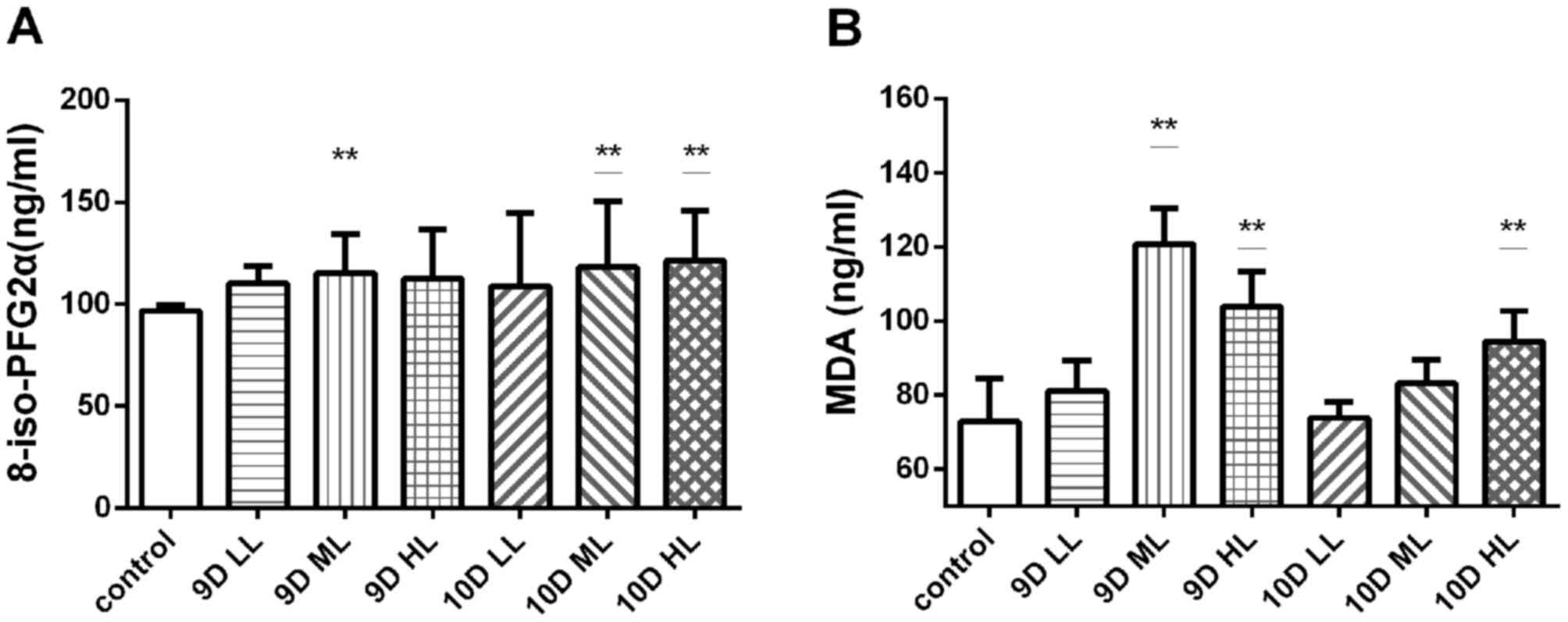 | Figure 5.Levels of 8-iso-PFG2α and MDA in the
plasma of pregnant rats in each group at the end of pregnancy
(GD20) were measured by ELISA or thiobarbituric acid reactive
substances. (A) Circulating 8-iso-PGF2α levels in pregnant rats.
(B) Circulating MDA levels in pregnant rats. **P<0.05 vs.
control group. Values are expressed as the mean ± standard
deviation. Groups: 9D LL, rats treated with low-dose L-NAME (40
mg/kg body weight/day) starting from GD 9; 9D ML, rats treated with
medium-L-NAME (75 mg/kg body weight/day) starting from GD 9; 9D HL,
rats treated with high-dose L-NAME (125 mg/kg body weight/day)
starting from GD 9; 10D LL, rats treated with low-dose L-NAME (40
mg/kg body weight/day) starting from GD 10; 10D ML, rats treated
with medium-L-NAME (75 mg/kg body weight/day) starting from GD 10;
10D HL, rats treated with high-dose L-NAME (125 mg/kg body
weight/day) starting from GD 10; control, normal pregnancy group.
GD, gestational day; L-NAME, N-nitro-L-arginine methyl ester; MDA,
malondialdehyde; PGF2, prostaglandin F2. |
MDA detection in the plasma of pregnant rats also
suggested that the level of oxidative stress in the L-NAME
treatment groups was higher than that in the control group. The
plasma MDA level of the 9D ML group (120.72±6.13 ng/ml), 9D HL
group (103.85±6.01 ng/ml) and 10D HL group (94.44±7.89 ng/ml) was
significantly higher than that of the control group (72.82±9.44
ng/ml; P<0.01; Fig. 5B).
L-NAME-treated pregnant rats exhibit a
more severe inflammatory reaction
The pathogenesis of preeclampsia involves
inflammation, oxidative stress, endothelial injury and numerous
other factors. In the present study, the BioLegend multifactor flow
assay was used to detect the plasma levels of TNF-α, IL-17A, MCP-1
and IL-1β in all groups (Fig.
6).
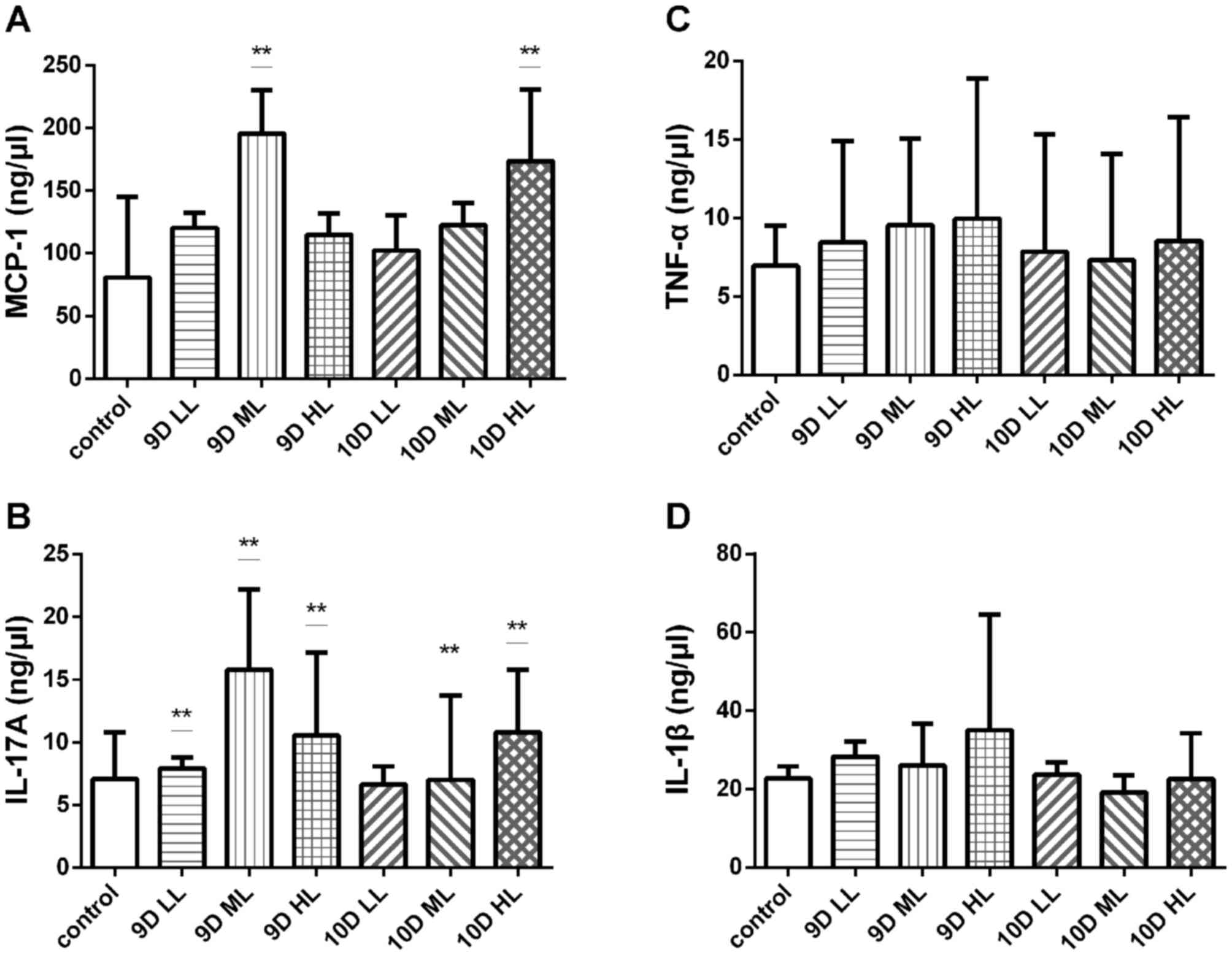 | Figure 6.Levels of inflammatory factors in the
plasma of pregnant rats in each group at the end of pregnancy
(GD20) were measured using the BioLegend multi-factor flow assay.
(A) Circulating MCP-1, (B) IL-17A, (C) TNF-α and (D) IL-1β levels.
**P<0.05 vs. control group. Values are expressed as the mean ±
standard deviation. Groups: 9D LL, rats treated with low-dose
L-NAME (40 mg/kg body weight/day) starting from GD 9; 9D ML, rats
treated with medium-L-NAME (75 mg/kg body weight/day) starting from
GD 9; 9D HL, rats treated with high-dose L-NAME (125 mg/kg body
weight/day) starting from GD 9; 10D LL, rats treated with low-dose
L-NAME (40 mg/kg body weight/day) starting from GD 10; 10D ML, rats
treated with medium-L-NAME (75 mg/kg body weight/day) starting from
GD 10; 10D HL, rats treated with high-dose L-NAME (125 mg/kg body
weight/day) starting from GD 10; control, normal pregnancy group.
GD, gestational day; L-NAME, N-nitro-L-arginine methyl ester; TNF,
tumor necrosis factor; IL, interleukin; MCP, monocyte
chemoattractant protein. |
A significant difference in the levels of MCP-1
compared with that in the control group (80.64±25.91 ng/µl) was
noted in the 9D ML group (195.55±13.97 ng/µl) and the 10D HL group
(173.73±22.92 ng/µl; P<0.05; Fig.
6A). However, there was no difference in the expression levels
of TNF-α among those groups (Fig.
6B). The expression levels of IL-17A in all experimental groups
except for the 10D LL group (6.64±0.57 ng/µl) were significantly
increased compared with those in the control group (7.08±1.50
ng/µl; P<0.05; Fig. 6C). However,
there was no difference in the expression levels of IL-1β among
those groups (Fig. 6D).
Collectively, the results of the flow cytometry assay indicated
that the levels of certain circulating inflammatory factors in
pregnant rats were increased.
Changes of circulating NO levels in
rats after L-NAME treatment
The effects of different treatment regimens with
L-NAME on the levels of NO in the circulation of pregnant rats in
each group were determined by serum analysis towards the end of
pregnancy (GD 20). The NO levels in the pregnant rats in each of
the L-NAME-treated groups were significantly decreased compared
with those in the control group (57.83±1.53 µg/ml; P<0.01),
except for those in the 10D LL group (54.49±2.94 µg/ml; Fig. 7).
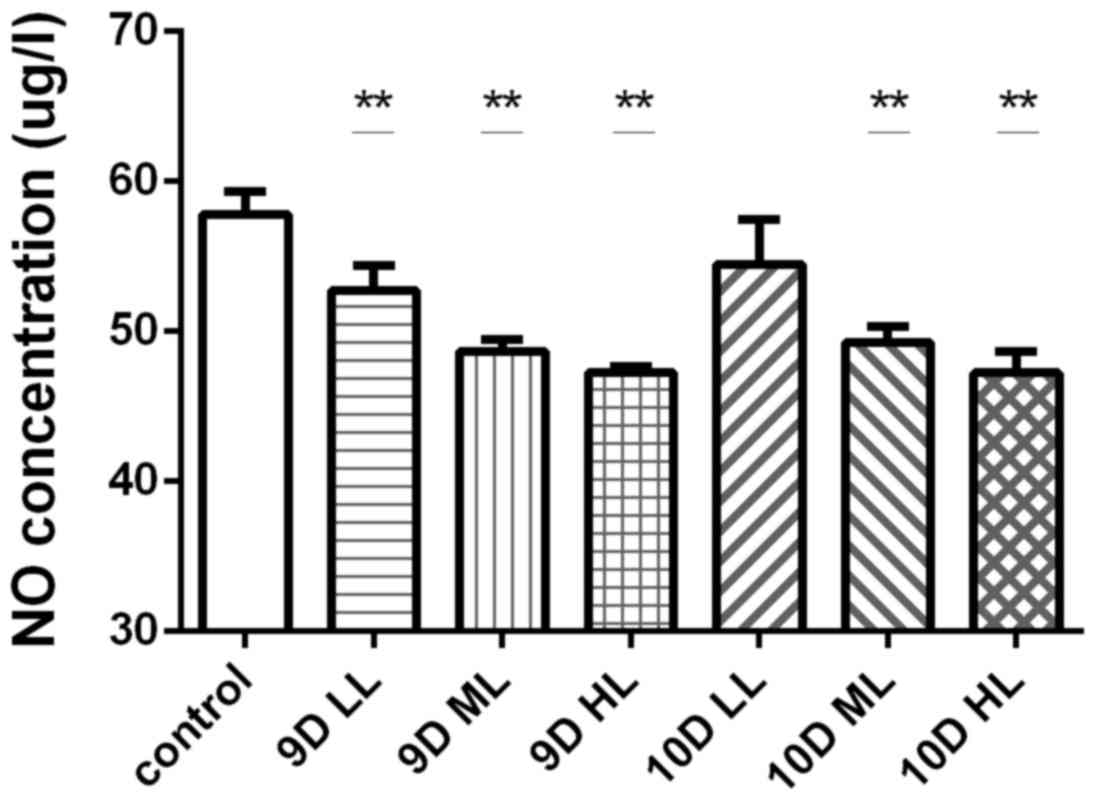 | Figure 7.Levels of circulating NO in pregnant
rats in each group at the end of pregnancy (GD20) were measured by
nitrate reductase method. **P<0.05 vs. control group. Values are
expressed as the mean ± standard deviation. Groups: 9D LL, rats
treated with low-dose L-NAME (40 mg/kg body weight/day) starting
from GD 9; 9D ML, rats treated with medium-L-NAME (75 mg/kg body
weight/day) starting from GD 9; 9D HL, rats treated with high-dose
L-NAME (125 mg/kg body weight/day) starting from GD 9; 10D LL, rats
treated with low-dose L-NAME (40 mg/kg body weight/day) starting
from GD 10; 10D ML, rats treated with medium-L-NAME (75 mg/kg body
weight/day) starting from GD 10; 10D HL, rats treated with
high-dose L-NAME (125 mg/kg body weight/day) starting from GD 10;
control, normal pregnancy group. GD, gestational day; L-NAME,
N-nitro-L-arginine methyl ester; NO, nitric oxide. |
Discussion
The L-NAME-induced SD rat model of preeclampsia
developed in the present study may be a promising means of
investigating the pathogenesis of preeclampsia and evaluating
therapies for this condition. Rats treated with L-NAME exhibited
elevated BP, increased urinary albumin excretion, severe
endotheliosis, mesangial expansion and increased sFlt-1/PLGF
ratios.
Preeclampsia is a serious complication in pregnancy.
Its major symptoms include high BP, proteinuria, endothelial
dysfunction, glomerular vascular endothelial cell proliferation and
trophoblast cell infiltration. However, the pathogenesis of
preeclampsia has remained to be fully elucidated.
Animal models are commonly used to study the
pathogenesis of preeclampsia and develop novel diagnostic methods
and treatments. At present, a wide range of preeclampsia models
exist, including the spontaneous preeclampsia model (24,25), the
NOS inhibition model (26), the
angiogenesis factor imbalance model (27,28), the
oxygen deprivation model (29,30), the
immune response model (31,32) and the chronic hypertension and
vascular injury model (33,34). However, an effective animal model is
still required to simulate the complex features of preeclampsia.
The present study aimed to explore the ideal dosing and timing of
L-NAME administration to pregnant rats for the establishment of a
model of preeclampsia.
During pregnancy, NO synthesis and its release in
endothelial cells have an important role in vascular relaxation and
the regulation of vascular tension (35). Chronic inhibition of NO production
increases the BP in a volume-dependent manner, and the associated
physiological and pathological characteristics are similar to those
of primary hypertension (36). In
addition, it is known that acute inhibition of NO biosynthesis by
the administration of L-NAME results in arterial hypertension and
vasoconstriction. Therefore, in the present study a novel
preeclampsia animal model was constructed via subcutaneous
injection of the NOS inhibitor L-NAME into rats starting at
mid-pregnancy. It was revealed all groups of pregnant rats injected
with L-NAME except for those in the 10D LL group had significantly
lower circulating NO levels than those in the control group. It is
suggested that L-NAME treatment inhibits NOS in pregnant rats and
induces the corresponding changes in preeclampsia (37,38).
The present study indicated that L-NAME successfully
induced preeclampsia in pregnant rats. The dosing and timing of
L-NAME injection are of great importance, as late drug
administration or low doses cannot induce a statistically
significant difference in BP to simulate the mid-pregnancy BP
increases observed in preeclampsia patients. In the present study,
L-NAME was injected from GD 9 or GD 10. Based on an extensive
literature search (17,19), L-NAME at 40, 75 and 125 mg/kg/day was
used to induce preeclampsia in pregnant rats. As expected, the SBP
and MAP were elevated in L-NAME-induced hypertensive rats, and the
pressure difference was 30 mmHg after the administration of L-NAME
to the 9D ML, 9D HL, 10D ML and 10D HL groups from GD12. The
difference in BP was observed until delivery at GD 20, particularly
in the 9D ML group. Furthermore, the rats in all groups except the
9D ML group exhibited normal pregnancy patterns, with a decrease in
SBP and MAP during late pregnancy, whereas the rats in the 9D ML
group did not exhibit any drop in BP; rather, these rats exhibited
a steady increase in BP during pregnancy. Thus, the results of the
present study indicate that the establishment of a pre-eclampsia
model for SD pregnant rats (starting from the 9th day of pregnancy
with a continuous subcutaneous injection of 75 mg/kg/day) is a
promising solution. Compared with the previous study (37), the current study assessed L-NAME
dosage, time and the effect of L-NAME in combination. Following
L-NAME treatment, significant changes in maternal urine protein
content, the placenta, the kidneys and the blood vessels were
observed in pregnant rats with preeclampsia, particularly those in
the D9 ML, 9D HL and 10D HL groups. In preeclampsia, maternal
uterine spiral artery remodeling disorders lead to placental
ischemia and placenta angiogenesis imbalances, the levels of sFlt-1
are elevated and the levels of PLGF, a subtype of vascular
endothelial growth factor, are decreased (39). In the present study, the plasma
concentration of sFlt-1 in rats of the 9D ML and 9D HL groups was
significantly increased compared with that in rats of the control
group, and the concentration of PLGF in rats of the 9D ML and 10D
ML groups was significantly decreased compared with that in rats of
the control group. The ratio of sFlt-1 to PLGF in the 9D ML and 9D
HL groups was significantly higher than that in the control group.
The above results indicated that L-NAME intervention on the 9th day
of pregnancy is more suitable to simulate pre-eclampsia symptoms.
In addition, it has been reported that the mechanism of urinary
protein aggravation may be associated with the release of
circulating factors, including TNF-α or the anti-angiogenic factor
sFlt-1, but this observation requires further study (32).
According to the present results, L-NAME-induced
preeclampsia-like pregnant rats also exhibited oxidative stress and
inflammatory changes. The plasma levels of 8-iso-PFG2α were
increased in all L-NAME-treated groups, and significant differences
from the control group were observed in the 9D ML, 10D ML and 10D
HL groups. Similarly, the plasma levels of MDA in the pregnant rats
after L-NAME treatment were substantially increased. As observed in
the development of human preeclampsia, L-NAME-treated pregnant rats
exhibited oxidative stress, which may be one of the causes of
preeclampsia or may lead to more serious premenstrual symptoms
(40). MCP-1 is a secreted
single-chain protein and a member of the chemokine family; as one
of the major chemokines, MCP-1 induces chemotaxis via the G
protein-coupled receptor pathway, as well as macrophage migration
and infiltration. MCP-1 releases chemokines to recruit monocytes
into the endodermis, which may be transformed into macrophages and
phagocytes with selectivity for oxidized low-density lipoprotein,
and are then transformed into foam cells. MCP-1 also participates
in cell growth, metabolism and apoptosis. In the present study, the
plasma levels of MCP-1 were identified to be elevated, indicating
that an excessive inflammatory reaction occurs in the rats with
preeclampsia. Excess macrophages may infiltrate the decidua, damage
the vascular endothelial cells, and prevent intravascular
trophoblast invasion and vascular remodeling in the placenta; at
the same time, MCP-1 may also affect inflammatory cell infiltration
at the maternal-fetal interface, and cause abnormal placental
trophoblastic cells, increases in apoptosis and acute
atherosclerosis of the small uterine spiral artery, all of which
induces the occurrence of preeclampsia.
IL-17A is a cytokine produced by numerous cell
types, including T-helper type 17 (Th17) cells (41). IL-17A regulates the expression of
cytokines, chemokines and adhesion molecules in the
microenvironment through distinct pathways, increases the levels of
inflammatory cells, particularly neutrophils, and induces an
inflammatory effect (42). IL-17A is
involved in inflammatory diseases. High levels of IL-17A, Th1/Th2
drift and the pro-inflammatory response are inseparable, and may
interact with a variety of cytokines to enhance small vascular
inflammation in the placenta. Elevated IL-17A also has a marked
ability to raise neutrophil levels, induce the release of active
substances to damage endothelial cells and at the same time, to
generate a large number of oxygen free radicals to cause damage to
vascular endothelial cells, increase the permeability of blood
vessels, and increase BP in preeclampsia, vasospasm, edema,
proteinuria and a series of pathological factors associated with
various clinical manifestations. Multifactor flow detection is an
effective method for detecting trace factors in tissues, plasma,
cell supernatants and other samples. The present indicated that the
levels of IL-17A and MCP-1 were elevated in the plasma of the
experimental groups, suggesting that an inflammatory response
occurs in the L-NAME-induced preeclampsia-like rat model.
Although numerous studies of L-NAME induced animal
models of preeclampsia have been established, due to the different
descriptions of this method, the present study aimed to optimize
the timing and dosing as approaches of molding (8,38). The
present study further indicated that oxidative stress and
inflammation have important roles in the development of
preeclampsia, which is based on numerous previous studies (43,44).
When assessing the clinical manifestations (hypertension,
proteinuria, and maternal and offspring outcomes) and pathological
changes (alterations in the pathological structure of placenta and
kidney) of preeclampsia, certain molecules that represent
significant oxidative stress and inflammatory responses were
assessed and compared. The similarity between the animal disease
model and real disease was further verified, which is in favor of
the experiment aimed to establish the model. The present
experiments led to the successful development of a model that
exhibits preeclampsia-like characteristics, including phenotype
(gestational hypertension and proteinuria), pregnancy outcome,
birth effects in offspring and serological markers in pregnant SD
rats. Due to the sustained action of the NOS inhibitor L-NAME, a
number of hemodynamic changes were observed in the pregnant rats,
including placental and renal ischemia, as well as hypoxia
symptoms, oxidative stress and an intensified inflammatory
response. Therefore, the L-NAME-induced preeclampsia model provides
a powerful tool with which to investigate the pathogenesis of
preeclampsia. The rat model of preeclampsia allows for the analysis
of time-dependent changes in the placenta, vasculature and kidney
throughout pregnancy, as well as the discovery of novel biomarkers
that are present during early pregnancy. The construction of this
model was simple and it may be used to study neonatal markers, the
pathophysiological mechanisms of preeclampsia and drug targets for
its prevention. The offspring with birth defects and dysplasia may
also be used for further study of preeclampsia progeny. It was
noted that this model may lead to fetal malformations (limb
reduction), which may be due to the side effects of NOS inhibition
by L-NAME. Of note, the present study had a certain limitation. The
obtainment of blood is required for the detection of plasma
markers, and frequent blood extraction may have affected the
experiment; furthermore, the availability of kidney and placenta
tissue is limited by the amount of experimental animals. Therefore,
the present study did not perform any other relevant tests on GD 14
and GD 18. This limitation of the present study will be further
improved in future experiments. In the future, animal models will
continue to enhance the current understanding of the physiological
and pathological conditions of trophoblast cell differentiation and
invasion during pregnancy.
Acknowledgements
Not applicable.
Funding
The study was supported by Tianjin Science and
Technology Major Projects (grant nos. 16ZXMJSY00130 and
15ZXJZSY00010).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
WS and HL participated in all experiments, and they
were major contributor in writing the manuscript. HG participated
in most experiments except TBARS, ELISA and LEGENDplexTM. MZ and XN
participated in the quality monitoring of all experimental methods
and results. YM participated in LEGENDplexTM. XZ participated in
blood pressure monitoring. WC participated in tissue collection. GY
participated in pathological staining of placenta and kidney. MW
analyzed and interpreted all experimental data. NY and YL
participated in the experimental design and guided the experiment.
All authors read and approved the final manuscript.
Ethical approval and consent to
participate
The present study was approved by the Ethics
Committee of the Logistics University of the Chinese People's Armed
Police Forces (Tianjin, China).
Consent for publication
Not applicable.
Competing interests
The authors declare no potential competing interests
with respect to the research, authorship and/or publication of this
article.
References
|
1
|
American College of Obstetricians and
Gynecologists; Task Force on Hypertension in Pregnancy:
Hypertension in pregnancy. Report of the American College of
Obstetricians and Gynecologists' Task Force on Hypertension in
Pregnancy. Obstet Gynecol. 122:1122–1131. 2013.PubMed/NCBI
|
|
2
|
Al-Jameil N, Aziz Khan F, Fareed Khan M
and Tabassum H: A brief overview of preeclampsia. J Clin Med Res.
6:1–7. 2014.PubMed/NCBI
|
|
3
|
Wang A, Rana S and Karumanchi SA:
Preeclampsia: The role of angiogenic factors in its pathogenesis.
Physiology (Bethesda). 24:147–158. 2009.PubMed/NCBI
|
|
4
|
ACOG Committee on Obstetric Practice: ACOG
practice bulletin. Diagnosis and management of preeclampsia and
eclampsia. Number 33, January 2002. American College of
Obstetricians and Gynecologists. Int J Gynaecol Obstet. 77:67–75.
2002.PubMed/NCBI
|
|
5
|
Khan KS, Wojdyla D, Say L, Gülmezoglu AM
and van Look PF: WHO analysis of causes of maternal death: A
systematic review. Lancet. 367:1066–1074. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Steegers EA, von Dadelszen P, Duvekot JJ
and Pijnenborg R: Pre-eclampsia. Lancet. 376:631–644. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
European Society of Gynecology (ESG);
Association for European Paediatric Cardiology (AEPC); German
Society for Gender Medicine (DGesGM); Regitz-Zagrosek V, Blomstrom
Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS,
et al: ESC Guidelines on the management of cardiovascular diseases
during pregnancy: The Task Force on the Management of
Cardiovascular Diseases during Pregnancy of the European Society of
Cardiology (ESC). Eur Heart J. 32:3147–3197. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soobryan N, Murugesan S, Phoswa W,
Gathiram P, Moodley J and Mackraj I: The effects of sildenafil
citrate on uterine angiogenic status and serum inflammatory markers
in an L-NAME rat model of pre-eclampsia. Eur J Pharmacol.
795:101–107. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jim B and Karumanchi SA: Preeclampsia:
Pathogenesis, prevention, and long-term complications. Semin
Nephrol. 37:386–397. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lisonkova S and Joseph KS: Incidence of
preeclampsia: Risk factors and outcomes associated with
early-versus late-onset disease. Am J Obstet Gynecol.
209:544.e1–544.e12. 2013. View Article : Google Scholar
|
|
11
|
Hladunewich M, Karumanchi SA and Lafayette
R: Pathophysiology of the clinical manifestations of preeclampsia.
Clin J Am Soc Nephrol. 2:543–549. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roberts JM and Gammill HS: Preeclampsia:
Recent insights. Hypertension. 46:1243–1249. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lam C, Lim KH and Karumanchi SA:
Circulating angiogenic factors in the pathogenesis and prediction
of preeclampsia. Hypertension. 46:1077–1085. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chaiworapongsa T, Chaemsaithong P, Yeo L
and Romero R: Pre-eclampsia part 1: Current understanding of its
pathophysiology. Nat Rev Nephrol. 10:466–480. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Powe CE, Levine RJ and Karumanchi SA:
Preeclampsia, a disease of the maternal endothelium: The role of
antiangiogenic factors and implications for later cardiovascular
disease. Circulation. 123:2856–2869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zeisler H, Hund M and Verlohren S: The
sFlt-1: PlGF ratio in women with suspected preeclampsia. N Engl J
Med. 374:1785–1786. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Motta C, Grosso C, Zanuzzi C, Molinero D,
Picco N, Bellingeri R, Alustiza F, Barbeito C, Vivas A and Romanini
MC: Effect of Sildenafil on Pre-Eclampsia-Like Mouse Model Induced
By L-Name. Reprod Domest Anim. 50:611–616. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Souza CO, Peraçoli MT, Weel IC, Bannwart
CF, Romão M, Nakaira-Takahagi E, Medeiros LT, Silva MG and Peraçoli
JC: Hepatoprotective and anti-inflammatory effects of silibinin on
experimental preeclampsia induced by L-NAME in rats. Life Sci.
91:159–165. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu W, Qiao F, Liu H, Gong X, Shi X, Li Y
and Wu Y: Low molecular weight heparin improves proteinuria in rats
with L-NAME induced preeclampsia by decreasing the expression of
nephrin, but not podocin. Hypertens Pregnancy. 34:24–35. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rowsell HC: Program and objectives of the
Canadian Council on Animal Care. Lab Anim Sci. 37:24–27.
1987.PubMed/NCBI
|
|
21
|
Song Y, Shi MM, Zhang YY, Mo XD, Wang Y,
Zhang XH, Xu LP, Huang XJ and Kong Y: Abnormalities of the bone
marrow immune microenvironment in patients with prolonged isolated
thrombocytopenia after allogeneic hematopoietic stem cell
transplantation. Biol Blood Marrow Transplant. 23:906–912. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chatziandreou N, Farsakoglu Y,
Palomino-Segura M, D'Antuono R, Pizzagalli DU, Sallusto F,
Lukacs-Kornek V, Uguccioni M, Corti D, Turley SJ, et al: Macrophage
death following influenza vaccination initiates the inflammatory
response that promotes dendritic cell function in the draining
lymph node. Cell Rep. 18:2427–2440. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu Y, Paniccia A, Schulick AC, Chen W,
Koenig MR, Byers JT, Yao S, Bevers S and Edil BH: Identification of
CD112R as a novel checkpoint for human T cells. J Exp Med.
213:167–176. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gille JH, Moore DG and Sedgwick CJ:
Placental infarction: A sign of pre-eclampsia in a patas monkey
(Erythrocebus patas). Lab Anim Sci. 27:119–121. 1977.PubMed/NCBI
|
|
25
|
Rogers JB, Klein R and Blumenthal HT:
Placental permeability in a toxemia-susceptible strain of guinea
pigs. Am J Obstet Gynecol. 88:495–501. 1964. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diket AL, Pierce MR, Munshi UK, Voelker
CA, Eloby-Childress S, Greenberg SS, Zhang XJ, Clark DA and Miller
MJ: Nitric oxide inhibition causes intrauterine growth retardation
and hind-limb disruptions in rats. Am J Obstet Gynecol.
171:1243–1250. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumasawa K, Ikawa M, Kidoya H, Hasuwa H,
Saito-Fujita T, Morioka Y, Takakura N, Kimura T and Okabe M:
Pravastatin induces placental growth factor (PGF) and ameliorates
preeclampsia in a mouse model. Proc Natl Acad Sci USA.
108:1451–1455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Venkatesha S, Toporsian M, Lam C, Hanai J,
Mammoto T, Kim YM, Bdolah Y, Lim KH, Yuan HT, Libermann TA, et al:
Soluble endoglin contributes to the pathogenesis of preeclampsia.
Nat Med. 12:642–649. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tal R, Shaish A, Barshack I, Polak-Charcon
S, Afek A, Volkov A, Feldman B, Avivi C and Harats D: Effects of
hypoxia-inducible factor-1alpha overexpression in pregnant mice:
Possible implications for preeclampsia and intrauterine growth
restriction. Am J Pathol. 177:2950–2962. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kanasaki K, Palmsten K, Sugimoto H, Ahmad
S, Hamano Y, Xie L, Parry S, Augustin HG, Gattone VH, Folkman J, et
al: Deficiency in catechol-O-methyltransferase and
2-methoxyoestradiol is associated with pre-eclampsia. Nature.
453:1117–1121. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kupferminc MJ, Peaceman AM, Wigton TR,
Rehnberg KA and Socol ML: Tumor necrosis factor-alpha is elevated
in plasma and amniotic fluid of patients with severe preeclampsia.
Am J Obstet Gynecol. 170:1752–1757; discussion 1757–1759. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
LaMarca BB, Bennett WA, Alexander BT,
Cockrell K and Granger JP: Hypertension produced by reductions in
uterine perfusion in the pregnant rat: Role of tumor necrosis
factor-alpha. Hypertension. 46:1022–1025. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wenzel K, Rajakumar A, Haase H, Geusens N,
Hubner N, Schulz H, Brewer J, Roberts L, Hubel CA, Herse F, et al:
Angiotensin II type 1 receptor antibodies and increased angiotensin
II sensitivity in pregnant rats. Hypertension. 58:77–84. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Takimoto-Ohnishi E, Saito T, Ishida J,
Ohnishi J, Sugiyama F, Yagami K and Fukamizu A: Differential roles
of renin and angiotensinogen in the feto-maternal interface in the
development of complications of pregnancy. Mol Endocrinol.
19:1361–1372. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Baksu B, Davas I, Baksu A, Akyol A and
Gulbaba G: Plasma nitric oxide, endothelin-1 and urinary nitric
oxide and cyclic guanosine monophosphate levels in hypertensive
pregnant women. Int J Gynaecol Obstet. 90:112–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vechoropoulos M, Ish-Shalom M, Shaklai S,
Sack J, Stern N and Tordjman KM: The proatherogenic effect of
chronic nitric oxide synthesis inhibition in ApoE-Null mice is
dependent on the presence of PPAR α. PPAR Res. 2014:1245832014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ribeiro MO, Antunes E, de Nucci G,
Lovisolo SM and Zatz R: Chronic inhibition of nitric oxide
synthesis. A new model of arterial hypertension. Hypertension.
20:298–303. 1992.
|
|
38
|
Wang Y, Zhang F, Liu Y, Yin S, Pang X, Li
Z and Wei Z: Nebivolol alleviates aortic remodeling through eNOS
upregulation and inhibition of oxidative stress in l-NAME-induced
hypertensive rats. Clin Exp Hypertens. 39:628–639. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zeisler H, Llurba E, Chantraine F, Vatish
M, Staff AC, Sennström M, Olovsson M, Brennecke SP, Stepan H,
Allegranza D, et al: Predictive value of the sFlt-1: PlGF ratio in
women with suspected preeclampsia. N Engl J Med. 374:13–22. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Amaral TAS, Ognibene DT, Carvalho LCRM,
Rocha APM, Costa CA, Moura RS and Resende AC: Differential
responses of mesenteric arterial bed to vasoactive substances in
L-NAME-induced preeclampsia: Role of oxidative stress and
endothelial dysfunction. Clin Exp Hypertens. 40:126–135. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cornelius DC, Hogg JP, Scott J, Wallace K,
Herse F, Moseley J, Wallukat G, Dechend R and LaMarca B:
Administration of interleukin-17 soluble receptor C suppresses TH17
cells, oxidative stress, and hypertension in response to placental
ischemia during pregnancy. Hypertension. 62:1068–1073. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Miossec P and Kolls JK: Targeting IL-17
and TH17 cells in chronic inflammation. Nat Rev Drug Discov.
11:763–776. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guerby P, Vidal F, Garoby-Salom S,
Vayssiere C, Salvayre R, Parant O and Negre-Salvayre A: Oxidative
stress and preeclampsia: A review. Gynecol Obstet Fertil.
43:751–756. 2015.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Walker JJ: Inflammation and preeclampsia.
Pregnancy Hyperte-ns. 1:43–47. 2011. View Article : Google Scholar
|





















