Introduction
As an opportunistic infection, Pneumocystis
jirovecii pneumonia (PJP) is a severe and life-threatening
complication experienced by immunocompromised patients (1). With the advent of highly active
antiretroviral therapy and prophylaxis strategies, the incidence of
PJP has decreased markedly among patients with human
immunodeficiency virus (HIV), with a mortality rate <10%
(2). By contrast, non-HIV patients
with PJP are characterized by advanced age, increased
comorbidities, non-classical clinical symptoms, rapid deterioration
and poor prognosis (3–7). Once acute respiratory failure (ARF)
develops, non-HIV patients with PJP most likely require intensive
care and ventilatory support and have a mortality rate of up to
75.6% (5). Despite the
aforementioned differences, treatment regimens remain the same for
HIV and non-HIV patients with PJP; for example, the administration
of trimethoprim/sulfamethoxazole (TMP-SMZ) as the first-line drug,
then an adjunctive steroid (standard dosage) for severe ARF,
followed by the second-line drug (8–15).
Therefore, other treatment options need to be investigated.
Due to the ability to inhibit the synthesis of β-1,
3-glucan, a major component of the cell wall of P.
jirovecii, echinocandins could be used in the treatment of PJP
(15–20). During the past two decades, the
prophylactic and therapeutic efficacies of echinocandins have been
investigated in animal models and clinical studies (21,22). A
cohort study demonstrated a high success rate (8/10) with
caspofungin salvage treatment in HIV-patients with PJP; however,
data regarding the treatment of PJP with echinocandins in non-HIV
patients remain limited and controversial (15,20).
The current report described two HIV-negative cases
of PJP in which echinocandins were used for salvage treatment at
the Medical Intensive Care Unit, Peking Union Medical College
Hospital (Beijing, China). Both patients provided written informed
consent. A review of previous reports involving the use of
echinocandins for the treatment of PJP in non-HIV patients was also
conducted.
Case report
Case 1
A 71-year-old male presented to the Emergency
Department of the Peking Union Medical College Hospital (Beijing,
China) in October 2017 with a 1-week history of fever,
non-productive cough and progressive dyspnea. The patient had been
diagnosed with IgG4-related disease 5 months prior to admission.
Prednisone (Shandong Luoxin Pharmaceutical Group Stock Co., Ltd.,
Linyi, China) was administered and the dosage was gradually reduced
to 20 mg/day after achieving remission. The patient also had
chronic renal failure with regular dialysis treatment. Examination
at the emergency room disclosed severe hypoxemia; chest computed
tomography (CT) exhibited diffuse bilateral ground-glass opacities
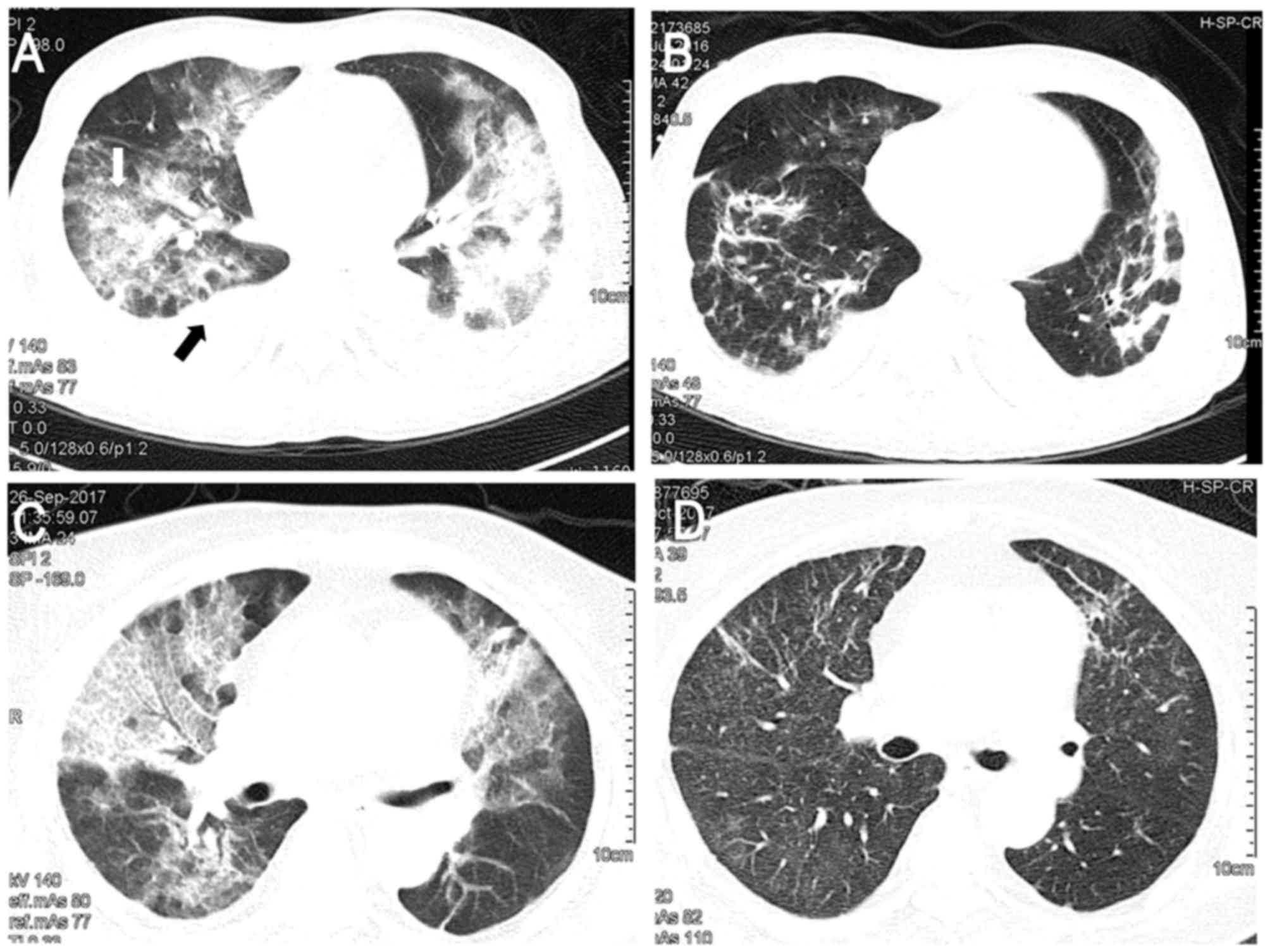
(GGO) of lung fields with pleural effusion (Fig. 1A). The patient was transferred to the
medical intensive care unit (MICU) for noninvasive positive
pressure ventilation and continuous renal replacement therapy.
Based on the radiographic findings, the patient was empirically
treated with TMP-SMZ (15 mg/kg/day TMP; Shandong Xinhua
Pharmaceutical Co., Ltd., Zibo, China) along with
methylprednisolone (40 mg, twice daily; Pfizer, Inc., New York, NY,
USA) for PJP, and moxifloxacin (400 mg/day; Bayer AG, Leverkusen,
Germany) for possible infections with atypical pathogens. The
patient's symptoms did not improve and required increased
respiratory support. Therefore, a regimen of clindamycin (600 mg/12
h; Hubei Shishun Biotechnology Co., Ltd., Hubei, China) and
primaquine (30 mg/day; Shanghai Zhongxi Pharmaceutical Co., Ltd.,
Shanghai, China) was initiated as salvage therapy on day 4 of the
ICU stay, the day PJP was confirmed by positive PJ DNA and
Gomorimethenamine silver staining in the bronchoalveolar lavage
(BAL) specimen. Despite this treatment, the patient's condition
further deteriorated and a repeated chest high-resolution CT scan
revealed aggravation of the bilateral diffuse GGO combined with
consolidation. Caspofungin (Merck & Co., Inc., Whitehouse
Station, NJ, USA) was administered at 70 mg on day 8 in the ICU,
followed by 50 mg daily. Treatment with moxifloxacin was
discontinued on the same day due to no evidence of atypical
pathogens. The patient's respiratory condition improved markedly
and respiratory support was gradually decreased following treatment
with caspofungin. The patient was weaned off non-invasive positive
pressure ventilation on day 19 in the ICU. A chest high-resolution
CT demonstrated that the majority of the patches were absorbed and
the pulmonary infiltrations were reduced after 14 days of treatment
with caspofungin (Fig. 1B). The
patient was transferred to the general ward on after 22 days in the
ICU and eventually recovered, and was discharged on day 54.
Case 2
A 68-year-old female with systemic lupus
erythematosus (SLE) was admitted to the Department of Rheumatology
of the Peking Union Medical College Hospital due to lupus disease
activity in August 2017. Prior to admission, the patient was
treated with methylprednisolone for nearly 3 months due to
hemolytic anemia associated SLE. During this hospitalization
period, the patient received methylprednisolone 0 mg/12 h; Pfizer,
Inc.) in addition to cyclophosphamide (4 mg/kg/day; Sinopharm
Shantou Jinshi Pharmaceutical Co., Ltd., Shantou, China). On day 11
after admission, the patient developed a fever of 38.5°C and
shortness of breath. A chest CT exhibited diffuse bilateral GGO
(Fig. 1C). Polymerase chain reaction
(PCR) revealed that P. jirovecii was detected within induced
sputum. Treatment with TMP-SMZ was initiated (15 mg/kg/day TMP
component, Shandong Xinhua Pharmaceutical Co., Ltd.).
Administration of immunosuppressive agents to the patient was
discontinued. Despite this treatment, the patient remained febrile
and exhibited increasing dyspnea requiring a reservoir face mask.
Given the severity of the condition, the patient was transferred to
the MICU to start high-flow nasal cannula (HFNC) oxygen therapy
(flow of 60 l/min and 60% FiO2) on day 16; caspofungin
(Merck & Co., Inc.) was administered as salvage therapy at a
loading dose of 70 mg, followed by a maintenance dose of 50 mg
daily. After 4 days of treatment with caspofungin, fever
alleviated, respiratory conditions improved and the HFNC was
replaced by conventional nasal prongs. The patient was transferred
to the ICU ward on day 7. A follow-up chest CT scan demonstrated a
partially normal appearance of the lung fields (Fig. 1D).
Quantitative polymerase chain reaction
(qPCR)
DNA was extracted using a commercial kit (QIAamp DNA
Mini kit; Qiagen GmbH, Hilden, Germany) following the manufacturers
protocol, but with elution using 30 µl Tris-EDTA buffer solution
(Beijing Leagene Biotech Co., Ltd., Beijing, China). Purified DNA
was then used as a template to amplify the part of the
mitochondrial gene, which encodes the large subunit of rRNA. PCR
was performed using the following primers: pAZ102-E forward,
5′-GATGGCTGTTTCCAAGCCCA-3′ and reverse, 5′-GTGTACGTTGCAAAGTACTC-3′.
Albumin forward, 5′-TGGTGAAATGGCTGACTGCT-3′ and reverse,
5′-CTCTGGTCTCACCAATCGGG-3′. PCR was performed with a total reaction
volume of 20 µl, comprising: Template DNA 2 µl (10–100 ng), 0.4 µM
of forward primer 0.4 µl, 0.4 Μm of reverse primer 0.4 µl,
ddH2O 6.8 µl and the SYBR Green Mix (2×) 10 µl
(SYBRGreen qPCR Master Mix, MedChemExpress USA, Monmouth Junction,
NJ, USA). The thermocycling conditions were as follows:
Pre-amplification denaturation at 95°C for 5 min; 40 cycles of 95°C
for 15 sec, 60°C for 30 sec The relative amount of gene expression
was normalized using Albumin and was calculated using the
2−ΔΔCq formula as previously described (23). PCR mixtures were prepared in a
laminar-flow cabinet and several controls were implemented. All
amplifications were performed in parallel with a negative control
(ultrapure distilled water) and a positive control (BAL samples of
patients with definite Pneumocystis pneumonia).
Gomorimethenamine silver staining
Slides were prepared using cytocentrifugation
(13,800 × g, 15 min, 4°C), cut to a thickness of 2–3 µm and
microwaved in a 10% chromic acid (Hubei XinRunde Chemical Co.,
Ltd., Hubei, China) solution at 65°C for 40 sec. Samples were then
washed with water and cleared using 1% sodium metabisulfite for 30
sec. After the slides were washed with distilled water, they were
placed in a Coplin jar containing 50 ml of methenamine working
solution and microwaved at 65°C for a further 65 sec. The slides
were rinsed again with distilled water and treated with 1% gold
chloride for 2–5 sec. Following further rinsing with distilled
water, samples were exposed to 5% sodium thiosulfate for 1 min,
counterstained with a light green working solution (Beijing Leagene
Biotech Co., Ltd.) and cleared using xylene. Samples were then
covered with cover slips and examined using routine light
microscopy at a magnification of ×70.
Through a literature review, the present study
identified 22 HIV-negative patients with PJP treated with
echinocandins (8–20). The demographic and clinical
characteristics of these cases, and the two patients included in
the present study, are summarized in Table I. Among these patients, the mean age
was 49.8 years. Underlying conditions varied between the included
cases, with solid organ transplant most commonly reported (11/24;
45.8%). Only three patients were given primary prophylaxis. All
enrolled cases exhibited concurrent bilateral pulmonary
infiltrations and were clinically treated for pneumonia. P.
jirovecii was the predominant pathogen that was confirmed by
methenamine silver staining or PCR. Of all patients, 8 and 16 were
treated with echinocandins as initial and salvage regimens,
respectively, and the mean duration of treatment with echinocandins
was 19 days. All included patients but one (11) were treated with caspofungin at 70 mg
on the first day with a maintenance dose of 50 mg/day. Associations
between the type of echinocandin treatment strategy, adjunctive
corticosteroids and the underlying disease are described in
Table II.
 | Table I.Clinical characteristics of the
reported cases of echinocandins use for Pneumocystis
jirovecii pneumonia in patients. |
Table I.
Clinical characteristics of the
reported cases of echinocandins use for Pneumocystis
jirovecii pneumonia in patients.
| Author, year | Patient no | Age/gender | Underlying
Disease | Initial
treatment | Cause of EC use | Salvage regimen | Time to use EC
(days) | Steroid used? | Duration of EC
(days) | End | (Refs.) |
|---|
| Zhang et al,
2006 | 1 | 93/M | COPD | TMP-SMZ | Adverse reaction | CA | 32 | No | 42 | S | (8) |
| Annaloro et
al, 2006 | 2 | 45/M | For HSCT | TMP-SMZ | Treatment
failure | CA+ TMP-SMZ | 11 | Yes | 45 | S | (9) |
| Beltz et al,
2006 | 3 | 5/M | ALL | CA+ TMP-SMZ | Empirical use | None used | NR | Yes | 22 | S | (10) |
| Kamboj et al,
2006 | 4 | 13/M | HSCT | CA | Antifungal | Pentamidine | NR | No | 18 | D | (11) |
| Kamboj et al,
2006 | 5 | 42/F | ALL | TMP-SMZ | Antifungal | MI + Others | 9 | No | 30 | D | (11) |
| Takeda et
al, 2009 | 6 | 47/M | Liver TP | MI+ TMP-SMZ | Empirical use | MI+ TMP-SMZ | NR | No | N/A | S | (12) |
| Zhang et al,
2012 | 7 | 58/M | Lung Cancer | N/A | Treatment
failure | CA+ TMP-SMZ +
CLI+PRI | NR | Yes | N/A | D | (13) |
| Li, 2016 | 8 | 46/M | CKD | TMP-SMZ | Adverse
reaction | CA+CLI | NR | Yes | 33 | S | (14) |
| Kim et al,
2013 | 9 | 1/M | SCID | TMP-SMZ | Treatment
failure | CA+ TMP-SMZ +AT+PRO
(CLI+PRI) | 26 | No | 26 | D | (15) |
| Kim et al,
2013 | 10 | 63/M | Liver TP | TMP-SMZ | Treatment
failure | CA+ TMP-SMZ | 9 | No | 5 | D | (15) |
| Kim et al,
2013 | 11 | 57/M | Kidney TP | TMP-SMZ | Treatment
failure | TMP-SMZ + PRI+CLI
(CA) | 18 | No | 11 | D | (15) |
| Kim et al,
2013 | 12 | 46/F | Liver TP | TMP-SMZ | Treatment
failure | CA+ TMP-SMZ
(CLI+PRI) | 2 | No | 7 | S | (15) |
| Tu et al,
2013 | 13 | 61/M | Kidney TP | TMP-SMZ | Adverse
reaction | CA+ TMP-SMZ | >10 | Yes | 14 | D | (16) |
| Tu et al,
2013 | 14 | 35/M | Kidney TP | TMP-SMZ | Adverse
reaction | CA+ TMP-SMZ | 10 | Yes | 14 | S | (16) |
| Tu et al,
2013 | 15 | 43/M | Kidney TP | CA+ TMP-SMZ | Empirical use | None used | 7 | No | 14 | S | (16) |
| Jiang et al,
2013 | 16 | 46/M | LBC-L | CA | Adverse
reaction | None used | 5 | No | NA | S | (17) |
| Mu et al,
2009 | 17 | 76/M | CML | CA | Adverse
reaction | CA+ TMP-SMZ | 9 | Yes | 21 | S | (18) |
| Hof and Schnülle,
2008 | 18 | 60/M | WG | TMP-SMZ | Treatment
failure | CA | 9 | No | 21 | S | (19) |
| Utili et al,
2007 | 19 | 57/F | Kidney TP | CA | Antifungal | CA+ TMP-SMZ | 1 | No | 14 | S | (20) |
| Utili et al,
2007 | 20 | 28/M | Kidney TP | TMP-SMZ | Treatment
failure | CA+ TMP-SMZ | 7 | Yes | 16 | S | (20) |
| Utili et al,
2007 | 21 | 59/M | Heart TP | TMP-SMZ | Treatment
failure | CA+ TMP-SMZ | 6 | Yes | 7 | S | (20) |
| Utili et al,
2007 | 22 | 58/F | Heart TP | CA+ TMP-SMZ | Empirical use | None used | 1 | Yes | 14 | S | (20) |
| Present study | 23 | 71/M | IgG4 | TMP-SMZ | Treatment
failure | CA+ TMP-SMZ | 14 | Yes | 21 | S | – |
| Present study | 24 | 68/F | SLE | TMP-SMZ | Treatment
failure | CA+ TMP-SMZ | NR | Yes | 7 | S | – |
 | Table II.Mortality of patients with
Pneumocystis jirovecii pneumonia treated with
echinocandins. |
Table II.
Mortality of patients with
Pneumocystis jirovecii pneumonia treated with
echinocandins.
| Author, year | Characteristic | Mortality (%) | (Refs.) |
|---|
| Zhang et al,
2006; Hof and Schnülle, 2008 | Used EC as
monotherapyin salvage regimen | 0/2 (0) | (8,19) |
| Kamboj et
al, 2006 | EC as initial
regimen | 1/8 (12.5) | (11) |
| Kamnoj et
al, 2006; Zhang et al, 2012; Kim et al, 2013; Tu
et al, 2013 | Total
mortality | 7/24 (29.2) | (11,13,15,16) |
| Kamnoj et
al, 2006; Zhang et al, 2012; Kim et al, 2013; Tu
et al, 2013 | Used EC as
combination therapy in salvage regimen | 5/14 (35.7) | (11,13,15,16) |
| Kamnoj et
al, 2006; Zhang et al, 2012; Kim et al, 2013; Tu
et al, 2013 | EC as salvage
regimen | 5/16 (31.5) | (11,13,15,16) |
| Kamboj et
al, 2006; Kim et al, 2013 | Treatment without
steroid regimen | 5/12 (41.7) | (11,15) |
| Zhang et al,
2012; Kim et al, 2013 |
EC+(TMP-SMZ+PRI+CLI) | 2/3 (66.7) | (13,15) |
| Zhang et al,
2012; Tu et al, 2013 | Treatment with
steroid regimen | 2/12 (16.7) | (13,16) |
| Kim et al,
2013 |
EC+(AT+PRO)/(PRI+CLI)a | 1/1 (100) | (15) |
| Kim et al,
2013; Tu et al, 2013 | EC+TMP-SMZin
salvage regimen | 2/9 (22.2) | (15,16) |
| Kamnoj et
al, 2006 | Used EC as
monotherapyin initial regimen | 1/2 (50.0) | (11) |
| – | Used
ECplusTMP-SMZin initial regimen | 0/6 (0) | – |
| – |
EC+(TMP-SMZ+CLI) | 0/1 (0) | – |
Discussion
The present study described two successful cases of
treatment with echinocandins for non-HIV patients with PJP. In
addition to the two cases in the present study, 24 non-HIV PJP
cases treated with echinocandins were identified in the literature
(8–20). This revealed that, although a
rationale exists for the use of echinocandins in the treatment of
PJP, the current clinical use appears to be low. Potential reasons
for this may include that fact that the use of echinocandins to
treat PJP has only appeared in recent years, the lack of convincing
efficacy data and that the use of echinocandins for PJP treatment
is currently off-label (11,19,20).
The present study analyzed the application strategy
of caspofungin in non-HIV patients with PJP. The results reported
in previous studies suggested that echinocandins were most
frequently considered as a salvage regimen (16/24), with a
mortality rate of 31.5% (5/16 patients) (8–9,11,13–16,19,20). As
of yet, no sufficient data for the outcome of echinocandins as a
salvage regimen in non-HIV patients with PJP have been reported. In
fact, for HIV patients who fail to respond to initial treatment,
several traditional salvage regimens have shown high therapeutic
effectiveness, including the combination of clindamycin and
primaquine [42-44/48 (88–92%)] or atovaquone [4/5 (80%)] (24). However, regarding non-HIV patients
with PJP, the data of these traditional salvage regimens is
limited. In a previous study, clindamycin-primaquine or pentamidine
were used as salvage regimens following treatment failure of the
first-line regimen in 12 non-HIV patients with PJP and the
mortality rate was 66.7% (8/12) (25). Therefore, the present results
indicated that use of echinocandins(caspofungin starting with a
loading dosage of 70 mg followed by 50 mg/day) as salvage therapy
resulted in favorable and comparative rates of mortality in non-HIV
patients compared with previous salvage regimens; however a
statistical comparison was not possible. This suggests that an
echinocandin-based salvage regimen maybe an option for the
treatment of PJP in non-HIV patients.
There were 8 cases from previously published studies
that used an echinocandin-based regimen for the initial treatment
(10–12,16–18,20) and
exhibited a good response with survival rates of 87.5%. When only
the 6/8 cases that used the combination regimen of echinocandins
and TMP-SMZ were considered, an excellent response was observed as
all 6 patients had survived the infection. When the cases that used
the combined regimen of echinocandins and TMP-SMZ in salvage
therapy were also included, the mortality rate was 13.3% (2/15).
These results appeared to favor the combination of echinocandins
and TMP-SMZ.
The combined regimen of echinocandins and TMP-SMZ
has been associated with high effectiveness in clearing the
invading P. jirovecii (26).
P. jirovecii is a fungus that exists in either trophic or
cyst forms (trophic/cyst 9:1) (27).
The primary component of the cyst cell wall is β-1, 3-glucan, which
is poorly expressed in the trophic forms. Therefore, echinocandins
mainly act on cyst forms by inhibiting the β-1, 3-glucan
synthaseenzyme and disturbing the integrity of the cell wall
(21,22). Previous experiments suggested that
the use of caspofungin alone was associated with a 90% decrease in
cyst forms after 4 days and a 66% reduction in trophic forms after
21 days of treatment. Furthermore, in an animal model of PJP, the
administration of low doses of caspofungin with TMP-SMX may provide
an improved clearance of Pneumocystis infection (28). On the other hand, echinocandins
exhibit reduced activity against the trophic forms of P.
jirovecii and the use of echinocandin alone may not completely
eradicate the infection. Therefore, the co-administration of
echinocandins and TMP/SMZ, which is primarily active against
trophic forms, may exert synergistic activity against P.
jirovecii by fully inhibiting the life cycle of the organism.
However, clinical data regarding this combined therapy regimen were
confined to case reports only (13,16,25).
In conclusion, positive clinical effects of
echinocandins were observed in the present study, which suggested
that the addition of echinocandins to TMP/SMZ was active against
trophic forms and may demonstrate activity against P.
jirovecii by inhibiting the organism's life cycle. However, the
present analysis was based exclusively on case reports. It is
possible that patients with favorable outcomes from the use of
echinocandins may have been selectively reported in these case
reports. Therefore, a large sample, well-designed randomized
trials, particularly in the use of echinocandins combined with
TMP-SMZ for the treatment of PJP are warranted to verify these
results.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HBH, JMP and BD contributed to the conception,
design and data interpretation. All authors read and approved the
manuscript.
Ethics approval and consent to
participate
Patients provided written informed consent to
participate.
Patient consent for publication
Consent for publication was obtained from the
patients.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ARF
|
acute respiratory failure
|
|
CT
|
computed tomography
|
|
GGO
|
ground-glass opacity
|
|
HFNC
|
high flow nasal cannula
|
|
HIV
|
human immunodeficiency virus
|
|
MICU
|
medical intensive care unit
|
|
PJP
|
Pneumocystis jirovecii
pneumonia
|
|
SLE
|
systemic lupus erythematosus
|
|
TMP-SMZ
|
trimethoprim/sulfamethoxazole
|
References
|
1
|
Li MC, Lee NY, Lee CC, Lee HC, Chang CM
and Ko WC: Pneumocystis jiroveci pneumonia in immunocompromised
patients: Delayed diagnosis and poor outcomes in
2014,non-HIV-infected individuals. J Microbiol Immunol Infect.
47:42–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mansharamani NG, Garland R, Delaney D and
Koziel H: Management and outcome patterns for adult pneumocystis
carinii pneumonia, 1985 to 1995: Comparison of HIV-associated cases
to other immunocompromised states. Chest. 118:704–711. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Miller RF, Allen E, Copas A, Singer M and
Edwards SG: Improved survival for HIV infected patients with severe
pneumocystis jirovecii pneumonia is independent of highly active
antiretroviral therapy. Thorax. 61:716–721. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yale SH and Limper AH: Pneumocystis
carinii pneumonia in patients without acquired immunodeficiency
syndrome: Associated illness and prior corticosteroid therapy. Mayo
Clin Proc. 71:5–13. 1996. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weng L, Huang X, Chen L, Feng LQ, Jiang W,
Hu XY, Peng JM, Wang CY, Zhan QY and Du B: Prognostic factors for
severe Pneumocystis jirovecipneumonia of non-HIV patients in
intensive care unit: A bicentric retrospective study. BMC
Infectious Dis. 16:5282016. View Article : Google Scholar
|
|
6
|
Monnet X, Vidal-Petiot E, Osman D,
Hamzaoui O, Durrbach A, Goujard C, Miceli C, Bourée P and Richard
C: Critical care management and outcome of severe Pneumocystis
pneumonia in patients with and without HIV infection. Crit Care.
12:R282008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Boonsarngsuk V, Sirilak S and Kiatboonsri
S: Acute respiratory failure due to pneumocystis pneumonia: Outcome
and prognostic factors. Int J Infect Dis. 13:59–66. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang JC, Dai JY, Fan J and Wu XP: The
treatment of pneumocystis Carinii pneumonia with caspofungin in
elderly patients: A case report and literature review. Zhonghua Jie
He He Hu Xi Za Zhi. 29:4632006.(In Chinese). PubMed/NCBI
|
|
9
|
Annaloro C, Volpe AD, Usardi P and
Lambertenghi Deliliers G: Caspofungin treatment of Pneumocystis
pneumonia during conditioning for bone marrow transplantation. Eur
J Clin Microbiol Infect Dis. 25:52–54. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Beltz K, Kramm CM, Laws HJ, Schroten H,
Wessalowski R and Göbel U: Combined trimethoprim and caspofungin
treatment for severe pneumocystis jiroveci pneumonia in a five year
old boy with acute lymphoblastic leukemia. Klin Pädiatr.
218:177–179. 2006. View Article : Google Scholar
|
|
11
|
Kamboj M, Weinstock D and Sepkowitz KA:
Progression of pneumocystis jiroveci pneumonia in patients
receiving echinocandin therapy. Clin Infect Dis. 43:e92–e94. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Takeda K, Morioka D, Kumamoto T, Matsuo K,
Tanaka K, Endo I, Togo S and Shimada H: A survival case of
ABO-incompatible liver transplantation complicated with severe
preoperative infection and subsequent overwhelming postsplenectomy
infection. Transplant Proc. 41:3941–3944. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang W, Wang GF, Nie LG, et al: Clinical
characteristics of Pneumocystis pneumonia during chemotherapy and
radiotherapy in lung cancer patients. J Prac Oncol. 27:175–179.
2012.
|
|
14
|
Li H, Huang H and He H: Successful
treatment of severe Pneumocystis pneumonia in an immunosuppressed
patient using caspofungin combined with clindamycin: A case report
and literature review. BMC Pulm Med. 16:1442016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim T, Hong HL, Lee YM, Sung H, Kim SH,
Choi SH, Kim YS, Woo JH and Lee SO: Is caspofungin really an
effective treatment for pneumocystis jirovecii pneumonia in
immunocompromised patients without human immunodeficiency virus
infection? Experiences at a single center and a literature review.
Scand J Infect Dis. 45:484–488. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tu GW, Ju MJ, Xu M, Rong RM, He YZ, Xue
ZG, Zhu TY and Luo Z: Combination of caspofungin and low-dose
trimethoprim/sulfamethoxazole for the treatment of severe
pneumocystis jirovecii pneumonia in renal transplant recipients.
Nephrology (Carlton). 18:736–742. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang XQ, Fang L, Mei XD, Wang XJ and Bao
MH: Pneumocystis jiroveci pneumonia in patients with non-Hodgkin's
lymphoma after Rituximab-containing regimen: Two cases of report
and literature review. J Thorac Dis. 5:E162–E166. 2013.PubMed/NCBI
|
|
18
|
Mu XD, Que CL, He B, Wang GF and Li HC:
Caspofungin in salvage treatment of severe pneumocystis pneumonia:
Case report and literature review. Chin Med J (Engl). 122:996–999.
2009.PubMed/NCBI
|
|
19
|
Hof H and Schnülle P: Pneumocystis
jiroveci pneumonia in a patient with Wegener's granulomatosis
treated efficiently with caspofungin. Mycoses. 1 Suppl:S65–S67.
2008. View Article : Google Scholar
|
|
20
|
Utili R, Durante-mangoni E, Basilico C,
Mattei A, Ragone E and Grossi P: Efficacy of caspofungin addition
to trimethoprim-sulfamethoxazole treatment for severe pneumocystis
pneumonia in solid organ transplant recipients. Transplantation.
84:685–688. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schmatz DM, Romancheck MA, Pittarelli LA,
Schwartz RE, Fromtling RA, Nollstadt KH, Vanmiddlesworth FL, Wilson
KE and Turner MJ: Treatment of pneumocystis carinii pneumonia with
1,3-beta-glucan synthesis inhibitors. Proc Natl Acad Sci USA.
87:5950–5954. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Armstrongjames D, Stebbing J, John L,
Murungi A, Bower M, Gazzard B and Nelson M: A trial of caspofungin
salvage treatment in PCP pneumonia. Thorax. 66:537–538. 2011.
View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Smego RA Jr, Nagar S, Maloba B and Popara
M: A Meta-analysis of salvage therapy for pneumocystis carinii
pneumonia. Arch Intern Med. 161:1529–1533. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim T, Kim SH, Park KH, Cho OH, Sung H,
Kim MN, Choi SH, Jeong JY, Woo JH, Kim YS and Lee SO:
Clindamycin-primaquine versus pentamidine for the second-line
treatment of pneumocystis pneumonia. J Infect Chemother.
15:343–346. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Powles MA, Liberator P, Anderson J,
Karkhanis Y, Dropinski JF, Bouffard FA, Balkovec JM, Fujioka H,
Aikawa M, McFadden D and Schmatz D: Efficacy of MK-991 (L-743,872),
a semisynthetic pneumocandin, in murine models of Pneumocystis
carinii. Antimicrob Agents Chemother. 42:1985–1989. 1998.PubMed/NCBI
|
|
27
|
Thomas CF Jr and Limper AH: Current
insights into the biology and pathogenesis of Pneumocystis
pneumonia. Nat Rev Microbiol. 5:298–308. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lobo ML, Esteves F, De SB, de Sousa B,
Cardoso F, Cushion MT, Antunes F and Matos O: Therapeutic potential
of caspofungin combined with trimethoprim-sulfamethoxazole for
pneumocystis pneumonia: A pilot study in mice. PLoS One.
8:e706192013. View Article : Google Scholar : PubMed/NCBI
|