Introduction
Bone defects, which may be caused by infections,
trauma, tumors or various congenital diseases, have become a major
challenge in the field of orthopedics (1). Current methods for treating bone
defects include autografts, allograft bone transplantation, tissue
engineering techniques and gene therapy. Autografts are recognized
as the ideal bone graft material, although these are associated
with a number of drawbacks, including a limited supply, pain at the
donor site and complications; furthermore, the sources of
allogeneic bone are limited, and a risk of infectious diseases and
varying degrees of immune response are present (2). Bone tissue engineering, which comprises
signaling molecule scaffold materials and seed cells, holds promise
for bone defect repair. Mesenchymal stem cells (MSCs) are adult
stem cells that are regarded as ideal seed cells due to their low
immunogenicity, as well as a high self-replicative ability and
multi-directional differentiation potential (3,4). These
stem cells may develop into multiple mesodermal lineages, including
bone and cartilage (5). Over the
course of the past few decades, MSCs have been isolated from
various types of tissue, including the bone marrow, umbilical cord
blood, umbilical cord tissue, placental tissue and adipose tissue
(6). However, BMSCs and UCMSCs
remain the major sources of MSCs, particularly in autologous
cell-based therapies, due to the ease of harvest and potential
autologous application (6–8). Indeed, BMSCs are recognized as the
ideal seed cells for bone tissue engineering therapies, and have
been successfully applied in the clinic. However, the negative
correlation between the viability and number of BMSCs with donor
age limits their applicability. Previous studies have indicated
that UCMSCs may be easily isolated, have a low immunogenicity and
are able to differentiate into mesenchymal lineages; therefore,
UCMSCs have been considered to have a high potential to be used as
seed cells (9,10). To confirm whether UCMSCs may be
employed as an alternative type of seed cell for bone regeneration,
the present study compared BMSCs and UCMSCs in vitro and in
ectopic bone formation and tibia bone defect models.
In addition to the seed cells, the signaling
molecule scaffold materials also serve an essential role in
cell-based bone regeneration. These scaffolds are named
‘biomimetic’, as they provide a suitable microenvironment for cell
attachment, growth, differentiation and reproduction (11,12).
In vivo studies have demonstrated de novo bone
formation or mineral deposition in MSC-implanted scaffolds, as well
as the direct involvement of transplanted cells in bone
regeneration (6,12,13).
Numerous studies have focused on the use of different types of
scaffold as basic tools for regenerative medicine (10,14).
Biofunctionalized macroporous calcium phosphate cement (CPC) has
demonstrated properties of excellent biocompatibility,
osteoconductivity, in situ hardening and molding
capabilities, as well as injectability, and may be resorbed and
replaced by newly formed bone in vivo (12,15,16). CPC
has the potential to fuse directly with the host bone and to guide
osteogenesis. However, to date, a comparison of UCMSCs and BMSCs
seeded on CPC scaffolds for bone regeneration has not been
provided, to the best of our knowledge.
In the present study, the osteogenic capacities of
BMSCs and UCMSCs in vitro were investigated by seeding them
on CPC, and a comparison of their osteogenic potential was made
using ectopic bone formation and tibia defect models in
vivo.
Materials and methods
Animals
A total of 27 male Sprague Dawley (SD) rats (220–240
g; 6–8 weeks) and 27 male Balb/c nude mice (18–22 g; 6–8 weeks)
were purchased from Beijing Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The SD rats received free
access to food and water and were housed at a temperature of
20–26°C, a humidity of 50–60% and a 12 h light/dark cycle. The
Balb/c nude mice were put in individual ventilated cages for mating
in the barrier system with ad libitum access to standard
murine food and water. The temperature, humidity and light/dark
cycle were the same as the rats as mentioned above.
The Institute of Radiation Medicine of the Chinese
Academy of Medical Sciences (Tianjin, China) provided an animal
experiment platform in which the animal experiments were performed.
The protocol was approved by the medical ethics committee of the
Institute of Radiation Medicine Chinese Academy of Medical Sciences
(Tianjin, China).
Culture of BMSCs and UCMSCs
BMSCs and UCMSCs were purchased from Cyagen
Biosciences, Inc. (Guangzhou, China). Cells were revived and seeded
in culture flasks, and maintained at 37°C in humidified atmosphere
containing 5% CO2 in Dulbecco's modified Eagle's medium
(DMEM)/F-12 (HyClone™; GE Healthcare, Logan, UT, USA)
containing 10% fetal bovine serum (FBS; Lanzhou Bailing
Biotechnology Co., Ltd., Lanzhou, China) and 1% penicillin and
streptomycin (P/S; North China Pharmaceutical Group Co., Ltd.,
Hebei, China). Cells were allowed to expand until they reached 80%
confluence. Passage-3 cells were used in the present study.
Routine characterization of the BMSCs and UCMSCs was
performed according to the manufacturer's protocol, as described
previously (17,18). After reaching 80% confluence, the
cells were treated with 0.25% trypsin-EDTA. Cells were incubated
with fluorochrome-conjugated primary antibodies against CD34 (cat.
no. 555822), CD44 (cat. no. 555479), CD45 (cat. no. 555483), CD73
(cat. no. 550257), CD90 (cat. no. 555596) and CD105 (cat. no.
560539; all from BD Biosciences, San Jose, CA, USA) at 4°C for 30
min. The samples were subsequently measured on a flow cytometer (BD
FACSCanto II; BD Biosciences) and data was analyzed with FlowJo
Version 7.6 software (Tree Star, Inc., Ashland, OR, USA).
A Cell Counting Kit-8 (CCK-8; Tongren Shanghai Co.,
Shanghai, China) was used to evaluate the proliferation of BMSCs
and UCMSCs. In brief, 2×103 cells were seeded onto
96-well plates and cultured for 8 days. Every day, designated wells
were stained with CCK-8 reagent according to the manufacturer's
protocols, the absorbance at 450 nm was determined using a
microplate reader.
Osteogenic differentiation
BMSCs and UCMSCs at passage 3 were cultured in
6-well plates with DMEM/F-12 medium. The next day, the media in the
experimental groups were replaced with osteo-inductive medium (OIM)
consisting of 50 µM ascorbate-2-phosphate, 10 mM
β-glycero-phosphate and 10 nM dexamethasone (all from
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) and control groups
were replaced with DMEM/high glucose medium (HyClone™; GE
Healthcare) with 10% FBS (6).
Alkaline phosphatase (ALP) staining was performed using the
Alkaline Phosphatase kit (cat. no. 86R-1KT; Sigma-Aldrich; Merck
KGaA) according to the manufacturer's instructions at day 9.
Staining with Alizarin red S (cat. no. A5533, Sigma-Aldrich; Merck
KGaA) was performed at day 18. Cells were washed with PBS, fixed
with 95% ethanol at room temperature for 15 min and stained with
0.1% Alizarin Red S (pH 4.2; Sigma-Aldrich; Merck KGaA) at 37 °C
for 30 min. The medium was replaced every 3 days during
culture.
Engineering of cell-scaffold
constructs
BMSCs and UCMSCs at passage 3 were used in the
subsequent experiments. First, the CPC scaffolds (Shanghai Rebone
Biomaterials Co., Ltd., Shanghai, China) were cut into an 8 mm
diameter round sheet with an average thickness of 2 mm, or to the
size of 2×2×6 mm. The CPCs were placed at the bottom of 24-well
plates in a 100-µl suspension (1×106 cells/ml) of BMSCs
or UCMSCs. Subsequently, the scaffold and cell complexes were
cultivated at 37°C with DMEM/F-12 for 24 h. As a control, certain
CPC pieces were immersed in medium lacking cells prior to
transplantation into the animal model.
Tibia defect model
A total of 27 mature SD rats were used for the
generation of the tibia bone defect model as described previously
(19,20). In brief, general anesthesia was
performed by administering sodium pentobarbital (40 mg/kg body
weight; intraperitoneal injection; Merck KGaA), and a 1–2 cm skin
incision was made to separate and expose the tibia following
disinfection. A unilateral 2×2×6-mm defect was created in the
middle part of the tibia. Rats were then randomly divided into
three groups (n=3 per group): The BMSCs+CPC scaffold, the
UCMSCs+CPC scaffold and the control group. For the control group,
the right side of the rats were implanted with the cell-free CPC
scaffold, whereas the left side remained untreated. Finally, the
surgical site was sutured. The grafts from each group were
harvested at 4, 6 and 8 weeks after the surgery, and these were
then evaluated using X-ray analysis, as well as histological and
immunohistochemical techniques.
Ectopic bone formation
A total of 27 BALB/c-nude mice aged 6–8 weeks were
used for the ectopic bone formation experiment (21). Mice were randomly divided into three
groups (n=3 per group): BMSCs+CPC scaffold, UCMSCs+CPC scaffold and
CPC scaffold (cell-free). Following anesthesia, an incision was
made in the dorsal side of the nude mouse and the scaffold
construct (8 mm in diameter) was implanted subcutaneously. The
wound was then closed in layers. The grafts were harvested at 4, 6
and 8 weeks following surgery and fixed in 4% paraformaldehyde.
Histological observations
Grafts were embedded in paraffin and cut into 5-µm
sections. These specimens were divided into two parts. One part was
stained with H&E, as described previously (22), whereas the other part was used for
immunofluorescence experiments. Osteopontin (OPN; cat. no.
sc-21742; 1:50; Santa Cruz Biotechnology, Inc., Dallas, TX, USA),
type I collagen (COL-1; cat. no. ab34710; 1:200; Abcam, Cambridge,
UK), Runt-related transcription factor 2 (Runx2; cat. no. sc-10758;
1:50; Santa Cruz Biotechnology, Inc.) and vascular endothelial
growth factor (VEGF; cat. no. ab46154; 1:200; Abcam) were assessed
according to protocols of previous studies (20,23).
Immunohistochemistry was performed using the
streptavidin-peroxidase method (SPlink Detection kit; cat. no.
SP-9001; OriGene Technologies, Inc., Rockville, MD, USA) according
to the manufacturer's protocol. The kit comprised Reagent A (goat
serum), B (goat anti-rabbit immunoglobulin G) and C (horseradish
peroxidase). Endogenous peroxidase activity was quenched using 1%
H2O2/methanol for 10 min and antigen
retrieval was performed with citrate buffer in the microwave for 10
min. Slides were blocked with reagent A at room temperature for 20
min and stained with the aforementioned primary antibodies
overnight at 4°C. Reagent B was then added and incubated at 37°C
for 20 min. Reagent C was added and incubated for 15 min at 37°C.
DAB (cat. no. ZLI-9019; OriGene Technologies, Inc.) was used for
further staining at room temperature for 6 min. Finally, sections
were counterstained with hematoxylin for 1 min at room temperature
and observed under an optical microscope (magnification, ×100;
Nikon Ti-S; Nikon Corporation, Tokyo, Japan) (20). In order to semi-quantitatively
analyze the immunoreactivity of OPN, COL-1 and VEGF, the H-score
was determined. The H-score was calculated by combining the
percentage of immunoreactive cells (%) with an estimate of the
staining intensity (0, 1, 2 or 3), as described previously
(24). An H-score of 9–12 was
considered to represent strong immunoreactivity (‘+++’), 5–8 was
considered moderate (‘++’), 1–4 was considered weak (‘+’) and 0 was
scored as negative (‘−’) (25,26).
Statistical analysis
Data were analyzed using SPSS 19.0 software (IBM
Corp., Armonk, NY, USA). Values are expressed as the mean ±
standard deviation. One-way analysis of variance and Tukey's post
hoc test were used to determine significant differences between the
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Expansion and characterization of the
cells
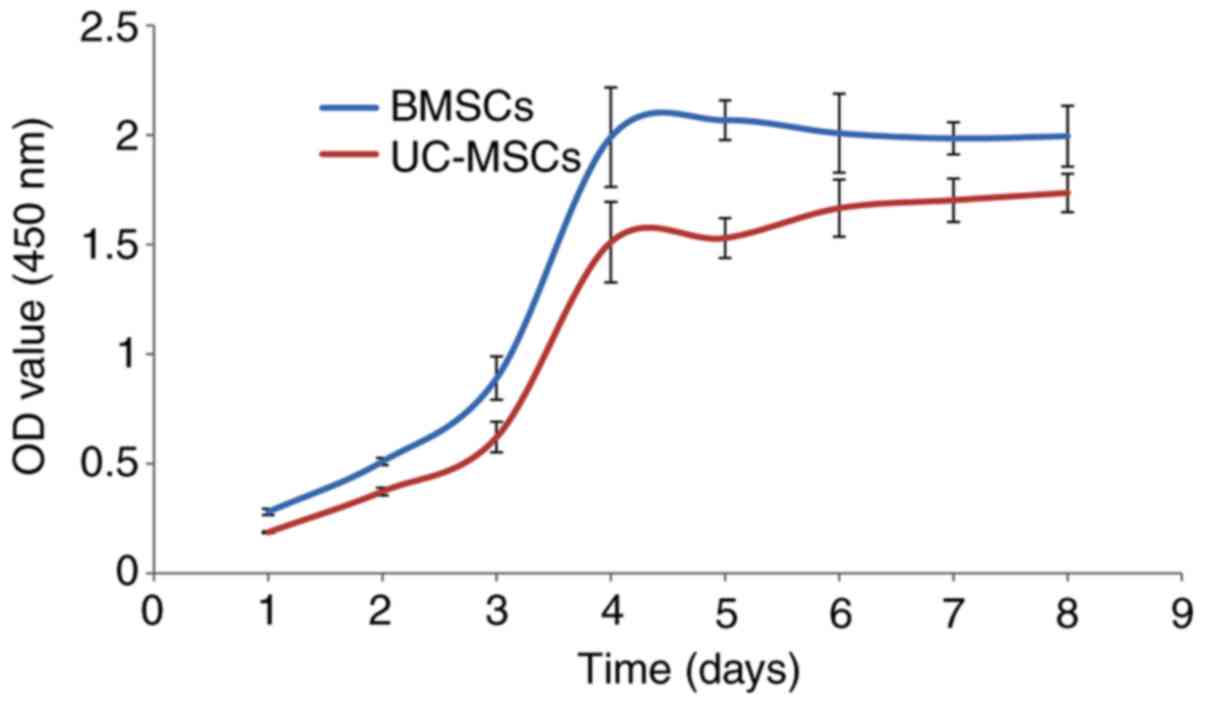
Assessment of the growth activities of BMSCs and
UCMSCs indicated that they exhibited a similar proliferation rate
within all phases of the growth period. In each of the two cases,
cells proliferated slowly at first, but then entered the
exponential growth phase, which continued for 3–5 days. Finally,
the cells became confluent and reached the period of stagnation
(Fig. 1).
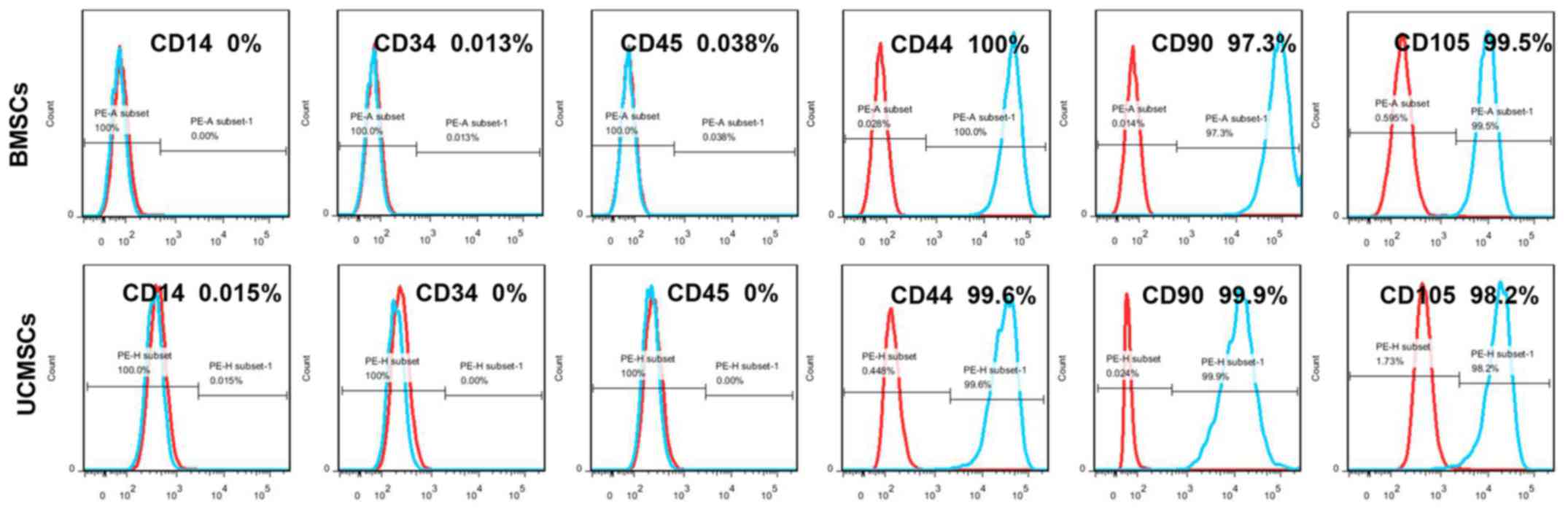
Flow cytometry was subsequently performed to
identify the typical cell-surface markers for MSCs. The results
indicated that BMSCs and UCMSCs were positive for the MSC markers
CD44, CD90 and CD105, but negative for the markers CD14, CD34 and
CD45 (Fig. 2). These results
suggested that the expression of cell markers was similar for BMSCs
and UCMSCs, and that the expression patterns conformed with the
typical surface markers for MSCs.
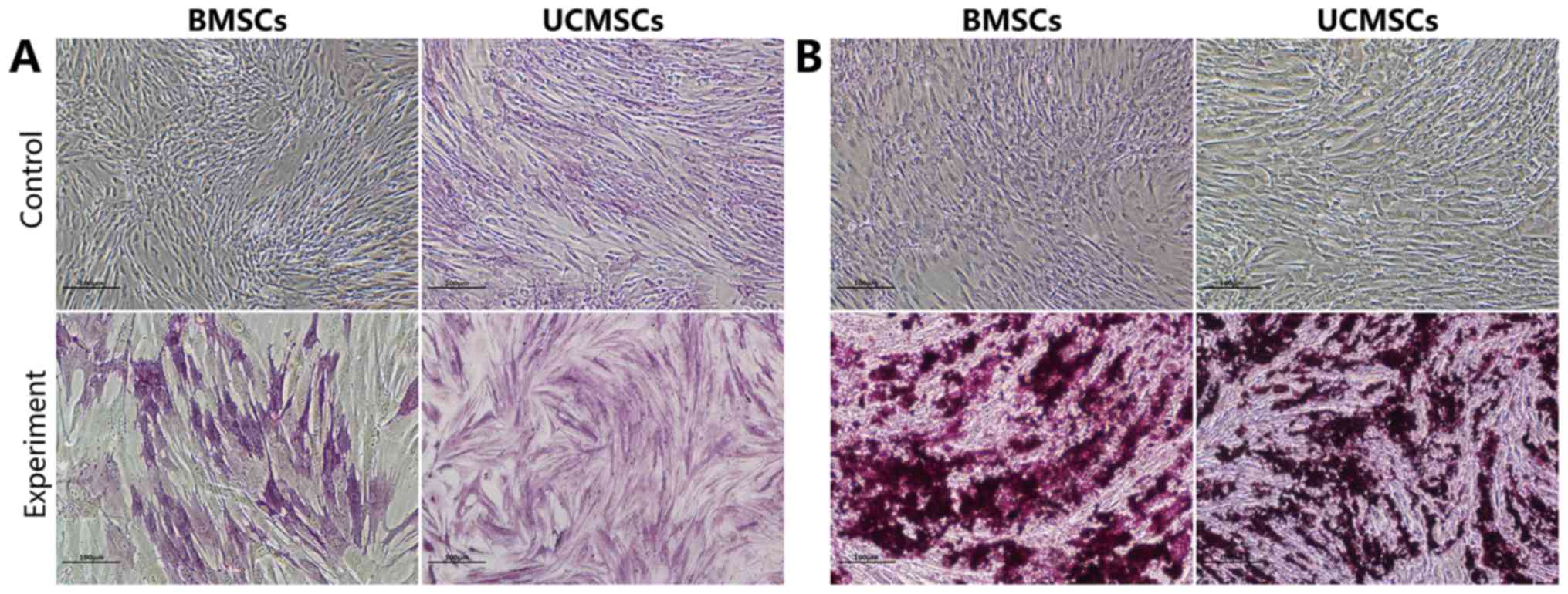
Osteogenesis of BMSCs and UCMSCs in
vitro
After having been cultured in OIM for 9 days, the
expression levels of ALP were high in the BMSCs and the UCMSCs.
After 18 days of culture in OIM, mineralized nodules were observed
in the BMSCs and UCMSCs, as demonstrated by Alizarin Red staining
(Fig. 3).
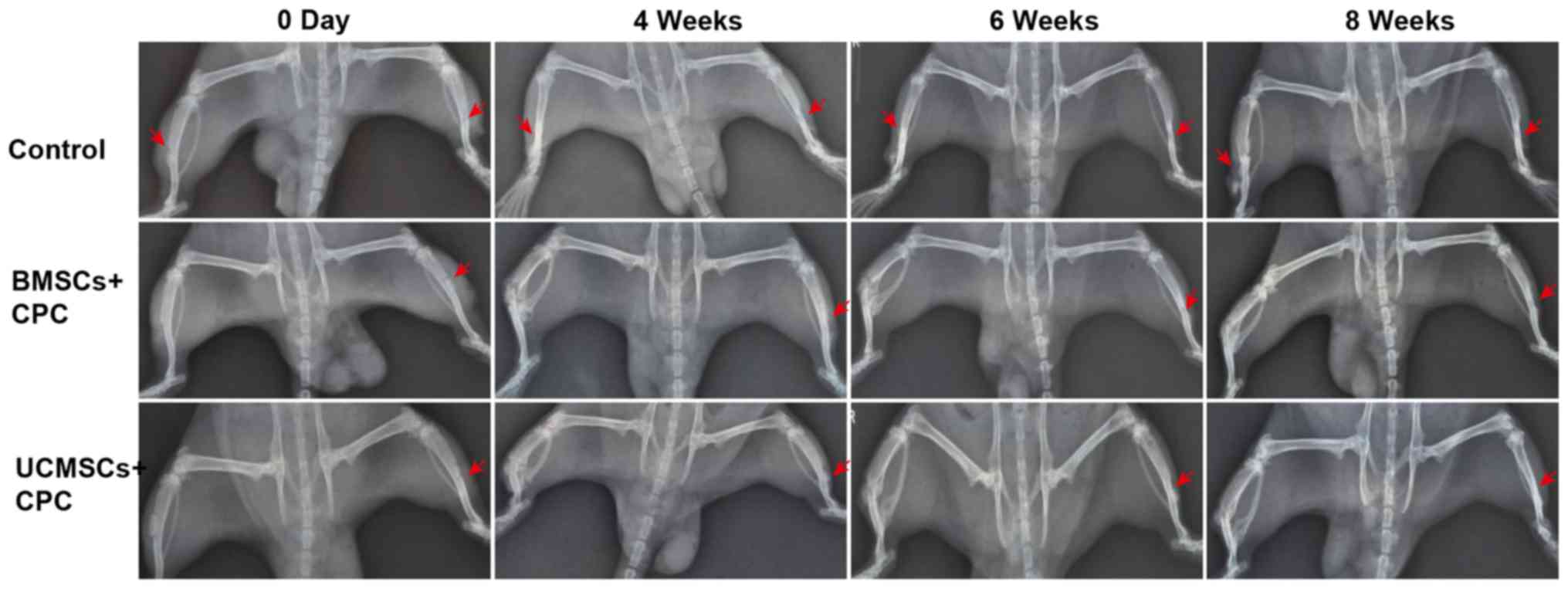
Bone regeneration using the tibia
defect model
To compare the effects of BMSCs and UCMSCs on bone
repair, the SD rat tibia defect model was constructed. The bone
structure in the defective area was observed by X-ray analysis at
4, 6 and 8 weeks after surgery. Fewer, or no new osseous were
observed in the defect area in control group (Fig. 4).
In addition, in the area of the defect, flaky
cartilage, bone-like matrixes and lacunae were observed in numerous
instances. A certain amount of fibrous tissue and vascular
hyperplasia were also observed. Lamellar bone was identified on
each side of the bone, which was densely arranged and close to
having reached maturity. The surface of the lamellar bone was lined
with osteoblasts, and a normal bone marrow cavity was observed at 8
weeks following the surgery in the BMSCs+CPC and UCMSCs+CPC groups.
However, in the CPC group, only a small amount of vascular
hyperplasia was observed, a large number of neutrophlis had
infiltrated into the area of the defect, and comparatively less
mature cancellous bone and normal bone tissue was observed in the
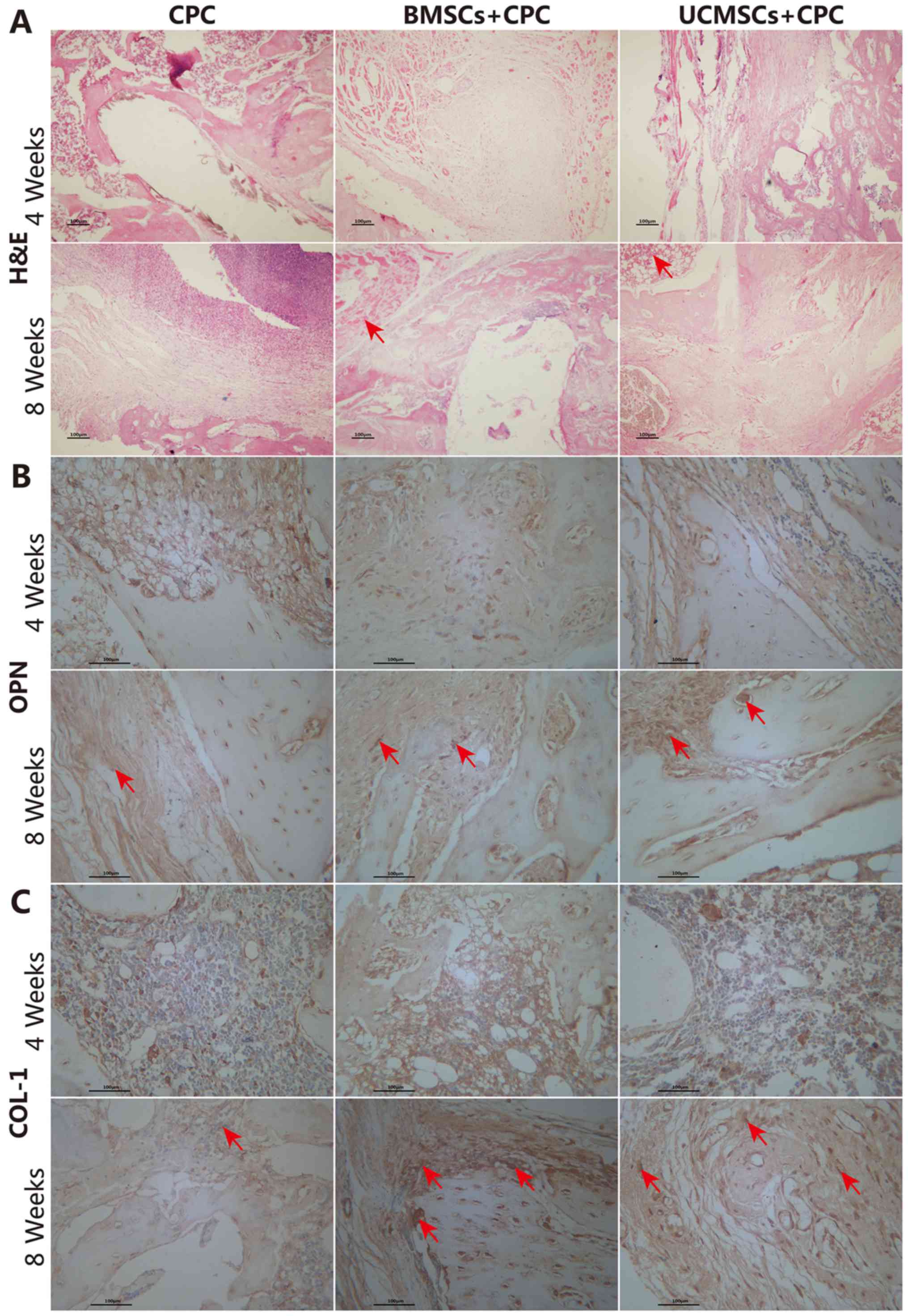
surrounding area on H&E staining (Fig. 5A).
At 4 weeks after the surgery, the expression of OPN
and COL-1 was detected in the BMSCs+CPC and UCMSCs+CPC groups,
although the staining lacked structure and definition (Fig. 5B and C). At 8 weeks after the
surgery, a markedly increased expression of OPN and COL-1 was
identified at the defect site of the experimental cell treatment
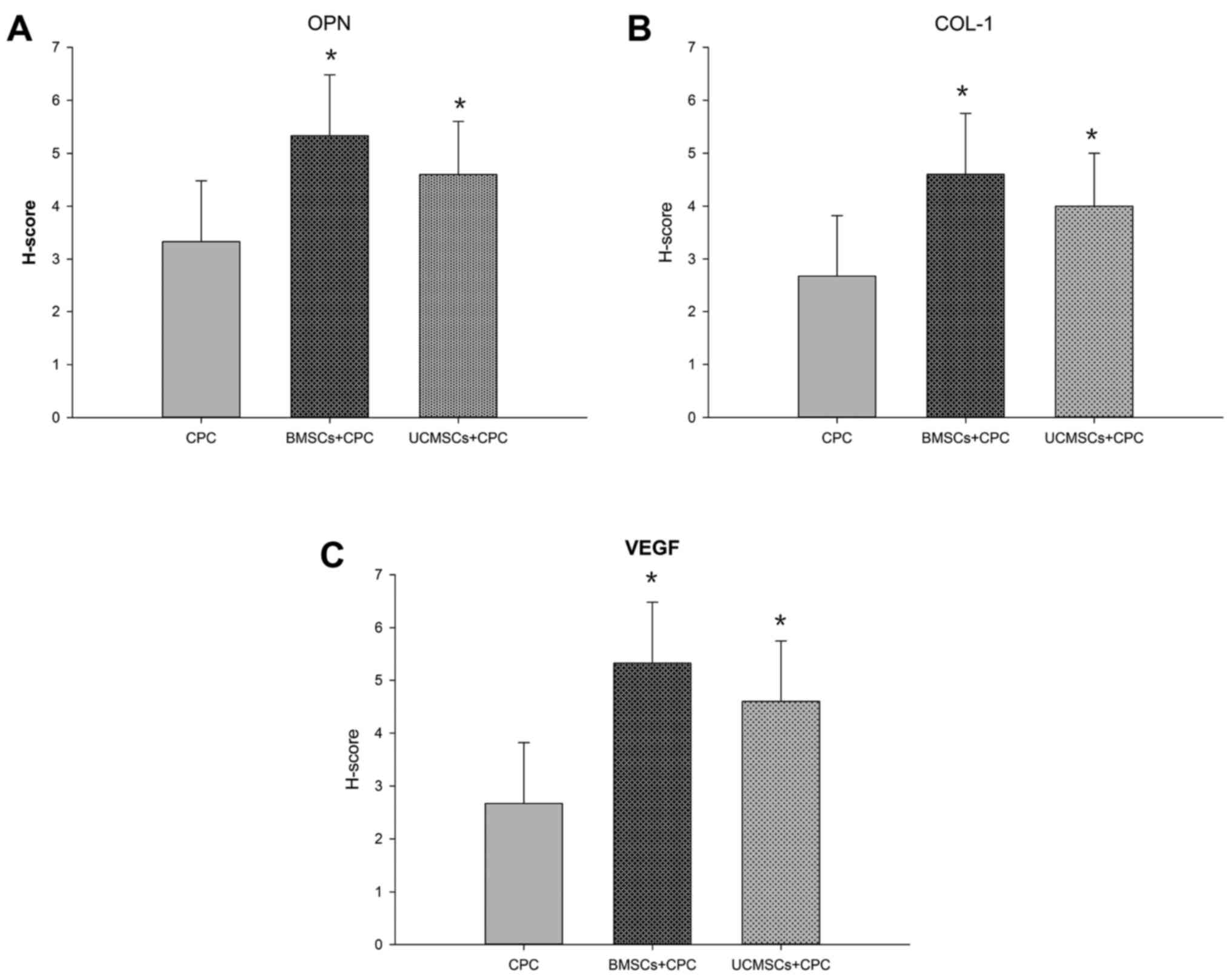
groups compared with that in the CPC group (Fig. 5B and C). The H-scores for OPN in the
BMSCs+CPC and the UCMSCs+CPC groups were greater compared with that
in the CPC group (P<0.05), while no significant differences were
identified between the BMSCs+CPC and UCMSCs+CPC groups (Fig. 6A). Furthermore, the H-scores for
COL-1 in the BMSCs+CPC and the UCMSCs+CPC groups were larger than
that in the CPC group (P<0.05), while no significant difference
was identified between the BMSCs+CPC and UCMSCs+CPC groups
(Fig. 6B). These results indicated
that BMSCs and UCMSCs did indeed accelerate the healing of the bone
defect, although no significant differences were observed between
the two cell types in terms of these effects.
Ectopic bone formation in the
BMSCs+CPC and UCMSCs+CPC groups in vivo
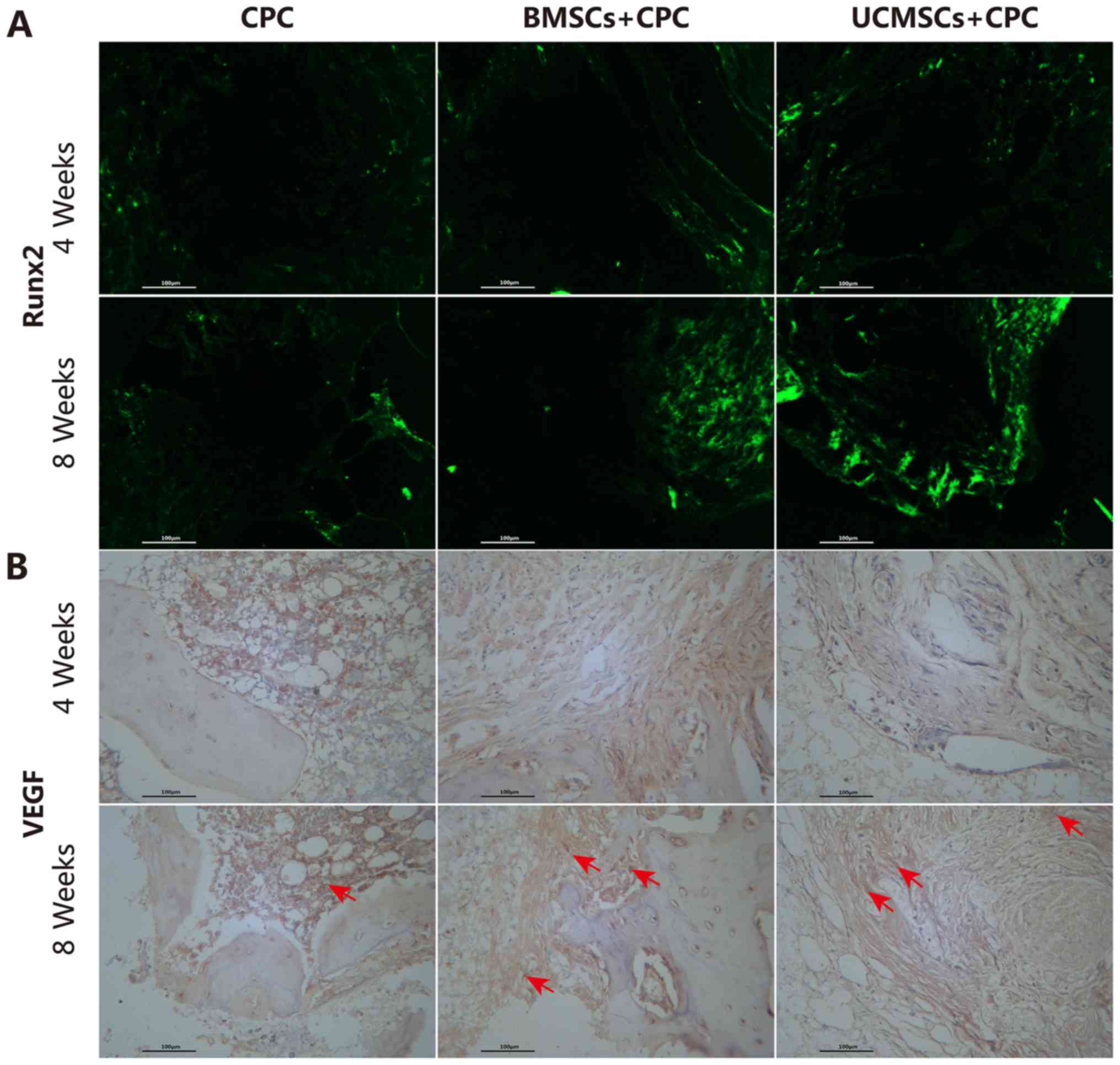
To further evaluate the osteogenic capacity of BMSCs
and UCMSCs in vivo, engineering of cell-scaffolds or
cell-free scaffolds were implanted under the skin of nude mice. The
expression of Runx2 and VEGF on the CPC was detected by
immunofluorescence and immunohistochemical analyses. Runx2 is the
transcription factor that regulates the differentiation and
maturation of osteogenic differentiation. For this reason, the
expression of Runx2 provides the hallmark of osteoblast
differentiation; it is the earliest one that may be observed and
the most specific marker gene in bone formation. In the present
study, a stronger intensity of fluorescence staining of Runx2 was
observed in the defect areas of the BMSCs+CPC and UCMSCs+CPC groups
compared with that in the CPC group (Fig. 7A). An increase in the levels of VEGF
was observed in all groups from 4–8 weeks post-surgery (Fig. 7B). At week 8 post-surgery, the
H-score of VEGF in the BMSCs+CPC and the UCMSCs+CPC groups were
greater compared with that in the CPC group (P<0.05), while no
significant differences were observed between the BMSCs+CPC group
and the UCMSCs+CPC group (Fig. 6C).
Therefore, these results suggested that the presence of BMSCs and
UCMSCs led to an elevation in the rate of angiogenesis, and that
this effect was not significantly different between the two cell
types. The results of the ectopic bone formation experiments
therefore exhibited the same trend as those of the experiments
using the tibia defect model of bone regeneration.
Discussion
Bone tissue engineering involves the signaling
molecule scaffold materials and the ideal seed cells (12,27).
Ideal seed cells should be safe and easy to obtain and to
proliferate, and have the capability of undergoing osteogenesis.
BMSCs and UCMSCs have been assessed for experimental periodontal
tissue regeneration in a variety of animal models (28–31). A
previous study by Shang et al (32) indicated that the osteogenic
capability of UCMSCs was similar to that of BMSCs in an animal
model of bone fracture healing. Furthermore, the above study
demonstrated that UCMSCs express osteogenic differentiation
markers, including ALP and Runx2. Given the limitations of BMSCs
that were mentioned in the Introduction, the advantages of UCMSCs
are even more pronounced. UCMSCs are derived from richly abundant
sources, have lower viral infection rates and are not associated
with any ethical obstacles. The aim of the present study was to
compare the promotional effects of BMSCs and UCMSCs on bone
regeneration, and to determine whether UCMSCs may be used as a
novel cell source for bone regeneration. The results of the present
study demonstrated that in vitro, BMSCs and UCMSCs have
numerous properties in common, including adherence ability,
proliferative capacity, immunophenotype and osteogenic
differentiation ability.
MSCs promote bone repair when implanted locally,
usually on a scaffold, e.g. CPC. Wang et al (12) have demonstrated that CPC has a good
affinity for cell attachment without having any negative effects on
cell viability. It was able to induce undifferentiated MSCs to
become osteoblasts, thereby serving an osteoinductive function.
Therefore, CPC was used as the carrier and scaffold of BMSCs and
UCMSCs to repair bone defects in the present study.
Zhang et al (33) investigated the proliferative and
osteogenic potential of MSCs from human fetal BMSCs, human UCMSCs,
as well as human adult adipose tissue MSCs and BMSCs, in in
vitro and in vivo models of ectopic bone formation. In
the present study, in vivo experiments of ectopic bone
formation and tibia bone defect were performed.
Osteogenesis-associated protein markers, including OPN, COL-1,
Runx2 and VEGF, were also detected. Therefore, the present study
was more comprehensive and clinical than the previous one.
Regarding the choice of animal model, nude mice are ideal for
assessing ectopic bone formation, as these immunocompromised
animals lack a thymus and do not reject the implant, so that the
cells may easily survive after transplantation (34,35).
However, rats are used for the classic animal model of bone defect
(36,37). In the present study, the X-ray
results revealed that the application of BMSCs+CPC and UCMSCs+CPC
in the tibia defect model led to improved levels of osteogenesis
compared with that in the CPC only group. OPN and COL-1 serve as
early markers of osteodifferentiation, whereas Runx2 is a key
transcription factor required for bone formation (23,38).
Achieving increased expression levels of OPN, COL-1 and Runx2 is an
essential prerequisite for bone formation. Angiogenesis is
postulated to have an essential role in new bone formation, from
the perspective of enhancing bone repair directly or indirectly by
promoting angiogenesis and bone metabolism (23,39,40). In
the present study, the increase in the expression levels of OPN and
COL-1 indicated that the BMSCs+CPC and UCMSCs+CPC treatments
accelerated the bone regeneration in the rat model of tibia bone
defect, and no significant differences were observed comparing
between the two groups. These results were consistent with those of
the X-ray analysis. In the ectopic bone formation experiments, the
expression levels of Runx2 and VEGF were increased in all groups
from 4–8 weeks. These results also suggested that the BMSCs and
UCMSCs accelerated the regeneration of the bone defect, while no
significant differences were observed between the two cell types.
However, more indicators associated with angiogenesis are required
to be detected in future studies, including CD31, which is a marker
of microvessel density. The combination of VEGF and CD31 would more
clearly indicate angiogenesis in the bone defect areas.
In conclusion, all of the results of the present
study demonstrated that BMSCs and UCMSCs improve bone regeneration
and angiogenesis in tibia defect and ectopic bone formation models,
and that UCMSCs may be used as an alternative to replace BMSCs as
an ideal type of seed cell for bone tissue engineering. The present
study may provide a novel lead for the selection of seed cells for
bone tissue engineering, and also enhances the current knowledge
regarding the treatment of bone defects. However, apart from the
MSC markers assessed in vitro, the expression of other types
of CD was not assessed, and the detailed mechanisms of the
osteogenesis of BMSCs and UCMSCs still require further
investigation; those will present the next challenge in future
studies by our group.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science
Foundation of Tianjin, China (grant no. 14JCYBJC43900).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JM and GZ conceived and designed the study. QW, GZ,
ZJX and JMZ performed the experiments. QW wrote the paper. QW, JM
and GZ reviewed and edited the manuscript. All authors have read
and approved the manuscript.
Ethics approval and consent to
participate
All animal experiments were reviewed and approved by
the Animal Experimentation Ethics Committee of Institute of
Radiation Medicine Chinese Academy of Medical Sciences (Tianjin,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Szpalski C, Barr J, Wetterau M, Saadeh PB
and Warren SM: Cranial bone defects: Current and future strategies.
Neurosurg Focus. 29:E82010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Terella A, Mariner P, Brown N, Anseth K
and Streubel SO: Repair of a calvarial defect with biofactor and
stem cell-embedded polyethylene glycol scaffold. Arch Facial Plast
Surg. 12:166–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meinel L, Betz O, Fajardo R, Hofmann S,
Nazarian A, Cory E, Hilbe M, McCool J, Langer R, Vunjak-Novakovic
G, et al: Silk based biomaterials to heal critical sized femur
defects. Bone. 39:922–931. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Megerdichian S: Adipose-derived
perivascular stem cells heal critical size mouse calvarial defects.
UCLA. ProQuest ID: Megerdichian_ucla_0031N_11204. Merritt ID:
ark:/13030/m5fr24h4. Dissertations and Theses-Gradworks.
2013.https://escholarship.org/uc/item/4zz5k216
|
|
5
|
Wang S, Qu X and Zhao RC: Mesenchymal stem
cells hold promise for regenerative medicine. Front Med. 5:372–378.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu L, Liu Y, Sun Y, Wang B, Xiong Y, Lin
W, Wei Q, Wang H, He W, Wang B and Li G: Tissue source determines
the differentiation potentials of mesenchymal stem cells: A
comparative study of human mesenchymal stem cells from bone marrow
and adipose tissue. Stem Cell Res Ther. 8:2752017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hoogduijn MJ and Dor FJ: Mesenchymal stem
cells: Are we ready for clinical application in transplantation and
tissue regeneration? Front Immunol. 4:1442013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee OK, Kuo TK, Chen WM, Lee KD, Hsieh SL
and Chen TH: Isolation of multipotent mesenchymal stem cells from
umbilical cord blood. Blood. 103:1669–1675. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Steinert AF, Rackwitz L, Gilbert F, Nöth U
and Tuan RS: Concise review: The clinical application of
mesenchymal stem cells for musculoskeletal regeneration: Current
status and perspectives. Stem Cells Transl Med. 1:237–247. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Caplan AI: Review: Mesenchymal stem cells:
Cell-based reconstructive therapy in orthopedics. Tissue Eng.
11:1198–1211. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Meyer U, Meyer TH, Handschel J and
Wiesmann HP: Fundamentals of Tissue Engineering and Regenerative
Medicine. Springer; Berlin Heidelberg: 2009, View Article : Google Scholar
|
|
12
|
Wang P, Liu X, Zhao L, Weir MD, Sun J,
Chen W, Man Y and Xu HH: Bone tissue engineering via human induced
pluripotent, umbilical cord and bone marrow mesenchymal stem cells
in rat cranium. Acta Biomater. 18:236–248. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Manferdini C, Cavallo C, Grigolo B,
Fiorini M, Nicoletti A, Gabusi E, Zini N, Pressato D, Facchini A
and Lisignoli G: Specific inductive potential of a novel
nanocomposite biomimetic biomaterial for osteochondral tissue
regeneration. J Tissue Eng Regen Med. 10:374–391. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Di Maggio N, Piccinini E, Jaworski M,
Trumpp A, Wendt DJ and Martin I: Toward modeling the bone marrow
niche using scaffold-based 3D culture systems. Biomaterials.
32:321–329. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Grover LM, Wright AJ, Gbureck U, Bolarinwa
A, Song J, Liu Y, Farrar DF, Howling G, Rose J and Barralet JE: The
effect of amorphous pyrophosphate on calcium phosphate cement
resorption and bone generation. Biomaterials. 34:6631–6637. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang J, Liu W, Schnitzler V, Tancret F
and Bouler JM: Calcium phosphate cements for bone substitution:
Chemistry, handling and mechanical properties. Acta Biomater.
10:1035–1049. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Calabrese G, Giuffrida R, Lo Furno D,
Parrinello NL, Forte S, Gulino R, Colarossi C, Schinocca LR,
Giuffrida R, Cardile V and Memeo L: Potential effect of CD271 on
human mesenchymal stromal cell proliferation and differentiation.
Int J Mol Sci. 16:15609–15624. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vicari L, Calabrese G, Forte S, Giuffrida
R, Colarossi C, Parrinello NL and Memeo L: Potential role of
activating transcription factor 5 during osteogenesis. Stem Cells
Int. 2016:52821852016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ai J, Ebrahimi S, Khoshzaban A, Kashi
Jafarzadeh TS and Mehrabani D: Tissue engineering using human
mineralized bone xenograft and bone marrow mesenchymal stem cells
allograft in healing of tibial fracture of experimental rabbit
model. Iran Red Crescent Med J. 14:962012.PubMed/NCBI
|
|
20
|
Chen Y, Xu J, Huang Z, Yu M, Zhang Y, Chen
H, Ma Z, Liao H and Hu J: An innovative approach for enhancing bone
defect healing using PLGA scaffolds seeded with
extracorporeal-shock-wave-treated bone marrow mesenchymal stem
cells (BMSCs). Sci Rep. 7:441302017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tsuchida H, Hashimoto J, Crawford E,
Manske P and Lou J: Engineered allogeneic mesenchymal stem cells
repair femoral segmental defect in rats. J Orthop Res. 21:44–53.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tanaka M, Fukushima N, Yamasaki F and
Ohshima K: Primary hepatic extranodal marginal zone lymphoma of
mucosa-associated lymphoid tissue type is associated with chronic
inflammatory process. Open J Hematol. 2010:1–5. 2010.
|
|
23
|
Huang Z, Xu J, Chen J, Chen H, Wang H,
Huang Z, Chen Y, Lu X, Lu F and Hu J: Photoacoustic stimulation
promotes the osteogenic differentiation of bone mesenchymal stem
cells to enhance the repair of bone defect. Sci Rep. 7:158422017.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cha YJ and Shim HS: PD-L1 expression and
CD8+ tumor-infiltrating lymphocytes are associated with
ALK rearrangement and clinicopathological features in inflammatory
myofibroblastic tumors. Oncotarget. 8:89465–89474. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Freitas MA, Rde Gomes O, Soares BL, Neto
Artigiani R, Montero EF and Martins JL: Effects of maternal
ischemic preconditioning in the colon of newborn rats submitted to
hypoxia-reoxygenation insult. Acta Cir Bras. 29:438–444. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
BM, Khan KN, Masferrer J and Koki AT: COX-2 is expressed in human
pulmonary, colonic, and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ye P, Yu B, Deng J, She RF and Huang WL:
Application of silk fibroin/chitosan/nano-hydroxyapatite composite
scaffold in the repair of rabbit radial bone defect. Exp Ther Med.
14:5547–5553. 2017.PubMed/NCBI
|
|
28
|
Yang Y, Rossi FM and Putnins EE:
Periodontal regeneration using engineered bone marrow mesenchymal
stromal cells. Biomaterials. 31:8574–8582. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tobita M, Uysal AC, Ogawa R, Hyakusoku H
and Mizuno H: Periodontal tissue regeneration with adipose-derived
stem cells. Tissue Eng Part A. 14:945–953. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guo W, Chen L, Gong K, Ding B, Duan Y and
Jin Y: Heterogeneous dental follicle cells and the regeneration of
complex periodontal tissues. Tissue Eng Part A. 18:459–470. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yan XZ, Yang F, Jansen JA, de Vries RB and
van den Beucken JJ: Cell-based approaches in periodontal
regeneration: A systematic review and meta-analysis of periodontal
defect models in animal experimental work. Tissue Eng Part B Rev.
21:411–426. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Shang F, Liu S, Ming L, Tian R, Jin F,
Ding Y, Zhang Y, Zhang H, Deng Z and Jin Y: Human umbilical cord
MSCs as new cell sources for promoting periodontal regeneration in
inflammatory periodontal defect. Theranostics. 7:4370–4382. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang ZY, Teoh SH, Chong MS, Schantz JT,
Fisk NM, Choolani MA and Chan J: Superior osteogenic capacity for
bone tissue engineering of fetal compared with perinatal and adult
mesenchymal stem cells. Stem Cells. 27:126–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Li Y, Chen YE, Chen J and Ma PX:
Cell-free 3D scaffold with two-stage delivery of miRNA-26a to
regenerate critical-sized bone defects. Nat Commun. 7:103762016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sensebé L and Fleury-Cappellesso S:
Biodistribution of mesenchymal stem/stromal cells in a preclinical
setting. Stem Cells Int. 2013:6780632013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang J, Gao Y, Cheng P, Li D, Jiang H, Ji
C, Zhang S, Shen C, Li J, Song Y, et al: CD31hiEmcnhi vessels
support new trabecular bone formation at the frontier growth area
in the bone defect repair process. Sci Rep. 7:49902017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Grewal BS, Keller B, Weinhold P and
Dahners LE: Evaluating effects of deferoxamine in a rat tibia
critical bone defect model. J Orthop. 11:5–9. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang T, Yang X, Qi X and Jiang C:
Osteoinduction and proliferation of bone-marrow stromal cells in
three-dimensional poly (ε-caprolactone)/hydroxyapatite/collagen
scaffolds. J Transl Med. 13:1522015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Geris L, Gerisch A, Sloten JV, Weiner R
and Oosterwyck HV: Angiogenesis in bone fracture healing: A
bioregulatory model. J Theor Biol. 251:137–158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tian XB, Sun L, Yang SH, Fu RY, Wang L, Lu
TS, Zhang YK and Fu DH: Ectopic osteogenesis of mouse bone marrow
stromal cells transfected with BMP 2/VEGF(165) genes in vivo.
Orthop Surg. 1:322–325. 2009. View Article : Google Scholar : PubMed/NCBI
|