Introduction
MicroRNAs (miRNAs) are small (18–25 nucleotides)
non-coding RNA harboring a post-transcriptional gene regulatory
function (1). Currently, miRNAs have
been detected in various body fluids, including serum (2), plasma (3), urine (4), cerebrospinal fluid (5), breast milk (6), saliva (7), bronchoalveolar lavage fluid (8), ascites (9), and pleural effusion (10). Noteworthy, miRNAs are stable under
non-physiological conditions, whether in body fluids or culture
media. Taylor et al (11)
reported that plasma miR-21 is stable for at least 28 days at
−30°C. We have reported that extracellular small RNAs are stable
for 4 weeks at room temperature, after 20 freeze-thaw cycles and
exposure to pH 2.0, and are resistant to ribonuclease A degradation
(12). In body fluids, miRNAs are
present in extracellular vesicles (EVs) (13) or high-density lipoproteins (14) and bind RNA-binding proteins (15). They are proposed to become novel
biomarkers of disorders including some cancers and
neurodegenerative diseases.
miR-375-3p is expressed in pancreatic β cells
(16), where this miRNA is involved
in pancreatic development, β cell proliferation, and insulin
secretion via gene regulation (17).
Overexpression of miR-375-3p suppresses insulin secretion (18), whereas inhibition of endogenous
miR-375-3p increases insulin secretion (19). Streptozotocin (STZ) is a nitrosourea
alkylating agent that induces tumor shrinkage and hypoglycemia and
causes the selective destruction of pancreatic β cells via a
glucose transporter 2 (20).
Therefore, STZ have been used as a therapeutic drug for the
treatment of neuroendocrine tumors in Japan (21). In mice and rats, the administration
of STZ induces diabetes after pancreatic β cells are injured
(22,23). Erener et al (24) reported that blood miR-375-3p
increased in STZ-treated mice.
We have previously shown that mice irradiated with a
lethal X-ray dose of 7 Gy present a significant serum increase of
miR-375-3p at 72 h after exposure (2). Since miR-375-3p is expressed the
highest in the pancreas among 20 types of cells and organs
examined, it was inferred that it derived from the pancreas. This
research suggested that radiation-induced death of pancreatic β
cells is associated with the release of EVs containing miR-375-3p.
Although miR-375-3p is expected to be released from injured
pancreatic β cells, no evidence has been obtained. Therefore, it is
necessary to investigate whether miR-375-3p is released from cells
by STZ treatment and 7 Gy X-ray irradiation, which is a different
mechanism to injure pancreatic β-cells. In this study, we
investigate the expression level of extracellular miR-375-3p
released from an insulinoma cell line exposed to 7 Gy X-ray
irradiation or STZ treatment.
Materials and methods
Cell line and culture
The rat pancreatic β cell line (RIN-5F) was
purchased from the American Type Culture Collection (ATCC,
Manassas, VA, USA). RIN-5F cells were cultured in RPMI-1640 medium
(Wako, Tokyo, Japan) supplemented with 10% fetal bovine serum
(Thermo Fisher Scientific, Inc., Waltham, MA, USA), 100 U/ml of
penicillin, and 100 µg/ml of streptomycin (Wako). Cells were
cultured at 37°C in a humidified atmosphere with 5%
CO2.
X-ray irradiation
RIN-5F cells were exposed to X-rays (MBR-1520R-3
X-ray machine, Hitachi Medical Corporation, Tokyo, Japan) at a dose
rate of 1.0 Gy/min (150 kVp, 20 mA, 0.5-mm aluminum, and 0.3-mm
copper filters).
STZ treatment
STZ and Dulbecco's phosphate-buffered saline
[D-PBS(−), pH 7.2] were purchased from Wako. STZ was diluted in
D-PBS(−).
RNA extraction
Total RNAs from RIN-5F cells were extracted using
Isogen II reagents (Nippongene, Tokyo, Japan) according to the
manufacturer's instruction. Cell culture medium samples were
centrifuged at 300 × g at 4°C for 3 min and floating cells removed.
Total RNAs from 200 µl culture supernatants added to 5 µl
cel-miR-39 (1 nM) were extracted using Isogen II reagents and
ethachinmate (Nippongene).
Reverse transcription quantitative
polymerase chain reaction (RT-qPCR)
The expression of rat insulin 1 (Ins 1) and β
actin (Actb) mRNAs in RIN-5F cells were determined by
RT-qPCR. Briefly, total RNAs were isolated from RIN-5F cells. cDNA
was synthesized from total RNA using the Applied Biosystems™ High
Capacity cDNA Reverse Transcription kit (Thermo Fisher Scientific,
Inc.), according to the manufacturer's instructions. qPCR was
performed using a FastStart Universal SYBR Green Master (Roche
Diagnostics, Basel, Switzerland), 10 µM of forward and reverse
primer pairs (Table I), and the
StepOne Plus Real-time PCR system (Thermo Fisher Scientific, Inc.)
in the following conditions: 10 min at 95°C, followed by 40 cycles
each of 95°C for 15 sec, and 60°C for 60 sec. Actb was used
as internal control. The PCR products were separated by
electrophoresis on 4% agarose gel and detected by ethidium bromide
staining.
 | Table I.Primers for reverse
transcription-polymerase chain reaction. |
Table I.
Primers for reverse
transcription-polymerase chain reaction.
| Primer name | Accession no. | Sequence (5′-3′) | Size (nt) | Amplicon size
(bp) |
|---|
| Ins1 forward
primer | NM_019129.3 |
TCATAGACCATCAGCAAGCAG | 21 | 95 |
| Ins1 reverse
primer |
|
CTTGGGCTCCCAGAGGAC | 18 |
|
| Actb forward
primer | NM_031144.3 |
CCCGCGAGTACAACCTTCT | 19 | 72 |
| Actb reverse
primer |
|
CGTCATCCATGGCGAACT | 18 |
|
RT-qPCR was used to determine the expression levels
of miR-375-3p in culture supernatants. The cDNAs were synthesized
using the TaqMan™ miRNA RT kit and the prescribed 5 × RT primer
(both from Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. qPCR for miRNAs was performed using a
FastStart TaqMan probe master (Roche Diagnostics), a 20 × probe,
and the StepOne Plus Real-Time PCR system (Thermo Fisher
Scientific, Inc.) under the following conditions: 10 min at 95°C,
followed by 45 cycles at 95°C for 15 sec, and 60°C for 60 sec.
Cel-miR-39 was used as an external control. The comparative Ct
method was used to determine expression levels.
Immunofluorescence staining
Immunofluorescence staining was performed for
detection of insulin in RIN-5F cells. RIN-5F cells were plated in
8-well chamber slides at a density of 2×104 cells per
well in culture media. After 48 h, the cells were washed three
times with D-PBS(−) and treated with 0.2% Triton X-100 in D-PBS(−)
at room temperature for 5 min. After washing three times in
D-PBS(−), the cells were incubated in an Image-iT FX signal
enhancer solution (Thermo Fisher Scientific, Inc.) at room
temperature for 30 min. Next, the cells were incubated with a
primary rabbit monoclonal antibody raised against insulin (C27C9)
(no. 3014; Cell Signaling Technology, Inc., Danvers, MA, USA) at
1:100 dilution in Immunostain Solution A (Toyobo, Osaka, Japan) at
room temperature for 60 min. After five times washing with
D-PBS(−), cells were incubated at room temperature for 60 min with
an anti-rabbit IgG Alexa Fluor 488-conjugated secondary antibody
(no. 4412; Cell Signaling Technology, Inc.) at 1:200 dilution in
Immunostain Solution B. The cells were washed five times with
D-PBS(−), and the nuclei were stained with
4′,6-diamidino-2-phenylindole (DAPI) in ProLong Gold Antifade
Reagent (Thermo Fisher Scientific, Inc.). The cells were visualized
under a confocal laser scanning microscope LSM710 (Carl Zeiss,
Oberkochen, Germany).
Flow cytometry
RIN-5F cells were plated on 3-cm dishes at a density
of 2×106 cells per dish in culture medium. After 96 h,
culture medium was discarded, the cells were washed three times in
D-PBS(−), and 10 ml culture medium was added to each dish. Then,
RIN-5F cells were exposed to none or 7 Gy of X-rays at a dose rate
of 1.0 Gy/min and treated with STZ of a concentration of 0, 1, 3,
10, or 30 mM. The RIN-5F cells were collected and were stained by
propidium iodide (PI) solution. PI-positive cells were detected
using a Cytomics FC500 (Beckman Coulter, Brea, CA, USA).
Cell proliferation assay
RIN-5F cells were seeded at the density of
2×104 cells per well of a 96-well flat-bottomed
microplate. After pre-culture for 48 h, media were discarded and
the cells washed with D-PBS(−). The cells were incubated with STZ
diluted in medium at various concentrations (0, 1, 3, 10, or 30 mM)
for 0, 24, or 48 h. The cells irradiated with 7 Gy of X-rays and
control cells not irradiated were cultured for 0, 24, 48, or 72 h
after irradiation. Viable cells were detected using the
alamarBlue® cell viability reagent (Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions. The
fluorescence intensity was measured after 6 h at an excitation
wavelength of 544 nm and an emission wavelength of 590 nm using a
Fluoroskan Ascent™ system (Thermo Fisher Scientific, Inc.).
Statistical analysis
Statcel 3 software (OMS Publishing Inc., Saitama,
Japan) was used to perform all statistical analyses. Student's
t-test was performed to compare the results of the two groups.
One-way analysis of variance (ANOVA) was performed followed by
Tukey-Kramer multiple comparisons test. P<0.05 was considered to
indicate a statistically significant difference.
Results
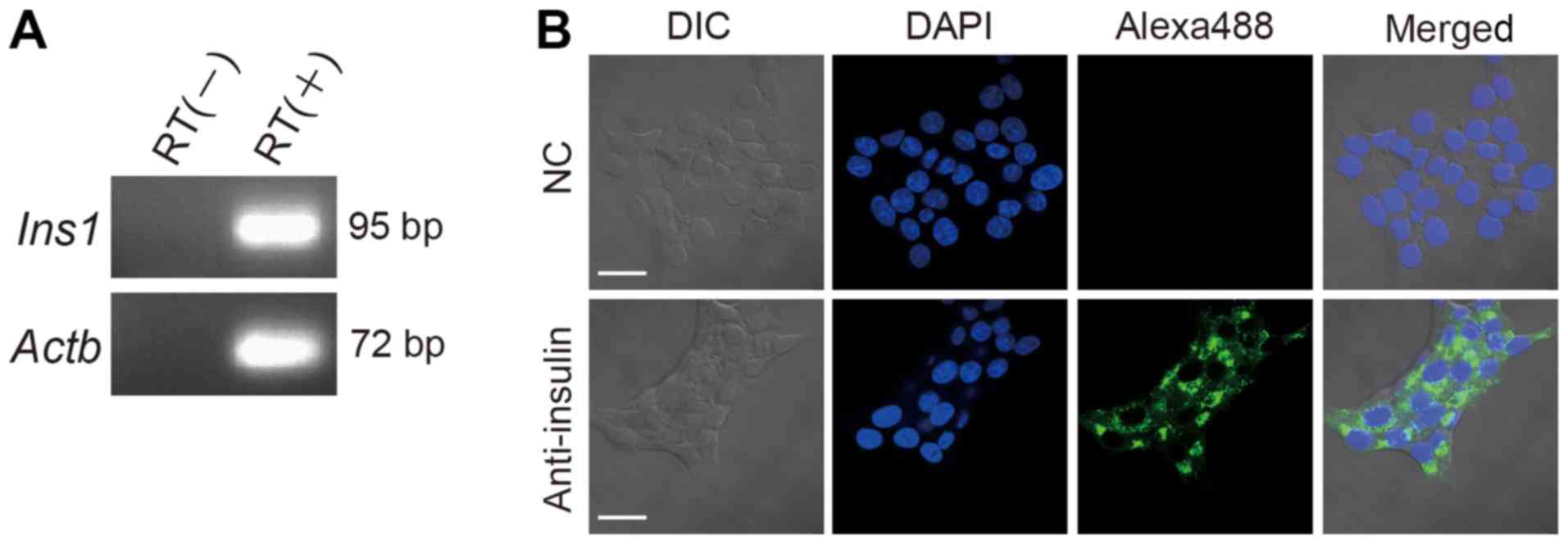
RIN-5F cells express insulin
To confirm the pancreatic β cells characteristics of
RIN-5F cells, the expression of insulin mRNA was determined by
RT-qPCR. Both Ins1 and Actb mRNAs were detected
(Fig. 1A). In addition, insulin
proteins were detected in RIN-5F cells by immunofluorescence
analysis using confocal laser scanning microscopy (Fig. 1B). These results indicate that RIN-5F
cells expressing insulin process characteristics of pancreatic β
cells.
X-ray irradiation and STZ treatment
suppress cell proliferation in RIN-5F cells
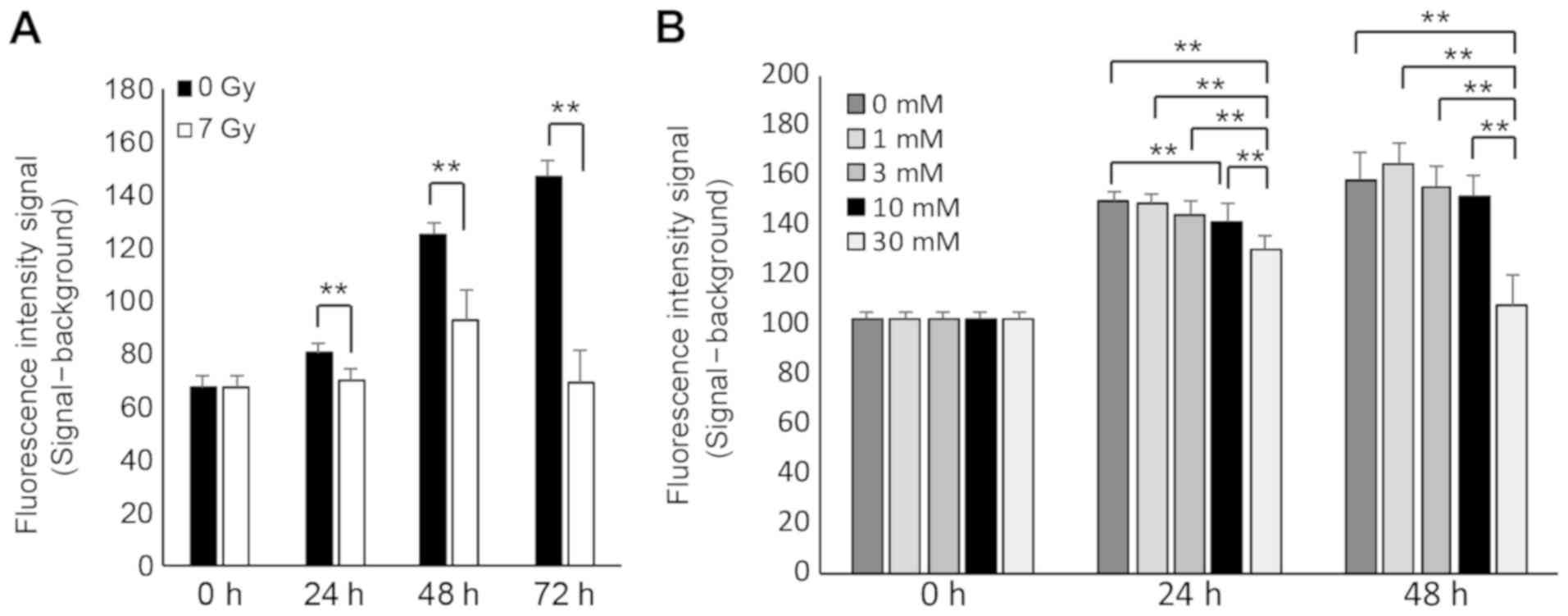
To assess the sensitivity of RIN-5F cells to
irradiation and STZ treatment, a cell proliferation assay was
performed. Cell proliferation of RIN-5F cells was significantly
suppressed at 24, 48, and 72 h after 7 Gy irradiation (Fig. 2A) and by treatment of 10 or 30 mM STZ
at 24 h (Fig. 2B) compared with
controls. These results indicate that cells exposed to 7 Gy
irradiation and high concentration of STZ cannot further
proliferate.
X-ray irradiation and STZ treatment
induce cell death in RIN-5F cells
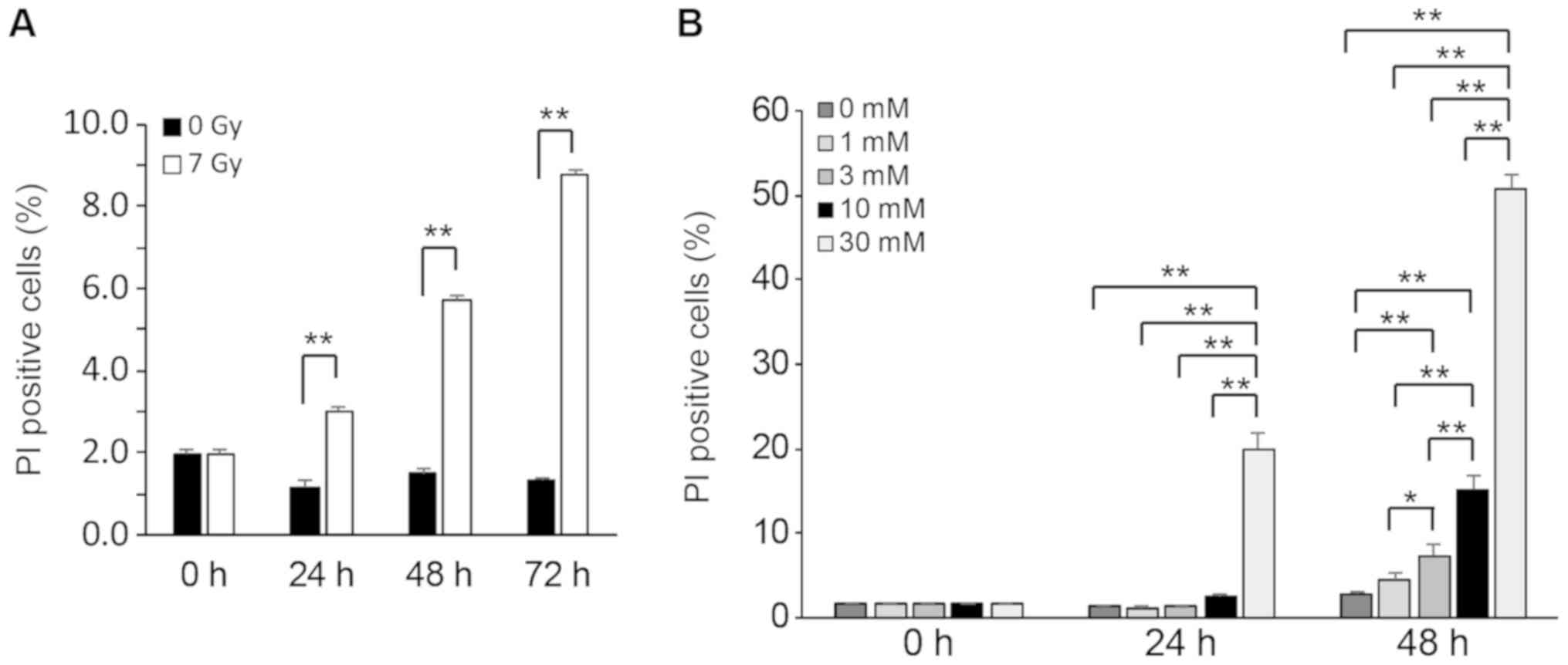
To evaluate cell death induced by 7 Gy irradiation
or STZ treatment in RIN-5F cells, we performed a flow cytometry
analysis in PI-stained RIN-5F cells. The number of PI-positive
cells significantly increased at 24, 48, and 72 h after 7 Gy
irradiation compared with non-irradiated (0 Gy) cells (Fig. 3A). PI-positive cells were
significantly increased at 24 h after treatment with 30 mM STZ and
at 48 h after treatment with 3, 10, or 30 mM STZ compared with
control cells (Fig. 3B). These
results indicate that within 24 h, cell death is induced in RIN-5F
cells either exposed to 7 Gy irradiation or 30 mM STZ
treatment.
X-ray irradiation and STZ treatment
enhance the release of miR-375-3p in RIN-5F cells
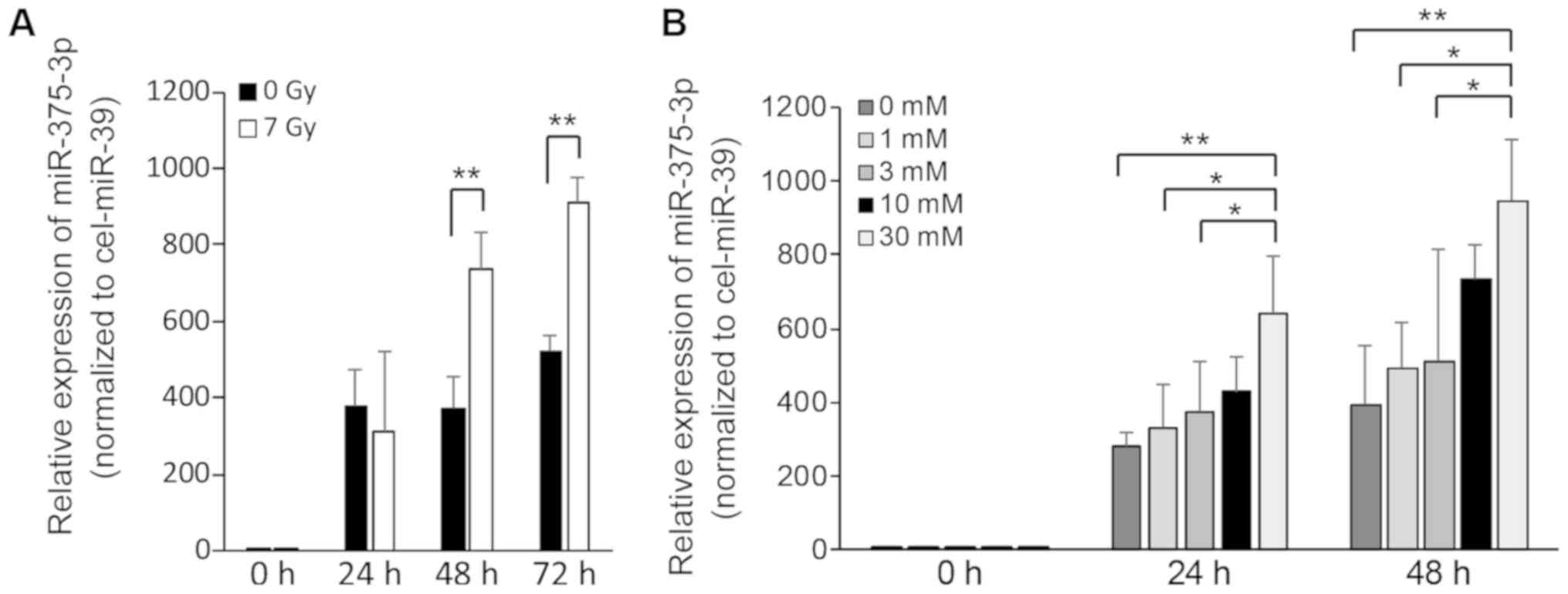
To examine the impairmentassociated release of
miR-375-3p from RIN-5F cells, we performed RT-qPCR on culture
supernatants of RIN-5F cells that were exposed to 7 Gy irradiation
or STZ treatment. The expression of miR-375-3p increased
significantly at 48 or 72 h after 7 Gy irradiation (Fig. 4A) and at 24 or 48 h after treatment
of 30 mM STZ compared with control supernatants (Fig. 4B). These results suggest that 7 Gy
irradiation and STZ treatment trigger the release of miR-375-3p
from RIN-5F cells into cell culture supernatants.
Discussion
We have previously shown that miR-375-3p increases
in the serum of mice exposed to 7 Gy X-rays causing cytotoxicity in
pancreatic β cells (2). However, the
direct relationship between miR-375-3p level increases in the blood
and the cytotoxicity caused by 7 Gy irradiation remained to be
clarified. In this study, employing an in vitro cell culture
model of pancreatic β cells, we investigated the direct release of
miR375-3p from damaged cells.
First, we confirmed that RIN-5F cells have traits
characteristics of pancreatic β cells. Pancreatic β cells are
insulin-producing cells and are known to express miR-375-3p
(16). The expression of insulin
mRNA was confirmed by RT-PCR, and insulin was detected by
fluorescent immunostaining (Fig. 1).
In this study, RIN-5F cells had traits of pancreatic β cells
including expression of insulin.
Next, we investigated the effects of 7 Gy X-ray
irradiation on cell proliferation and cell death in RIN-5F cells.
The suppression of cell proliferation and the increase of
PI-positive cells were observed at 24 h after irradiation (Figs. 2A and 3A). Although the expression of
extracellular miR-375-3p was not significantly different after 24 h
of irradiation, it was significantly increased after 48 and 72 h
(Fig. 4A), suggesting that
miR-375-3p requires over 24 h after irradiation to be released in
the medium. On the other hand, 30 mM STZ treatment inhibited cell
proliferation and increased cell death at 24 or 48 h, when treated
with 30 mM STZ (Figs. 2B and
3B), and the expression of
extracellular miR-375-3p was concomitantly and significantly
increased at these times (Fig. 4B).
Therefore, the mechanism of miR-375-3p release may be different
after X-ray irradiation compared with after STZ treatment.
STZ causes the selective destruction of pancreatic β
cells via a glucose transporter 2 (20). The release of miR-375-3p from
cellular to extracellular space is enhanced by the treatment of STZ
(22,23). Therefore, STZ have been used as a
therapeutic drug for the treatment of insulinoma (21). We propose that the miR-375-3p
increase in blood is due to leakage from damaged pancreatic β cells
caused by STZ. High-dose irradiation induces a programmed cell
death called apoptosis (25).
Apoptotic cell death minimizes leakage of cell contents by forming
EVs such as apoptotic bodies (26).
In Fig. 4A, extracellular miR-375-3p
did not increase at 24 h after 7 Gy irradiation compared with
control probably due to cell death by apoptosis. To clarify whether
extracellular miR-375-3p is contained in EVs such as apoptotic
bodies of culture supernatant after X-ray irradiation and STZ
treatment, we aim to analyze in detail apoptosis and EV contents
following X-ray irradiation and STZ treatment in the future.
In this study, we clarified that pancreatic β-cell
injury induced an extracellular miR-375-3p increase. PI is only
taken up by the cell when the membrane is damaged and decreased
metabolism, and binds to the nuclear DNA. Therefore, cells
undergoing late apoptosis or necrosis can be detected with PI. In
the future, it is necessary to assess the degree of membrane injury
using Annexin V staining binding to membrane phospholipid.
Acknowledgements
Not applicable.
Funding
This work was supported in part by JSPS KAKENHI
(grant nos. JP25670264, JP17H04761, and JP17K19779), and a grant
from the Takeda Science Foundation.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MC was a major contributor in performing experiments
and writing the manuscript. IN, HU, and HK helped perform
experiments. MC was the leader of this study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chiba M, Monzen S, Iwaya C, Kashiwagi Y,
Yamada S, Hosokawa Y, Mariya Y, Nakamura T and Wojcik A: Serum
miR-375-3p increase in mice exposed to a high dose of ionizing
radiation. Sci Rep. 8:13022018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen Y, Han J, Chen J, Dong J, Xia Y, Liu
J, Jiang Y, Dai J, Lu J, Jin G, et al: Plasma miRNAs as early
biomarkers for detecting hepatocellular carcinoma. Int J Cancer.
137:1679–1690. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ben-Dov IZ, Tan YC, Morozov P, Wilson PD,
Rennert H, Blumenfeld JD and Tuschl T: Urine microRNA as potential
biomarkers of autosomal dominant polycystic kidney disease
progression: Description of miRNA profiles at baseline. PLoS One.
9:e868562014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akers JC, Ramakrishnan V, Kim R, Phillips
S, Kaimal V, Mao Y, Hua W, Yang I, Fu CC, Nolan J, et al: miRNA
contents of cerebrospinal fluid extracellular vesicles in
glioblastoma patients. J Neurooncol. 123:205–216. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rubio M, Bustamante M, Hernandez-Ferrer C,
Fernandez-Orth D, Pantano L, Sarria Y, Piqué-Borras M, Vellve K,
Agramunt S, Carreras R, et al: Circulating miRNAs, isomiRs and
small RNA clusters in human plasma and breast milk. PLoS One.
13:e01935272018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Z, Zhang J, Wei W, Zhou D, Luo H,
Chen X and Hou Y: Identification of saliva using MicroRNA
biomarkers for forensic purpose. J Forensic Sci. 60:702–706. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Molina-Pinelo S, Suárez R, Pastor MD,
Nogal A, Márquez-Martín E, Martín-Juan J, Carnero A and Paz-Ares L:
Association between the miRNA signatures in plasma and
bronchoalveolar fluid in respiratory pathologies. Dis Markers.
32:221–230. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Záveský L, Jandáková E, Weinberger V,
Minář L, Hanzíková V, Dušková D, Drábková LZ, Svobodová I and
Hořínek A: Ascites-derived extracellular microRNAs as potential
biomarkers for ovarian cancer. Reprod Sci. Jan 1–2018.(Epub ahead
of print). View Article : Google Scholar
|
|
10
|
Han HS, Yun J, Lim SN, Han JH, Lee KH, Kim
ST, Kang MH, Son SM, Lee YM, Choi SY, et al: Downregulation of
cell-free miR-198 as a diagnostic biomarker for lung
adenocarcinoma-associated malignant pleural effusion. Int J Cancer.
133:645–652. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Taylor DD and Gercel-Taylor C: MicroRNA
signatures of tumor-derived exosomes as diagnostic biomarkers of
ovarian cancer. Gynecol Oncol. 110:13–21. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chiba M, Kimura M and Asari S: Exosomes
secreted from human colorectal cancer cell lines contain mRNAs,
microRNAs and natural antisense RNAs, that can transfer into the
human hepatoma HepG2 and lung cancer A549 cell lines. Oncol Rep.
28:1551–1558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu R, Greening DW, Zhu HJ, Takahashi N and
Simpson RJ: Extracellular vesicle isolation and characterization:
Toward clinical application. J Clin Invest. 126:1152–1162. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wagner J, Riwanto M, Besler C, Knau A,
Fichtlscherer S, Röxe T, Zeiher AM, Landmesser U and Dimmeler S:
Characterization of levels and cellular transfer of circulating
lipoprotein-bound microRNAs. Arterioscler Thromb Vasc Biol.
33:1392–1400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang K, Zhang S, Weber J, Baxter D and
Galas DJ: Export of microRNAs and microRNA-protective protein by
mammalian cells. Nucleic Acids Res. 38:7248–7259. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kloosterman WP, Lagendijk AK, Ketting RF,
Moulton JD and Plasterk RH: Targeted inhibition of miRNA maturation
with morpholinos reveals a role for miR-375 in pancreatic islet
development. PLoS Biol. 5:e2032007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li X: MiR-375, a microRNA related to
diabetes. Gene. 533:1–4. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Poy MN, Eliasson L, Krutzfeldt J, Kuwajima
S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P
and Stoffel M: A pancreatic islet-specific microRNA regulates
insulin secretion. Nature. 432:226–230. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eliasson L: The small RNA miR-375-a
pancreatic islet abundant miRNA with multiple roles in endocrine
beta cell function. Mol Cell Endocrinol. 456:95–101. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lenzen S: The mechanisms of alloxan- and
streptozotocin-induced diabetes. Diabetologia. 51:216–226. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Okusaka T, Ueno H, Morizane C, Kondo S,
Sakamoto Y, Takahashi H, Ohno I, Shimizu S, Mitsunaga S and Ikeda
M: Cytotoxic chemotherapy for pancreatic neuroendocrine tumors. J
Hepatobiliary Pancreat Sci. 22:628–633. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Latreille M, Herrmanns K, Renwick N,
Tuschl T, Malecki MT, McCarthy MI, Owen KR, Rülicke T and Stoffel
M: miR-375 gene dosage in pancreatic β-cells: Implications for
regulation of β-cell mass and biomarker development. J Mol Med
(Berl). 93:1159–1169. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bathina S, Srinivas N and Das UN:
Streptozotocin produces oxidative stress, inflammation and
decreases BDNF concentrations to induce apoptosis of RIN5F cells
and type 2 diabetes mellitus in Wistar rats. Biochem Biophys Res
Commun. 486:406–413. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Erener S, Mojibian M, Fox JK, Denroche HC
and Kieffer TJ: Circulating miR-375 as a biomarker of β-cell death
and diabetes in mice. Endocrinology. 154:603–608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Meyn RE, Stephens LC and Milas L:
Programmed cell death and radioresistance. Cancer Metastasis Rev.
15:119–131. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mathivanan S, Ji H and Simpson RJ:
Exosomes: Extracellular organelles important in intercellular
communication. J Proteomics. 73:1907–1920. 2010. View Article : Google Scholar : PubMed/NCBI
|