Introduction
Cardiopulmonary bypass (CPB) is a technique that
temporarily takes over the function of the heart and lungs during
surgery, maintaining the circulation of blood and oxygen supply for
the body. This technique is widely used during heart surgery due to
the difficulty of operating on a beating heart. Surgery opens the
chambers of the heart, which requires the use of CPB to support the
circulation. However, since the body is under an abnormal status
during CPB, an inflammatory response may be induced by multiple
stimulants, including ischemia-perfusion injury, low temperature
and CPB time. This is likely to result in the occurrence of
multiple organ dysfunction syndrome (MODS) (1,2).
Therefore, studies on the systemic inflammatory response during CPB
are crucial for the prevention and treatment of complications
following CPB. Additionally, pro-inflammatory cytokines not only
reflect the condition of the systemic inflammatory response, but
are also involved in the occurrence of complications (3).
High mobility group box-1 (HMGB1) is a
multifunctional protein, described as a non-histone DNA-binding
nuclear protein. HMGB1 binds to DNA in a sequence-independent
manner and modifies DNA structure to facilitate transcription,
replication and repair (4). This
function is essential for survival, as HMGB1-deficient mice die of
hypoglycemia within 24 h of birth (5). Previously, HMGB1 was identified as a
cytokine mediator for lethal systemic inflammation, arthritis and
local inflammation (6,7). In a previous study, it was observed
that the addition of purified recombinant HMG-1 to human monocyte
cultures significantly stimulated the release of tumor necrosis
factor (TNF), which is an important pro-inflammatory mediator. The
HMG-1-activated TNF synthesis occurred in a biphasic pattern, with
an early peak at 3 h after the addition of HMG-1, followed by
another peak at 8-10 h (8).
However, the LPS-induced TNF release only peaked 1 h after LPS
administration. As for the changes in TNF level during CPB, Hennein
et al identified that arterial TNF-α increases in a bimodal
manner, peaking at 2 and 18–24 h after surgery, then declines to
the level before bypass (9).
However, the expression of HMGB1 during CPB remains
unclear. Therefore, in the present study, the levels of HMGB1
expression before, during and after surgery were characterized, as
well as the levels of TNF-α. Given the close association between
HMGB1 and TNF-α, the correlation between HMGB1 and TNF-α was
analyzed. Exploring the role of HMGB1 in inflammation may provide
information beneficial in further intervention to reduce the
related inflammatory response.
Patients and methods
Patients
Seventy-eight patients (41 males and 37 females)
aged 24–59 years with an American Society of Anesthesiologists
(ASA) grade of II–IV for heart function and rheumatic heart disease
requiring valve replacement surgery were admitted to the Department
of Cardiothoracic Surgery, Second Xiangya Hospital, Central South
University between May 1, 2007 and November 31, 2008. Informed
consent was obtained from patients or close relatives of patients.
The study was conducted in accordance with the Declaration of
Helsinki and was approved by the research ethics committee at the
Central South University.
The patients had no medical history of endocarditis,
diabetes, hypertension, neurological diseases, immune diseases or
psychiatric diseases, as well as infectious diseases, including
tuberculosis, hepatitis B and syphilis. The function of the liver,
kidneys and lungs was normal prior to surgery. There were three
types of surgery, including mitral valve replacement (MVR, n=40),
aortic valve replacement (AVR, n=14) and double valve replacement
(DVR, n=24).
Collection of blood samples
The CPB was performed as previously described
(10). Blood or urine samples were
collected after anesthesia prior to surgery (T1), before aortic
cross-clamping (T2), after CPB (completion of cross-clamping; T3)
and the first (T4), second (T5) and third (T6) day after surgery
between 6 and 7 a.m. Levels of HMGB1, TNF-α, serum alanine
aminotransferase (ALT), serum creatinine (Cr), blood urea nitrogen
(BUN), urinary N-acetyl-β-D-glucosamidase (NAG), urinary Cr and
urinary β2-microglobulin (β2-MG) were determined. In order to
exclude the effect of blood dilution during CPB on the detection
values, the final results were corrected as follows: Corrected
value = actual value x hematocrit (HCT) before aortic
cross-clamping/HCT when clamping.
Measurement of cytokines
Serum TNF-α was quantified using an enzyme-linked
immunosorbent assay (ELISA) kit (Boster Biological Technology Ltd.,
Wuhan, China) and expressed in picograms per milliliter. Absorbance
was determined at 450 nm using a microplate reader (BioTek
Instruments Inc., Winooski, VT, USA).
Western blot assay
Following routine laboratory tests, an equal volume
of blood serum was collected and filtered through Millex-GP
(Millipore, Bedford, MA, USA) to remove debris and other
macromolecular complexes. Samples were then concentrated 40-fold
with Amicon Ultra-4-10000 NMWL (Millipore) following the
manufacturer’s instructions, as previously described (11,12).
Proteins were then resolved by 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene difluoride (PVDF) membranes
(Schleicher & Schuell Bioscience Inc., Keene, NH, USA).
Membranes were blocked with 2% bovine serum albumin for 6 h and
incubated for 2 h with a primary antibody specific for HMGB1
(1:1000; 556528; BD Biosciences, San Jose, CA, USA). Following
incubation with peroxidase-conjugated secondary antibody for 1 h at
room temperature (RT), the bands were visualized by
diaminobenzidine detection (Boster Biological Technology Ltd.)
according to the manufacturer’s instructions and
semi-quantitatively determined using Gel-Pro Analyzer densitometry
software (Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
All descriptive data are expressed as mean ±
standard error of the mean (SEM) for continuous variables.
Comparisons of two groups were performed by student’s t-test.
Multiple comparisons were performed by one- or two-way analysis of
variance (ANOVA) followed by Bonferroni’s test. The Pearson
correlation coefficient test was used to evaluate associations
between two quantitative variables. P<0.05 was considered to
indicate a statistically significant difference.
Results
Changes to HMGB1 expression level during
CPB
The results of western blot analysis revealed that
the protein level of HMGB1 at T3 was much higher than at T1 and T2.
The level at T4 was significantly lower than at T3 (P<0.01).
Subsequently, the protein levels of HMGB1 were increased at T4, T5
and T6 (Fig. 1A).
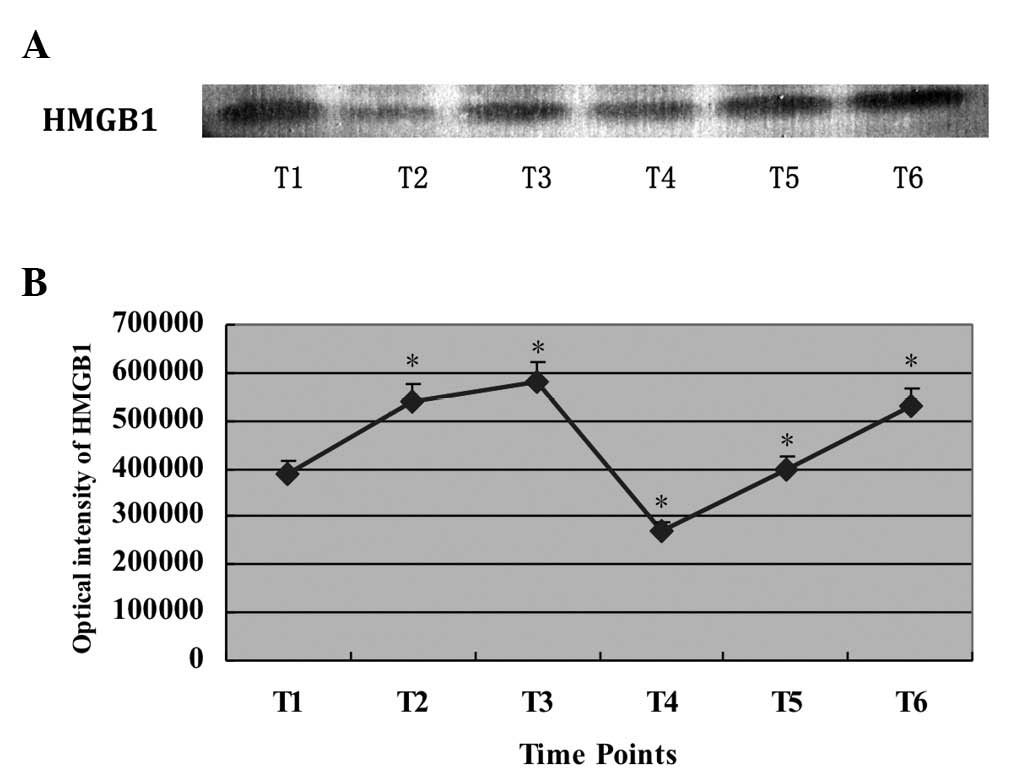 | Figure 1Expression level of HMGB1 during CPB.
(A) Representative bands of HMGB1 protein expression at different
time points. (B) Corrected relative optical intensity values of
HMGB1 at different time points. T1, before surgery (after
anesthetization); T2, before aortic cross-clamp; T3, after CPB; T4,
first day after surgery (between 6 and 7 a.m.); T5, second day
after surgery (between 6 and 7 a.m.); T6, third day after surgery
(between 6 and 7 a.m.). *P<0.05, vs. T1. Data are
expressed as mean ± SEM, n=78. HMGB1, high mobility group box 1;
CPB, cardiopulmonary bypass; SEM, standard error of the mean. |
The relative optical intensity values of the results
were corrected based on the HCT using the formula described in
Materials and methods. The values at T2 and T3 were not corrected
due to the CPB. Summarized data in Fig. 1B revealed that HMGB1 was expressed
as early as T1 (before surgery, after anesthesia), increased after
T2 (cross-clamping) and peaked at T3 (completion of
cross-clamping). After surgery (T4), the HMGB1 level was rapidly
decreased to the level measured at T1; however, it increased again
at T5 and further increased on the third day after surgery (T6), at
which time it was close to the level at T2.
Changes to TNF-α level during CPB
As shown in Fig. 2,
the level of TNF-α increased from the beginning of CPB and peaked
at the end of cross-clamping (T3). The level of TNF-α decreased
significantly after surgery (T4), and the reduction lasted to the
second day after surgery; however, the TNF-α levels remained higher
than the level recorded before surgery (T1). On the third day after
surgery (T6), the level of TNF-α was increased slightly; however,
it remained lower than the level at T2.
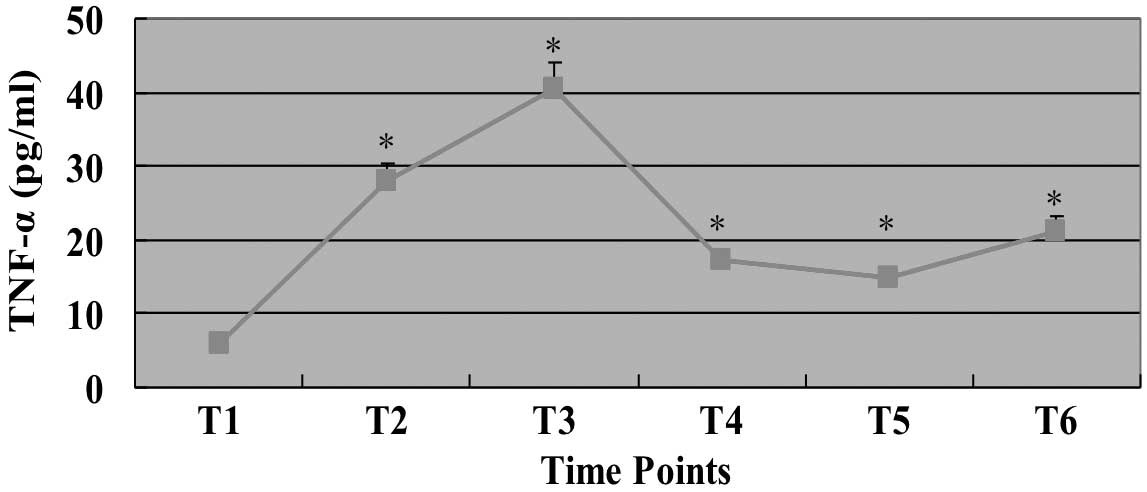 | Figure 2Serum level of TNF-α during CPB. The
serum level of TNF-α at each time point is shown. T1, before
surgery (after anesthetization); T2, before aortic cross-clamping;
T3, after CPB; T4, first day after surgery (between 6 and 7 a.m.);
T5, second day after surgery (between 6 and 7 a.m.); T6, third day
after surgery (between 6 and 7 a.m.). *P<0.05, vs.
T1. Data are expressed as mean ± SEM, n=78. TNF, tumor necrosis
factor; CPB, cardiopulmonary bypass; SEM, standard error of the
mean. |
The merged curves of HMGB1 and TNF-α are shown in
Fig. 3. The curve profile of HMGB1
level was similar to the curve of TNF-α level.
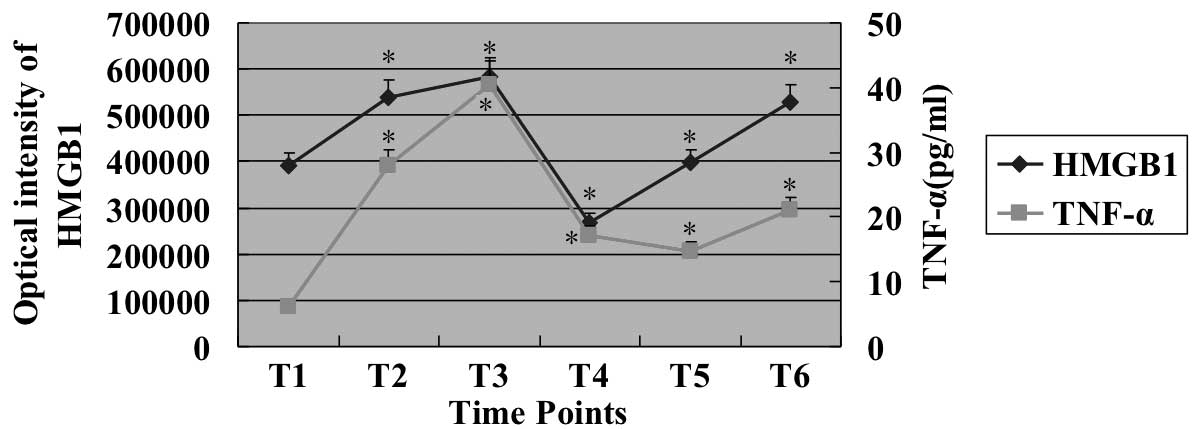 | Figure 3Merged curve of HMGB1 and TNF-α
levels. The overlapped figure shows that profiles of HMGB1 and
TNF-α are similar. T1, before surgery (after anesthetization); T2,
before aortic cross-clamping; T3, after CPB; T4, first day after
surgery (between 6 and 7 a.m.); T5, second day after surgery
(between 6 and 7 a.m.); T6, third day after surgery (between 6 and
7 a.m.). *P<0.05, vs. T1. Data are expressed as mean
± SEM, n=78. HMGB1, high mobility group box 1; TNF, tumor necrosis
factor; CPB, cardiopulmonary bypass; SEM, standard error of the
mean. |
Changes to other parameters during
CPB
The clinical parameters were determined and
presented in Table I, together
with the levels of HMGB1 and TNF-α. As shown in Table I, changes of white blood cell (WBC)
levels, neutrophils (N), lymphocytes (L), BUN, Cr and ALT during
CBP were detected and the results were consistent with those of a
previous study (13). No
significant changes of kidney function were observed following
surgery. ALT increased following CPB and reached a peak at T6
without any decrease. Changes in liver function following surgery
appeared to be correlated with the expression of HMGB1. Therefore,
a correlation analysis between these parameters was performed.
 | Table IClinical characteristics of patients
during CPB. |
Table I
Clinical characteristics of patients
during CPB.
| Parameters | T1 | T2 | T3 | T4 | T5 | T6 |
|---|
| WBC (×109
cells/l) | 5.56±1.50 | 5.59±1.56 | 13.05±7.69a | 18.74±6.62a | 29.29±9.07a | 22.96±7.91a |
| N (%) | 61.78±14.30 | 58.75±10.47 | 75.22±13.57a | 89.71±3.50a | 87.89±3.40a | 81.72±5.88a |
| L (%) | 24.93±5.93 | 31.95±7.58a | 22.61±12.58 | 5.73±2.82a | 5.95±2.46a | 10.99±5.25a |
| BUN (mmol/l) | 6.25±2.61 | 8.36±2.16b | 9.36±2.59a | 11.02±3.64a | 12.53±6.38a | 10.98±6.81a |
| Serum Cr
(μmol/l) | 63.87±13.43 | 94.07±24.17a | 103.42±30.24a | 111.45±38.63a | 91.52±41.07a | 85.73±31.18b |
| ALT (U/l) | 23.43±10.17 | 19.67±9.10 | 22.38±8.22 | 32.24±8.50a | 34.85±9.65a | 43.67±25.87a |
| Urinary NAG/Cr
(u/g.cr) | 0.019±0.019 | 0.043±0.070a | 0. 62±0.610a | 0.013±0.006 | 0.045±0.030a | 0.044±0.026a |
| Urinary β2-MG/Cr
(mg/mmol) | 0.01±0.01 | 0.93±0.26b | 0.51±0.64a | 0.20±0.29a | 0.13±0.15 | 0.08±0.08 |
| TNF-α (pg/ml) | 6.01±5.51 | 27.96±17.80a | 40.37±25.41b | 17.18±12.26a | 14.81±11.92a | 21.19±19.52a |
| HMGB1 (pixels) | 390228±205.5 | 539322±212.5a | 583262±388.5a | 268621±136.6a | 397748±196.9 |
529287±278.5a |
Correlations between HMGB1, TNF-α and
other parameters
HMGB1 was positively correlated with TNF-α (r=
0.237, P= 0.002). ALT was positively correlated with HMGB1
(r=0.559, P=0.02) but not with TNF-α (r=-0.49; P=0.858) at T3. The
correlations between NAG/Cr and HMGB1 (r=0.376; P=0.379) or TNF-α
were negative (r=0.236; P=0.134) at T3.
Discussion
In this study, we demonstrated the significant
changes in serum levels of HMGB1 and TNF-α following CPB, as well
as other parameters. This is the first study to track the changes
of HMGB1 level during CPB at six time points: before surgery,
before aortic cross-clamping, after CPB and one, two and three days
after surgery. The HMGB1 levels were significantly different in the
patients with CPB and may be correlated with the level of
TNF-α.
It is well known that CPB is characteristically
associated with a systemic inflammatory response, which is
initiated by the synthesis of various cytokines, including TNF,
inter-leukin-1, interleukin-6 and interleukin-8 (14). Inflammatory cytokines, endothelial
activation and endothelial-leukocyte interactions have been
reported to play an important role in the induction of systemic
inflammatory response syndrome (SIRS), causing large fluid shifts,
coagulation disturbances and increased concentrations of
catecholamines and stress hormones (15). Several reports have shown that
marked changes in serum cytokine levels occur during and after CPB
in children and adults (16,17).
An increased inflammatory response due to elevated sequestration of
inflammatory mediators is considered responsible for the high
incidence of poor outcomes following bypass (16).
HMGB1 was first identified as a nuclear protein;
then, it was revealed that HMGB1 activates inflammatory responses
through multiple pathways, including macrophage activation and
release of pro-inflammatory cytokines, endothelial cell activation
and expression of adhesion molecules. These effects of HMGB1 lead
to a cascade of inflammatory responses that cause tissue damage and
even mortality (11). HMGB1 is
secreted as a late mediator during inflammation and participates in
the pathogenesis of systemic inflammation once the early mediator
response has resolved. In vitro, extracellular HMGB1
activates macrophages and monocytes and promotes dendritic cell
maturation (18,19). In vivo, HMGB1 causes acute
lung inflammation, epithelial-cell barrier leakage and even
mortality (20). Extracellular
HMGB1 translocation during inflammatory responses in vivo
leads to significantly increased serum levels in patients with
arthritis, sepsis and other inflammatory disorders (21). Moreover, increased levels of HMGB1
are observed in patients with sepsis and other major inflammatory
diseases, including rheumatoid arthritis (22). In the current study, we identified
that HMGB1 is expressed initially before surgery after
anesthetization (T1). Then, it increases following cross-clamping
(T2) and peaks at the completion of cross-clamping (T3). A factor
involved in the increase of HMGB1 may be stress. Additionally, this
change may result from myocardial ischemia/reperfusion during
cardiac surgery. Following surgery, the HMGB1 level rapidly
decreased to the level before surgery (T4) and then increased again
(T5), and the elevation continued until the third day after surgery
(T6). The level of HMGB1 was high prior to surgery, possibly as a
result of the long-term chronic inflammatory stimulation of
rheumatic heart disease.
The cytokines that are significantly increased
following bypass are also associated with SIRS/MODS. TNF-α is
strongly associated with SIRS/MODS (23). Hypotension, followed by
coagulopathy and renal dysfunction, is strongly associated with the
increase of TNF-α. TNF-α may be a direct inducer of hypotension,
coagulopathy and renal dysfunction (24–27).
It was suggested that during bypass, an elevation of TNF-α may
participate in capillary leakage and subsequently pulmonary edema,
which may lead to respiratory dysfunction (28). In the present study, the level of
TNF-α was monitored during CPB. Our data revealed that the TNF-α
level was highest at T3 (after CPB) and the highest inflammatory
reaction was observed at T3. These data suggest that
anti-inflammatory treatment may be most beneficial after CPB.
Moreover, we identified that the variation profile of TNF-α level
with time was consistent with that of HMGB1, suggesting that HMGB1
and TNF-α are indicators of an inflammatory response. Due to the
important role of TNF-α in pathophysiological processes during CPB,
intervention targeting these two mediators may be effective. For
example, potent inhibitors of TNF-α or HMGB1 prior to bypass may be
useful in ameliorating the damage resulting from inflammatory
responses.
In addition to the correlation between HMGB1 and
TNF-α, we also investigated the correlations of HMGB1 and TNF-α
with other important biochemical parameters during CPB. We
identified that ALT was positively correlated with HMGB1, which may
suggest that HMGB1 plays a role in hepatic injury during CPB. The
mechanism may be the ischemia-reperfusion of multiple organs during
CPB. This further supports a previous study suggesting that HMGB1
mediates hepatic injury following murine liver ischemiareperfusion
(29).
The results of the present study demonstrate a
positive correlation between HMGB1 and TNF-α, suggesting that HMGB1
is an indicator of inflammation during CPB. These data provide
useful evidence to support further in vivo and in
vitro studies aimed at therapy targeting HMGB1 to attenuate
inflammation-related damage following surgery. In addition,
considering the time-curve of TNF-α level during CPB, the optimal
timing for treatment is after CPB at the completion of
cross-clamping (T3).
References
|
1.
|
Davies SW, Duffy JP, Wickens DG, et al:
Time-course of free radical activity during coronary artery
operations with cardio-pulmonary bypass. J Thorac Cardiovasc Surg.
105:979–987. 1993.PubMed/NCBI
|
|
2.
|
Picone AL, Lutz CJ, Finck C, et al:
Multiple sequential insults cause post-pump syndrome. Ann Thorac
Surg. 67:978–985. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Marin ML, Veith FJ, Cynamon J, et al:
Human transluminally placed endovascular stented grafts:
preliminary histopathologic analysis of healing grafts in
aortoiliac and femoral artery occlusive disease. J Vasc Surg.
21:595–603; discussion 603–604, 1995.
|
|
4.
|
Bustin M: Regulation of DNA-dependent
activities by the functional motifs of the high-mobility-group
chromosomal proteins. Mol Cell Biol. 19:5237–5246. 1999.PubMed/NCBI
|
|
5.
|
Calogero S, Grassi F, Aguzzi A, et al: The
lack of chromosomal protein Hmg1 does not disrupt cell growth but
causes lethal hypoglycaemia in newborn mice. Nat Genet. 22:276–280.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Wang H, Bloom O, Zhang M, et al: HMG-1 as
a late mediator of endotoxin lethality in mice. Science.
285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Wang H, Yang H and Tracey KJ:
Extracellular role of HMGB1 in inflammation and sepsis. J Intern
Med. 255:320–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Andersson U, Wang H, Palmblad K, et al:
High mobility group 1 protein (HMG-1) stimulates proinflammatory
cytokine synthesis in human monocytes. J Exp Med. 192:565–570.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Hennein HA, Ebba H, Rodriguez JL, et al:
Relationship of the proinflammatory cytokines to myocardial
ischemia and dysfunction after uncomplicated coronary
revascularization. J Thorac Cardiovasc Surg. 108:626–635.
1994.PubMed/NCBI
|
|
10.
|
Lotan D, Zilberman D, Dagan O, et al:
Beta-chemokine secretion patterns in relation to clinical course
and outcome in children after cardiopulmonary bypass: continuing
the search to abrogate systemic inflammatory response. Ann Thorac
Surg. 71:233–237. 2001. View Article : Google Scholar
|
|
11.
|
Liu J, Liu Y, Zhang H, et al: KLF4
promotes the expression, translocation and release of HMGB1 in
RAW264.7 macrophages in response to LPS. Shock. 30:260–266.
2008.PubMed/NCBI
|
|
12.
|
Tang D, Shi Y, Kang R, et al: Hydrogen
peroxide stimulates macrophages and monocytes to actively release
HMGB1. J Leukoc Biol. 81:741–747. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Roth-Isigkeit A, Hasselbach L, Ocklitz E,
et al: Inter-individual differences in cytokine release in patients
undergoing cardiac surgery with cardiopulmonary bypass. Clin Exp
Immunol. 125:80–88. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Miller BE and Levy JH: The inflammatory
response to cardio-pulmonary bypass. J Cardiothorac Vasc Anesth.
11:355–366. 1997. View Article : Google Scholar
|
|
15.
|
Tsuchida M, Watanabe H, Watanabe T, et al:
Effect of cardio-pulmonary bypass on cytokine release and adhesion
molecule expression in alveolar macrophages. Preliminary report in
six cases. Am J Respir Crit Care Med. 156:932–938. 1997. View Article : Google Scholar
|
|
16.
|
Casey LC: Role of cytokines in the
pathogenesis of cardiopulmonary-induced multisystem organ failure.
Ann Thorac Surg. 56(Suppl 5): S92–S96. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Hill GE: Cardiopulmonary bypass-induced
inflammation: is it important? J Cardiothorac Vasc Anesth. 12(Suppl
2): S21–S25. 1998.
|
|
18.
|
Raucci A, Palumbo R and Bianchi ME: HMGB1:
a signal of necrosis. Autoimmunity. 40:285–289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Messmer D, Yang H, Telusma G, et al: High
mobility group box 1 (HMGB1) is an endogenous Th1 polarizing signal
for dendritic cell maturation. J Immunol. 173:307–313. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Abraham E, Arcaroli J, Carmody A, et al:
HMG-1 as a mediator of acute lung Inflammation. J Immunol.
165:2950–2924. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Yang H, Wang H, Czura CJ and Tracey KJ:
The cytokine activity of HMGB1. J Leukoc Biol. 78:1–8. 2005.
View Article : Google Scholar
|
|
22.
|
Pisetsky DS: The role of nuclear
macromolecules in innate immunity. Proc Am Thorac Soc. 4:258–262.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Khabar KS, elBarbary MA, Khouqeer F, et
al: Circulating endotoxin and cytokines after cardiopulmonary
bypass: differential correlation with duration of bypass and
systemic inflammatory response/multiple organ dysfunction
syndromes. Clin Immunol Immunopathol. 85:97–103. 1997. View Article : Google Scholar
|
|
24.
|
Bertani T, Abbate M, Zoja C, et al: Tumor
necrosis factor induces glomerular damage in the rabbit. Am J
Pathol. 134:419–430. 1989.PubMed/NCBI
|
|
25.
|
Gómez-Chiarrri M, Ortiz A,
González-Cuadrado S, et al: Interferon-inducible protein-10 is
highly expressed in rats with experimental nephrosis. Am J Pathol.
148:301–311. 1996.PubMed/NCBI
|
|
26.
|
Brennan DC, Yui MA, Wuthrich RP and Kelley
VE: Tumor necrosis factor and interleukin 1 in New Zealand
Black/White mice. Enhanced gene expression and acceleration of
renal injury. J Immunol. 143:3470–3475. 1999.PubMed/NCBI
|
|
27.
|
Brady HR, Lamas S, Papayianni A, et al:
Lipoxygenase product formation and cell adhesion during
neutrophil-glomerular endothelial cell interaction. Am J Physiol.
268(1 Pt 2): F1–F12. 1995.PubMed/NCBI
|
|
28.
|
Miakotina OL and Snyder JM: TNF-alpha
inhibits SP-A gene expression in lung epithelial cells via P38
MAPK. Am J Physiol Lung Cell Mol Physiol. 283:L418–L427. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Tsung A, Sahai R, Tanaka H, et al: The
nuclear factor HMGB1 mediates hepatic injury after murine liver
ischemia-reperfusion. J Exp Med. 201:1135–1143. 2005. View Article : Google Scholar : PubMed/NCBI
|

















