Introduction
Ischemia-reperfusion (I/R)-induced kidney injury
results from a sudden transient reduction in blood flow, arising
from shock, trauma, abdominal surgery or kidney transplantation,
and often leads to acute kidney injury, chronic renal failure or
failure of the kidney transplant (1–3).
Previous studies have identified that I/R leads to the activation
and infiltration of neutrophils and macrophages, which release
numerous pro-inflammatory mediators that trigger acute inflammatory
responses; this has been demonstrated to be crucial in I/R-induced
renal injury (4–7). In addition, renal cell apoptosis may
be involved in I/R-induced renal injury.
Glycyrrhizin is the predominant active component
that is extracted from the roots of Glycyrrhiza glabra and
exhibits anti-inflammatory effects (8). Furthermore, glycyrrhizin has been
reported to attenuate I/R-induced gut, spinal cord, liver and heart
damage (9–14). However, to the best of our
knowledge, the effect of glycyrrhizin on I/R-induced renal injury
has not been investigated.
Materials and methods
Renal I/R procedure
The animal experiments in the present study were
approved by the Ethics Committee of the Third Xiangya Hospital,
Central South University (Changsha, China). Male C57BL/6 mice (age,
12 weeks; weight, 20–25 g) were housed at Xiangya Medical
Experimental Animal Center, Central South University in a laminar
flow, temperature-controlled, pathogen-free environment under a 12
h light/dark cycle. The mice were fasted for 24 h prior to the
experiments and provided with tap water ad libitum. During
surgery, an intraperitoneal injection of 50 mg/kg pentobarbital was
administered to anesthetize the mice. Bilateral flank incisions
were made, the right kidney was removed and the left kidney was
subjected to ischemia using a microvascular clamp, which was
removed after 30 min and the wound was closed. To induce ischemia
of the left kidney, a microvascular clip was used to clamp the
renal artery after a transverse incision was made to the abdomen.
Half an hour later, the clamp was removed, and the wound was
closed. The mice were divided into three groups of six; in the
sham-operated group, the mice underwent anesthesia, bilateral flank
incisions and a right nephrectomy. In the glycyrrhizin-treated
group, the mice were injected with 60 mg/kg glycyrrhizin using an
infusion pump (Chizhou Kangyuan Medical Equipment Co., Ltd.
Chizhou, China) 1 h prior to ischemia. In the saline-treated group,
the mice were administered with 60 mg/kg saline. The mice were
sacrificed by cervical dislocation under anesthesia 6 h after
reperfusion and the blood and kidney samples were immediately
collected.
Assessment of kidney function
Renal function was assessed via observation of serum
creatinine (Cr) and blood urea nitrogen (BUN) levels at the
Clinical Laboratory of the Third Xiangya Hospital, Central South
University (Changsha, China).
Assessment of the renal tissue superoxide
anion (SOA) level
An SOA assay kit (Sigma, St. Louis, MO, USA) was
used to determine the level of SOA in the soluble fraction of renal
tissue in accordance with the manufacturer’s instructions and the
reaction was analyzed using a spectrophotometer (Shimadzu, Kyoto,
Japan) at a wavelength of 550 nm.
Assessment of the renal tissue superoxide
dismutase (SOD) activity
The SOD activity was measured using the SOD activity
assay kit (Biovision, Milpitas, CA, USA) based on the inhibition of
adenochrome production by SOD during epinephrine auto-oxidation.
Changes in fluorescence were read at a wavelength of 480 nm
(Microplate Reader; Bio-Rad, Hercules, CA, USA).
Caspase-3 activity measurement
Caspase-3 colorimetric assay kit (Biovision, San
Francisco Bay Area, CA, USA) was used to determine the activity of
caspase-3, according to the manufacturer’s instructions. Briefly,
20 μg kidney cytosolic protein was extracted and incubated in a
solution buffer at room temperature for 30 min. The reaction was
initiated by the addition of 200 μM
N-acetyl-Asp-Glu-Val-Asp-7-amino-4-trifluoromethylcoumarin and
incubated at 37°C for 2 h. The change in absorbance was measured
spectrophotometrically at a wavelength of 400 nm.
Myeloperoxidase (MPO) activity
measurement
MPO is a neutrophil-specific enzyme, the presence of
which is considered to be an indicator of neutrophil infiltration
in the kidney. Renal tissues were homogenized on ice in
phosphate-buffered saline (PBS) with 0.5% hexadecyltrimethyl
ammonium bromide (Sigma) and 0.146% EDTA (pH 6.0). The homogenates
were centrifuged at 13,400 × g for 30 min at 4°C and the
supernatant was incubated at 60°C for 2 h. Hydrogen peroxide
(0.005%) and 0.167 mg/ml O-dianisidine dihydrochloride (Sigma) in
PBS (pH 6.0) was added and the change in absorbance was measured
spectrophotometrically at a wavelength of 460 nm.
Serum pro-inflammatory cytokine
measurements
Enzyme-linked immunosorbent assay kits (mouse TNF-α
ELISA kit, Mouse IFN-γ ELISA kit, mouse IL-1β ELISA kit, mouse IL-6
ELISA kit; Sigma) were used to determine the serum levels of the
key pro-inflammatory cytokines, including tumor necrosis factor
(TNF)-α, interferon (IFN)-γ, interleukin (IL)-1β and IL-6, in
accordance with the manufacturer’s instructions.
Western blot assay
Cytoplasmic extraction reagents (Pierce
Biotechnology, Inc., Rockford, IL, USA) were used to extract the
cytosolic proteins from the mice kidney tissues, in accordance with
the manufacturer’s instructions. After determining the
concentration of protein using the Enhanced BCA Protein Assay kit
(Beyotime, Shanghai, China), 20 μg protein was separated using 10%
SDS-PAGE, which was transferred to nitrocellulose membranes and
maintained at room temperature for 1 h in a buffer solution
containing 5% dried skimmed milk. The membrane was incubated at
room temperature for 3 h with mouse anti-total-p38 MAPK, mouse
anti-phospho-p38 MAPK, mouse anti-cleaved caspase-3 or mouse
anti-GAPDH monoclonal antibodies (Abcam, Cambridge, UK), and
subsequently with goat anti-mouse secondary antibody (Abcam) for 1
h. The signals on the membranes were detected using an enhanced
chemiluminescence reagent (PerkinElmer, Waltham, MA, USA) and the
densitometry was analyzed by Image-Pro plus software 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA).
Statistical analysis
All of the data were expressed as means ± standard
deviation and analyzed by one-way analysis of variance, followed by
Student’s t-test to assess the statistical significance. P<0.05
was considered to indicate a statistically significant
difference.
Results
Administration of glycyrrhizin protects
mice from I/R-induced renal injury
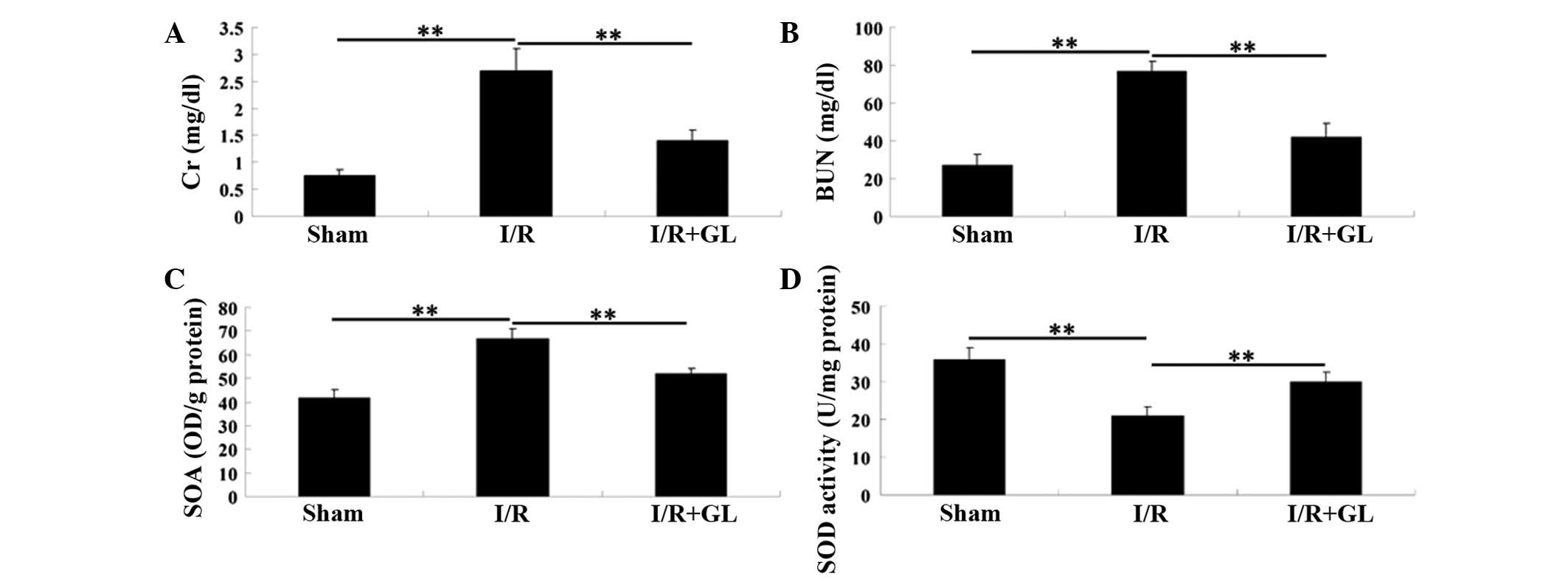
To investigate the effect of glycyrrhizin on
I/R-induced renal injury in mice, the serum levels of Cr and BUN
were examined 6 h following reperfusion. The mice in the
saline-treated group showed significantly higher serum levels of Cr
and BUN compared with the mice in the sham-operated group, which
indicated marked I/R-induced renal damage. However, the mice that
were administered with glycyrrhizin exhibited notably lower serum
levels of Cr and BUN, when compared with those in the
saline-treated group. These findings indicate that pretreatment
with glycyrrhizin provides effective protection against I/R-induced
renal injury in mice (Fig. 1A and
B).
The SOA level that was observed in the soluble
fraction of the renal tissue 6 h after reperfusion was
significantly higher in the saline-treated control group, when
compared with that observed in the sham-operated and
glycyrrhizin-treated groups (Fig.
1C). In addition, the SOD activity in the kidney tissue
following I/R was examined and it was demonstrated that the SOD
activity was greater in the glycyrrhizin-treated group than in the
saline-treated group (Fig.
1D).
Administration of glycyrrhizin attenuates
I/R-induced renal cell apoptosis in mice
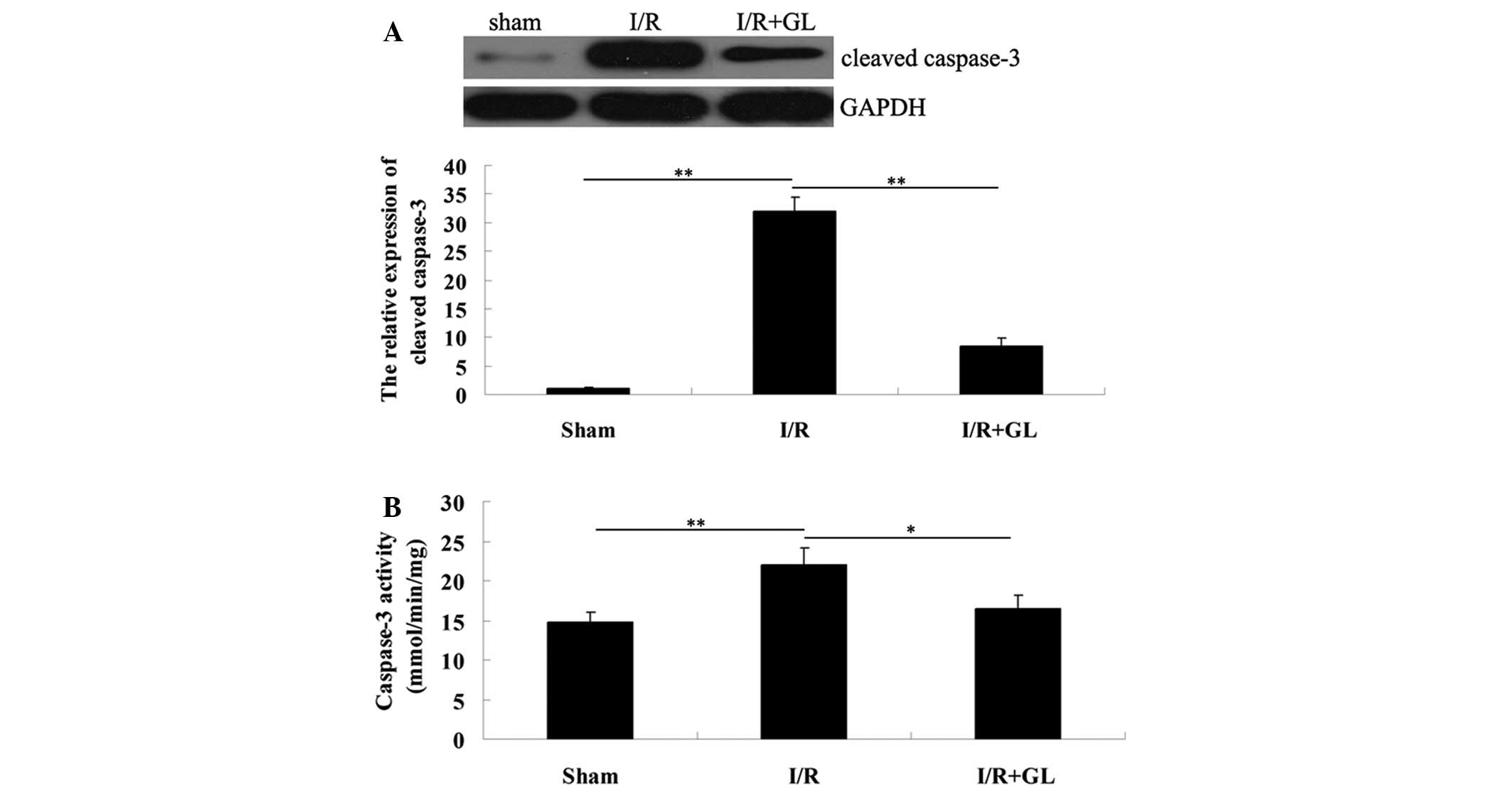
To further investigate the effect of renal cell
apoptosis in I/R-induced renal injury, western blot analysis was
performed to determine the expression of cleaved caspase-3, 6 h
after reperfusion. The expression level was notably reduced in the
mice that were pretreated with glycyrrhizin when compared with that
of the mice pretreated with saline. For further confirmation, the
activity of caspase-3 in the renal tissues was determined in each
group (Fig. 2A). Consistently, the
activity of caspase-3 was downregulated in the mice from the
glycyrrhizin-treated group compared with the saline-treated group.
Therefore, it was hypothesized that glycyrrhizin protects against
I/R-induced renal damage in mice, partially via the inhibition of
renal cell apoptosis.
Administration of glycyrrhizin attenuates
I/R-induced renal inflammation in mice
As neutrophil and macrophage infiltration is key in
I/R-induced tissue inflammation, and MPO activity is a key
indicator of neutrophil and macrophage infiltration, the MPO
activity in the renal tissue of each group was assessed. The
activity of MPO was identified to be significantly upregulated in
the saline-treated group compared with the sham-operated group.
However, the activity of MPO in the glycyrrhizin-treated group was
significantly reduced compared with the saline-treated group with a
value that was comparable to the sham-operated group (Fig. 3A).
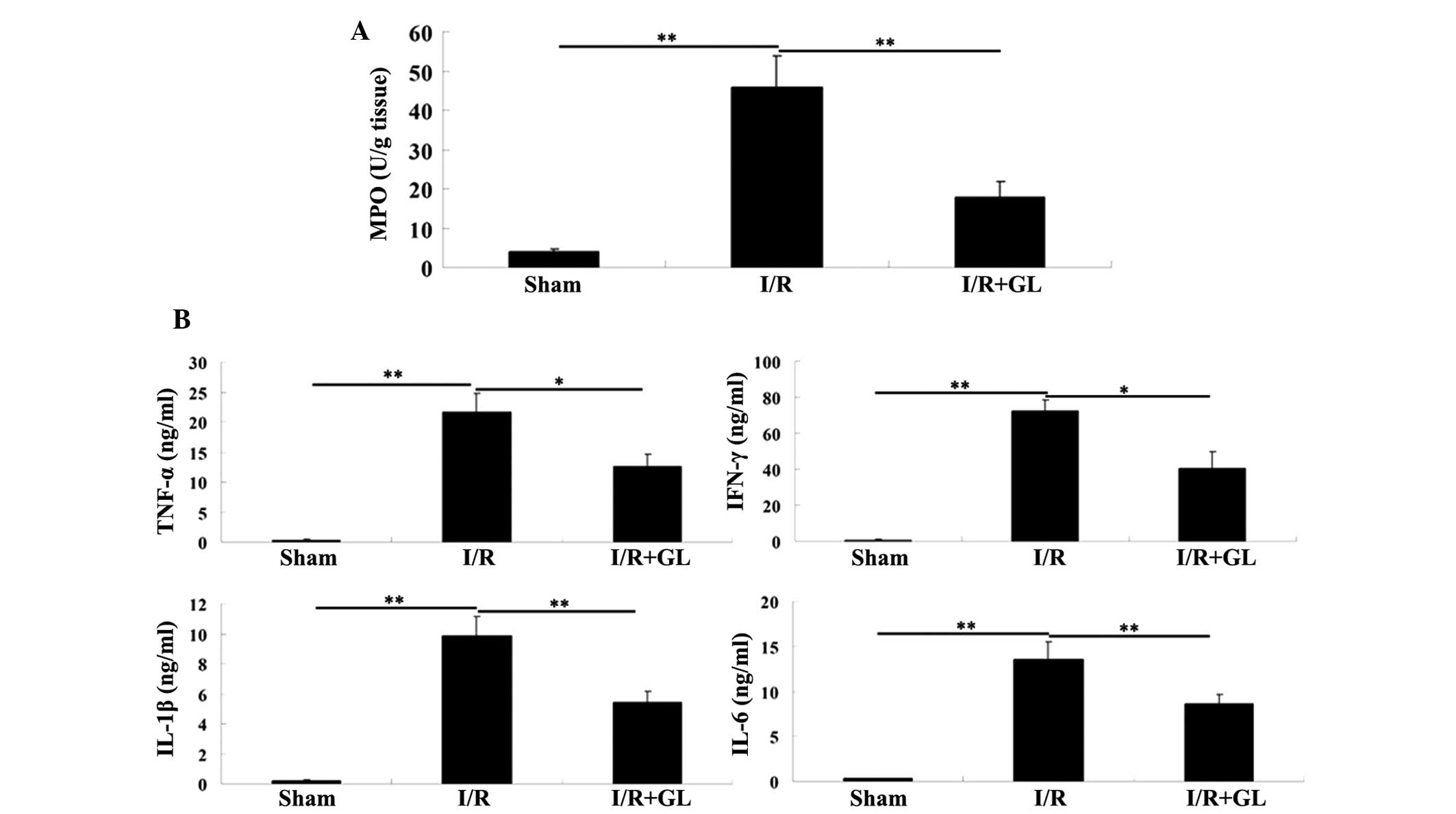 | Figure 3(A) Activity of MPO in the renal
tissue was determined in the three groups. (B) The serum levels of
key pro-inflammatory cytokines, including TNF-α, IFN-γ, IL-1β and
IL-6, were examined in each group using enzyme-linked immunosorbent
assay kits. *P<0.05 and **P<0.01. MPO,
myeloperoxidase; I/R, ischemia-reperfusion; Gl, glycyrrhizin; TNF,
tumor necrosis factor; IFN, interferon; IL, interleukin. |
To identify the mechanism involved, the effect of
glycyrrhizin on the production of inflammatory cytokines, including
TNF-α, IFN-γ, IL-1β and IL-6 was examined; these inflammatory
cytokines have been demonstrated to be significant in the induction
of renal I/R injury (15,16). The production of pro-inflammatory
TNF-α, IFN-γ, IL-1β and IL-6 was significantly reduced in the
glycyrrhizin-treated mice, when compared with that observed in the
saline-treated mice 6 h after reperfusion (Fig. 3B). Accordingly, these findings
indicate that pretreatment with glycyrrhizin ameliorates
I/R-induced renal injury via the mediation of inflammatory cell
infiltration in addition to the production of inflammatory
cytokines.
Administration of glycyrrhizin suppresses
p38 MAPK activation during I/R
As glycyrrhizin has been reported to exhibit a
suppressive effect on the activation of the p38 MAPK pathway in
I/R-induced myocardial injury in rabbits, it was hypothesized that
it may be similarly involved in the regulation of the p38 MAPK
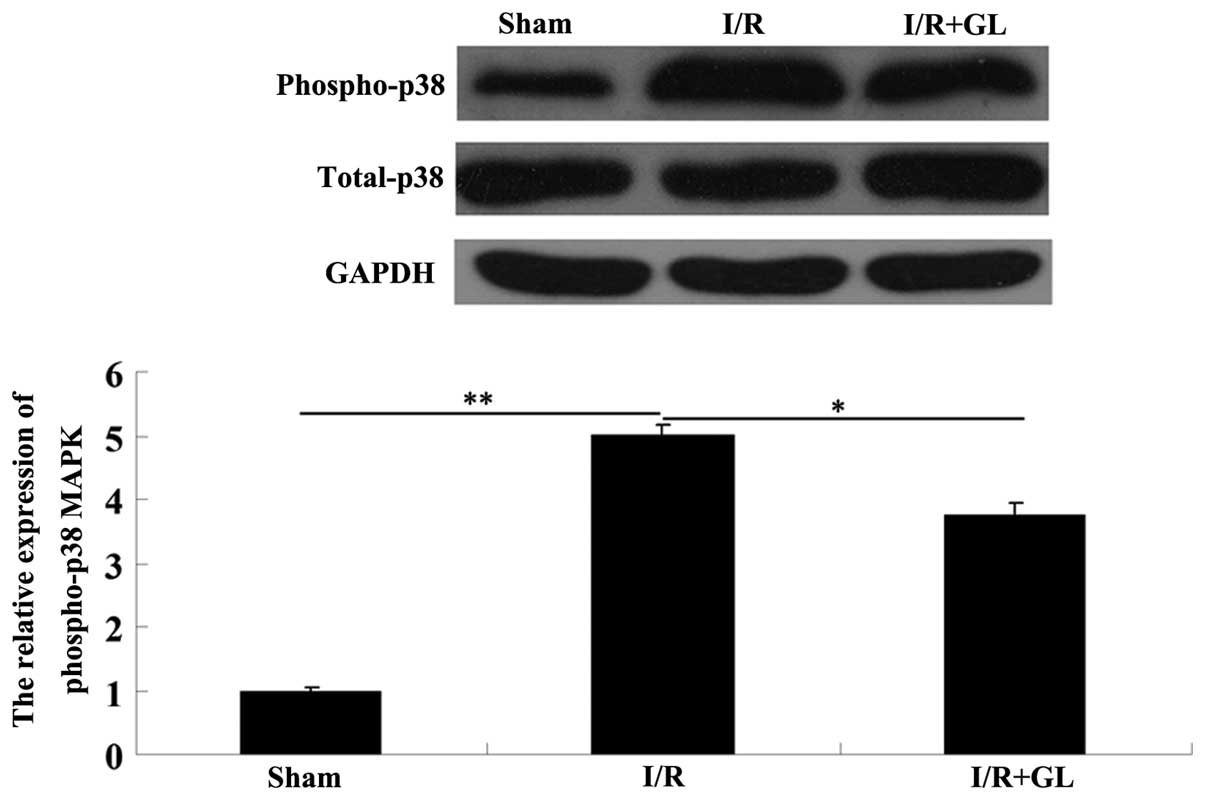
pathway in renal I/R damage in mice. Thus, the activity of the p38
MAPK pathway in the renal tissues was determined in each group via
western blot assay. The phosphorylated-p38 MAPK protein level in
the saline-treated group was significantly increased compared with
the sham-operated group; however, the phosphorylated-p38 protein
level in the glycyrrhizin-treated group was notably reduced
compared with that in the saline-treated group (Fig. 4). These findings confirmed the
hypothesis that administration of glycyrrhizin suppressed the
activation of p38 MAPK in I/R-induced renal damage in mice.
Discussion
Glycyrrhizin has been identified to exhibit a
protective effect on I/R-induced organ damage, including damage to
the gut, liver and heart (9–13).
However, whether glycyrrhizin protects against I/R-induced renal
injury has not previously been analyzed. In the present study, the
effect of glycyrrhizin on I/R-induced kidney injury in mice was
investigated. To the best of our knowledge, this is the first study
to show that pretreatment with glycyrrhizin attenuates renal I/R
injury via inhibition of tissue inflammation as well as protection
against cell apoptosis, indicating that glycyrrhizin may be used
for the prevention of I/R-induced kidney injury in clinical
settings.
Renal cell apoptosis has been shown to be key in
I/R-induced renal damage (17).
Among the apoptotic mediators, activated caspase-3 acts as a final
executor, as it regulates the extrinsic and intrinsic pathways of
apoptosis (18,19). Furthermore, caspase-3-mediated cell
apoptosis has been demonstrated to be essential in I/R-induced
organ damage (20); thus, the
effect of glycyrrhizin on I/R-induced renal cell apoptosis was
investigated in the present study. The findings showed that
administration of glycyrrhizin notably inhibited the protein level
of cleaved caspase-3, (the activated form of caspase-3) within the
renal tissues. Moreover, the activity of caspase-3 was
downregulated as a result of glycyrrhizin administration.
Therefore, it is hypothesized that glycyrrhizin may protect renal
cells against I/R-induced apoptosis by suppressing caspase-3.
Tissue I/R damage has been identified to
predominantly result from I/R-induced excessive inflammatory
responses (21). When renal I/R
occurs, neutrophils and macrophages initially infiltrate into the
damaged kidney and secrete large quantities of pro-inflammatory
mediators, which promote the infiltration of neutrophils and
macrophages into the damaged tissues and further promote tissue
inflammatory responses (22,23).
Furthermore, infiltrated neutrophils and macrophages reduce renal
blood flow, which leads to microcirculatory failure (24,25).
To investigate the effect of glycyrrhizin on I/R-induced neutrophil
and macrophage infiltration into damaged renal tissues, the
activity of MPO, an enzyme specific to neutrophils and macrophages,
was examined. The findings indicated that the administration of
glycyrrhizin protects against I/R-induced macrophage and neutrophil
infiltration in the renal tissue of mice. Consistently, the
following findings demonstrated that the secretion of
pro-inflammatory cytokines, including TNF-α, IFN-γ, IL-1β and IL-6,
was markedly lower in the glycyrrhizin-treated mice compared with
the saline-treated mice. Similarly, the production of IL-10, a key
anti-inflammatory cytokine, was significantly higher in
glycyrrhizin-treated mice compared with saline-treated mice. In
addition, glycyrrhizin has been demonstrated to protect against
I/R-induced tissue damage by suppressing the inflammatory response.
Liu et al (11) identified
that administration of glycyrrhizin reduced the serum levels of
pro-inflammatory cytokines, including TNF-α, IL-6 and IL-8, in
I/R-induced myocardial injury in rabbits. Zhai et al
(13) indicated that the
administration of glycyrrhizin significantly decreased the levels
of serum TNF-α and IL-6, which protected the rat hearts against I/R
injury. In accordance with these previous findings, the data from
the present study indicated that glycyrrhizin protects mice against
I/R-induced renal injury, via the inhibition of inflammatory
responses.
The activation of p38 MAPK signaling is known to be
significant in I/R-induced cell apoptosis as well as in
inflammatory responses (26,27);
for example, administration of C-phycocyanin protected against
I/R-induced cardiomyocyte apoptosis in rats by inhibiting
I/R-induced p38 activation (28).
Furthermore, it has been reported that glycyrrhizin attenuated
I/R-induced myocardial damage in rabbits by suppressing the
production of certain inflammatory cytokines through the p38 MAPK
pathway (11). Thus, it was
hypothesized in the present study that the protective function of
glycyrrhizin against I/R-induced renal cell apoptosis and
inflammatory responses may occur through inhibition of p38 MAPK
activation. The results of the western blot analysis conducted in
the present study showed that glycyrrhizin significantly suppressed
the phosphorylated-p38 protein level, demonstrating that
glycyrrhizin attenuated the I/R-induced activation of p38 MAPK
signaling, which resulted in a protective effect against renal cell
apoptosis and inflammatory responses in I/R-induced kidney injury
in mice.
In conclusion, the present study demonstrated that
pretreatment with glycyrrhizin provided marked protection for mice
against I/R-induced renal injury via inhibition of inflammatory
responses and renal cell apoptosis. Therefore, the administration
of glycyrrhizin may be effective in the prevention of I/R-induced
renal injury in abdominal surgery and kidney transplantation.
Acknowledgements
The present study was supported by the Fundamental
Research Funds for the Central Universities (grant nos. 303275111
and 303275899).
References
|
1
|
Arumugam TV, Shiels IA, Woodruff TM,
Granger DN and Taylor SM: The role of the complement system in
ischemia-reperfusion injury. Shock. 21:401–409. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ichimaru N, Yazawa K and Takahara S:
Kidney transplantation: how shall we deal with marginal cases?
Future prospects from basic research. Hinyokika Kiyo. 56:481–484.
2010.(In Japanese).
|
|
3
|
Versteilen AM, Di Maggio F, Leemreis JR,
Groeneveld AB, Musters RJ and Sipkema P: Molecular mechanisms of
acute renal failure following ischemia/reperfusion. Int J Artif
Organs. 27:1019–1029. 2004.PubMed/NCBI
|
|
4
|
Jin R, Yang G and Li G: Inflammatory
mechanisms in ischemic stroke: role of inflammatory cells. J Leukoc
Biol. 87:779–789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kupiec-Weglinski JW and Busuttil RW:
Ischemia and reperfusion injury in liver transplantation.
Transplant Proc. 37:1653–1656. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
De Greef KE, Ysebaert DK, Ghielli M, et
al: Neutrophils and acute ischemia-reperfusion injury. J Nephrol.
11:110–122. 1998.PubMed/NCBI
|
|
7
|
Thurman JM: Triggers of inflammation after
renal ischemia/reperfusion. Clin Immunol. 123:7–13. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Okda FA, Yassein S, Ahmed AR, Soufy H and
Nasr SM: Some haematological and biochemical investigations on duck
virus hepatitis following administration of glycyrrhizin. ISRN
Pharmacol. 2013:8494122013.PubMed/NCBI
|
|
9
|
Di Paola R, Menegazzi M, Mazzon E, et al:
Protective effects of glycyrrhizin in a gut hypoxia
(ischemia)-reoxygenation (reperfusion) model. Intensive Care Med.
35:687–697. 2009.PubMed/NCBI
|
|
10
|
Mabuchi A, Wake K, Marlini M, Watanabe H
and Wheatley AM: Protection by glycyrrhizin against warm
ischemia-reperfusion-induced cellular injury and derangement of the
microcirculatory blood flow in the rat liver. Microcirculation.
16:364–376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu L, Zhou HY, Ran K and Wang JB:
Glycyrrhiznatis ameliorates rabbit myocardial ischemia-reperfusion
injury through P38MAPK pathway. Nan Fang Yi Ke Da Xue Xue Bao.
30:298–300. 2010.(In Chinese).
|
|
12
|
Ogiku M, Kono H, Hara M, Tsuchiya M and
Fujii H: Glycyrrhizin prevents liver injury by inhibition of
high-mobility group box 1 production by Kupffer cells after
ischemia-reperfusion in rats. J Pharmacol Exp Ther. 339:93–98.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhai CL, Zhang MQ, Zhang Y, et al:
Glycyrrhizin protects rat heart against ischemia-reperfusion injury
through blockade of HMGB1-dependent phospho-JNK/Bax pathway. Acta
Pharmacol Sin. 33:1477–1487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ni B, Cao Z and Liu Y: Glycyrrhizin
protects spinal cord and reduces inflammation in spinal cord
ischemia-reperfusion injury. Int J Neurosci. 123:745–751. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fang F, Liu GC, Zhou X, et al: Loss of
ACE2 exacerbates murine renal ischemia-reperfusion injury. PLoS
One. 8:e714332013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amura CR, Renner B, Lyubchenko T, Faubel
S, Simonian PL and Thurman JM: Complement activation and toll-like
receptor-2 signaling contribute to cytokine production after renal
ischemia/reperfusion. Mol Immunol. 52:249–257. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang YP, Li G, Ma LL, et al: Penehyclidine
hydrochloride ameliorates renal ischemia-reperfusion injury in
rats. J Surg Res. 186:390–397. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
McIlwain DR, Berger T and Mak TW: Caspase
functions in cell death and disease. Cold Spring Harb Perspect
Biol. 5:a0086562013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang A, Fu S, Chen L, et al: Lacidipine
attenuates apoptosis via a caspase-3 dependent pathway in human
kidney cells. Cell Physiol Biochem. 32:1040–1049. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Daemen MA, de Vries B and Buurman WA:
Apoptosis and inflammation in renal reperfusion injury.
Transplantation. 73:1693–1700. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou X, Luo YC, Ji WJ, et al: Modulation
of mononuclear phagocyte inflammatory response by
liposome-encapsulated voltage gated sodium channel inhibitor
ameliorates myocardial ischemia/reperfusion injury in rats. PLoS
One. 8:e743902013. View Article : Google Scholar
|
|
22
|
Ysebaert DK, De Greef KE, Vercauteren SR,
et al: Identification and kinetics of leukocytes after severe
ischaemia/reperfusion renal injury. Nephrol Dial Transplant.
15:1562–1574. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Facio FN Jr, Sena AA, Araújo LP, et al:
Annexin 1 mimetic peptide protects against renal
ischemia/reperfusion injury in rats. J Mol Med (Berl). 89:51–63.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bolisetty S and Agarwal A: Neutrophils in
acute kidney injury: not neutral any more. Kidney Int. 75:674–676.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jing XX, Wang ZG, Ran HT, et al:
Evaluation of renal ischemia-reperfusion injury in rabbits using
microbubbles targeted to activated neutrophils. Clin Imaging.
32:178–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang S, Qi Y, Xu Y, et al: Protective
effect of flavonoid-rich extract from Rosa laevigata Michx
on cerebral ischemia-reperfusion injury through suppression of
apoptosis and inflammation. Neurochem Int. 63:522–532.
2013.PubMed/NCBI
|
|
27
|
Lai EW, Toledo-Pereyra LH, Walsh J,
Lopez-Neblina F and Anaya-Prado R: The role of MAP kinases in
trauma and ischemia-reperfusion. J Invest Surg. 17:45–53. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Khan M, Varadharaj S, Ganesan LP, et al:
C-phycocyanin protects against ischemia-reperfusion injury of heart
through involvement of p38 MAPK and ERK signaling. Am J Physiol
Heart Circ Physiol. 290:H2136–H2145. 2006. View Article : Google Scholar : PubMed/NCBI
|