Introduction
Sphingolipids (SLs) are a class of lipids, which are
structural cell components involved in the regulation of cellular
processes such as cell proliferation, differentiation, apoptosis
and inflammation (1–5). The basic structure of SLs contains a
sphingoid base that is N-acylated with a fatty acid and C-1
linked to a polar head group. SLs are found in eukaryotes and
prokaryotes and are well studied in mammals (6). The mammalian SL metabolites
ceramide, sphingosine and sphingosine 1-phosphate have drawn
attention as bioactive signaling molecules.
Inflammation is important in the healing processes
of tissue injury or infections. Tumor necrosis factor-α (TNF-α) and
lipopolysaccharide (LPS) are essential pro-inflammatory stimuli
involved in the pathogenesis of chronic inflammatory diseases.
Chronic inflammation, however, is harmful to tissue and is
considered to underlie the onset and development of a wide variety
of pathological responses causing tissue damage. Diseases such as
cancer, atherosclerosis, insulin resistance and diabetes are
associated with chronic inflammation (7). Therefore the timely shut down of
inflammatory processes is of utmost importance to maintain
individual health.
Dietary SLs, such as glucocerebrosides (GluCer)
derived from plants, are enzymatically hydrolyzed by brush border
enzymes in the gut lumen into metabolites namely ceramides and
sphingoid bases. This results in the uptake of the derived
ceramides and sphingoid bases by intestinal epithelial cells
(8–10). Sphingoid bases or ‘sphingosines’
occur in a great variety in type and chain length, such as
sphingosine (d18:1) in mammals, and sphingomyelin (d16:1) in milk
(11,12). Several studies have reported on
SLs regulating inflammatory responses at multiple levels (4,13).
Research of anti-inflammatory effects of plant
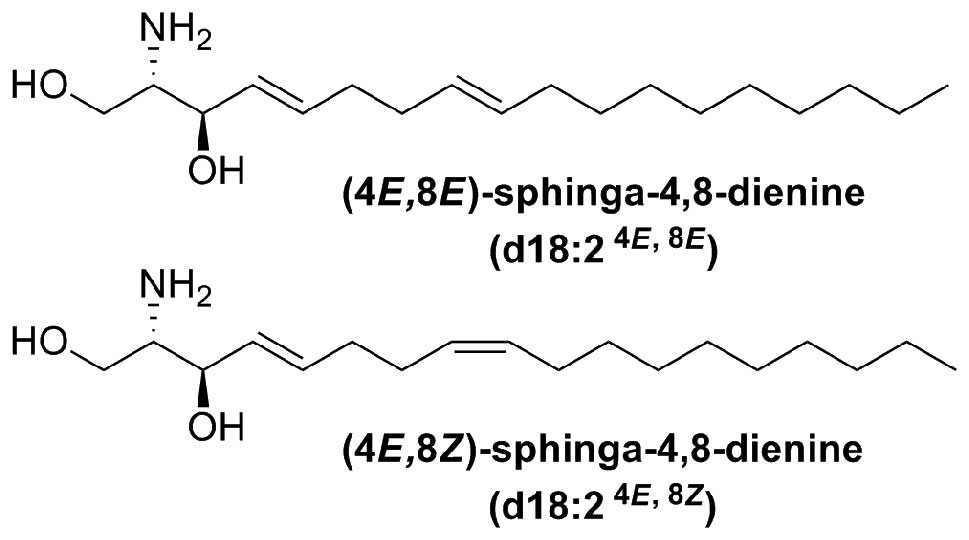
GluCer and sphingoid bases is very limited. 4,8-Sphingadienine
(4,8-SD) (d18:2) is the sphingoid backbone of the characteristic
GluCer present in the Araceae species Arisaema amurense
Maxim. (14) and Pinellia
ternata (Thunb.) Breit., as well as in plants in human diet
such as spinach, soybean and eggplant (11,15).
To the best of our knowledge, this is the first time
the effect of the plant-derived sphingoid base on inflammatory
responses was tested by investigation of the regulation of TNF-α-
and LPS-induced expression of IL-8 and E-selectin.
Materials and methods
Materials
Fetal calf serum (FCS) was purchased from HyClone
(Logan, UT, USA) and TNF-α was from Genzyme (Cambridge, MA, USA).
BAY 11–7085 (BAY) (purity ≥98%), medium 199, o-phenylenediamine and
LPS from E. coli serotype 055:B5 were purchased from
Sigma-Aldrich (Vienna, Austria). The 4,8-SD obtained after
hydrolysis of GluCer was isolated from Arisaema amurense
Maxim. (Araceae) and purified by silica column chromatography (CC).
Polyclonal antibodies were purchased from R&D Systems
(Minneapolis, MN, USA) and peroxidase-conjugated secondary
antibodies from Amersham Life Science (Amersham, UK). For
extraction, fractionation and isolation by CC solvents of highest
available purity were used (VWR, Vienna, Austria). All other
chemicals were obtained from Sigma-Aldrich.
Plant material
Dried, processed rhizomes of Arisaema
amurense Maxim. were purchased from Plantasia (Oberndorf,
Austria). A voucher specimen, encoded ER-I, was deposited at the
Department of Pharmacognosy, University of Vienna.
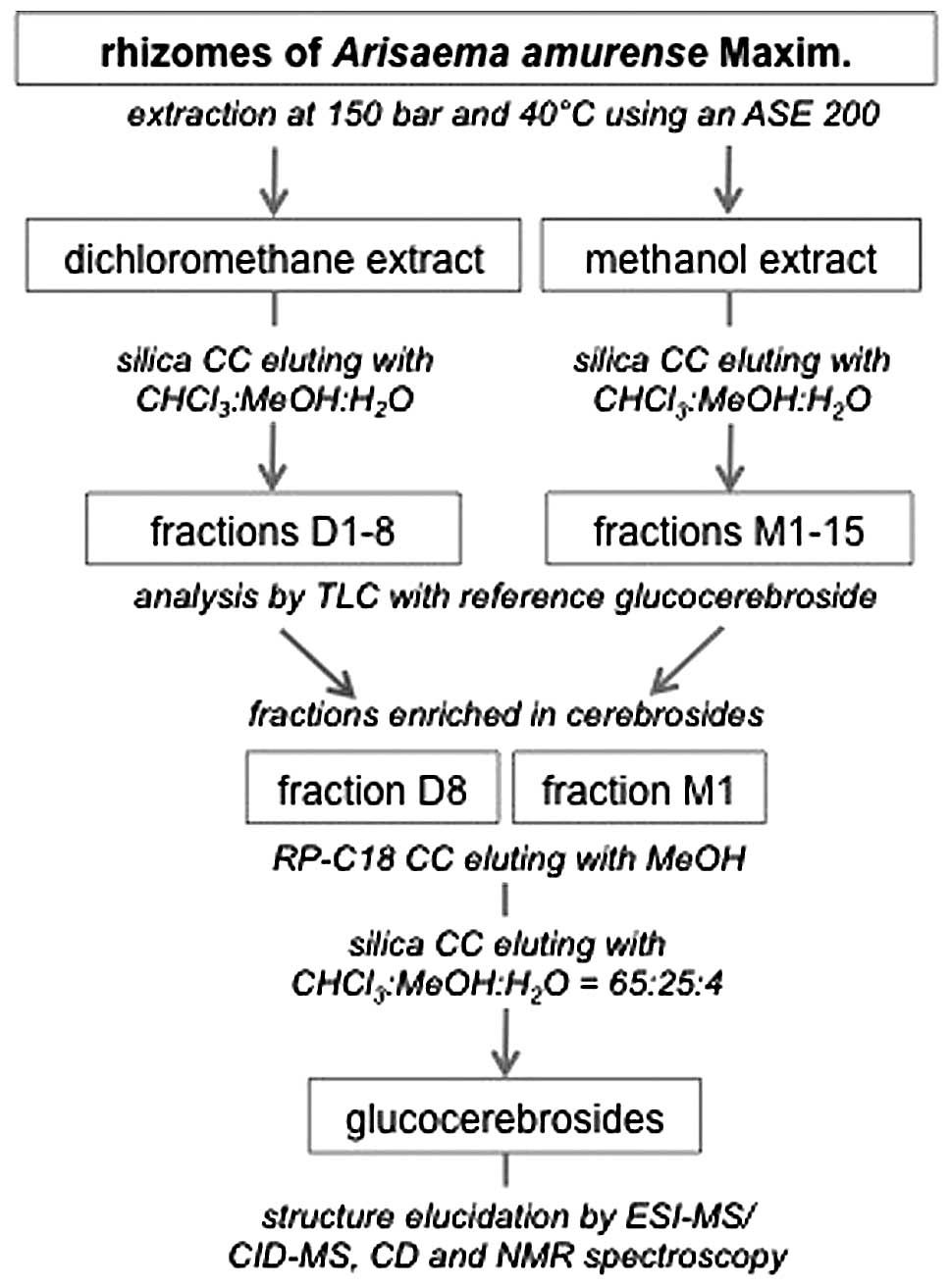
Isolation of GluCer and 4,8-SD
The dried, processed rhizomes of A. amurense
(1.9 kg) were pulverized and extracted by dichloromethane and
subsequently 1.4 kg of this material was extracted with methanol.
The dried extracts were chromatographed over a silica column eluted
by CHCl3:MeOH:H2O 98:2:1 to 60:38:8.5 to
obtain fractions D1–8 for the dichloromethane extract (9.8 g) and
eluted by CHCl3:MeOH:H2O 70:22:3.5 to
60:40:10 for the methanol extract (10.7 g) to obtain fractions
M1–15. GluCer were isolated from fractions D8 (3.6 g) and M1 (2.5
g) by RP C-18 CC with methanol as mobile phase and subsequent
silica CC with CHCl3:MeOH:H2O (65:25:4) as
mobile phase (Fig. 1). A mixture
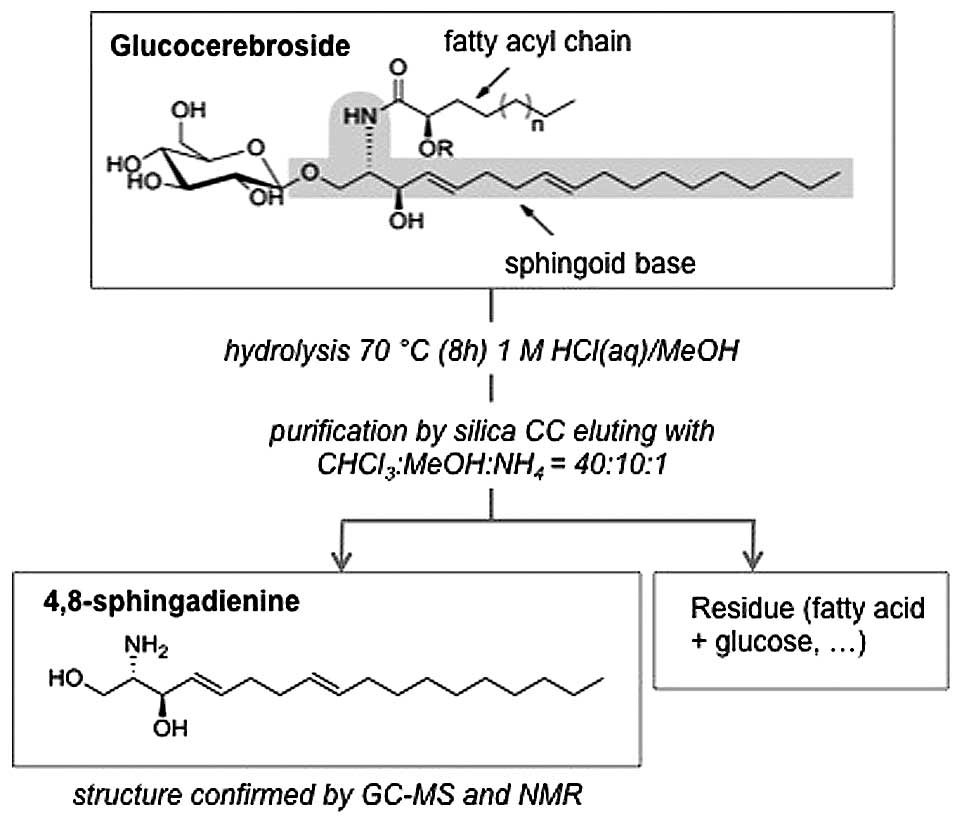
of GluCer (40 mg) was hydrolyzed in methanolic 1 M HCl for 7 h at
70˚C under reflux. The hydrolyzed sample was neutralized and
evaporated to dryness. The liberated sphin-goid base was purified
by silica CC with CHCl3:MeOH:NH4 (40:10:1) as
mobile phase (Fig. 2), with a
yield of 5.5 mg.
GC-MS and NMR analysis
The pure compound was further converted to the
N-acetyl-di-O-trimethylsilyl derivative and analyzed
by GC-MS and NMR and identified as 4,8-sphingadienine (4,8-SD)
(Fig. 3). The amide group was
N-acetylated with acetic anhydride:MeOH (v/v 1:4) overnight
at 25˚C. The sample was brought to dryness and further derivatized
by BSTFA and TMCS (v/v 99:1) and incubated at 50˚C for 30 min. The
analyses were performed on an Agilent Technologies 6890N Network GC
equipped with an Agilent Technologies 5973 inert Mass selective
Detector and a CombiPAL autosampler (CTC Analytics). The column
used was a DB-5 with dimensions of 30 m x 0.25 mm (inner diameter),
0.23 μm (film thickness) (Agilent Technologies). The software used
was MSD Chemstation 2004.
NMR spectra were recorded on a Bruker Avance 500 NMR
spectrometer. 4,8-SD was dissolved in CDCl3 (99.96 atom
% D). The 1H and 13C NMR spectra were
operated at 500 and 125 MHz, respectively. The compound has a
purity of at least 95% judging from the NMR-spectra.
4,8-SD was a brownish, yellow, amorphous solid.
Electron impact mass spectrometry (EIMS) of the
N-acetyl-di-O-trimethylsilyl derivative showed ions
at m/z 73, 174, 309, 334, 378, 468 (16). For biological testing 4,8-SD was
dissolved in DMSO.
Cells
The study was performed using immortalized human
umbilical vein endothelial (HUVECtert) cells (17). In contrast to primary HUVECs,
HUVECtert have the advantage of an indefinite cell division
potential. However, the tert transfection alters the cells’ gene
expression profile (18)
affective the response to the dual treatment. HUVECtert were
cultured in M199 medium supplemented with endothelial cell growth
supplement (Technoclone), heparin, penicillin/streptomycin and 20%
FCS. The cells were grown in a humidified atmosphere at 37˚C and 5%
CO2 and passaged twice a week using trypsin-EDTA
solution. Experiments were performed using cells up to passage
5.
Cell ELISA
HUVECtert cells (1x105 cells/well) were
seeded in a 96-well plate and grown for 24 h overnight in a cell
culture incubator. Monolayers of the grown HUVECtert cells were
then treated with 4,8-SD or GluCer and agonists in medium 199
containing 5% FCS. 4,8-SD (0–45 μM) or BAY 11–7082 (5 μM), which
served as positive control, were added to the cells 20 min before
the addition of TNF (100 ng/ml) or LPS (100 ng/ml). BAY 11-7085 is
an anti-inflammatory agent acting by inhibiting NF-κB thus
decreasing expression of inflammatory molecules including
E-selectin and IL-8 (19). The
solvent vehicle (0.2% DMSO) served as the negative control. After 6
h, the medium was collected for quantification of IL-8, and the
cells were washed and fixed with 4% glutaraldehyde for
determination of E-selectin. Cell surface-expressed E-selectin was
detected using corresponding antibodies, secondary
peroxidase-conjugated antibodies and o-phenylenediamine as
substrate essentially as described before (20). Concentrations of IL-8 in cell
culture medium were determined using human CXCL8/IL-8 ELISA DuoSet
ELISA Development kit (R&D Systems) and the TMB 2-Component
Microwell Peroxidase Substrate kit (VWR International, Radnor, PA,
USA). The absorbance was measured at 490 nm.
Cell proliferation/viability assays
The HUVECtert cells (1x105 cells/well)
were seeded in 96-well plates. After 24 h test compounds (4,8-SD or
GluCer) or vehicle (DMSO) were added. Cell viability and metabolic
activity were assessed after 6 h either by the LDH (Sigma Aldrich,
St. Louis, MO, USA) or WST-1 (Roche Applied Science, Mannheim,
Germany) colorimetric assays. The absorbance was measured at 340 nm
after 55 min for the LDH assay at 450 nm for the WST1-assay.
Data and statistical analysis
Statistical analysis and calculation of
IC50 values were performed using the Prism Software
(ver. 4.03; GraphPad Software Inc., San Diego, CA, USA). Data were
normalized to the mean value from three experiments of DMSO treated
control. The data shown represent the mean values out of at least
three experiments ± SEM. Statistical significance was determined by
a one-way analysis of variance combined with a Dunnett’s multiple
comparison post test. Results with P<0.05 were considered
significant.
Results
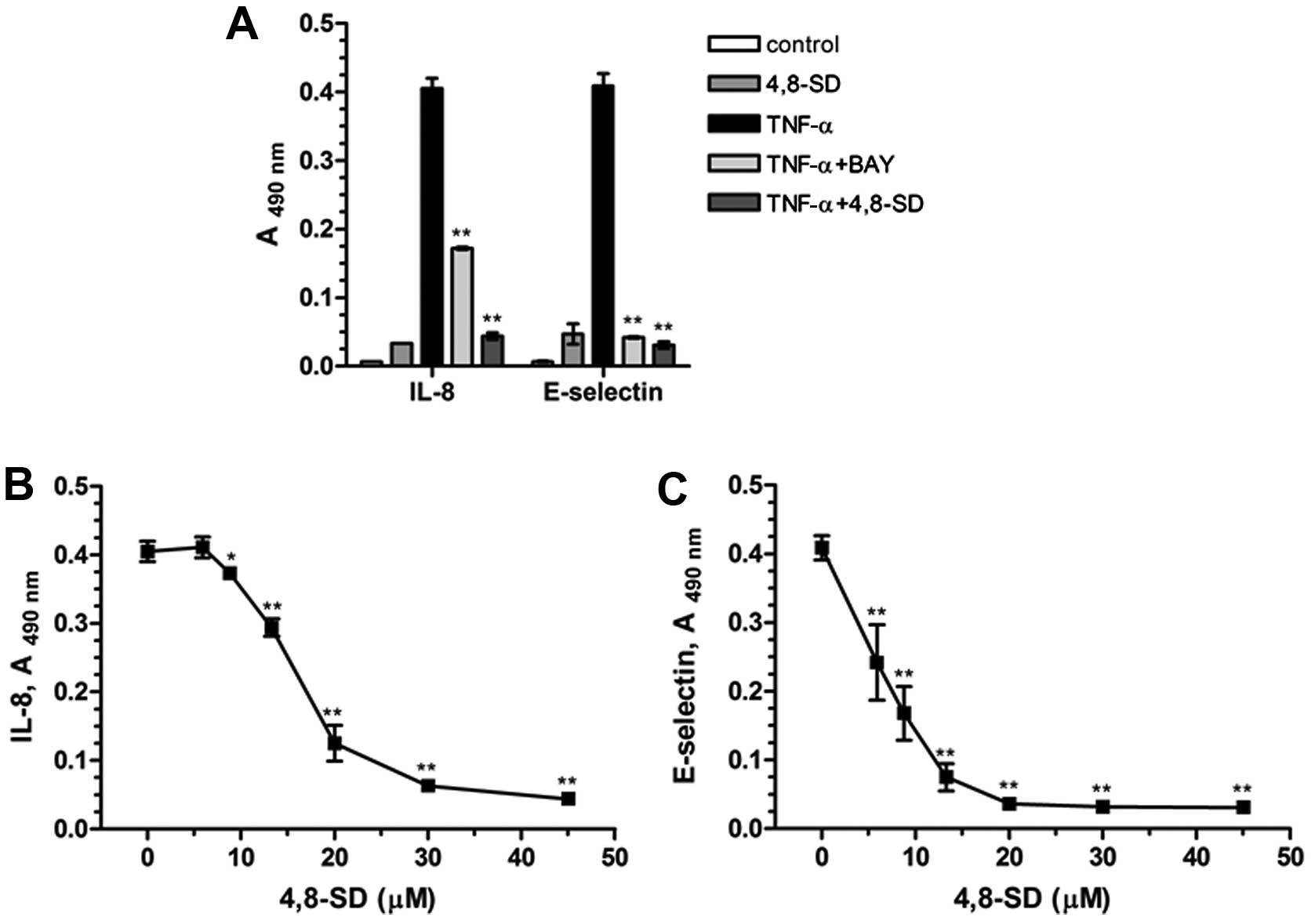
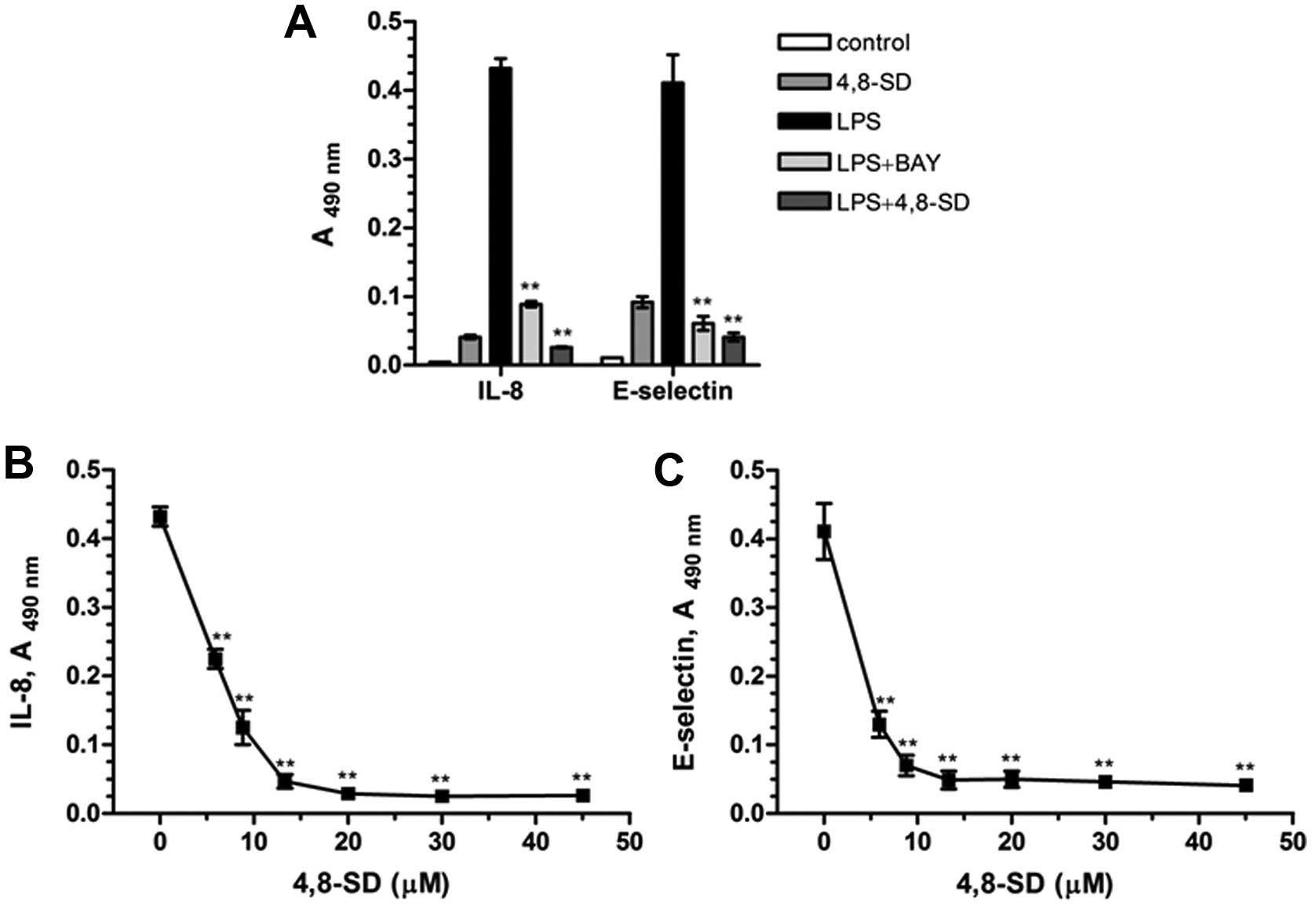
In our studies 4,8-SD (Fig. 3) blocked TNF-α- (Fig. 4) and LPS-induced (Fig. 5) upregulation of the inflammatory
adhesion molecule E-selectin and the cytokine IL-8 in HUVECtert
cells. 4,8-SD showed this inhibitory effect in a
concentration-dependent manner. Half-maximal inhibition of
TNF-α-induced upregulation of IL-8 (Fig. 4B) and E-selectin (Fig. 4C) was observed at 15.4 and 6.8 µM
4,8-SD, respectively. Half-maximal inhibition of LPS-induced
upregulation of IL-8 (Fig. 5B)
and E-selectin (Fig. 5C) was
observed at 6.0 and 4.2 μM 4,8-SD, respectively. 4,8-SD exerted an
inflammatory response by the unstimulated HUVECtert cells at the
cytotoxic concentration of 30 μM, which exceeds the control
(Figs. 4A and 5A).
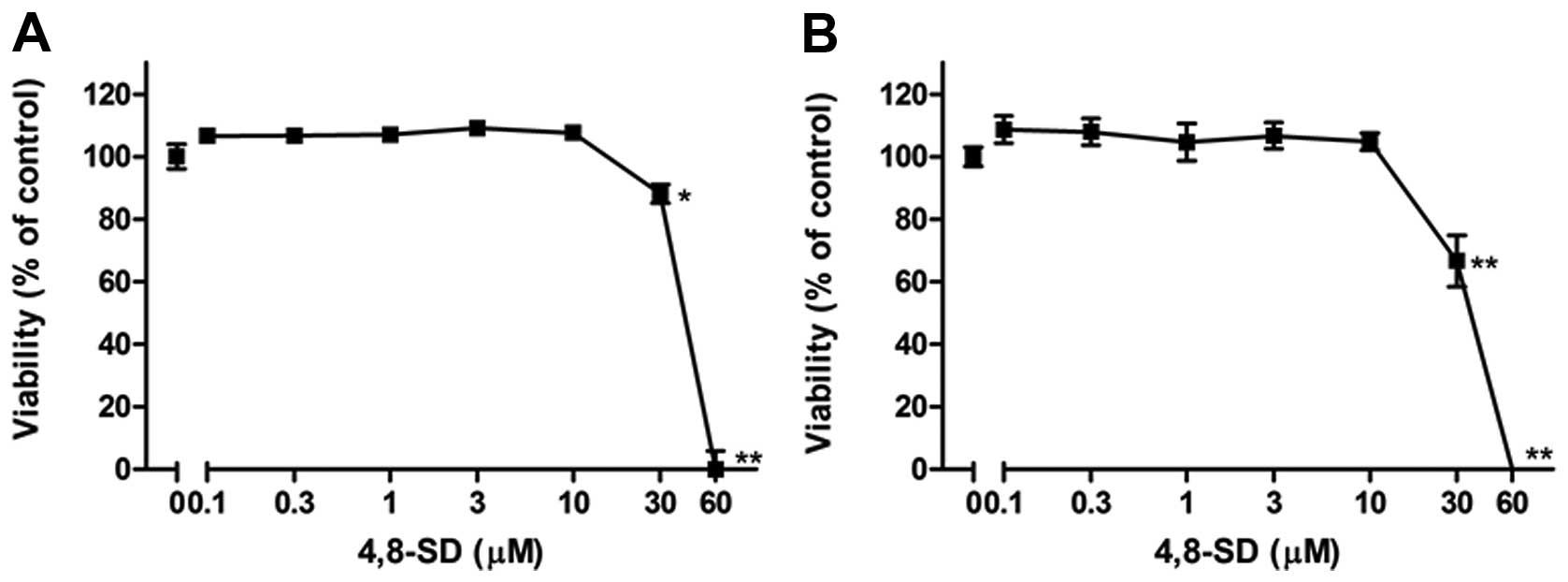
In addition, it was investigated whether the
treatment of the cells with 4,8-SD induced cell death that could
influence TNF-α- and LPS-induced IL-8 and E-selectin expression. To
this end, cytotoxic effects of 4,8-SD towards HUVECtert cells were
investigated using the LDH and the WST-1 colorimetric assays. The
viability of the cells decreased at concentrations greater than 20
μM 4,8-SD after 6 h of treatment (Fig. 6). In contrast to 4,8-SD, GluCer
were poorly soluble in DMSO and lacked activity in both the ELISA
and cell viability studies (data not shown).
Discussion
We observed that the sphingoid base 4,8-SD of GluCer
from A. amurense inhibits the inflammatory responses induced
by TNF-α and LPS in HUVECtert cells. The anti-inflammatory effects
were observed at significantly lower concentrations of 4,8-SD
compared to the cytotoxic effects. The GluCer however lacked
effects on the HUVECtert cells in the presented in vitro
bioassays. These findings confirm that GluCer may be metabolized to
4,8-SD in the gut lumen by enzymes in order to acquire cytotoxic
and/or anti-inflammatory properties (5). The daily intake by humans of total
SLs from plant sources is estimated to be as little as 50 mg
(9,15). It is unknown what portion of
4,8-SD becomes bioavailable in the mucosal cells of the intestines
after ingestion of complex SLs. However, it was reported that
sphingadienines in general have the advantage over other SLs of
being slowly metabolized and of having a long half-life in
intestinal epithelial cells (21).
Previously, pro-apoptotic effects of 4,8-SD and
various other sphingoid bases were reported which were regulated by
activation of caspases thereby explaining the cytotoxic effects
(22–25). Although there appears to be a
difference between concentrations inhibiting IL-8 and E-selectin
production and cytotoxicity, the effects induced by TNF-α and LPS
could still be toxic to the cells. Therefore, deeper in
vitro and in vivo studies regarding the
anti-inflammatory and cytotoxic effects of 4,8-SD are required.
Studies on the effect of 4,8-SD on a wider spectrum of pro- (e.g.
IL-1β, IL-6, IL-12, IFNγ) and anti-inflammatory cytokines and
mediators (e.g. IL-4, IL-10, IL-13), will provide insight in the
compounds’ specificity and sensitivity. Furthermore, the effects of
long-term exposure by 4,8-SD on its anti-inflammatory and cytotoxic
activities and studies pertaining to the involved molecular
mechanisms of this compound are needed.
Taken together, the present findings revealed that
4,8-SD alters the inflammatory responses of endothelial cells in
vitro in a favorable way. Since the source of 4,8-SD can be
found in the human diet, consecutive studies on the nutritional and
therapeutical function of 4,8-SD merit attention.
Acknowledgements
This study was supported by the
Sino-Austrian Research Project (to B.K.) funded by the Austrian
Federal Ministry of Science and Research and Federal Ministry of
Health, Women and Youth, and NFN-project ‘Drugs from Nature
Targeting Inflammation - DNTI’, Subproject S10713 from the Austrian
Science Fund (to V.B.).
Abbreviations:
|
CC
|
column chromatography;
|
|
CD
|
circular dichromism;
|
|
ELISA
|
enzyme-linked immunosorbent assay;
|
|
FCS
|
fetal calf serum;
|
|
GC-MS
|
gas chromatography mass
spectrometry;
|
|
GluCer
|
glucocerebrosides;
|
|
IL-8
|
interleukin 8;
|
|
LPS
|
lipopolysaccharide;
|
|
NMR
|
nuclear magnetic resonance;
|
|
4,8-SD
|
4,8-sphingadienine;
|
|
SL
|
sphingolipid;
|
|
TNF-α
|
tumor necrosis factor-α
|
References
|
1.
|
N BartkeYA HannunBioactive sphingolipids:
metabolism and functionJ Lipid ResSuppl 50S91S96200919017611
|
|
2.
|
YA HannunLM ObeidPrinciples of bioactive
lipid signalling: lessons from sphingolipidsNat Rev Mol Cell
Biol9139150200810.1038/nrm232918216770
|
|
3.
|
RX TanJH ChenThe cerebrosidesNat Prod
Rep20509534200310.1039/b307243f14620845
|
|
4.
|
M El AlwaniBX WuLM ObeidYA HannunBioactive
sphingolipids in the modulation of the inflammatory
responsePharmacol Ther112171183200616759708
|
|
5.
|
S LahiriAH FutermanThe metabolism and
function of sphingolipids and glycosphingolipidsCell Mol Life
Sci6422702284200710.1007/s00018-007-7076-017558466
|
|
6.
|
DV LynchTM DunnAn introduction to plant
sphingolipids and a review of recent advances in understanding
their metabolism and functionNew
Phytol161677702200410.1111/j.1469-8137.2004.00992.x
|
|
7.
|
LM CoussensZ WerbInflammation and
cancerNature420860867200210.1038/nature0132212490959
|
|
8.
|
A NilssonRD DuanAbsorption and lipoprotein
transport of sphingomyelinJ Lipid
Res47154171200610.1194/jlr.M500357-JLR20016251722
|
|
9.
|
H VesperEM SchmelzMN
Nikolova-KarakashianDL DillehayDV LynchAH Merrill JrSphingolipids
in food and the emerging importance of sphingolipids to nutritionJ
Nutr12912391250199910395583
|
|
10.
|
T SugawaraM KinoshitaM OhnishiJ NagataM
SaitoDigestion of maize sphingolipids in rats and uptake of
sphingadienine by Caco-2 cellsJ Nutr13327772782200312949364
|
|
11.
|
H ImaiM OhnishiK HotsuboM KojimaS
ItoSphingoid base composition of cerebrosides from plant
leavesBiosci Biotechnol Biochem61351353199710.1271/bbb.61.351
|
|
12.
|
ST PruettA BushnevK HagedornBiodiversity
of sphingoid bases (‘sphingosines’) and related amino alcoholsJ
Lipid Res49162116392008
|
|
13.
|
GF NixonSphingolipids in inflammation:
pathological implications and potential therapeutic targetsBr J
Pharmacol158982993200910.1111/j.1476-5381.2009.00281.x19563535
|
|
14.
|
JH JungCO LeeYC KimSS KangNew bioactive
cerebro-sides from Arisaema amurenseJ Nat
Prod59319322199610.1021/np960201+8882436
|
|
15.
|
T SugawaraT MiyazawaSeparation and
determination of glycolipids from edible plant sources by
high-performance liquid chromatography and evaporative
light-scattering detectionLipids3412311237199910606047
|
|
16.
|
A HayashiT MatsubaraDetermination of the
structure of sphinga-4,8-dienine from oyster glycolipids by gas
chromatography and mass spectrometryBiochim Biophys
Acta248306314197110.1016/0005-2760(71)90019-14331787
|
|
17.
|
HB SchillerA SzekeresBR BinderH
StockingerV LeksaMannose 6-phosphate/insulin-like growth factor 2
receptor limits cell invasion by controlling alphaVbeta3 integrin
expression and proteolytic processing of urokinase-type plasminogen
activator receptorMol Biol
Cell20745756200910.1091/mbc.E08-06-0569
|
|
18.
|
H TakanoS MurasawaT AsaharaFunctional and
gene expression analysis of hTERT overexpressed endothelial
cellsBiologics2547554200819707384
|
|
19.
|
JW PierceR SchoenleberG JesmokNovel
inhibitors of cytokine-induced IkappaBalpha phosphorylation and
endothelial cell adhesion molecule expression show
anti-inflammatory effects in vivoJ Biol
Chem2722109621103199710.1074/jbc.272.34.21096
|
|
20.
|
VN BochkovA KadlJ HuberF GruberBR BinderN
LeitingerProtective role of phospholipid oxidation products in
endotoxin-induced tissue
damageNature4197781200210.1038/nature0102312214235
|
|
21.
|
H FyrstB OskouianP BandhuvulaNatural
sphingadienines inhibit Akt-dependent signaling and prevent
intestinal tumorigenesisCancer
Res6994579464200910.1158/0008-5472.CAN-09-234119934323
|
|
22.
|
H OhtaEA SweeneyA MasamuneY YatomiS
HakomoriY IgarashiInduction of apoptosis by sphingosine in human
leukemic HL-60 cells: a possible endogenous modulator of apoptotic
DNA fragmentation occurring during phorbol ester-induced
differentiationCancer Res556916971995
|
|
23.
|
MT ParkJA KangJA ChoiPhytosphingosine
induces apoptotic cell death via caspase 8 activation and Bax
translocation in human cancer cellsClin Cancer
Res9878885200312576463
|
|
24.
|
T SugawaraN ZaimaA YamamotoS SakaiR
NoguchiT HirataIsolation of sphingoid bases of sea cucumber
cerebro-sides and their cytotoxicity against human colon cancer
cellsBiosci Biotechnol
Biochem7029062912200610.1271/bbb.6031817151482
|
|
25.
|
K AidaM KinoshitaT SugawaraJ OnoT
MiyazawaM OhnishiApoptosis inducement by plant and fungus sphingoid
bases in human colon cancer cellsJ Oleo
Sci53503510200410.5650/jos.53.503
|