Introduction
Osteoporosis is a common disease characterized by a
reduction of bone mass resulting from the negative balance between
bone formation and bone destruction (1). It can occur at any age and in any
ethnic or racial group, although it is more common in
post-menopausal women, and affects millions of people worldwide
(2,3). The most serious consequence of
osteoporosis is fracture, which is associated with an increase in
substantial morbidity, mortality and social costs. Early
intervention, now possible with the help of some effective
medications, may reduce the risk of first and recurrent fractures
(4). However, the lack of
reliable and effective drugs to cure osteoporosis-related fragility
fractures remains an important global issue. Therefore, there is a
clear clinical need to develop new bone anabolic agents for the
prevention and treatment of osteoporosis. Natural products that
have relatively fewer side-effects have been used clinically.
Epimedium, one of the most frequently prescribed herbs, has
been utilized in traditional Chinese medicine for the treatment of
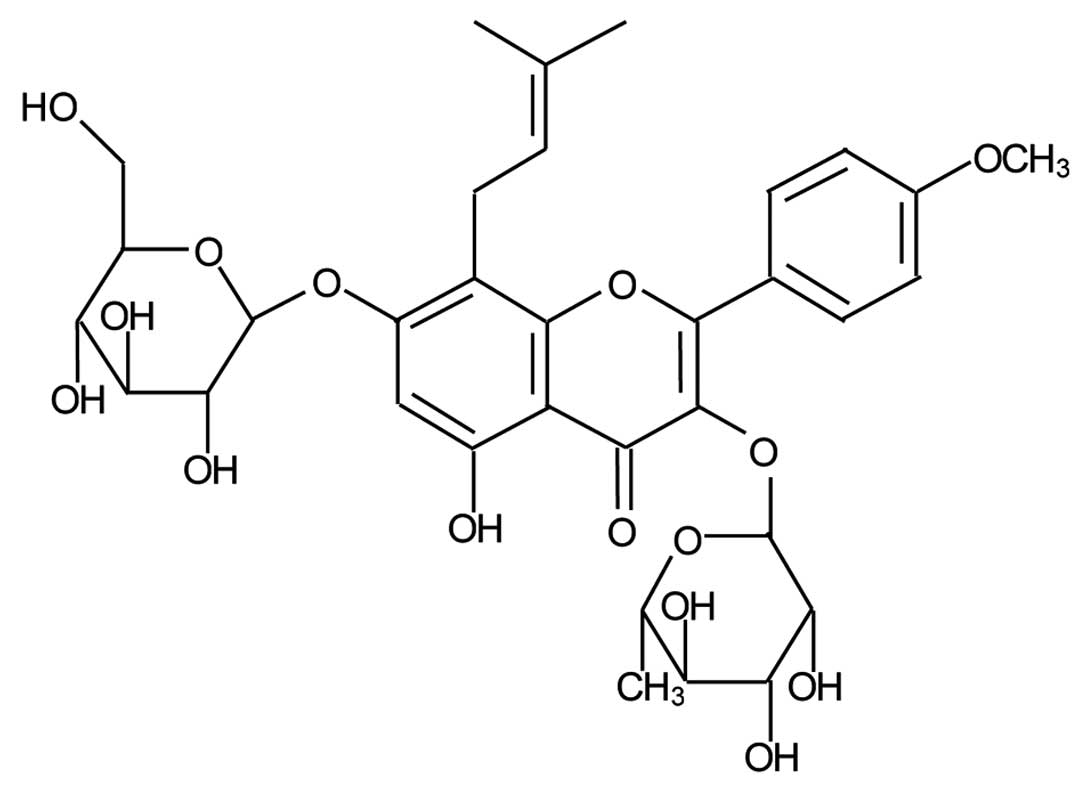
osteoporosis. Icariin (C33H40O15;
molecular weight, 676.67), a flavonol glycoside obtained from this
herb, is believed to be the major active compound that accounts for
its bone protective actions (Fig.
1) (5,6).
Recently, icariin was found to be therapeutically
effective in ovariectomized rats. It increases trabecular bone
mineral density (BMD) and bone strength and prevents the
suppression of serum Ca levels (7,8).
In addition, icariin has been shown to increase cell proliferation,
differentiation, mineralization, osteocalcin secretion, as well as
the expression levels of bone-related proteins in a dose-dependent
manner in primary osteoblastic cells (9–11).
These results suggest that icariin prevents bone loss by
suppressing bone resorption and stimulating bone formation.
However, the understanding of its precise mechanism of biological
action in bone formation remains incomplete.
Bone is an active tissue that undergoes constant
remodeling in which old bone is degraded by osteoclasts
(bone-resorbing cells), and subsequently replaced by new bone
formed by osteoblasts (bone-forming cells), via a process known as
remodeling (12). Bone
remodeling, an active and dynamic process, facilitates the repair
of microdamage and provides calcium from bone stores for cellular
functions. Osteoblasts are the obvious target for agents that aim
to mediate bone anabolism (13,14). A key signaling component in bone
formation is bone morphogenic protein-2 (BMP-2), a member of the
transforming growth factor-β (TGF-β) superfamily (15,16). BMP-2 signals via Smad4, which is a
nuclear transcription factor that regulates the activity of TGF-β
ligands and plays an important role in bone formation (17). In the present study, we
hypothesized that icariin can modulate the process of bone
formation by the BMP-2/Smad4 signal transduction pathway. We
assessed the direct effect of icariin on cell viability, alkaline
phosphatase (ALP) levels and the amount of calcified nodules, as
well as the expression of BMP-2, Smad4, Cbfa1/Runx2,
osteoprotegerin (OPG), receptor activator of nuclear factor κ-B
ligand (RANKL) and the OPG/RANKL ratio in hFOB 1.19 cells in
vitro, and investigated the possible molecular mechanism
mediating its biological effect. We found that icariin enhanced the
cell viability in a dose- and time-dependent manner. In addition,
icariin treatment promoted the amount of calcified nodules in the
hFOB 1.19 cells, which was accompanied by the upregulation of the
expression of ALP, BMP-2, Smad4, Cbfa1/Runx2, OPG, RANKL and the
OPG/RANKL ratio. Our findings suggest that icariin promotes the
process of bone formation.
Materials and methods
Materials and reagents
Fetal bovine serum (FBS), Dulbecco’s modified
Eagle’s medium (DMEM), and trypsin-EDTA were purchased from Hyclone
Laboratories, Inc. (Logan, UT, USA).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
was obtained from the Sigma Chemical Co. (St. Louis, MO, USA).
TRIzol reagent was purchased from Inc. (Grand Island, NY, USA).
Monoclonal rabbit anti-human Smad4, Cbfa1/Runx2, and β-actin HRP
secondary goat anti-rabbit antibodies were purchased from Abcam
(Cambridge, MA, USA). Primers were synthesized by Sangon Biotech
(Shanghai, China). Icariin (HPLC ≥98%) was produced by Nanjing
Zelang Medical Technological Co., Ltd. (Nanjing, China). Stock
solutions of icariin were prepared by dissolving the icariin powder
in DMSO to a concentration of 10−3 M, and stored at
−20°C. The working concentrations of icariin were obtained by
diluting the stock solution with the culture medium. The final
concentration of DMSO in the medium was <0.5%.
Cell culture
The hFOB 1.19 human osteoblastic cell line obtained
from the Insitute of Biochemistry and Cell Biology, Chinese Academy
of Sciences (Shanghai, China) was cultured in DMEM, supplemented
with 10% (v/v) FBS, penicillin (100 U/ml) and streptomycin (100
μg/ml) at 37°C in a humidified incubator with 5%
CO2. The medium was replaced every 3 days. At 80–90%
confluence, the cells were seeded in 96- or 6-well plates at a
density of 1x105 or 3x103 cells/well,
respectively, for different assays. The cells used in this study
were subjected to ≥30 cell passages.
Evaluation of cell viability by MTT
assay
The hFOB 1.19 cell viability was assessed by MTT
colorimetric assay. The cells were seeded in 96-well plates at a
density of 1.0x105 cells/well, cultured for 24 h and
starved for 24 h in serum-free DMEM medium. The cells were treated
with icariin at various final concentrations (10−15,
10−12, 10−9 and 10−6 M) and the
vehicle control cells were treated with 0.5% DMSO for 48 h. In some
experiments, the cells were treated with 10−9 M of
icariin for different periods of time. The medium was discarded and
replaced with 10 μl MTT [5 mg/ml in phosphate-buffered
saline (PBS)]. After incubation at 37°C for 4 h, the purple-blue
MTT formazan precipitate was dissolved in 100 μl DMSO and
the cells were agitated for 10 min. The absorbance at 490 nm was
measured on an ELISA reader (Model EXL800; BioTek, Winooski, VT,
USA).
Alizarin red S staining for
mineralization
Calcified nodules of the hFOB 1.19 cells were
demonstrated by Alizarin red S staining. The cells were seeded in
48-well plates at a density of 2x105 cells/well and
cultured for 24 h, and then treated with or without icariin. The
medium was replaced every 3 days. After 14 days, the cell cultures
were washed 3 times with PBS, fixed with formalin:methanol:
H2O (1:1:1.5) 0.5 ml/well for 30 min at room
temperature, and then washed 3 times with double distilled water.
The cells were stained with 0.1% Alizarin red S at 37°C for 30 min,
and washed 5 times with double distilled water and air-dried. The
stained calcified nodules that appeared bright red in color were
identified by light microscopy.
ALP activity assay
The cells were seeded in 48-well plates at a density
of 2x105 cells/well and cultured for 24 h, and then
treated with or without icariin for 48 h. Cells were harvested
after treatment and lysed with 100 μl Nonidet P-40 lysis
buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1 mM MgCl2,
1 mM CaCl2, 10% glycerol, 1% Nonidet P-40) supplemented
with protease inhibitors [2 μg/ml leupeptin, 2 μg/ml
aprotinin and 1 mM phenylmethylsulfonyl fluoride (PMSF)] by
incubation on ice for 30 min. The supernatant, centrifuged at
12,000 x g and 4°C for 5 min, was stored at −80°C until analysis.
Intracellular ALP activity was determined according to the
manufacturer’s instructions. Briefly, the sample was mixed with 1
ml ALP reagent (Hitachi, Tokyo, Japan) and the absorbance change at
405 nm in 3 min was recorded. ALP activity was calculated as: ALP
(U/l) = (total volume/sample volume) x (absorbance change in 3
min/0.01875). To normalize the result, bicinchoninic acid (BCA)
protein assay was carried out and ALP activity was expressed as
units of activity (U)·l−1·(mg protein)−1.
RNA extraction and real-time PCR
analysis
After icariin treatment for 48 h, total RNA from the
cells was isolated with TRIzol reagent (Invitrogen).
Oligo(dT)-primed RNA (5 μg) was reverse-transcribed with
SuperScript II reverse transcriptase (Promega) according to the
manufacturer’s instructions. The sequences of the PCR primers for
the amplification of BMP-2, Smad4, OPG, RANKL and β-actin
transcripts were as follows: BMP-2 forward, 5′-CGG ACT GCG GTC TCC
TAA-3′ and reverse, 5′-GGA AGC AGC AAC GCT AGA AG-3′, 68 bp; Smad4
forward, 5′-AAA GGT GAA GGT GAT GTT TGG GTC-3′ and reverse, 5′-CTG
GAG CTA TTC CAC CTA CTG ATC C-3′, 268 bp; OPG forward, 5′-AGT ACG
TCA AGC AGG AGT GCA AT-3′ and reverse, 5′-CCA GCT TGC ACC ACT CCA
A-3′, 129 bp; RANKL forward, 5′-AGA GCG CAG ATG GAT CCT AA-3′ and
reverse, 5′-TTC CTT TTG CAC AGC TCC TT-3′, 180 bp; and GAPDH
forward, 5′-CAA CTA CAT GGT TTA CAT GTT C-3′ and reverse, 5′-GCC
AGT GGA CTC CAC GAC-3′, 163 bp. PCR was carried out in a 20
μl reaction mixture containing 10 μl iQTM SYBR-Green
Supermix (Bio-Rad Laboratories, Hercules, CA, USA) and 0.5
μl of cDNA template. PCR was performed in an ABI 7900 HT
fast real-time PCR system (Applied Biosystems) using the following
cycle parameters: 1 cycle of 95°C for 1 min, and 40 cycles of 95°C
for 20 sec, different temperatures for 20 sec and 72°C for 18 sec.
Upon completion, a melting curve was examined. Standard curves were
generated using serially diluted solutions of cDNA derived from the
control group sample. The target gene transcripts in each group
sample were normalized on the basis of GAPDH.
Western blot analysis
The treated cells were harvested and lysed with
Nonidet P-40 lysis buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1
mM MgCl2, 1 mM CaCl2, 10% glycerol, 1%
Nonidet P-40) supplemented with protease inhibitors (2 μg/ml
aprotinin, 2 μg/ml leupeptin and 1 mM PMSF) and phosphatase
inhibitors (1 mM sodium orthovanadate, 10 mM NaF). Protein
concentrations were determined using the BCA protein assay. Equal
amounts of proteins were separated by SDS-PAGE on a 12% reducing
gel at a constant voltage (110 V) for approximately 2 h, and
transblotted onto PVDF membranes. The non-specific binding sites on
the membranes were blocked with 5% skimmed milk. The blots were
probed with monoclonal rabbit anti-human Smad4 (1:2,000),
Cbfa1/Runx2 (1:1,000) and β-actin (1:1,000) antibodies overnight at
4°C with rocking, followed by incubation with goat anti-rabbit
conjugated with horseradish peroxidase (1:2,000). The
antigen-antibody complexes were then detected with enhanced
chemiluminescence (ECL) reagent (Santa Cruz Biotechnology, Inc.,
USA). Bands were then quantified by scanning densitometry (170–8070
Molecular Imager ChemiDoc XRS System; Bio-Rad). Protein
concentrations were determined using the Tocan 190 protein assay
system and normalized to β-actin in the sample.
Statistical analysis
Data were analyzed using the SPSS package for
Windows (version 13.0). Quantitative data were expressed as the
means ± standard deviation (SD). Statistical analysis of the data
was performed with the Student’s t-test and ANOVA. P-value <0.05
was considered to indicate a statistically significant
difference.
Results
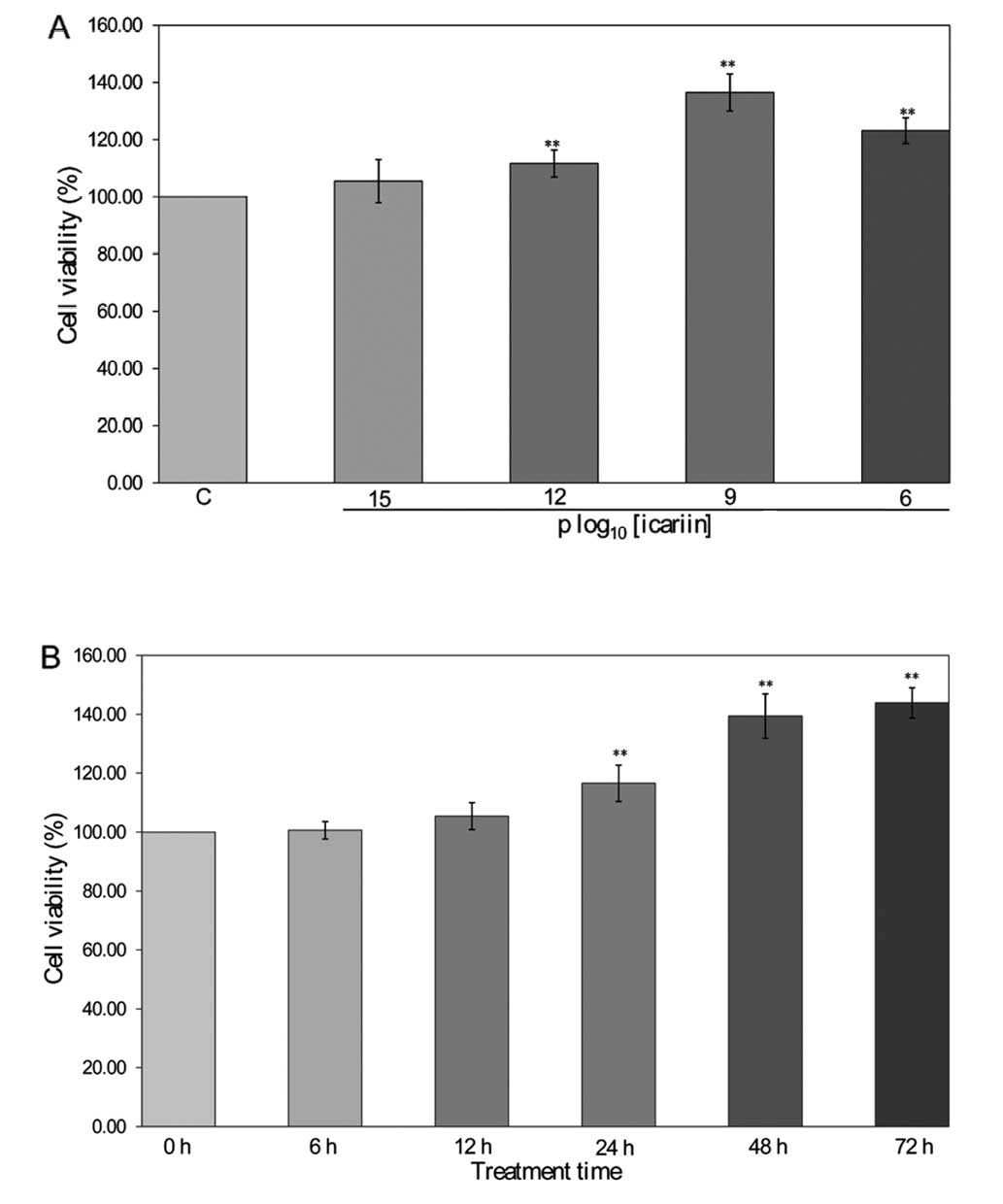
Effect of icariin on cell viability in
hFOB 1.19 cells
Icariin (10−12, 10−9 and
10−6 M) significantly increased cell viability by
approximately 111.67±4.72, 136.50±6.47 and 123.17±4.49% in hFOB
1.19 cells compared to the control cells (100±0.00%, P<0.01)
(Fig. 2A). The cell viability
with 10−9 M of icariin at 24 h (116.57±6.16%), 48 h
(139.42±7.53%) and 72 h (143.91±5.13%) was significantly higher
than that at 0 h (100±0.00%, P<0.01) (Fig. 2B). These results indicate that
icariin enhances osteoblastic cell viability in a dose- and
time-dependent manner.
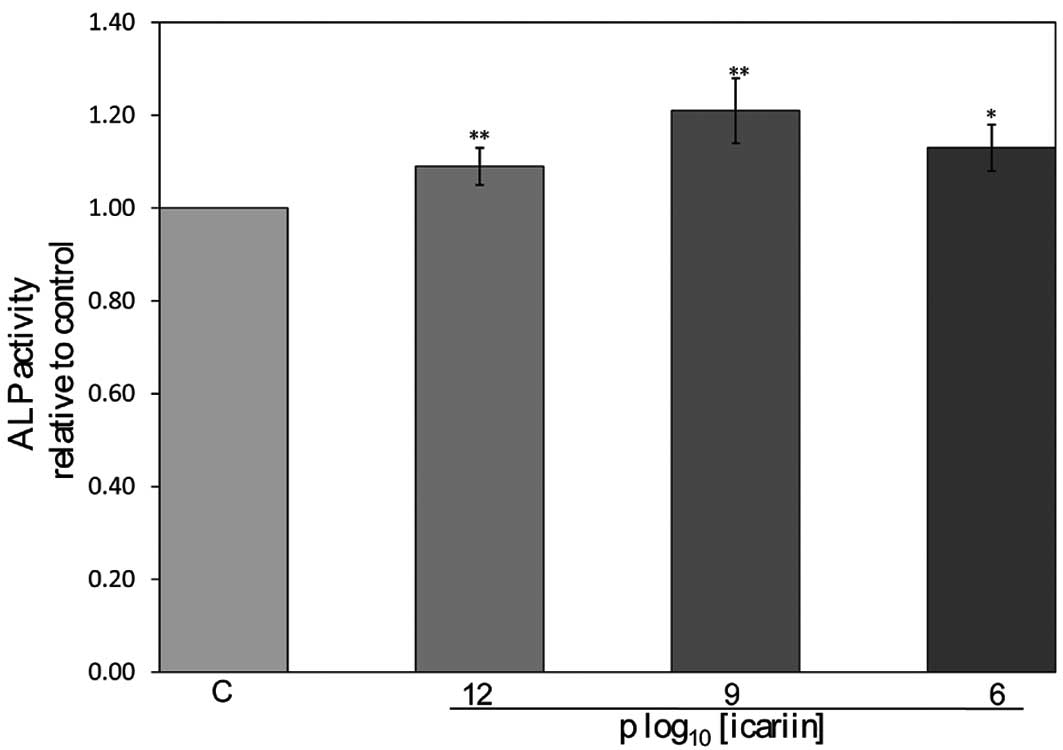
Effect of icariin on ALP activity and
calcified nodules in hFOB 1.19 cells
The ALP activity in the hFOB 1.19 cells was
increased by 1.09-, 1.21- and 1.13-fold when the cells were treated
with icariin at the final concentrations of 10−12,
10−9 and 10−6 M, respectively, significantly
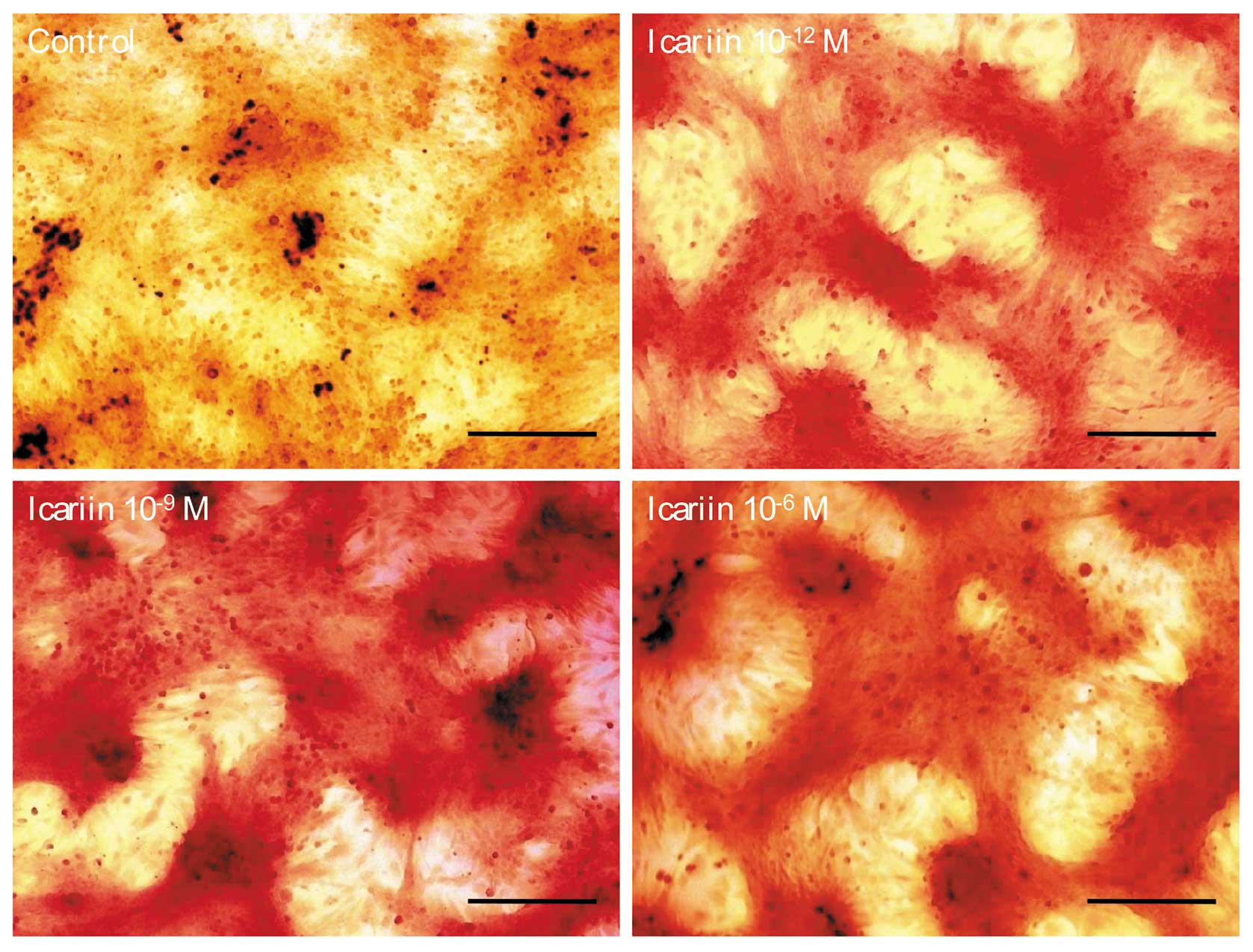
higher than that of the control cells (P<0.05) (Fig. 3). The calcified nodules appeared
bright red in color by Alizarin red S staining (Fig. 4). Icariin at 10−12,
10−9 and 10−6 M stimulated the formation of
calcified nodules significantly compared to the control cells.
These results suggest that icariin promotes ALP expression and the
formation of calcified nodules in hFOB 1.19 cells.
Effect of icariin on BMP-2, Smad4, OPG
and RANKL mRNA expression in hFOB 1.19 cells
Icariin induced a 1.24-, 1.38- and 1.32-fold
increase in BMP-2 mRNA expression (P<0.01) (Fig. 5A) and a 1.08-, 1.23- and 1.15-fold
increase in Smad4 mRNA expression (P<0.05) (Fig. 5B). Furthermore, icariin not only
significantly increased OPG mRNA expression in hFOB 1.19 cells
(P<0.01) (Fig. 5C), but also
significantly increased RANKL mRNA expression in hFOB 1.19 cells at
all concentrations tested (P<0.01) (Fig. 5D). The overall effects of icariin
on the OPG/RANKL mRNA expression ratio in hFOB 1.19 cells are shown
in Fig. 5E. The results clearly
indicated that icariin significantly increased the OPG/RANKL ratio
in hFOB 1.19 cells (P<0.05), suggesting that icariin enhances
bone formation via its actions on OPG and RANKL expression.
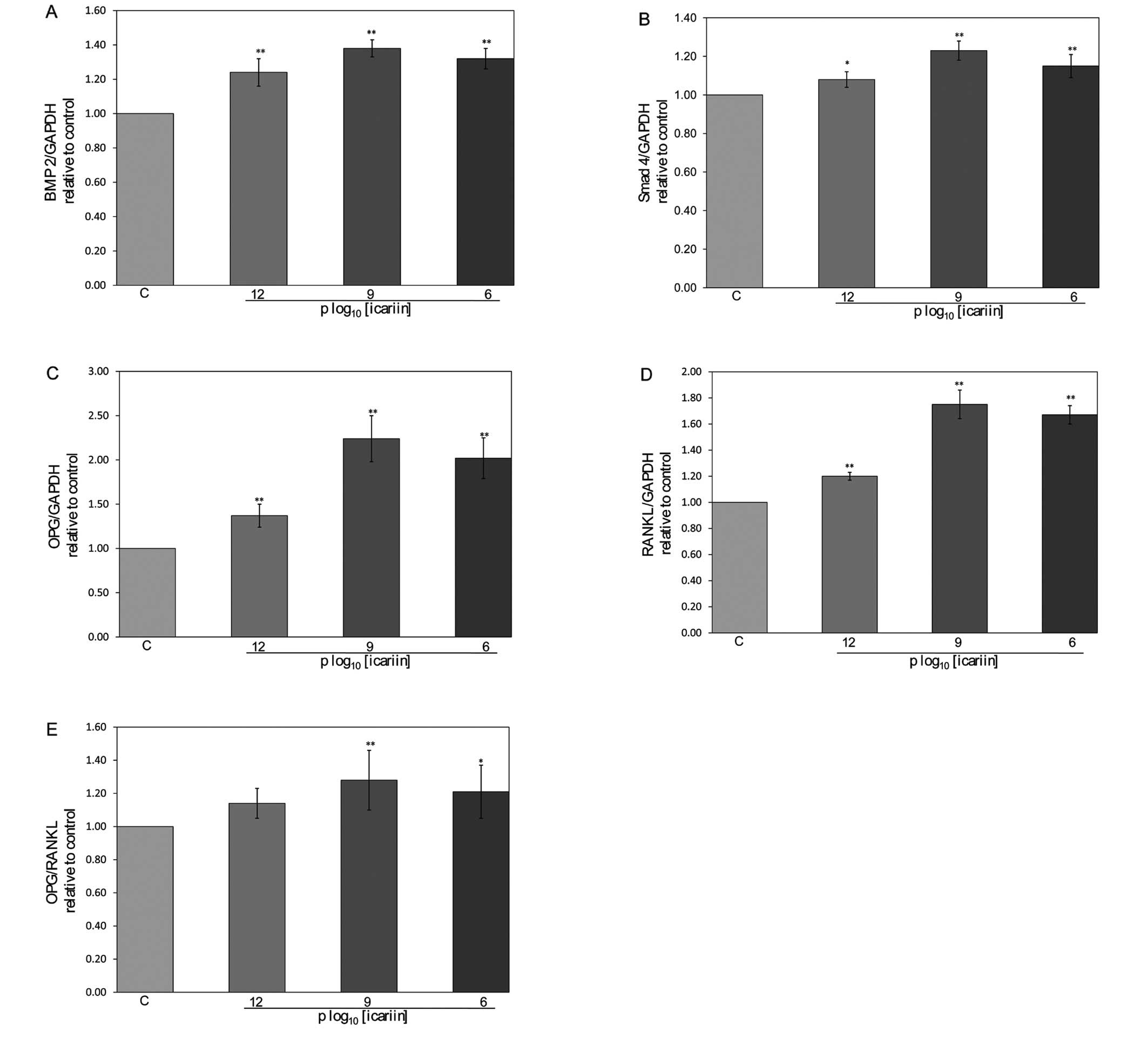 | Figure 5Effect of icariin on BMP-2, Smad4,
OPG, RANKL mRNA expression and the OPG/RANKL ratio in hFOB 1.19
cells. The hFOB 1.19 cells were treated with or without icariin for
48 h. Total RNA was isolated and real-time PCR was performed to
determine the mRNA expression levels of (A) BMP-2, (B) Smad4, (C)
OPG, (D) RANKL and (E) OPG/RANKL, which were normalized to those of
GAPDH. Results were obtained from 3 independent experiments, and
data are averages ± SD (error bars), *P<0.05;
**P<0.01 vs. control. C, control cells. |
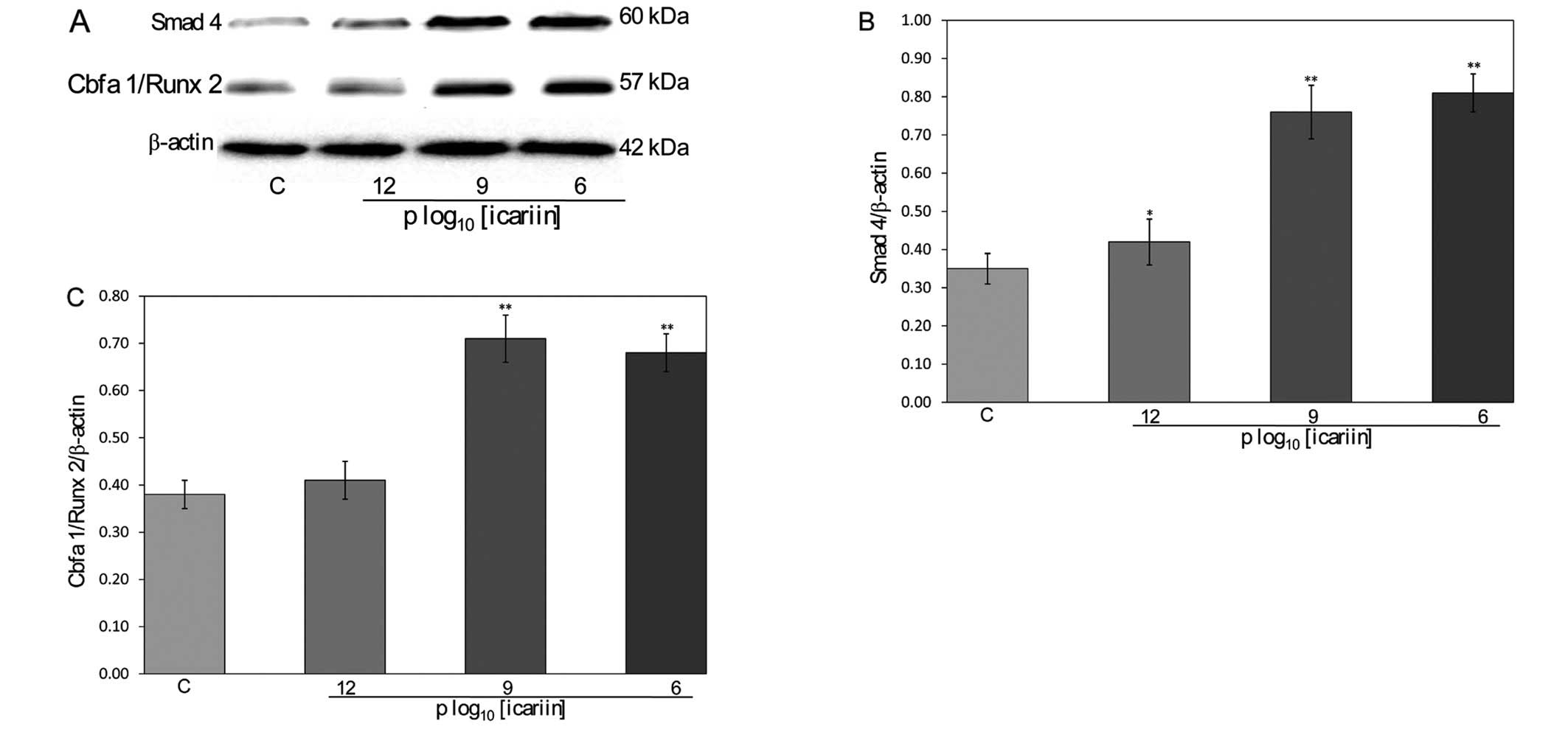
Effect of icariin on Smad4 and
Cbfa1/Runx2 protein expression in hFOB 1.19 cells
To further explore the mechanism by which icariin
regulates bone formation, we analyzed the protein expression levels
of Smad4 and Cbfa1/Runx2 after icariin treatment using western blot
analysis (Fig. 6A). Icariin
(10−12, 10−9 and 10−6 M)
significantly increased Smad4 expression (0.42±0.06, 0.76±0.07 and
0.81±0.05) in the hFOB 1.19 cells compared to the control cells
(0.35±0.04, P<0.05) (Fig. 6B).
Icariin (10−12, 10−9 and 10−6 M)
significantly increased Cbfa1/Runx2 expression (0.41±0.04,
0.71±0.05 and 0.68±0.04) in the hFOB 1.19 cells compared to the
control cells (0.38±0.03, P<0.01) (Fig. 6C). Taken together, these results
suggest that icariin modulates the process of bone formation via
its effects on Smad4 and Cbfa1/Runx2 expression.
Discussion
In the present study, we systematically evaluated
the osteoprotective effects and mechanism of actions of icariin in
the hFOB 1.19 human osteoblastic cell line. Our results clearly
demonstrate that icariin enhances the cell viability and increases
the amount of calcified nodules, as well as increasing the
expression ratio of OPG/RANKL in hFOB 1.19 cells. In addition, our
results show that icariin upregulates the expression of ALP, BMP-2,
Smad4, Cbfa1/Runx2, OPG and RANKL, suggesting that it promotes
osteoblastic bone formation by the BMP-2/Smad4 signal transduction
pathway.
Osteoporosis, a progressive disorder of aging bone,
is a worldwide health problem with a high prevalence. Bone is a
dynamic tissue whereby old bone is removed by osteoclasts and new
bone is formed continuously by osteoblasts. Bone generation,
maintenance and healing are complicated processes in which
osteoblasts, osteoclasts, and osteocytes are known to play
important roles (18). Multiple
factors can cause the loss of bone mass, including increased bone
turnover, which results in an imbalance of osteoclasts and
osteoblasts at the bone remodeling process (19). Therefore, agents with an anabolic
action on the bone may be effective in increasing the activity of
osteoblasts and treating osteoporosis.
The study of Chinese herbs is worthwhile as this may
lead to the discovery of certain agents which can stimulate the
proliferation and differentiation of osteoblasts. Recently,
Epimedium has received increased attention since many
studies on animals and cell culture systems have indicated that
icariin plays an important role in the prevention of osteoporosis
(20,21). Hence, in the present study, we
explored the effect of icariin on the hFOB 1.19 human osteoblastic
cell line.
The results of the present study confirm that
icariin stimulates the proliferation of hFOB 1.19 cells in a dose-
and time-dependent manner. Osteoblasts are derived from mesenchymal
stem cells. The sequential expression of type I collagen, ALP and
the deposition of calcium are known as molecular markers. Human
osteoblasts cultured for 48 h in the presence of 10−12,
10−9 and 10−6 M of icariin exhibited a
significant increase in ALP activity, and the formation of
mineralized nodules increased significantly after the cells were
cultured for 14 days in the presence of 10−12,
10−9 and 10−6 M icariin. As the appearance of
ALP activity is an early phenotypic marker for mature osteoblasts,
and mineralized nodule formation is a phenotypic marker for a later
stage of osteoblast differentiation, our results suggested that
icariin stimulated bone formation. Further study is required to
clarify the possible additional effects of icariin on renal Ca
transport that contribute to the conservation of bone mass in
animal models of osteoporosis.
BMPs play important roles in the regulation of bone
induction, repair and maintenance (22). BMP-2 has demonstrated a strong
osteo-inductive capacity, and has been shown to induce the
osteoblastic differentiation of various types of cells, including
pre-osteoblasts, undifferentiated mesenchymal cells and bone marrow
stromal cells (23). The function
of BMP-2 is mediated by heterotetrameric serine/threonine kinase
receptors and the downstream transcription factors, Smad1, 5 and 8
(24). After these transcription
factors are phosphorylated on serine residues, they form a complex
with Smad4 (a common mediator), and subsequently the complex is
translocated into the nucleus to activate the transcription of
Cbfa1/Runx2, thereby regulating bone metabolism (25). Our results indicated that the
BMP-2, Smad4 and Cbfa1/Runx2 expression increased in the
icariin-treated hFOB 1.19 cells.
Osteoblasts are recruited to the resorption area and
osteoclasts are activated to resorb old bone in the bone remodeling
process. OPG and RANKL are critical in determining
osteoclastogenesis and bone homeostasis (26,27). OPG blocks these effects and
prevents bone resorption, whereas RANKL represents the
osteoblast-derived factor required for osteoclast formation
(28,29). The levels of OPG and RANKL play
important roles in the regulation of the formation of hFOB 1.19
cells. Our study indicates that OPG and RANKL mRNA expression
increases after treatment with icariin. As its stimulatory effects
on OPG mRNA expression were stronger than those on RANKL mRNA
expression, the effects of icariin at 10−9 and
10−6 M on the OPG/RANKL ratio were also stimulatory.
These results suggest that icariin promotes bone formation through
its direct actions on modulating the expression of OPG and
RANKL.
Icariin, an active ingredient identified in
Epimedium, is commonly used for the treatment of
osteoporosis in traditional Chinese medicine. The present study
clearly demonstrates that icariin modulates the process of bone
formation via the regulation of the BMP-2/Smad4 signal transduction
pathway in hFOB 1.19 cells. Our study provides the evidence to
support the use of icariin as an effective candidate for the
management of osteoporosis. Further studies are required to
elucidate the mechanisms by which icariin protects against bone
loss.
Acknowledgements
This study was supported by the
National Natural Science Foundation of China (Grant no. 81102609),
the Natural Science Foundation of Fujian Province (Grant no.
2011J05076) and the Youth Foundation of Fujian Provincial Health
Bureau (Grant no. 2011-2-31).
References
|
1.
|
D NarayananA AnithaR JayakumarSV NairKP
ChennazhiSynthesis, characterization and preliminary in
vitro evaluation of PTH 1–34 loaded chitosan nanoparticles for
osteoporosisJ Biomed Nanotechnol8981062012
|
|
2.
|
J PrzedlackiStrontium ranelate in
post-menopausal osteoporosisEndokrynol Pol626572201121365582
|
|
3.
|
G PadovaG BorzìL IncorvaiaPrevalence of
osteoporosis and vertebral fractures in acromegalic patientsClin
Cases Miner Bone Metab83743201122461828
|
|
4.
|
NS DattaOsteoporotic fracture and
parathyroid hormoneWorld J
Orthop26774201110.5312/wjo.v2.i8.6722474638
|
|
5.
|
G QianX ZhangL LuX WuS LiJ MengRegulation
of Cbfa1 expression by total flavonoids of Herba
epimediiEndocr J538794200610.1507/endocrj.53.8716543677
|
|
6.
|
L QinT HanQ ZhangAntiosteoporotic chemical
constituents from Er-Xian Decoction, a traditional Chinese herbal
formulaJ
Ethnopharmacol118271279200810.1016/j.jep.2008.04.00918501540
|
|
7.
|
KM ChenHP MaBF GeIcariin enhances the
osteogenic differentiation of bone marrow stromal cells but has no
effects on the differentiation of newborn calvarial osteoblasts of
ratsPharmazie627857892007
|
|
8.
|
J HuangL YuanX WangTL ZhangK WangIcaritin
and its glycosides enhance osteoblastic, but suppress osteoclastic,
differentiation and activity in vitroLife
Sci81832840200710.1016/j.lfs.2007.07.01517764702
|
|
9.
|
SK MokWF ChenWP LaiIcariin protects
against bone loss induced by oestrogen deficiency and activates
oestrogen receptor-dependent osteoblastic functions in UMR 106
cellsBr J
Pharmacol159939949201010.1111/j.1476-5381.2009.00593.x20128811
|
|
10.
|
HP MaLG MingBF GeIcariin is more potent
than genistein in promoting osteoblast differentiation and
mineralization in vitroJ Cell
Biochem112916923201110.1002/jcb.2300721328465
|
|
11.
|
J ZhangY LiJ SunC LiuD ZhangSynergistic or
antagonistic effect of MTE plus TF or icariin from Epimedium
koreanum on the proliferation and differentiation of primary
osteoblasts in vitroBiol Trace Elem
Res14317461757201110.1007/s12011-011-8987-z21301987
|
|
12.
|
J XiongCA O’BrienOsteocyte RANKL: new
insights into the control of bone remodelingJ Bone Miner
Res27499505201210.1002/jbmr.154722354849
|
|
13.
|
SJ MlakarJ PrezeljJ MarcTesting GSTP1
genotypes and haplotypes interactions in Slovenian
post-/pre-menopausal women: novel involvement of glutathione
S-transferases in bone remodeling
processMaturitas71180187201210.1016/j.maturitas.2011.11.02322221655
|
|
14.
|
S SchmidtTM PostLA PeletierMA BoroujerdiM
DanhofCoping with time scales in disease systems analysis:
application to bone remodelingJ Pharmacokinet
Pharmacodyn38873900201110.1007/s10928-011-9224-222028207
|
|
15.
|
H CaoY KeY ZhangCJ ZhangW QianGL
ZhangIcariin stimulates MC3T3-E1 cell proliferation and
differentiation through up-regulation of bone morphogenetic
protein-2Int J Mol Med29435439201222109711
|
|
16.
|
T MatsubaraK KidaA YamaguchiBMP2 regulates
Osterix through Msx2 and Runx2 during osteoblast differentiationJ
Biol Chem2832911929125200810.1074/jbc.M80177420018703512
|
|
17.
|
TP HsiehSY SheuJS SunMH ChenMH LiuIcariin
isolated from Epimedium pubescens regulates osteoblasts
anabolism through BMP-2, SMAD4, and Cbfa1
expressionPhytomedicine174144232010
|
|
18.
|
G SwarnkarK SharanJA SiddiquiA novel
flavonoid isolated from the steam-bark of Ulmus wallichiana
planchon stimulates osteoblast function and inhibits osteoclast and
adipocyte differentiationEur J Pharmacol6586573201121376034
|
|
19.
|
M TabuchiK MiyazawaM KimuraEnhancement of
crude bone morphogenetic protein-induced new bone formation and
normalization of endochondral ossification by bisphosphonate
treatment in osteoprotegerin-deficient miceCalcif Tissue
Int77239249200510.1007/s00223-004-0223-9
|
|
20.
|
S PengG ZhangY HeEpimedium-derived
flavonoids promote osteoblastogenesis and suppress adipogenesis in
bone marrow stromal cells while exerting an anabolic effect on
osteoporotic boneBone45534544200910.1016/j.bone.2009.09.004
|
|
21.
|
TP HsiehSY SheuJS SunMH ChenIcariin
inhibits osteoclast differentiation and bone resorption by
suppression of MAPKs/NF-κB regulated HIF-1α and PGE(2)
synthesisPhytomedicine18176185201120554188
|
|
22.
|
J NojimaK KanomataY TakadaDual roles of
smad proteins in the conversion from myoblasts to osteoblastic
cells by bone morphogenetic proteinsJ Biol
Chem2851557715586201010.1074/jbc.M109.02801920231279
|
|
23.
|
JE Sotillo RodriguezKC ManskyED
JensenEnhanced osteoclastogenesis causes osteopenia in twisted
gastrulation-deficient mice through increased BMP signalingJ Bone
Miner Res2419171926200919419314
|
|
24.
|
L WangX ZhangY GuoInvolvement of BMPs/Smad
signaling pathway in mechanical response in osteoblastsCell Physiol
Biochem2610931102201010.1159/00032398721220940
|
|
25.
|
J ZhaoS OhbaM ShinkaiUI ChungT
NagamuneIcariin induces osteogenic differentiation in vitro in a
BMP- and Runx2-dependent mannerBiochem Biophys Res
Commun369444448200810.1016/j.bbrc.2008.02.05418295595
|
|
26.
|
Q XiaoA ChenF GuoEffects of Icariin on
expression of OPN mRNA and type I collagen in rat osteoblasts in
vitroJ Huazhong Univ Sci Technolog Med
Sci25690692200510.1007/BF0289617216696327
|
|
27.
|
B MaQ ZhangD WuStrontium fructose
1,6-diphosphate prevents bone loss in a rat model of postmenopausal
osteoporosis via the OPG/RANKL/RANK pathwayActa Pharmacol
Sin33479489201210.1038/aps.2011.17722426695
|
|
28.
|
JW LeeA IwahashiS HasegawaCoptisine
inhibits RANKL-induced NF-κB phosphorylation in osteoclast
precursors and suppresses function through the regulation of RANKL
and OPG gene expression in osteoblastic cellsJ Nat
Med66816201221656335
|
|
29.
|
A EnjuanesS Ruiz-GaspàP PerisThe effect of
the alendronate on OPG/RANKL system in differentiated primary human
osteoblastsEndocrine37180186201010.1007/s12020-009-9285-920963568
|