Introduction
Mast cells are broadly distributed throughout
mammalian tissues and play an important role as regulators of
allergic inflammation in various allergy-related disorders, such as
asthma, atopic dermatitis, eczema and sinusitis. Mast cells have
been considered not only in association with immediate-type
hypersensitivity, but also in delayed hypersensitivity reactions,
such as inflammatory response (1,2).
Immediate-type hypersensitivity is mediated by histamine in
response to the antigen cross-linking of immunoglobulin E (IgE)
bound to FcɛRI on mast cells (3).
After the activation of mast cells, degranulation is triggered,
which results in the release of mediators, such as products of
arachidonic acid metabolism, cytokines, proteases and histamine
(4,5). In mast cell-mediated inflammatory
responses, histamine is one of the most well-characterized and
important mediators involved in the acute phase of immediate
hypersensitivity (6,7).
Mast cell activation is initiated by the
phosphorylation of tyrosine kinase which leads to the activation of
protein kinase C, nuclear factor (NF)-κB and the expression of
pro-inflammatory cytokines (4,8).
Activated mast cells can release histamine and other inflammatory
mediators, such as eicosanoids, proteoglycans and a number of
pro-inflammatory cytokines, including tumor necrosis factor
(TNF)-α, interleukin (IL)-1β, IL-6 and IL-13 (5,9).
Although these inflammatory cytokines have beneficial effects on
the host defense process, they cause pathological conditions when
overexpressed. Therefore, the inhibition of these inflammatory
cytokines produced by mast cells is one of the most important
targets for the reduction of allergic inflammatory symptoms.
Diospyros kaki (D. kaki) has been
cultivated throughout Eastern Asia for hundreds of years. D.
kaki contains various biological active compounds, such as
amino acids, carotenoids, flavonoids, tannins, catechins and
vitamin A (10,11). The leaves of D. kaki are
commonly used for tea in Asia. Previous studies have shown that
D. kaki has beneficial effects on homeostasis, constipation,
hypertension, atherosclerosis and allergic dermatitis and it has
been broadly applied in the medicinal area (12–16). D. kaki is also a good
source of antioxidants, polyphenols and dietary fiber (17). However, the anti-allergic and
anti-inflammatory effects of D. kaki have not yet been
elucidated.
In the present study, we investigated the effects of
the aqueous extract of D. kaki (AEDK) on allergic
inflammation by using in vitro and in vivo mast
cell-based models. cAMP levels and the intracellular calcium
concentration were investigated to clarify the mechanisms by which
AEDK inhibits the release of histamine from mast cells. The effects
of AEDK on gene expression and the secretion of pro-inflammatory
cytokines and the role of NF-κB in these effects were also
investigated. In addition, we examined the effects of AEDK on
systemic and local allergic reaction to assess its anti-allergic
effects in vivo.
Materials and methods
Reagents and cell culture
Compound 48/80, anti-dinitrophenyl (DNP) IgE,
DNP-human serum albumin (HSA), phorbol 12-myristate 13-acetate
(PMA), calcium ionophore A23187 and disodium cromoglycate (DSCG)
were purchased from Sigma (St. Louis, MO, USA). The human mast cell
line (HMC-1) was grown in Iscove’s medium (Life Technologies, Grand
Island, NY, USA) supplemented with 10% (v/v) fetal bovine serum at
37°C in 5% CO2. HMC-1 cells of passage 4–8 were used in
all the experiments.
Animals
The original stock of male imprinting control region
(ICR) mice (6 weeks of age) was purchased from Dae Han Bio Link
Co., Ltd (Eumsung-Gun, Korea). The animals were housed 5 per cage
in a laminar air flow room maintained under a temperature of 22±2°C
and a relative humidity of 55±5% throughout the study. The care and
treatment of the mice were in accordance with the guidelines
established by the Public Health Service Policy on the Humane Care
and Use of Laboratory Animals and were approved by the Animal Care
and Use Committee at Kyungpook National University.
Preparation of AEDK, isolation and
identification of active compound
D. kaki was supplied from the Korea National
Arboretum. A voucher specimen (no. WSP-10-38) was deposited at the
herbarium of the College of Pharmacy, Woosuk University, Jeonju,
Korea. D. kaki (600 g) was shade dried and powdered. It was
then extracted 3 times with purified water (500 ml) at 70°C for 5 h
in a water bath and filtered through a Whatman no. 1 filter. The
extracts were combined and evaporated. The yield of dried extract
from the crude materials was approximately 4.16% (w/w). The dried
extract of AEDK was dissolved in saline or Tyrode buffer and was
filtered using a 0.45 μm syringe filter. To isolate the active
components responsible for the anti-allergic and anti-inflammatory
effects of AEDK, the resultant water extract (70 g) was
successively partitioned as chloroform, ethyl acetate, n-butanol
and water soluble fractions. The ethyl acetate soluble fraction
exhibited the highest anti-allergic activity. The ethyl acetate
soluble fraction was chromatographed on a Sephadex LH-20 column
(MeOH) (GE Healthcare Bio-Sciences, Uppsala, Sweden) to yield 5
fractions (E1–E5). E2 was subjected to chromatography on a Sephadex
LH-20 gel column (MeOH) to yield compound 1 (25 mg). The molecular
formulae of compound 1 were characterized by nuclear magnetic
resonance (NMR). By comparing the NMR data and literature values
(18,19), compound 1 was identified as a
catechin.
Histamine and β-hexosaminidase
levels
The degranulation of mast cells was monitored by
measuring the release of histamine and β-hexosaminidase. The
histamine content in serum and HMC-1 cells was measured using the
o-phthaldialdehyde spectrofluorometric procedure as
previously described (20). HMC-1
cells (1×106 cells/ml) were pre-incubated with AEDK for
30 min, and then incubated for 30 min with PMA (20 nM) and calcium
ionophore A23187 (PMACI) (1 μM) as previously described (21). The release of β-hexosaminidase was
measured as previously described (22). In brief, the HMC-1 cells were
treated with AEDK at 37°C for 30 min. The cells were sensitized
with PMACI for 30 min and placed on ice for 10 min to terminate the
reaction.
cAMP and intracellular calcium
levels
The cAMP level was measured as previously described
(4). The intracellular calcium
concentration was measured with the use of the fluorescence
indicator Fluo-3/AM (Molecular Probes, Eugene, OR, USA). The HMC-1
cells were pre-incubated with Fluo-3/AM for 30 min at 37°C. After
washing the dye from the cell surface, the cells were treated with
AEDK for 5 min prior to the addition of PMACI. They were excited at
488 nm, the emission was filtered with 515 nm by a flow cytometer
(BD Biosciences Pharmingen, San Diego, CA, USA) and visualized
under a fluorescence microscope (Olympus BX51; Olympus, Center
Valley, PA, USA).
Real-time PCR and enzyme-linked
immunosorbent assay (ELISA)
Total cellular RNA was isolated from the cells
(1×106 cells/well in a 24-well plate) following
stimulation with PMACI with or without AEDK for 4 h. Real-time
polymerase chain reaction (PCR) was used to analyze the mRNA
expression of TNF-α, IL-1β and β-actin (internal control) as
previously described (23). The
band intensity was normalized to that of β-actin in the same
sample. The secretion of TNF-α and IL-1β was measured by ELISA as
previously described (4). The
HMC-1 cells were cultured in medium and resuspended in Tyrode
buffer A. The cells were sensitized with PMACI for 8 h in the
absence or presence of AEDK.
Western blot analysis and luciferase
activity assay
The samples were electrophoresed using 12% sodium
dodecyl sulfate-polyacrylamide gel electrophoresis, as previously
described (24) and then
transferred onto a nitrocellulose membrane. Nuclear and cytosolic
p65 NF-κB and IκBα were assayed using anti-NF-κB (p65) and
anti-IκBα antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Luciferase activity was measured as previously described (21). The HMC-1 cells were seeded at
2×106 in a 6-well plate 1 day prior to transient
transfection. After the transfected cells were incubated for 18 h,
they were stimulated with PMACI at 37°C for 2 h. The cells were
pre-treated with AEDK for 30 min prior to stimulation with PMACI.
Luciferase activity was determined using a luminometer.
Systemic anaphylaxis
Mice were administered an intraperitoneal injection
[8 mg/kg of body weight (BW)] of the mast cell degranulator,
compound 48/80. AEDK was dissolved in saline and administered
intraperitoneally (1–100 mg/kg BW) 1 h prior to the injection of
compound 48/80 (n=10/group). Mortality was monitored for 1 h after
the induction of anaphylactic shock. After the mortality test,
blood was obtained from the heart of each mouse to measure the
serum histamine content.
Passive cutaneous anaphylaxis (PCA)
An IgE-dependent cutaneous reaction was carried out
as previously described (23).
The mice were injected intradermally with 0.5 μg of anti-DNP IgE.
After 48 h, each mouse (n=10/group) received an injection of 1 μg
of DNP-HSA containing 4% Evans blue (1:4) via the tail vein. Thirty
minutes after the challenge, the mice were sacrificed and the
dorsal skin (diameter, 1 cm) was removed in order to measure the
pigmented area. The amount of dye was then determined
colorimetrically.
Statistical analysis
Statistical analyses were performed using SAS
statistical software (SAS Institute, Cary, NC). The effects of
treatment were analyzed using an analysis of variance, followed by
Duncan’s multiple range tests. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Effects of AEDK on the release of
histamine and β-hexosaminidase
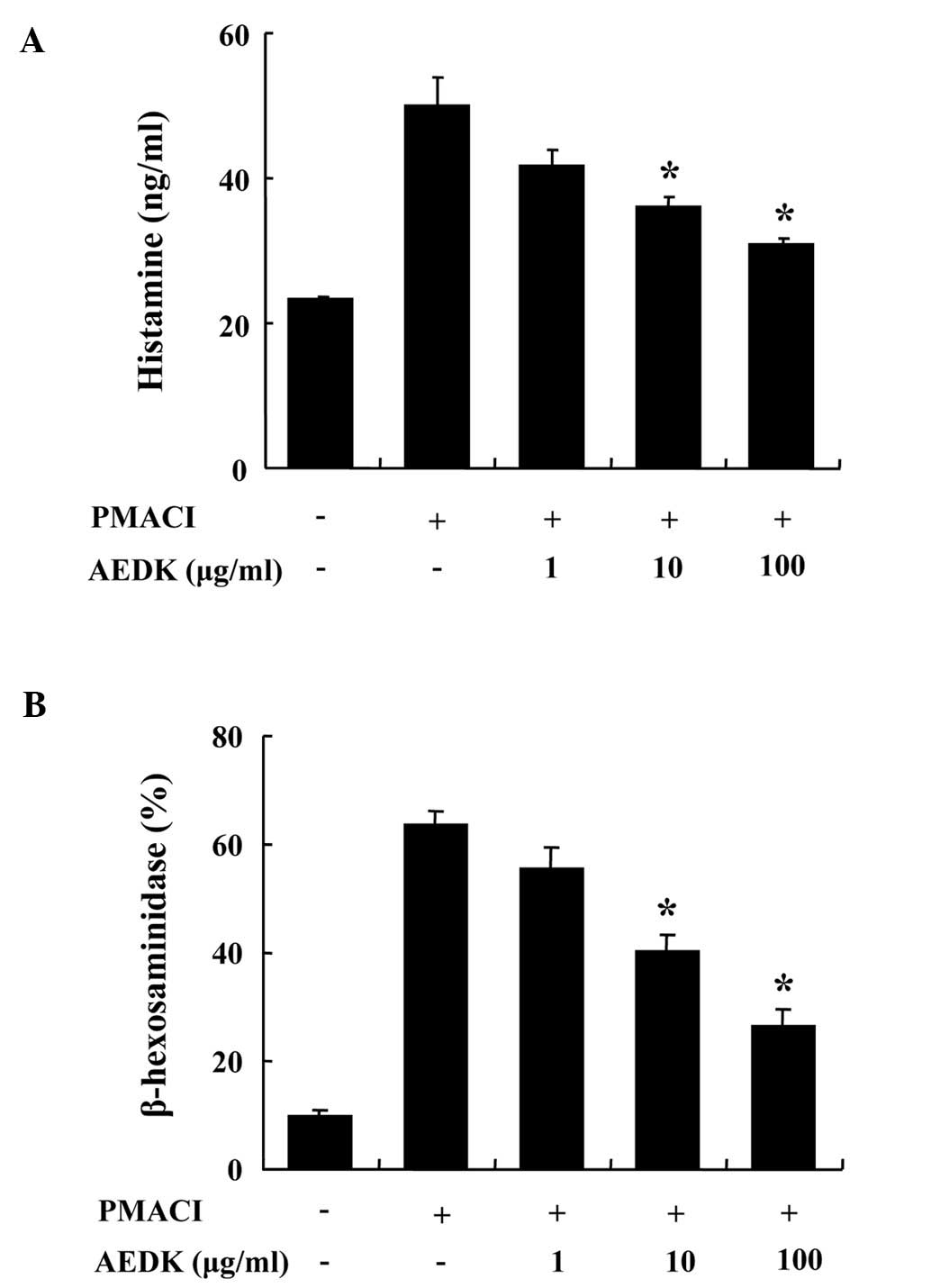
We determined the effects of AEDK on the
degranulation of mast cells. The release of histamine and
β-hexosaminidase from activated mast cells is a hallmark of
degranulation. HMC-1 cells released a high level of histamine when
stimulated with PMACI (Fig. 1A).
When pre-treated with AEDK (1–100 μg/ml) for 30 min, histamine
levels were dose-dependently inhibited in the PMACI-stimulated
HMC-1 cells. To confirm the inhibitory effects of AEDK on the
degranulation of mast cells, we investigated the effects of AEDK on
the release of β-hexodaminidase (Fig.
1B). The results revealed that 10 to 100 μg/ml AEDK
significantly decreased the PMACI-stimulated release of
β-hexodaminidase from HMC-1 cells. The concentration and duration
of AEDK treatment used in these experiments had no significant
effect on the cell viability of HMC-1 cells (data not shown).
Effects of AEDK on cAMP and intracellular
calcium levels
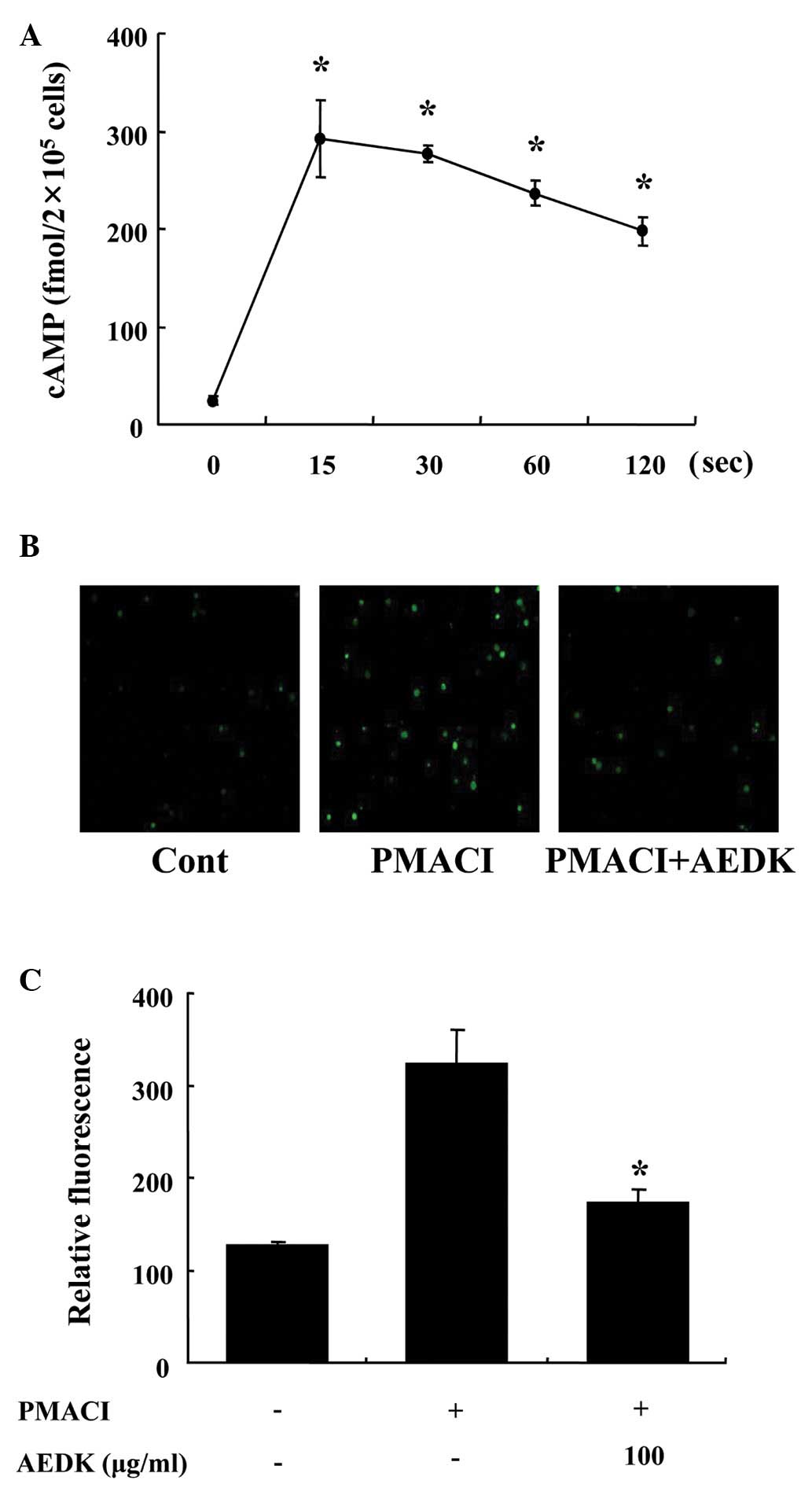
To investigate the mechanisms responsible for the
reduction in the levels of histamine following treatment with AEDK,
we assayed the cAMP and intracellular calcium levels. When the
HMC-1 cells were incubated with AEDK (100 μg/ml), the cAMP content
increased within 15 sec and remained at relatively high levels for
120 sec (Fig. 2A). Calcium
movements across the membranes of mast cells are critical to
histamine release (25). To
further investigate the mechanisms responsible for the reduction of
the release of histamine by AEDK, we assayed the intracellular
calcium levels. When the HMC-1 cells were stimulated with PMACI,
the intracellular calcium level was significantly elevated
(Fig. 2B). However, the
pre-incubation of HMC-1 cells with AEDK (100 μg/ml) decreased the
intracellular calcium level induced by PMACI. The level of
intracellular calcium was also depicted by the relative
fluorescence intensity (Fig.
2C).
Effects of AEDK on the expression and
secretion of pro-inflammatory cytokines
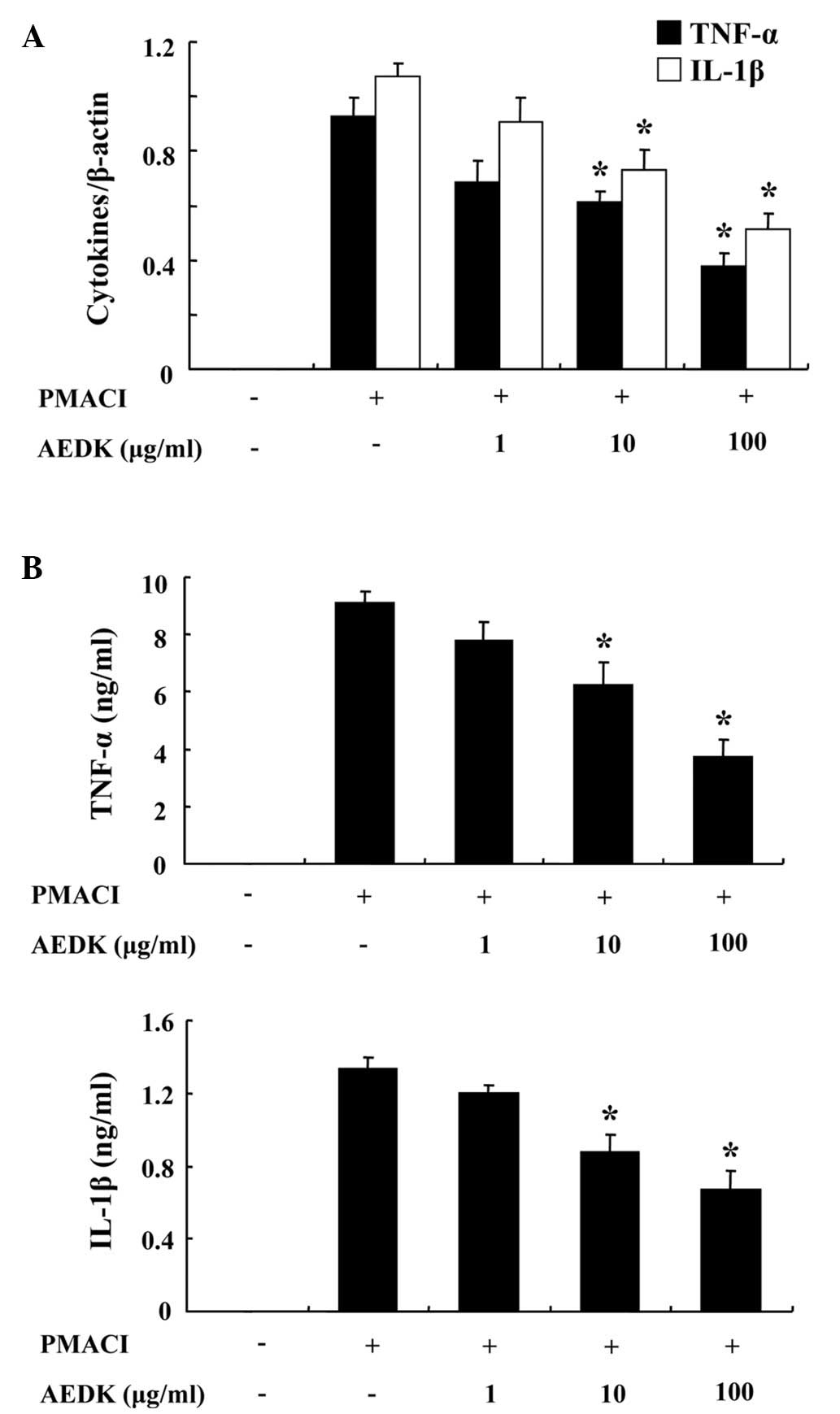
We investigated the inhibitory effects of AEDK on
the expression of pro-inflammatory cytokines, such as TNF-α and
IL-1β. Previously, we reported that the gene expression of TNF-α
and IL-1β peaked 4 h after treatment with PMACI (26). Consequently, the HMC-1 cells were
stimulated with PMACI for 4 h following pre-treatment with AEDK for
30 min. Fig. 3A illustrates that
the expression of pro-inflammatory cytokines was inhibited by AEDK.
To confirm the correlation of mRNA expression with protein
production, we evaluated the secretion of TNF-α and IL-1β with
ELISA. When the HMC-1 cells were stimulated with PMACI for 8 h, the
secretion of cytokines was markedly induced. The secretion of TNF-α
and IL-1β was significantly inhibited by AEDK (10 and 100 μg/ml) in
the PMACI-stimulated HMC-1 cells (Fig. 3B).
Effects of AEDK on the activation of
NF-κB
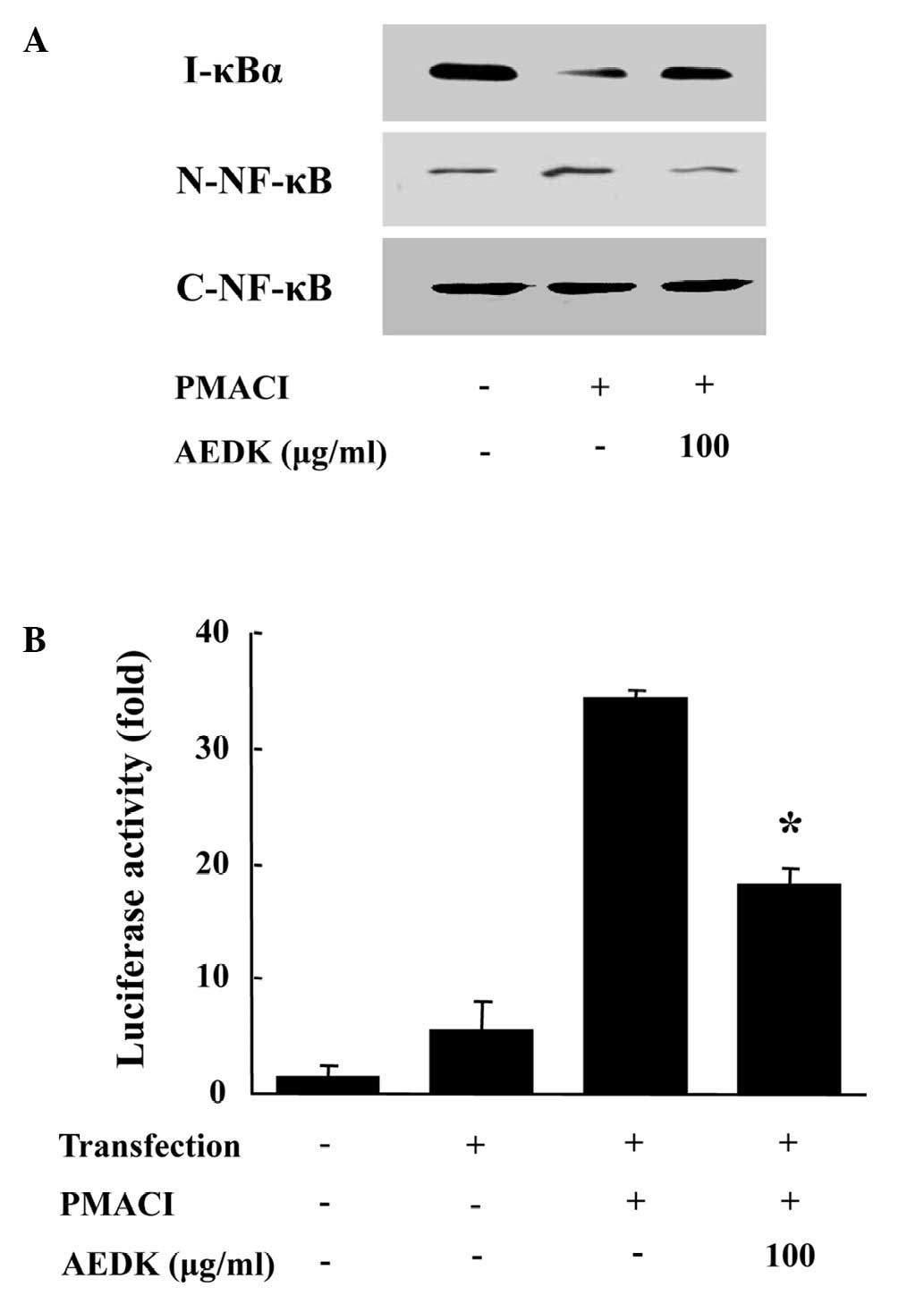
To investigate the intracellular mechanisms
responsible for the inhibitory effects of AEDK on the expression of
pro-inflammatory cytokines, we examined the effects of AEDK on the
activation of the transcription factor, NF-κB. NF-κB is an
important transcriptional regulator of inflammatory cytokines and
plays a crucial role in immune and inflammatory responses (4). The stimulation of HMC-1 cells with
PMACI induced the nuclear translocation of p65 NF-κB and the
degradation of IκBα. AEDK inhibited the PMACI-induced nuclear
translocation of NF-κB and the degradation of IκBα (Fig. 4A). To confirm the inhibitory
effects of AEDK on NF-κB activation, we used an NF-κB-dependent
gene reporter assay. The HMC-1 cells were transiently transfected
with a NF-κB-luciferase reporter construct or an empty vector.
Exposure of the cells to PMACI increased the luciferase activity in
the cells transfected with the NF-κB-luciferase reporter construct
(Fig. 4B). AEDK (100 μg/ml)
significantly reduced the PMACI-induced luciferase activity.
Effects of AEDK on systemic and local
allergic reactions
To determine the effects of AEDK on allergic
reaction, an in vivo model of systemic anaphylaxis was used.
Compound 48/80 (8 mg/kg, BW) was used as a model of induction for a
systemic fatal allergic reaction. Groups of mice (n=10/group) were
intraperitoneally injected with 200 μl of saline or drugs at
various doses, 1 h before the intraperitoneal injection of compound
48/80. After the intraperitoneal injection of compound 48/80, the
mice were monitored for 1 h, after which the mortality rate was
determined as the number of dead mice ×100/total number of
experimental mice. All mice underwent fatal shock after the
injection of compound 48/80. We compared the anti-anaphylactic
effects of AEDK with DSCG, a known anti-allergic drug. When AEDK
and DSCG were intraperitoneally administered, at doses ranging from
1 to 100 mg/kg (BW) for 1 h, the mortality rate was
dose-dependently reduced (Table
I). The effect of AEDK on the compound 48/80-induced release of
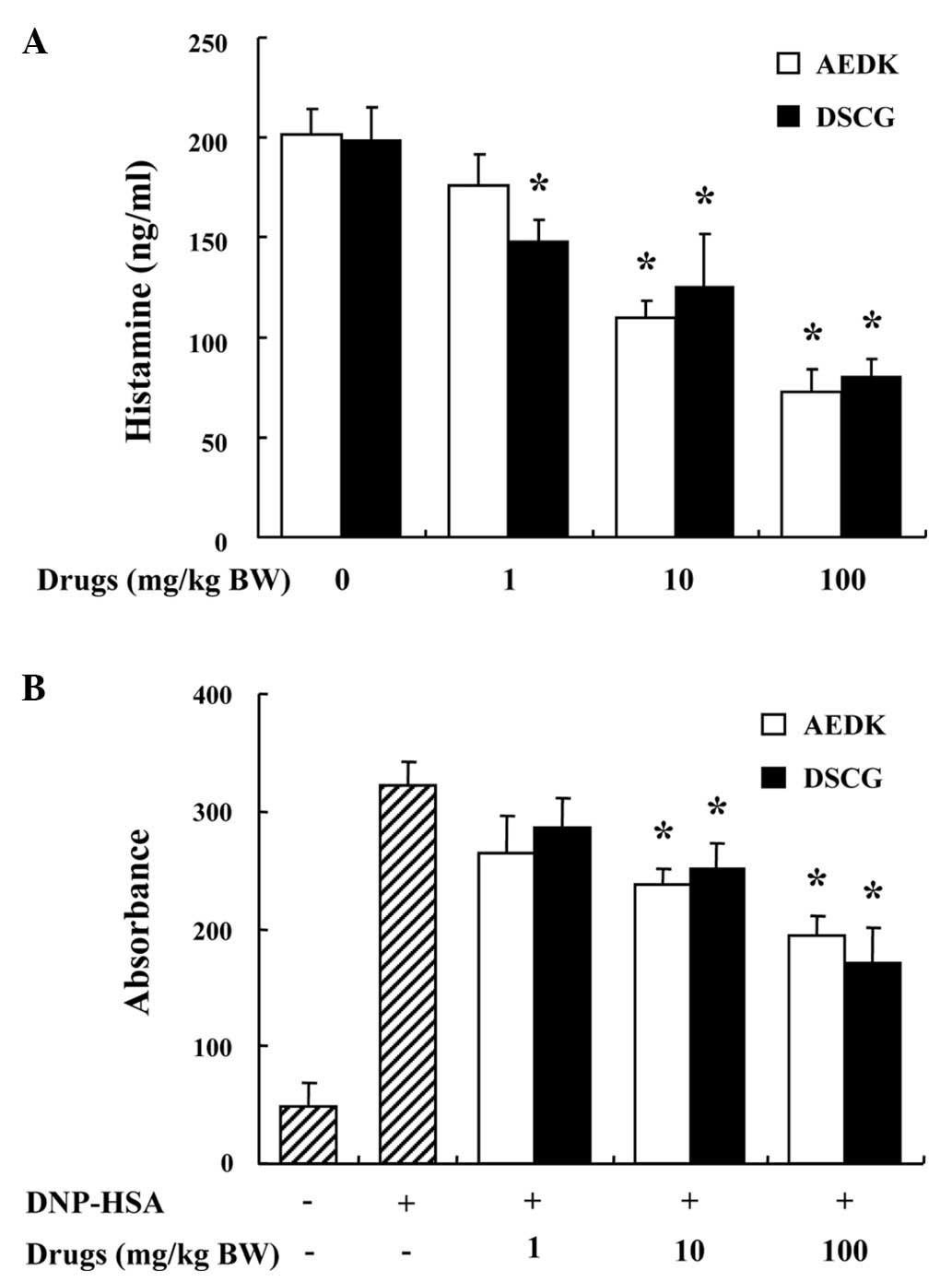
histamine in serum was also investigated. AEDK and DSCG were
administered 1 h prior to the injection of compound 48/80. The
injection of compound 48/80 induced a marked increase in the
release of histamine in serum, which was inhibited by treatment
with AEDK in a dose-dependent manner (Fig. 5A).
 | Table IEffects of AEDK on compound
48/80-induced systemic anaphylaxis. |
Table I
Effects of AEDK on compound
48/80-induced systemic anaphylaxis.
| AEDK treatment
(mg/kg BW) | Compound 48/80 (8
mg/kg BW) | Mortality rate
(%) |
|---|
| None (saline) | + | 100 |
| AEDK |
| 1 | + | 90 |
| 10 | + | 30 |
| 100 | + | 0 |
| 100 | − | 0 |
| DSCG |
| 1 | + | 80 |
| 10 | + | 40 |
| 100 | + | 0 |
| 100 | − | 0 |
Another way to examine the anaphylactic reaction is
to induce PCA. A local extravasation was induced by the local
injection of IgE followed by an antigenic challenge. AEDK and DSCG
were intraperitoneally administered at 1 to 100 mg/kg (BW) 1 h
prior to the antigen challenge. AEDK dose-dependently reduced the
IgE-mediated PCA reaction (Fig.
5B). The inhibitory effects of AEDK on systemic and local
anaphylaxis were similar to those of DSCG.
Discussion
Immediated-type hypersensitivity is a
life-threatening syndrome induced by the sudden systemic release of
inflammatory mediators such as histamine and cytokines (27). A number of studies have
established that the stimulation of mast cells initiates the
activation of signal transduction pathways, which leads to
degranulation. In this study, we demonstrate that AEDK inhibits
systemic allergic reaction and the release of histamine in serum,
which is an index of mast cell degranulation. In addition, the
administration of AEDK to mice protected them from IgE-mediated
PCA, one of the most important in vivo modes of acute local
anaphylaxis. In both systemic and local anaphylaxis, the inhibitory
effects of AEDK were comparable to those of DSCG, a clinically used
medication for treating asthma and allergies. These findings
suggest that AEDK may prove useful in the treatment of allergic
diseases.
cAMP and intracellular calcium pathways are critical
to the release of allergic inflammatory mediators, such as
histamine from mast cells (4).
Calcium movements across the membranes of mast cells represent a
major target for anti-allergic drugs, as these are essential events
linking stimulation to secretion (28). The transduction pathways
modulating cAMP and intracellular calcium are modified by the
ADP-ribosylation of G-protein (29). The release of histamine is known
to be reduced by an increase in the intracellular cAMP level due to
the activation of adenylate cyclase or the inhibition of cAMP
phosphodiesterase (30).
cAMP-elevating drugs, such as AEDK, inhibit the release of calcium
from intracellular calcium stores, indicating the regulatory role
of cAMP in the release of histamine. According to these results, we
suggest that the increase in cAMP and the decrease in calcium
levels may be involved in the inhibitory effects of AEDK on the
release of histamine.
TNF-α and IL-1β play an important role in triggering
and sustaining allergic inflammation in mast cells (2,31).
Mast cells are a principal source of TNF-α and IL-1β in the human
dermis. TNF-α and IL-1β promote inflammation, leukocyte
infiltration, the chemotaxis of neutrophils and stimulate T cells,
thereby contributing to chronic inflammation. Therefore, the
reduction in the levels of pro-inflammatory cytokines from mast
cells is one of the key indicators of reduced allergic inflammatory
symptoms. The expression of TNF-α and IL-1β is regulated by the
activation of the transcription factor, NF-κB (32). NF-κB regulates the expression of
multiple inflammatory- and immune-related genes and plays a
critical role in chronic inflammatory diseases. In PMACI-stimulated
mast cells, AEDK inhibited the expression of TNF-α and IL-1β and
decreased the activation of NF-κB. These data demonstrated that
AEDK attenuates the activation of NF-κB and the expression of
downstream cytokines, such as TNF-α and IL-1β.
To isolate the active components responsible for the
anti-allergic and anti-inflammatory effects of AEDK, we partitioned
AEDK as shown in Materials and methods and defined catechin as an
active component of AEDK. The anti-allergic and anti-inflammatory
properties of epigallocatechin-3-gallate (EGCG) are well known
(33). Due to the structural
similarity of EGCG and catechin, we hypothesized that catechin may
be one of the compounds responsible for the anti-allergic and
anti-inflammatory effects of AEDK. In conclusion, the present study
demonstrates that AEDK significantly reduces mast cell-mediated
allergic inflammation in in vitro and in vivo models.
We suggest that AEDK reduces the release of histamine by modulating
cAMP and intracellular calcium levels. AEDK inhibits the expression
and secretion of inflammatory cytokines by suppressing NF-κB. We
provide evidence that AEDK may contribute to the prevention or
treatment of mast cell-mediated allergic inflammatory diseases.
Acknowledgements
This study was supported by NRF funded by the
Ministry of Science, ICT & Future Planning
(2012M3A9B6055416).
References
|
1
|
Caughey GH: Mast cell proteases as
protective and inflammatory mediators. Adv Exp Med Biol.
716:212–234. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Galli SJ, Kalesnikoff J, Grimbaldeston MA,
Piliponsky AM, Williams CM and Tsai M: Mast cells as ‘tunable’
effector and immunoregulatory cells: recent advances. Annu Rev
Immunol. 23:749–786. 2005.
|
|
4
|
Kim SH, Jun CD, Suk K, et al: Gallic acid
inhibits histamine release and pro-inflammatory cytokine production
in mast cells. Toxicol Sci. 91:123–131. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Amin K: The role of mast cells in allergic
inflammation. Respir Med. 106:9–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee DH, Kim SH, Eun JS and Shin TY:
Mosla dianthera inhibits mast cell-mediated allergic
reactions through the inhibition of histamine release and
inflammatory cytokine production. Toxicol Appl Pharmacol.
216:479–484. 2006. View Article : Google Scholar
|
|
7
|
Tagen M, Elorza A, Kempuraj D, et al:
Mitochondrial uncoupling protein 2 inhibits mast cell activation
and reduces histamine content. J Immunol. 183:6313–6319. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gwack Y, Feske S, Srikanth S, Hogan PG and
Rao A: Signalling to transcription: store-operated Ca2+
entry and NFAT activation in lymphocytes. Cell Calcium. 42:145–156.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sismanopoulos N, Delivanis DA,
Alysandratos KD, et al: Mast cells in allergic and inflammatory
diseases. Curr Pharm Des. 18:2261–2277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mallavadhani UV, Panda AK and Rao YR:
Pharmacology and chemotaxonomy of Diospyros. Phytochemistry.
49:901–951. 1998.PubMed/NCBI
|
|
11
|
Duan J, Zheng Y, Dong Q and Fang J:
Structural analysis of a pectic polysaccharide from the leaves of
Diospyros kaki. Phytochemistry. 65:609–615. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Matsumoto N, Okushio K and Hara Y: Effect
of black tea polyphenols on plasma lipids in cholesterol-fed rats.
J Nutr Sci Vitaminol (Tokyo). 44:337–342. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kotani M, Matsumoto M, Fujita A, et al:
Persimmon leaf extract and astragalin inhibit development of
dermatitis and IgE elevation in NC/Nga mice. J Allergy Clin
Immunol. 106:159–166. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kameda K, Takaku T, Okuda H, et al:
Inhibitory effects of various flavonoids isolated from leaves of
persimmon on angiotensin-converting enzyme activity. J Nat Prod.
50:680–683. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Funayama S and Hikino H: Hypotensive
principles of Diospyros kaki leaves. Chem Pharm Bull
(Tokyo). 27:2865–2868. 1979. View Article : Google Scholar
|
|
16
|
Sun L, Zhang J, Lu X, Zhang L and Zhang Y:
Evaluation to the antioxidant activity of total flavonoids extract
from persimmon (Diospyros kaki L.) leaves. Food Chem
Toxicol. 49:2689–2696. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steinmetz KA and Potter JD: Vegetables,
fruit, and cancer prevention: a review. J Am Diet Assoc.
96:1027–1039. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rattmann YD, Cipriani TR, Sassaki GL, et
al: Nitric oxide-dependent vasorelaxation induced by extractive
solutions and fractions of Maytenus ilicifolia Mart ex
Reissek (Celastraceae) leaves. J Ethnopharmacol. 104:328–335. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ali K, Maltese F, Toepfer R, Choi YH and
Verpoorte R: Metabolic characterization of Palatinate German white
wines according to sensory attributes, varieties, and vintages
using NMR spectroscopy and multivariate data analyses. J Biomol
NMR. 49:255–266. 2011. View Article : Google Scholar
|
|
20
|
Kim SH, Lee S, Kim IK, et al: Suppression
of mast cell-mediated allergic reaction by Amomum
xanthioides. Food Chem Toxicol. 45:2138–2144. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim HH, Yoo JS, Lee HS, Kwon TK, Shin TY
and Kim SH: Elsholtzia ciliata inhibits mast cell-mediated
allergic inflammation: role of calcium, p38 mitogen-activated
protein kinase and nuclear factor-{kappa}B. Exp Biol Med (Maywood).
236:1070–1077. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Itoh T, Tsukane M, Koike M, et al:
Inhibitory effects of whisky congeners on IgE-mediated
degranulation in rat basophilic leukemia RBL-2H3 cells and passive
cutaneous anaphylaxis reaction in mice. J Agric Food Chem.
58:7149–7157. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bae Y, Lee S and Kim SH: Chrysin
suppresses mast cell-mediated allergic inflammation: involvement of
calcium, caspase-1 and nuclear factor-kappaB. Toxicol Appl
Pharmacol. 254:56–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee S, Yun HS and Kim SH: The comparative
effects of mesoporous silica nanoparticles and colloidal silica on
inflammation and apoptosis. Biomaterials. 32:9434–9443. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eisenhut M and Wallace H: Ion channels in
inflammation. Pflugers Arch. 461:401–421. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Park HH, Lee S, Oh JM, et al:
Anti-inflammatory activity of fisetin in human mast cells (HMC-1).
Pharmacol Res. 55:31–37. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boden SR and Wesley Burks A: Anaphylaxis:
a history with emphasis on food allergy. Immunol Rev. 242:247–257.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Beaven MA, Rogers J, Moore JP, Hesketh TR,
Smith GA and Metcalfe JC: The mechanism of the calcium signal and
correlation with histamine release in 2H3 cells. J Biol Chem.
259:7129–7136. 1984.PubMed/NCBI
|
|
29
|
Alfonso A, Cabado AG, Vieytes MR and
Botana LM: Functional compartments in rat mast cells for cAMP and
calcium on histamine release. Cell Signal. 12:343–350. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Makino H, Saijo T, Ashida Y, Kuriki H and
Maki Y: Mechanism of action of an antiallergic agent, amlexanox
(AA-673), in inhibiting histamine release from mast cells.
Acceleration of cAMP generation and inhibition of
phosphodiesterase. Int Arch Allergy Appl Immunol. 82:66–71. 1987.
View Article : Google Scholar
|
|
31
|
Walsh LJ, Trinchieri G, Waldorf HA,
Whitaker D and Murphy GF: Human dermal mast cells contain and
release tumor necrosis factor alpha, which induces endothelial
leukocyte adhesion molecule 1. Proc Natl Acad Sci USA.
88:4220–4224. 1991. View Article : Google Scholar
|
|
32
|
Karin M: NF-kappaB as a critical link
between inflammation and cancer. Cold Spring Harb Perspect Biol.
1:a0001412009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bani D, Giannini L, Ciampa A, et al:
Epigallocatechin-3-gallate reduces allergen-induced asthma-like
reaction in sensitized guinea pigs. J Pharmacol Exp Ther.
317:1002–1011. 2006. View Article : Google Scholar : PubMed/NCBI
|