Introduction
Growth factors are important in anti-aging and wound
healing processes (1–4). Wound healing processes involve
coagulation, inflammation, migration/proliferation, granulation,
and remodeling phases (5). The
migration and proliferation of keratinocytes and fibroblasts play
key roles in initiating wound healing (6). The transition from the inflammatory
phase of wound healing to granulation phases is mediated by a
variety of growth factors and cytokines (7,8).
Multiple signaling pathways induce the formation of new collagen
and repair of extracellular matrix during the granulation
phase.
Growth factors are crucial in the initiation of
proliferation and migration of dermal fibroblasts during wound
healing phases. The migration and proliferation of fibroblasts are
precisely regulated by positive and negative cell cycle regulatory
proteins (9–11). Positive cell cycle regulatory
proteins include cyclin D1, cyclin E, cyclin A, and their kinase
partners, cyclin-dependent protein kinases (Cdk). Of these, cyclin
D1-Cdk4/6 and cyclin E-Cdk2 are the key positive regulatory
proteins in the progression of G1/S transition phase, and cyclin
A-Cdk2 plays an important role in the G2/M transition phase
(12).
Fibroblasts, which are derived from chronic wounds,
showed a decreased proliferation rate, senescence-like morphology,
and decreased expressions of extracellular matrix proteins
(13). Several growth factors,
including platelet-derived growth factors (PDGF), interleukin-1 and
transforming growth factor (TGF)-β have been shown to exert an
effect on the proliferation and activation of dermal fibroblasts
leading to the regeneration and remodeling of dermal extracellular
matrix (14).
The aim of this study was to evaluate the potential
use of recombinant growth factor mixtures (RGFM) for wound healing
or an anti-aging agent by investigating the effect of RGFM on cell
migration, proliferation, expressions of cell cycle regulatory
proteins as well as type I collagen in fibroblasts and animal wound
healing model.
Materials and methods
Materials
Antibodies against cell cycle regulatory proteins
(Cdk2, Cdk4, cyclin A, cyclin D1 and cyclin E), type I collagen,
matrix metalloproteinase (MMP)-1, MMP-2, and tissue inhibitor
metalloproteinase-1 (TIMP-1), were obtained from Santa Cruz
Biotechnology, Inc. (Santa Cruz, CA, USA). Anti-RB antibody was
purchased from BD Biosciences Pharmingen (San Diego, CA, USA).
Smad-2, Smad-3, phospho-Smad2 (p-Smad2) and p-Smad3 were obtained
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Antibodies
against phospho-extracellular signal-regulated kinases (p-ERK),
ERK, phospho-p38 (p-p38), p38, phospho-c-Jun N-terminal kinases
(p-JNK), and JNK were purchased from Cell Signaling Technology,
Inc.
Preparation of recombinant growth
factors
The ingredients of RGFM, recombinant human epidermal
growth factor (EGF), recombinant human basic fibroblast growth
factor (bFGF), recombinant human keratinocyte growth factor (KGF),
recombinant human insulin-like growth factor-1 (IGF-1), and
recombinant human superoxide dismutase (SOD), were provided by
Nutrex Technology Co., Ltd. (Seoul, Korea). The RGFM was prepared
by mixing the same ratios of recombinant human EGF, recombinant
human bFGF, recombinant human KGF, recombinant human IGF-1, and
recombinant human SOD. For the in vitro and in vivo
experiments, RGFM was dissolved in water containing 5 mM sodium
phosphate buffer and 80 mM NaCl at pH 7.4.
Cell cultures
Human skin fibroblasts (HSF) were maintained at 37°C
in a humidified atmosphere of 95% air and 5% CO2 in
Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10%
heat-inactivated fetal bovine serum (FBS), 2 mM glutamine, 100 U/ml
penicillin and 100 μg/ml streptomycin. For experiments, cells
(5×104 cells/ml) were seeded in a culture dish, and
maintained in the tissue culture incubator.
Cell proliferation assay
Cell proliferation was determined using a
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT)
assay. Briefly, human fibroblasts were seeded at a density of
2×104 cells/well in 24-well plates and cultured for 18
h. After twice washing with phosphate-buffered saline (PBS), cells
were cultured for 72 h with various concentrations of RGFM in
serum-free medium. Five hundred microliters of MTT solution (5
mg/ml) in serum-free medium were added and incubated at 37°C for 4
h. The supernatant was removed and formazan crystals were dissolved
in 200 μl of dimethyl sulfoxide (DMSO). The plate was agitated at
room temperature for 30 min, and the optical density was measured
at 540 nm using an enzyme-linked immunosorbent assay (ELISA) reader
(VersaMax; Molecular Devices, Sunnyvale, CA, USA). Cell morphology
was evaluated using phase contrast microscopy after treatment for
72 h. Phase contrast images were captured using an Olympus CKX41
inverted microscope with QCapture Pro-software (Olympus Optical,
Tokyo, Japan).
Migration assay
For the measurement of cell migration, confluent
fibroblasts kept in serum-free medium for 24 h were wounded with a
plastic micropipette tip that had a large orifice. Subsequent to
washing, the medium was replaced with various concentrations of
RGFM without FBS. Images of the wounded area were captured every 24
h by phase-contrast microscopy under crystal violet staining.
Western blot analysis
Whole cell extracts were prepared in the lysis
buffer [10 mM Tris (pH 7.4), 5 mM ethylenediaminetetraacetic acid
(EDTA), 130 mM NaCl, 1% Triton X-100, phenylmethylsulphonyl
fluoride (PMSF, 10 g/ml), aprotinin (10 g/ml), leupeptin (10 g/ml),
5 mM phenanthroline and 28 mM benzamidine-HCl]. The protein
concentration of extracts was estimated with Bradford reagent
(Bio-Rad, Hercules, CA, USA) using bovine serum albumin as the
standard. Equal amounts of protein (40 μg/lane) were resolved by
6.5–12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis,
and transferred onto a nitrocellulose membrane. The membrane was
washed with Tris-buffered saline (10 mM Tris, 150 mM NaCl)
containing 0.05% Tween-20 (TBST) and blocked in TBST containing 5%
non-fat dry milk. The membrane was then incubated with the
respective specific antibodies. The membrane was continuously
incubated with appropriate secondary antibodies coupled with
horseradish peroxidase, and developed in the ECL Western detection
reagents (Amersham Pharmacia Biotech, Inc., Piscataway, NJ,
USA).
Wound healing studies in the animal
model
Six-week-old hairless male mice (Japan SLC,
Shizuoka, Japan) weighing 18–20 g were used for all the experiments
(n=18). Anesthesia was performed by an intraperitoneal injection of
a 30–40 μl mixture of Rompun and Zoletil 50 (1 ml anesthetic is
composed of 0.5 ml Zoletil 50, 0.22 ml Rompun, and 0.28 ml
ddH2O). The anesthetized animals received two
full-thickness round wounds (~1.5 cm in diameter) created on the
back of each hairless mouse using the Er:YAG laser (CB Erbium/2.94;
Continuum Biomedical, Inc., Dublin, CA, USA). The energy density of
each wound site was 60 J/cm2.
The dissolved RGFM in distilled water was applied to
the wound. In order to evaluate the wound healing in each group of
RGFM- and PBS-treated mice after 24 h, the wound closure rate was
observed at days 0, 7 and 11 after the wounds were inflicted and
the rates were compared among the differently treated groups of
mice. Three groups of mice were used: non-treated (which received
irradiation) (n=6), PBS-treated (n=6), and RGFM-treated (n=6) mice.
Image analysis of the wound sites was performed by an image
analysis device, the 3D LifeViz (Quantificare, Sophia Antipolis,
France). Following scarification of mice, skin specimens were taken
from the center area of each wound, 11 days following creation of
the wound.
Hematoxylin and eosin (H&E)
staining
Skin tissue samples from the center of each wound,
were fixed in a 10% formalin solution for 24 h at room temperature
and processed for conventional paraffin embedding. Cross-sections
(5 μm) were deparaffinized in xylene and dehydrated in an ethanol
concentration series of 100, 95, 90, 80 and 70%. For morphological
observations, the sections were stained with H&E. Slide
staining results were evaluated using a DX70 microscope, Olysia
Soft Imaging System (Olympus Optical).
Statistical analysis
Data are presented as the means ± SD. One-way ANOVA
followed by Dunnett’s T3 test was used to assess statistical
significance with thresholds of P<0.05, P<0.01 and P<0.001
for significant and highly significant, respectively.
Results
Effect of RGFM on cell
migration/proliferation rates of fibroblasts
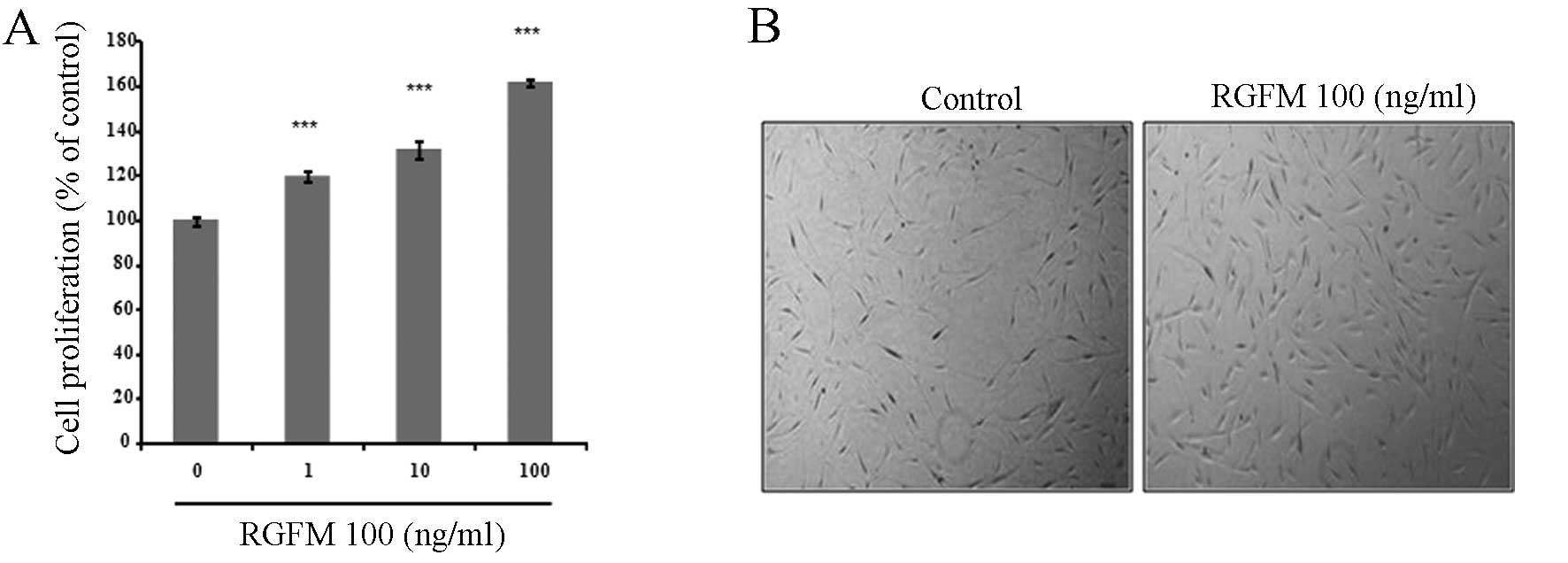
We analyzed the effect of RGFM on the proliferation
and migration rates of the fibroblast cell line. RGFM resulted in a
dose-dependent increase of proliferation rates of fibroblasts
(Fig. 1). In addition, migration
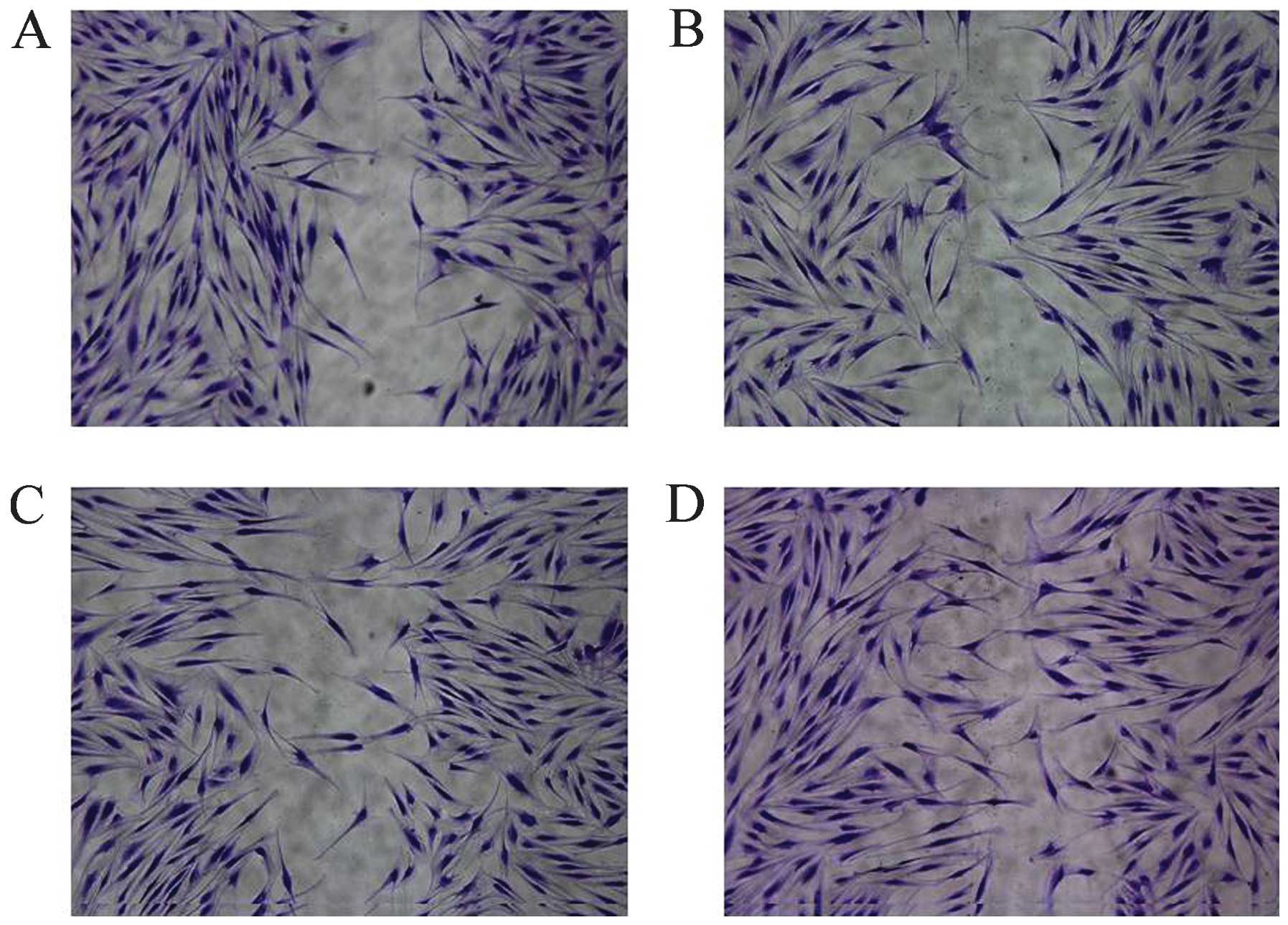
of fibroblasts is important in the promotion of wound healing. In
order to examine whether RGFM is more effective on the migration of
fibroblasts, confluent fibroblasts were scratched using a plastic
micropipette and were then cultured for 1 day under various
concentrations of RGFM. RGFM treatment resulted in markedly
increased rates of the migration of fibroblasts (Fig. 2).
Effect of RGFM on type I collagen, MMP-1
and TIMP-1 in HSF
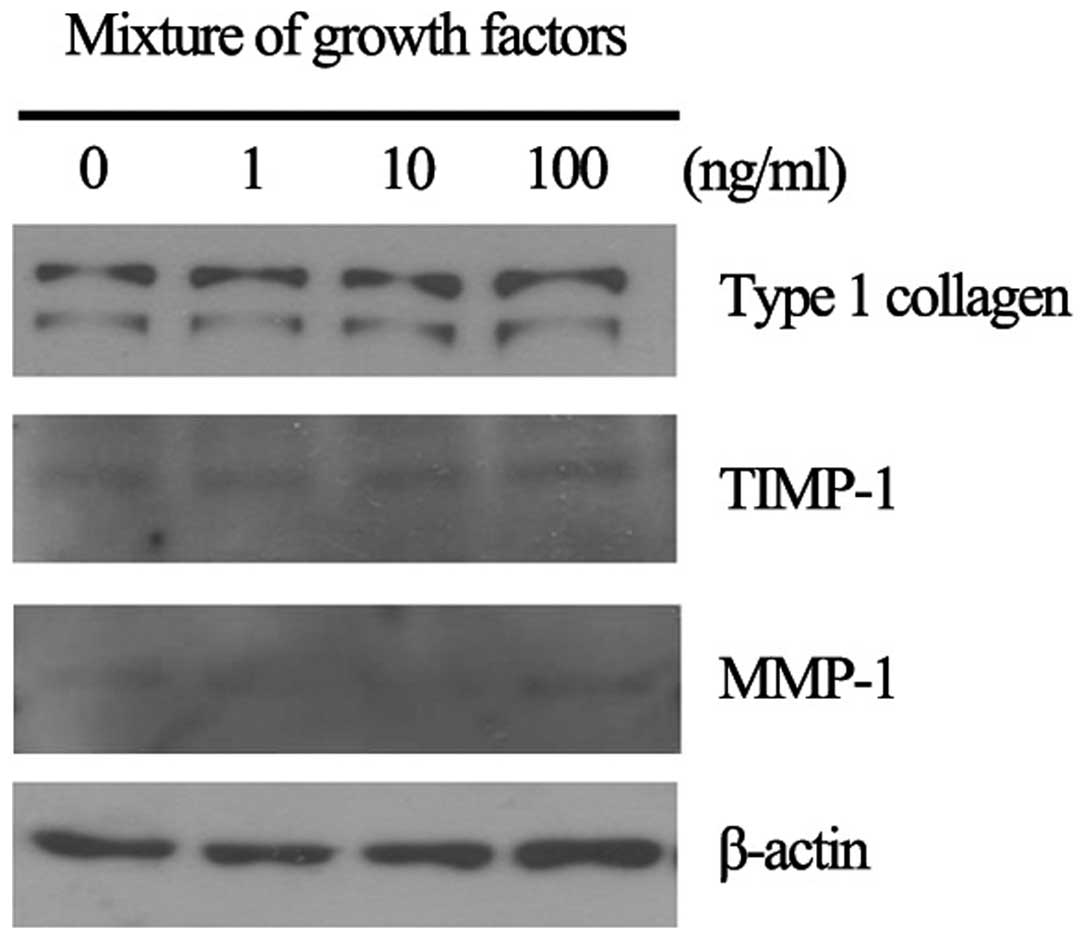
We analyzed the effect of RGFM on the expression of
type I collagen, MMP-1 and TIMP-1 in culture fibroblasts. RGFM
induced the increased expression of type I collagen in a
dose-dependent manner, however, the expression of MMP-1 and TIMP-1
was not markedly altered (Fig.
3). β-actin was used as the loading control. The increased
expression of type I collagen was clearly observed in RGFM-treated
fibroblasts (100 ng/ml). RGFM induced the increased expression of
type I collagen as well as the proliferation and migration rates of
fibroblasts. Thus, we focused on the role of RGFM in Smad2 and
Smad3 in cultured HSF.
Effect of RGFM on Smad2 and Smad3
expression in HSF
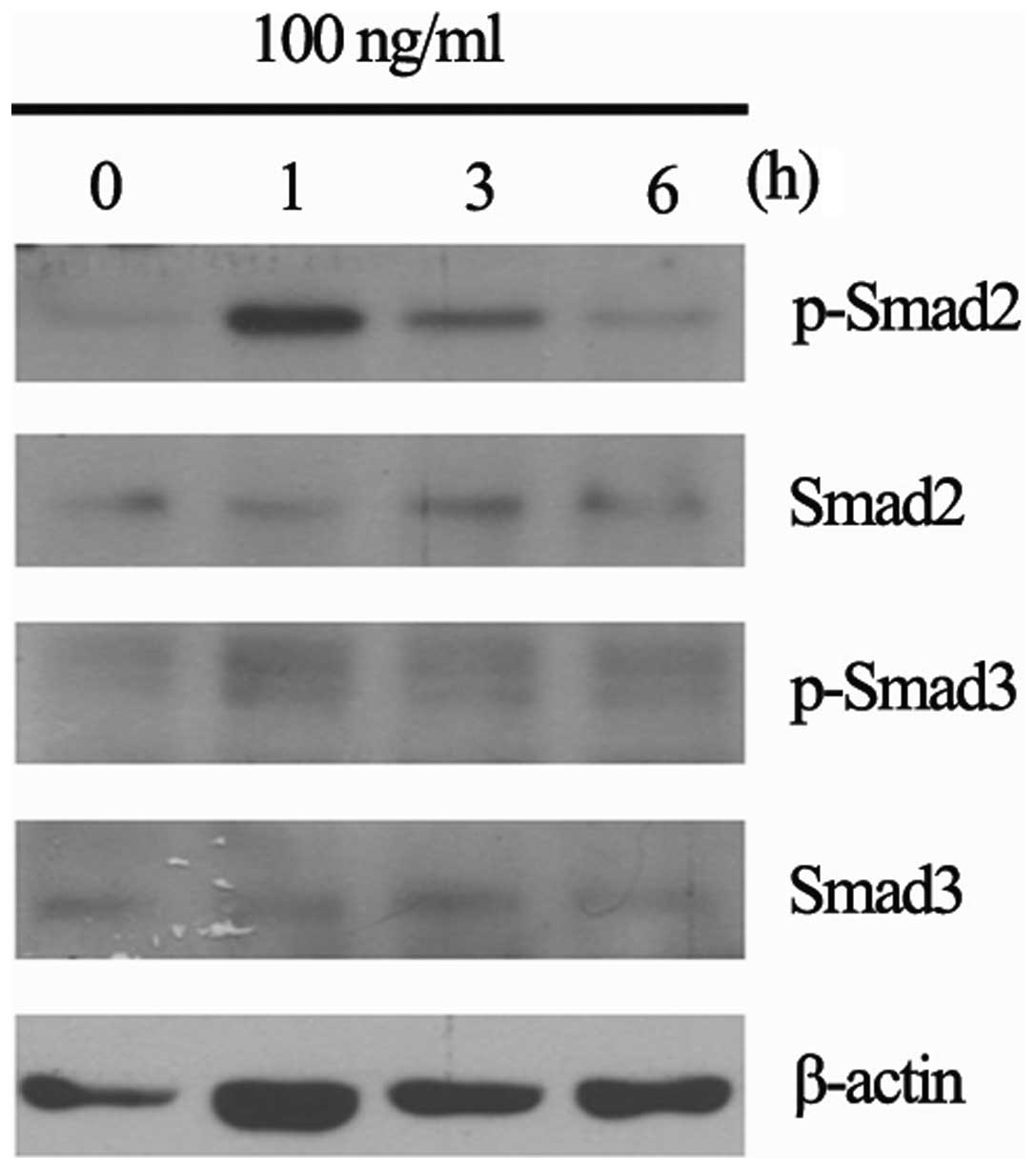
We analyzed the effect of RGFM on the expression of
Smad2 and Smad3 in cultured skin fibroblasts. RGFM modulated the
activity of Smad2 and Smad3 (Fig.
4). The phosphorylations of Smad2 and Smad3 were clearly
observed at 1 h after treatment.
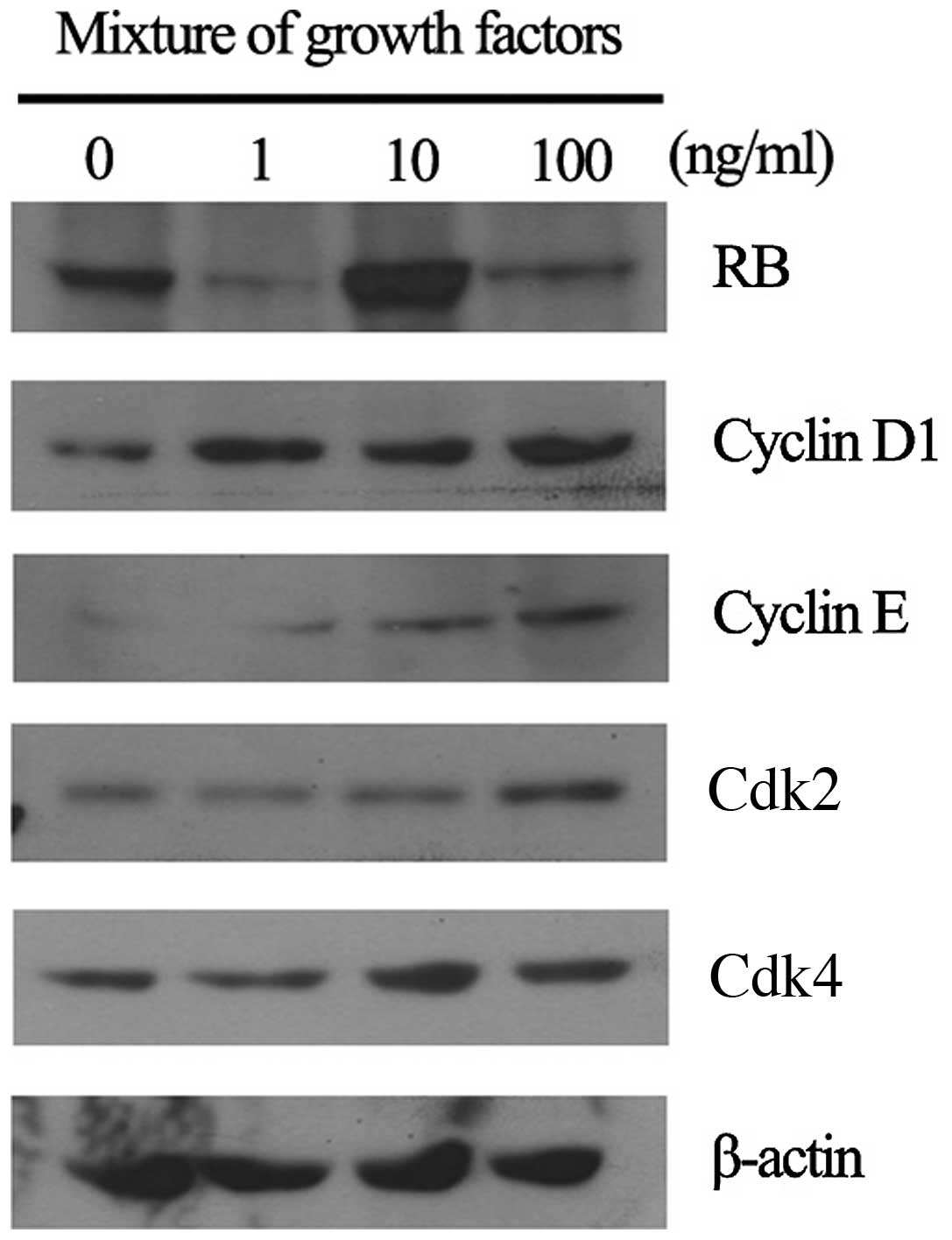
Expression of cell cycle regulatory
proteins in fibroblast cell line by RGFM treatment
Cell cycle regulatory proteins play important roles
in proliferation and migration in fibroblast cell lines. We have
investigated whether RGFM treatment promotes proliferation and
migration activities of fibroblasts through the upregulation of
G1/S or G2/M transition regulatory proteins. Expression levels of
G1/S transition regulatory proteins, i.e., cyclin D1, cyclin E,
Cdk2 and Cdk4, were increased in RGFM-treated fibroblasts,
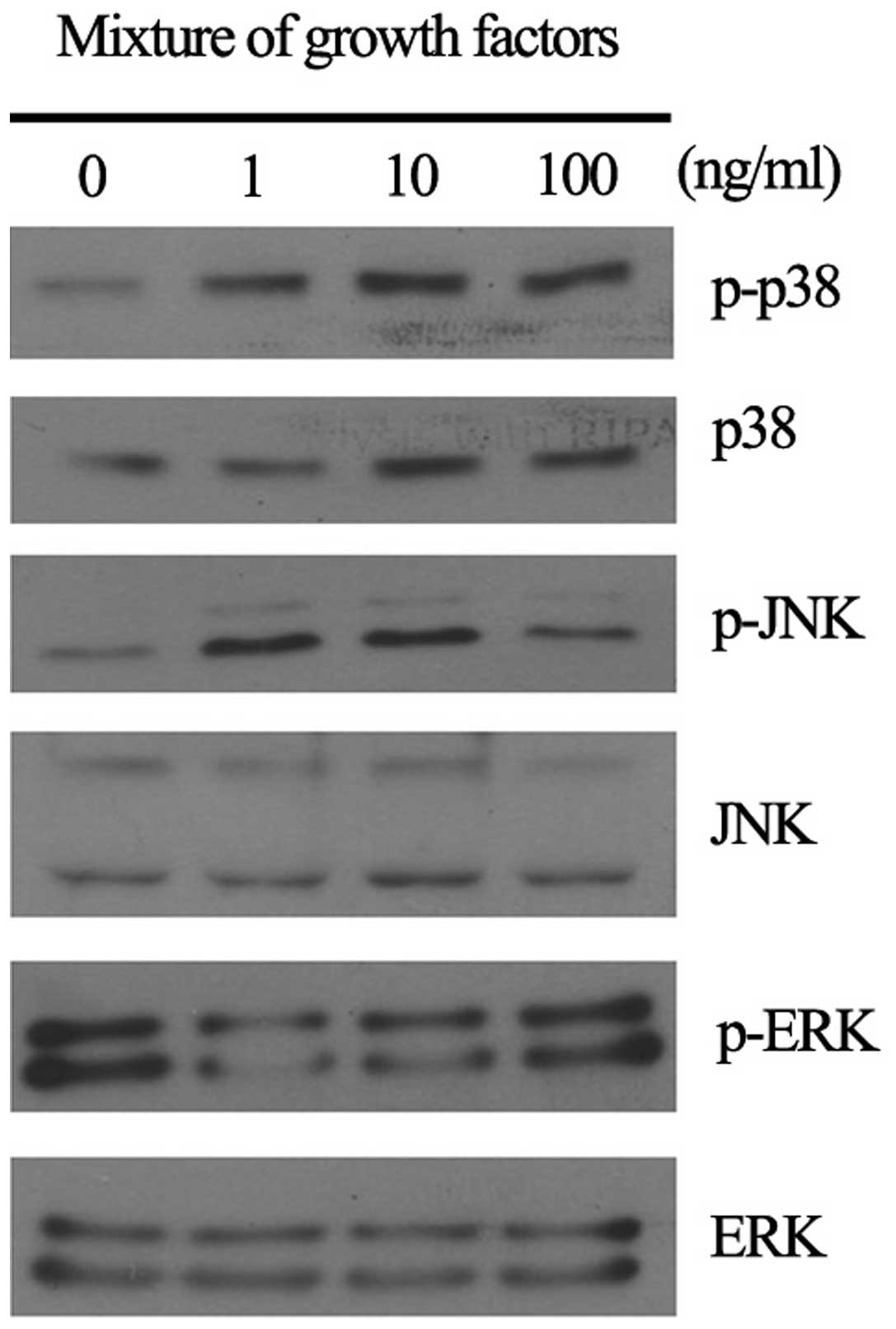
resulting in increased phosphorylation of RB protein (Fig. 5). In addition, RGFM induced the
activation of JNK, p38 and ERK, in cultured skin fibroblasts at 15
min after treatment (Fig. 6).
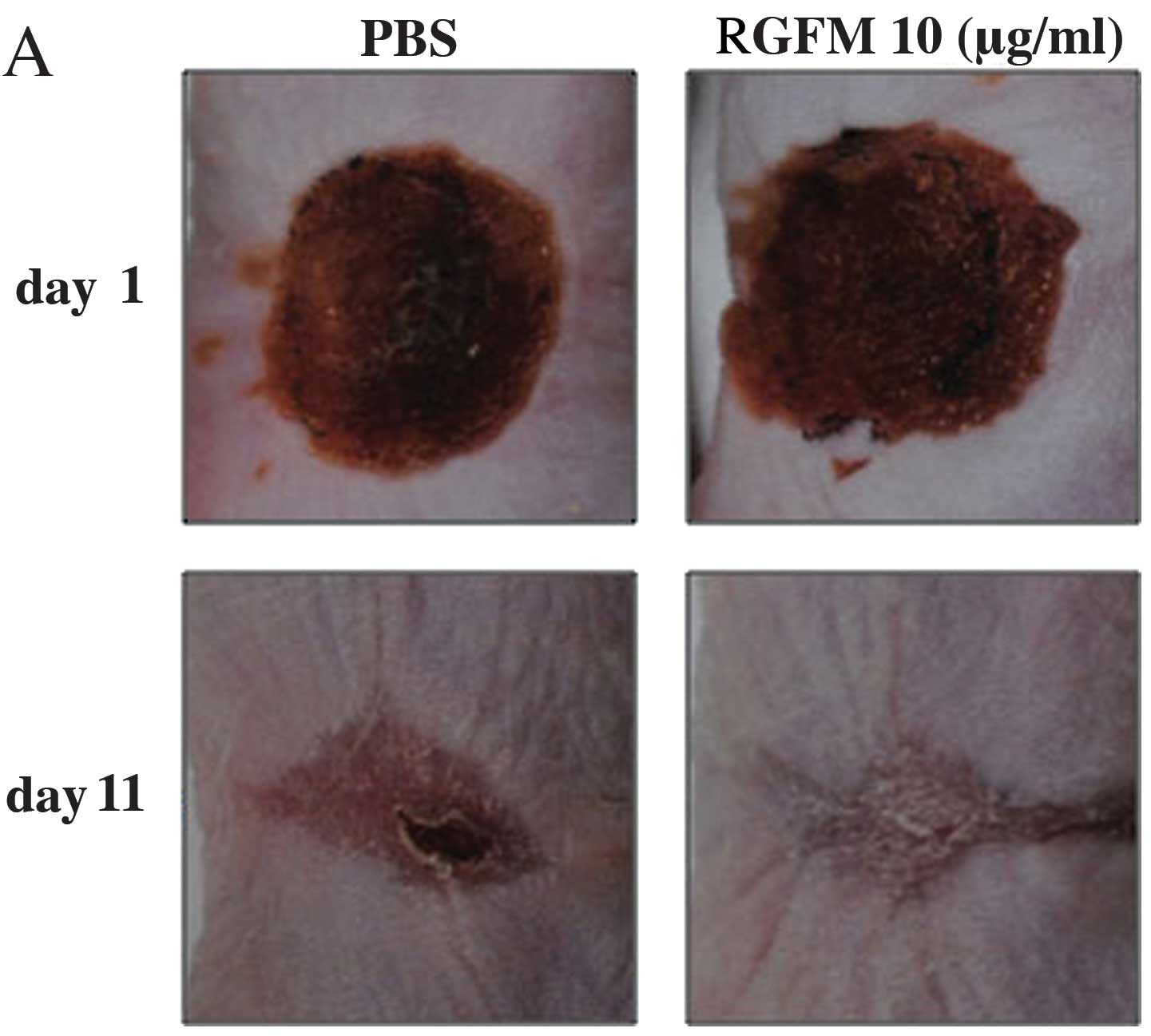
Wound healing effect of RGFM on the
animal wound model
Topical application of RGFM on wound healing was
carried out by irradiation on the back skins of hairless mice for
up to 11 days. The RGFM-treated mice exhibited accelerated wound
closure at day 7 after injury. This accelerated wound closure was
observed until day 11 (Fig. 7A and
B). The PBS-treated mice exhibited delayed wound closure
compared to the RGFM-treated mice (Fig. 7A and B). At 11 days, the mice were
sacrificed, and wound histology was analyzed by H&E staining.
The histological parameters were re-epithelialization, dermal
regeneration, and granulation tissue formation. The PBS-treated
mice showed similarly sufficient epidermal layers and discontinuity
of dermal regeneration with a large amount of inflammatory cells
infiltrating the dermal region (Fig.
7C). On the other hand, the RGFM-treated mice found complete
re-epithelialization of the epidermal region. Additionally,
continuity of dermal regeneration included decreased levels of
inflammatory cell infiltration in the wound (Fig. 7C).
Discussion
The wound healing process involves the phases of
inflammation, tissue formation, and tissue remodeling (15). Successful wound healing requires a
balance between development of inflammation and its rapid
resolution (16). Furthermore,
transition from the inflammatory phase of wound healing to the
granulation phase is mediated by a variety of growth factors and
cytokines including PDGF, TGF-β, FGF, IGF-1 and TNF-α (8).
In the present study, we prepared recombinant
synthetic growth factors (RGFM) for the wound healing agent. RGFM
contain bioactive proteins including EGF, bFGF and KGF, which have
fundamental roles in wound healing. These factors are known to
regulate processes such as cell migration, attachment,
proliferation and differentiation, and promote extracellular matrix
accumulation by binding to specific cell surface receptors. Thus,
RGFM may be a promising agent for acceleration of the wound healing
process through high concentrations of growth factors.
Skin fibroblasts play an important role during the
wound healing process through extracellular modulation (17). Synthesis, remodeling and
deposition of structural extracellular matrix molecules, are
indispensable for initiating repair and progression into the
healing state (15). In this
study, we investigated the effect of RGFM on the activation of
fibroblasts including migration, proliferation, and expression of
type I collagen and MMP-1 as well as cell cycle regulatory
proteins. Our data clearly indicate that RGFM accelerated the
migration of fibroblasts. In addition, cell proliferation rates of
RGFM-treated fibroblasts were increased by the upregulation of
cyclin D1, cyclin E, Cdk2 and Cdk4 expression. Furthermore,
mitogen-activated protein kinases, such as p38, JNK and ERK, were
clearly activated by RGFM treatment in fibroblasts. Upregulation of
type I collagen by RGFM treatment in fibroblasts was mediated by
the activation of Smad2/3. Phosphorylation and nuclear
translocation of Smad2 and Smad3 in response to TGF-β1 are critical
for the synthesis of type I collagen in fibroblasts (18). It is well known that fibroblasts,
which are isolated from chronic wound, are senescent and show a
decreased proliferative response to growth factors (19,20). Fibroblasts from chronic wounds
show a decreased expression of type II TGF-β receptors, with
impaired phosphorylation of transduction signals, including Smad2,
Smad3 and mitogen-activated protein kinase (13,21). Thus, RGFM-induced fibroblast
migration, cell proliferation, and upregulation of type I collagen
by the activation of Smad2/3 may contribute to the rapid wound
healing process under chronic wounds.
Findings of the in vivo study showed that the
RGFM-treated mice exhibited complete re-epithelialization of the
epidermal region, while continuity of dermal regeneration included
decreased levels of inflammatory cell infiltration in the wound.
Persistent inflammation has been associated with delayed wound
healing and eventually resulted in scar formation (16).
In conclusion, RGFM has the potential to accelerate
wound healing through the upregulation of type I collagen which is
partly mediated by the activation of Smad2/3-dependent signaling
pathway as well as the cell cycle progression in HSF. Topical
application of RGFM to acute and chronic skin wound may accelerate
the epithelization process through these molecular mechanisms.
Acknowledgements
This study was financially supported by the Ministry
of Knowledge Economy (MKE), the Korea Institute for Advancement of
Technology (KIAT) through the Inter-ER Cooperation Projects
References
|
1
|
Taguchi M, Moran SL, Zobitz ME, et al:
Wound-healing properties of transforming growth factor β (TGF-β)
inducible early gene 1 (TIEG1) knockout mice. J Musculoskelet Res.
11:63–69. 2008.
|
|
2
|
Rosen PS: Using recombinant
platelet-derived growth factor to facilitate wound healing. Compend
Contin Educ Dent. 27:520–525. 2006.PubMed/NCBI
|
|
3
|
Frechette JP, Martineau I and Gagnon G:
Platelet-rich plasmas: growth factor content and roles in wound
healing. J Dent Res. 84:434–439. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mehta RC and Fitzpatrick RE: Endogenous
growth factors as cosmeceuticals. Dermatol Ther. 20:350–359. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Martin P: Wound healing - aiming for
perfect skin regeneration. Science. 276:75–81. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spiekstra SW, Breetveld M, Rustemeyer T,
Scheper RJ and Gibbs S: Wound-healing factors secreted by epidermal
keratinocytes and dermal fibroblasts in skin substitutes. Wound
Repair Regen. 15:708–717. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kiritsy CP, Lynch AB and Lynch SE: Role of
growth factors in cutaneous wound healing: a review. Crit Rev Oral
Biol Med. 4:729–760. 1993.PubMed/NCBI
|
|
8
|
Moulin V: Growth factors in skin wound
healing. Eur J Cell Biol. 68:1–7. 1995.PubMed/NCBI
|
|
9
|
Obeyesekere MN, Zimmerman SO, Tecarro ES
and Auchmuty G: A model of cell cycle behavior dominated by
kinetics of a pathway stimulated by growth factors. Bull Math Biol.
61:917–934. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Andreeva V, Prudovsky I and Thomas M:
Stimulation of quiescent cells by individual polypeptide growth
factors is limited to one cell cycle. Eur J Cell Biol. 83:327–335.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Baserga R, Porcu P and Sell C: Oncogenes,
growth factors and control of the cell cycle. Cancer Surv.
16:201–213. 1993.PubMed/NCBI
|
|
12
|
Hunt T, Nasmyth K and Novak B: The cell
cycle. Philos Trans R Soc Lond B Biol Sci. 366:3494–3497. 2011.
View Article : Google Scholar
|
|
13
|
Loots MA, Lamme EN, Mekkes JR, Bos JD and
Middelkoop E: Cultured fibroblasts from chronic diabetic wounds on
the lower extremity (non-insulin-dependent diabetes mellitus) show
disturbed proliferation. Arch Dermatol Res. 291:93–99. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Werner S, Krieg T and Smola H:
Keratinocyte-fibroblast interactions in wound healing. J Invest
Dermatol. 127:998–1008. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fu X, Cheng B and Sheng Z: Growth factors
and wound healing: review and prospect in recent ten years.
Zhongguo Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 18:508–512.
2004.(In Chinese).
|
|
16
|
Eming SA, Krieg T and Davidson JM:
Inflammation in wound repair: molecular and cellular mechanisms. J
Invest Dermatol. 127:514–525. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mansbridge JN, Liu K, Pinney RE, Patch R,
Ratcliffe A and Naughton GK: Growth factors secreted by
fibroblasts: role in healing diabetic foot ulcers. Diabetes Obes
Metab. 1:265–279. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ghosh AK, Yuan W, Mori Y and Varga J:
Smad-dependent stimulation of type I collagen gene expression in
human skin fibroblasts by TGF-beta involves functional cooperation
with p300/CBP transcriptional coactivators. Oncogene. 19:3546–3555.
2000. View Article : Google Scholar
|
|
19
|
Moreo K: Understanding and overcoming the
challenges of effective case management for patients with chronic
wounds. Case Manager. 16:62–63. 67:2005
|
|
20
|
Mustoe T: Understanding chronic wounds: a
unifying hypothesis on their pathogenesis and implications for
therapy. Am J Surg. 187:S65–S70. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Loot MA, Kenter SB, Au FL, et al:
Fibroblasts derived from chronic diabetic ulcers differ in their
response to stimulation with EGF, IGF-I, bFGF and PDGF-AB compared
to controls. Eur J Cell Biol. 81:153–160. 2002. View Article : Google Scholar : PubMed/NCBI
|