Introduction
Prion diseases, also known as transmissible
spongiform encephalopathies (TSEs), are a group of transmissible
and rapidly progressive neurodegenerative diseases including
Creutzfeldt-Jakob disease (CJD), fatal familial insomnia (FFI) in
humans, bovine spongiform encephalopathy (BSE) in cattle, scrapie
in sheep and chronic wasting disease (CWD) in cervids, that can
affect a series of species in mammals (1). The key pathogens of prion diseases
are thought to be prions, which are completely different from all
known microorganisms since nucleic acids are not found in them
(2,3). Prion proteins are found naturally in
normal brains and certain other organs (4,5).
However, the accumulation of an alternative or abnormal isoform of
prion protein known as PrPSc, which is partially
resistant to the digestion of proteinase K (PK), leads to
neurodegeneration. PrPSc stimulates the conversion of
normal prion protein (PrPC) into nascent
PrPSc (6,7). Both PrPSc and its normal
form, PrPC, share identical amino acid sequences
(8); the differences between the
two proteins are in their secondary structure. Compared to
PrPC, PrPSc has a higher β-sheet and lower
α-helix content in terms of the PrP secondary structure (9,10).
Generally, prions selectively replicate themselves
in the central nervous system (CNS) (11,12), where there are high levels of
PrPC expression. Certain strains of prions can also
propagate in peripheral tissues, such as the lymph tissue (13). Usually, it is extremely difficult
to get prions to directly replicate themselves in cultured cells.
Several cell lines, mostly neuron-derived cells, however, have been
described as being able to host the replications of a few special
prion strains, either temporally or continually in vitro
(14,15). The SMB-S15 cell line was
originally established when it was cultured from the brain of a
mouse affected by the Chandler scrapie strain (16). SMB-S15 cells help scrapie prions
to replicate continuously in vitro and possess almost the
same biochemical characteristics as the original PrPSc
in brain tissues. However, the effect of the infectivity of the
cell culture-derived prions on experimental animals has not yet
been well documented.
To address this lacuna in the research, the lysates
of SMB-S15 cells were intracerebrally inoculated into three
different strains of mice, namely C57BL/6, Balb/c and CD1. We found
that typical experimental TSEs were induced in all challenged mice,
and similar incubation times were noted. Although the major
neuropathological abnormalities and biochemical features of brain
PrPSc were similar in all three strains of mice, the
clinical manifestations and PrPSc deposits in the
cerebellum regions of the tested rodents exhibited certain
differences.
Materials and methods
Cell culture
The cell line infected with the scrapie agent
Chandler and its cured cell line SMB-PS were obtained from The
Roslin Institute (Scotland, UK). SMB-S15 was originally taken from
the brain of a mouse infected by the Chandler scrapie strain
(17). SMB-PS denotes SMB cells
which have been permanently cured by pentosan sulfate (PS)
(16). Cells were cultured in
DMEM with 10% fetal calf serum, in an atmosphere with 5%
CO2 and at 33°C. Cells were collected, counted and
stored at −20°C for further study.
Animal bioassay
Approximately 1×108 SMB-S15 and SMB-PS
cells were homogenized in 2 ml phosphate-buffered saline (PBS; pH
7.4), respectively. We verified that the content of
PrPSc in the cell homogenates was comparable with that
in ME7- or 139A-infected brain homogenates by western blot analysis
(data not shown). Cell debris was removed with low-speed
centrifugation at 2,000 × g for 10 min, and the supernatants were
collected as inoculums prior to challenging. Five microliters of
SMB-S15 or SMB-PS cell homogenates were intracerebrally injected
into 3 to 4-week-old CD1, C57BL/6 and Balb/c mice, respectively.
Each of the 6 groups (the CD1, C57BL/6 and Balb/c mice injected
with either SMB-S15 or SMB-PS) consisted of 10 female mice. Before
the injection, all mice were narcotized with halothane. The animals
were monitored twice a week before the appearance of clinical
symptoms by experienced staff, but once per day after the
appearance of clinical symptoms, until the animals died or were
sacrificed. The clinical symptoms and signs were scored as
previously described (18). The
incubation time was calculated from the inoculation to the onset of
clinical manifestations, and clinical course was evaluated from the
onset of clinical manifestations to death at the terminal stage of
the disease. The main clinical manifestations at the end of the
disease included progressive ataxia, sluggishness, loss of weight
and extreme emaciation. At the end of the clinical phase, the
animals were sacrificed using ether and exsanguinated, and the
brains were surgically removed from the mice. Three brains were
fixed with formalin, and the others were stored at −80°C for
further analyses. In addition, two untreated 3 to 4-week-old mice
from each strain were sacrificed using ether, and 200 µl
blood was collected and anticoagulated with
ethylenediaminetetraacetic acid (EDTA) for gene sequencing.
For successive passage, each brain from 3
SMB-S15-inoculated strains and each from SMB-PS-inoculated strains
were homogenized (1:10, w/v). Before injection, western blot
analysis indicated that the 3 S15-inoculated brains were positive
for PrPSc, while the SMB-PS-inoculated brains were negative. One
microliter of each brain homogenate was intracerebrally injected
into corresponding 3 to 4-week-old CD1, C57BL/6 and Balb/c mice.
Each group consisted of 10 female mice. These mice were termed
second passage SMB-inoculated mice.
Polymerase chain reaction (PCR) protocol
for the prion protein (PRNP) gene
Total DNA was extracted from the blood of the mice
(the 2 untreated mice from each strain) with a DNeasy Blood &
Tissue kit (cat. no. 69504; Qiagen, Hilden, Germany) according to
the manufacturer's instructions. The mouse PRNP gene was
amplified using PCR in a Bio-Rad S1000 Thermal Cycler (Bio-Rad,
Hercules, CA, USA). Two pairs of primers were used for better
accuracy. The information of the primers was as follows: pair 1
forward, TCAGCCT AAATACTGGGCAC and reverse, AGATGAGGAGGATG ACAGGA;
and pair 2 forward, GCCTAAATACTGGGC ACTGATAC and reverse,
AGGAGATGAGGAGGATGACA; the size of both amplicons was 791 bp. The
reaction mixtures consisted of a total of 50 µl, containing
1 µl DNA, 20 pmol sense and antisense primers, 21 µl
RNase-free water and 25 µl 2X Taq Master Mix (CW0682; CWBio,
Beijing, China). The touchdown method was adopted to increase the
specificity of the PCR products. Details of the PCR conditions are
as follows: denaturing at 94°C for 40 sec, annealing at 59°C for 45
sec with a decrease of 0.5°C every cycle in the first 8 cycles and
55°C for the other 30 cycles, and a final extension at 72°C for 55
sec. All PCR assays were carefully carried out in the PCR
laboratory in four separate rooms to avoid DNA contamination.
Direct sequencing
The PCR products were analyzed in 1.2% agarose gel
and recovered from gel with a QIAquick Gel Extraction kit (cat. no.
28706; Qiagen) according to the manufacturer's instructions. Direct
sequencing was performed using the same PCR primers and an ABI
Prism™ 3730XL DNA Analyzer.
Preparation of brain homogenates and PK
digestion
The brain samples of the SMB-S15- and
SMB-PS-inoculated mice were homogenized in 10% lysis buffer (100 mM
NaCl, 10 mM EDTA, 0.5% Nonidet P-40, 0.5% sodium deoxycholate, and
10 mM Tris, pH 7.5) according to a previously described protocol
(19). Briefly, tissue debris was
removed with low-speed centrifugation at 2,000 × g for 10 min, and
the supernatants were collected. To detect the presence of
PK-resistant PrPSc in brain tissues, the brain
homogenates were firstly digested with a final concentration of 50
µg/ml PK at 37°C for 60 min prior to western blot analyses.
To evaluate the PK resistances of PrPSc from the three
mouse strains, the brain homogenates of the SMB-S15-inoculated mice
were treated with different amounts of PK (70663-4; Merck KGaA,
Darmstadt, Germany) at final concentrations of 100, 200, 500,
1,000, 2,000 and 5,000 µg/ml at 37°C for 60 min. PK
digestion was terminated by heating the samples at 100°C for 10
min.
Western blot analysis
Aliquots of brain homogenates were separated on 15%
SDS-PAGE and electroblotted onto nitrocellulose membranes using a
semi-dry blotting system (Bio-Rad). Membranes were blocked with 5%
(w/v) non-fat milk powder (NFMP) in 1X Tris-buffered saline
containing 0.1% Tween-20 (TBST) at room temperature for 1 h and
probed with anti-PrP monoclonal antibody (mAb 6D11, sc-58581; Santa
Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4°C overnight.
After washing with TBST, blots were incubated with horseradish
peroxidase (HRP)-conjugated goat anti-mouse (cat. no. 31460; Thermo
Fisher Scientific, Waltham, MA, USA) at room temperature for 2 h.
Blots were developed using an enhanced chemiluminescence system
(ECL) (NEL103E001EA; PerkinElmer, Waltham, MA, USA) and visualized
on autoradiography films. Images were captured by the ChemiDoc™
XRS+ Imager (Bio-Rad).
Deglycosylation assay
After being mixed with equal volumes of glycoprotein
denaturing buffer (New England Biolabs, Ipswich, MA, USA), various
PK-treated brain homogenates were heated at 100°C for 10 min.
Subsequently, 50 mM sodium phosphate, pH 7.5, containing 1% NP-40
and 2 µl N-glycosidase F (1,800,000 U/mg; New England
Biolabs) were added to the samples, and the mixtures were incubated
at 37°C for 2 h. PrP signals in each preparation were detected by
western blot analysis, as described above.
Pathological assays
Brain tissues of the differently inoculated mice
were fixed in 10% buffered formalin solution. Before histological
processing, all fixed tissues were immersed in 98% formic acid for
at least 1 h for inactivation and paraffin-embedded. The
paraffin-embedded sections (5 µm thickness) were then
subjected to conventional staining with hematoxylin and eosin
(H&E). The spongiform degeneration in the brain regions
infected with various scrapie strains was monitored using a light
microscope (BX41; Olympus, Tokyo, Japan), and the severity and
distribution of vacuolation were measured according to previously
described protocol (20).
Briefly, 0 denotes no lesions; 0.5, minimum vacuolation (2–3
vacuoles in half an ×40 objective field); 1.0, little vacuolation
(3–5 vacuoles in half a field); 2.0, moderate vacuolation (several
vacuoles evenly scattered); 3.0, extensive vacuolation (many
vacuoles distributed in half a field); and 4.0, severe vacuolation
(numerous vacuoles, often coalescing).
Immunohistochemical (IHC) assays
Paraffin-embedded sections (5 µm thickness)
of brain tissues were prepared, and IHC assays were performed
according to protocol described in a previous study (18). Prior to the staining with PrP mAb,
brain sections were treated with 6M GdnHCl at 4°C for 2 h. In
sections, endogenous peroxidases were quenched in 3%
H2O2 in methanol for 15 min, and then
sections were pretreated for enzyme digestion antigen retrieval for
1 min. After blocking in 1% normal goat serum, the sections were
incubated at 4°C overnight with anti-PrP mAb (6D11), rabbit
anti-glial fibrillary acidic protein (GFAP) polyclonal antibody
(pAb; Boster Biological Tech Ltd.) or rabbit anti-Iba1 pAb,
respectively. The sections were then incubated with HRP-conjugated
goat anti-mouse or rabbit secondary antibody (cat nos. 31460 and
31430; Thermo Fisher Scientific) at 37°C for 60 min, and visualized
by incubation with 3,3-diaminobenzidine tetrahydrochloride (DAB).
The sections were counterstained with hematoxylin, dehydrated and
mounted in permount (ZLI-9559, ZSGB-BIO, Beijing, China).
Statistical analysis
Statistical analysis was performed using SPSS 17.0
statistical package (SPSS, Inc., Chicago, IL, USA). Quantitative
analysis of the western blots was carried out using ImageJ
software. The gray values of each target blot were evaluated.
Statistical analyses were performed using the Kruskal-Wallis test
and Student's t-test, as appropriate. A P-value <0.05 was
considered to indicate a statistically significant difference.
Ethical statement
The present study was approved by the Ethical
Committee of the National Institute for Viral Disease Control and
Prevention, China (CDC) under protocol no. 2009ZX10004-101.
Results
Homogeneity of the PRNP gene in three
different strains of mice
C57BL/6, CD1 and Balb/c mice are commonly used
experimental mice strains with clearly distinct phenotypes. In
order to study the homogeneity of the PRNP gene in the three
different types of mice, genomic DNA was extracted from peripheral
blood cells, and the PRNP genes were obtained using a
specific PCR technique. Sequencing assays of the 791-bp PCR
products revealed 100% homogeneity between the C57BL/6 and Balb/c
mice, which were also identical to the mouse PRNP sequences
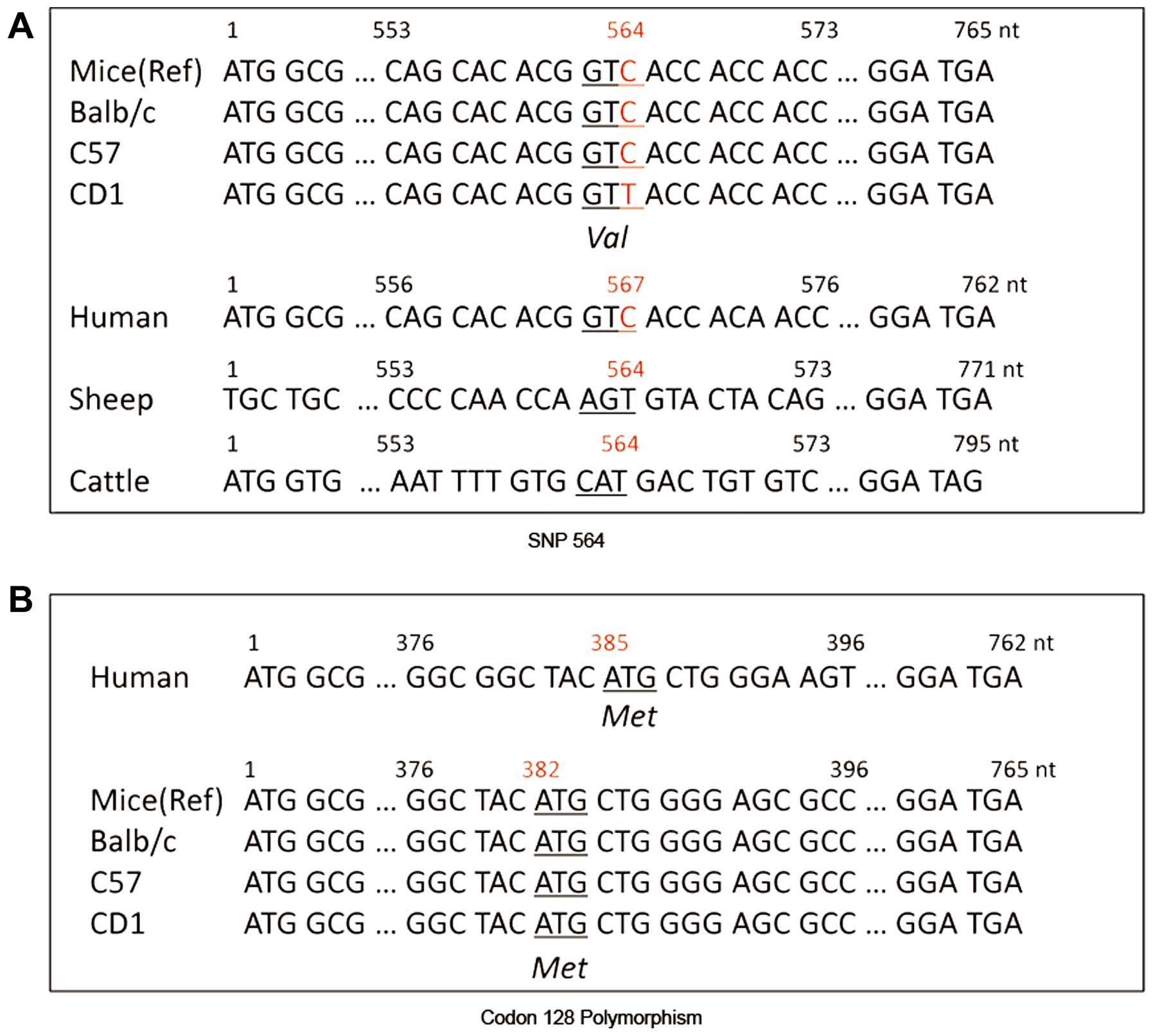
found on NCBI (CT010345.1), whereas only one different nucleotide
at the position of nt 564 (C-T exchange) in CD1 mice was noted
(Fig. 1A). According to the codon
table, it was confirmed that this C564T variation in CD1 mice was a
synonymous single nucleotide polymorphism (SNP) which did not cause
any change of the encoded amino acid (valine). The polymorphisms of
codon 128 of PrP proteins of those three strains of mice, which
corresponded to the polymorphism of codon 129 in humans, were all
methionine/methionine (Met/Met) homozygote (Fig. 1B). These data indicate a
homogeneity in the PRNP gene among the three strains of
mice.
Inoculation of SMB-S15 cell lysates into
CD1, Balb/c, and C57BL/6 mice induced experimental TSEs
Cell lysates of SMB-S15 with detectable PK-resistant
PrPSc were intracerebrally inoculated into 10 CD1,
Balb/c and C57BL/6 mice, respectively, and cell lysates of SMB-PS
without detectable PrPSc were also intracerebrally
inoculated into the mice of each strain as controls. One mouse in
each SMB-S15-inoculated group died within a week of inoculation,
and we considered this to be due to the acute mechanical brain
damage suffered during inoculation. Animals infected with SMB-S15
lysates began to exhibit abnormal signs 165 days after inoculation,
and the onset times of diseases within the groups, or among the
different strains, were quite similar. The average incubation times
of CD1, Balb/c and C57BL/6 mice were 175.4±1.0, 175.3±1.2 and
172.8±1.8 days, respectively, and no statistical difference was
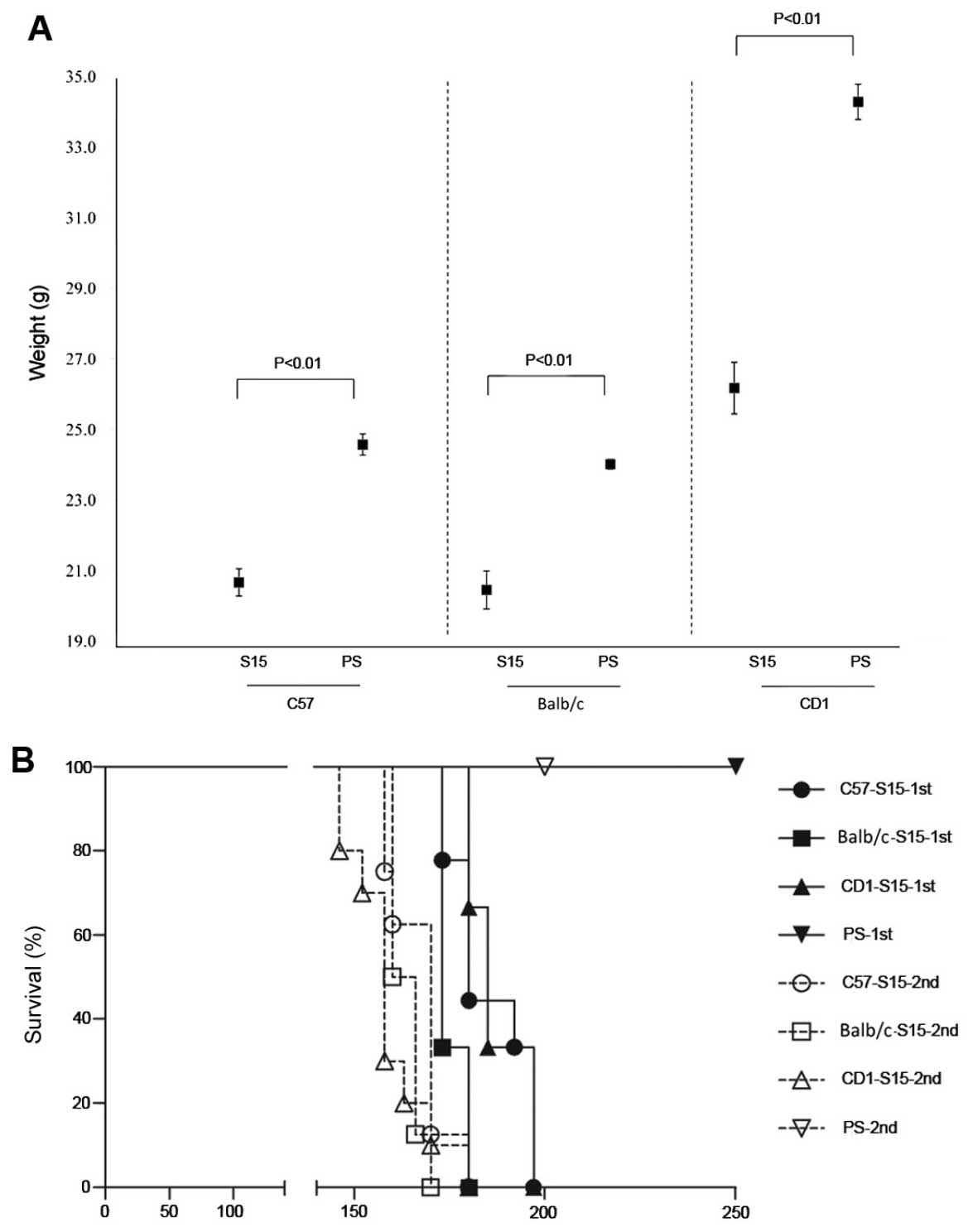
noted (P=0.382) (Table I). Severe
weight loss, extreme emaciation and sluggishness were observed in
all infected animals. Compared with the individual
SMB-PS-inoculated mice at the same survival time,
SMB-S15-inoculated mice were of obviously lower weight. The average
weight (in 95% CI) of the S15-infected CD1, Balb/c and C57BL/6 mice
dropped down to 76, 85 and 84%, respectively, thus exhibiting
significant differences (see Table
I and Fig. 2A). Ataxia (such
as moving unsteadily with uncoordinated movements) was easily
identified in the S15-inoculated C57BL/6 and Balb/c mice, but less
frequently in CD1 mice. A small proportion of C57BL/6 and Balb/c
mice, but not CD1 mice, trembled (Table I). The clinical manifestations
progressed so quickly that almost all S15-treated mice died within
7–20 days after the onset of symptoms. The average clinical courses
of S15-inoculated mice were 11.9±5.1 (CD1), 11.4±4.6 (Balb/c) and
12.7±6.6 (C57BL/6) days (Table
I). The survival times of the S15-inoculated mice were
187.3±2.5 (CD1), 186.8±2.6 (Balb/c) and 185.4±3.4 (C57BL/6) days,
respectively (Fig. 2B). Animals
inoculated with SMB-PS lysates did not show any abnormality until
the end of observation (>250 days after inoculation).
 | Table IClinical characteristics and brain
PrPSc deposits of SMB-S15-inoculated C57BL/6, CD1 and
Balb/c mice. |
Table I
Clinical characteristics and brain
PrPSc deposits of SMB-S15-inoculated C57BL/6, CD1 and
Balb/c mice.
| Mice | No. of
ill/inoculated mice | Incubation
time
(means ± SE)
(days) | Clinical
course
(means ± SD)
(days) | Major clinical
manifestations/No. of positive/inoculated mice
| No. of
PrPSc positive/inoculated mice |
|---|
| Weight loss | Ataxia | Sluggishness | Trembling |
|---|
| C57BL/6 | 9/9 | 165–180
(172.8±1.8) | 7–24
(12.7±6.6) | 9/9 | 7/9 | 8/9 | 3/9 | 9/9 |
| Balb/c | 9/9 | 173–180
(175.3±1.2) | 7–17
(11.4±4.6) | 9/9 | 7/9 | 7/9 | 2/9 | 9/9 |
| CD1 | 9/9 | 173–180
(175.4±1.0) | 7–20
(11.9±5.1) | 9/9 | 4/9 | 7/9 | 0/9 | 9/9 |
Successive inoculation of brain
homogenates of SMB-S15-infected mice into various types of
mice
In order to successively passage the scrapie agents
from the brains of SMB-S15-infected mice (first passage) onto the
same strain of mice, 10% brain homogenates with detectable
PK-resistant PrPSc were intracerebrally inoculated into
ten CD1, Balb/c and C57BL/6 mice, respectively. Brain homogenates
of SMB-PS-inoculated mice without detectable PrPSc were
also intracerebrally inoculated into the mice of each strain as
controls. Two mice from the groups of C57BL/6 and Balb/c died
within a week of inoculation, which was attributed to acute
mechanical brain damage. The mice of second passage showed abnormal
signs 140 days after inoculation. The clinical manifestations of
the second passage S15-inoculated mice were almost the same as
those of the first passage animals: among them, loss of body
weight, ataxia and sluggishness were the most noticeable signs. The
average incubation times of the second-passage S15-inoculated CD1,
Balb/c and C57BL/6 mice were 148.0±2.3, 146.8±1.8 and 153.0±2.2
days, respectively, and no statistical difference between the three
strains of mice was noted (P=0.166) (Table II). However, compared with those
of first passage mice, all second passage mice exhibited
significantly shorter incubation times. The average clinical
courses of second passage mice were 10.9±4.2 (CD1), 16.8±3.0
(Balb/c) and 14.0±3.2 (C57BL/6) days, respectively. The survival
times of the second passage mice were significantly shorter than
those of the first passage mice: 158.9±3.3 (CD1, P<0.001),
163.5±1.4 (Balb/c, P<0.001) and 167.0±2.7 days (C57BL/6,
P=0.001), respectively (Fig. 2B).
Moreover, no abnormality was noted in PS-inoculated animals until
the end of observation (>200 days after inoculation).
 | Table IIClinical characteristics and brain
PrPSc deposits of second passage of SMB-S15-inoculated
C57BL/6, CD1 and Balb/c mice. |
Table II
Clinical characteristics and brain
PrPSc deposits of second passage of SMB-S15-inoculated
C57BL/6, CD1 and Balb/c mice.
| Mice | No. of
ill/inoculated mice | Incubation
time
(means ± SE)
(days) | Clinical
course
(means ± SD)
(days) | Major clinical
manifestations/No. of positive/inoculated mice
| No. of
PrPSc positive/inoculated mice |
|---|
| Weight loss | Ataxia | Sluggishness | Trembling |
|---|
| C57BL/6 | 8/8 | 146–160
(153.0±2.2) | 12–20
(14.0±3.2) | 8/8 | 8/8 | 8/9 | 2/8 | 8/8 |
| Balb/c | 8/8 | 140–152
(146.8±1.8) | 14–20
(16.8±3.0) | 8/8 | 7/8 | 7/8 | 1/8 | 8/8 |
| CD1 | 10/10 | 140–160
(148.0±2.3) | 6–20
(10.9±4.2) | 10/10 | 8/10 | 8/10 | 1/10 | 10/10 |
Presence of PK-resistant PrPSc
in the brain tissues of S15-inoculated mice
To assess the presence of PrPSc in the
brains of S15-inoculated mice, the brain homogenates of all
diseased animals of the first passage were subjected to PK
digestion and subsequently to western blot analysis with
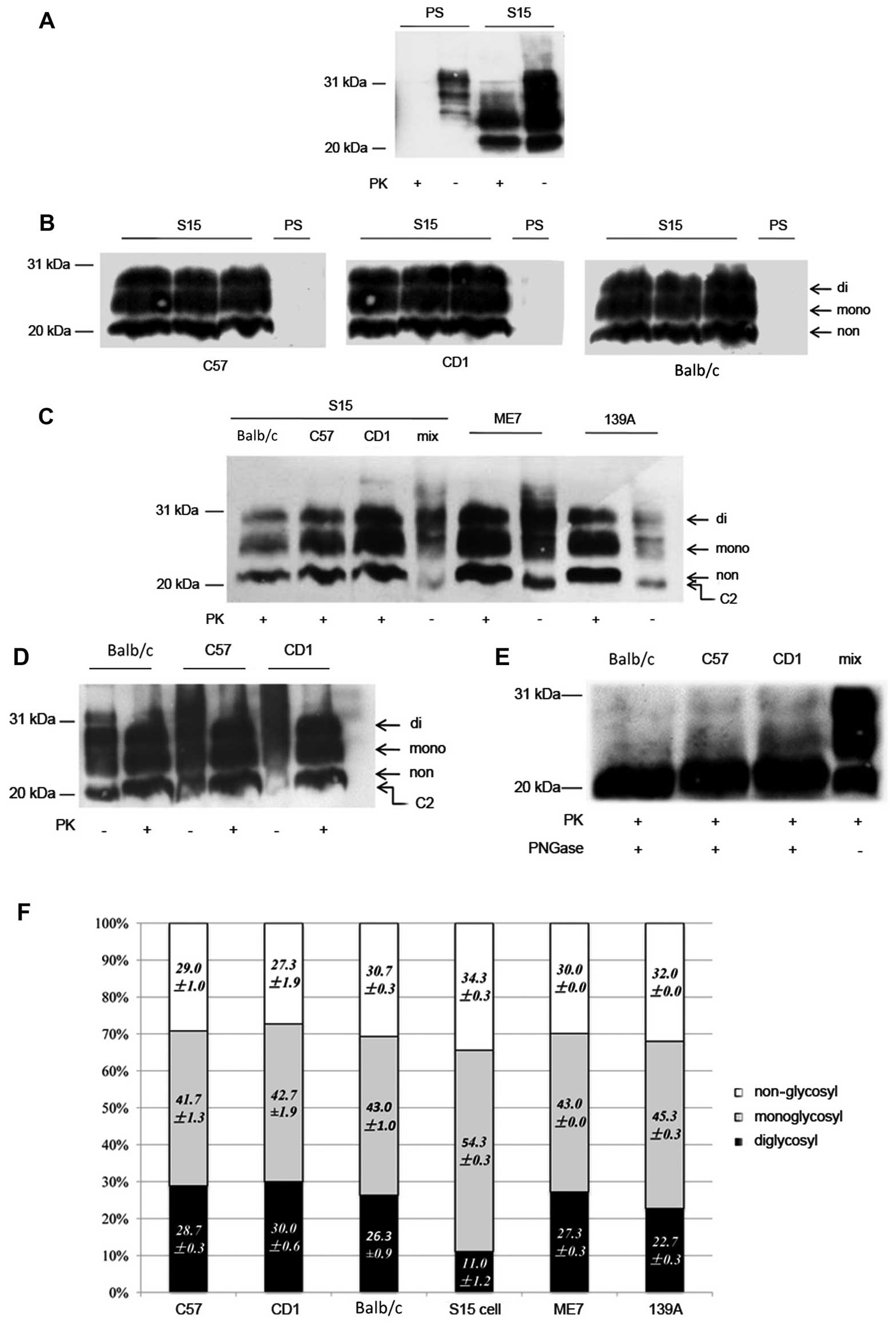
PrP-specific mAb 6D11. As with the SMB cell lysates (Fig. 3A), PK-resistant PrP signals were
detected in the brain homogenates of all diseased mice of three
different strains (Table I),
which presented three predominant bands and migrated from 21–27 kDa
(Fig. 3B). On the contrary, no
trace of PK-resistant PrP signal was observed in the brain tissues
of SMB-PS inoculated mice, regardless of whether they were CD1,
Balb/c or C57BL/6 mice.
To address the similarity in electrophoresis and
glycosylation of PrPSc molecules in the brains of the
strains of mice inoculated with SMB-S15 cell lysates, the
PK-treated and PK-untreated brain homogenates of the three
different types of mice were separated on one SDS-PAGE together
with those of scrapie strains 139A- and ME7-infected C57BL/6 mice
and evaluated by PrP-specific western blot analysis. A band of
roughly 21-kDa was observed in all preparations of S15-inoculated
mice that had not been treated with PK, which was located in the
same location as those of 139A- and ME7-infected mice (Fig. 3C and D), possibly indicating that
the same size C2 fragments of PrP were generated in brains infected
with those three scrapie strains. Three PK-resistant PrP bands in
three strains of S15-inoculated mice mobilized at the same
positions as those in 139A- and ME7-infected mice, in which the
monoglycosyl PrPSc was the predominant form, followed by
non-glycosyl and diglycosyl PrPSc (Fig. 3C and D). Further deglycosylation
of the PrPSc molecules in the brains of S15-inoculated
C57BL/6, CD1, Balb/c mice with PNGase F revealed a signal
PrP-specific band that was located in the same positions (Fig. 3E).
Calculating the relative gray values of each
glycosyl PrPSc band in the western blots showed that the
percentages of di-, mono- and non-glycosyl forms in the brains of
three S15-inoculated mice were 29, 42 and 29% in C57BL/6; 30, 43
and 27% in CD1; and 26, 43 and 31% in Balb/c mice. Compared with
the glycosylating distributions of PrPSc molecules in
139A-infected mice (23, 45 and 32%) and ME7-infected mice (27, 43
and 30%). No significant differences were noted between the three
strains of S15-inoculated mice or between the mice infected with
139A, ME7 and S15 (Fig. 3F).
PrPSc molecules in the brain
tissues of SMB-S15-infected mice possess strong PK-resistance
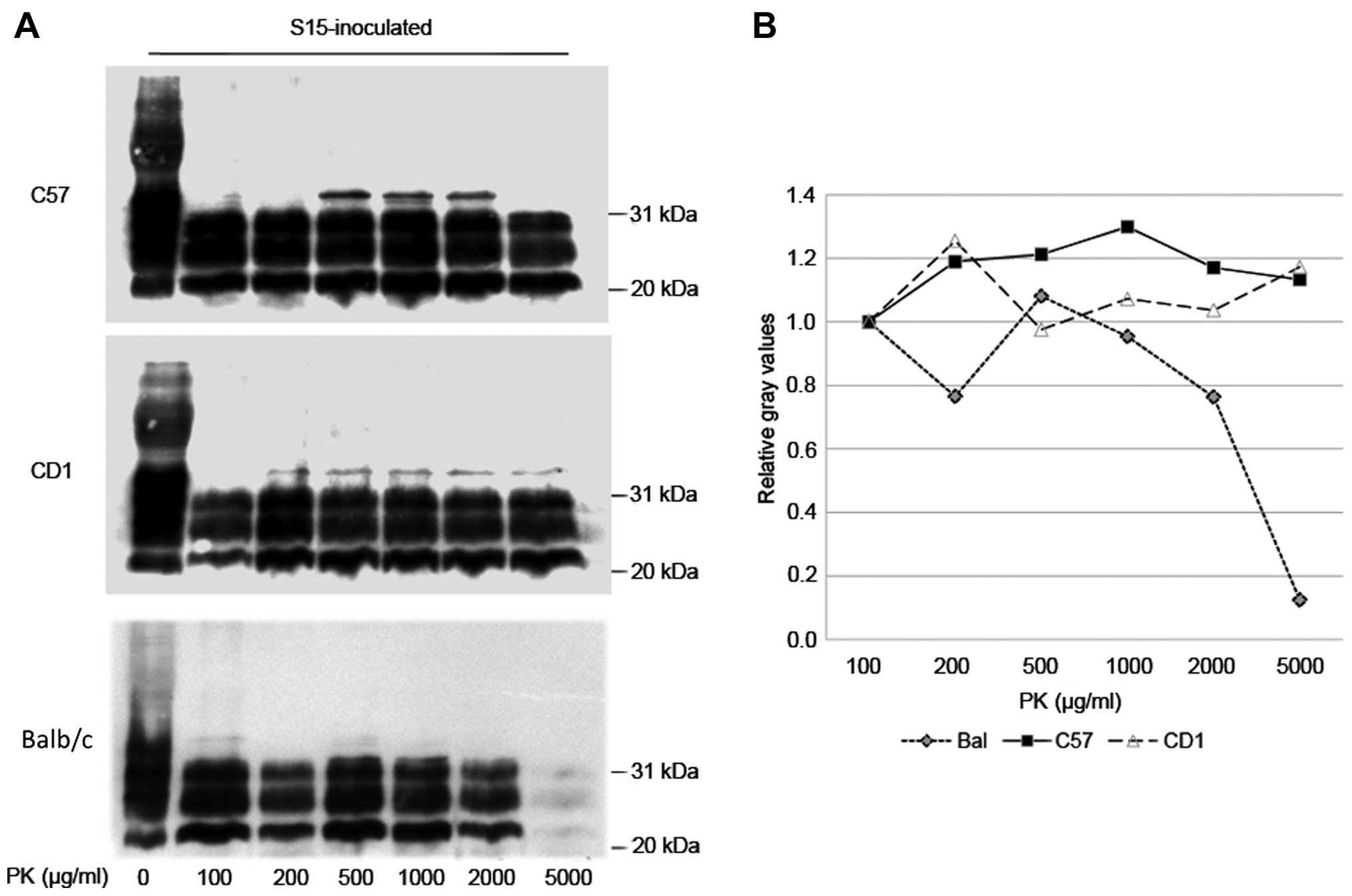
To compare the potential differences in the levels
of PK resistance of PrPSc in the brains of
SMB-15-inoculated C57BL/6, CD1 and Balb/c mice, the brain
homogenates from 3 randomly selected mice of first passage were
selected from each group and pooled as the representative samples.
The samples were exposed to digestion with different amounts of PK,
ranging from 100–5,000 µg/ml. Western blot analysis revealed
clear and similar PK-resistant PrP signals in all three types of
mice in the preparations treated with a low concentration of PK
(from 100–2,000 µg/ml). However, as the concentration of PK
increased to 5,000 µg/ml, the PrPSc signals in
Balb/c mice clearly became weaker, whereas PrPSc in the
other two strains of mice remained almost unchanged (Fig. 4). These results indicate the
strong PK resistance of PrPSc in the brains of
S15-inoculated mice. The PrPSc formed in the infected
Balb/c mice seems to have slightly weaker PK-resistant properties
than PrPSc in C57BL/6 and CD1 mice.
Large quantities of PrPSc are
deposited in the brains of the infected mice
In order to examine the characteristics of
PrPSc deposits in the brains of mice infected with
SMB-S15, brain sections from SMB-S15-inoculated mice of the first
passage were analyzed by PrPSc-specific IHC assays.
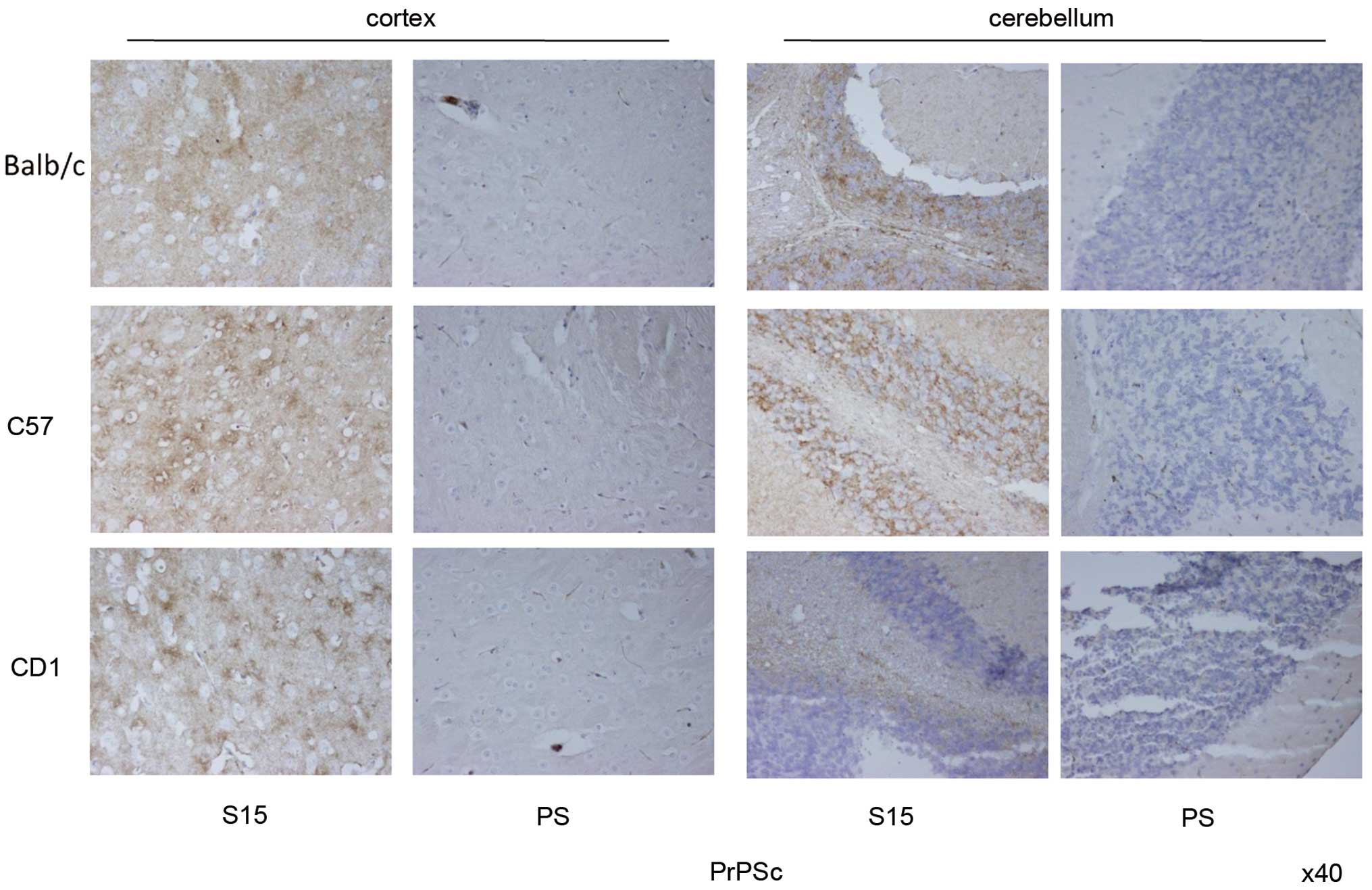
After treatment with GdnHCl, large amounts of brown
PrPSc deposits were detected in the cortex regions of
S15-inoculated C57BL/6, CD1 and Balb/c mice, accompanied by various
sizes of vacuoles, whereas no PrPSc signal was observed
in the cortex tissues of SMB-PS inoculated mice (Fig. 5). PrPSc in the
S15-infected mice mainly appeared in a dispersed manner. In the
regions with more vacuoles, PrPSc accumulated and formed
relatively dark-stained particles around the vacuoles. Three
S15-inoculated mice presented quite similar PrPSc
deposit features in the cortex, regardless of the signal intensity
or accumulating type. Obvious PrPSc signals were also
observed in the cerebellum regions of S15-inoculated mice, which
presented as numerous granular structures on a background of
dispersive deposits, particularly in the regions of the gray layer.
Noticeably, the PrPSc deposits in the cerebellum of the
infected CD1 mice were significantly less than the other two types
of mice (Fig. 5).
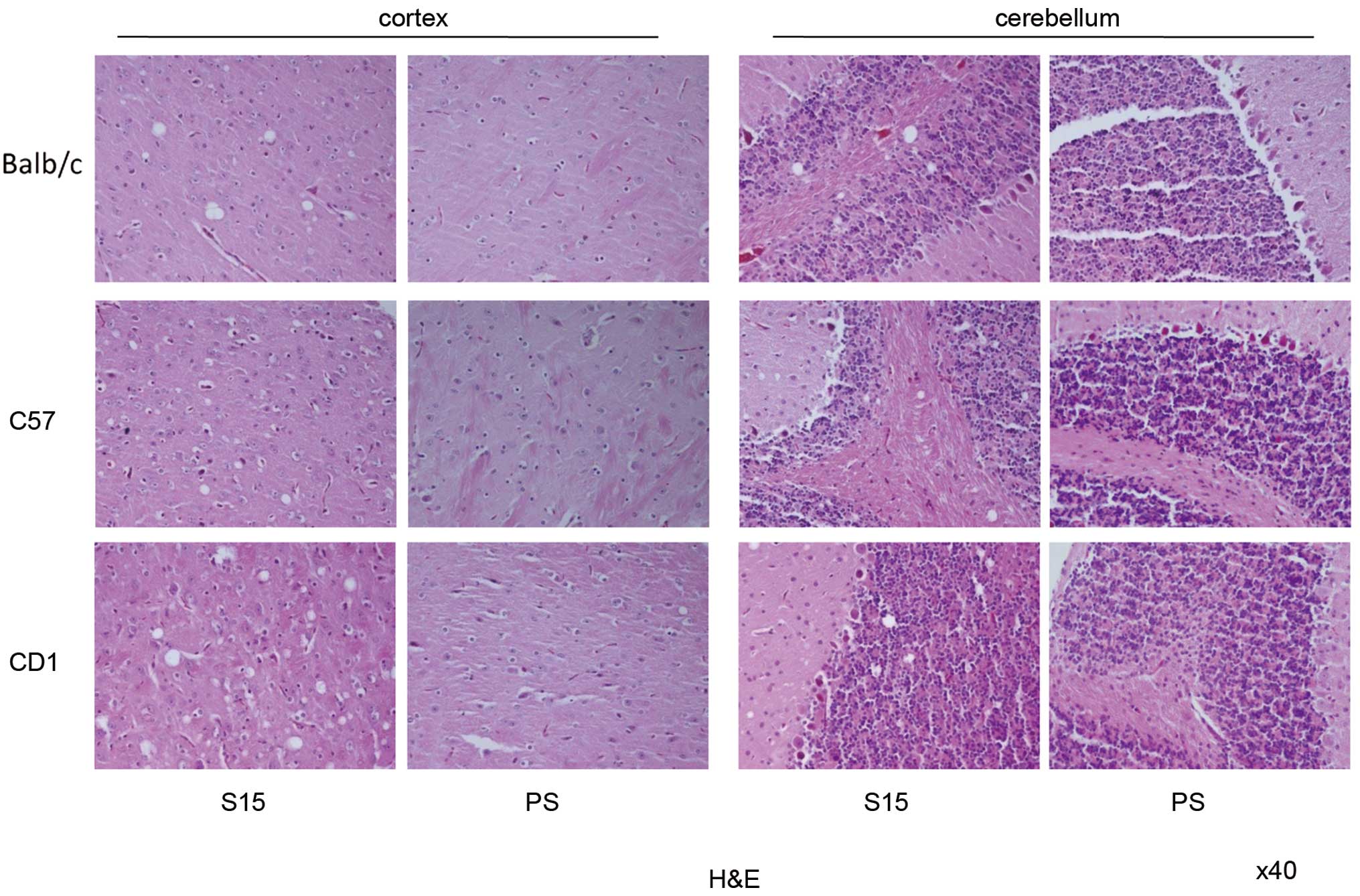
Similar pathological changes noted in the
brains of SMB-S15 infected mice
To study the neuropathological characteristics of
infected mice, brain sections of the S15- and PS-inoculated
C57BL/6, CD1 and Balb/c mice of the first passage were analyzed by
H&E staining, and GFAP- and Iba1-specific IHC tests. Numerous
various-sized vacuoles were observed in the brain tissues of
S15-inoculated mice, but not in those of PS-inoculated mice
(Fig. 6). The majority of the
vacuoles were round or oval and varied in terms of size. Generally,
spongiform changes in the cortex were more obvious than those in
the cerebellum. CD1 mice seemed to have more and larger vacuoles
than the other two groups of mice. Calculations of the severity and
distribution of vacuoles in the cortex and cerebellum of the
infected mice revealed that the average lesion scores in the cortex
of C57BL/6, CD1 and Balb/c mice were 3.3, 3.7 and 3.0,
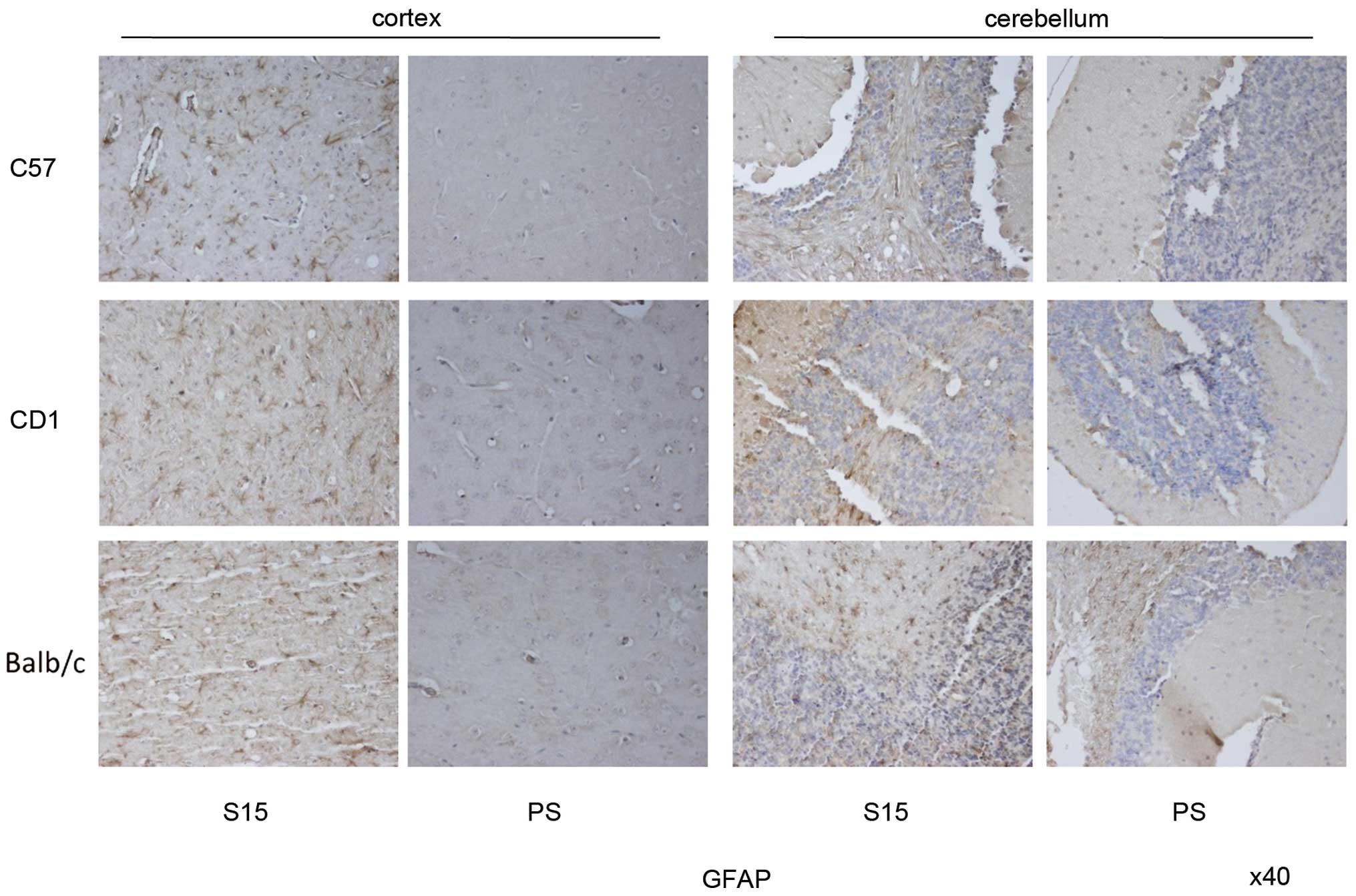
respectively, while those in cerebellum were all 0.5. Abundant,
large GFAP positive-stained astrocytes were noted in the brain
tissues of all three strains of infected mice, and astrogliosis in
the cortex region was more notable than that in cerebellum region
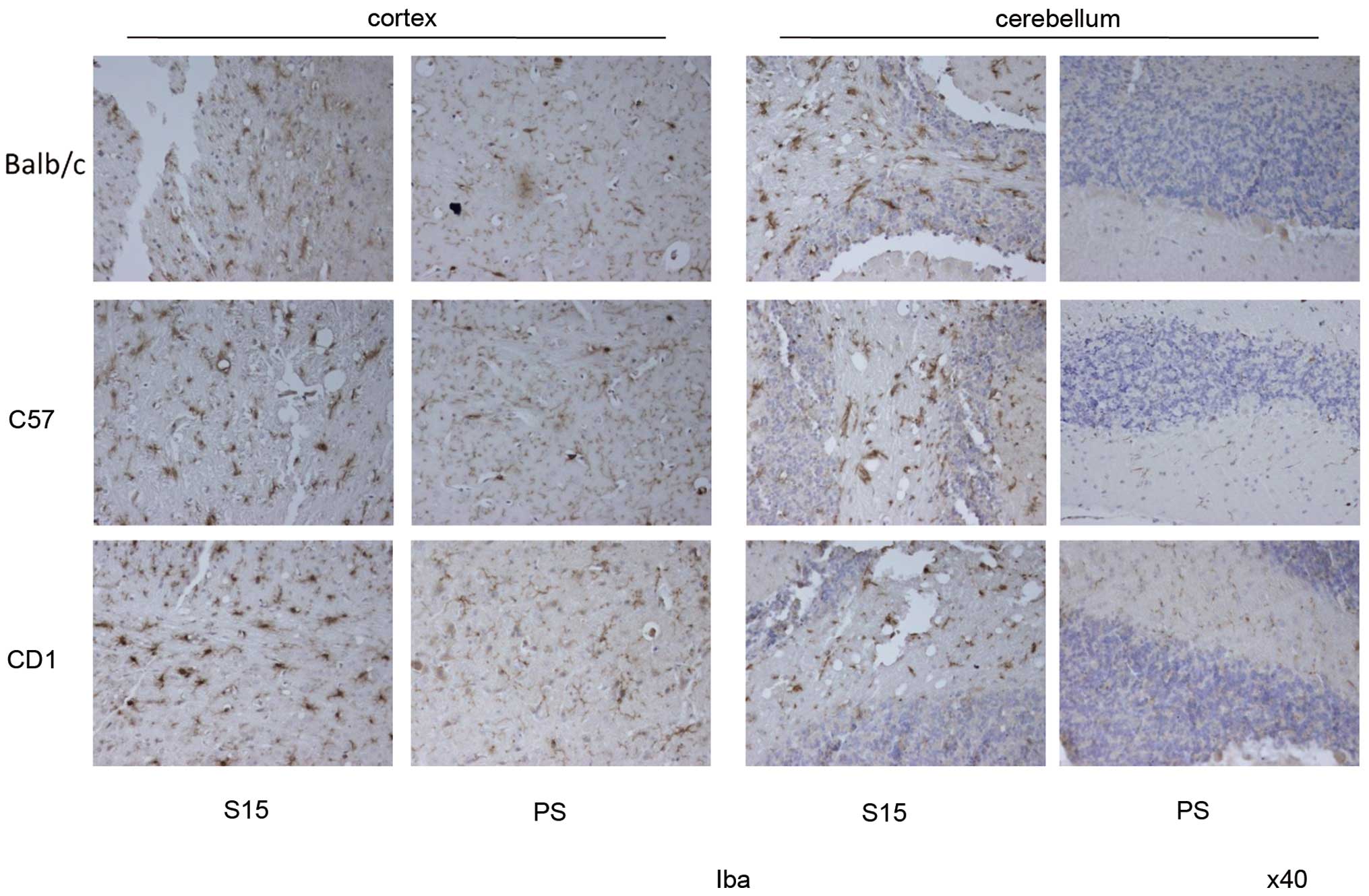
(Fig. 7). Iba1-specific IHC
assays identified abundances of microglia with much larger round-
or amoeboid-shaped cell bodies in the sections of S15-infected
mice, and no significant difference between the cortex and
cerebellum was noted between the three strains of mice (Fig. 8). These data confirm that all
three types of mice infected with SMB-S15 lysates exhibit typical
neuropathological abnormalities representative of experimental
TSEs
Discussion
The SMB-S15 cell line was established originally
using a culture from the brain of a mouse affected by the Chandler
scrapie strain. This cell line consistently expresses
PrPSc during cell passage, whereas the SMB-PS cell line
is an SMB-S15 cell line cured by PS, in which PrPSc is
completely removed. In the present study, we successfully set up
three experimental scrapie infections with SMB-S15 cell lysates
using three different phenotypic mice, C57BL/6, CD1 and Balb/c,
respectively, and gained pathogenic and pathological confirmation.
The infectivity of the brain homogenate of SMB-inoculated mice has
been also confirmed in this study by successively inoculating three
strains of mice. Moreover, the mice inoculated with the lysates of
SMB-PS cells were all healthy, with no detectable abnormality in
the brains. Thus, the findings of this study verify that the prion
agent in SMB-S15 remains ineffective in experimental mice after
long-term propagation in vitro.
Chandler is a commonly used scrapie strain, which
was originally adapted using CD1 mice decades ago (21). The incubation time of strain
Chandler via intracerebral inoculation is 166±5 days (22). Coincidentally, in a previous study
it was noted that three strains of mice infected with SMB-S15
lysates had similar incubation times, approximately 175 days in the
first passage, highlighting again that the incubation time is
strongly influenced by the original prion strain (23). Furthermore, as demonstrated in the
present study, the incubation times of the second passages of all
infected mice were shorter than the first passages. It has been
previously observed, when the TSE agents are passaged in new,
sensitive host species, that the incubation period will shorten
during the first passage and become stable in the later passages
(24). Although SMB cells are
mouse neuron-derived cells, the relatively shorter incubation times
of the second passages of S15-infected mice noted in the present
study indicate that the scrapie agents propagating in the cultured
cells in vitro need to adapt a little when replicating in
the brain tissues of the same species in vivo. By contrast,
our previous studies have verified that interspecies transmissions
of mouse-adapted scrapie strains 139A and ME7 into hamsters require
much longer incubation periods (395 days in 139A and 496 days in
ME7), but the incubation times become much shorter in the
successive passages (176 days in 139A and 183 days in ME7), which
are comparable with that of their parent mouse strains, as noted in
a previous study on C57BL/6 mice (Shi et al, unpublished
data). Obviously, TSE agents need much longer times for adaption in
different species hosts.
The main biochemical characteristics of
PrPSc, such as the electrophoretic positions, the
glycosylating patterns, PK resistance and the length of C2
fragments, in the brains of three strains of the infected mice are
quite comparable. Furthermore, the neuropathological features, such
as PrPSc deposits, spongiform degeneration, reactive
gliosis and activated microglia, are also similar in the three
types of infected mice. However, the molecular profiles of
PrPSc and some neuropathological features change during
interspecies transmission and were maintained stably afterwards
(24). This implies that prions
possess stable pathogenic and pathological features when they adapt
in a particular species, regardless of whether they propagate in
vivo or in vitro.
CD1, C57BL/6 and Balb/c mice are distinct phenotypic
animals, with different color body hair (white in CD1 and Balb/c,
and black in C57BL/6) and body weight (CD1 mice are clearly larger
than Balb/c and C57BL/6). However, the induced experimental scrapie
in those mice, regardless of clinical or pathological aspects, is
quite similar. In the present study, PRNP gene analysis
verified the level of homogeneity between the three strains of
mice, with only one nucleotide difference (C564T) in CD1 mice but a
synonymous SNP. The polymorphisms of codon 128, corresponding to
codon 129 in humans, of three types of mice are all Met/Met
homozygote. The PRNP similarities in the three types of mice
provide genetic support for the theory that there are similar
phenotypes of experimental scrapie after inoculation with the same
scrapie agent. On the other hand, our data in the present study
also highlight that no other factor from the host side, except
PRNP, influences susceptibility to prion infection.
In addition to severe loss of weight, extreme
emaciation and sluggishness, that were commonly seen in all
infected animals, ataxia and trembling were also frequently
identified in the S15-inoculated C57BL/6 and Balb/c mice, but much
less frequently in CD1 mice. Interestingly, the deposits of
PrPSc in the cerebellum of the infected CD1 mice were
also significantly less than in the other two types of mice. As
well as the difference in PrPSc deposits in the
cerebellum, other neuropathological abnormalities, including
spongiform degeneration, gliosis and activated microglia, were also
comparable between the three types of the infected mice. Thus, it
seems that the fewer cerebellum-associated symptoms in CD1 mice are
related to less deposition of PrPSc in the cerebellum
region. However, the exact mechanism for such diversity remains
unknown. One may postulate that there are some slight differences
in the brain microenvironments of those three strains of mice. High
levels of microglia proliferation in S15-infected mice, which have
been repeatedly observed in the brains of naturally occurring
sporadic CJD (25) and
experimentally scrapie-infected rodents (26), reflect the activation of the
innate immune system during prion infection.
The electrophoresis and glycosylation patterns of
PrPSc are used as indexes for distinguishing prion
strains (27). The brain
PrPSc levels from three different strains of
S15-infected mice demonstrate the exact same electrophoretic and
glycosylation profiles, and profile patterns have also been noted.
The stable electrophoresis and glycosylation patterns of
PrPSc from brain to cultured cells (Chandler strain to
SMB-S15 cells) and from cells to brain (SMB-S15 cells to
S15-inoculated mice) supply strong molecular evidence for the
maintenance of prion-strain characteristics during passage in the
same biological species. Moreover, the PrPSc molecules
from three S15-infected mice reveal similar electrophoresis and
glycosylation patterns as the PrPSc molecules in the
brains of two other mouse-adapted stains, 139A- and ME7-infected
mice. In our previous study, it was proposed that the glycosylating
profiles of PrPSc are altered during interspecies
transmissions of scrapie agents 139A and ME7 from mouse to hamster,
generating hamster strain 263K-like patterns with predominate
diglycosyl PrPSc (28). Such changed glycosylating patterns
are stably maintained during the subsequent passage in hamsters
(Shi et al, unpublished data). The transitions of
glycosylating patterns of PrPSc molecules of
mouse-adapted strain 139A and hamster-adapted strain 263K have
recently been observed in interspecies protein misfolding cyclic
amplification (PMCA) (Gao et al, unpublished data). Taken
together, our data suggest that as well as the prion strains, the
host microenvironment, particularly host PrPC, also
contributes greatly to the molecular features of
PrPSc.
In conclusion, we have successfully re-infected the
prions of a prion-infected cell line into three different strains
of mice. The majority of the clinical, pathogenic and pathological
characteristics in three types of mice are similar. The
PrPSc deposits in the cerebellum region are different,
an aspect possibly linked with the slight diversity in the
cerebellum-associated symptoms. In addition, the present study
provides us with more choices of scrapie rodent models which may be
used in further studies.
Acknowledgments
The present study was supported by the Chinese
National Natural Science Foundation Grants (no. 81301429 and
81572048), the China Mega-Project for Infectious Diseases (nos.
2011ZX10004-101 and 2012ZX10004215) and the SKLID Development Grant
(nos. 2012SKLID102 and 2015SKLID503).
References
|
1
|
Liberski PP: Historical overview of prion
diseases: a view from afar. Folia Neuropathol. 50:1–12.
2012.PubMed/NCBI
|
|
2
|
Prusiner SB: Novel proteinaceous
infectious particles cause scrapie. Science. 216:136–144. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Prusiner SB: The prion diseases. Brain
Pathol. 8:499–513. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Caughey B, Race RE and Chesebro B:
Detection of prion protein mRNA in normal and scrapie-infected
tissues and cell lines. J Gen Virol. 69:711–716. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chesebro B, Race R, Wehrly K, Nishio J,
Bloom M, Lechner D, Bergstrom S, Robbins K, Mayer L, Keith JM, et
al: Identification of scrapie prion protein-specific mRNA in
scrapie-infected and uninfected brain. Nature. 315:331–333. 1985.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bolton DC, McKinley MP and Prusiner SB:
Molecular characteristics of the major scrapie prion protein.
Biochemistry. 23:5898–5906. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meyer RK, McKinley MP, Bowman KA,
Braunfeld MB, Barry RA and Prusiner SB: Separation and properties
of cellular and scrapie prion proteins. Proc Natl Acad Sci USA.
83:2310–2314. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stahl N, Baldwin MA, Teplow DB, Hood L,
Gibson BW, Burlingame AL and Prusiner SB: Structural studies of the
scrapie prion protein using mass spectrometry and amino acid
sequencing. Biochemistry. 32:1991–2002. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Colby DW and Prusiner SB: Prions. Cold
Spring Harb Perspect Biol. 3:a0068332011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dolby DW and SB P: Prions. Cold Harb
Perspect Biol. 3:a0068332011. View Article : Google Scholar
|
|
11
|
Kretzschmar HA, Prusiner SB, Stowring LE
and DeArmond SJ: Scrapie prion proteins are synthesized in neurons.
Am J Pathol. 122:1–5. 1986.PubMed/NCBI
|
|
12
|
McLennan NF, Rennison KA, Bell JE and
Ironside JW: In situ hybridization analysis of PrP mRNA in human
CNS tissues. Neuropathol Appl Neurobiol. 27:373–383. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oesch B, Westaway D, Wälchli M, McKinley
MP, Kent SB, Aebersold R, Barry RA, Tempst P, Teplow DB, Hood LE,
et al: A cellular gene encodes scrapie PrP 27–30 protein. Cell.
40:735–746. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Race RE, Fadness LH and Chesebro B:
Characterization of scrapie infection in mouse neuroblastoma cells.
J Gen Virol. 68:1391–1399. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Butler DA, Scott MR, Bockman JM, Borchelt
DR, Taraboulos A, Hsiao KK, Kingsbury DT and Prusiner SB:
Scrapie-infected murine neuroblastoma cells produce
protease-resistant prion proteins. J Virol. 62:1558–1564.
1988.PubMed/NCBI
|
|
16
|
Birkett CR, Hennion RM, Bembridge DA,
Clarke MC, Chree A, Bruce ME and Bostock CJ: Scrapie strains
maintain biological phenotypes on propagation in a cell line in
culture. EMBO J. 20:3351–3358. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Clarke MC and Haig DA: Multiplication of
scrapie agent in cell culture. Res Vet Sci. 11:500–501.
1970.PubMed/NCBI
|
|
18
|
Zhang J, Chen L, Zhang BY, Han J, Xiao XL,
Tian HY, Li BL, Gao C, Gao JM, Zhou XB, et al: Comparison study on
clinical and neuropathological characteristics of hamsters
inoculated with scrapie strain 263K in different challenging
pathways. Biomed Environ Sci. 17:65–78. 2004.PubMed/NCBI
|
|
19
|
Gao JM, Gao C, Han J, Zhou XB, Xiao XL,
Zhang J, Chen L, Zhang BY, Hong T and Dong XP: Dynamic analyses of
PrP and PrP(Sc) in brain tissues of golden hamsters infected with
scrapie strain 263K revealed various PrP forms. Biomed Environ Sci.
17:8–20. 2004.PubMed/NCBI
|
|
20
|
Deleault NR, Harris BT, Rees JR and
Supattapone S: Formation of native prions from minimal components
in vitro. Proc Natl Acad Sci USA. 104:9741–9746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chandler RL: Encephalopathy in mice
produced by inoculation with scrapie brain material. Lancet.
1:1378–1379. 1961. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moda F, Vimercati C, Campagnani I,
Ruggerone M, Giaccone G, Morbin M, Zentilin L, Giacca M, Zucca I,
Legname G and Tagliavini F: Brain delivery of AAV9 expressing an
anti-PrP monovalent antibody delays prion disease in mice. Prion.
6:383–390. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Luers L, Bannach O, Stöhr J, Wördehoff MM,
Wolff M, Nagel-Steger L, Riesner D, Willbold D and Birkmann E:
Seeded fibrillation as molecular basis of the species barrier in
human prion diseases. PLoS One. 8:e726232013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi Q, Xiao K, Zhang BY, Zhang XM, Chen
LN, Chen C, Gao C and Dong XP: Successive passaging of the scrapie
strains, ME7-ha and 139A-ha, generated by the interspecies
transmission of mouse-adapted strains into hamsters markedly
shortens the incubation times, but maintains their molecular and
pathological properties. Int J Mol Med. 35:1138–1146.
2015.PubMed/NCBI
|
|
25
|
Shi Q, Xie WL, Zhang B, Chen LN, Xu Y,
Wang K, Ren K, Zhang XM, Chen C, Zhang J and Dong XP: Brain
microglia were activated in sporadic CJD but almost unchanged in
fatal familial insomnia and G114V genetic CJD. Virol J. 10:2162013.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xie WL, Shi Q, Zhang J, Zhang BY, Gong HS,
Guo Y, Wang SB, Xu Y, Wang K, Chen C, et al: Abnormal activation of
microglia accompanied with disrupted CX3CR1/CX3CL1 pathway in the
brains of the hamsters infected with scrapie agent 263K. J Mol
Neurosci. 51:919–932. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Aguzzi A, Heikenwalder M and Polymenidou
M: Insights into prion strains and neurotoxicity. Nat Rev Mol Cell
Biol. 8:552–561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shi Q, Zhang BY, Gao C, Zhang J, Jiang HY,
Chen C, Han J and Dong XP: Mouse-adapted scrapie strains 139A and
ME7 overcome species barrier to induce experimental scrapie in
hamsters and changed their pathogenic features. Virol J. 9:632012.
View Article : Google Scholar : PubMed/NCBI
|