Introduction
The PC12 cell line is traceable to a
pheochromocytoma from the rat adrenal medulla (1–4).
When exposed to nerve growth factor (NGF), PC12 cells present an
observable change in sympathetic neuron phenotype and properties.
Neural differentiation of PC12 has been widely used as a neuron
cell model in neuroscience, such as in the nerve injury-induced
neuropathic pain model (5) and
nitric oxide-induced neurotoxicity model (6). NGF induces differentiation of PC12
cells by acting through the TrkA receptor (7).
Differentiation of PC12 cells is assessed by
semi-quantitative or quantitative morphological methods. These
methods can include the measurement of the cell size, neurite
number and neurite length. Additionally, neurotypic and gliotypic
proteins have been used as biochemical markers of neurotoxicity
(8). In general, differentiated
PC12 cells have been widely used in both neurobiological and
neurotoxicological studies as a model of neuronal
differentiation.
At present, there are many different differentiation
methods. Greene and Tischler (9)
first demonstrated that PC12 cells generated in RPMI-1640 medium
retained their tumorigenic properties and were sensitive to nerve
growth factor protein, but the PC12 neuronal processes only reached
500–1,000 μm in length. Cell differentiation was increasing
following replacing the Dulbecco's modified Eagle's medium (DMEM)
containing 10% FBS and 10% horse serum (HS) with fresh medium
containing 50 ng/ml NGF. The results reported that PC12 cells did
not proliferate and started to differentiate into neuron-like
cells, but the increase in cell number reached
51.09±9.3×104 cells/ml (10). PC12 cells grown in DMEM medium
containing 1% HS and 0.5% FBS then were treated with 100 ng/ml NGF,
100 ng/ml basic fibroblast growth factor (bFGF) and 1 mM cAMP in
serum-starved media containing antibiotics and 2 mg/ml BSA for 2
days. The results indicated that Tau-1 and synaptotagmin were
localized at the neurite tips (11).
The problems with the traditional methods are the
limited neurite outgrowth, high proliferation rate, low
differentiation rate and lack of synapse-like structures. Methods
with which to improve the morphological differentiation and
physiological function of PC12 cells in order to make them more
similar to neurons are urgently required. In the present study, by
comparing traditional induction method and observed the neurite
length, differentiation, adhesion, cell proliferation and action
potential of the induced PC12 cells in addition to the protein
levels of axonal GAP-43 and synaptic protein synapsin-1. The
research introduces a novel, improved induction method involving
the use of Opti-MEM medium containing 0.5% FBS to induce PC12
cells, resulting in a better neuron cell model than the traditional
method of induction.
Materials and methods
Reagents
The following materials were used in this study,
Cy3-conjugated Goat Anti-Rabbit secondary antibody (Abcam,
Cambridge, UK). MTT (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany), Matrigel (BD Biosciences, Franklin Lakes, NJ, USA),
poly-L-lysine (PLL; Sigma-Aldrich; Merck KGaA), fetal bovine serum
(FBS) and HS (both from Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), GAP-43 antibody (ab75810; Abcam), synapsin-1
antibody (SAB1412529; Sigma-Aldrich; Merck KGaA), PBS tablet (BD
Biosciences), NGF (Hiteck Biological Pharma Co., Ltd., Wuhan,
China), Protease Inhibitor Cocktail Set I (539131; Millipore,
Billerica, MA, USA), and anti-tubulin-βIII antibody (Sigma-Aldrich;
Merck KGaA).
Cell culture
PC12 cells were preserved in our laboratory
(Institute of Brain Sciences, Jinan University). PC12 cells were
cultured in RPMI-1640 medium supplemented with 10% HS, 5% FBS, 100
U/ml penicillin and 100 mg/l streptomycin. PC12 cells were all
maintained at 37°C in a 95% humidified incubator with 5%
CO2 before the experiments.
Differentiation
PC12 cells were plated at low density
(2×103 cells/cm2) in RPMI-1640 medium
supplemented with 10% normal HS, 5% FBS and
penicillin-streptomycin. At 2 h after plating, the medium was
replaced with serum-free 1640/Opti-MEM medium containing 50 ng/ml
NGF. Cells treated with culture medium alone were used as the
control. Cells were fed 1640/Opti-MEM medium on days 0, 2, 4 and 6
containing 50 ng/ml NGF and 0.5% FBS or 1% HS and 0.5% FBS.
Neurite outgrowth assay
Chamber slides were coated with 0.01% PLL at room
temperature overnight. PC12 cells were plated at a density of
1×104 cells/well in a volume of 100 μl. After 24
h, fresh media with NGF (25, 50 and 100 ng/ml) was added and
incubated for 24 h. Cells were fixed with 4% paraformaldehyde and
incubated with a mouse anti-tubulin-βIII antibody (1:800). Each
group was evaluated in duplicate, and the experiments were repeated
four times. The percentage of neurite outgrowth was quantified, and
neurons with neurites longer than the diameter of the cell body
were considered to be 'neurite-bearing'. The average neurite length
of each neuron was measured with Image-Pro Plus software (Media
Cybernetics, Inc., Rockville, MD, USA). A total of 100 neurons were
measured from each group. Morphometric analysis was performed on
digitized images. Images were taken with an average of 10
cells/field. The number of differentiated cells was expressed as a
percentage of the total cells in the field and counting cells that
had at least one neurite with a length equal to the cell body
diameter. Experiments were repeated at least three times using
cultures prepared on separate days.
Cell adhesion
A total of 96 wells were treated with 50 μl
of Matrigel at 37°C and 5% CO2 for 1 h, and incubated in
1% BSA for 1 h after washing twice with PBS. The 96 wells were
washed two more times with PBS before plating with
1.0×105/ml cell suspension density in a volume of 100
μl at 37°C and 5% CO2 for different times (0.5,
1, 2, 3, 4, 5 and 6 h). In each group, half of the wells without
PBS washing and the other half of the wells that were treated with
warm PBS wash demonstrated cell adhesion disruption. Cell viability
was evaluated with the MTT experiment after measuring the well
absorbance value (A value). The ratio = (A value/total adhesion
cells A value) × 100%. Following 2 h of incubation, the medium was
replaced with Opti-MEM/1640.
Cell counts
PC12 cells (7×104 cells/ml) were plated
on a 35 mm2 plastic dish and counted daily. Cells were
incubated under the conditions described above for various time of
durations, and the medium was replaced every 2 days. Cells was
centrifuged and harvested after trypsinization (0.025%
trypsin-EDTA). The collected cells were transferred to a new tube,
the suspension was mixed uniformity, and an aliquot of
predetermined volume was placed to quantify the number of
cells.
Western blot analysis
The expression levels of GAP-43 and synapsin-1 were
quantified with western blot analysis. Cells (1.3×104
cells/cm2) were plated on 12-well plates. The cells were
collected by centrifugation at 1,000 × g for 5 min at 4°C. The
supernatant was discarded, and cell pellets were sonicated for 15
sec in ice-cold lysate buffer (20 mM Tris, pH 7.5; 150 mM NaCl, 1
mM EDTA, 10% glycerol, 1% Triton X-100, 1 mM NaF, 1 mM
Na3VO4 and 1:200 dilution of Protease
Inhibitor Cocktail Set I). The cell lysate was centrifuged at
10,000 × g for 10 min at 4°C, and the supernatant was saved for
analyses. Total protein was determined by bicinchoninic acid (BCA
Protein Assay kit; Thermo Fisher Scientific, Inc.) method. For
western blot analysis, the supernatant was added to an equal volume
of loading buffer and heated to 95°C for 8 min. Equal amounts of
proteins (0.125–2 mg/ml) were separated by 12% SDS-PAGE and were
transferred onto nitrocellulose membranes. The membranes were
incubated with a monoclonal mouse GAP-43 antibody (1:1,000) or a
polyclonal rabbit synapsin-1 antibody (1:1,000) overnight at 4°C.
Immunoreactivity was detected using a secondary antibody (1:5,000,
ARG65351; Arigobio, Hsinchu, Taiwan) room temperature for 1 h.
Images were collected, and the band density was analyzed using a
Fluor-S MultiImager and Quantity One software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Experiments were repeated
four times on separate days.
Electrophysiology
Cells grown on coverslips with a recording chamber.
Action potentials were recorded with a current-clamp whole-cell
configuration. Patch micropipettes with resistance values of 8–10
MΩ were pulled with an electrode puller (P-97; Sutter Instrument
Co., Novato, CA, USA). Pipettes were filled with solution
containing 140 mM KCl, 5 mM NaCl, 1 mM CaCl2, 10 mM
HEPES, 5 mM EGTA and 2 mM Na-ATP, and the pH was adjusted to 7.3.
The standard external solution was composed of (in mM) 140 NaCl, 5
KCl, 2.5 CaCl2, 1 MgCl2, 10 glucose and 10
HEPES, and the pH was adjusted to 7.3. Cells were perfused at
22–24°C, and whole-cell currents were recorded using an EPC-10
patch clamp amplifier (HEKA Elektronik Dr. Schulze GmbH, Lambrecht,
Germany). The membrane currents were low-pass filtered at 2.9 kHz
and sampled at 50 kHz, and the current injections ranged from −50
to +90 pA within 10 pA steps. Data were collected with PatchMaster
acquisition software (HEKA Elektronik Dr. Schulze GmbH); then,
traces of the action potentials were displayed by Igor software
(version 6.3; WaveMetrics, Lake Oswego, OR, USA).
Statistical analysis
All data are expressed as the mean ± standard error
of the mean. Analyses were performed using SPSS statistical
software (version 16.0; SPSS, Inc., Chicago, IL, USA). Comparisons
between groups were analyzed by one-way analysis of variance. The
statistical significance was determined by Student's t-test for two
groups. Multiple comparison between the groups was performed using
SNK method. P<0.05 was considered to indicate a statistically
significant difference.
Results
The neurite length and differentiation
rate of PC12 cells in different NGF-induced differentiation culture
conditions
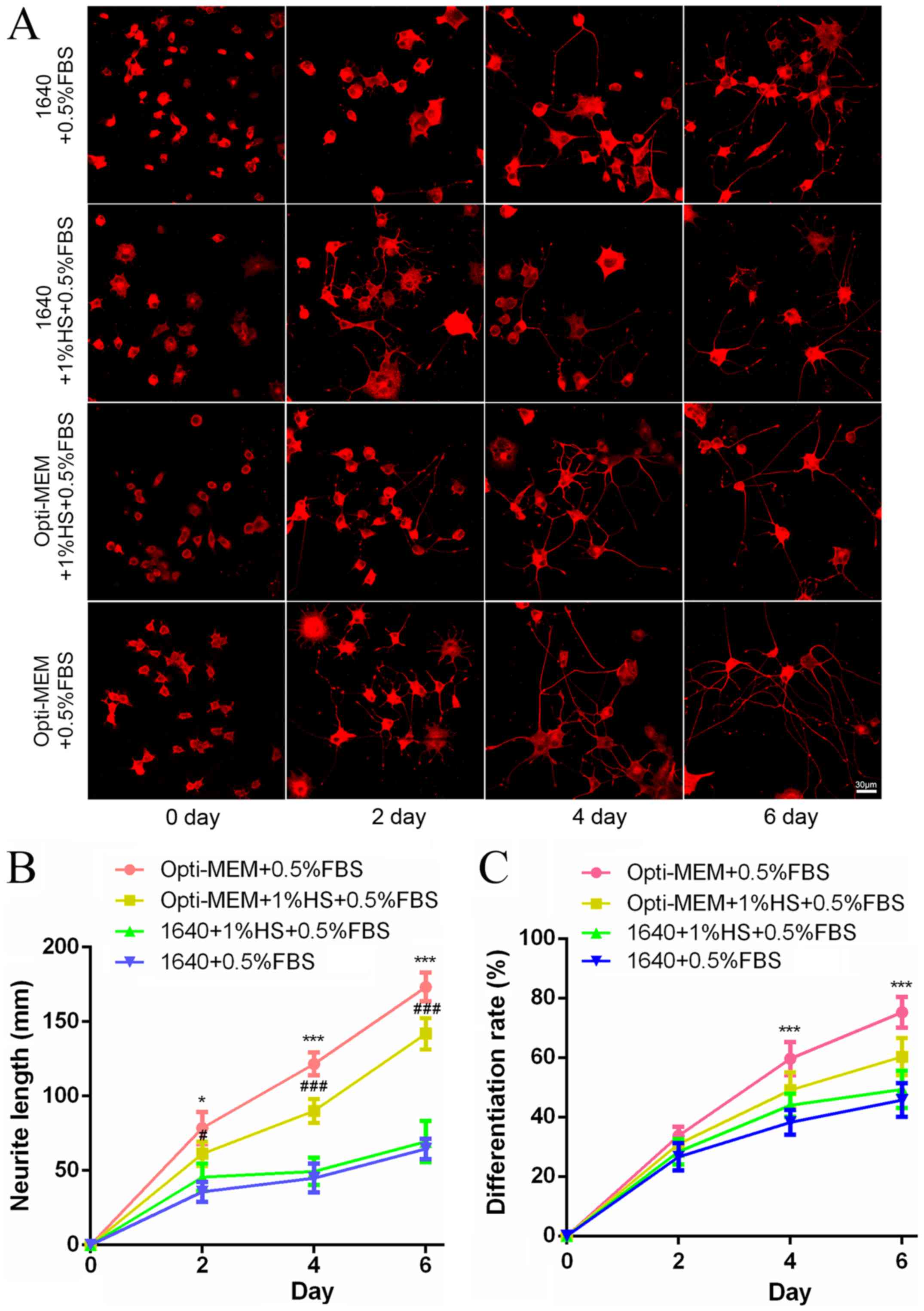
The effects of NGF (50 ng/ml) culture of PC12 cells
is shown in Fig. 1. PC12 cells
were fed 50 ng/ml NGF under different culture conditions on 0, 2, 4
and 6 days. NGF-induced differentiation of PC12 cells based on
morphology are presented in Fig.
1A. For all culture conditions, the neurite length and cell
differentiation were time-dependently increased (Fig. 1B and C). Compared with RPMI-1640
groups, the Opti-MEM medium group for 6 days resulted in an
increased neurite length, and Opti-MEM medium containing 0.5% FBS
was the best culture condition (Fig.
1B). The number of differentiated cells in Opti-MEM groups were
higher than that in RPMI-1640, and the maximal extent of
differentiated cells appeared in Opti-MEM medium containing 0.5%
FBS (Fig. 1C).
Neurite length of induced PC12 cells with
different concentrations of NGF using Opti-MEM for different
days
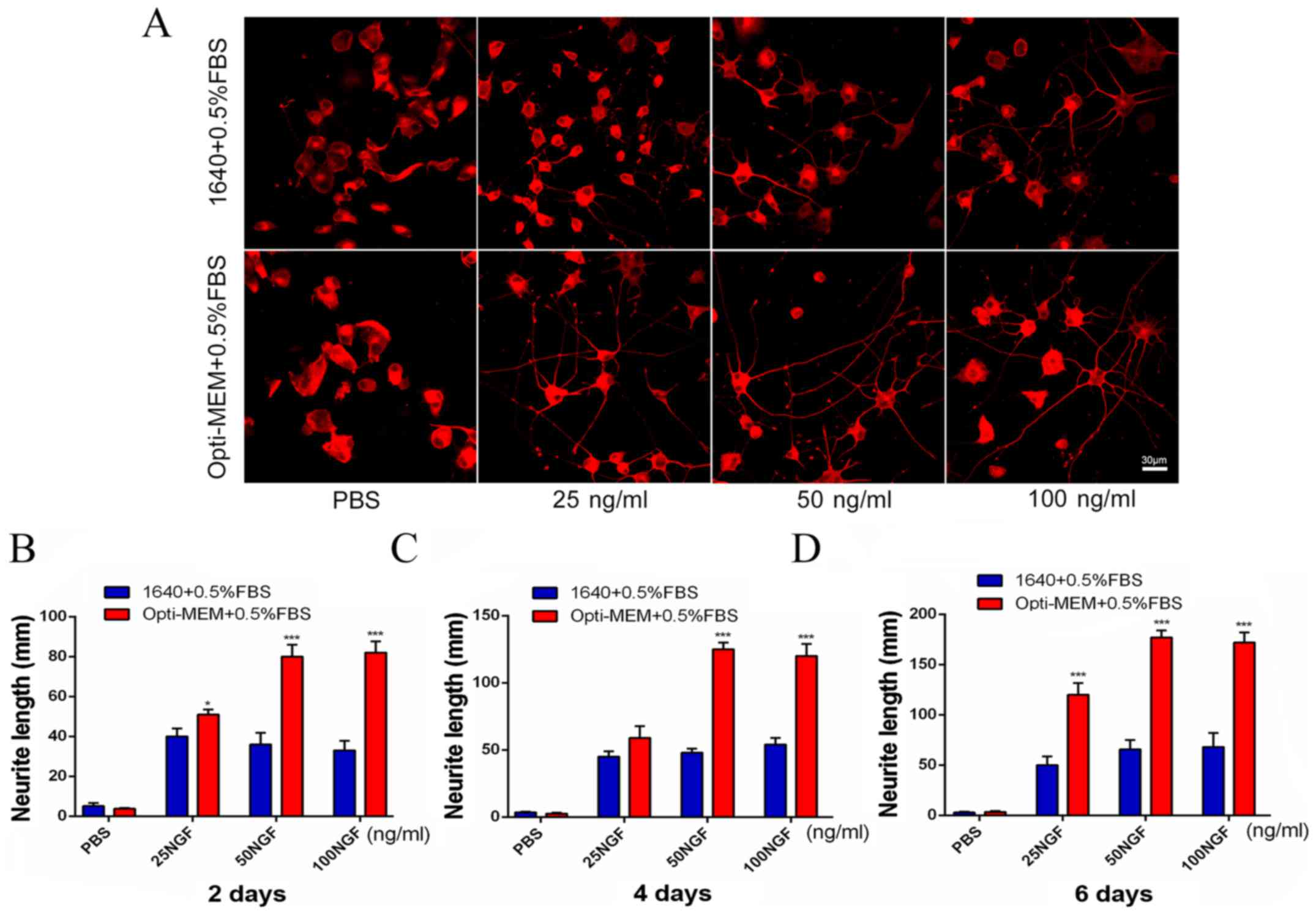
The two different conditions used induction in
Opti-MEM medium containing 0.5% FBS or RPMI-1640 medium
supplemented with 0.5% FBS. The effects of NGF at different
concentrations and different time-points are presented in Fig. 2. The effects of 0, 25, 50 and 100
ng/ml NGF on the PC12 cell morphology was observed in the two
groups on day 6 (Fig. 2A).
Exposure of PC12 cells to different concentrations of NGF on day 2
resulted in concentration-dependent increases in the neurite length
in Opti-MEM group, which was significant at 25 ng/ml. However, the
1640 group results were the opposite (Fig. 2B). Exposure of PC12 cells to
different concentrations of NGF on day 4 caused
concentration-dependent increases in the neurite length. There was
a significant increase in the neurite length in the Opti-MEM group
with 50 ng/ml NGF (Fig. 2C). With
the same culture and induction conditions for PC12 as indicated in
Fig. 2B and C, the authors found
that the experimental group exposure to 50 ng/ml NGF on day 6
resulted in the greatest neurite length (Fig. 2D).
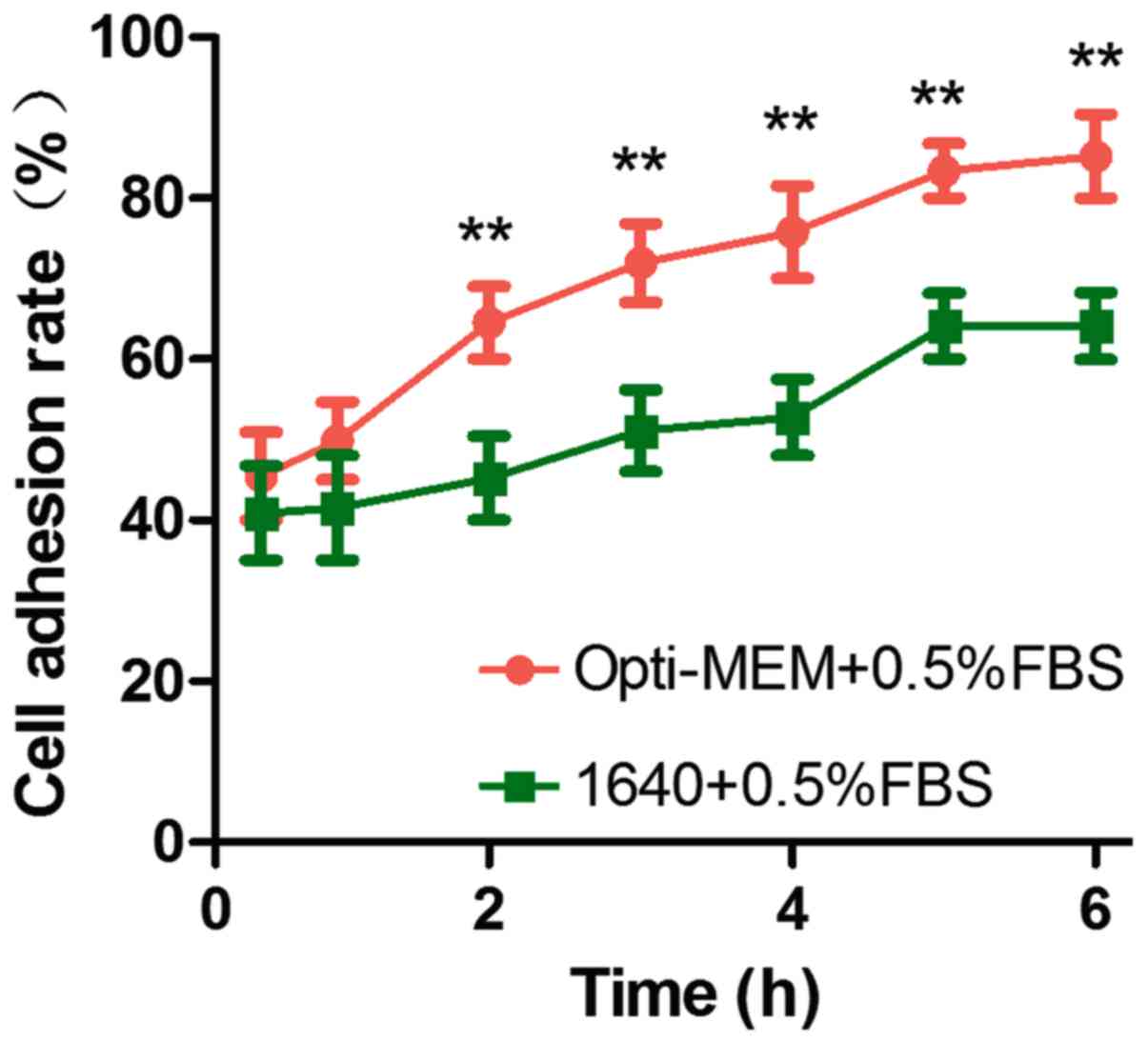
Comparison of the cell adhesion rate of
induced PC12 cells in the Opti-MEM and 1640 groups
PC12 cells were treated with 50 ng/ml NGF at
different time-points (0.5, 1, 2, 3, 4, 5 and 6 h) resulted in a
time-dependent increase in the cell adhesion rate over the next 6
h. It can be seen that Opti-MEM group compared with RPMI-1640 group
resulted in a more obvious cell adhesion rate (Fig. 3).
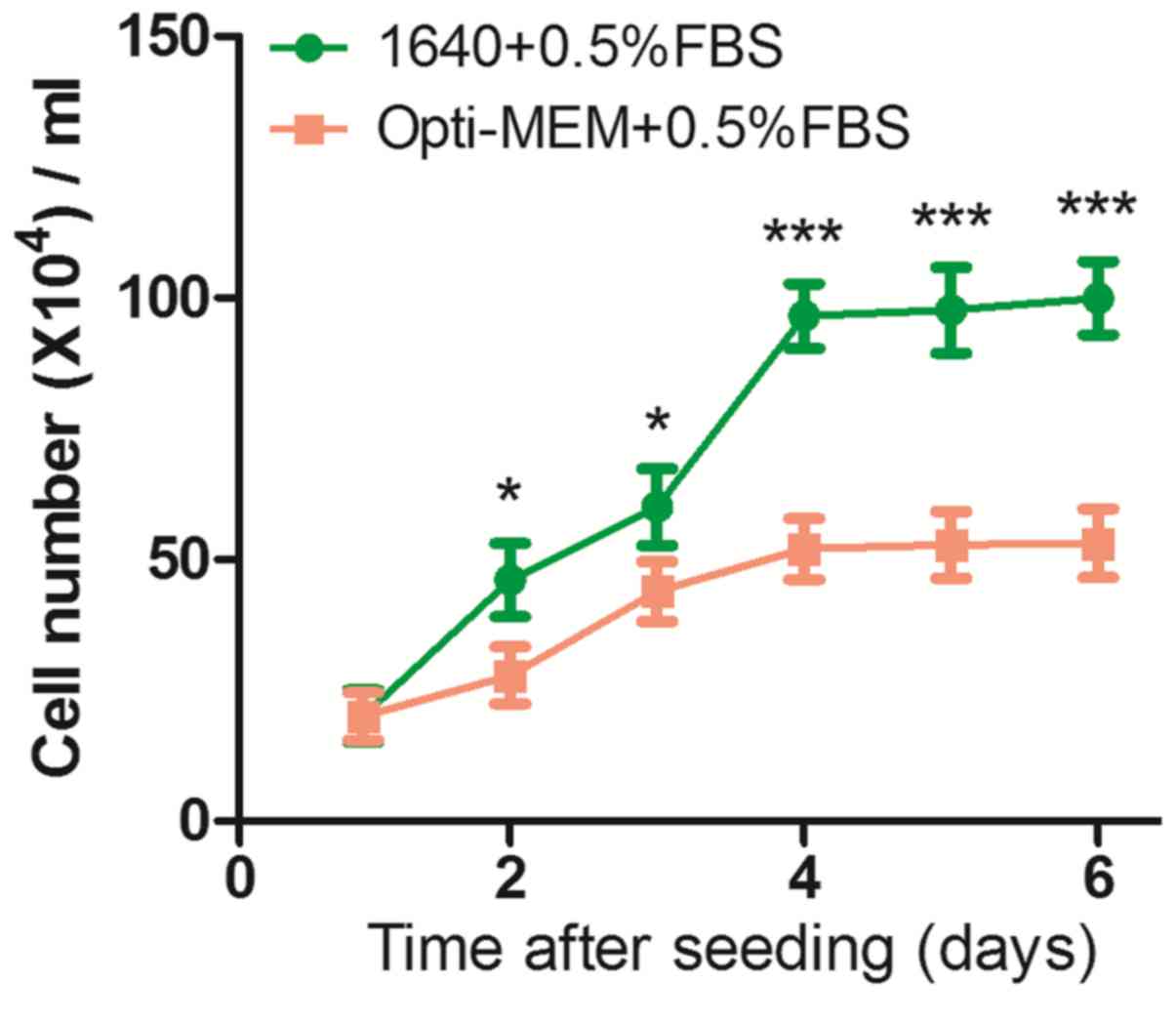
Assessment of PC12 cell proliferation in
the Opti-MEM and 1640 groups
Cells were cultured in the presence of serum and 50
ng/ml NGF, which enabled continuous proliferative activity. Cell
growth rates were estimated and counting cell numbers every other
day for 6 days. The authors found that the cell proliferation rate
on RPMI-1640 group was higher than the Opti-MEM group during the
experiment (Fig. 4). On day 2,
there were significantly more cells in the 1640 groups
(46.11±1.06×104 cells/ml) than the Opti-MEM group
(27.85±5.45×104 cells/ml), and the difference was
maintained from day 4 to day 6 (1640 groups,
99.88±7.02×104; Opti-MEM group,
53.11±6.55×104 cells/ml), which indicated that Opti-MEM
medium containing 0.5% FBS can provide a better environment for
inducing PC12 cells to become neurons.
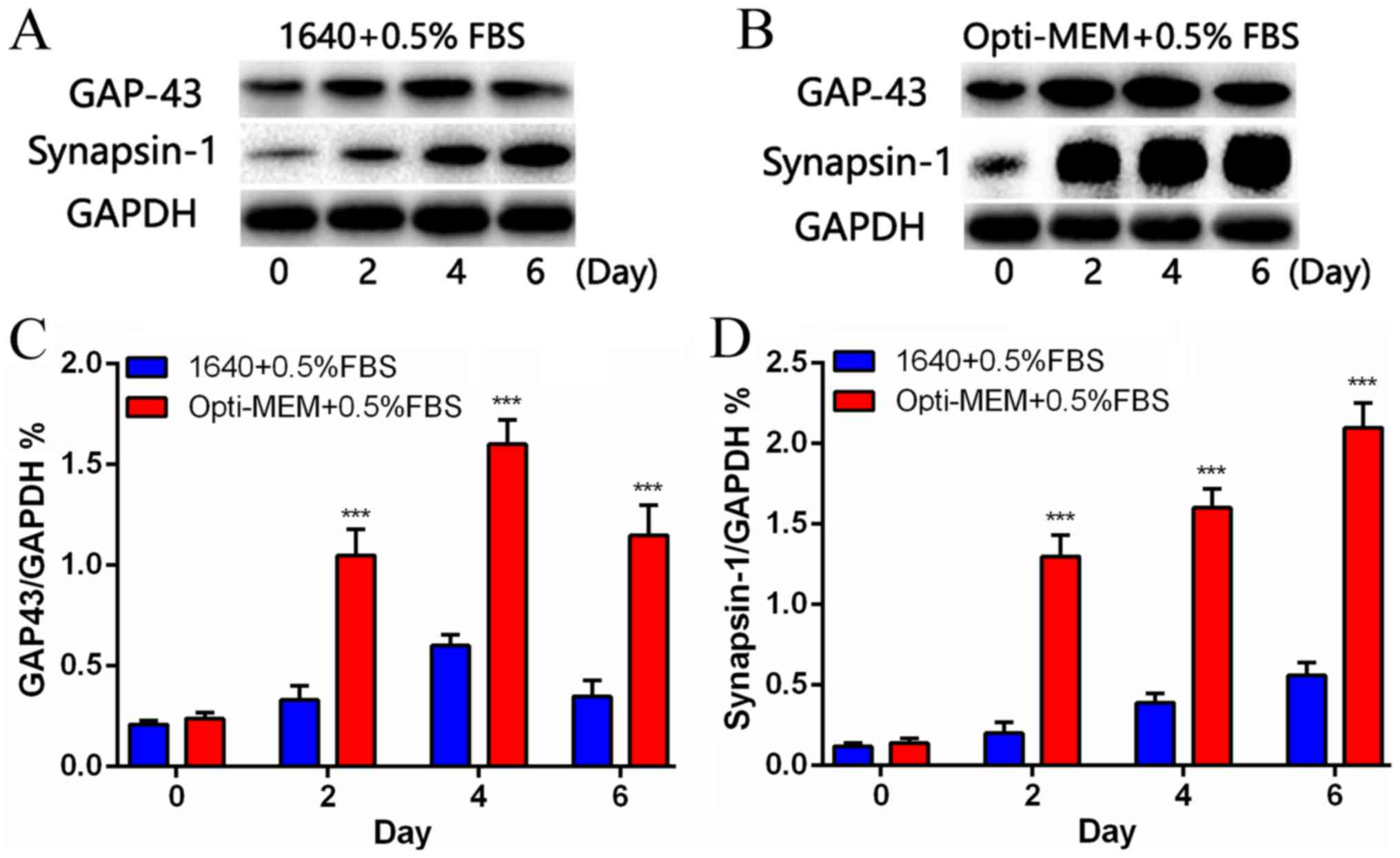
The expression of synapsin-1 and GAP-43
in the Opti-MEM and 1640 groups
Differentiation of PC12 cells with 50 ng/ml NGF
increased the expression of synapsin-1 and GAP-43 (Fig. 5). Western blot analysis indicated
that synapsin-1 levels were dramatically increased in the Opti-MEM
groups than 1640 groups on days 2, 4 and 6 (Fig. 5A). The expression of GAP-43 was
consistent with the synapsin-1 levels on days 2, 4 and 6, but that
all the levels were dramatically increased in the Opti-MEM groups
than 1640 groups (Fig. 5B).
Quantification of the western blot data from the two groups is
presented in Fig. 5C and D.
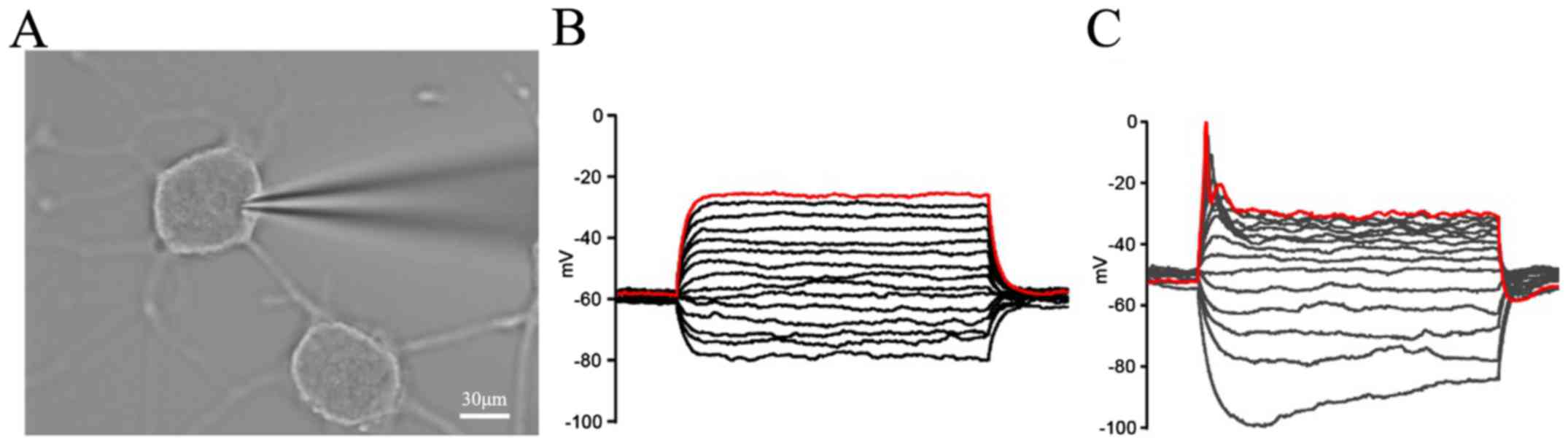
Action potential of the induced PC12
cells using Opti-MEM as an induction medium
Differentiation of PC12 cells with 50 ng/ml NGF had
active membrane properties (Fig.
6). Whole-cell recordings were performed on day 6 following
treatment with or without 50 ng/ml NGF (Fig. 6A). When the induced PC12 cells
were step-depolarized, compared with the control cells without
induction (Fig. 6B), the action
potentials could be recorded in the induction of PC12 cells on day
6 with the 50 ng/ml NGF treatment (Fig. 6C).
Discussion
The PC12 cell line was first separated from a tumor
in the adrenal medulla of a rat (12). It can reversibly react to the
addition of NGF by differentiating and growing neurites (13). PC12 cells have been widely used as
a neuronal in vitro model system (14), including studies on the effects of
neurotoxicants on differentiation (15,16). Previous studies have used
different training and induction methods to transform PC12 cells
into neurons, but there are some limitations that exist. First,
although cells do not generate axons or dendrites or form real
synapses with each other. In addition, they have the potential for
gene mutation resulting in a phenotype change (17). Induced PC12 cells have a low
differentiation rate, short neurite length and low adhesion rate
(10–12).
In the present study, the authors used Opti-MEM
medium containing 0.5% FBS and/or 1% HS compared with RPMI-1640
medium containing 0.5% FBS and/or 1% HS. With the novel method of
PC12 neural differentiation, the authors observed a significant
increase in both cell differentiation number and neurite length on
day 6. The low variability morphological measurements were highly
consistent between cultures. In addition, the study also
demonstrated that adhesion of PC12 cells was significantly improved
and proliferation was significantly decreased by Opti-MEM group
with 50 ng/ml NGF. The Opti-MEM group with 50 ng/ml NGF showed a
higher adhesive and slower proliferation effect than RPMI-1640
group. The results also demonstrated the interaction between the
supplemented medium and serum in inducing PC12 to become
neurons.
Axonal growth and formation of synaptic vesicles is
modulated by the expression of neuronal proteins and synaptic
proteins (18–23). GAP-43 and synapsin-1 are related
to PC12 cell differentiation and neurite outgrowth. As an
endogenous substrate for PKC, phosphorylated GAP-43 is stimulated
by NGF in PC12 cells (24–26),
and upregulation of GAP-43 mRNA and protein is related to the
differentiation of PC12 cells (27–29). Both proteins have been identified
at increased levels during the formation of mature synapses in cell
development (30,31). A previous report verified that
GAP-43 and synapsin-1 are sensitive to chemical disruption of
differentiation and neurite outgrowth (32). GAP-43 was absent on day 0 and
plateaued at high levels by day 6, and was correlated with axonal
outgrowth and neurite outgrowth (33,34). However, synapsin-1 increased
during the differentiation of PC12 cells, and increased most
prominently on day 4 following differentiation (35). Therefore, the expression of GAP-43
and synapsin-1 were evaluated as markers of axons and presynaptic
vesicles (36). The current data
of GAP-43 and synapsin-1 suggest that this improved method induces
differentiated PC12 cells to mimic sympathetic neurons.
To identify whether induced PC12 cells had active
membrane properties, whole-cell recordings were performed. When the
induced PC12 cells were step-depolarized, action potentials were
only detected in many NGF+ cells. The cells appeared
remarkably similar to neuroblastoma cells, but their results were
somewhat smaller than those reported for rat sympathetic neurons.
Previous studies have reported that the resting potentials of
NGF+ cells were −50 to −65 (37) the authors demonstrated that PC12
cells cultured in Opti-MEM medium containing 0.5% FBS are suitable
for electrophysiological studies. Because Opti-MEM medium has more
hypoxanthine and thymine than RPMI-1640, it was speculated that
these nutrients affected the PC12 cell neuron differentiation
potential.
In conclusion, compared with the conventional
RPMI-1640 induction method, the new approach with Opti-MEM could
significantly increase the induced cell neurite length,
differentiation rate, adhesion rate and expression of GAP-43 and
synapsin-1. The resulting morphology was more like neurons.
Therefore, the present study provided an improved induction method
for neural differentiation of PC12 cells using Opti-MEM medium
containing 0.5% FBS, an approach that can be widely used in
neurobiology and neuropharmacology research models. Admittedly,
there are some limitations to this study. As previous studies have
demonstrated that pathways, such as the mitogen-activated protein
kinase/extracellular regulated kinase (MAPK/ERK) and the
phosphatidylinositol-3 kinase (PI3K)/AKT pathways are closely
related to the proliferation, differentiation and apoptosis of PC12
cells (38–41), the examination of such pathways
would be valuable and this was not carried out in this study. Thus,
further studies on these pathways are required to explore the full
potential of using Opti-MEM medium containing 0.5% FBS induction of
PC12 cell differentiation.
Acknowledgments
The present study was funded by the Ocean and
Fisheries Foundation of Guangdong Province (grant no. B201601-04),
the Natural Science Foundation of China (grant nos. 81202519 and
81403202), the Science and Technology Program of Guangzhou (grant
nos. 201607010216, 201510010074 and 201607010063), and was
supported by Natural Science Foundation of Guangdong Province
(grant nos. 2014A030313362 and 2014A030313416) and the Science and
Technology Planning Project of Guangdong Province (grant nos.
2014A010105029, 2014A020211022 and 2016A020214012) and the Youth
Elite Project of GUCM (grant no. QNYC20140103).
References
|
1
|
Uceda G, Artalejo AR, López MG, Abad F,
Neher E and García AG: Ca(2+)-activated K+ channels
modulate muscarinic secretion in cat chromaffin cells. J Physiol.
454:213–230. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou Z and Neher E: Calcium permeability
of nicotinic acetylcholine receptor channels in bovine adrenal
chromaffin cells. Pflugers Arch. 425:511–517. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nooney JM, Peters JA and Lambert JJ: A
patch clamp study of the nicotinic acetylcholine receptor of bovine
adrenomedullary chromaffin cells in culture. J Physiol.
455:503–527. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Horrigan FT and Bookman RJ: Releasable
pools and the kinetics of exocytosis in adrenal chromaffin cells.
Neuron. 13:1119–1129. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shao J, Cao J, Wang J, Ren X, Su S, Li M,
Li Z, Zhao Q and Zang W: MicroRNA-30b regulates expression of the
sodium channel Nav1.7 in nerve injury-induced neuropathic pain in
the rat. Mol Pain. 12:17448069166715232016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng W, Chong CM, Wang H, Zhou X, Zhang
L, Wang R, Meng Q, Lazarovici P and Fang J: Artemisinin conferred
ERK mediated neuroprotection to PC12 cells and cortical neurons
exposed to sodium nitroprusside-induced oxidative insult. Free
Radic Biol Med. 97:158–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu L, Sun T, Xin F, Cui W, Guo J and Hu
J: Nerve growth factor protects against alcohol-induced
neurotoxicity in PC12 cells via I3K/Akt/mTOR pathway. Alcohol
Alcohol. 52:12–18. 2017. View Article : Google Scholar
|
|
8
|
O'Callaghan JP: Neurotypic and gliotypic
proteins as biochemical markers of neurotoxicity. Neurotoxicol
Teratol. 10:445–452. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Greene LA and Tischler AS: Establishment
of a noradrenergic clonal line of rat adrenal pheochromocytoma
cells which respond to nerve growth factor. Proc Natl Acad Sci USA.
73:2424–2428. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Medina Benavente JJ, Mogami H, Sakurai T
and Sawada K: Evaluation of silicon nitride as a substrate for
culture of PC12 cells: an interfacial model for functional studies
in neurons. PLoS One. 9:e901892014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jeon CY, Jin JK, Koh YH, Chun W, Choi IG,
Kown HJ, Kim YS and Park JB: Neurites from PC12 cells are connected
to each other by synapse-like structures. Synapse. 64:765–772.
2010.PubMed/NCBI
|
|
12
|
Chen ZA, Wang JL, Liu RT, Ren JP, Wen LQ,
Chen XJ and Bian GX: Liquiritin potentiate neurite outgrowth
induced by nerve growth factor in PC12 cells. Cytotechnology.
60:125–132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Daniele S, Lecca D, Trincavelli ML, Ciampi
O, Abbracchio MP and Martini C: Regulation of PC12 cell survival
and differentiation by the new 2Y-like receptor GPR17. Cell Signal.
22:697–706. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Das KP, Freudenrich TM and Mundy WR:
Assessment of PC12 cell differentiation and neurite growth: a
comparison of morphological and neurochemical measures.
Neurotoxicol Teratol. 26:397–406. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thiel G: Synapsin I, synapsin II, and
synaptophysin: marker proteins of synaptic vesicles. Brain Pathol.
3:87–95. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Webb SJ, Monk CS and Nelson CA: Mechanisms
of postnatal neurobiological development: implications for human
development. Dev Neuropsychol. 19:147–171. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen J, Venkat P, Zacharek A and Chopp M:
Neurorestorative therapy for stroke. Front Hum Neurosci. 8:3822014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Benowitz LI and Routtenberg A: GAP-43: an
intrinsic determinant of neuronal development and plasticity.
Trends Neurosci. 20:84–91. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Goslin K, Schreyer DJ, Skene JH and Banker
G: Changes in the distribution of GAP-43 during the development of
neuronal polarity. J Neurosci. 10:588–602. 1990.PubMed/NCBI
|
|
20
|
McGuire CB, Snipes GJ and Norden JJ:
Light-microscopic immunolocalization of the growth- and
plasticity-associated protein GAP-43 in the developing rat brain.
Brain Res. 469:277–291. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meiri KF, Willard M and Johnson MI:
Distribution and phosphorylation of the growth-associated protein
GAP-43 in regenerating sympathetic neurons in culture. J Neurosci.
8:2571–2581. 1988.PubMed/NCBI
|
|
22
|
Costello B, Meymandi A and Freeman JA:
Factors influencing GAP-43 gene expression in PC12 pheochromocytoma
cells. J Neurosci. 10:1398–1406. 1990.PubMed/NCBI
|
|
23
|
Dani JW, Armstrong DM and Benowitz LI:
Mapping the development of the rat brain by GAP-43
immunocytochemistry. Neuroscience. 40:277–287. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ramakers GM, De Graan PN, Urban IJ, Kraay
D, Tang T, Pasinelli P, Oestreicher AB and Gispen WH: Temporal
differences in the phosphorylation state of pre- and postsynaptic
protein kinase C substrates B-50/GAP-43 and neurogranin during
long-term potentiation. J Biol Chem. 270:13892–13898. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ramakers GM, Gerendasy DD and de Graan PN:
Substrate phosphorylation in the protein kinase Cgamma knockout
mouse. J Biol Chem. 274:1873–1874. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van Hooff CO, De Graan PN, Boonstra J,
Oestreicher AB, Schmidt-Michels MH and Gispen WH: Nerve growth
factor enhances the level of the protein kinase C substrate B-50 in
pheochromocytoma PC12 cells. Biochem Biophys Res Commun.
139:644–651. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
de la Torre JC, Mallory M, Brot M, Gold L,
Koob G, Oldstone MB and Masliah E: Viral persistence in neurons
alters synaptic plasticity and cognitive functions without
destruction of brain cells. Virology. 220:508–515. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jap Tjoen San ER, Schmidt-Michels MH,
Spruijt BM, Oestreicher AB, Schotman P and Gispen WH: Quantitation
of the growth-associated protein B-50/GAP-43 and neurite outgrowth
in PC12 cells. J Neurosci Res. 29:149–154. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jap Tjoen San ER, Schmidt-Michels M,
Oestreicher AB, Schotman P and Gispen WH: Dexamethasone-induced
effects on B-50/GAP-43 expression and neurite outgrowth in PC12
cells. J Mol Neurosci. 3:189–195. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ehrhart-Bornstein M, Treiman M, Hansen GH,
Schousboe A, Thorn NA and Frandsen A: Parallel expression of
synaptophysin and evoked neurotransmitter release during
development of cultured neurons. Int J Dev Neurosci. 9:463–471.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fletcher TL, Cameron P, De Camilli P and
Banker G: The distribution of synapsin I and synaptophysin in
hippocampal neurons developing in culture. J Neurosci.
11:1617–1626. 1991.PubMed/NCBI
|
|
32
|
Eik LF, Naidu M, David P, Wong KH, Tan YS
and Sabaratnam V: Lignosus rhinocerus (Cooke) ryvarden: a medicinal
mushroom that stimulates neurite outgrowth in PC-12 cells. Evid
Based Evid Based Complement Alternat Med. 320308:2012.
|
|
33
|
Greene LA and Tischler AS: Establishment
of a noradrenergic clonal line of rat adrenal pheochromocytoma
cells which respond to nerve growth factor. Proc Natl Acad Sci U S
A. 73:2424–2428. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Greene LA and Tischler AS: PC12
pheochromocytoma cultures in neurobiological research. Adv Cell
Neurobiol. 3:373–414. 1982. View Article : Google Scholar
|
|
35
|
Peterman MC, Bloom DM, Lee C, Bent SF,
Marmor MF, Blumenkranz MS and Fishman HA: Localized
neurotransmitter release for use in a prototype retinal interface.
Invest Ophthalmol Vis Sci. 44:3144–3149. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Berger-Sweeney J and Hohmann CF:
Behavioral consequences of abnormal cortical development: insights
into developmental disabilities. Behav Brain Res. 86:121–142. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
O'Lague PH and Huttner SL: Physiological
and morphological studies of rat pheochromocytoma cells (PC12)
chemically fused and grown in culture. Proc Natl Acad Sci USA.
77:1701–1705. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Marques-Fernandez F, Planells-Ferrer L,
Gozzelino R, Galenkamp KM, Reix S, Llecha-Cano N, Lopez-Soriano J,
Yuste VJ, Moubarak RS and Comella JX: TNFα induces survival through
the FLIP-L-dependent activation of the MAPK/ERK pathway. Cell Death
Dis. 4:e4932013. View Article : Google Scholar
|
|
39
|
Wu PK, Hong SK, Yoon SH and Park JI:
Active ERK2 is sufficient to mediate growth arrest and
differentiation signaling. FEBS J. 282:1017–1030. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mufti RE, Sarker K, Jin Y, Fu S, Rosales
JL and Lee KY: Thrombin enhances NGF-mediated neurite extension via
increased and sustained activation of p44/42 MAPK and p38 MAPK.
PLoS One. 9:e1035302014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu SD, Xia F, Lin XM, Duan KL, Wang F, Lu
QL, Cao H, Qian YH and Shi M: Ginsenoside-Rd promotes neurite
outgrowth of PC12 cells through MAPK/ERK- and I3K/AKT-dependent
pathways. Int J Mol Sci. 17:172016.
|