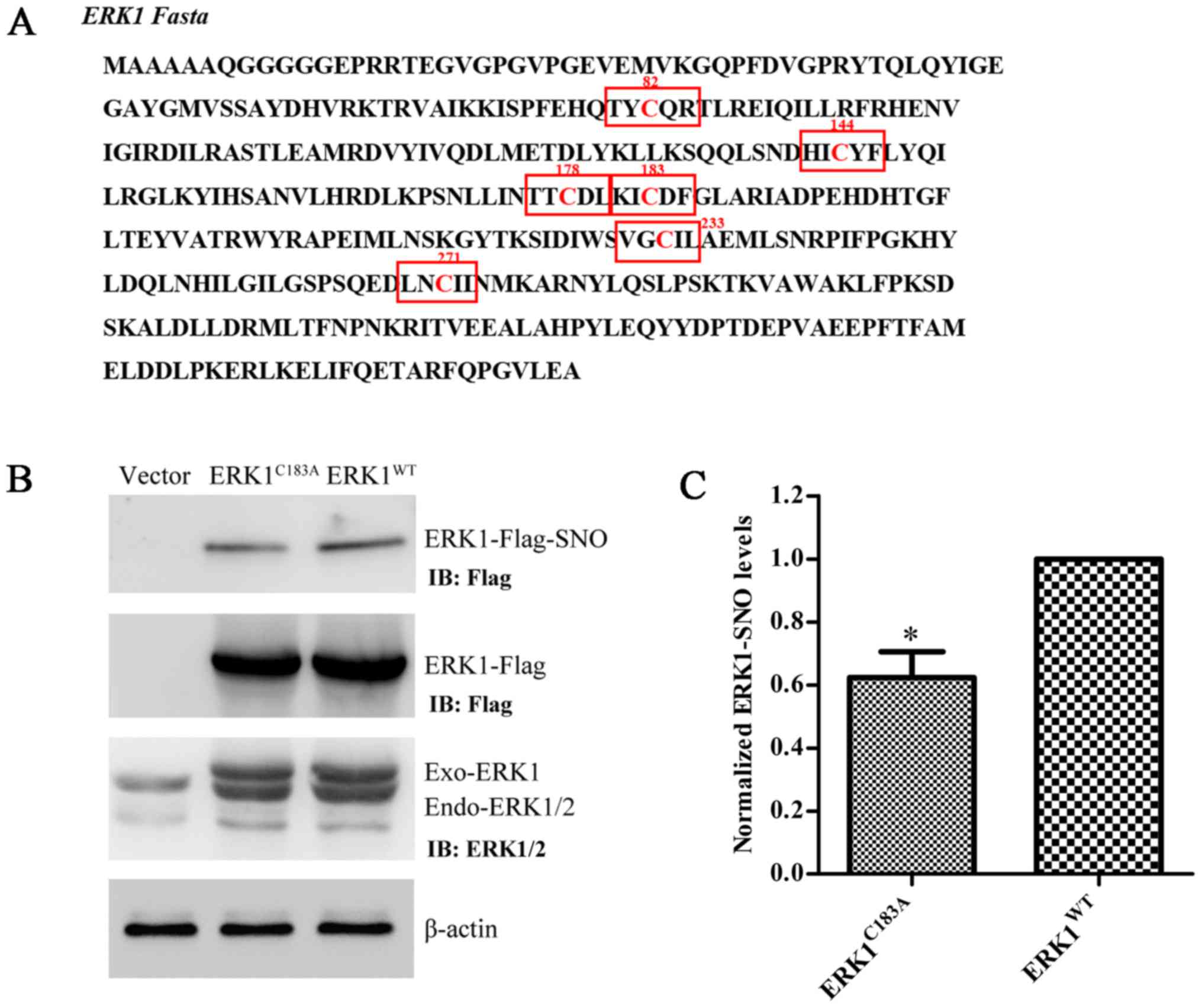
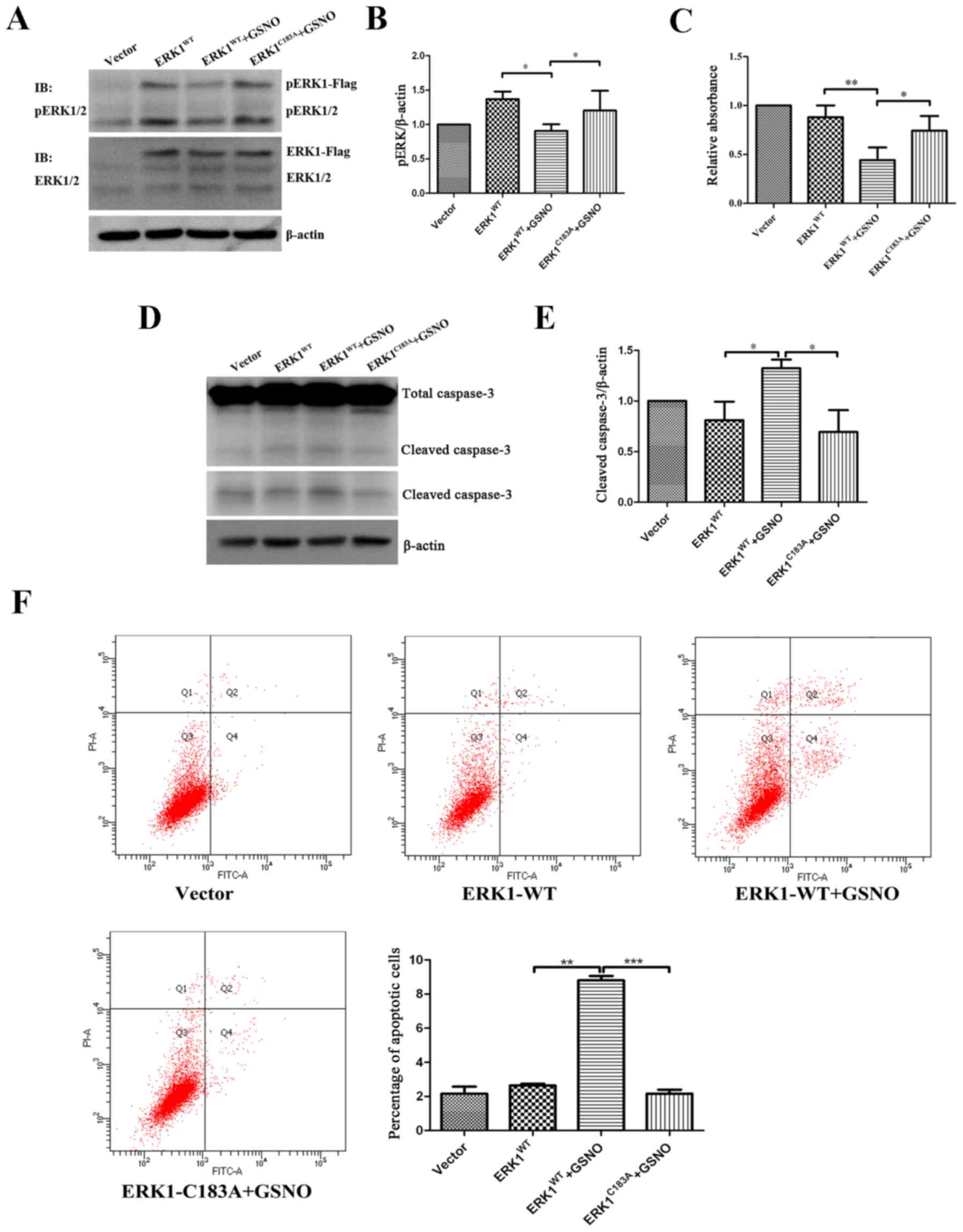
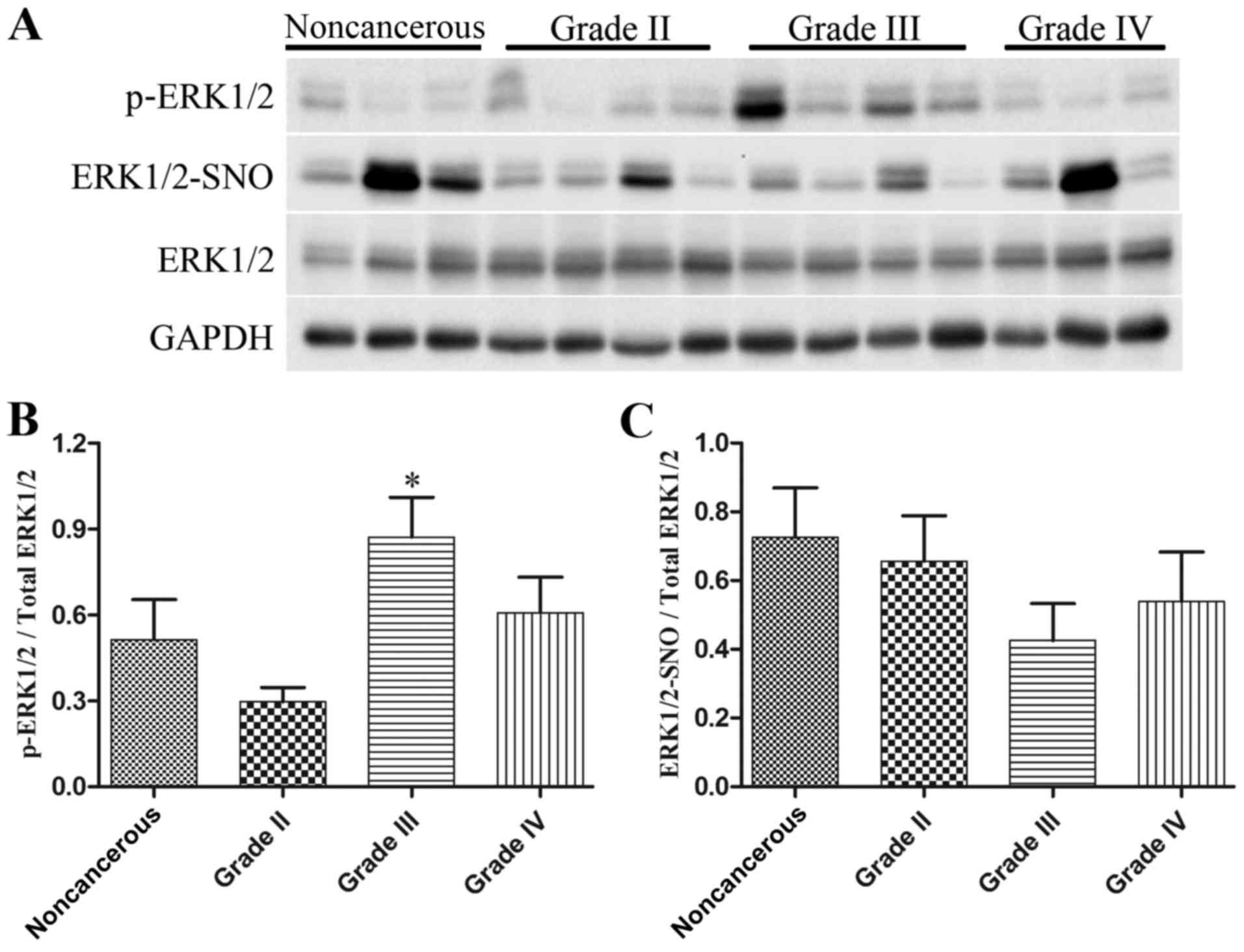
|
1
|
Stupp R, Hegi ME, Gilbert MR and
Chakravarti A: Chemoradiotherapy in malignant glioma: Standard of
care and future directions. J Clin Oncol. 25:4127–4136. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sanai N, Alvarez-Buylla A and Berger MS:
Neural stem cells and the origin of gliomas. N Engl J Med.
353:811–822. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wen PY and Kesari S: Malignant gliomas in
adults. N Engl J Med. 359:492–507. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Roberts PJ and Der CJ: Targeting the
Raf-MEK-ERK mitogen-activated protein kinase cascade for the
treatment of cancer. Oncogene. 26:3291–3310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pandey V, Bhaskara VK and Babu PP:
Implications of mitogen-activated protein kinase signaling in
glioma. J Neurosci Res. 94:114–127. 2016. View Article : Google Scholar
|
|
6
|
Dong Chen, Waters SB, Holt KH and Pessin
JE: SOS phosphorylation and disassociation of the Grb2-SOS complex
by the ERK and JNK signaling pathways. J Biol Chem. 271:6328–6332.
1996. View Article : Google Scholar
|
|
7
|
Bhaskara VK, Panigrahi M, Challa S and
Babu PP: Comparative status of activated ERK1/2 and PARP cleavage
in human gliomas. Neuropathology. 25:48–53. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie H, Xue YX, Liu LB, Wang P, liu YH and
Ying HQ: Expressions of matrix metalloproteinase-7 and matrix
metalloproteinase-14 associated with the activation of
extracellular signal-regulated kinase1/2 in human brain gliomas of
different pathological grades. Med Oncol. 28(Suppl 1): S433–S438.
2011. View Article : Google Scholar
|
|
9
|
Lundberg JO, Gladwin MT and Weitzberg E:
Strategies to increase nitric oxide signalling in cardiovascular
disease. Nat Rev Drug Discov. 14:623–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakamura T and Lipton SA: Protein
S-nitrosylation as a therapeutic target for neurodegenerative
diseases. Trends Pharmacol Sci. 37:73–84. 2016. View Article : Google Scholar :
|
|
11
|
Wang Z: Protein S-nitrosylation and
cancer. Cancer Lett. 320:123–129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng X, Sun T, Bei Y, Ding S, Zheng W, lu
Y and Shen P: S-nitrosylation of ERK inhibits ERK phosphorylation
and induces apoptosis. Sci Rep. 3:18142013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen A, Gao S, Ben Z, Wang H, Jia J, Tao
T, Niu S, Li X and Cheng C: Identification and potential role of
PSD-95 in Schwann cells. Neurol Sci. 29:321–330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Louis DN, Ohgaki H, Wiestler OD and
Cavenee WK: WHO Classification of Tumours of the Central Nervous
System. IARC WHO Classification of Tumours; Lyon: 2016
|
|
15
|
Kurimoto M, Endo S, Hirashima Y, Hamada H,
Ogiichi T and Takaku A: Growth inhibition and radiosensitization of
cultured glioma cells by nitric oxide generating agents. J
Neurooncol. 42:35–44. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Stamler JS, Toone EJ, Lipton SA and Sucher
NJ: (S)NO signals: Translocation, regulation, and a consensus
motif. Neuron. 18:691–696. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Weyerbrock A, Baumer B and Papazoglou A:
Growth inhibition and chemosensitization of exogenous nitric oxide
released from NONOates in glioma cells in vitro. J Neurosurg.
110:128–136. 2009. View Article : Google Scholar
|
|
18
|
Maejima Y, Adachi S, Morikawa K, Ito H and
Isobe M: Nitric oxide inhibits myocardial apoptosis by preventing
caspase-3 activity via S-nitrosylation. J Mol Cell Cardiol.
38:163–174. 2005. View Article : Google Scholar
|
|
19
|
Lechner M, Lirk P and Rieder J: Inducible
nitric oxide synthase (iNOS) in tumor biology: The two sides of the
same coin. Semin Cancer Biol. 15:277–289. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lancaster JR Jr and Xie K: Tumors face NO
problems. Cancer Res. 66:6459–6462. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang Y, Chen C, Loake GJ and Chu C: Nitric
oxide: Promoter or suppressor of programmed cell death. Protein
Cell. 1:133–142. 2010. View Article : Google Scholar
|
|
22
|
Azad N, Vallyathan V, Wang L,
Tantishaiyakul V, Stehlik C, leonard SS and Rojanasakul Y:
S-nitrosylation of Bcl-2 inhibits its ubiquitin-proteasomal
degradation. A novel antiapoptotic mechanism that suppresses
apoptosis. J Biol Chem. 281:34124–34134. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chanvorachote P, Nimmannit U, Stehlik C,
Wang L, Jiang BH, Ongpipatanakul B and Rojanasakul Y: Nitric oxide
regulates cell sensitivity to cisplatin-induced apoptosis through
S-nitrosylation and inhibition of Bcl-2 ubiquitination. Cancer Res.
66:6353–6360. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leon-Bollotte L, Subramaniam S, Cauvard O,
Plenchette-Colas S, Paul C, Godard C, Martinez-Ruiz A, Legembre P,
Jeannin JF and Bettaieb A: S-nitrosylation of the death receptor
fas promotes fas ligand-mediated apoptosis in cancer cells.
Gastroenterology. 140:2009–2018. 2018.e2001–2004. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sebolt-Leopold JS and Herrera R: Targeting
the mitogen-activated protein kinase cascade to treat cancer. Nat
Rev Cancer. 4:937–947. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Murillo-Carretero M, Torroglosa A, Castro
C, Villalobo A and Estrada C: S-nitrosylation of the epidermal
growth factor receptor: A regulatory mechanism of receptor tyrosine
kinase activity. Free Radic Biol Med. 46:471–479. 2009. View Article : Google Scholar
|
|
27
|
Kaliyaperumal K, Sharma A K, McDonald DG,
Dhindsa JS, Yount C, Singh AK, Won JS and Singh I:
S-nitrosoglutathione-mediated STAT3 regulation in efficacy of
radiotherapy and cisplatin therapy in head and neck squamous cell
carcinoma. Redox Biol. 6:41–50. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chattopadhyay M, Goswami S, Rodes DB,
Kodela R, Velazquez CA, Boring D, Crowell JA and Kashfi K:
NO-releasing NSAIDs suppress NF-κB signaling in vitro and in vivo
through S-nitrosylation. Cancer Lett. 298:204–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Szabo C: Gasotransmitters in cancer: From
pathophysiology to experimental therapy. Nat Rev Drug Discov.
15:185–203. 2016. View Article : Google Scholar
|