Introduction
Breast cancer is the most common type of cancer and
the second leading cause of cancer-related mortality in women
(1,2). Although several effective options,
including radiation, chemotherapy, endocrine therapy and surgery,
may be selected for treatment, the mortality rate of breast cancer
remains high. Numerous studies have been conducted to investigate
the pathogenesis of breast cancer; however, the precise mechanism
remains unclear. Genetic alterations in normal cells are considered
to be involved in the occurrence of breast cancer.
Breast cancer-specific gene 1 (BCSG1) is not
expressed in normal breast tissue or benign breast diseases, but is
highly expressed in human infiltrating breast carcinomas, and its
expression is stage-specific (3–5).
When overexpressed, BCSG1 leads to a significant increase in the
proliferation, invasiveness and metastasis of breast cancer cells
(6), whereas downregulation of
BCSG1 expression sensitizes breast cancer cells to antimicrotubule
agent-induced cytotoxicity (7–9).
These findings indicate that BCSG1 may act as a tumor marker, and
downregulation of BCSG1 may be an effective strategy in breast
cancer treatment.
As a type of retrovirus, lentiviral vectors can
infect both dividing and non-dividing cells due to their
preintegration complex (virus 'shell') (10,11). It has been reported that
lentiviruses can change the expression levels of target genes for
up to 6 months (12); thus, it
possible to provide highly effective gene therapy by using
lentiviruses. These properties make lentiviral vectors attractive
vehicles for delivering small interfering RNAs (siRNAs) into
mammalian cells (13,14).
RNA interference (RNAi) inhibits gene expression by
reducing mRNA stability or inhibiting translation (15). Since the discovery of siRNA in
gene silencing (16), RNAi has
become a powerful research tool in gene function studies. Compared
with genetic deletion, RNAi-mediated gene silencing has several
advantages (17). Numerous
studies have demonstrated the applications of RNAi in cancer
research (18,19). In the present study, a BCSG1 RNAi
lentiviral vector was initially constructed, followed by
transfection of MDA-MB-231 breast cancer cells. The proliferation,
migration and apoptosis of MDA-MB-231 cells were then evaluated and
the underlying mechanisms were investigated.
Material and methods
Cell lines
The 293T cell line was selected for lentivirus
packaging and titer determination, while the human breast cancer
line MDA-MB-231 was used for functional experiments. All the cells
were purchased from American Type Culture Collection (Manassas, VA,
USA) and were cultured in Dulbecco's modified Eagle's medium
(Gibco; Thermo Fisher Scientific, Carlsbad, CA, USA) supplemented
with 10% heat-inactivated fetal bovine serum, 100 mg/l streptomycin
and 100 u̸̸ml penicillin (Gibco; Thermo Fisher Scientific) under 5%
Co2, at 37°C in a humidified incubator.
Construction of the BCSG1 RNAi lentiviral
vector
Based on the gene sequence of BCSG1 in GenBank (Gene
ID: 6623), primers of BCSG1 siRNA and negative control were
designed and cloned into a PGLV3/H1/GFP + Puro vector. The
interference primers for BCSG1 (Si-BCSG1) were as follows: Forward,
5′-GAT CCG CCC ACT TAT GCT GCT GTG AAT TTC AAG AGA ATT CAC AGC AGC
ATA AGT GGG CTT TTT TG-3′ and reverse, 5′-AAT TCA AAA AAG CCC ACT
TAT GCT GCT GTG AAT TCT CTT GAA ATT CAC AGC AGC ATA AGT GGG CG-3′.
The primers for the interference negative control (NC) were as
follows: Forward, 5′-GAT CCG TTC TCC GAA CGT GTC ACG TTT CAA GAG
AAC GTG ACA CGT TCG GAG AAC TTT TTT G-3′ and reverse, 5′-GTT CTC
CGA ACG TGT CAC GTT TCA AGA GAA CGT GAC ACG TTC GGA GAA CTT-3′.
Cell transformation and plasmid sequencing of positive cell clones
were used to confirm the successful construction of the lentiviral
vector.
Lentivirus packaging and titer
determination
After reaching a confluence of ~70–80%, 293T cells
were transfected with NC and BCSG1 RNAi lentiviral vectors. After
48 h, the viruses were harvested and concentrated, and their titers
were detected. MDA-MB-231 cells at a confluence of ~90% were
transfected with NC lentivirus (NC group), BCSG1 lentivirus siRNA
(siRNA group) or not transfected (control group). Lipofectamine
2000 (Invitrogen; Thermo Fisher Scientific, Carlsbad, CA, USA) was
used for transfection according to the manufacturer's
instructions.
Quantitative polymerase chain reaction
(qPCR)
Total RNA was extracted from MDA-MB-231 cells and
the quality was evaluated by agarose gel electrophoresis. The
concentration of the extracted RNA was estimated by optical density
measurement (A260/A280 ratio) with the Q5000 Spectrophotometer
(Quawell, Sunnyvale, CA, USA). qPCR was then performed using the
SYBR-Green-based PCR master mix. The ABI PRISM 7500 system (ABI,
Grand Island, NY, USA) was used for all amplification reactions in
a total volume of 25 μl. The cycling conditions were as
follows: an initial 10 min of pre-denaturation at 95°C, followed by
40 cycles of 95°C for 10 sec, 60°C for 20 sec, and 72°C for 15 sec.
The specificity of the amplification products was confirmed by
melting curve analysis. All products were normalized to β-actin
mRNA levels. Each specimen was repeated 3 times.
CCK-8 assay
At 72 h after transfection, MDA-MB-231 cells were
seeded in the 96-well plates at a density of 2,000 cells/well and
incubated for 0, 24, 48 and 72 h. At the end of the incubation, 20
μl CCK-8 (Dojindo Molecular Technologies, Inc., Xiongben,
Japan) were added to each well. The plates were then incubated in a
humidified incubator at 37°C under 5% Co2 for 1 h, and
the absorbance was measured at 450 nm.
Transwell assay
After 72 h of transfection, MDA-MB-231 cells were
seeded in the 6-well Transwell upper chambers at a density of
25,000 cells/well. The Transwell assay was performed according to
the manufacturer's instructions. The Transwell chambers were then
incubated for 48 h at 37°C in a humidified incubator with 5%
Co2, and then the lower chamber was stained with
hematoxylin and photographed.
Flow cytometry analyses
MDA-MB-231 cells in the logarithmic phase of growth
were seeded in 6-well plates at a density of 500,000 cells̸well and
incubated overnight. After 72 h of transfection, the cells were
collected, washed with Dulbecco's phosphate-buffered saline (DPBS;
Genview, El Monte, CA, USA), fixed in 70% ethanol, and incubated
overnight at −20°C; ethanol was then removed by centrifugation at
3,000 × g. The cell pellets were washed with DPBS, followed by
incubation with 100 μl propidium iodide (PI) solution
(Sigma-Aldrich; Merck KGaA, St. Louis, Mo, USA) for 5–10 min in the
dark at 37°C and were then analyzed by flow cytometry (Beckman
Coulter, Brea, CA, USA).
Flow cytometeric analysis for apoptosis was
performed using an Annexin V-FITC apoptosis detection kit (Shanghai
Genechem Biotech Co., Ltd., Shanghai, China) and PI. Cells were
harvested 72 h after transfection, followed by staining with the
binding buffer, 5 μl Annexin V̸ fluorescein isothiocyanate
(FITC) for 15 min in the dark at room temperature. PI was then
added and incubated in the dark at room temperature for a further
15 min. Apoptosis was then detected by flow cytometry.
Western blotting
Cell lysates were harvested and samples (50
μg protein/lane) were fractionated by 12% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and transferred to
polyvinylidene fluoride membranes. The membranes were incubated in
5% skimmed milk for 1 h at room temperature, and overnight at 4°C
with primary antibodies; glyceraldehyde 3-phosphate dehydrogenase
was used as the control. The bands were visualized using an ECL
chemiluminescence kit (Genview) and quantitated by Quantity one
(Bio-Rad, Hercules, CA, USA).
Results
BCSG1 mRNA level in MDA-MB-231 cells
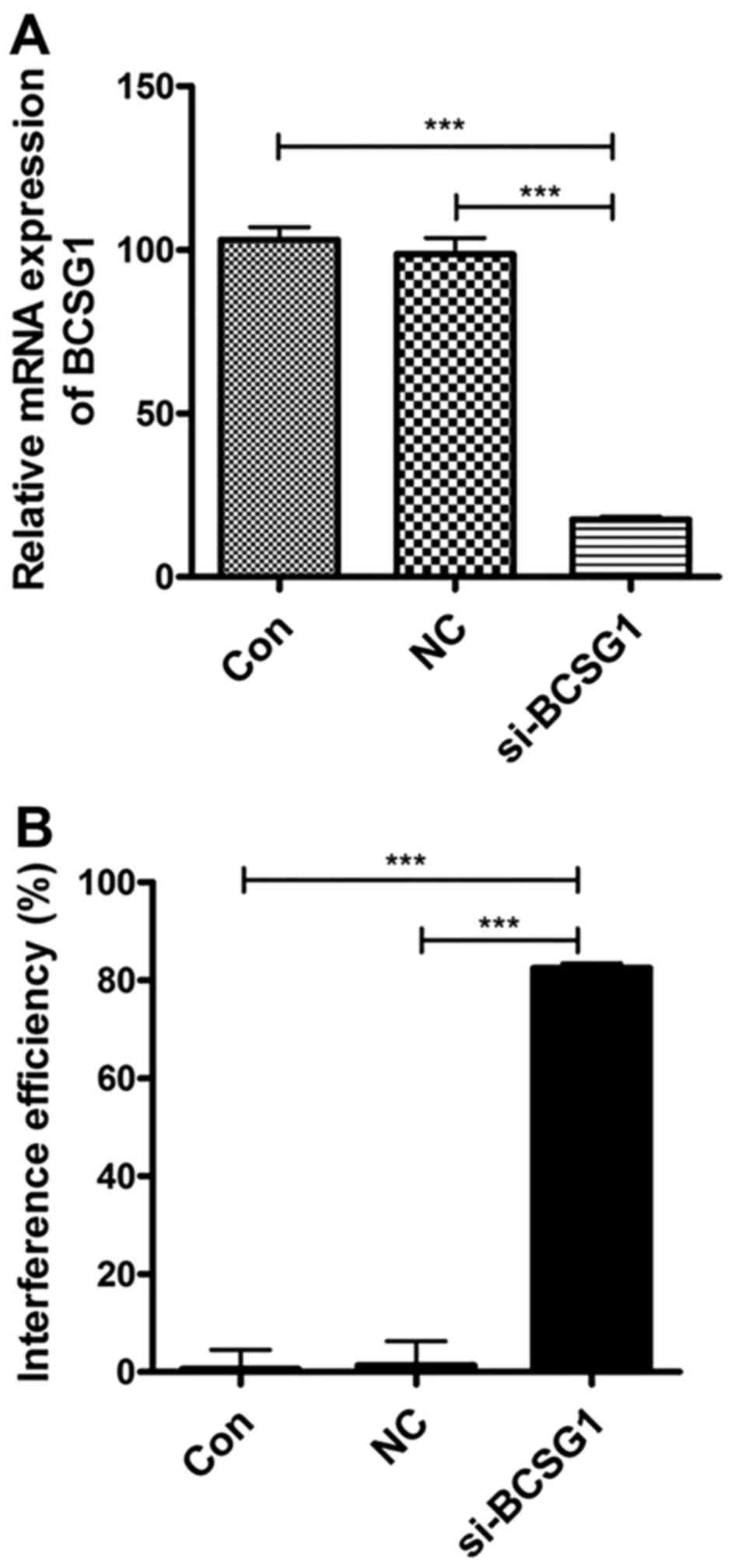
As shown in Fig.
1A, the levels of BCSG1 mRNA were found to be significantly
lower in the siRNA group compared with those in the NC
(P<0.0001) and control groups (P<0.0001). These results
suggest that BCSG1 lentivirus siRNA significantly downregulated the
BCSG1 mRNA levels in breast cancer cells; the interference
efficiency reached 82.45% (Fig.
1B).
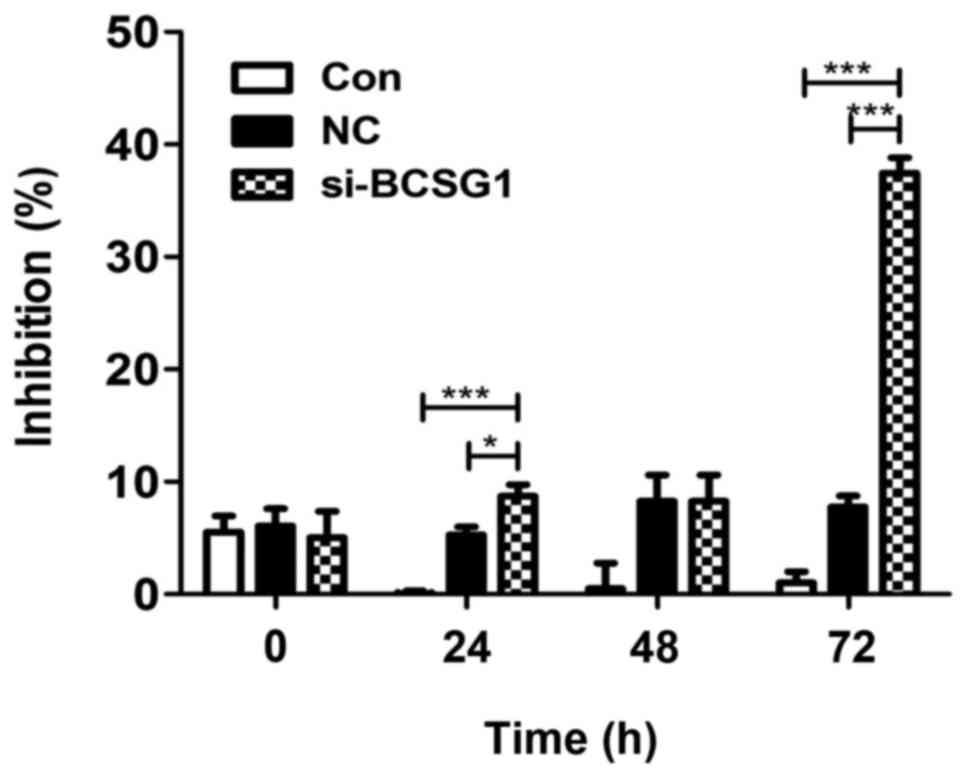
Proliferation of MDA-MB-231 cells
The CCK-8 assay demonstrated that cell proliferation
decreased significantly in the siRNA group (P<0.0001),
particularly after 72 h of treatment; no significant difference was
observed between the NC and control groups (P>0.05; Fig. 2). The CCK-8 assay demonstrated
that BCSG1 lentivirus siRNA inhibited the proliferation of breast
cancer cells.
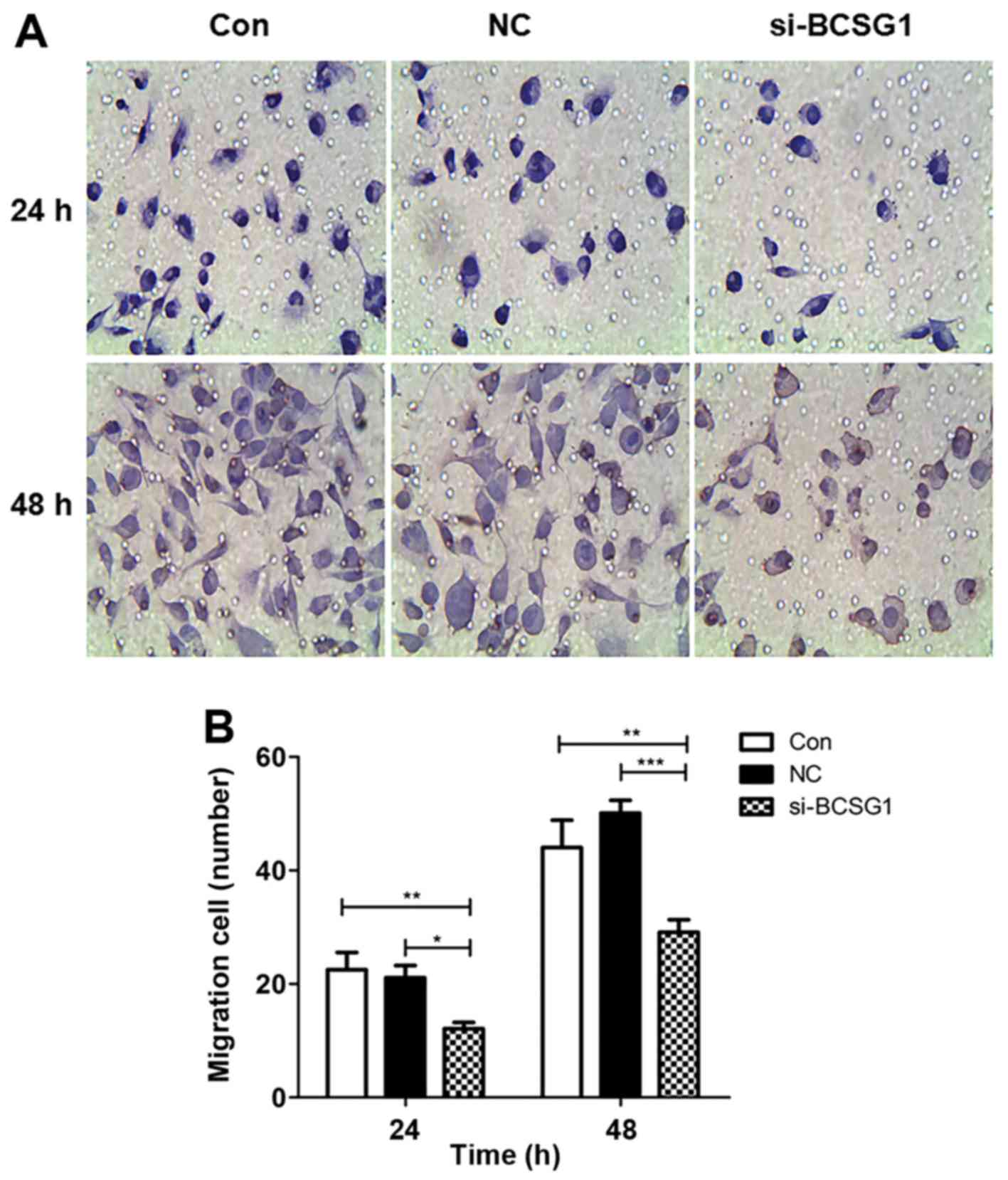
Migration of MDA-MB-231 cells
Cells that migrated through the membrane were
counted in five random fields for each group, and the relative
migration rate was calculated as follows: Relative migration rate =
number of migrated cells/number of migrated cells in the control
group. The migrated cell number in the siRNA group decreased
significantly, particularly after 48 h of treatment (Fig. 3A). The relative migration rates of
the siRNA group were significantly lower compared with those in the
control and NC groups (P<0.001 and P<0.0001, respectively;
Fig. 3B). The results indicated
that BCSG1 lentivirus siRNA inhibited breast cancer cell
migration.
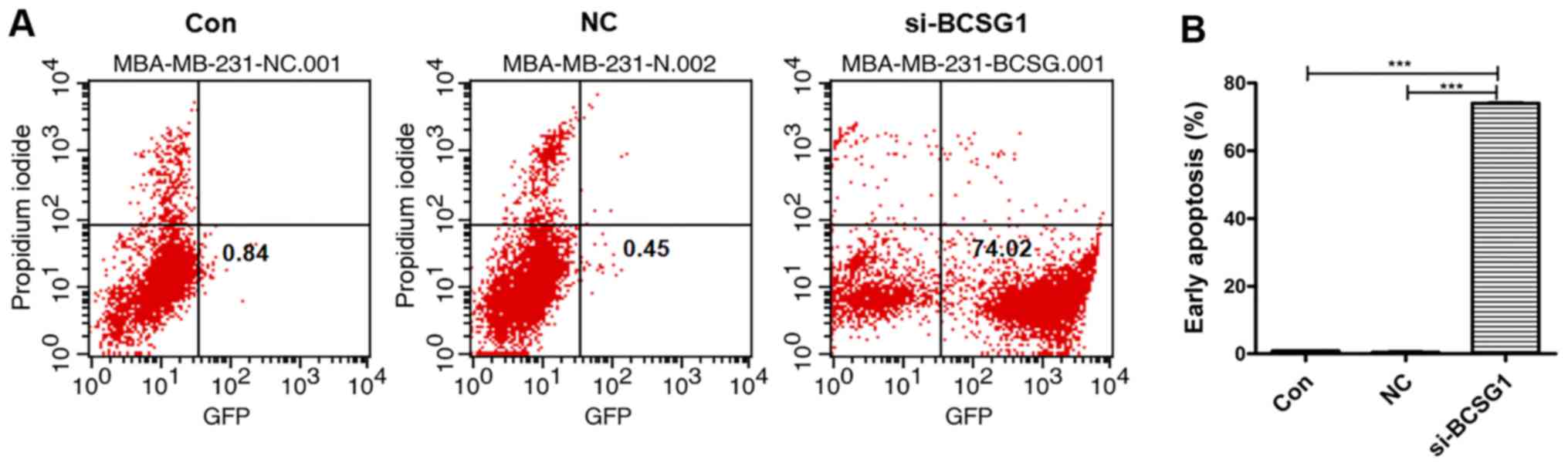
Apoptosis of MDA-MB-231 cells
As shown in Fig.
4, 74.02% of the cells in the siRNA group were Annexin
V/FITC-positive, which was significantly higher compared with the
NC (0.45%) and control groups (0.84%). These results suggested that
the BCSG1 lentivirus siRNA decreased the proliferation of breast
cancer cells through induction of apoptosis.
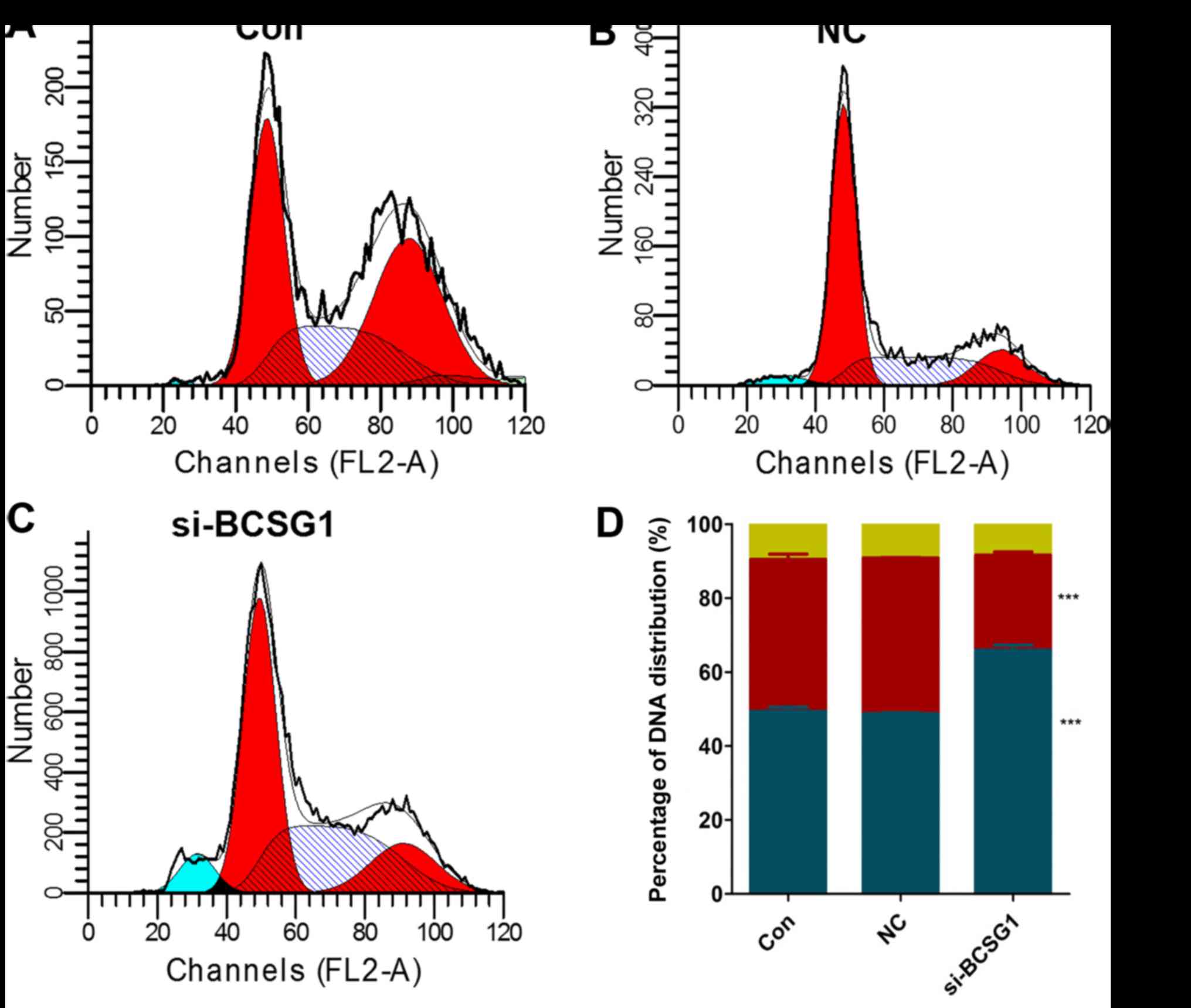
MDA-MB-231 cell cycle
As shown in Fig.
5, a higher percentage of cells in the siRNA group
(67.25±0.93%) were in the G0/G1 phase when compared with those in
the NC and control groups (48.90±0.40%, P<0.05; and 50.50±0.89%,
P<0.05, respectively). A lower percentage of cells in the siRNA
group (25.69±1.57%) were in the S phase. And ~8.42±0.87% of cells
were in the G2̸M phase. These results indicated that transfection
with BCSG1 lentivirus siRNA led to breast cancer cell cycle
arrest.
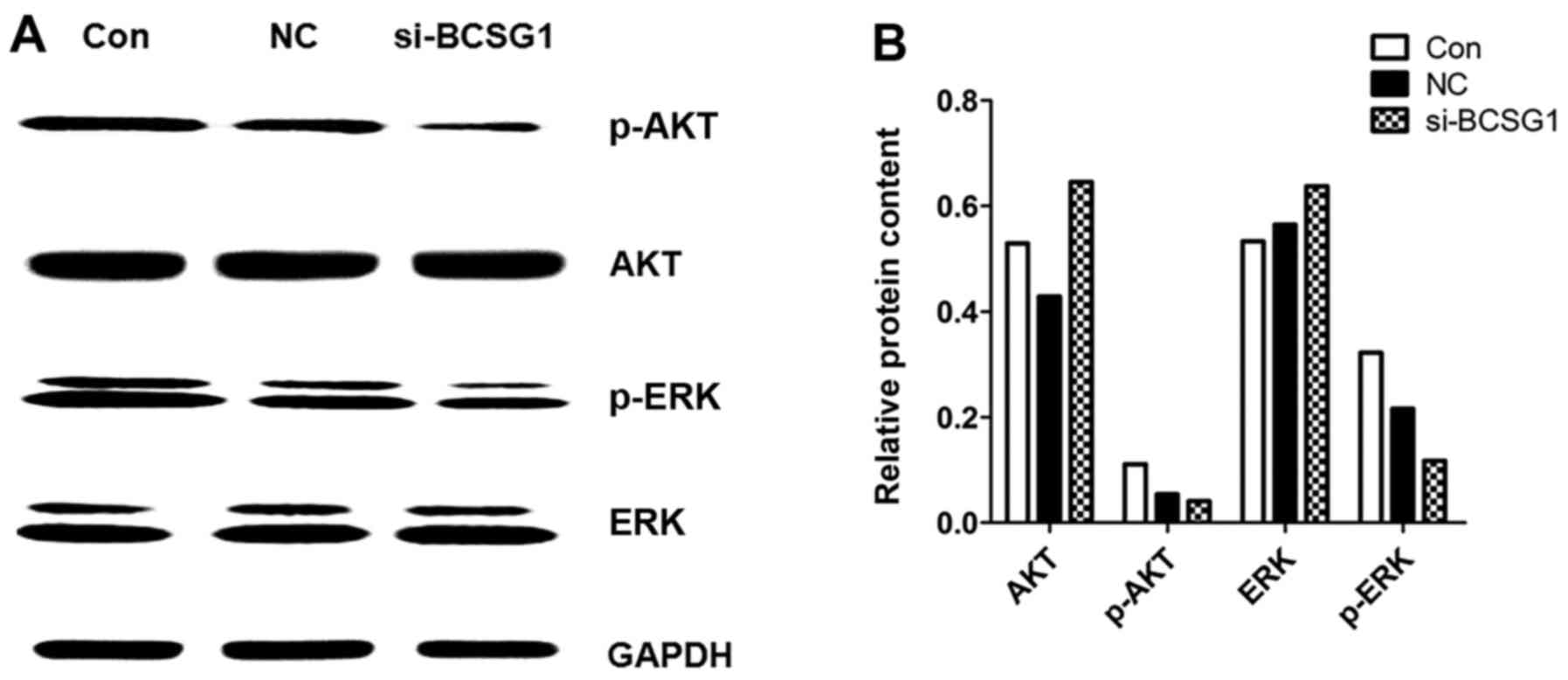
Protein expression in MDA-MB-231
cells
After transfection of BCSG1 lentivirus siRNA,
MDA-MB-231 cells exhibited a relative downregulation of p-AKT and
p-extracellular signal-regulated kinase (ERK) levels (Fig. 6), while there were no significant
differences in the expression levels of AKT and ERK among the three
groups. These data suggested that the BCSG1 lentivirus siRNA
downregulated the levels of p-AKT and p-ERK, which, in turn, may be
involved in the process of cell apoptosis induced by BCSG1
lentivirus siRNA.
Discussion
BCSG1, also referred to as γ-synuclein gene (SNCG),
was identified by Ji et al (3) in 1997 by direct sequencing of cDNA
in breast cancer. BCSG1 is not expressed in normal or benign breast
tissues, but is highly expressed in advanced and metastatic breast
tumors (4). Abnormal expression
of BCSG1 has been implicated in various types of cancer, including
ovarian, hepatic, glial tumors, esophageal, prostatic, pancreatic,
colon, gastric, lung, bladder and cervical cancers (5,20–27). In breast cancer, BCSG1 expression
was found to be closely correlated with disease stage, lymph node
involvement, metastasis, tumor size and human epidermal growth
factor receptor 2 status; however, BCSG1 expression was found to be
independent of the expression of estrogen receptor (ER) and
progesterone receptor (28).
overexpression of BCSG1 in breast cancer cells may facilitate cell
proliferation (29), increase
migration and promote metastasis in nude mice (6). Moreover, BCSG1 is associated with
ERα overexpression (30),
antimicrotubule drug resistance (31), and an accelerated rate of
chromosomal instability (32).
All these studies suggest that BCSG1 knockdown may be an effective
therapy in breast cancer treatment.
In the present study, a constructed siRNA lentiviral
vector was used to effectively suppress BCSG1 expression in human
breast cancer. BCSG1 mRNA expression was found to be significantly
suppressed (up to 84.2%) in MDA-MB-231 cells; cell migration and
proliferation decreased significantly and the cell cycle was
arrested. In accordance with our previous study, western blot
analysis indicated that overexpression of BCSG1 may enhance the
migration and viability of breast cancer cells through regulating
the AKT and ERK pathways. In addition, the induction of apoptosis
of breast cancer cells was more prominent compared with that in our
previous study (74.02 vs. 33.2%, respectively) (33). We hypothesized that this may due
to prolonged expression of BCSG1 siRNA delivered by lentiviral
vector in breast cancer cells.
RNAi is a powerful new tool, which may be used to
perform loss-of-function genetic screens in lower organisms and may
greatly facilitate the identification of components of cellular
signaling pathways. In mammalian cells, such screens have been
hampered by a lack of suitable tools that can be used on a large
scale (34). RNAi lentiviral
vectors may be a potential biological method for the short-term
treatment of breast cancer.
In conclusion, our results demonstrated that BCSG1
siRNA delivered by a lentiviral vector was able to significantly
reduce BCSG1 expression, suppress cell migration and proliferation
and lead to cell cycle arrest; reduced protein levels of p-AKT and
p-ERK may contribute to these phenomena.
Acknowledgments
The present study was funded by the Science and
Technology Planning Project of Guangdong Province (grant nos.
2013B021800096, 2013B021800096 and 2014A020212038), the Shenzhen
City Science and Technology Innovation International Cooperation
Projects 2014 (grant no. GJHZ20140414170821180), the Basic Research
Program of Shenzhen (grant nos. JCYJ 20130329110955809 and
JCYJ20150330102720122) and the Science and Technology Foundation of
Shenzhen (grant nos. CXZZ20150430092951135 and
KQTD20140630100658078), Shenzhen City Science and Technology
Innovation International Cooperation Projects 2016 (grant no.
GJHZ20160301164637011) and the Natural Science Foundation of
Guangdong (grant nos. 2016A030313029 and 2017A030313668).
References
|
1
|
Donepudi MS, Kondapalli K, Amos SJ and
Venkanteshan P: Breast cancer statistics and markers. J Cancer Res
Ther. 10:506–511. 2014.PubMed/NCBI
|
|
2
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLoBoCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar
|
|
3
|
Ji H, Liu YE, Jia T, Wang M, Liu J, Xiao
G, Joseph BK, Rosen C and Shi YE: Identification of a breast
cancer-specific gene, BCSG1, by direct differential cDNA
sequencing. Cancer Res. 57:759–764. 1997.PubMed/NCBI
|
|
4
|
Wu K, Weng Z, Tao Q, Lin G, Wu X, Qian H,
Zhang Y, Ding X, Jiang Y and Shi YE: Stage-specific expression of
breast cancer-specific gene gamma-synuclein. Cancer Epidemiol
Biomarkers Prev. 12:920–925. 2003.PubMed/NCBI
|
|
5
|
Bruening W, Giasson BI, Klein-Szanto AJ,
Lee VM, Trojanowski JQ and Godwin AK: Synucleins are expressed in
the majority of breast and ovarian carcinomas and in preneoplastic
lesions of the ovary. Cancer. 88:2154–2163. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia T, Liu YE, Liu J and Shi YE:
Stimulation of breast cancer invasion and metastasis by synuclein
gamma. Cancer Res. 59:742–747. 1999.PubMed/NCBI
|
|
7
|
Pan ZZ, Bruening W, Giasson BI, Lee VM and
Godwin AK: Gamma-synuclein promotes cancer cell survival and
inhibits stress- and chemotherapy drug-induced apoptosis by
modulating MAPK pathways. J Biol Chem. 277:35050–35060. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Singh VK, Zhou Y, Marsh JA, Uversky VN,
Forman-Kay JD, Liu J and Jia Z: Synuclein-gamma targeting peptide
inhibitor that enhances sensitivity of breast cancer cells to
antimicrotubule drugs. Cancer Res. 67:626–633. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Inaba S and Liu J: Inhibition of
synuclein-gamma expression increases the sensitivity of breast
cancer cells to paclitaxel treatment. Int J Oncol. 29:289–295.
2006.PubMed/NCBI
|
|
10
|
Naldini L: Lentiviruses as gene transfer
agents for delivery to non-dividing cells. Curr Opin Biotechnol.
9:457–463. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vodicka MA: Determinants for lentiviral
infection of non-dividing cells. Somat Cell Mol Genet. 26:35–49.
2001. View Article : Google Scholar
|
|
12
|
Cockrell AS and Kafri T: Gene delivery by
lentivirus vectors. Mol Biotechnol. 36:184–204. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li MJ and Rossi JJ: Lentiviral vector
delivery of recombinant small interfering RNA expression cassettes.
Methods Enzymol. 392:218–226. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dropulić B: Lentiviral vectors: Their
molecular design, safety, and use in laboratory and preclinical
research. Hum Gene Ther. 22:649–657. 2011. View Article : Google Scholar
|
|
15
|
Diederichs S, Jung S, Rothenberg SM,
Smolen GA, Mlody BG and Haber DA: Coexpression of argonaute-2
enhances RNA interference toward perfect match binding sites. Proc
Natl Acad Sci USA. 105:9284–9289. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elbashir SM, Harborth J, Lendeckel W,
Yalcin A, Weber K and Tuschl T: Duplexes of 21-nucleotide RNAs
mediate RNA interference in cultured mammalian cells. Nature.
411:494–498. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Stovall DB, Wan M, Zhang Q, Dubey P and
Sui G: DNA vector-based RNA interference to study gene function in
cancer. J Vis Exp. 64:e41292012.
|
|
18
|
Sui G, Soohoo C, Affar B, Gay F and Shi Y,
Forrester WC and Shi Y: A DNA vector-based RNAi technology to
suppress gene expression in mammalian cells. Proc Natl Acad Sci
USA. 99:5515–5520. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Brummelkamp TR, Bernards R and Agami R: A
system for stable expression of short interfering RNAs in mammalian
cells. Science. 296:550–553. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu H, Liu W, Wu Y, Zhou Y, Xue R, Luo C,
Wang L, Zhao W, Jiang JD and Liu J: Loss of epigenetic control of
synuclein-gamma gene as a molecular indicator of metastasis in a
wide range of human cancers. Cancer Res. 65:7635–7643. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lavedan C, Leroy E, Dehejia A, Buchholtz
S, Dutra A, Nussbaum RL and Polymeropoulos MH: Identification,
localization and characterization of the human gamma-synuclein
gene. Hum Genet. 103:106–112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhao W, Liu H, Liu W, Wu Y, Chen W, Jiang
B, Zhou Y, Xue R, Luo C, Wang L, et al: Abnormal activation of the
synuclein-gamma gene in hepatocellular carcinomas by epigenetic
alteration. Int J Oncol. 28:1081–1088. 2006.PubMed/NCBI
|
|
23
|
Zhou CQ, Liu S, Xue LY, Wang YH, Zhu HX,
Lu N and Xu NZ: Downregulation of gamma-synuclein in human
esophageal squamous cell carcinoma. World J Gastroenterol.
9:1900–1903. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu C, Guo J, Qu L, Bing D, Meng L, Wu J
and Shou C: Applications of novel monoclonal antibodies specific
for synu-clein-gamma in evaluating its levels in sera and cancer
tissues from colorectal cancer patients. Cancer Lett. 269:148–158.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Linhart W, Briem D, Amling M, Rueger JM
and Windolf J: Mechanical failure of porous hydroxyapatite ceramics
7.5 years after implantation in the proximal tibia. Unfallchirurg.
107:154–157. 2004.In German. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwaki H, Kageyama S, Isono T, Wakabayashi
Y, Okada Y, Yoshimura K, Terai A, Arai Y, Iwamura H, Kawakita M, et
al: Diagnostic potential in bladder cancer of a panel of tumor
markers (calreticulin, gamma-synuclein, and
catechol-O-meth-yltransferase) identified by proteomic analysis.
Cancer Sci. 95:955–961. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fung KM, Rorke LB, Giasson B, Lee VM and
Trojanowski JQ: Expression of alpha-, beta-, and gamma-synuclein in
glial tumors and medulloblastomas. Acta Neuropathol. 106:167–175.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo J, Shou C, Meng L, Jiang B, Dong B,
Yao L, Xie Y, Zhang J, Chen Y, Budman DR, et al: Neuronal protein
synuclein gamma predicts poor clinical outcome in breast cancer.
Int J Cancer. 121:1296–1305. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jiang Y, Liu YE, Goldberg ID and Shi YE:
Gamma synuclein, a novel heat-shock protein-associated chaperone,
stimulates ligand-dependent estrogen receptor alpha signaling and
mammary tumorigenesis. Cancer Res. 64:4539–4546. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang Y, Liu YE, Lu A, Gupta A, Goldberg
ID, Liu J and Shi YE: Stimulation of estrogen receptor signaling by
gamma synuclein. Cancer Res. 63:3899–3903. 2003.PubMed/NCBI
|
|
31
|
Gupta A, Inaba S, Wong OK, Fang G and Liu
J: Breast cancer-specific gene 1 interacts with the mitotic
checkpoint kinase BubR1. Oncogene. 22:7593–7599. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Inaba S, Li C, Shi YE, Song DQ, Jiang JD
and Liu J: Synuclein gamma inhibits the mitotic checkpoint function
and promotes chromosomal instability of breast cancer cells. Breast
Cancer Res Treat. 94:25–35. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He J, Xie N, Yang J, Guan H, Chen W, Wu H,
Yuan Z, Wang K, Li G, Sun J, et al: siRNA-mediated suppression of
Synuclein γ inhibits MDA-MB-231 cell migration and proliferation by
downregulating the phosphorylation of AKT and ERK. J Breast Cancer.
17:200–206. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Berns K, Hijmans EM, Mullenders J,
Brummelkamp TR, Velds A, Heimerikx M, Kerkhoven RM, Madiredjo M,
Nijkamp W, Weigelt B, et al: A large-scale RNAi screen in human
cells identifies new components of the p53 pathway. Nature.
428:431–437. 2004. View Article : Google Scholar : PubMed/NCBI
|