Introduction
Macrophages play important roles in the host immune
defense system during infection and disease development; their
activation by different stimuli, including bacterial
lipopolysaccharide (LPS) (1,2),
causes production of pro-inflammatory cytokines [tumor necrosis
factor (TNF)-α, interleukin (IL)-1β and IL-6], inflammatory
mediators [nitric oxide (NO), prostaglandin (PG) E2],
and reactive oxygen species (ROS), which contribute to the
progression of several inflammatory diseases (3). In turn, the inflammatory burden of
ROS is increased by the formation of reactive nitrogen species
(RNS) from the rapid combination of NO with superoxide
radical-induced nitrosative stress. Therefore, an imbalanced redox
milieu may result from either or both overproduction of ROS and
decreased antioxidant capacity (2). It is well-known that useful
therapeutic strategies for various inflammatory diseases include
the control of production of free radicals and inflammatory
mediators.
In complementary and alternative medicine, diseases
are considered to represent an imbalance between Yin and Yang. In
traditional Chinese medicine and traditional Korean medicine (TKM),
various treatment methods, including acupuncture, pharmacopuncture
and herbal medicines, are used to rebalance the Yin and Yang of
individual patients; in particular, the mechanism underlying their
therapeutic effect has been reported to be associated with
anti-inflammatory and immunomodulatory responses.
A growing body of scientific evidence supports
acupuncture as an effective treatment of various inflammatory
conditions in several diseases (4,5).
Recent studies have reported on the mediation of the
anti-inflammatory and immunomodulatory effects of acupuncture by
the downregulation of pro- inflammatory cytokines, including TNF-α,
IL-1β, IL-6, IL-8 and IL-10, along with C-reactive protein,
erythrocyte sedimentation rate, and the modulation of
immunoglobulin (Ig) M and IgA in chronic obstructive pulmonary
disease, spastic cerebral palsy and periodontitis, as well as in
clinical studies of rheumatoid arthritis and acute pancreatitis
(4,6-10).
Pharmacopuncture therapy, which includes stimulating
acupoints along with the injection at the acupoints of herbal
extracts, is a new form of acupuncture applied in TKM. This therapy
is commonly used to regulate immune imbalance in the clinical
setting and is considered as a good candidate for the treatment of
inflammatory diseases. Among pharmaco-puncture medicines, MOK is
used for the clinical treatment of the fire meridians and symptoms
related to diseases of the heart and thyroid gland. It is also used
for a Korean somatization disorder, Hwa-Byung, which is a mental
illness associated with the inability to control anger (11). MOK, a pharmacopuncture medicine
consisting of ten herbs, is used to treat these conditions due to
its anti-inflammatory and antioxidant properties, in addition to
its immunomodulatory functions (12-14). Specifically, MOK has been reported
to exert anti-inflammatory effects in cell-based assays (14). Moreover, its in vivo
anti-hypothyroidism and anti-hyperthyroidism effects have been
confirmed in previous studies (15,16). In addition, its inhibitory
function on the production of inflammatory mediators by primary
peritoneal macrophages has been reported (17). However, there is little scientific
evidence regarding the efficacy of MOK therapy in inflammation.
The aim of the present study was to investigate the
anti-inflammatory and antioxidant effects of MOK on RAW264.7
macrophages stimulated by LPS and identify the mechanism
responsible for these effects on signaling pathways.
Materials and methods
Reagents
The present study used Dulbecco’s modified Eagle’s
medium (DMEM) obtained from Invitrogen; Thermo Fisher Scientific
(Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific), 2 mm L-glutamine
(Invitrogen; Thermo Fisher Scientific), and 0.1 mm (0.7
µl/100 ml final media volume) 2-mercaptoethanol
(Sigma-Aldrich; Merck KGaA, St. Louis, MO, USA). LPS (Isotype
055:B5), sulphanilamide, dimethyl sulphoxide and
N-(1-napthyl)ethylenediamine dihydrochloride (NED) were purchased
from Sigma-Aldrich; Merck KGaA. Penicillin and streptomycin were
obtained from GenDEPOT (Barker, TX, USA). The WST-1-based cell
cytotoxicity assay kit was obtained from Roche Diagnostics GmbH
(Mannheim, Germany). Primary monoclonal antibodies against iNOS
(1:200, cat. no. 13120, Cell Signaling Technology, Danvers, MA,
USA), NF-κB (1:200, sc-109, Santa Cruz Biotechnology, Dallas, TX,
USA), TBP (1:200, sc-273, Santa Cruz Biotechnology), β-actin
(1:2,000, cat. no. A-5316-2ML, Sigma-Aldrich; Merck KGaA), ERK1/2
(1:200, cat. no. 9102, Cell Signaling Technology), JNK (1:200, cat.
no. 9252, Cell Signaling Technology), p38 (1:200, cat. no. 9212,
Cell Signaling Technology) MAPKs and their phosphorylated forms
(1:200, cat. no. 9101 for p-ERK, cat. no. 9251 for p-JNK, and cat.
no. 9211 for p-p38), superoxide dismutase (SOD) 2 (1:1,000, cat.
no. 13141, Cell Signaling Technology), catalase (CAT; 1:1,000, cat.
no. 14097, Cell Signaling Technology), and COX-2 (1:1,000, cat no.
sc-7951, Santa Cruz Biotechnology) were used. Goat anti-mouse
(1:2,000, cat. no. 1721011, Bio-Rad Laboratories, Hercules, CA,
USA) or rabbit IgE (H+L)-horseradish peroxidase-conjugated
secondary antibody (1:2,000, cat. no. 1721019, Bio-Rad
Laboratories) were used. All other chemicals were purchased from
Sigma-Aldrich; Merck KGaA, the Taq-based PCR enzyme was supplied by
Toyobo (Osaka, Japan), and the OxiSelect in vitro ROS/RNS
assay kit was obtained from Cell Biolabs (San Diego, CA, USA).
MOK extract preparation
The MOK extract, a prescription containing ten herbs
(Table I), was obtained in a
sealing vial (53.1 mg/ml) from Namsangcheon Herbal Medicine
Dispensary, an extramural facility meeting the Korean Good
Manufacturing Practice standards. All raw materials of MOK were
authenticated by the Korean Food and Drug Administration.
 | Table IConstituents of MOK extract. |
Table I
Constituents of MOK extract.
| Herbal name | Scientific
name | Content
(mg/ml) |
|---|
| Hominis
Placenta | Hominis
Placenta | 2 |
| Moschus | Moschus
berezovskii | 0.5 |
| Fel Ursi | Ursus
arctos | 0.3 |
| Calculus Bovis
Cow | Bos
taurus | 0.3 |
| bezoar | | |
| Scutellariae
Radix | Scutellaria
baicalensis | 10 |
| Phellodendri
Cortex | Phellodendron
amurense | 10 |
| Pulsatilla
Koreana | Pulsatilla
koreana | 10 |
| Sophorae
Subprostratae | Sophora
tonkinensis | 10 |
| Radix | | |
| Aucklandiae
Radix | Aucklandia
lappa | 5 |
| Aquilaria
agallocha | Aquilaria
agallocha | 5 |
| Total | 53.1 |
Cell culture
RAW 264.7 cells, a mouse macrophage cell line
obtained from the American Type Culture Collection (Manassas, VA,
USA), were maintained in DMEM supplemented with 10% FBS (HyClone;
GE Healthcare Life Sciences, Logan, UT, USA), 100 U/ml penicillin
and 100 µg/ml streptomycin at 37°C in a 5% CO2
incubator.
Cell viability assay
The commercially available WST-1 cell viability
assay kit was employed to evaluate the cytotoxic effects of MOK
extract in accordance with the manufacturer’s protocol. Briefly,
RAW 264.7 cells (5×104) were seeded into 96-well
microtiter plates (Nunc, Roskillde, Denmark) with different
concentrations of MOK extract and cultured for 24 h at 37°C in an
incubator with 5% CO2. The WST-1 reagent was added to
each well at the end of the treatment, and the plates were
incubated for a further 2 h. Finally, absorbance was measured at
450 nm with a microtiter plate reader (Asys, Cambridge,
England).
NO level measurement
RAW 264.7 cells (1×105 cells/ml) were
pretreated with various concentrations of MOK extract for 30 min
and then stimulated for 24 h with or without 1 µg/ml LPS at
37°C in an incubator with 5% CO2. The NO level in the
culture supernatants was measured using Griess reagents by adding
50 µl 1% sulphanilamide and 50 µl 0.1% NED in 5%
phosphoric acid to 100 µl of culture supernatant in each
well and incubating at room temperature for 15 min in the dark.
Subsequently, absorbance at 540 nm was measured with a Spectramax
250 microplate reader (GENios; Tecan, Männedorf, Switzerland). A
standard curve was prepared using NaNO2 as a standard
solution in the same manner, and was used to calculate the
concentration of NO.
PGE2 level measurement
RAW 264.7 cells were pretreated with various
concentrations of MOK extract for 30 min followed by stimulation
with or without LPS for 18 h at 37°C in an incubator with 5%
CO2. PGE2 levels were assessed by a
commercially available PGE2 enzyme immunoassay kit
(Cayman Chemical Co., Ann Arbor, MI, USA) in accordance with the
instructions of the manufacturer. The PGE2 concentration
was analyzed in accordance with the formula obtained from the
standard curve generated using the PGE2 standard
solution provided in the kit.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis
RAW 264.7 cells were pretreated with various
concentrations of MOK extract for 30 min prior to incubation for 5
h at 37°C in an incubator with 5% CO2, with or without
LPS. Total RNA was extracted from the cells using TRIzol reagent
(Gibco-BRL Life Technologies; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and used for cDNA synthesis along with an
oligo-dT primer, ImProm-II reverse transcriptase (2 U), 0.5 mM
dNTP, 3 mM MgCl2, and RNase inhibitor in 5X Reverse
Transcriptase Buffer (Promega Co., Madison, WI, USA). cDNA was
synthesized at 25°C for 5 min and 42°C for 60 min. PCR was
performed with the incubation mixture [2 µl cDNA, 4
µM 5′ and 3′ specific primers (Table II), 10X buffer (10 mM Tris-HCl,
pH 8.3, 50 mM KCl, 25 mM MgCl2, 0.1% Triton X-100, 250
µM dNTP, and 1 U Taq polymerase (TaKaRa Bio Inc., Shiga,
Japan)] under the following conditions: 30 sec at 94°C
(denaturation), 30 sec at 60°C (annealing), 1 min for extension,
and a final extension for 10 min at the end of 35 cycles. The band
intensities were quantified by densitometric analysis (ChemiDoc MP
Imaging System; Bio-Rad Laboratories) and were expressed relative
to the intensity of the GAPDH band.
 | Table IIPrimers for PCR analysis. |
Table II
Primers for PCR analysis.
| Name | Forward
(5′→3′) | Reverse
(5′→3′) |
|---|
| iNOS |
GGTGTTGAAGGCGTAGCTGA |
ATCATGGACCACCACACAGC |
| COX-2 |
ATGCTCCTGCTTGAGTATGT |
CACTACATCCTGACCCACTT |
| IL-1β |
ATGGCAACTGTTCCTGAACTCAACT |
CAGGACAGGTATAGATTCTTTCCTTT |
| IL-6 |
GAGGATACCACTCCCAACAGACC |
TTCACAGAGGATACCACTCC |
| TNF-α |
AGCCCCCAGTCTGTATCCTT |
CTCCCTTTGCAGAACTCAGG |
| MnSOD |
GTGACTTTGGGTCTTTTGA |
GCTAACATTCTCCCAGTT |
| HO-1 |
AAGATTGCCCAGAAAGCCCTGGAC |
AACTGTCGCCACCAGAAAGCTGAG |
| GAPDH |
GACATCATACTTGGCAGG |
CTCGTGGAGTCTACTGGT |
Western blot analysis
RAW 264.7 cells were pretreated for 30 min with MOK
extract at various concentrations, followed by stimulation with or
without LPS for 24 h (in the case of iNOS, COX-2, SOD2 and CAT),
for 30 min (in the case of NF-κB, I-κB, JNK, and p38 MAPK), or for
5 min (in the case of ERK MAPK) at 37°C in a 5% CO2
incubator. The cells were lysed by adding 0.1 ml 50 mm Tris-HCl (pH
7.2), including 0.1% sodium dodecyl sulphate, 1% sodium
deoxycholate, 1% NP-40 and 0.15 M NaCl. Nuclear extracts were
prepared using the NE-PER Nuclear and Cytoplasmic Extraction Kit
(Pierce Biotechnology Inc., Rockford, IL, USA) in accordance with
the manufacturer’s instructions, and the protein concentration was
determined using the Bradford assay. Equal amounts of protein (20
µg/ml) were electrophoresed on 10% sodium dodecyl
sulfate-polyacrylamide gels. After electrophoresis, an electric
transfer system was used to transfer proteins from the gel onto a
nitrocellulose membrane. Non-specific binding was blocked with 3%
skimmed milk in TBST buffer (5 mm Tris-HCl, pH 7.6, 136 mm NaCl and
0.1% Tween-20) for 1 h. The blots were then incubated for 1 h at
room temperature with primary antibodies against iNOS, COX-2,
β-actin, all forms of ERK1/2, JNK, p38 and their phosphorylated
forms, SOD2, CAT, p65 NF-κB, or I-κB, and washed three times with
1X TBST. The blots were incubated for 1 h at room temperature with
horseradish peroxidase-labeled anti-mouse IgG (Santa Cruz
Biotechnology), washed with 1X TBST three times and developed using
ECL western blotting detection reagents (GE Healthcare
Bio-Sciences, Pittsburgh, PA, USA) prior to analysis with a
ChemiDoc MP Imaging System (Bio-Rad Laboratories). The intensity of
western blot bands was quantified and values were expressed
relative to that of β-actin or total forms of the MAPKs.
Immunocytochemistry
To confirm the nuclear translocation of NF-κB, cells
were seeded in four-well slide chambers and pretreated with MOK
extract at various concentrations with or without LPS (1
µg/ml) for 2 h. The cells were washed twice with 1X PBS and
fixed with 1% paraformaldehyde for 15 min, followed by blocking
with 1% bovine serum albumin in PBST to reduce non-specific
immunoreactivity. The cells were then incubated overnight with
anti-NF-κB p65 antibody at 4°C, washed three times with 1X PBS, and
incubated with goat anti-rabbit Alexa Fluor 488 (green)-labelled
secondary antibody (Abcam, Cambridge, UK) for 2 h at room
temperature in the dark. The stained cells were washed with 1X PBS
and then mounted with fluorescence mounting medium containing
4′,6-diamidino-2-phenylindole (DAPI). Next, the fluorescent-stained
cells were examined under a fluorescence microscope (Leica, Solms,
Germany) at a magnification of ×200.
Intracellular ROS measurement
RAW 264.7 cells (5×105 cells/ml) were
pre-incubated for 20 h, followed by incubation with or without MOK
extract (2.5, 5 or 10 mg/ml) with or without LPS for 24 h. The ROS
levels were then analyzed using an OxiSelect in vitro
ROS/RNS assay kit (Cell Biolabs) based on the conversion of
2′,7′,7-diacetate to 2′,7′-dichlorodihydrofluorescein by ROS. The
cells were homogenized, and the fluorescence spectrum (excitation:
480 nm, emission: 530 nm) was measured with a fluorescence plate
reader (LS 55 Luminescence spectrometer; Perkin Elmer, Wellesley,
MA, USA).
Statistical analysis
GraphPad Prism (GraphPad Software, Inc., San Diego,
CA, USA) was used for the statistical analyses. The data were
summarized as means ± standard error of the mean of three
independent experiments and analyzed by one-way analysis of
variance followed by Tukey’s post hoc test (a null hypothesis of no
difference was rejected with a P-value <0.05).
Results
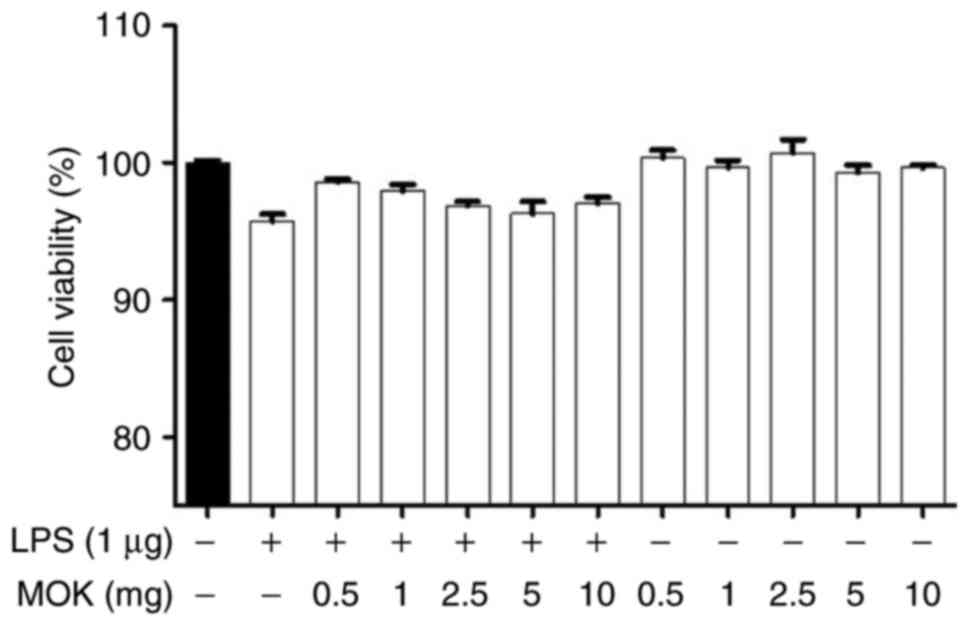
Effect of MOK on cell viability
The cytotoxic effects of MOK extract were
investigated by measuring cell viability using the WST-1 assay.
Treatment of RAW 264.7 cells stimulated by LPS with MOK extract at
a concentration of 10 mg/ml exerted no effect on cell viability
(Fig. 1). Therefore, MOK extract
was used at concentrations of 2.5-10 mg/ml to investigate its
effects on inflammation induced by LPS in RAW 264.7 cells.
Effects of MOK on NO production and iNOS
expression
The inhibitory effect of MOK extract on
pro-inflammatory mediator production was investigated by measuring
NO levels and iNOS expression in RAW 264.7 cells stimulated by LPS.
NO production (Fig. 2A) and iNOS
expression at the mRNA (Fig. 2B)
and protein (Fig. 2C) levels
exhibited a statistically significant (P<0.05, P<0.01 and
P<0.01, respectively) increase following stimulation of the
cells by LPS. Ttreatment with MOK extract at concentrations of 2.5,
5 and 10 mg/ml achieved a statistically significant decrease
(P<0.05) of NO production in cells stimulated by LPS, in a
dose-dependent manner. Moreover, treatment with 10 mg/ml MOK
extract achieved a statistically significant inhibition of iNOS
expression at the mRNA (Fig. 2B)
and protein (Fig. 2C) levels
(P<0.01 and P<0.05, respectively) in cells stimulated by LPS,
indicating that MOK extract inhibited NO production by
downregulating iNOS transcription in the activated macrophages.
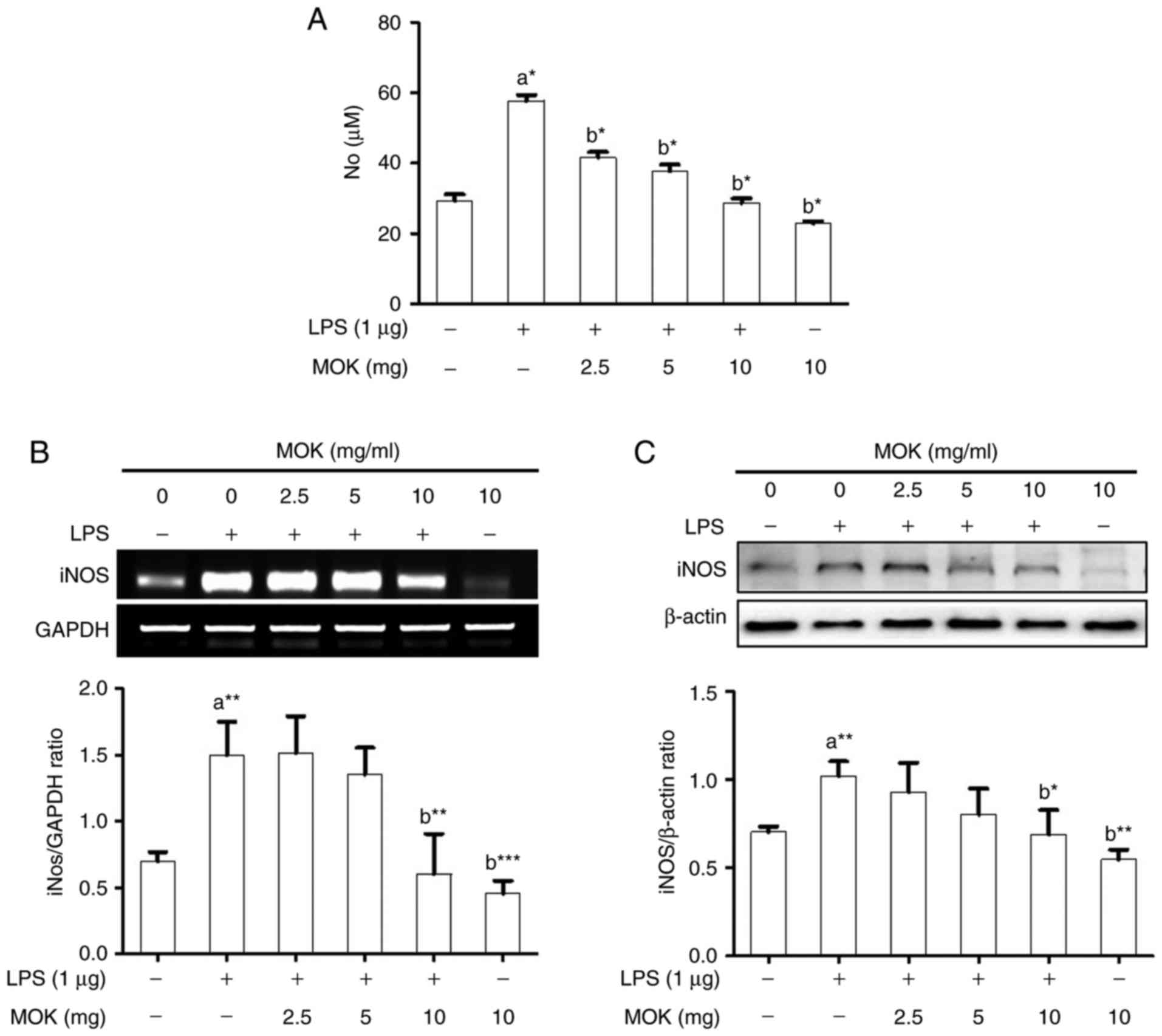 | Figure 2Effects of MOK extract on NO
production and iNOS expression in RAW 264.7 cells stimulated by
LPS. (A) After treatment with the indicated MOK extract
concentrations for 30 min, cells were stimulated for 24 h with LPS
(1 µg/ml). Griess reaction was used to measure NO
concentrations in the culture media. Data represent means ±
standard error of the mean of three independent experiments.
*P<0.05, **P<0.01 and
***P<0.001 vs. 1st bar, untreated control cells (a)
or 2nd bar, cells treated with LPS only (b). (B) After treatment
with MOK extract for 30 min, cells were stimulated with LPS for an
additional 5 h. Total RNA was isolated, and then RT-PCR was used to
measure the mRNA levels of iNOS with GAPDH expression as an
internal control. (C) After treatment with MOK extract for 30 min,
cells were stimulated with LPS for an additional 24 h. The cell
lysates were extracted and western blotting was used to analyze
iNOS protein levels with β-actin expression as an internal control.
Mean densitometric values of the three independent experiments were
analyzed and are expressed as bar charts. NO, nitric oxide; iNOS,
inducible nitric oxide synthase; LPS, lipopolysaccharide; RT-PCR,
reverse transcription-polymerase chain reaction; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase. |
Effects of MOK extract on PGE2
production and COX-2 expression
Next, the inhibitory effects of MOK extract on
PGE2 production and COX-2 expression were examined in
RAW 264.7 cells stimulated by LPS. PGE2 production
(Fig. 3A) and COX-2 expression at
the mRNA (Fig. 3B) and protein
(Fig. 3C) levels exhibited a
statistically significant increase (P<0.001, P<0.01 and
P<0.05, respectively) following stimulation of the cells by LPS.
Treatment of RAW 264.7 cells with MOK extract at concentrations of
2.5, 5 and 10 mg/ml achieved a statistically significant decrease
(P<0.05, P<0.01 and P<0.001, respectively) in LPS-induced
PGE2 production in a concentration-dependent manner
(Fig. 3A). MOK extract (10 mg/ml)
also achieved a statistically significant inhibition of COX-2 mRNA
(Fig. 3B) and protein (Fig. 3C) expression (P<0.05 and
P<0.01, respectively) in cells stimulated by LPS, indicating
that MOK extract inhibited PGE2 production by
downregulating the transcription of COX-2 in activated
macrophages.
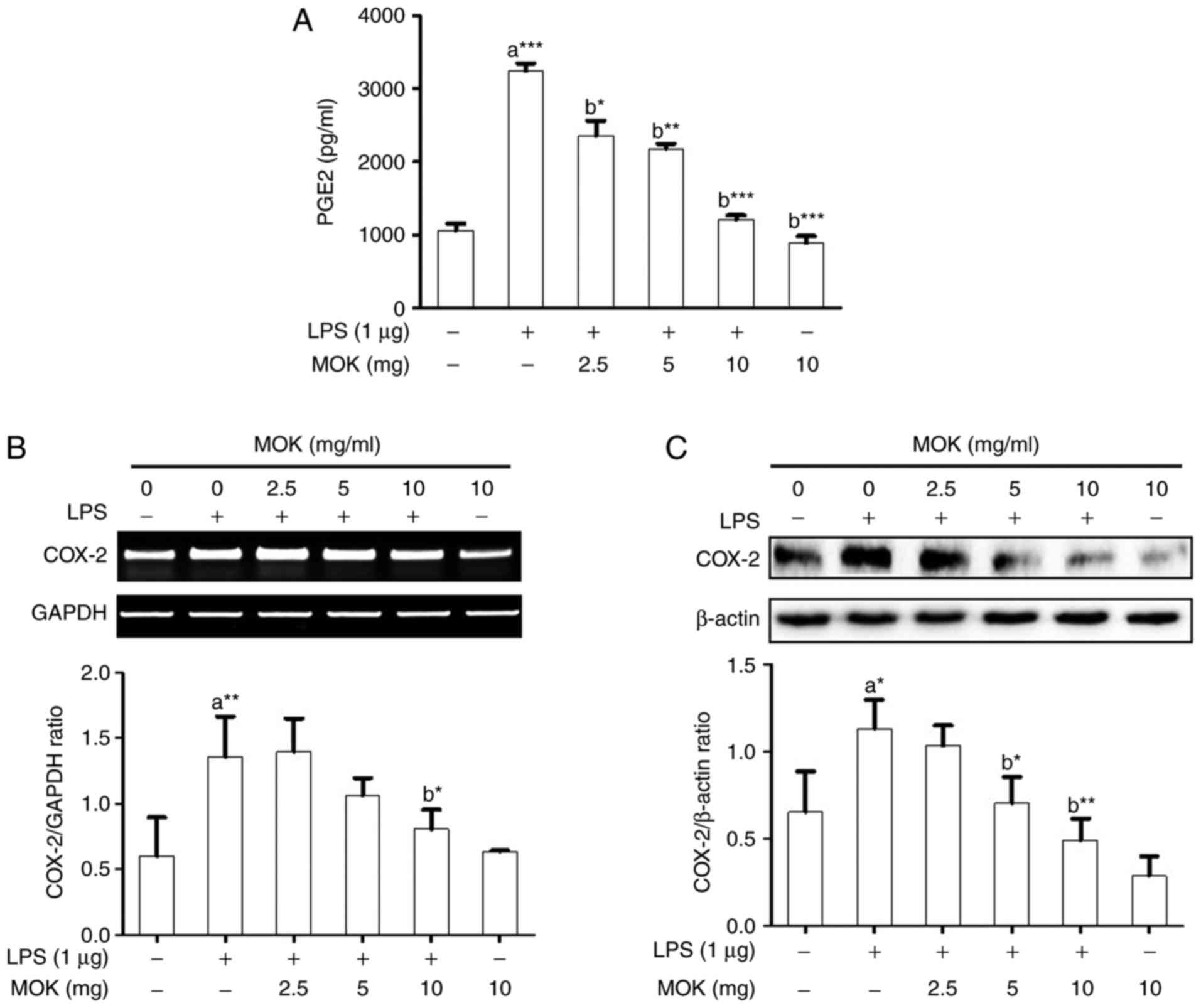 | Figure 3Effects of MOK extract on the
production of PGE2 and expression of COX-2 in RAW 264.7
cells stimulated by LPS. (A) After treatment with various
concentrations of MOK extract for 30 min, cells were then
stimulated with LPS. At 16 h, culture media were collected and
measured by an enzyme immunoassay for PGE2
concentration. Data represent means ± standard error of the mean of
three independent experiments. *P<0.05,
**P<0.01 and ***P<0.001 vs. 1st bar,
untreated control cells (a) or 2nd bar, cells treated with LPS only
(b). (B) After treatment with MOK extract for 30 min, cells were
stimulated with LPS for an additional 5 h. RT-PCR was used to
measure the mRNA levels of COX-2 with GAPDH expression as an
internal control. (C) After treatment with MOK extract for 30 min,
cells were stimulated with LPS for an additional 24 h. Western
blotting was used to analyze the protein level of COX-2 in total
cell lysates with β-actin as an internal control. Mean
densitometric values of the three independent experiments were
analyzed and are expressed as bar charts. PGE2,
prostaglandin E2; COX, cyclooxygenase; LPS,
lipopolysaccharide; RT-PCR, reverse transcription-polymerase chain
reaction; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
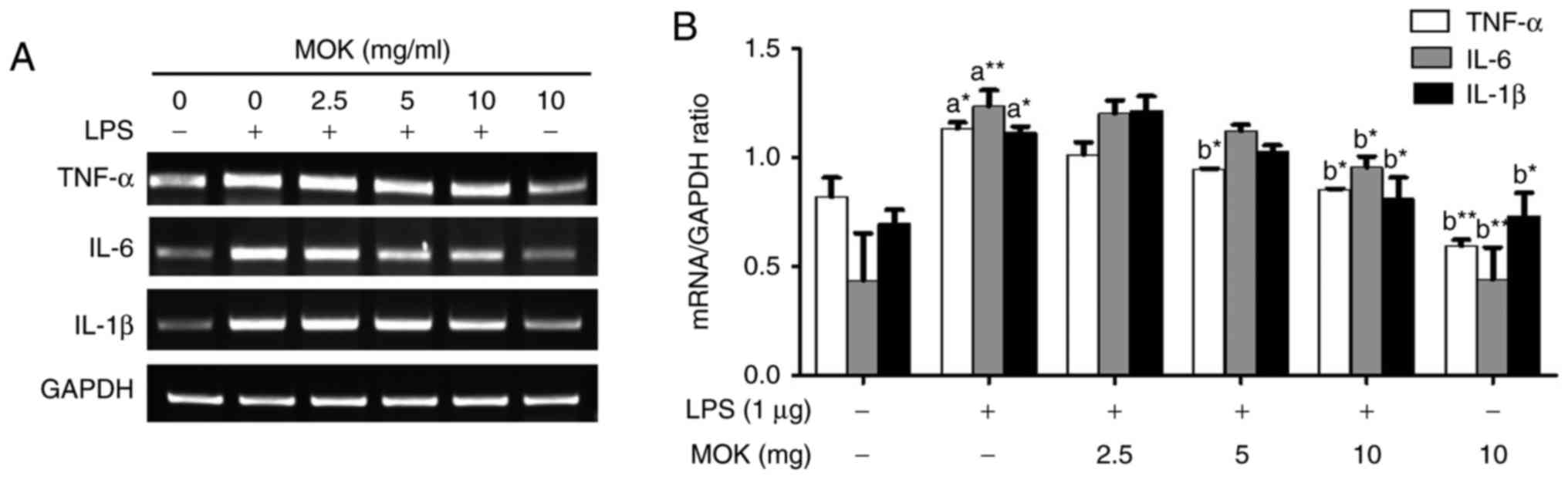
Effect of MOK extract on the LPS-induced
production of pro-inflammatory cytokines
To better understand the inhibitory effects of MOK
extract on inflammation, mRNA expression of pro-inflammatory
cytokines (IL-6, IL-1β and TNF-α) was investigated via RT-PCR in
RAW 264.7 cells stimulated by LPS. The expression of
pro-inflammatory cytokines (IL-6, IL-1β and TNF-α) at the mRNA
level exhibited a statistically significant increase (P<0.05,
P<0.01 and P<0.05, respectively) following stimulation of the
cells by LPS. Treatment with MOK extract was associated with
inhibition of LPS-induced mRNA expression of IL-6, IL-1β and TNF-α
in RAW 264.7 cells (Fig. 4A); a
statistically significant decrease (P<0.05) in the expression of
these mRNAs at a concentration of 10 mg/ml MOK (Fig. 4B) was observed, indicating that
MOK extract inhibits the synthesis of pro-inflammatory cytokines in
activated macrophages.
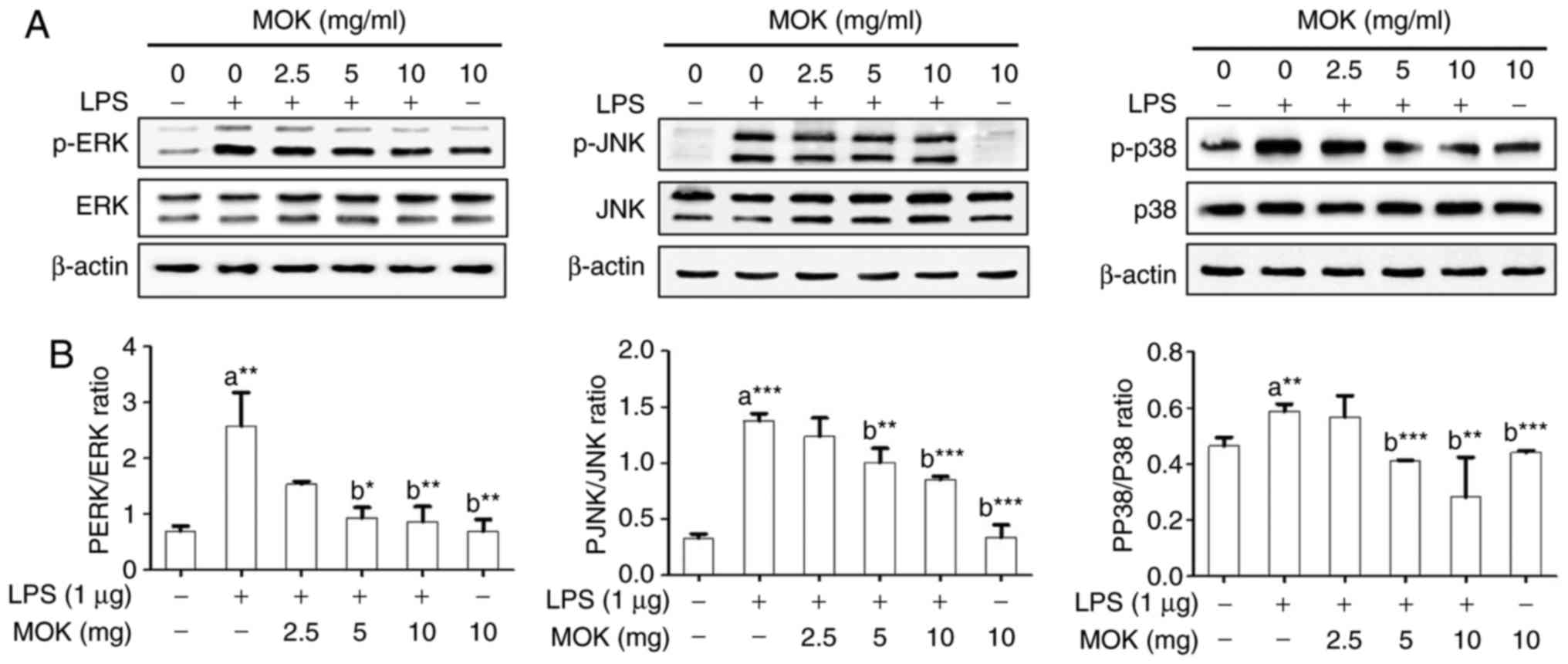
Effect of MOK extract on the MAPK/NF-κB
pathway
To confirm the effects of MOK extract on
inflammatory signaling in RAW 264.7 cells, the phosphorylation of
ERK1/2, JNK and p38 MAPK, and the expression of NF-κB p65, were
investigated by western blot analysis. Phosphorylation of MAPK
(ERK1/2, JNK and p38) exhibited a statistically significant
increase (P<0.01, P<0.001 and P<0.01, respectively)
following stimulation of the cells by LPS. Treatment with 5 mg/ml
(P<0.05, P<0.01 and P<0.001, respectively) and 10 mg/ml
MOK extract (P<0.01, P<0.001 and P<0.001, respectively)
was associated with a statistically significant inhibition of
phosphorylation of MAPK (ERK1/2, JNK and p38) in RAW 264.7 cells
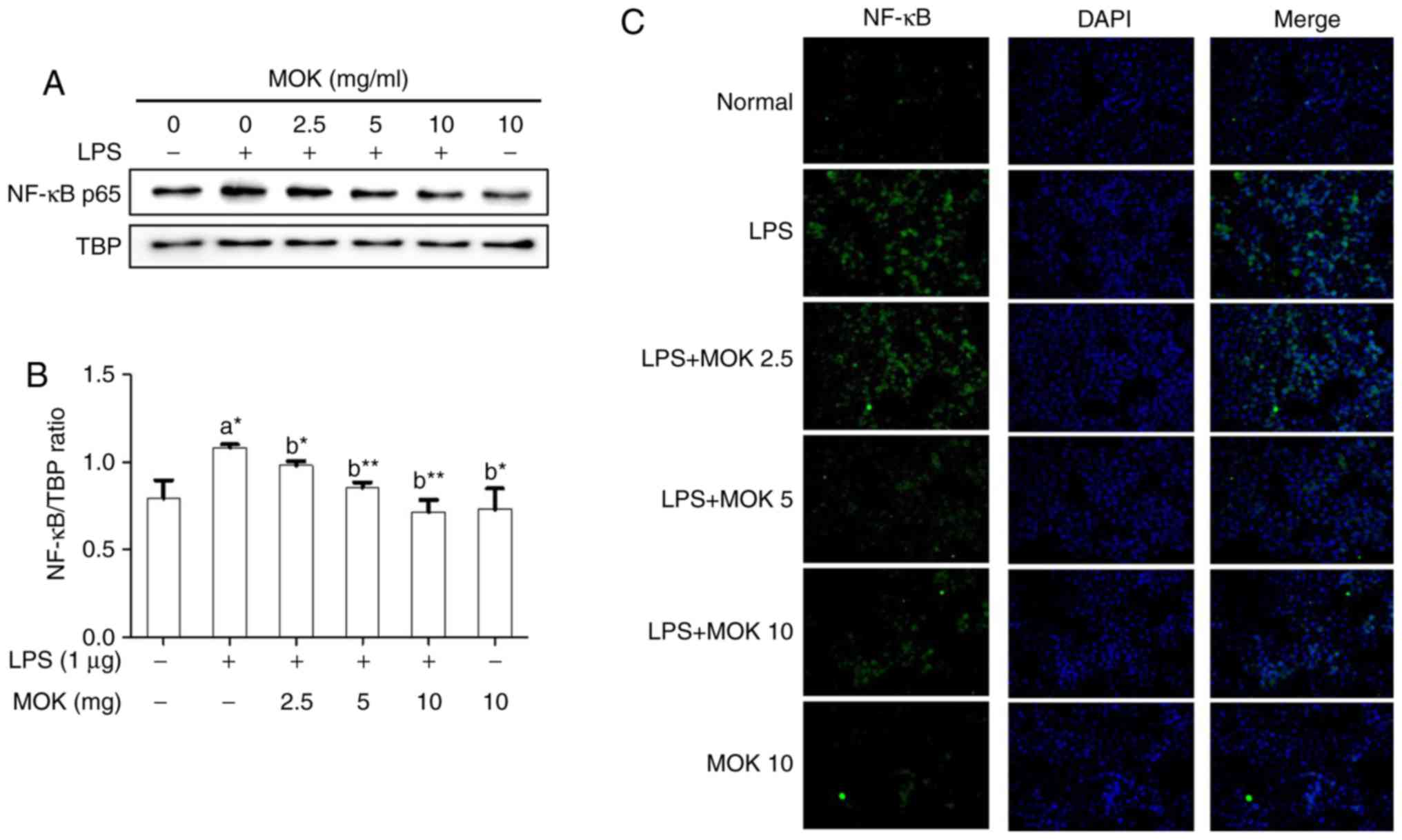
stimulated by LPS (Fig. 5). NF-κB
p65 nuclear expression statistically significantly increased
(P<0.05) following stimulation of the cells by LPS (Fig. 6). Additionally, MOK extract
treatment at concentrations of 2.5, 5 and 10 mg/ml resulted in a
significant decrease (P<0.05, P<0.01 and P<0.01,
respectively) of NF-κB p65 nuclear expression in LPS-stimulated
cells (Fig. 6A and B); this
inhibitory effect of MOK was also observed via immunohistochemistry
(Fig. 6C). Therefore, it was
demonstrated that MOK treatment inhibited nuclear translocation of
NF-κB p65 in RAW 264.7 cells stimulated by LPS. In addition, the
results indicated that the inhibitory effects of MOK extract on the
production of pro-inflammatory factors are mediated by inhibition
of the MAPK/NF-κB pathway in activated macrophages.
Effect of MOK extract on oxidative
damage
Next, the inhibitory effect of MOK extract on
oxidative damage was investigated by measuring ROS production and
antioxidant enzyme expression in RAW 264.7 cells stimulated by LPS.
Following stimulation of the cells by LPS, ROS production exhibited
a significant increase (Fig. 7A),
along with a signifi-cant decrease in antioxidant enzyme
expression, except HO-1 production (Fig. 7B and C). Treatment by 5 or 10
mg/ml MOK extract achieved a statistically significant decrease
(P<0.05 and P<0.001, respectively) in the production of ROS
in LPS-stimulated cells in a concentration-dependent manner
(Fig. 7A). Additionally, with
concentrations of MOK extract of 2.5, 5 and 10 mg/ml, there was a
statistically significant increase in the mRNA expression of HO-1
(P<0.05, P<0.05 and P<0.01, respectively) and MnSOD
(P<0.01, P<0.05 and P<0.01, respectively), as well as an
increase in the expression of SOD2 (P<0.01, P<0.01 and
P<0.05, respectively) and CAT (P<0.05 for 5 mg/ml and
P<0.01 for 10 mg/ml) proteins in RAW 264.7 cells stimulated by
LPS (Fig. 7B and C). These
results indicate that MOK extract may prevent oxidative damage by
inhibiting ROS production and activating antioxidant enzymes in
activated macrophages.
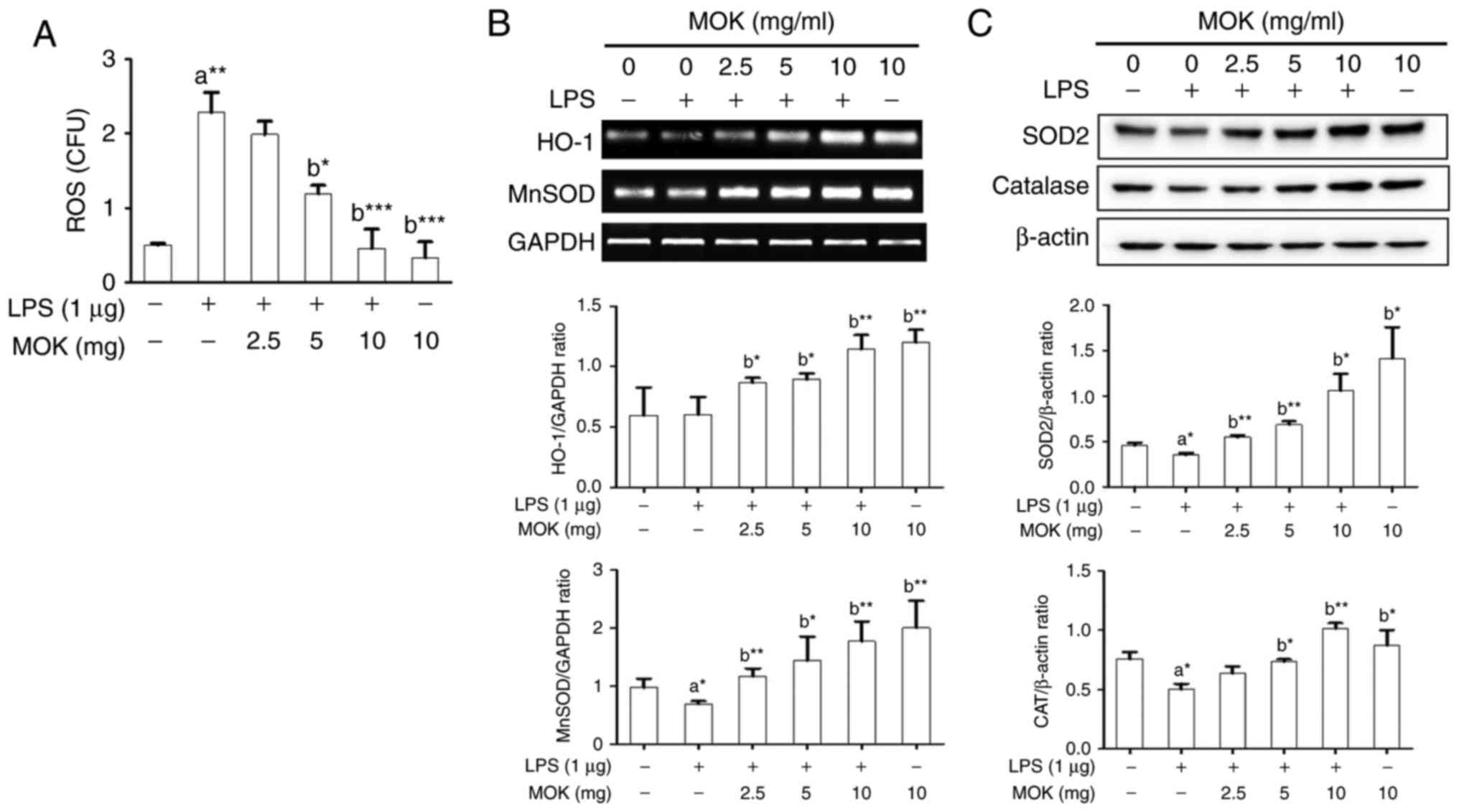 | Figure 7Effects of MOK extract on ROS
production and expression of antioxidant genes and proteins (MnSOD,
HO-1, SOD2 and CAT) in RAW 264.7 cells stimulated with LPS. (A)
After treatment with graded concentrations of MOK extract for 30
min, cells were stimulated for 24 h with or without LPS. Then, the
cells were homogenized, and an in vitro ROS/RNS assay kit
used to measure ROS levels in RAW 264.7 cells stimulated with LPS.
Data represent means ± standard error of the mean of three
independent experiments. *P<0.05,
**P<0.01 and ***P<0.001 vs. 1st bar,
untreated control cells (A) or 2nd bar, cells treated with LPS only
(b). (B) After treatment with MOK extract for 30 min, the cells
were stimulated for an additional 5 h with LPS. RT-PCR was used to
measure the mRNA levels of MnSOD and HO-1 with GAPDH
expression as an internal control. (C) After treatment with MOK
extract for 30 min, the cells were stimulated for an additional 24
h with LPS. Western blotting was used to analyze the protein levels
of SOD2 and CAT in total cell lysates with β-actin as an internal
control. Mean densitometric values of the three independent
experiments were analyzed and are expressed as bar charts. ROS,
reactive oxygen species; RNS, reactive nitrogen species; LPS,
lipopolysaccharide; RT-PCR, reverse transcription-polymerase chain
reaction; SOD, superoxide dismutase; HO-1, heme oxygenase-1; CAT,
catalase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. |
Discussion
Pharmacopuncture is a frequently used practice in
clinical TKM, as it acts faster and is considered to be more
effective compared with orally administered medicines. MOK is a
polyherbal pharmacopuncture medicine that consists of ten herbs
(Table I) and is frequently used
in clinical practice for the treatment of heart and thyroid
diseases. Although scientific evidence regarding the therapeutic
aspects of MOK is lacking, it is expected that the clinical
applications of MOK will be justified based on efficacy studies of
the MOK constituents.
RAW264.7 is a functional macrophage line transformed
by Abelson leukemia virus (18)
and requires LPS for full activation. RAW264.7 cells are commonly
used to study the anti-inflammatory properties of drugs. In the
present study, in order to investigate the effects of MOK extract
on inflammatory and oxidative responses, RAW264.7 cells were
stimulated with LPS (1 µg/ml). We first determined the
non-toxic concentrations of MOK extract with or without LPS in
RAW264.7 macrophages using a WST viability assay. The decrease of
RAW264.7 cell viability following LPS stimulation due to the
release of inflammatory substances, which may act as cytotoxic
agents, has been previously demonstrated, but an increase in cell
viability (19) or little change
in cell viability (20-23) have also been reported. However, in
our cell viability assay, LPS (1 µg/ml) stimulation of
RAW263.7 cells was not associated with a decrease in their
viability, similar to non-treated cells or cells treated with MOK
alone.
MOK did not decreased cell viability at 10 mg/ml;
therefore, MOK extract at 2.5, 5 and 10 mg/ml was used for the
efficacy study. MOK extract is applied in pharmacopuncture therapy
at concentrations in the range of 0.2 ml (10.62 mg) to 0.4 ml
(21.24 mg) that are known to be safe (10) for patients with various
conditions, such as Hwa-Byeong (11), tension headache (12), functional dyspepsia (13) and herpes zoster (24).
Among the ten MOK constituent herbs, Moschus
berezovskii (Moschus), Bos taurus (Bovis Calculus) and
Ursus arctos (Ursi Fel) comprise the main active components.
Specifically, M. berezovskii is a representative
orifice-opening medicine used in unconscious patients and U.
arctos, B. taurus, Scutellaria baicalensis,
Phellodendron amurense, Pulsatilla koreana and
Sophora tonkinensis act as heat-clearing medicines for
patients with fever. Aucklandia lappa and Aquilaria
agallocha are medicines regulating qi, whereas Hominis
plancenta is a medicine for tonifying and replenishing.
Notably, these ten herbs have individually been reported to have
anti-inflammatory and antioxidant properties (25-32). Additionally, in our previous
high-performance liquid chromatography analysis of MOK extract, we
detected several compounds, such as bilirubin, ursodeoxycholic
acid, baicalein and muscone, that were also reported to have
anti-inflammatory and antioxidant properties (15). Therefore, various pharmaceutical
effects of MOK extract in pharmacopuncture therapy may be predicted
based on the studies on the individual effects of MOK components
in vitro and in vivo. Furthermore, in the present
study, MOK was found to exert anti-inflammatory and antioxidant
effects on RAW 264.7 macrophages stimulated by LPS by
downregulating inflammatory mediators and upregulating antioxidant
enzymes. Unavoidable noxious stimuli that adversely affect normal
tissue function induce inflammation as a protective response
(33). Inflammation, as one of
several immune responses against infection, is implicated in
various human diseases, including cancer, neurological disorders,
metabolic syndromes, inflammatory bowel disease, arthritis,
cardiovascular and infectious diseases (34,35).
Although inflammatory mediators (NO, iNOS,
PGE2 and COX-2) and pro-inflammatory cytokines (TNF-α,
IL-1β and IL-6) produced by activated macrophages have important
functions regarding host survival and tissue repair in the normal
state (36), whereas their
overproduction contributes to the induction and progression of
several inflammatory diseases (37,38). Accordingly, the regulation of
inflammatory mediators is recognized as a beneficial therapeutic
strategy for inflammatory diseases. The present study demonstrated
that MOK extract inhibited NO and PGE2 production by
downregulating the expression of their synthetic enzymes, including
iNOS and COX-2, as well as pro-inflammatory cytokines, including
IL-6, IL-1β and TNF-α, in RAW 264.7 cells stimulated by LPS. We
previously reported that MOK extract also inhibits the expression
of iNOS, COX-2 and pro-inflammatory cytokines in primary
macrophages isolated from the mouse peritoneal cavity (18). These results indicate that MOK
extract effectively improves inflammatory conditions, as it can
suppress the overproduction of inflammatory mediators by activated
macrophages.
Oxidative stress caused by ROS overproduction
damages cellular lipids, DNA and proteins, and is implicated in a
variety of acute and chronic inflammatory diseases, cardiovascular
disease, diabetes, central nervous system disorders, age-related
disorders, neurodegenerative disorders and cancer (39,40). Notably, macrophage stimulation by
LPS also causes production of ROS (41,42). Cells have two defense systems that
react to oxidative stress from exogenous and endogenous sources,
with compounds related to the latter including antioxidant enzymes,
such as SODs (CuZnSOD and MnSOD), CAT and HO-1 (39). Therefore, the downregulation of
ROS expression levels or the upregulation of antioxidant enzyme
activity is important for the treatment of oxidative damage. In
previous studies, it was reported that LPS stimulation induces
oxidative stress by increasing ROS production and decreasing the
expression of antioxidant enzymes, SOD, MnSOD, HO-1 and the levels
of GSH in RAW264.7 macrophages (43,44). In the present study, MOK extract
inhibited ROS production by upregulating the expression of SOD and
CAT in RAW 264.7 cells stimulated by LPS. These results suggest
that MOK exerts antioxidant effects during macrophage activation.
Additionally, we also reported that MOK extract inhibited mRNA
expression of HO-1 and MnSOD in mouse peritoneal macrophages
without LPS stimulation (17).
To elucidate the underlying mechanism responsible
for the anti-inflammatory and antioxidant effects of MOK extract,
its regulatory effects on the MAPK/NF-κB inflammatory signaling
pathway were investigated in RAW 264.7 cells stimulated by LPS, as
the therapeutic effects of several anti-inflammatory agents are
associated with inhibition of inflammatory gene expression, which
often occurs through blockade of the MAPK/NF-κB pathway in
macrophage activation. Antioxidant effects also depend on an
increase in antioxidant enzyme expression and a decrease in ROS
levels. ERK1/2, JNK and p38 MAPKs along with NF-κB comprise two
cellular pathways involved in macrophage-mediated inflammation.
Specifically, MAPKs are a family of proteins associated with
serine/threonine kinases, which play an important role in cell
proliferation and differentiation and cellular responses to
cytokines or stress inducers. These are activated by
phosphorylation and then induce activation of the transcription
factor NF-κB. NF-κB also plays a key role in the pathogenesis and
regulation of inflammatory responses, and its activation can
regulate inflammatory cytokines (40,45,46). In the present study, MOK extract
inhibited the phosphorylation of ERK1/2, JNK and p38 MAPKs, as well
as the nuclear translocation of NF-κB p65 in RAW 264.7 cells
stimulated by LPS. These findings indicate that MOK extract exerts
anti-inflammatory effects in activated macrophages by blocking the
MAPK/NF-κB pathway. In a future study, we will consider the
application of specific inhibitors, e.g., MAPK inhibitors, to
investigate the underlying mechanism in detail.
In conclusion, MOK extract, a pharmacopuncture
medicine, inhibited the production of the inflammatory mediators NO
and PGE2 in RAW 264.7 cells stimulated by LPS by
downregulating their synthetic enzymes, iNOS and COX. The
expression of pro-inflammatory cytokines, such as IL-6, IL-1β and
TNF-α, was also inhibited. In addition, MOK extract inhibited the
phosphorylation of ERK1/2, JNK and p38 MAPKs and the nuclear
translocation of NF-κB p65 in RAW 264.7 cells stimulated by LPS.
Moreover, MOK extract decreased ROS production by inducing the
expression of the antioxidant enzymes MnSOD and CAT in RAW 264.7
cells stimulated by LPS. These results indicate that MOK exerts
anti-inflammatory and antioxidant effects in activated macrophages,
and it may be useful for the treatment of inflammatory conditions
as a pharmacopuncture medicine.
Acknowledgments
Not applicable.
Funding
This study was supported by the National Research
Foundation of Korea (NRF), funded by the Korean government (MSIT)
(grant no. NRF-2017R1C1B5076224).
Authors’ contributions
JHH, HWJ and YKP made substantial contributions to
the conception and design of the present study; JHH, JNM and JHP
performed the experiments for data acquisition; JHH, JNM and HWJ
performed the statistical analysis; JHH and HWJ interpreted the
experimental results and wrote the manuscript; JHH, HWJ, JHP and
YKP revised the manuscript. The final version of the manuscript was
read and approved by all authors.
Availability of data and materials
The corresponding author will make available the
data generated and analyzed during this study upon reasonable
request. All materials used are included in Materials and
methods.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Z, Tang L, Zou P, Zhang Y, Wang Z,
Fang Q, Jiang L, Chen G, Xu Z, Zhang H and Liang G: Synthesis and
biological evaluation of allylated and prenylated mono-carbonyl
analogs of curcumin as anti-inflammatory agents. Eur J Med Chem.
74:671–682. 2014. View Article : Google Scholar
|
|
2
|
Mittal M, Siddiqui MR, Tran K, Reddy SP
and Malik AB: Reactive oxygen species in inflammation and tissue
injury. Antioxid Redox Signal. 20:1126–1167. 2014. View Article : Google Scholar :
|
|
3
|
Zhang L and Wang CC: Inflammatory response
of macrophages in infection. Hepastobiliary Pancreat Dis Int.
13:138–152. 2014. View Article : Google Scholar
|
|
4
|
Cho SY, Yang SB, Shin HS, Lee SH, Koh JS,
Kwon S, Jung WS, Moon SK, Park JM, Ko CN and Park SU:
Anti-inflammatory and immune regulatory effects of acupuncture
after craniotomy: Study protocol for a parallel-group randomized
controlled trial. Trials. 18:4812017. View Article : Google Scholar
|
|
5
|
Zijlstra FJ, van den Berg-de Lange I,
Huygen FJ and Klein J: Anti-inflammatory actions of acupuncture.
Mediators Inflamm. 12:59–69. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lisboa MR, Gondim DV, Ervolino E, Vale ML,
Frota NP, Nunes NL, Mariguela VC, Taba M Jr, Messora MR and
Furlaneto FA: Effects of electroacupuncture on experimental
periodontitis in rats. J Periodontol. 86:801–811. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
McDonald JL, Cripps AW, Smith PK, Smith
CA, Xue CC and Golianu B: The anti-inflammatory effects of
acupuncture and their relevance to allergic rhinitis: A narrative
review and proposed model. Evid Based Complement Alternat Med.
2013.591796:2013.
|
|
8
|
Song Q, Hu S, Wang H, Lv Y, Shi X, Sheng Z
and Sheng W: Electroacupuncturing at Zusanli point (ST36)
attenuates pro-inflammatory cytokine release and organ dysfunction
by activating cholinergic anti-inflammatory pathway in rat with
endotoxin challenge. Afr J Tradit Complement Altern Med.
11:469–474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wei Y, Dong M, Zhang H, Lv Y, Liu J, Wei
K, Luo Q, Sun J, Liu F, Xu F and Dong J: Acupuncture attenuated
inflammation and inhibited Th17 and treg activity in experimental
asthma. Evid Based Complement Alternat Med. 2015.340126:2015.
|
|
10
|
Jung C, Jung JH and Lee MS: A Clinical
Study of Immune Pharmacopuncturology. Kyungrak Medical Publishing
Co.; Chungnam: 2011, In Korean.
|
|
11
|
Hwang JH: A case report of Hwa-byeong with
MOK herbal acupuncture therapy. J Immuno-Pharmacopuncture. 2:43–55.
2013.In Korean.
|
|
12
|
Ha TD and Jung C: A case report on severe
tension-type headache treated with pharmacopuncture (Yakchim). J
Immuno-Pharmacopuncture. 5:37–45. 2016.In Korean.
|
|
13
|
Kim MJ, Kim SK, Ko SJ and Park JW: A case
study of MOK and V Yakchim on functional dyspepsia. J
Immuno-Pharmacopuncture. 3:41–49. 2014.In Korean.
|
|
14
|
Kim HJ, Gwan R, Han JW, Jung C and Park
KH: Analysis of physioactivities on MOK Yakchim. J
Immuno-Pharmacopuncture. 2:17–25. 2013.In Korean.
|
|
15
|
Hwang JH, Kang SY, Kang AN, Jung HW, Jung
C, Jeong JH and Park YK: MOK, a pharmacopuncture medicine,
regulates thyroid dysfunction in L-thyroxin-induced hyperthyroidism
in rats through the regulation of oxidation and the TRPV1 ion
channel. BMC Complement Altern Med. 17:5352017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hwang JH, Jung HW, Kang SY, Kang AN, Ma
JN, Meng XL, Hwang MS and Park YK: Therapeutic effects of
acupuncture with MOK, a polyherbal medicine, on PTU-induced
hypothyroidism in rats. Exp Ther Med. 16:310–320. 2018.PubMed/NCBI
|
|
17
|
Hwang JH, Hwang MS and Park YK: MOK, a
pharmacopuncture medicine, reduces inflammatory response through
inhibiting the proinflammatory cytokine production in
LPS-stimulated mouse peritoneal macrophages. J Acupunct Res.
34:11–21. 2017. View Article : Google Scholar
|
|
18
|
Raschke WC, Baird S, RalpH P and Nakoinz
I: Functional macrophage cell lines transformed by Abelson leukemia
virus. Cell. 15:261–267. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Xiong H and Liu L: Effects of
taraxasterol on inflammatory responses in
lipopolysaccharide-induced RAW 264.7 macrophages. J Ethnopharmacol.
141:206–211. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi HS, Seo HS, Kim SR, Choi YK, Shin YC
and Ko SG: Anti-inflammatory and anti-proliferative effect of
herbal medicines (APR) in RAW264.7 cells. Mol Med Rep. 9:1569–1574.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li T, Gao D, Du M, Cheng X and Mao X:
Casein glycomacropeptide hydrolysates inhibit PGE2
production and COX2 expression in LPS-stimulated RAW264.7
macrophage cells via Akt mediated NF-κB and MAPK pathways. Food
Funct. 9:2524–2532. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang X, Kang MC, Li Y, Kim EA, Kang SM and
Jeon YJ: Anti-inflammatory activity of questinol isolated from
marine-derived fungus Eurotium amstelodami in
lipopoly-saccharide-stimulated RAW 264.7 macrophages. J Microbiol
Biotechnol. 24:1346–1353. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim HJ, Tsoyi K, Heo JM, Kang YJ, Park MK,
Lee YS, Lee JH, Seo HG, Choi HS and Chang KC: Regulation of
lipopolysaccha-ride-induced inducible nitric-oxide synthase
expression through the nuclear factor-κB pathway and
interferon-β/tyrosine kinase 2/Janus tyrosine kinase 2-signal
transducer and activator of transcription-1 signaling cascades by
2-naphthylethyl-6, 7-dihydroxy-1, 2, 3, 4-tetrahydroisoquinoline
(THI 53), a new synthetic isoquino-line alkaloid. J Pharmacol Exp
Ther. 320:782–789. 2007. View Article : Google Scholar
|
|
24
|
Gong HM, Jun SA, Lee HJ and Kim JS: A case
report of Herpes zoster patient treated with additional CS, V
pharmacopuncture. J Immuno-Pharmacopuncture. 1:27–36. 2016.
|
|
25
|
De D, Datta Chakraborty P, Mitra J, Sharma
K, Mandal S, Das A, Chakrabarti S and Bhattacharyya D:
Ubiquitin-like protein from human placental extract exhibits
collagenase activity. PLoS One. 8:e595852013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng Y, Siu K, Wang N, Ng KM, Tsao SW,
Nagamatsu T and Tong Y: Bear bile: Dilemma of traditional medicinal
use and animal protection. J Ethnobiol Ethnomed. 5:22009.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jang SY, Park JW, Bu Y, Kang JO and Kim J:
Protective effects of hominis placenta hydrolysates on radiation
enteropathy in mice. Nat Prod Res. 25:1988–1992. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, Xu Y, Zhang C, Deng L, Chang M, Yu Z
and Liu D: Protective effect of calculus bovis sativus on dextran
sulphate sodium-induced uicerative colitis in mice. Evid Based
Complement Alternat Med. 2015.469506:2015.
|
|
29
|
The National College of Oriental Medicine
Herbology Classroom: Herbology: Youngrimsa, Seoul. 2016.In
Korean.
|
|
30
|
Park SY, Phark S, Lee M, Lim JY and Sul D:
Anti-oxidative and anti-inflammatory activities of placental
extracts in benzo[a] pyrene-exposed rats. Placenta. 31:873–879.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Park JY, Lee J, Jeong M, Min S, Kim SY,
Lee H, Lim Y and Park HJ: Effect of Hominis Placenta on cutaneous
wound healing in normal and diabetic mice. Nutr Res Pract.
8:404–409. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thevis M, Schänzer W, Geyer H, Thieme D,
Grosse J, Rautenberg C, Flenker U, Beuck S, Thomas A, Holland R and
Dvorak J: Traditional Chinese medicine and sports drug testing:
Identification of natural steroid administration in doping control
urine samples resulting from musk (pod) extracts. Br J Sports Med.
47:109–114. 2013. View Article : Google Scholar
|
|
33
|
Okin D and Medzhitov R: Evolution of
inflammatory diseases. Curr Biol. 22:R733–R740. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Monaco C, Andreakos E, Kiriakidis S,
Feldmann M and Paleolog E: T-cell-mediated signalling in immune,
inflammatory and angiogenic processes: the cascade of events
leading to inflammatory diseases. Curr Drug Targets Inflamm
Allergy. 3:35–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yamamoto Y and Gaynor RB: IkappaB kinases:
Key regulators of the NF-kappaB pathway. Trends Biochem Sci.
29:72–79. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adib-Conquy M, Scott-Algara D, Cavaillon
JM and Souza- Fonseca-Guimaraes F: TLR-mediated activation of NK
cells and their role in bacterial/viral immune responses in
mammals. Immunol Cell Biol. 92:256–262. 2014. View Article : Google Scholar
|
|
37
|
Korhonen R, Lahti A, Kankaanranta H and
Moilanen E: Nitric oxide production and signaling in inflammation.
Curr Drug Targets Inflamm Allergy. 4:471–479. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sweet MJ and Hume DA: Endotoxin signal
transduction in macrophages. J Leukoc Biol. 60:8–26. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Davies KJ: Oxidative stress: The paradox
of aerobic life. Biochem Soc Symp. 61:1–31. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Thalhamer T, McGrath MA and Harnett MM:
MAPKs and their relevance to arthritis and inflammation. Rheumatol
(Oxford). 47:409–414. 2008. View Article : Google Scholar
|
|
41
|
Chang LY, Wan HC, Lai YL, Chou IC, Chen YT
and Hung SL: Areca nut extracts increased the expression of
cyclooxygenase-2, prostaglandin E2 and interleukin-1α in
human immune cells via oxidative stress. Arch Oral Biol.
58:1523–1531. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Satoh M, Minami Y, Takahashi Y and
Nakamura M: Immune modulation: Role of theinflammatory cytokine
cascade in the failing human heart. Curr Heart Fail Rep. 5:69–74.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang L, Xu Ml, Liu J, Wang Y, Hu JH and
Wang MH: Sonchus asper extract inhibits 7 macrophages. Nutr Res
Pract. 9:579–585. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jin Y, Yang J, Lin L, Lin Y and Zheng C:
The attenuation of scutellariae radix extract on oxidative stress
for colon injury in lipopolysaccharide-induced RAW264.7 Cell and
2,4,6-trinitro-benzene sulfonic acid-induced ulcerative colitis
rats. Pharmacogn Mag. 12:153–159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Broom OJ, Widjaya B, Troelsen J, Olsen J
and Nielsen OH: Mitogen activated protein kinases: A role in
inflammatory bowel disease. Clin Exp Immunol. 158:272–280. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Shan J, Fu J, Zhao Z, Kong X, Huang H, Luo
L and Yin Z: Chlorogenic acid inhibits lipopolysaccharide-induced
cyclo-oxygenase-2 expression in RAW264.7 cells through suppressing
NF-kappaB and JNK/AP-1 activation. Int Immunopharmacol.
9:1042–1048. 2009. View Article : Google Scholar : PubMed/NCBI
|