Introduction
Rectal cancer is one of the most common cancers in
Japan as well as the western world. Preoperative CRT followed by
total mesorectal excision (TME) has improved the rates of survival,
sphincter preservation, and local pelvic control (1–4).
Despite significant improvements in the management of rectal
cancer, tumor relapse remains the major cause of mortality in
patients with preoperative CRT followed by TME. Further
improvements in the survival rate cannot be achieved without better
control of post-surgical local and distal recurrence.
Leucine-rich repeat-containing G protein-coupled
receptor 5 (LGR5), also known as GPR49 (G-protein-coupled receptor
49), is closely related to members of the glycoprotein hormone
receptor subfamily with seven transmembrane domains and is a target
of Wnt signaling. LGR5 is a potential marker for stem cells in the
small intestine and colon (5–7).
Sato et al demonstrated that a single LGR5-positive cell
could initiate a crypt villus-like structure and generate a
continuously expanding, self-organizing epithelial structure
reminiscent of normal gut without stroma tissue (8). On the other hand, it has been
reported that LGR5 expression is implicated in colorectal
carcinogenesis (7,9). Uchida et al reported that LGR5
might be implicated not only in early events but also in late
events in colorectal tumori-genesis (10). From these findings, we hypothesized
that LGR5 expression might participate in the maintenance and
proliferation of residual cancer cells after CRT.
The transmembrane glycoprotein CD44, a hyaluronan
receptor, is an adhesion molecule with multiple splice variant
isoforms, which facilitates both cell-cell and cell-extracellular
matrix (ECM) interactions (11,12).
Additionally, CD44 is an important cell surface marker for
isolating colon cancer stem cells (CSCs) (13–15).
CD44 is expressed by most human cell types and is implicated in a
wide variety of physiological and pathological process, including
lymphocyte homing and activation, and cell migration (16,17).
CD44 is consistently overexpressed in many types of carcinomas
including colon cancer and participates in tumor proliferation,
invasion, and metastasis (16,18,19).
CD44 activation is important to consider in the metastasis cascade
due to stimulating tumoral ECM (12,18).
Lakshman et al demonstrated that CD44 expression, and, more
importantly the v3–10 isoform, promoted resistance to apoptosis
in vitro (20). Therefore,
we hypothesized that CD44 might be implicated in resistance to CRT
and distant relapse in rectal cancer after preoperative CRT. To the
best of our knowledge, CD44 expression after CRT has scarcely been
evaluated.
Both LGR5 and CD44 have been reported to be Wnt
signal targets (7,21–23)
and known as stemness markers (21). In this study of locally advanced
rectal cancer patients treated with preoperative CRT, we used
transcriptional and immunohistochemical analyses to investigate the
correlation between LGR5 and CD44 expression, and the association
of their expression with clinical outcomes. For CD44, we examined
expression in both cancer and stromal cells.
Materials and methods
Patients and specimens
From 2001 to 2008, 64 patients with rectal cancer
received preoperative CRT followed by surgery at our institute. The
criteria for induction of preoperative CRT in our institute are as
follows. Patients must be ≤80 years old, in clinical stage II/III
based on the International Union Against Cancer’s TNM
classification, with no evidence of distant metastases, no invasion
of external sphincter muscle nor elevator muscle of the anus, and
no evidence of deep venous thrombosis. Five patients without
curative surgery were excluded in this study. Additionally, we
excluded 5 patients with pathological complete response after
preoperative CRT because cancer cells were not obtained. A total of
54 formalin-fixed, paraffin-embedded (FFPE) specimens were
investigated for this study. The study design was approved by the
ethics review board of Mie University Hospital. All patients signed
informed consent forms for their tissues to be used in this
study.
5-fluorouracil-based chemoradiotherapy
regimen
Patients with rectal cancer were treated with
short-course (a dose of 20 Gy in 4 fractions) or long-course (a
dose of 45 Gy in 25 fractions) radiotherapy using a 4-field box
technique with concurrent chemotherapy to take advantage of
5-fluorouracil (5-FU) radio-sensitization. Patients underwent
concurrent pharmacokinetic modulation chemotherapy (intravenous
infusion of 5-FU: 600 mg/m2 for 24 h, and tegafur-uracil
(UFT) given as 400 mg/m2 orally for 5 days. This regimen
was based on the previously tested combination of continuous
infusion of 5-FU and oral administration of UFT (24). Short-course radiotherapy in our
institute is different from standard short-course radiotherapy, 25
Gy in 5 fractions. There are several reasons that we designed the
present regimen. We calculated a biologically equivalent dose (BED)
of 20 Gy in 4 fractions using a linear quadratic model (25) and its BED was 30 Gy (α/β ratio: 10
Gy). We understand that BED: 30 Gy had a sufficient efficacy
reducing the local failure of radiotherapy (26). Forty-two patients received
short-course radiotherapy with chemotherapy over 1 week. The
remaining 10 patients received long-course radiotherapy with
chemotherapy for 4 weeks. The time interval between preoperative
CRT and surgery was 2–3 weeks in short-course irradiation patients,
and 4–6 weeks in long-course irradiation patients. All patients
underwent standard surgery including total mesorectal excision, and
received 5-FU based adjuvant chemotherapy after surgery for 6
months to 1 year.
Histopathological tumor regression after
CRT
The histo-pathological response of CRT was evaluated
using Rödel tumor regression grading (TRG) system (27) and 3-point Ryan system (28). Each TRG was classified by two
investigators in a blinded fashion without knowledge of the
clinical and pathological information. Rödel TRG system is
classified into five categories: grade 0, no regression; grade 1,
minor regression (dominant tumor mass with obvious fibrosis in ≤25%
of the tumor mass); grade 2, moderate regression (dominant tumor
mass with obvious fibrosis in 26–50% of the tumor mass); grade 3,
good regression (dominant fibrosis outgrowing the tumor mass; i.e.,
>50% tumor regression); and grade 4, total regression (no viable
tumor cells, only fibrotic mass). We categorized responders as
patients with TRG 3 to 4, while non-responders were TRG 0–2.
Three-point Ryan system is devised by combining TRG1 (no viable
cancer cells) and TRG2 (single cells or small groups of cancer
cells) to form one category as 3-point TRG1, and TRG3 (residual
cancer outgrown by fibrosis) into 3-point TRG2, combining TRG4
(significant fibrosis outgrown by cancer) and TRG5 (no fibrosis
with extensive residual cancer) into 3-point TRG3. Patients with
3-point TRG1 and 2 were categorized as responders and patients with
3-point TRG were categorized as non-responders.
Microdissection and RNA extraction from
formalin-fixed paraffin-embedded (FFPE) specimens
Microdissection of FFPE was performed as previously
described (29). Micro-dissected
specimens were digested with proteinase K in lysis buffer
containing Tris-HCl, ethylenediaminetetraacetic acid, and sodium
dodecyl sulfate, as previously published (30), with minor modifications. RNA was
purified by phenol and chloroform extraction. Isolated RNA was
purified using ethanol precipitation. The concentration and quality
of RNA was measured with UV absorbance at 260 and 280 nm (A260/280
ratio).
cDNA synthesis
To reverse transcribe the fragmented mRNA from FFPE
tissue materials, we used random hexamer priming, instead of
oligo(dT)-based priming. cDNA was synthesized with random hexamer
and Superscript III reverse transcriptase (Invitrogen, Carlsbad,
CA) according to the manufacturer’s instructions.
Quantitative real-time polymerase chain
reaction (qRT-PCR
qRT-PCR analysis was carried out with the SYBR Green
PCR Master Mix (Applied Biosystems, Foster City, CA) using the
Applied Biosystems 7500 Real-Time PCR system according to the
manufacturer’s instructions. Primers for LGR5, CD44, CTNNB1
(β-catenin) and ACTB (β-actin) were designed with Primer3
software (Biology Workbench Version 3.2, San Diego Supercomputer
Center, at the University of California, San Diego). Sequences were
as follows: LGR5-specific primers (sense,
GATGTTGCTCAGGGTGGACT, and antisense, GGG AGCAGCTGACTGATGTT);
CD44-specific primers (sense, CGGACACCATGGACAAGTTT, and
antisense, CACGTGGA ATACACCTGCAA), CTNNB1-specific primer (sense,
TGTT CGTGCACATCAGGATAC and antisense, GCTCCGGTACA ACCTTCAAC) and
ACTB (sense, ACAGAGCCTCGCCTT TGC, and antisense,
GCGGCGATATCATCATCC). PCR was performed in a final volume of 25 μl
with a SYBR Green PCR Master Mix, using 1 μl cDNA, and 400 nM of
each primer for the respective genes. Cycling conditions were 50°C
for 2 min and 95°C for 10 min, followed by 40 cycles at 95°C for 15
sec and 60°C for 1 min.
Relative gene expression levels of LGR5
and CD44
Relative gene expression levels were determined by
the standard curve method. The standard curves and line equations
were generated using 5-fold serially diluted solutions of cDNA from
qPCR Human Reference Total RNA (Clontech, Mountain View, CA) for
LGR5 and CD44. All standard curves were linear in the
analyzed range with an acceptable correlation coefficient
(R2). The amount of target gene expression was
calculated from the standard curve followed by quantitative
normalization of cDNA in each sample using ACTB gene
expression as an internal control. Target gene mRNA levels are
given as ratios to ACTB mRNA levels. RT-PCR assays were done
in duplicate for each sample and the mean value was used for
calculation of the mRNA expression levels.
Immunohistochemistry for LGR5 and
CD44
Immunohistochemistry was performed as previously
described (29). LGR5 (GPR49,
rabbit monoclonal antibody, clone EPR3065Y, Epitomics, CA, USA) and
human CD44H antibody (monoclonal mouse IgG2A, clone no.
2C5, R&D Systems, MN, USA) as primary antibodies were used at
dilutions of 1:100 and 1:1000 respectively. All sections were
counterstained with hematoxylin, and were dehydrated and mounted.
Negative controls were also run simultaneously. LGR5 and CD44
expression was evaluated semi-quantitatively in a blinded fashion
without knowledge of clinical and pathological information. We
defined the high expression group as cases in which >50% of
cancer cells. For the evaluation of immunoreactivity of CD44 in
stroma, we defined the high expression group as cases that its
strong expressions were localized in cancer stroma surrounding
cancer nests.
Statistical analyses
All statistical analyses were performed using
StatView 5.0 for Windows (SAS Institute Inc., Cary, NC). Values of
each target gene are expressed as median values (inter-quartile
range) in tables. Associations between continuous variables and
categorical variables were evaluated using Mann-Whitney U tests for
two groups. The χ2 test was also used to assess the
significance of the correlation between categorical variables. A
non-parametric receiver operating characteristic (ROC) analysis was
performed to calculate the best cutoff value for each gene
expression level that would be predictive of recurrence and
survival, using Medcalc 7.2 for Windows (Mariakerke, Belgium).
Recurrence-free survival (RFS) and overall survival (OS) time were
calculated from the date of surgery to the date of disease
recurrence and patients’ death, respectively. RFS and OS
probabilities were calculated using the Kaplan-Meier product limit
method; intergroup differences were determined using a log-rank
test. The influence of distant recurrence and survival predictors
identified univariate analysis was accessed by multivariate
analysis using Cox’s proportional hazards model. Two-sided P-values
<0.05 were considered statistically significant.
Results
Patient and tumor characteristics
A total of 54 total-RNA were obtained from FFPE
specimens of rectal cancer with preoperative CRT. Two specimens
were excluded as the ACTB expressions were very low and
without reproducibility. Therefore, 52 patients were included in
this study. Table I shows patient
characteristics and the association of the gene expression levels
of LGR5 and CD44 with clinicopathological variables.
The median age was 64.5 years (range 37–78 years) and the male to
female ratio was 3.7:1. The post-CRT pathological T stages were
ypT1 (n=5), ypT2 (n=12), ypT3 (n=33), and ypT4 (n=2). Seventeen
patients (33%) had lymph node metastases. Forty-four tumors (85%)
showed well or moderately differentiated adenocarcinoma histology.
Three patients (6%) had local recurrence alone. A total of 12
patients (23%) had distant recurrence. Patterns of distant
recurrence were seen as liver and lung metastases in 2 patients,
lung metastasis alone in 6 patients, and peritoneal metastasis in 2
patients. Rödel TRG system was as follows: grade 0, 0 patients;
grade 1, 10 patients; grade 2, 22 patients; grade 3, 19 patients;
and grade 4, 1 patient. 3-point Ryan TRG was as follows: TRG1, 14
patients; TRG2, 23 patients; TRG3, 15 patients. The median
follow-up period was 67 months (range 22–129 months).
 | Table I.Tumor characteristics and association
of LGR5 and CD44 gene expression levels with
clinicopathological variables. |
Table I.
Tumor characteristics and association
of LGR5 and CD44 gene expression levels with
clinicopathological variables.
| Cancer
| Stroma
|
|---|
| Variables | No. (%) | LGR5 | P-value | CD44 | P-value | CD44
Positive (n=16) | CD44
(n=36) | P-value |
|---|
| Gender | | | | | | | | |
| Male | 41 (79) | 6.394 | 0.796 | 0.128 | 0.536 | 13 | 28 | 0.7772 |
| Female | 11 (21) | 2.568 | | 0.214 | | 3 | 8 | |
| Age (median
64.5) | | | | | | | | |
| <65 | 26 (50) | 6.477 | 0.640 | 0.139 | 0.701 | 7 | 19 | 0.5479 |
| ≥65 | 26 (50) | 6.295 | | 0.130 | | 9 | 17 | |
| ypT
classification | | | | | | | | |
| 1/2 | 17 (33) | 7.216 | 0.506 | 0.213 | 0.165 | 5 | 12 | 0.8825 |
| 3/4 | 35 (67) | 6.169 | | 0.108 | | 11 | 24 | |
| ypN
classification | | | | | | | | |
| Absent | 35 (67) | 7.216 | 0.845 | 0.153 | 0.405 | 9 | 26 | 0.2571 |
| Present | 17 (33) | 6.169 | | 0.107 | | 7 | 10 | |
| Postoperative
stage | | | | | | | | |
| I/II | 33 (64) | 7.253 | 0.429 | 0.150 | 0.654 | 9 | 24 | 0.4716 |
| III | 19 (36) | 2.934 | | 0.108 | | 7 | 12 | |
| Lymphatic
invasion | | | | | | | | |
| Absent | 13 (25) | 7.253 | 0.504 | 0.185 | 0.119 | 2 | 11 | 0.1652 |
| Present | 39 (75) | 6.132 | | 0.116 | | 14 | 25 | |
| Vascular
invasion | | | | | | | | |
| Absent | 21 (40) | 7.499 | 0.043a | 0.163 | 0.150 | 6 | 15 | 0.7775 |
| Present | 31 (60) | 2.927 | | 0.107 | | 10 | 21 | |
| Histology | | | | | | | | |
|
Well/moderate | 44 (85) | 7.499 | 0.009a | 0.152 | 0.346 | 13 | 31 | 0.6539 |
|
Poor/signet/mucinous | 8 (15) | 1.814 | | 0.099 | | 3 | 5 | |
| Rödel TRG | | | | | | | | |
| Non-responder,
1/2 | 32 (62) | 9.708 | 0.011a | 0.174 | 0.103 | 12 | 20 | 0.1835 |
| Responder,
3/4 | 20 (38) | 2.032 | | 0.105 | | 4 | 16 | |
| Ryan 3-point
TRG | | | | | | | | |
| Non-responder,
1/2 | 37 (71) | 10.667 | 0.004a | 0.150 | 0.9354 | 14 | 23 | 0.1064 |
| Responder, 3 | 15 (29) | 2.012 | | 0.108 | | 2 | 13 | |
| Radiotherapy | | | | | | | | |
| Short | 42 (81) | 13.398 | 0.204 | 0.139 | 0.852 | 10 | 32 | 0.0258a |
| Long | 10 (19) | 6.151 | | 0.134 | | 6 | 4 | |
| Recurrence | | | | | | | | |
| Absent | 37 (71) | 2.934 | 0.043a | 0.116 | 0.341 | 5 | 31 | 0.0004a |
| Present | 15 (29) | 12.363 | | 0.197 | | 10 | 6 | |
Association of LGR5 and CD44 gene
expression levels in residual cancer cells with clinicopathological
variables
Elevated LGR5 gene expression was
significantly correlated with the absence of vascular invasion
(P=0.043), well differentiated tumor (P=0.009), and poor
pathological response (Rödel system, P=0.011; Ryan system,
P=0.0037). On the other hand, no significant associations of CD44
gene expression in residual cancer cells with clinicopathological
variables were found (Table
I).
Correlation of stromal CD44 gene
expression with clinico-pathological variables
qRT-PCR revealed that 16 of the 52 (30.8%) total
patients showed detectable CD44 mRNA expression in residual
cancer stroma, whereas the remaining patients had no detectable
expression despite positive gene expression of ACTB. The
right side of Table I shows
significant correlations of stromal CD44 gene expression with long
course radiation (P=0.0258) and recurrence after curative operation
(P=0.0004) (Table I).
Positive correlation between LGR5 and
CD44 gene expression
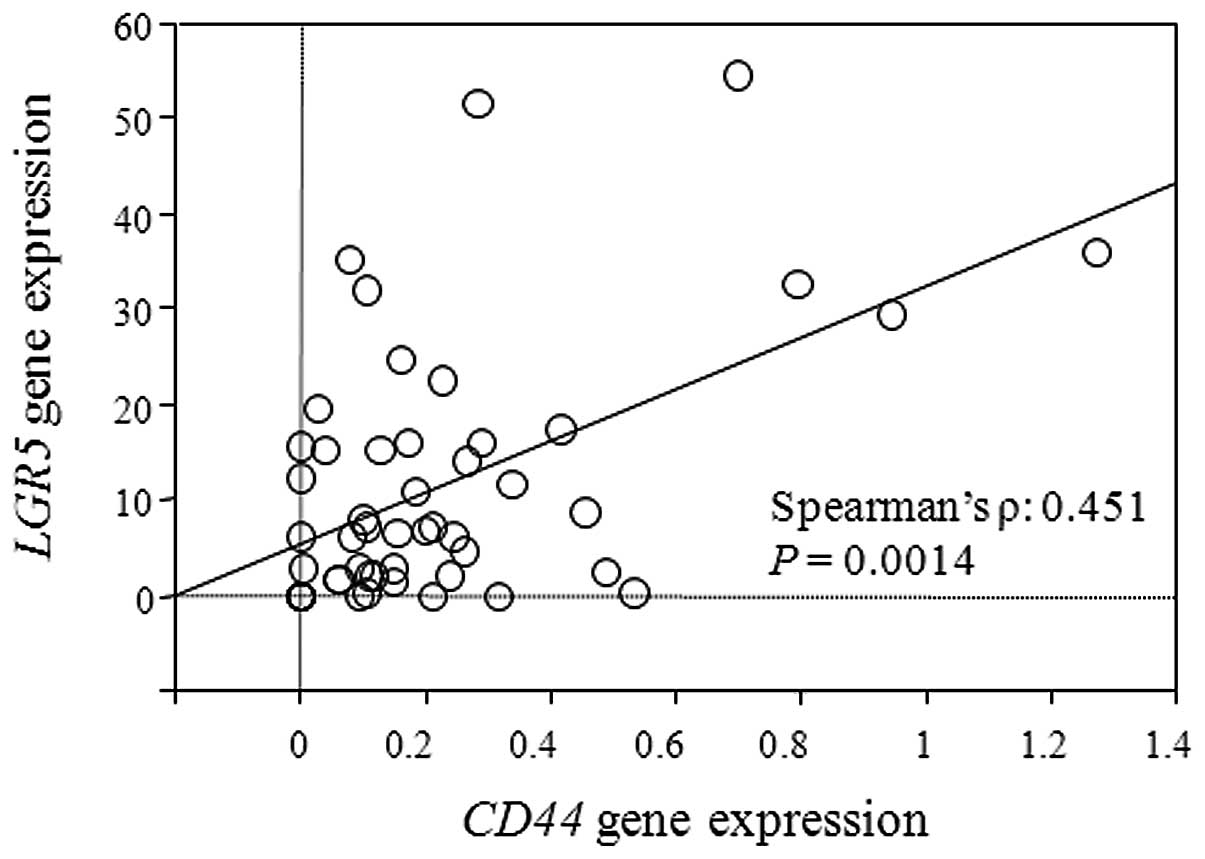
As shown in Fig. 1,
there was a significant positive correlation between expression
levels of LGR5 and CD44 in rectal cancer after CRT
(Spearman’s ϱ = 0.451, P=0.0014).
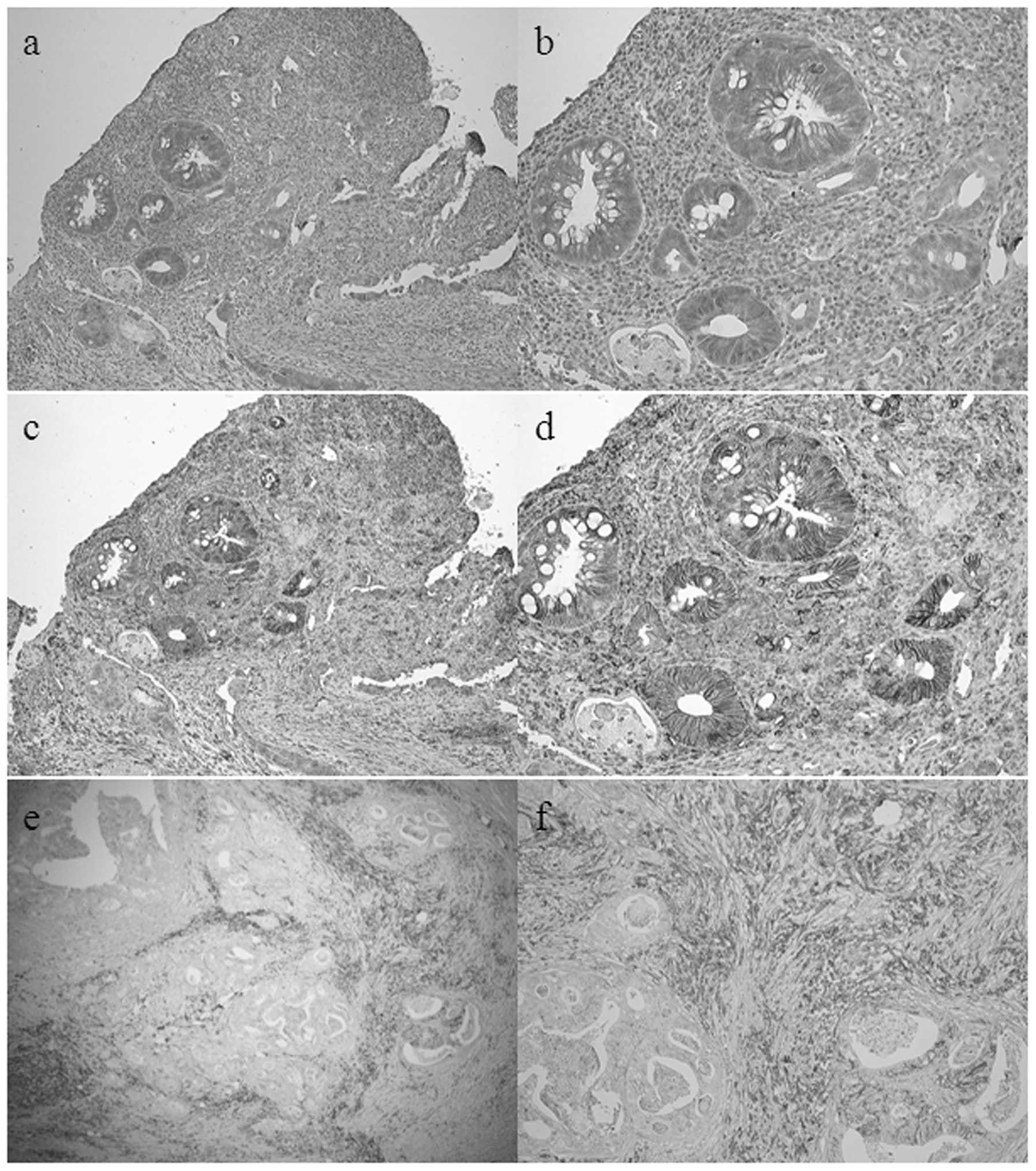
Immunohistochemical analysis of LGR5 and
CD44
LGR5 expression was detected immunohistochemically
at the membrane and in the cytoplasm of cancer cells. CD44
expression was observed at the membrane of cancer cells and in
stromal tissue surrounding residual cancer nests (Fig. 2). We divided expression into two
groups, high or low, according to immunoreactivity of LGR5 and
CD44. There was significant correlation between their
immunoreactivities using the χ2 test (P=0.0356) (data
not shown).
Correlation of immunoreactivity of LGR5
and CD44 with clinicopathological variables
The correlation of LGR5 and CD44 immunoreactivities
with clinical outcome was evaluated. The LGR5 immunoreactivity of
cancer cells was correlated with well differentiated tumor
(P=0.0196). On the other hand, the CD44 immunoreactivity of cancer
cells was correlated with the absence of lymph node metastasis and
low postoperative stage (P=0.0092 and 0.00046, respectively). The
strong immunoreactivity of CD44 in stromal cells was correlated
with tumor recurrence (Rödel, P=0.012; Ryan, P=0.042, respectively)
(Table II).
 | Table II.Correlation of immunoreactivity of
LGR5 and CD44 with clinicopathological variables. |
Table II.
Correlation of immunoreactivity of
LGR5 and CD44 with clinicopathological variables.
| LGR5 in cancer
| CD44 in cancer
| CD44 in stroma
|
|---|
| Variables | High (n=19) | Low (n=33) | P-value | High (n=11) | Low (n=41) | P-value | High (n=32) | Low (n=20) | P-value |
|---|
| Gender | | | | | | | | | |
| Male | 17 | 24 | 0.155 | 8 | 33 | 0.576 | 24 | 17 | 0.390 |
| Female | 2 | 9 | | 3 | 8 | | 8 | 3 | |
| Age (median
64.5) | | | | | | | | | |
| <65 | 10 | 16 | 0.773 | 5 | 21 | 0.734 | 15 | 11 | 0.569 |
| ≥65 | 9 | 17 | | 6 | 20 | | 17 | 9 | |
| ypT
classification | | | | | | | | | |
| 1/2 | 4 | 13 | 0.175 | 3 | 14 | 0.666 | 12 | 5 | 0.350 |
| 3/4 | 15 | 20 | | 8 | 27 | | 20 | 15 | |
| ypN
classification | | | | | | | | | |
| Absent | 15 | 20 | 0.175 | 11 | 24 | 0.009a | 19 | 16 | 0.123 |
| Present | 4 | 13 | | 0 | 17 | | 13 | 4 | |
| Postoperative
stage | | | | | | | | | |
| I/II | 15 | 18 | 0.079 | 11 | 22 | 0.005a | 19 | 14 | 0.439 |
| III | 4 | 15 | | 0 | 19 | | 13 | 6 | |
| Lymphatic
invasion | | | | | | | | | |
| Absent | 4 | 9 | 0.618 | 5 | 8 | 0.078 | 6 | 7 | 0.188 |
| Present | 15 | 24 | | 6 | 33 | | 26 | 13 | |
| Vascular
invasion | | | | | | | | | |
| Absent | 8 | 13 | 0.848 | 7 | 14 | 0.077 | 12 | 9 | 0.592 |
| Present | 11 | 20 | | 4 | 27 | | 20 | 11 | |
| Histology | | | | | | | | | |
|
Well/moderate | 19 | 25 | 0.020a | 10 | 34 | 0.515 | 28 | 16 | 0.466 |
|
Poor/signet/mucinous | 0 | 8 | | 1 | 7 | | 4 | 4 | |
| Rödel TRG | | | | | | | | | |
| Non-responder,
1/2 | 13 | 19 | 0.439 | 8 | 24 | 0.390 | 24 | 8 | 0.012a |
| Responder,
3/4 | 6 | 14 | | 3 | 17 | | 8 | 12 | |
| Ryan 3-point
TRG | | | | | | | | | |
| Non-responder,
1/2 | 16 | 21 | 0.115 | 10 | 27 | 0.103 | 26 | 11 | 0.042a |
| Responder, 3 | 3 | 12 | | 1 | 14 | | 6 | 9 | |
| Radiotherapy | | | | | | | | | |
| Short | 13 | 29 | 0.087 | 9 | 33 | 0.921 | 26 | 16 | 0.911 |
| Long | 6 | 4 | | 2 | 8 | | 6 | 4 | |
| Recurrence | | | | | | | | | |
| Absent | 13 | 24 | 0.741 | 9 | 28 | 0.379 | 20 | 17 | 0.082 |
| Present | 6 | 9 | | 2 | 13 | | 12 | 3 | |
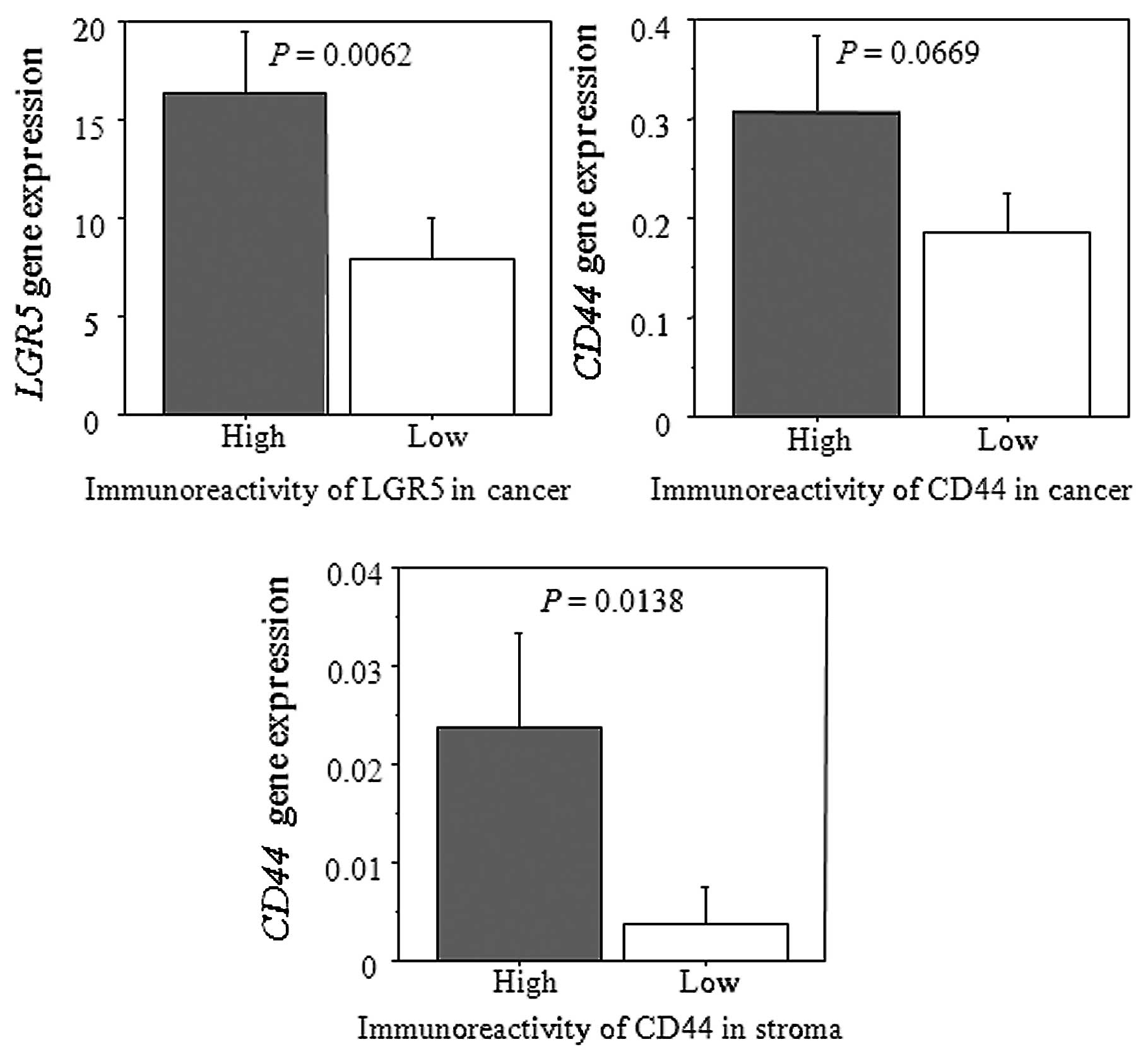
Correlation of gene expression levels of
LGR5 and CD44 with their immunoreactivity
A significant correlation of gene expression of
LGR5 with LGR5 immunoreactivity was observed (P= 0.0062). On
the other hand, there was a significant correlation between
CD44 mRNA levels and its immunoreactivity in cancer stroma
(P=0.0138). Without reaching statistical significance, there was a
correlation between CD44 mRNA levels and its
immunoreactivity in cancer cells (P=0.0669) (Fig. 3).
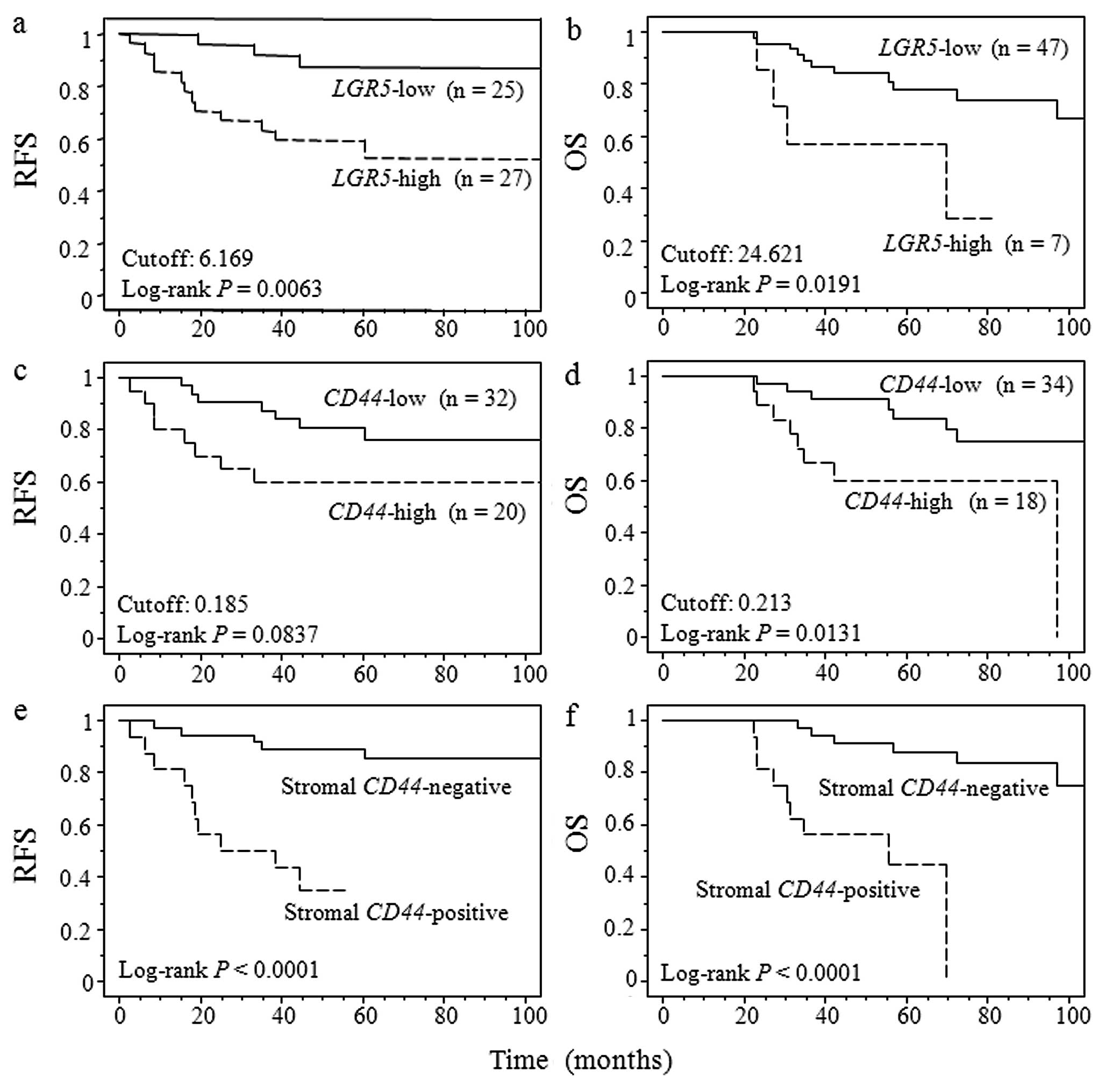
Recurrence-free and overall survival
after curative surgery
On the basis of these results, receiver operating
curve (ROC) analysis was used to identify the cutoff value for
LGR5 and for CD44 gene expression levels that were
predictive of disease recurrence and patients’ death. A
nonparametric ROC analysis determined that the optimal cutoff
values of LGR5 and CD44 were 6.169 and 0.185 for RFS,
and 24.621 and 0.213 for OS. Fig.
4 shows the survival curve for RFS and OS according to
LGR5 and CD44 gene expression using Kaplan-Meier
method. Patients with LGR5 gene expression levels above
cutoff values showed a significantly poorer RFS and OS than did
patients with expression levels below cutoff values (RFS; P=0.0063,
OS; P=0.0191). While, CD44 gene expression levels above
cutoff values showed a significantly poorer OS than did patients
with expression levels below cutoff values although CD44
gene expression level did not have a significant association with
RFS (RFS; P=0.0837, OS; P=0.0131). On the other hand, patients with
positive CD44 gene expression in cancer stroma showed
significantly worse RFS and OS (P<0.0001). RFS and OS according
to the immunoreactivity of LGR5 and CD44 did not show the
significant difference (data not shown).
Predictive value of LGR5 and CD44 gene
expression levels for tumor recurrence and survival
Table III shows the
results of univariate and multivariate analyses of factors
influencing patients’ prognosis using Cox’s proportional hazards
model. univariate analysis showed that cancer LGR5
expression and positivity of stromal CD44 gene expression
levels were significantly associated with a higher rate of
developing recurrence after preoperative CRT (P=0.0136 and 0.0005,
respectively). In a multivariate analysis, a positivity of stromal
CD44 gene expression was found to be an independent
predictive marker for disease recurrence after preoperative CRT
(P=0.0028) (Table IIIA). On the
other hand, univariate analysis showed that the following factors
were significantly related to postoperative overall survival:
disease recurrence, cancer LGR5, CD44 gene expression
level and positivity of stromal CD44 gene expression
(P=0.0044, 0.0287, 0.0192 and 0.0004, respectively). Multivariate
analysis indicated that positivity of stromal CD44 gene
expression was an independent prognostic factor for the overall
survival of patients with rectal cancer after preoperative CRT
(P=0.0491) (Table IIIB). The
clinical variables before CRT seemed not to be influenced in
patient prognosis after CRT.
 | Table III.Prognostic value. |
Table III.
Prognostic value.
| A, Univariate and
multivariate analyses for tumor recurrence after preoperative
CRT |
|
| Variables | HR | 95% CI | P-value |
|
| Univariate | | | |
| Age (< 65 vs
≥65 ) | 1.596 | 0.568–4.486 | 0.3751 |
| Rodel TRG
(responder vs non-responder) | 3.114 | 0.877–11.064 | 0.0790 |
| Ryan 3-point TRG
(responder vs non-responder) | 1.851 | 0.522–6.566 | 0.3407 |
| Pre-stage (I /II
vs III) | 2.227 | 0.126–1.591 | 0.2145 |
| Pre-T
classification (SI-negative vs -positive) | 1.938 | 0.176–1.512 | 0.2275 |
| Pre-N
classification (absent vs present) | 2.227 | 0.126–1.591 | 0.2145 |
| Post-stage (I /II
vs III) | 2.375 | 0.153–1.163 | 0.0951 |
| ypT
classification (T1/2 vs T3/4) | 0.917 | 0.372–3.193 | 0.8743 |
| ypN
classification (absent vs present) | 2.188 | 0.165–1.261 | 0.1305 |
| LGR5 (<
cutoff vs ≥ cutoff ) | 4.942 | 1.390–17.577 | 0.0136a |
| CD44 (<
cutoff vs ≥ cutoff ) | 2.389 | 0.863–6.611 | 0.0936 |
| Stroma
CD44 (negative vs positive) | 8.065 | 0.039–0.398 | 0.0005a |
| Stroma CD44 (low
vs high) | 3.163 | 0.891–11.232 | 0.0749 |
| Multivariate | | | |
| LGR5 (<
cutoff vs ≥ cutoff ) | 3.350 | 0.911–12.321 | 0.0688 |
| Stroma
CD44 (negative vs positive) | 6.173 | 0.049–0.534 | 0.0028a |
|
| B, Univariate and
multivariate analyses for survival after preoperative CRT |
|
| Variables | HR | 95% CI | P-value |
|
| Univariate | | | |
| Age (<65 vs
≥65 ) | 1.855 | 0.673–5.111 | 0.2319 |
| Rodel TRG
(responder vs non-responder) | 1.594 | 0.551–4.613 | 0.3899 |
| Ryan 3-point
TRG | 1.915 | 0.545–6.731 | 0.3112 |
| Pre-stage (I /II
vs III) | 0.724 | 0.233–2.251 | 0.5768 |
| Pre-T
classification (SI-negative vs -positive) | 0.638 | 0.211–1.844 | 0.4070 |
| Pre-N
classification (absent vs present) | 0.311 | 0.233–2.251 | 0.5768 |
| Post-stage (I /II
vs III) | 0.382 | 0.141–1.037 | 0.0588 |
| ypT
classification (T1/2 vs T3/4) | 0.875 | 0.276–2.772 | 0.8208 |
| ypN
classification (absent vs present) | 0.575 | 0.212–1.561 | 0.2778 |
| Recurrence
(absent vs present) | 4.292 | 0.085–0.635 | 0.0044a |
| LGR5 (<
cutoff vs ≥ cutoff ) | 3.684 | 1.146–11.846 | 0.0287a |
| CD44 (<
cutoff vs ≥ cutoff ) | 3.476 | 1.225–9.861 | 0.0192a |
| Stroma
CD44 (negative vs positive) | 9.743 | 0.032–0.370 | 0.0004a |
| Stroma
CD44 (low vs high) | 2.213 | 0.710–6.895 | 0.1708 |
| Multivariate | | | |
| Recurrence
(absent vs present) | 2.160 | 0.144–1.487 | 0.1958 |
| LGR5 (<
cutoff vs ≥ cutoff ) | 1.061 | 0.299–3.771 | 0.9267 |
| CD44 (<
cutoff vs ≥ cutoff ) | 2.105 | 0.667–6.645 | 0.2044 |
| Stroma
CD44 (negative vs positive) | 4.630 | 0.047–0.994 | 0.0491a |
Discussion
Preoperative CRT for locally advanced rectal cancer
is an effective tool for local control because it induces cancer
cell apoptosis and death, and inhibits cell growth in various
malignancies (2,31). However, the mechanism of tumor
relapse in rectal cancer after preoperative CRT has been not fully
elucidated. Actually, clinicopathological variables including TNM
classification in pre-CRT was not influenced in patient prognosis
after CRT in the present study. To clarify the characteristics of
cancer cells after CRT, it is necessary to reevaluate the
expression of genes and proteins associated with clinical outcome
because the characteristics of cancer cells after CRT may be
different from those of primary cancer cells prior to treatment.
The identification of predictive markers for recurrence or poor
prognosis should improve both clinical outcome and potential
treatment stratification for such patients. Therefore, we focused
the expression of genes and proteins after preoperative CRT.
CSCs are a small sub-population of cancer cells that
possess stem cell-like properties such as self-renewal and the
ability to differentiate into multiple cell types. Recent research
suggests that CSCs are particularly resistant to conventional CRT
compared with non-CSCs (13–15,32).
These lines of evidence prompted us to hypothesize that CSCs
survive CRT and are associated with resistance to CRT and tumor
relapse after CRT. CD44 is a candidate marker for colon CSCs
(13–15), while LGR5 is a potential marker for
stem cells in the small intestine and colon (5–7). We
found a significant positive correlation between LGR5 and CD44
protein expression and between LGR5 and CD44 gene
expression in cancer cells after CRT using transcriptional and
immunohistochemical analyses. Both these markers have been known as
targets of Wnt signaling. Wnt signaling has emerged as a critical
regulator of stem cells and the its pathway is integrally involved
in both stem cell and cancer cell maintenance and growth (33). Kanwar et al reported that
Wnt/β-catenin signaling plays a critical role in the growth and
maintenance of colonospheres and the inhibition of β-catenin
results in a marked reduction in CD44-positive cells as well as
colonospheres formation (34).
While, it has been reported that LGR5 expression is associated with
activation of the Wnt pathway (35). We immunohistochemically examined
the expression of β-catenin as Wnt target molecule and observed its
expression in residual cancer cells with both LGR5 and CD44
expression (data not shown). Although our study did not demonstrate
the direct correlation between LGR5 and CD44, these expressions
might have any interaction via Wnt signaling pathway.
We observed that elevated LGR5 expression in
cancer cells and CD44 expression in cancer stroma, but not cancer
cells were significantly correlated with poor pathological
response. Additionally, elevated gene expression of LGR5 in
cancer cells and positive gene expression of CD44 in cancer
stroma were significantly associated with poor RFS, and elevated
gene expression of LGR5 and CD44 in cancer cells and
positive gene expression of CD44 in cancer stroma were
significantly associated with poor OS. These results suggested that
both LGR5 and CD44 gene expression were useful
prognostic markers of patients with rectal cancer after
preoperative CRT. Especially, CD44 gene expression in both
cancer cells and stroma was an independent prognostic factor for
the RFS and OS. Without reaching statistical significance, there
was the association of poor recurrence-free survival with CD44
immunoreactivity in cancer stroma (log-rank test; P=0.060). CD44 is
an important mediator in regulating interaction between ECM and the
intra-cellular actin cytoskeleton. CD44 are considered to generate
a number of cellular signals which play critical roles in not only
cancer invasion and metastasis, but also various physiological and
pathological processes (12,16,18).
In the present study, our results emphasized the significance of
CD44 expression in not only cancer cells but also cancer stromal
tissue. However, there were some discrepancy of the results between
gene expression level and immunoreactivity of these markers
although the correlation between these gene and protein expressions
was observed. Immunohistochemistry, western blotting, and other
protein-quantification methods do not always corroborate RT-qPCR
data. For CD44, we used the monoclonal antibody for CD44
immunostaining included variant 3–10. Previous reports demonstrated
that overexpression of the standard CD44 isoform resulted in
decreased tumorigenesis and tumor progression in vitro
(36). Some studies indicated that
high expression of CD44v6 was associated with primary tumors and
was a predictor of metastasis, including colon cancer (11,37).
Taken together, whether CD44 promotes or protects against tumor
progression may depend on the isoform. Hence, to clarify the
function of CD44, we plan to further investigate the differences of
expression according to each splice variant.
In conclusion, there was a significant positive
correlation between LGR5 and CD44 expression and
elevated these expressions were associated with poor prognosis. Our
results suggest that LGR5 and CD44 may contribute to tumor relapse
in locally advanced rectal cancer treated with preoperative CRT.
However, data in this study should be interpreted with some
caution. First of all, the major limitations were the small number
of patients (n=52), especially those with a recurrence (n=15), and
the retrospective nature of the study. Second, this study included
two neoadjuvant radiation regimens with different time interval
between pretreatment and surgery. Third, our short-course regimen
was different from standard one. A larger study population, a
long-term follow-up and the unification of pretreatments are needed
to validate these results.
Acknowledgements
The authors would like to thank Motoko
Ueeda, Yuka Kato, and Chihiro Hibi for providing excellent
technical assistance.
References
|
1.
|
Sauer R, Becker H, Hohenberger W, et al:
Preoperative versus postoperative chemoradiotherapy for rectal
cancer. N Engl J Med. 351:1731–1740. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Sebag-Montefiore D, Stephens RJ, Steele R,
et al: Preoperative radiotherapy versus selective postoperative
chemoradiotherapy in patients with rectal cancer (MRC CR07 and
NCIC-CTG C016): a multicentre, randomised trial. Lancet.
373:811–820. 2009. View Article : Google Scholar
|
|
3.
|
Bosset JF, Collette L, Calais G, et al:
Chemotherapy with preoperative radiotherapy in rectal cancer. N
Engl J Med. 355:1114–1123. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Guillem JG, Chessin DB, Cohen AM, et al:
Long-term oncologic outcome following preoperative combined
modality therapy and total mesorectal excision of locally advanced
rectal cancer. Ann Surg. 241:829–838. 2005. View Article : Google Scholar
|
|
5.
|
Barker N, van Es JH, Kuipers J, et al:
Identification of stem cells in small intestine and colon by marker
gene Lgr5. Nature. 449:1003–1007. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Becker L, Huang Q and Mashimo H:
Immunostaining of Lgr5, an intestinal stem cell marker, in normal
and premalignant human gastrointestinal tissue. Sci World J.
8:1168–1176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Haegebarth A and Clevers H: Wnt signaling,
lgr5, and stem cells in the intestine and skin. Am J Pathol.
174:715–721. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Sato T, Vries RG, Snippert HJ, et al:
Single Lgr5 stem cells build crypt-villus structures in vitro
without a mesenchymal niche. Nature. 459:262–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Barker N, Ridgway RA, van Es JH, et al:
Crypt stem cells as the cells-of-origin of intestinal cancer.
Nature. 457:608–611. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Uchida H, Yamazaki K, Fukuma M, et al:
Overexpression of leucine-rich repeat-containing G protein-coupled
receptor 5 in colorectal cancer. Cancer Sci. 101:1731–1737. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Ponta H, Sherman L and Herrlich PA: CD44:
from adhesion molecules to signalling regulators. Nat Rev Mol Cell
Biol. 4:33–45. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Nagano O and Saya H: Mechanism and
biological significance of CD44 cleavage. Cancer Sci. 95:930–935.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Reya T, Morrison SJ, Clarke MF, et al:
Stem cells, cancer, and cancer stem cells. Nature. 414:105–111.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Vermeulen L, Sprick MR, Kemper K, et al:
Cancer stem cells - old concepts, new insights. Cell Death Differ.
15:947–958. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Thenappan A, Li Y, Shetty K, et al: New
therapeutics targeting colon cancer stem cells. Curr Colorectal
Cancer Rep. 5:2092009. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Marhaba R and Zoller M: CD44 in cancer
progression: adhesion, migration and growth regulation. J Mol
Histol. 35:211–231. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Subramaniam V, Vincent IR, Gardner H, et
al: CD44 regulates cell migration in human colon cancer cells via
Lyn kinase and AKT phosphorylation. Exp Mol Pathol. 83:207–215.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Jothy S: CD44 and its partners in
metastasis. Clin Exp Metastasis. 20:195–201. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Lopez JI, Camenisch TD, Stevens MV, et al:
CD44 attenuates metastatic invasion during breast cancer
progression. Cancer Res. 65:6755–6763. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Lakshman M, Subramaniam V, Rubenthiran U,
et al: CD44 promotes resistance to apoptosis in human colon cancer
cells. Exp Mol Pathol. 77:18–25. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Zeki SS, Graham TA and Wright NA: Stem
cells and their implications for colorectal cancer. Nat Rev
Gastroenterol Hepatol. 8:90–100. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Wielenga VJ, Smits R, Korinek V, et al:
Expression of CD44 in Apc and Tcf mutant mice implies regulation by
the WNT pathway. Am J Pathol. 154:515–523. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Kim BM, Mao J, Taketo MM, et al: Phases of
canonical Wnt signaling during the development of mouse intestinal
epithelium. Gastroenterology. 133:529–538. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Yoshikawa R, Kusunoki M, Yanagi H, et al:
Dual antitumor effects of 5-fluorouracil on the cell cycle in
colorectal carcinoma cells: a novel target mechanism concept for
pharmacokinetic modulating chemotherapy. Cancer Res. 61:1029–1037.
2001.
|
|
25.
|
Fowler JF: The linear-quadratic formula
and progress in fractionated radiotherapy. Br J Radiol. 62:679–694.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Viani GA, Stefano EJ, Soares FV, et al:
Evaluation of biologic effective dose and schedule of fractionation
for preoperative radiotherapy for rectal cancer: meta-analyses and
meta-regression. Int J Radiat Oncol Biol Phys. 80:985–991. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27.
|
Rodel C, Martus P, Papadoupolos T, et al:
Prognostic significance of tumor regression after preoperative
chemoradiotherapy for rectal cancer. J Clin Oncol. 23:8688–8696.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Ryan R, Gibbons D, Hyland JM, et al:
Pathological response following long-course neoadjuvant
chemoradiotherapy for locally advanced rectal cancer.
Histopathology. 47:141–146. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Saigusa S, Tanaka K, Toiyama Y, et al:
Correlation of CD133, OCT4, and SOX2 in rectal cancer and their
association with distant recurrence after chemoradiotherapy. Ann
Surg Oncol. 16:3488–3498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Bijwaard KE, Aguilera NS, Monczak Y, et
al: Quantitative real-time reverse transcription-PCR assay for
cyclin D1 expression: utility in the diagnosis of mantle cell
lymphoma. Clin Chem. 47:195–201. 2001.PubMed/NCBI
|
|
31.
|
Watanabe T: Chemoradiotherapy and adjuvant
chemotherapy for rectal cancer. Int J Clin Oncol. 13:488–497. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Bao S, Wu Q, McLendon RE, et al: Glioma
stem cells promote radioresistance by preferential activation of
the DNA damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Kanwar SS, Yu Y, Nautiyal J, et al: The
Wnt/beta-catenin pathway regulates growth and maintenance of
colonospheres. Mol Cancer. 9:2122010. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Takahashi H, Ishii H, Nishida N, et al:
Significance of Lgr5(+ve) cancer stem cells in the colon and
rectum. Ann Surg Oncol. 18:1166–1174. 2011.
|
|
36.
|
Choi SH, Takahashi K, Eto H, et al: CD44s
expression in human colon carcinomas influences growth of liver
metastases. Int J Cancer. 85:523–526. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Mulder JW, Wielenga VJ, Polak MM, et al:
Expression of mutant p53 protein and CD44 variant proteins in
colorectal tumorigenesis. Gut. 36:76–80. 1995. View Article : Google Scholar : PubMed/NCBI
|