Introduction
Cervical carcinoma, the most common malignant tumor
of the female reproductive system, is a serious threat to the
physical and mental health of women worldwide (1). Despite advances in surgical
techniques, including conservative treatments, such as radical
trachelectomy, the fertility of patients is still highly affected
(2). In recent years, non-invasive
treatment with preserved fertility has increasingly become the
expectation of doctors as well as patients.
In 1992, Okino et al(3) first originated the concept of
electrochemotherapy (ECT) on the basis of electroporation. The
lipid bilayer of cells is temporarily rearranged, followed by the
formation of aqueous channels in the cell membrane when exposed to
long pulses (millisecond to microsecond), termed electroporation,
which was proposed by Weaver (4).
Over the past two decades, electrochemical therapy has received a
gret deal of attention and tremendous progress has been made in
this field. Electrochemical therapy is based on a pulsed electric
field (PEF). According to the pulse duration, PEF can be classified
into millisecond (msec), microsecond (μsec), nano second
(nsec) and picosecond (psec). Previous studies have shown that the
main effect of the msec and μsec pulses occurs in the cell
membrane. PEF causes a tremendous increase in molecular
transportation across the cell membrane, thus, many electroporation
techniques are applied in cell trans fection for gene expression
and drug delivery. For example, Hofmann et al(5) and Dev et al(6) applied electricochemotherapy in
conjuction with the administration of bleomycin for the treatment
of tumors, which significantly reduced the side-effects of the
drugs. When the pulse duration decreases to nsec and psec, it
mainly affects organelles and leads to intracellular
electromanipulations such as apoptosis, an intracellular calcium
burst, as well as cytoskeletal, nuclear membrane and DNA damage,
with the outer membrane remaining intact (7–9).
Apoptosis, a genetically controlled mode of cell
death, is of critical importance for the removal of potentially
dangerous cells, including precursor tumor cells (10). It usually occurs through two major
pathways. One is the extrinsic pathway, in which the ligation of
death receptors by death ligands is followed by the recruitment of
adaptor molecules and the activation of caspase-8 or -10 (11). The other is the intrinsic pathway,
in which pro-apoptotic signals provoke the release of cytochrome
c from the mitochondrial inter-membranous space into the
cytosol, which forms a complex with Apaf-1, pro-caspase-9 and dATP,
known as apoptosome. Apoptosome formation leads to the subsequent
activation of executioner caspases, such as caspase-3, -6 and -7,
which in turn stimulates a series of apoptotic events, eventually
leading to cell death (12,13).
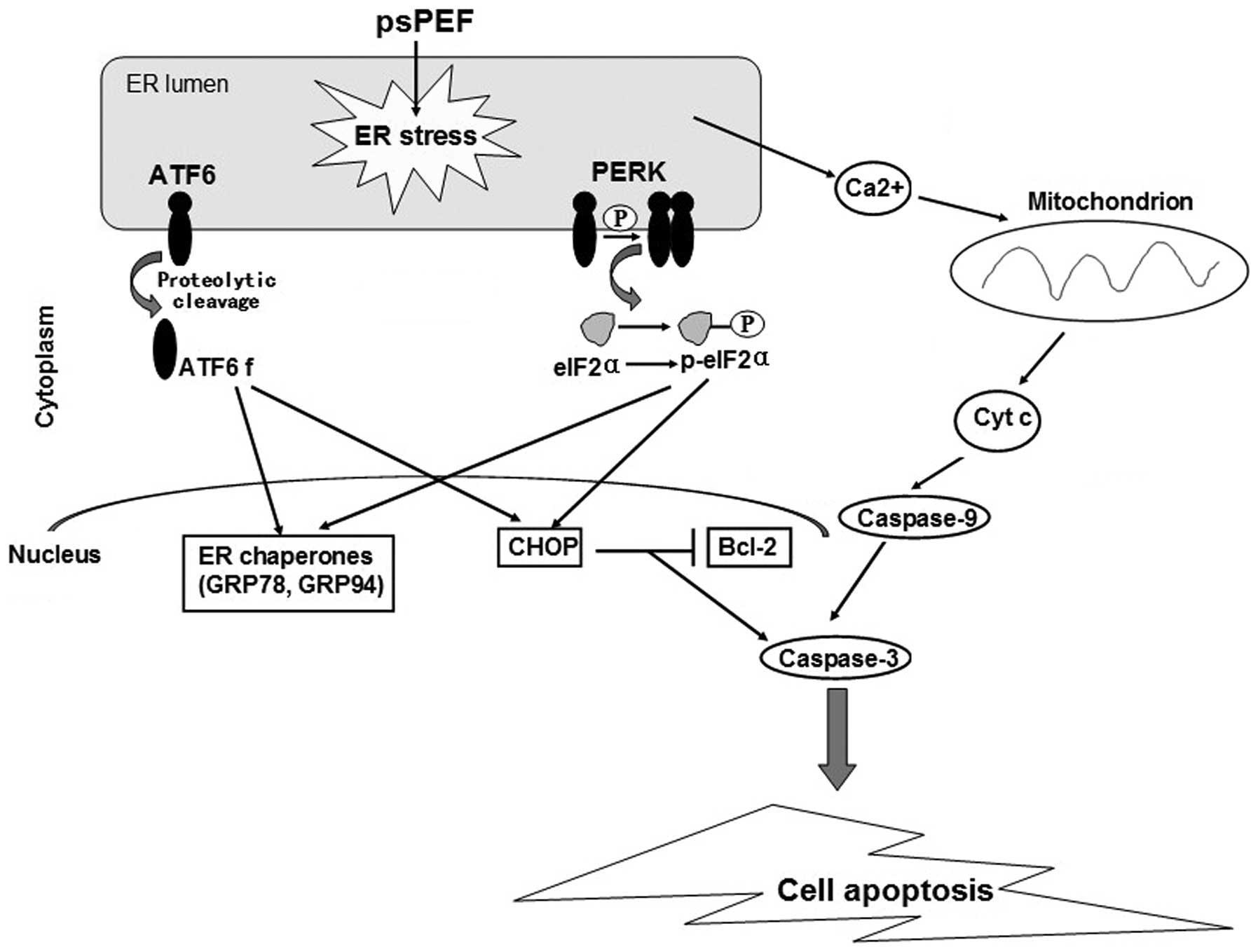
A previous study suggested that the mitochondria and endoplasmic
reticulum (ER) play important roles in the intrinsic pathways of
apoptosis (14).
ER serves as a critical site responsible for
regulating protein synthesis, protein folding and intra cellular
calcium (Ca2+) homeostasis (15). The abnormalities in the ER function
can cause ER stress and ultimately trigger apoptosis through a
variety of mechanisms, including redox imbalance, alteration in
Ca2+ levels and the activation of the Bcl-2 family of
proteins (16). ER stress is
mediated by three ER sensors: double-stranded RNA-activated protein
kinase-like ER kinase (PERK), activating transcription factor 6
(ATF6) and inositol requiring enzyme-1 (IRE1) (17). Additionally, CCAAT enhancer-binding
protein (C/EBP) homologous protein (CHOP), a transcription factor,
is believed to play an important role in promoting ER
stress-induced apoptosis (18).
Our group has been dedicated to studying the
antitumor effects of microsecond PEF (μsPEF) or nanosecond
PEF (nsPEF) for a number of years. Our previous studies showed that
the mitochondrial and ER stress pathways cooperate in nsPEF-induced
human ovarian cancer SKOV-3 cell apoptosis (19–21).
In addition, we have previously demonstrated that psPEF induces
apoptosis through a mitochondrial-mediated pathway in HeLa cells
(22). To determine whether
psPEF-induced apoptosis in cervical carcinoma cells is mediated
through the ER stress pathway, we chose the human cervical
carcinoma cell line,HeLa, as an in vitro model to explore
the effects of psPEF.
Materials and methods
Chemicals and reagents
RPMI-1640 medium and fetal bovine serum (FBS) were
purchased from Gibco (Grand Island, NY, USA);
3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT)
and dimethylsulfoxide (DMSO) were from Sigma (St. Louis, MO, USA);
TRIzol and Fluo-3 AM were from Invitrogen Corp. (Carlsbad, CA,
USA); and the Annexin V-fluorescein isothiocyanate (FITC) apoptosis
detection kit and Hoechst 33258 were obtained from Key-Gen Biotech
Co., Ltd. (Nanjing, China). Takara Taq™ and the
PrimeScript® RT reagent kit (Perfect Real-Time) were
purchased from Takara Biotech Co., Ltd. (Dalian, China); rabbit
anti-Bax, rabbit anti-caspase-12, rabbit anti-caspase-9, mouse
anti-Bcl-2, mouse anti-cytochrome c and mouse anti-caspase-3
antibodies and SuperSignal-enhanced chemiluminescent (ECL)
substrate were from Millipore Co. (Billerica, MA, USA). The goat
anti-GRP78, goat anti-GRP94, mouse anti-PERK, mouse
anti-phospho-PERK (p-PERK) and mouse anti-phosphorylated-eukaryotic
translation initiation factor 2α (p-eIF2α) antibodies were from
Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA); goat
anti-CHOP, mouse anti-poly(ADP-ribose) polymerase (PARP), rabbit
anti-eIF2α, rabbit anti-ATF6 and rabbit anti-β-actin antibodies
were obtained from Cell Signaling Technology (Danvers, MA,
USA).
Cell culture and exposure to picosecond
pulsed electric field (psPEF)
The HeLa human cervical carcimona cells were a gift
from the Institute of Ultrasound Engineering in Medicine of
Chongqing Medical University. The cells were maintained in
RPMI-1640 supplemented with 10% fetal bovine serum (FBS), 100 U/ml
penicillin and 100 μg/ml streptomycin and incubated at 37°C
in a humidified atmosphere 5% CO2. When the cells grew
to ∼80% confluence, they were subcultured or treated with psPEF. In
the following experiments, cells loaded into cuvettes and merely
placed into the circuit without being pulsed were used as the
normal controls. A total amount of 100 μl of cell suspension
was exposed to 2,000 pulses for 800 psec with various electric
field amplitudes.
Cell viability assay
Cell viability was evaluated by the MTT reduction
assay. After treatment with various amplitudes of psPEF, HeLa cells
were seeded at a density of 5×103 cells/well in 96-well
microtiter plates and routinely cultured for 12 and 24 h and then
incubated with MTT (5 mg/ml) in culture medium for 3 h at 37°C.
Subsequently, the medium was discarded and formazan blue, which
formed in the cells, was dissolved in 100 μl of DMSO. The
absorbance was measured at 490 nm using a Sunrise Remote Microplate
Reader (Grodlg, Austria) and then normalized to the value of the
control (untreated cells).
Apoptosis analysis with flow
cytometer
After treatment with pulses, HeLa cells were grown
in 25 cm2 culture flasks for 12 h. Cells were
trypsinized, centrifuged, washed in PBS and then double-stained by
using an Annexin V-FITC apoptosis detection kit according to the
manufacturer’s instructions. Samples were incubated at room
temperature for 15 min in the dark with Annexin V and PI. The
fluorescence in the cells was quantitatively analyzed at an
emission wavelength of 530 nm and an excitation wavelength of 480
nm using a Vantage SE flow cytometer with a fluorescence activated
cell sorting (FACS) system (Becton-Dickinson, San Jose, CA,
USA).
Flow cytometric assessment of cell
cycle
HeLa cells were harvested and fixed with ice-cold
alcohol (75%) for over a day and a night, following treatment with
pulses and growth in 25 cm2 culture flasks for 12 h.
After being washed twice, the cells were incubated with PBS (pH
7.4) containing RNase (5 U) and PI (50 μg/ml) for 15 min at
37°C. Flow cytometry was performed using a FACS vantage SE flow
cytometer.
Hoechst staining
Morphological changes occurring during apoptosis
were detected by fluorescence microscopy after staining with 1
μg/ml Hoechst 33258 for 30 min at 37°C in the dark. More
than 300 cells were counted for each well by three independent
experiments.
Laser scanning confocal microscopy (LSCM)
evaluation of intracellular Ca2+ levels
The HeLa cells were treated with psPEF and then
seeded into 6-well plates for 12 h. After incubation, they were
washed and loaded with 0.5 μM of Fluo-3-AM for 1 h at 37°C
in the dark. The cells were washed twice with PBS and then
subjected to LSCM (Leica TCS-SP2, Wetzlar, Germany) analysis by
detecting the green fluorescence signals.
RNA extraction and gene expression
analysis
Total RNA was extracted from the treated HeLa cells
using TRIzol. Reverse transcription was performed in 20 μl
of reaction mixture containing 2 μg of total RNA, 5 U of AMV
reverse transcriptase, 50 pmol of oligo(dT) primer, 40 nmol of dNTP
mixture, 40 U of RNase inhibitor, 4 μl of 5X RT buffer
(Hangzhou Bioer Technology Co., Ltd, Hangzhou, China) at 42°C for 1
h and 95°C for 5 min. Reverse transcription-polymerase chain
reaction (RT-PCR) was used to examine the expression of caspase-12,
-9 and -3. PCR amplification was performed in 20 μl of PCR
reaction mixture containing 1 μl of the cDNA reaction
mixture, 10 nmol of the dNTP mixture, 10 pmol of the upstream and
downstream primers and 2 U of rTaq polymerase. Real-time
quantitative polymerase chain reaction was used to examine the
expression of ER stress-associated genes, namely glucose-regulated
protein-78 (GRP78), glucose-regulated protein-94 (GRP94) and CHOP.
For determination, SYBR-Green detection was used and the values
were normalized to those of β-actin.
Western blot analysis
After treatment with pulses and routine culture for
12 h, the HeLa cells were washed with ice-cold PBS and lysed in
RIPA lysis buffer (50 mM Tris with pH 7.4, 150 mM NaCl, 1% Triton
X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulphate and
0.05 mM EDTA) for 15 min on ice and the cell lysate was centrifuged
at 12,000 × g for 15 min at 4°C. The supernatant was collected and
the protein content of the extracted samples was measured using the
bicinchoninic acid protein assay kit (Bio-Med, Beijing, China). All
samples were stocked at −80°C for further experiments. The levels
of target proteins including Bax, Bcl-2, cytochrome c,
procaspase-12, -9 and -3, PARP, GRP78, GRP94, CHOP, PERK, p-PERK,
eIF2α, p-eIF2α and activating transcription factor 6 (ATF6) were
determined by western blot analysis using the respective antibodies
stated above. Briefly, total cell lysate was boiled in 5X loading
buffer (125 mM Tris-HCl, pH 6.8, 10% SDS, 8% dithiothreitol, 50%
glycerol and 0.5% bromochlorophenol blue) for 10 min. Equal amounts
of protein (60 μg) were subjected to 8–15% sodium dodecyl
sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and
transferred onto polyvinylidene fluoride membranes. The membranes
were blocked with 5% non-fat milk in PBS with 0.1% Tween-20 (PBST)
for 1 h and incubated with primary antibodies overnight at 4°C.
Antibodies were detected using HRP-conjugated secondary antibody
for 1 h at room temperature. Immunoreactive bands were visualized
using enhanced chemiluminescence reagents (ECL) and densitometric
analysis was performed with the use of a ChemiDoc image analyzer
(Bio-Rad, Hercules, CA, USA).
Statistical analysis
The results were expressed as the means ± SD of at
least three independent experiments performed in triplicate.
Comparisons between groups were performed using the Student’s
t-test and one-way analysis of variance (ANOVA). A value of
p<0.05 was considred to indicate a statistically significant
difference.
Results
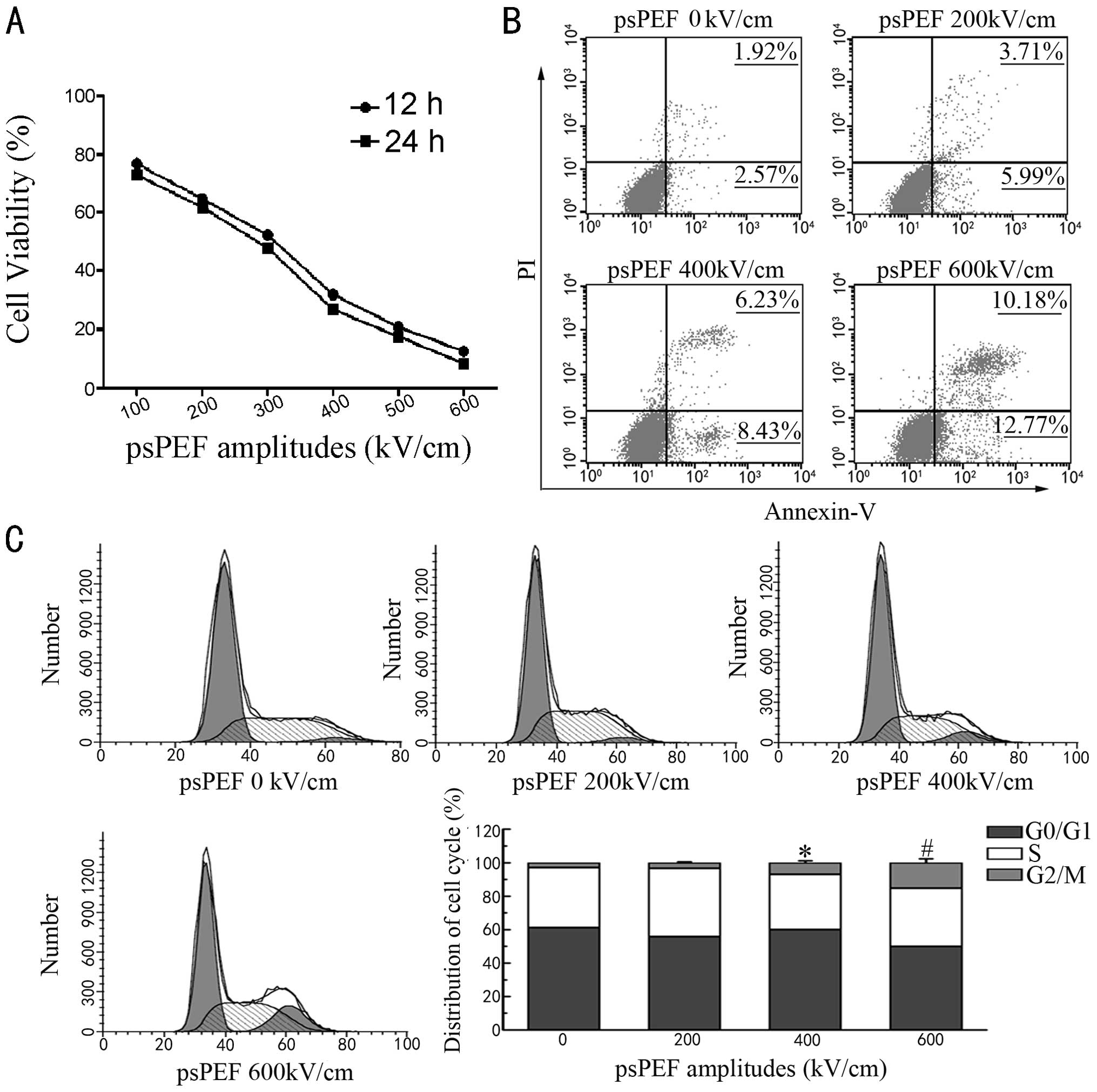
Effect of psPEF on HeLa cell
viability
HeLa cell viability was examined by MTT assay
following treatment with various amplitudes of psPEF at 12 and 24
h. As shown in Fig. 1A, a gradual
decrease in cell viability was observed with the increased
amplitudes of psPEF. However, compared to the 12 h time-point, the
percentage of cell viability was not significantly reduced at 24 h
at the given amplitude of pulses. On the basis of these data, the
experimenatl condition in which the HeLa cells were treated with
the amplitude of 200, 400 and 600 kV/cm of psPEF and routinely
cultured for 12 h after treatment was applied to the following
experiments.
HeLa cells show characteristics of
apoptosis with psPEF treatment
In order to assess whether the proliferation
inhibition induced by psPEF in the HeLa cells was associated with
apoptosis, Annexin V-FITC/PI fluorescence was measured by flow
cytometric analysis. The appearance of the cells with a high
Annexin signal and a low PI signal is characteristic of early
apoptosis. The progression of apoptosis results in cells with a
high Annexin signal and a high PI signal characteristic of late
apoptosis. As presented in Fig.
1B, for the vehicle-treated control group, 4.49% cells were
negative for PI and positive for Annexin V-FITC binding, which
represents apoptotic cells. Following exposure to psPEF (200, 400
and 600 kV/cm), the percentage of apoptotic cells increased to 9.7,
14.66 and 22.95%, respectively. To examine the role of psPEF in
HeLa cell cycle progression, the cell cycle distribution was
assessed by monitoring the intensity of PI fluorescence. The
results indicated that psPEF blocked cell cleavage at the
G2/M phase of the cell cycle (Fig. 1C).
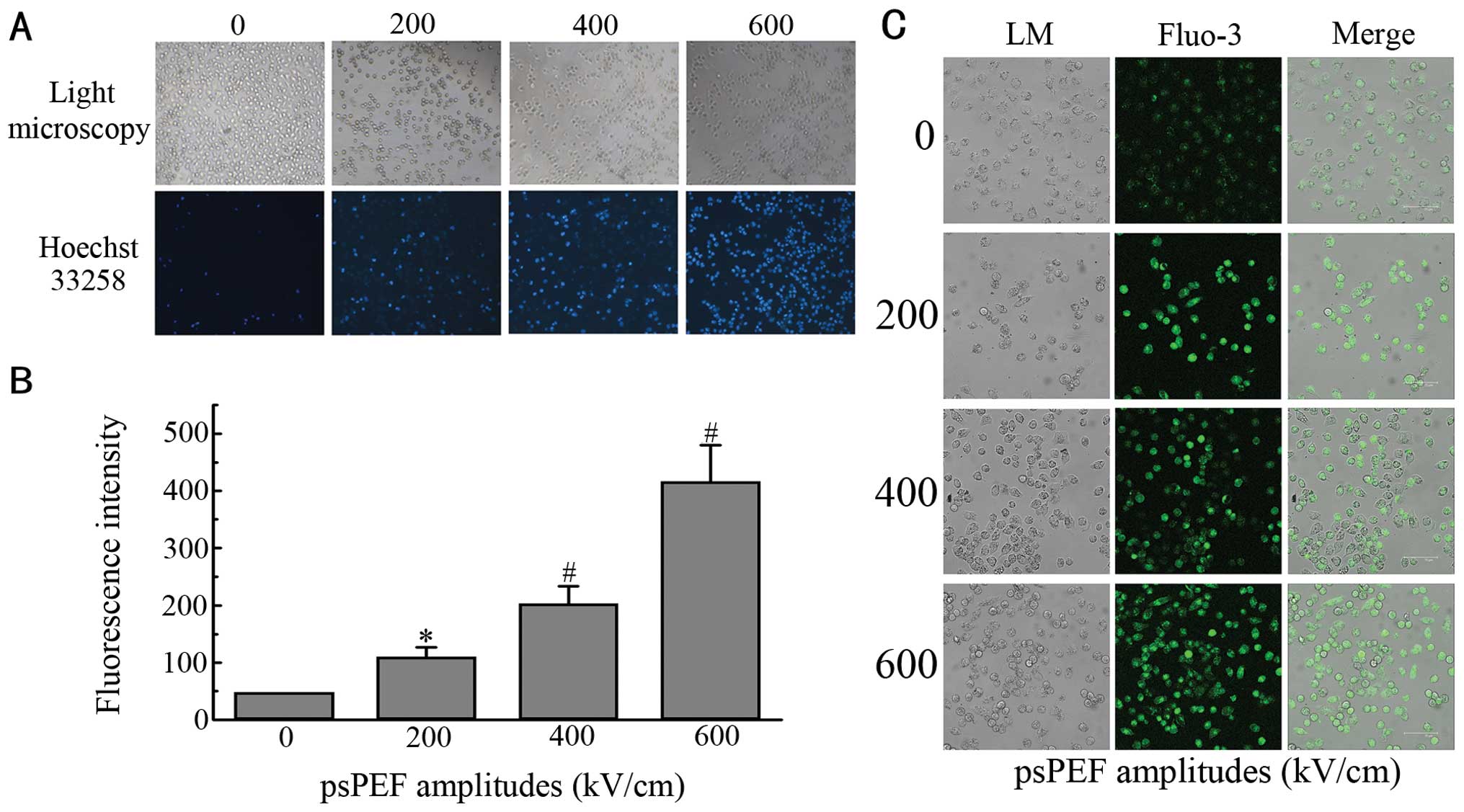
Following treatment with different amplitudes of
psPEF and culture for 12 h, typical apoptotic morphological changes
in the HeLa cells were detected by Hoechst 33258 staining (Fig. 2A and B). Fig. 2B clearly shows that the quantity of
positive cells increased significantly compared to the control
group (p<0.05).
psPEF induces the activation of the ER
stress pathway
It is well-established that there are three ER
transmembrane proteins identified as sensors of ER stress. These
include PERK, IRE1 and ATF6. They activate the unfolded protein
response (UPR) under ER dysfunction. Phospho-PERK phosphorylates
eIF2α, decreasing gene expression. The IRE1 and ATF6 pathways
promote the expression of ER chaperones and the pro-apoptotic
transcription factor, CHOP (23,24).
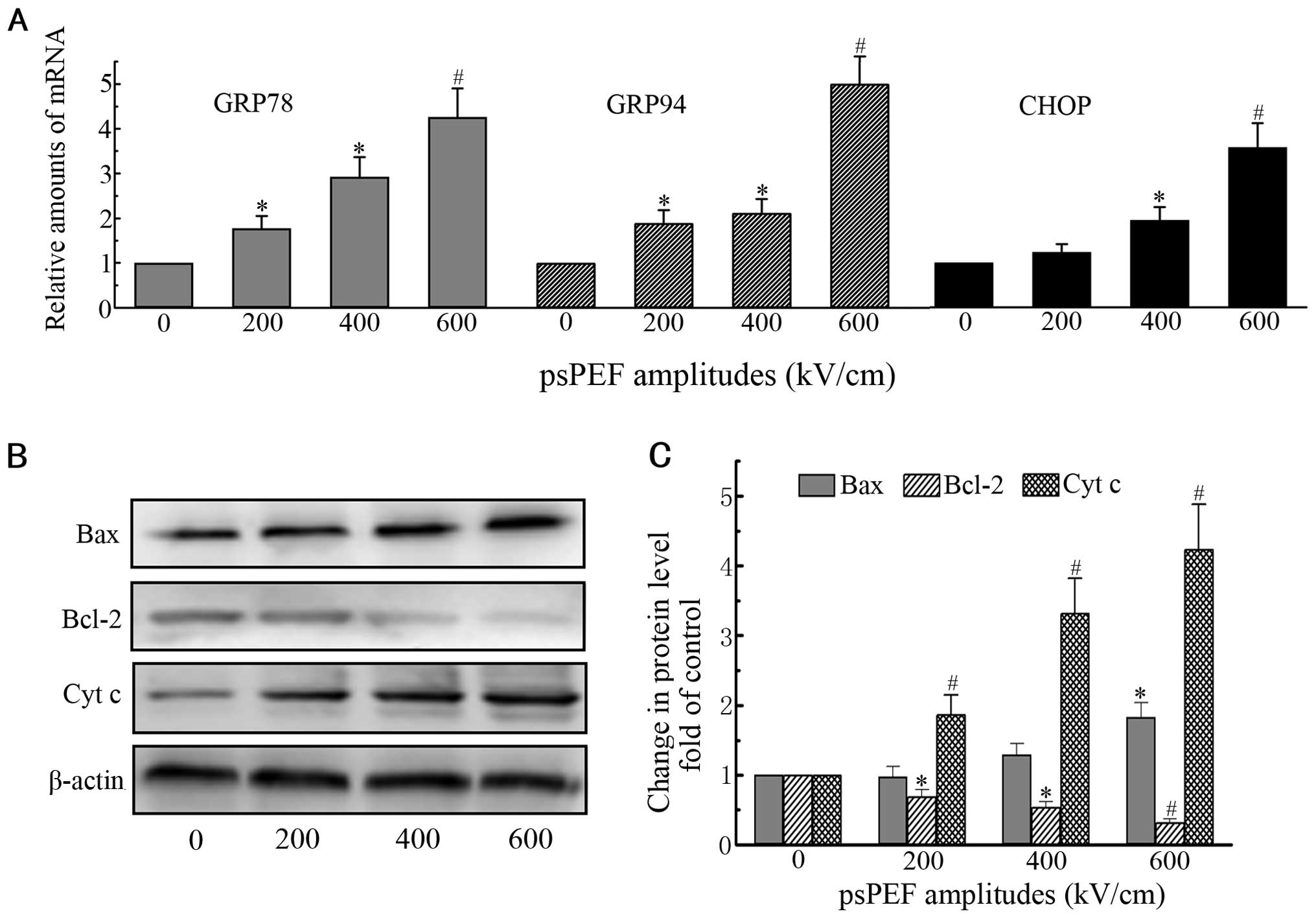
In the current study, GRP78, GRP94 and CHOP expression was
evaluated by real-time PCR and western blot analysis. There was a
significant increase in expression 12 h after the pulses,
regardless of the level of gene or protein, compared with the
control group (Figs. 3A and B and
4A). p-PERK, p-eIF2α and ATF6
fragments increased during the early period (6 h after the pulses),
whereas their normal forms were not altered (Fig. 3C).
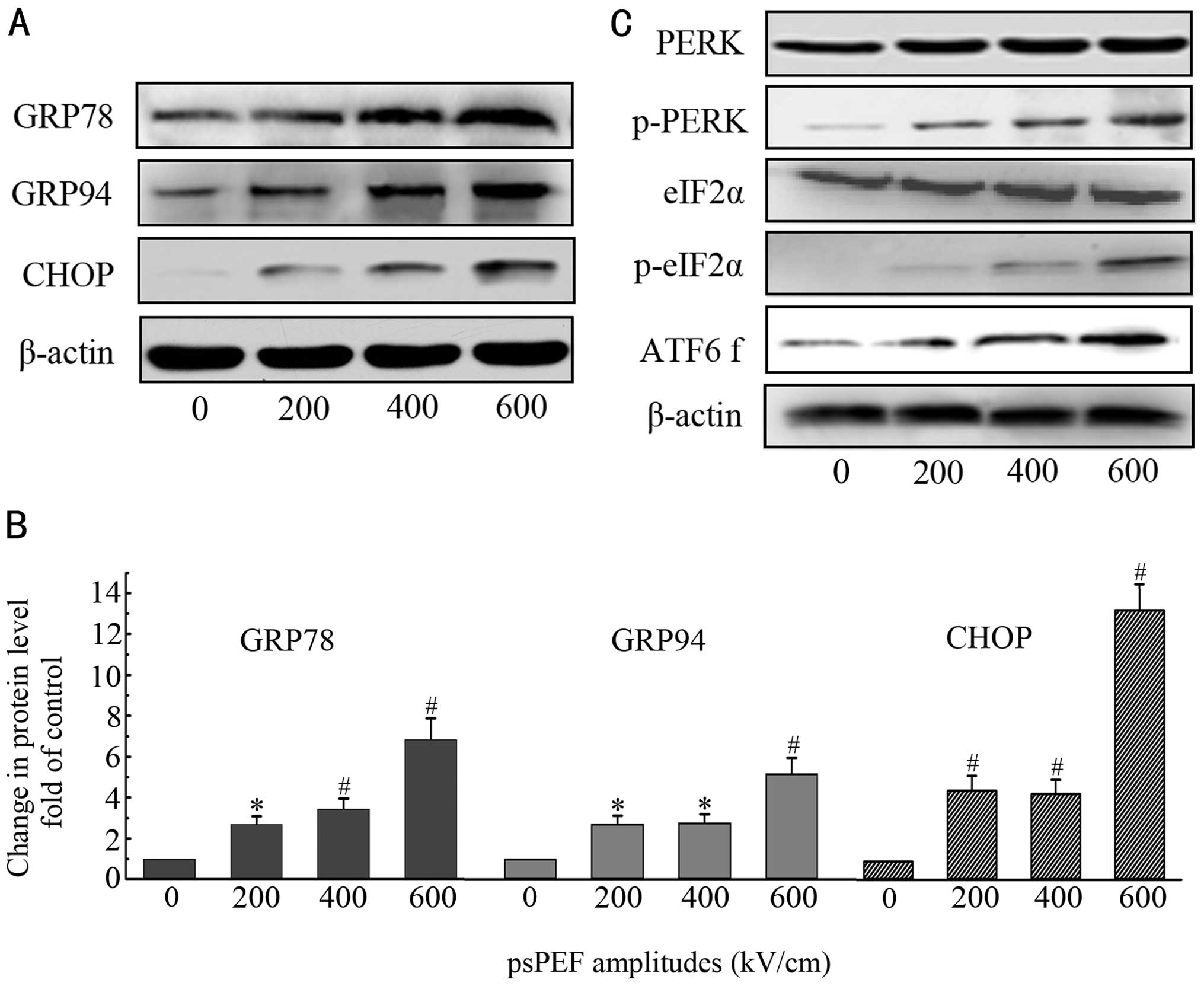 | Figure 3Effect of psPEF on the endoplasmic
reticulum stress-related pathway. HeLa cells were pre-treated with
various amplitudes of psPEF (200, 400 and 600 kV/cm) and cultured
for 12 h (A and B) or 6 h (C). Subsequently, the proteins were
extracted. (A and B) Western blot analysis of the ER stress-related
chaperones, GRP78 and GRP94, and the pro-apoptotic transcription
factor, CHOP. (C) Western blot analysis of the ER stress sensors,
PERK, p-PERK, eIF2α, p-eIF2α and the ATF6 fragment (ATF6 f).
*p<0.05, #p<0.01 indicate a
statistically significant difference compared with the
vehicle-treated control group. |
Cytosolic Ca2+ levels in
psPEF-treated HeLa cells
Increases in intracellular Ca2+ levels
have been found in ER-stressed cells (25). As indicated above, the apoptosis of
HeLa cells induced by psPEF also involved ER stress; therefore,
Ca2+ levels were measured. Cells were loaded with the
calcium probe, Fluo-3-AM, and subsequently examined by
immunofluorescence using confocal microscopy. Fig. 2C shows the fluorescence images of
the control and treated groups 12 h after the pulses. The green
fluorescence intensity increased after the pulses, which indicated
the elevation of Ca2+ concentrations.
Effect of psPEF on the release of
cytochrome c and the activation of proteins from the Bcl-2
family
The development of apoptosis has been shown to be
associated with an increase in cytosolic Ca2+ levels,
which subsequently leads to mitochon-, which subsequently leads to
mitochondrial depolarization and initiates a cell death cascade
(26). The depolarization of the
mitochondrial membrane induces the release of cytochrome c
into the cytosol, which is a prominent downstream manifestation of
the evolution of apoptotic cell death (27). In our study, treatment with psPEF
resulted in an increase in cytosolic cytochrome c in a
dose-dependent manner in the HeLa cells (Fig. 4B and C).
The Bcl-2 family, and in particular, the ratio of
Bax (proapoptotic member) to Bcl-2 (anti-apoptotic member) is
critical for regulating cell death. The balance between Bax and
Bcl-2 decides the destiny of cells (28). In our study, western blot analysis
revealed that the expression of Bax was upregulated. By contrast,
the expression of Bcl-2 was significantly down-regulated (Fig. 4B and C).
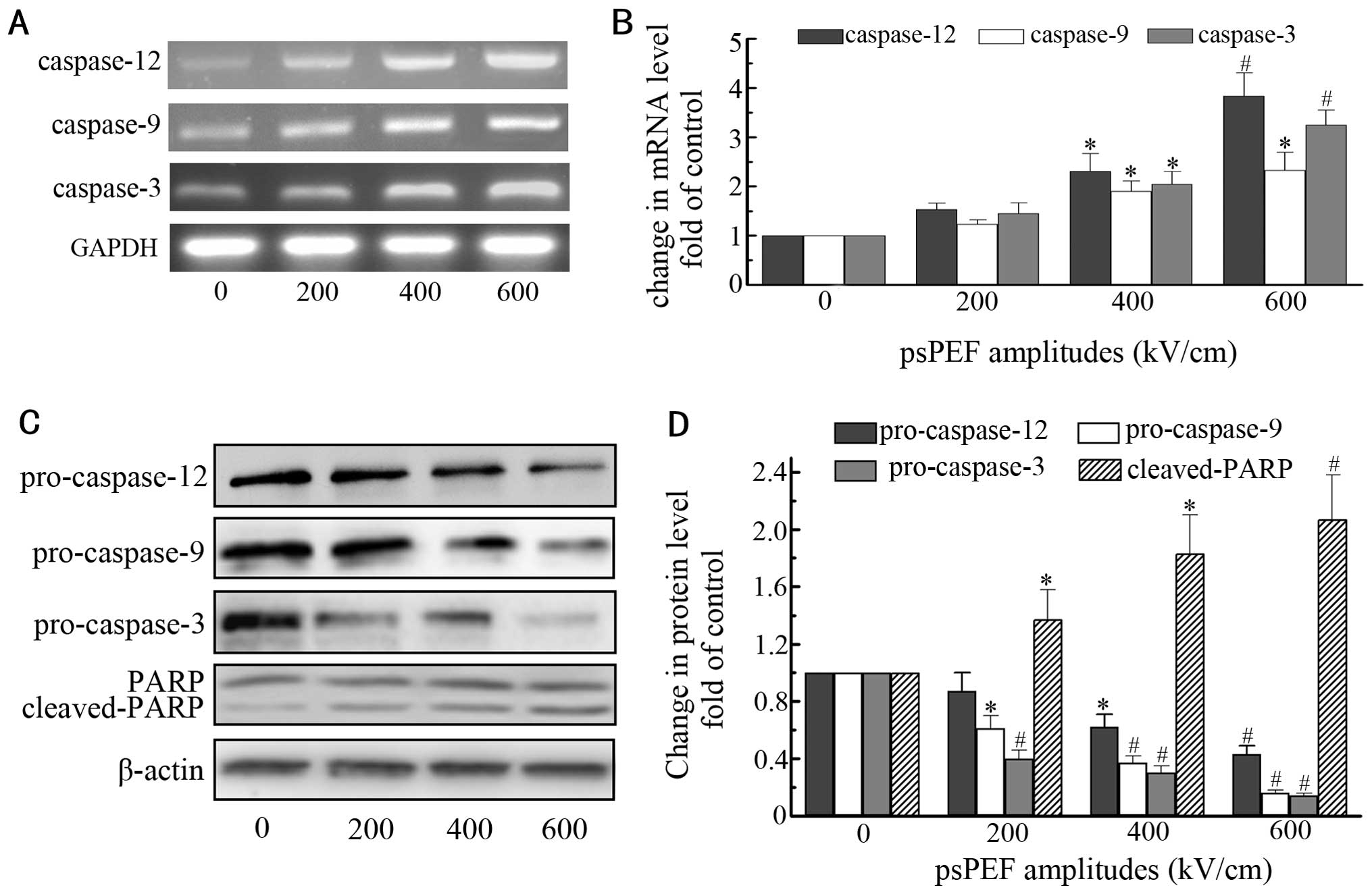
Caspase-dependent pathway is activated by
psPEF treatment
Caspases, a conserved enzymatic family, serve as the
executioners of apoptosis. In response to apoptotic stimuli, they
become activated (29). RT-PCR
analysis demonstrated the psPEF-induced activation of caspase-12,
-9 and -3 (Fig. 5A and B). Western
blot analysis showed the downregulation of pro-caspase-12, -9 and
-3 (Fig. 5C and D). To further
identify the activation of the caspase cascade, PARP (116 kDa), one
of the caspase downstream effectors, was examined by western blot
analysis. In Fig. 5C and D, we
show that the cleaved fragment of PARP (89 kDa) was increased.
Discussion
The discovery of a safe, effective and non-invasive
treatment with preserved fertility for patients is becoming the
most promising research direction. PEF is a new biomedical
engineering technique which can be used as electrochemotherapy,
tumor ablation and intracellular electromanipulation. In previous
studies, the effectiveness of msPEF, μsPEF or nsPEF has been
well proven. However, the application of msPEF, μsPEF or
nsPEF requires the use of an invasive or minimally invasive needle
or plate electrodes, to guide the puncture of tumor tissue, which,
to a certain extent, limits the clinical applica tion of this
method. psPEF has an ultra-broadband optical spectrum, with
extended time and spatial resolution and low signal distortion. It
can be transferred to target deep tissue non-invasively and
precisely with wide band antennas (30,31).
These features make the implementation of non-invasive treatment
possible.
msPEF and μsPEF mainly target the outer
membrane, with few effects on the cell nucleus, mitochondria, ER
and other organelles; thus, it causes electroporation to the outer
membrane. As the pulse duration decreases, plasma membrane
electroporation decreases and the electroporation effect changes
gradually from the outer membrane to the intracellular
substructures. It has been demonstrated nsPEF-induced apoptosis is
not dependent on plasma membrane electroporation (32).
A previous study pointed out that ER is a
subcellular compartment involved in the intrinsic apoptotic pathway
(33). Under certain conditions,
ER dysfunction leads to an accumulation of unfolded or misfolded
proteins in the ER lumen and activates the compensatory mechanism,
which has been referred to as ER stress response or unfolded
protein response (34). The
alterations of cellular protein expression related to psPEF
stimulation can provide valuable information to point the
occurrence of stress and also to help elucidate the mechanisms of
action of pluses. Our study showed the upregulation of GRP78 and
GRP94 (Fig. 3A and B). They are
the chaperones of the heat shock protein family and responsible for
the folding and maturation of non-glycosylated proteins. GRP78 is a
key element in maintaining normal ER function and protecting the ER
from dangerous stimulation. It controls the activation of
transmembrane ER stress sensors through a binding-release mechanism
and activates the downstream signaling pathways (35). In the present study, ER stress
sensors were also assayed to validate the involvement of ER stress
in the apoptotic process. Following psPEF exposure, PERK and eIF2α
were phosphorylated; however, the total protein level of PERK and
eIF2α was not affected, as shown in Fig. 3C. At the same time, ATF6 was
cleaved to the activated form ATF6 fragmentation (Fig. 3C). These signaling transduction
pathways are the adaptation responses to ER stress which can
suppress the protein synthesis to relieve the burden of ER and
increase the transcription of ER-associated chaperone genes
(15). CHOP, a downstream
pro-apoptotic component of the IRE1α, PERK and ATF6 stress
responder pathways, is minimally expressed under physiological
conditions; however, it is strongly induced in response to ER
stress (36). The data presented
in our study showed that CHOP expression was significantly
upregulated. CHOP overexpression has been shown to lead to a
decrease in the pro-survival protein, Bcl-2 (37), providing evidence that the
pro-apoptotic functions of CHOP are associated with the
mitochondrial-dependent mecha nisms of cell death. Bax, a
pro-apoptotic protein in the Bcl-2 family, forms channels at the
outer mitochondrial membrane to facilitate the release of
cytochrome c. Under normal conditions, Bcl-2 forms
heterodimers with the pro-apoptotic element, Bax. Under stress
conditions, Bax enters the mitochondrial outer membrane, increasing
membrane permeability and subsequently leading to cytochrome
c release by forming pores on the outer mitochondrial
membrane (38). In the present
study, psPEF treatment resulted in a significant increase in Bax
expression and a decrease in Bcl-2 expression. Changes in the ratio
of pro-apoptotic and anti-apoptotic Bcl-2 family proteins can
activate downstream signaling, for example, caspase-3, eventually
leading to apoptosis (39). Our
data support this conclusion and are substantiated by observations
of increased caspase 3 activity in HeLa cells treated with
psPEF.
The regulation of intracellular calcium homeostasis
is one of the main functions of ER. In our study, Ca2+
levels increased in the cytosol as shown in Fig. 2C, suggesting the involvement of ER
stress in psPEF-treated HeLa cells. The abnormality of
Ca2+ concentration in the ER can directly activate
caspase-12 following cleavage by calpain-dependent apoptosis in the
ER stress pathway (40).
Ca2+ is released from the ER into the cytoplasm and the
mitochondria (Fig. 6). The
pathological overload of calcium in the mitochondria triggers the
opening of the mitochondrial permeability transition pore (mPTP),
which leads to mitochondrial dysfunction, thus, resulting in the
release of the mitochondrial apoptogenic factor, cytochrome
c(41). Therefore, as shown
in our study, the expression of cytochrome c was increased
(Fig. 4B and C). Cytochrome
c is released from the mitochondrial intermembrane spaces to
initiate the subsequent activation of caspase-9, further activating
downstream caspase-3. They are the executioners of apoptosis. In
this study, we detected the downregulation of pro-caspase-9 and -3
by western blot analysis (Fig. 5C and
D). The decreased intensity of the pro-enzymes reflected the
emergence of the cleaved form. Caspase-3 has been demonstrated to
cleave its substrate, PARP, inducing characteristic apoptotic
changes, such as chromatin condensation and DNA chromatin
fragmentation (42).
In this study, to our knowledge, we demonstrate for
the first time, the effect of ps-PEF on ER stress. Our current
findings may lead to the discovery of a novel, non-invasive
treatment for tumors. An understanding of the mechanism of
ps-PEF-activated HeLa cells death is a basic step in clinical
therapeutic approaches. Additional functional studies to identify
the specific targets of psPEF are undergoing.
Acknowledgements
We are grateful for the support from
The National Nature Science Foundation of China (project no.
81172123) and the Health Bureau of Chongqing (project no.
2012-2-068).
References
|
1
|
Jemal A, Siegel R, Ward E, Hao Y, Xu J and
Thun MJ: Cancer statistics, 2009. CA Cancer J Clin. 59:225–249.
2009. View Article : Google Scholar
|
|
2
|
Karimi Zarchi M, Mousavi A, Malekzadeh M,
Dehghani A, Behnamfar Z and Godarzi A: Conservative treatment in
young patients with cervical cancer: a review. Asian Pac J Cancer
Prev. 11:589–594. 2010.PubMed/NCBI
|
|
3
|
Okino M, Tomie H, Kanesada H, et al:
Optimal electric conditions in electrical impulse chemotherapy. Jpn
J Cancer Res. 83:1095–1101. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Weaver JC: Electroporation: a general
phenomenon for manipulating cells and tissues. J Cell Biochem.
51:426–435. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hofmann GA, Dev SB, Dimmer S, et al:
Electroporation therapy: a new approach for the treatment of head
and neck cancer. IEEE Trans Biomed Eng. 46:752–759. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dev SB, Rabussay DP, Widera G, et al:
Medical applications of electroporation. IEEE Trans Plasma Sci.
28:206–223. 2000. View Article : Google Scholar
|
|
7
|
Stacey M, Stickley J, Fox P, Statler V,
Schoenbach K, Beebe SJ and Buescher S: Differential effects in
cells exposed to ultra-short, high intensity electric fields: cell
survival, DNA damage and cell cycle analysis. Mutat Res. 542:65–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Craviso GL, Chatterjee P, Maalouf G, et
al: Nanosecond electric pulse-induced increase in intracellular
calcium in adrenal chromaffin cells triggers calcium-dependent
catecholamine release. IEEE Trans Dielect El In. 16:1294–1301.
2009. View Article : Google Scholar
|
|
9
|
Stacey M, Fox P, Buescher S and Kolb J:
Nanosecond pulsed electric field induced cytoskeleton, nuclear
membrane and telomere damage adversely impact cell survival.
Bioelectrochemistry. 82:131–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Thorburn A: Death receptor-induced cell
killing. Cell Signal. 16:139–144. 2004. View Article : Google Scholar
|
|
12
|
Budihardjo I, Oliver H, Lutter M, Luo X
and Wang X: Biochemical pathways of caspase activation during
apoptosis. Annu Rev Cell Dev Biol. 15:269–290. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li UX, Kim CN, Yang J, Jemmerson R and
Wang X: Induction of apoptotic program in cell-free extracts:
requirement for dATP and cytochrome c. Cell. 86:147–157. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yokouchi M, Hiramatsu N, Hayakawa K, Kasai
A, Takano Y, Yao J and Kitamura M: Atypical, bidirectional
regulation of cadmiuminduced apoptosis via distinct signaling of
unfolded protein response. Cell Death Differ. 14:1467–1474. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Boyce M and Yuan J: Cellular response to
endoplasmic reticulum stress: a matter of life or death. Cell Death
Differ. 13:363–373. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yamaguchi H and Wang HG: CHOP is involved
in endoplasmic reticulum stress-induced apoptosis by enhancing DR5
expression in human carcinoma cells. J Biol Chem. 279:45495–45502.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moenner M, Pluquet O, Bouchecareilh M and
Chevet E: Integrated endoplasmic reticulum stress responses in
cancer. Cancer Res. 67:10631–10634. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Schroder M and Kaufman RJ: ER stress and
the unfolded protein response. Mutat Res. 569:29–63. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yao CG, Guo F, Wang J, Li CX, Wen YQ and
Tang JY: Study of tumor cell mitochondrial apoptosis signaling
pathway induced by nanosecond pulsed electric fields. Chin J Biomed
Eng. 29:724–730. 2010.
|
|
20
|
Liu LJ, Zhao DY, Wang J, Yao CG, Sun CX
and Tang JY: Ca2+ is an important mediator of nanosecond
steep pulse-induced apoptosis in human ovarian cancer SKOV3 cells.
J Southern Med Univ. 31:772–776. 2011.(In Chinese).
|
|
21
|
Tang JY, Wu XJ, Yao CG, et al: The effects
of nanosecond steep pulse on apoptosis and the concentration of
intracellular calcium of human ovarian carcinoma cell Line SKOV3.
Prog Obstet Gynecol. 16:827–831. 2007.
|
|
22
|
Hua YY, Wang XS, Zhang Y, Yao CG, Zhang XM
and Xiong ZA: Intense picosecond pulsed electric fields induce
apoptosis through a mitochondrial-mediated pathway in HeLa cells.
Mol Med Rep. 5:981–987. 2012.PubMed/NCBI
|
|
23
|
Hamanaka RB, Bennett BS, Cullinan SB and
Diehl JA: PERK and GCN2 contribute to eIF2alpha phosphorylation and
cell cycle arrest after activation of the unfolded protein response
pathway. Mol Biol Cell. 16:5493–5501. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davenport EL, Moore HE, Dunlop AS, Sharp
SY, Workman P, Morgan GJ and Davies FE: Heat shock protein
inhibition is associated with activation of the unfolded protein
response pathway in myeloma plasma cells. Blood. 110:2641–2649.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang D and Armstrong JS: Bax and the
mitochondrial permeability transition cooperate in the release of
cytochrome c during endoplasmic reticulum-stress-induced apoptosis.
Cell Death Differ. 14:703–715. 2007. View Article : Google Scholar
|
|
26
|
Crow MT, Mani K, Nam YJ and Kitsis RN: The
mitochondrial death pathway and cardiac myocyte apoptosis. Circ
Res. 95:957–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Degli Esposti M and Dive C: Mitochondrial
membrane permeabilisation by Bax/Bak. Biochem Biophys Res Commun.
304:455–461. 2003.PubMed/NCBI
|
|
28
|
Zhou J, Zhang S, Ong CN and Shen HM:
Critical role of proapoptotic Bcl-2 family members in
andrographolide-induced apoptosis in human cancer cells. Biochem
Pharmacol. 72:132–144. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schlegel J, Peters I, Orrenius S, et al:
CPP32/apopain is a key interleukin 1 beta converting enzyme-like
protease involved in Fas-mediated apoptosis. J Biol Chem.
271:1841–1844. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Baum CE, Stone AP and Tyo JS:
Ultra-Wideband, Short-Pulse Electromagnetics 8. Springer Press; New
York, NY: 2007
|
|
31
|
Bajracharya C, Shu X, Baum CE and
Schoenbach KH: Target detection with impulse radiating antenna.
IEEE Antennas Wireless Propag Lett. 10:496–499. 2011. View Article : Google Scholar
|
|
32
|
Beebe SJ, Fox PM, Rec LJ, Willis EL and
Schoenbach KH: Nanosecond, high-intensity pulsed electric fields
induce apoptosis in human cells. FASEB J. 17:1493–1495.
2003.PubMed/NCBI
|
|
33
|
Rao RV, Ellerby HM and Bredesen DE:
Coupling endoplasmic reticulum stress to the cell death program.
Cell Death Differ. 11:372–380. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xu C, Bailly-Maitre B and Reed JC:
Endoplasmic reticulum stress: cell life and death decision. J Clin
Invest. 115:2656–2664. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ni M and Lee AS: ER chaperones in
mammalian development and human diseases. FEBS Lett. 581:3641–3651.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oyadomari S and Mori M: Roles of
CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ.
11:381–389. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
McCullough KD, Martindale JL, Klotz LO, Aw
TY and Holbrook NJ: Gadd153 sensitizes cells to endoplasmic
reticulum stress by down-regulating Bcl-2 and perturbing the
cellular redox state. Mol Cell Biol. 21:1249–1259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yang JC and Cortopassi GA: Induction of
the mitochondrial permeability transition causes release of the
apoptogenic factor cytochrome c. Free Radic Biol Med. 24:624–631.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakagawa T and Yuan J: Cross-talk between
two cysteine protease families. Activation of caspase-12 by calpain
in apoptosis. J Cell Biol. 150:887–894. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Boitier E, Rea R and Duchen MR:
Mitochondria exert a negative feedback on the propagation of
intracellular Ca2+ waves in rat cortical astrocytes. J
Cell Biol. 145:795–808. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Nicholson DW and Thornberry NA: Apoptosis.
Life and death decisions. Science. 299:214–215. 2003. View Article : Google Scholar : PubMed/NCBI
|