Glaucoma is a leading cause of permanent blindness
and is characterized by progressive retinal ganglion cell (RGC)
death that produces characteristic optic nerve head damage and
visual field loss (1,2). Some risk factors are related with
glaucoma pathogenesis. These include intraocular pressure (IOP),
age, family history, clinical appearance of the optic nerve, race
and potential vascular disease (3–6). Of
these, elevated IOP is considered as a major risk factor for
glaucoma, and lowering IOP is the most effective treatment method
available for glaucoma (1,2).
Several prospective randomized multi-center studies
have identified that IOP reduction with either medicines or surgery
can reduce the development and progression of vision loss in
glaucoma patients (7–13). The precise mechanisms that lead to
the death of RGCs in glaucoma have not been identified
conclusively, but might involve the blockade of both anterograde
and retrograde axonal transport leading to the deprivation of
neurotrophic signals (2). If IOP
is beyond the tolerable range of the optic nerve, RGCs axons
degenerate at the optic nerve head in the region of the lamina
cribrosa, a process that occurs in parallel to the apoptotic death
of RGCs. The glaucomatous neuropathy might occur in parallel to a
remodeling of the extracellular matrix (ECM) of the optic nerve
head (2,14,15).
IOP is determined by the equilibrium between the
secretion of aqueous humor by the ciliary body and the drainage of
aqueous humor from the eye. There are two main aqueous humor
outflow pathways, trabecular (conventional) and uveoscleral
(unconventional). The unconventional route of this drainage is
through the interstitial spaces of the ciliary muscle and the
supraciliary space, whose physiological role is not fully
understood. The conventional outflow pathway is composed of the
trabecular meshwork (TM), juxtacanalicular tissue (JCT), Schlemm’s
canal (SC), and the episcleral veins on a continuous basis, and in
humans, this pathway represents a predominant route of aqueous
humor drainage (16,17). The ciliary secretion of aqueous
humor usually remains normal in glaucoma (18), therefore, it is thought that
impaired drainage through the trabecular pathway caused by
increased resistance is the primary cause for increased IOP in
primary open-angle glaucoma (POAG) (18). The normal aqueous humor outflow
resistance resides in the inner wall region of the trabecular
meshwork outflow pathways (19,20).
The site of highest resistance remains uncertain (21,22),
but likely resides at the confluence of the TM, JCT and SC inner
wall (21,23). It has been proposed that abnormal
accumulation of extracellular material/ECM (ECM hypothesis), and
changes in contractile activity and cell adhesive interactions of
the cells of aqueous outflow pathway (contractility hypothesis) are
contributed to increases resistance to drainage of aqueous humor
through the conventional pathway (16–18,24–26).
The ECM hypothesis is supported by the observation that perfusion
of anterior eye segments in organ cultures with metalloproteinases
that digest ECM components leads to a reversible increase in
outflow facility (27). The
contractility hypothesis is supported by the observation that
experimental disruption of the actin cytoskeleton of the trabecular
meshwork decreases outflow resistance (28,29)
and by recent findings which provide evidence that the trabecular
meshwork of patients with primary open angle glaucoma is stiffer
than that of age-matched controls (30). The two hypotheses can exist
simultaneously, since it is possible that trabecular meshwork cells
that increase their contractile capabilities simultaneously
synthesize more fibrillar matrix to transmit more force.
Over the past few years, many studies have shed
light on the important role of Rho/Rho-associated kinase (ROCK)
pathway in the pathogenesis and treatment of glaucoma. The purpose
of this review is to summarize the role of Rho/ROCK pathway in the
IOP modulation, subconjunctival scarring of the filtering bleb and
neuroprotection of glaucoma.
Rho is a member of Rho family of small molecular
guanosine triphosphatase (GTPase) superfamily related to Ras. Rho
has three isomer types: RhoA, RhoB and RhoC (31). ROCK is a serine/threonine kinase
and one of the major downstream effectors of Rho GTPases (32). ROCK has two isomer types: ROCK1 and
ROCK2. The structures of ROCK1 and ROCK2 are conserved with 64%
overall amino acid identity (33,34).
The kinase domain containing both extension segments is more highly
conserved between these two proteins (83% identical), suggesting
that they may have similar substrate specificity (33,34).
Both Rho-kinase proteins are ubiquitously expressed in most
tissues; however, higher levels of ROCK2 are found in brain and
muscles whereas higher levels of ROCK1 are found in non-neuronal
tissues including liver, lung and testis (33,35).
ROCK1 is specifically cleaved by caspase-3, whereas ROCK2 is
cleaved by granzyme B (36–38).
The Rho GTPases act as molecular switches by cycling between an
active GTP-bound and an inactive GDP-bound form. In the GTP-bound
form, the Rho GTPase interact with specific downstream effector
proteins - ROCK, which include Rho kinase, regulators of actin
polymerization and adaptor proteins (32). The activity of Rho GTPase is
regulated by signaling input originating from different classes of
cell surface receptors, including the heterotrimeric G
protein-coupled receptors tyrosine kinase receptors, cytokine
receptors, frizzled receptors, and adhesion receptors (32,39).
Rho/ROCK pathway has critical functions in the formation of actin
stress fibers and focal adhesions (40–43),
and the regulation of actomyosin cytoskeletal organization, cell
adhesion, cell morphology, cell motility, smooth muscle
contraction, neurite elongation and neuronal architecture and
cytokinesis (44–55).
Rho/ROCK pathway is involved in various cellular
functions through phosphorylation of their specific substrates. The
main substrates of Rho/ROCK pathway is the myosin light chain
(MLC), LIM kinase 1 (LIMK1), LIMK2 and myosin phosphatase target
subunit 1 (MYPT1) (56–58). Gene silencing experiments suggest
ROCK1 appears to be essential for the formation of stress fibers,
whereas ROCK2 appears to be necessary for cytoskeletal
rearrangements, cell motility and cell contraction, both of which
are dependent on MLC phosphorylation (59,60).
The phosphorylation status of MLC is controlled not only by myosin
light chain kinase (MLCK), but also by myosin light chain
phosphatase (MLCP). MLC is phosphorylated by
Ca2+/calmodulin-dependent MLCK and dephosphorylated by
Ca2+-independent MLCP, and the balance between these two
enzyme activities is a critical determinant of MLC phosphorylation
(61–63). Phosphorylation of MLC subsequently
results in stimulation of the myosin-actin interactions. Increased
and decreased MLC phosphorylation induces contraction and
relaxation responses of the cell and influences the formation of
actin stress fibers and smooth muscle contraction. ROCK is
implicated in the RhoA-mediated inhibition of MLCP (64). Inhibition of ROCK results in an
increased activity of MLCP and dephosphorylation of MLC. Thus,
Rho/ROCK pathway is a master regulator of the actin cytoskeleton
and cell contractility (32,51,65,66).
Growth factors, mechanical stretch, cytokines and ECM can activate
Rho GTPase through guanine nucleotide exchange factors. This
subsequently activates ROCK, which then leads to MLC
phosphorylation that enhances actomyosin cross-bridging and
contractility, thereby regulating many cell processes including
contraction, cytoskeleton organization, adhesive interactions,
trafficking and permeability (48,51,65,67–70).
The TM beams have been characterized as connective
tissue containing elastic and collagen fibers surrounded by
endothelial-like trabecular cells resting on a basement membrane
(16). The outermost JCT or
cribriform region has no collagenous beams, but rather several cell
layers immersed in a loose web of ECM fibrils. The adjacent SC is a
continuous endothelium-lined channel that drains aqueous humor to
the general venous circulation (29). The TM cells exhibit a smooth
muscle-like phenotype, based on their expression of various smooth
muscle-specific proteins, including α-smooth muscle actin (α-SMA)
and CPI-17 (the 17 kDa protein kinase C-potentiated protein
phosphatase 1 inhibitor protein) (24,28,29,75,76).
The actomyosin system, composed of actin microfilaments and
associated proteins, is present in essentially all cells, and is
highly organized in TM and SC cells. There are numerous
microfilament-based structures in cells along the trabecular
outflow pathway. These structures primarily include focal contacts,
adherens cell-cell junctions and bundles of microfilaments
(77). A physiologically
contracted state of the JCT-SC region is required to maintain the
microfilament-related structures in the outflow pathway (29). Microfilaments are involved in a
variety of cellular processes from cell adhesion and motility to
organelle traffic to adhesion-mediated signal transduction. As
discussed above, ROCK mainly promotes myosin II activity by
inhibiting MLCP as well as by phosphorylating the myosin regulatory
light chain. This, in turn, induces the assembly of contractile
actomyosin bundles that generate strong tensile forces (65). A specific Rho kinase inhibitor,
Y-27632, induces reversible changes in cell shape and decreases in
actin stress fibers, focal adhesions, and protein phosphotyrosine
staining in human TM cells and SC cells (72,73).
In isolated bovine TM strips, Y-27632 completely blocks
Ca2+-independent phorbol myristate acetate or
endothelin-1-induced contraction (71,78,79).
A morphological study in bovine eyes indicates that, with Y-27632,
the inner wall of SC and the JCT are significantly distended
compared to control eyes, with discernible separation between the
inner wall of SC and JCT, which suggests that the structural
correlate to the increase in outflow facility of non-human eyes
after Y-27632 is physical separation between the JCT and inner wall
of SC (80).
Regulation of mechanical and contractile properties
of the pressure-sensitive TM cells is recognized to play a
significant role in modulation of aqueous humor outflow and ocular
pressure homeostasis (20,24,81–83).
There is growing evidence, that contraction of TM reduces aqueous
humor outflow and thus enhances intraocular pressure, whereas
relaxation exerts the opposite effect (24,29,72,75,76,84).
The activation of Rho/ROCK pathway could result in TM contraction,
and the inhibition of this pathway would provoke relaxation of TM
with subsequent increase in outflow facility (53,72,73,82).
As expected, ROCK inhibitors, such as Y-27632, Y-39983, HA-1077,
H-1152, increase outflow facility and/or decrease IOP in animals
(72,85–88).
Conversely, agents that activate Rho GTPase and myosin II activity,
including lysophosphatidic acid (LPA), sphingosine-1-phosphate,
TGF-β2, and endothelin-1, decrease aqueous humor outflow facility
concomitant with increased contractile activity of the TM cells,
indicating a potential importance of actomyosin organization and
the contractile force generated by the actomyosin system in the
regulation of aqueous humor drainage (76,84,89–91).
In addition to the effect on the contractility of cells in
trabecular (conventional) outflow, Rho/ROCK pathway may also
modulate the contractility of tissues in uveoscleral
(unconventional) outflow (72,92).
CM is one of the main tissues in the uveoscleral outflow pathway,
and CM cells morphologically and electrophysiologically express
properties that are typical of smooth muscle cells (93). The ROCK inhibitor Y-27632 has been
shown to induce inhibition of smooth muscle contraction and alter
various cellular behavior (94,95).
Moreover, Y-27632 can relax the excised ciliary muscle which is
previously constricted by carbachol, suggesting that the inhibitor
acts to increase the uveoscleral outflow (92). However, there is also evidence to
the contrary. ROCK and its substrates show higher expression in TM
compared to CM (53), and ROCK
inhibitor Y-39983 leads to relaxation of TM, but Y-39983 is only
slightly effective in CM (86).
Honjo et al also reported that only a modest increase in the
uveoscleral outflow was found in rabbit eyes by Y-27632, and its
effects were not statistically significant (72). These results suggest that the
mechanism for decreased IOP by ROCK inhibitor is largely mediated
by enhancement of aqueous outflow facility through relaxation of TM
in the conventional outflow pathway (24).
Alterations in ECM content and organization have
been found to be associated with increased resistance in the
outflow pathway of human glaucomatous eyes (96–100). Rho/ROCK pathway has an important
role for modulating the synthesis of ECM components in the
trabecular pathway. Pattabiraman and Rao found that human TM cells
expressing a constitutively activated form of RhoA (RhoAV14)
demonstrated increased levels of fibronectin, fibronectin fibril
formation, laminin, tenascin C and α-SMA (101). Furthermore, the changes in
expression of ECM proteins could be suppressed by the Rho GTPase
inhibitor (C3 transferase) and ROCK inhibitor (Y-27632), in
association with decreased MLC phosphorylation, actin stress
fibers, focal adhesions and fibronectin fibrils (101). Zhang et al reported that
TM cells expressing a constitutively activated form of RhoA had
increased expression of various ECM-related genes and cytokines
such as TGF-β, interleukin-1, and connective tissue growth factor
(CTGF) in TM cells (91). The
stimulation of TM cells with physiological agonists such as LPA and
TGF-β2, which are known to induce Rho GTPase activation and MLC
phosphorylation in TM (90,102),
leads to an increase in levels of fibronectin, fibronectin fibrils,
laminin and α-SMA in a RhoA- and Rho kinase-dependent manner. In
the case of TGF-β2, increased resistance to aqueous humor outflow
is reported to be associated with increased levels of synthesis of
ECM components (25,98,103). CTGF has also an important role in
ECM synthesis, Iyer et al reported that stimulation of human
TM cells with CTGF treatment for 24 h led to an increase in the
levels of laminin, fibronectin, and in the levels of phosphorylated
MLC in human TM cells, and that the expression of CTGF is regulated
closely by Rho GTPase (104).
There is a potential interplay among the contractile
activity, ECM synthesis and Rho GTPase activation (105–109). As mentioned above, the activation
of Rho GTPase and ROCK was able to promote myosin II
phosphorylation and contractile activity (53,72,73,82),
and to induce ECM synthesis/assembly in TM cells (91,101,104). On the other hand, the
actomyosin-derived contractile force induced ECM synthesis/assembly
and, conversely, ECM assembly/rigidity could influence actomyosin
contraction and induce Rho GTPase activation (91). ECM rigidity has been reported to
increase fibronectin fibril formation, Erk activation, focal
adhesion kinase activity, α-SMA, and actin stress fibers in TM
cells (110). The interplay among
contractile activity, ECM synthesis/assembly and Rho GTPase
activation in the cells of aqueous humor outflow pathway, including
TM, JCT and SC cells, represents a crucial regulatory component in
the homeostasis of aqueous humor outflow resistance (101).
The permeability of SC endothelial cells is
suggested to play important roles in the regulation of aqueous
outflow (17,111). Breaks have been found in the
endothelial lining of the SC and aqueous plexus after perfusion
with certain cytoskeletal drugs (112–114). Additionally, SC endothelial cells
have transcellular pores accompanied by giant vacuoles (111,115). ROCK inhibitor Y-27632 resulted in
Rho/ROCK-dependent filamentous actin reorganization and disruption
of proteins associated with tight junction, increased SC
endothelial-cell monolayer permeability, which may lead to
increased aqueous humor outflow facility (73,111).
Rho/ROCK pathway has a crucial role in IOP
modulation. In general, the activation of Rho/ROCK pathway in the
outflow tissue results in reduction of aqueous humor outflow, and
thereby increase IOP, whereas the inhibition of Rho/ROCK pathway
tissue results in increase of aqueous humor outflow, and thereby
decrease IOP. Organ-cultured anterior segments from porcine eyes
expressing RhoAV14 exhibited significant reduction of aqueous humor
outflow (91). However, inhibiting
RhoA expression in TM with siRNA is effective in suppressing
elevated IOP in mice (116).
Furthermore, several ROCK inhibitors, such as Y-27632, Y-39983,
HA-1077 and H-1152, increase outflow facility and/or decrease IOP
in living rabbits, mouse, rat, monkeys, human and enucleated
porcine eyes (72,73,76,85–88,92,117–120). In monkey eyes, 0.05% Y-39983
induces significant IOP reduction almost equal to that obtained
with 0.005% latanoprost (86).
SNJ-1656, an ophthalmic solution of Y-39983, has been proved as a
safe topical agent that is effective in reducing IOP in healthy
adult volunteers (87). Thus, ROCK
inhibitors might be a candidate for the next generation of glaucoma
therapy (53,119).
Filtration surgery, such as trabeculectomy, is the
most widely used anti-glaucoma surgery. The most frequent cause of
failure of glaucoma filtration surgery is postoperative scarring in
the filtering bleb. Fibroblasts from the subconjunctival space play
a key role in the scarring process. Perioperative administration of
antimetabolites such as 5-fluorouracil and mitomycin C (MMC) is
effective in limiting the scarring process. However, use of these
antiproliferative agents is accompanied by severe side-effects
(121,122). Therefore, alternative
anti-scarring agents that do not cause extensive tissue damage are
needed.
Subconjunctival scarring of the filtering bleb site
is mainly mediated by tenon fibroblasts (TFs) proliferation,
migration, and contraction (123–125). Transdifferentiation of
fibroblasts into myofibroblasts is a crucial step in wound healing
and scar formation (126), which
is associated with expression of α-SMA (127). Enhanced α-SMA expression
indicates the presence of activated fibroblasts with increased
synthesis of ECM proteins, growth factors and integrins (128,129). Myofibroblasts are responsible for
fibrosis via increased ECM synthesis, for granulation tissue
formation, wound contraction and scar formation (126,130–132). TFs are stimulated by growth
factors to differentiate into myofibroblasts both in vitro
(133), and in vivo
(134). LPA and serum, as well as
TGF-β, could activate myofibroblast differentiation (135–138), which is supposedly one of the
most potent stimulators of TFs (124). After glaucoma filtration surgery,
TFs are likely to be exposed to LPA via serum and/or plasma,
because the blood-aqueous humor barrier breaks down, and
circulating aqueous humor bathes the wound site (139).
There is increasing evidence demonstrating the
protective effects of RhoA/ROCK-inhibition on adult retinas. The
intraocular injection of the RhoA antagonist C3 is reported to
increase both axonal regeneration and RGC survival after optic
nerve axotomy in rats (170). The
inhibition of ROCK (fasudil) was shown to decrease the extent of
N-methyl-D-aspartic acid (NMDA)-induced neurotoxicity in rat
retinas (171). In an in
vivo rat model of glaucoma, intraperitoneal injection of the
Rho kinase inhibitor fasudil protected against neuronal loss
(172,173), which suggested that abnormal
activity of Rho/Rho kinase pathway may participate in the
pathophysiology of glaucoma (82).
Inactivation of Rho/ROCK signaling also contributes to the
neuroprotectivity of neuronal cells in the retinal
ischemia/reperfusion injury. Retinal ischemia/reperfusion injury
leads to a loss of neuronal cells in the inner retinal layers such
as RGCs and amacrine cells (174,175), and neuronal cell apoptosis
induced by transient retinal ischemia progresses through the
reperfusion phase rather than the ischemic phase. Injury during
reperfusion is caused by the infiltration of leukocytes into the
neural tissue through vascular endothelial cells (176). The Rho/Rho kinase pathway
contributes to leukocyte extravasation by regulating the leukocyte
cytoskeleton and tight junction of endothelial cells (177,178). ROCK inhibitors attenuate the
ischemia/reperfusion induced apoptosis of retinal cells in the
inner retinal layers (including RGCs) by decreasing Bax/Bcl-2 mRNA
ratio and the expression of caspase-3 and iNOS (179), and by regulating leukocyte
infiltration in the neural tissue (180,181). The treatment with the ROCK
inhibitor Y-27632 could promote the viability of primary RGCs, the
RGCs-5 cell line (182).
Moreover, ROCK inhibition also rescues RGCs from axotomy-induced
apoptosis in vivo, and the neuroprotective effects of the
ROCK inhibitor Y-27632 are mediated by the activation of
well-established cell survival pathways, such as the Akt and MAPK
pathways (182). Furthermore,
Tura et al found that the neuroprotective effect of H-1152P
on retinal cells, particularly in RGCs, was associated with a
decrease in the reactivity of astrocytes, Müller cells, and
microglia both in retinas cultured under serum deprivation and
after optic nerve crush, which suggested the neuroprotective effect
of H-1152P-mediated ROCK-inhibition on retinal cells under stress
may rely partly on the attenuation of glial cell reactivity
(183).
The failure of axon regeneration after injury to
the mammalian CNS is attributable to the limited availability of
neurotrophic factors (NTF) which promote neuron survival and axon
regeneration, and the presence of myelin- and scar-derived
inhibitory molecules such as Nogo-A, myelin associated glycoprotein
(MAG), oligodendrocyte-myelin glycoprotein (Omgp), chondroitin
sulphate proteoglycan (CSPG), ephrins and semaphorins (184–191). After binding to their cognate
receptors, these inhibitory molecules converge on the Rho/ROCK
pathway to change actin dynamics and initiate growth cone collapse
(184,186,187,192). The Rho/ROCK pathway has been
mainly associated with inhibitory signaling for neurite elongation
(193), and inactivation of
Rho/ROCK pathway can promote the regeneration (193). As discussed above, ONH expresses
RhoA, ROCK1 and ROCK2 (74), and
their presence in the optic nerve suggests a potential role for the
Rho/ROCK pathway in neurite outgrowth and axon regeneration through
actin cytoskeletal reorganization (147). Inhibition of Rho and ROCK has
been shown to increase RGC axon regeneration (193,194). It has been shown that intraocular
delivery of C3-exoenzyme, an inactivator of Rho GTPase, can promote
the regeneration of RGC axons in the optic nerve after a microcrush
lesion (193,195). On the other hand, ROCK inhibitors
have also been shown to increase regeneration in different optic
nerve lesion models (182,195–198).
In terms of their intensity, the effect of Y-39983 in promotion of
neurite outgrowth was stronger than that of Y-27632, fasudil and
dimethylfasudil (194,196). In addition to a ROCK inhibitor
used alone, the treatment with ROCK inhibitor (Y-27632) in
combination with CNTF and/or raised cAMP levels has additive
effects, and promotes robust RGCs axon regeneration (182,197). We recently found that RhoA/ROCK
signaling pathway was involved in the erythropoietin (EPO) effect
to promote RGC axon regeneration after optic nerve crush, and EPO
and Y-27632 had additive effects in promoting RGC axon regeneration
(199).
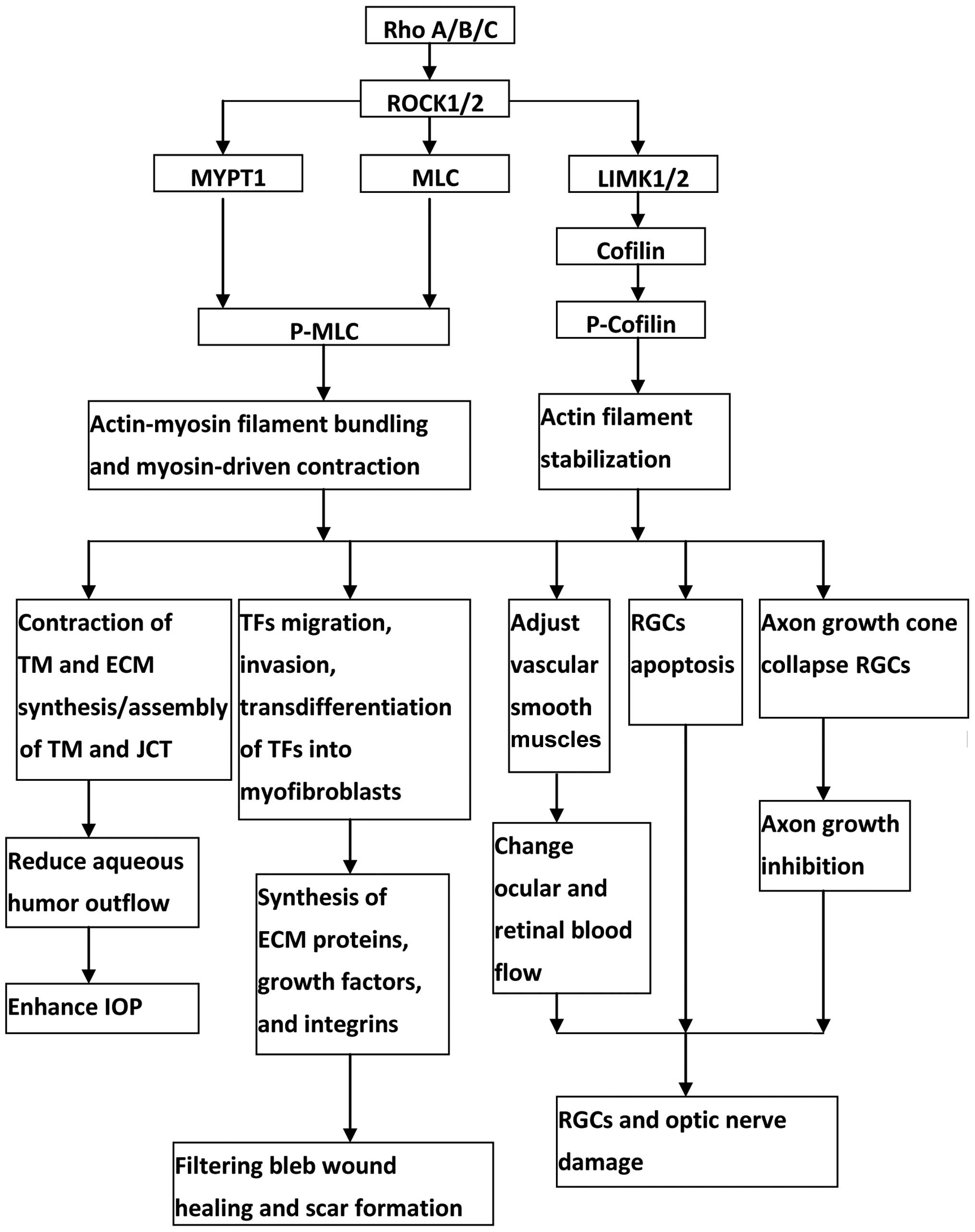
Rho/ROCK pathway has important roles for modulating
the cytoskeletal integrity of cells, the synthesis of ECM
components in the outflow tissue, and the permeability of the SC
endothelial cells. The activation of Rho/ROCK pathway in the
outflow tissue results in reduction of aqueous humor outflow, and
thereby increase IOP, whereas the inhibition of Rho/ROCK pathway in
the outflow tissue results in increase of aqueous humor outflow,
and thereby decrease IOP. ROCK inhibitors also serve as a potent
anti-scarring agent via inhibition of transdifferentiation of TFs
into myofibroblasts. Furthermore, RhoA/ROCK pathway is involved in
optic nerve neuroprotection. Inactivation of Rho/ROCK signaling
could increase ocular blood flow, improve RGCs survival and promote
RGCs axon regeneration. Considering the IOP modulation, potent bleb
anti-scarring effect and neuroprotective properties of ROCK
inhibitors (Fig. 1), Rho/ROCK
pathway is an attractive target for anti-glaucoma therapy, and it
may be used for human therapy in the near future.
This study was funded by the National
Natural Science Foundation of China (nos. 81070728, 81371014 and
81000373), Shanghai leading Academic Discipline Project (no.
S30205) and Shanghai ‘Science and Technology Innovation Action
Plan’ Basic Research Key Project (nos. 11JC1407700 and
11JC1407701).
|
1.
|
Weinreb RN and Khaw PT: Primary open-angle
glaucoma. Lancet. 363:1711–1720. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Quigley HA: Glaucoma. Lancet.
377:1367–1377. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Sommer A: Intraocular pressure and
glaucoma. Am J Ophthalmol. 107:186–188. 1989. View Article : Google Scholar
|
|
4.
|
Tielsch JM, Sommer A, Katz J, Royall RM,
Quigley HA and Javitt J: Racial variations in the prevalence of
primary open-angle glaucoma. The Baltimore Eye Survey. JAMA.
266:369–374. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
5.
|
Klein BE, Klein R, Sponsel WE, Franke T,
Cantor LB, Martone J and Menage MJ: Prevalence of glaucoma. The
Beaver Dam Eye Study. Ophthalmology. 99:1499–1504. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Mitchell P, Smith W, Attebo K and Healey
PR: Prevalence of open-angle glaucoma in Australia. The Blue
Mountains Eye Study. Ophthalmology. 103:1661–1669. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Collaborative Normal-Tension Glaucoma
Study Group: Comparison of glaucomatous progression between
untreated patients with normal-tension glaucoma and patients with
therapeutically reduced intraocular pressures. Am J Ophthalmol.
126:487–497. 1998. View Article : Google Scholar
|
|
8.
|
Collaborative Normal-Tension Glaucoma
Study Group: The effectiveness of intraocular pressure reduction in
the treatment of normal-tension glaucoma. Am J Ophthalmol.
126:498–505. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
The AGIS Investigators: The advanced
glaucoma intervention study (AGIS): 7. The relationship between
control of intraocular pressure and visual field deterioration. Am
J Ophthalmol. 130:429–440. 2000. View Article : Google Scholar
|
|
10.
|
Lichter PR, Musch DC, Gillespie BW, Guire
KE, Janz NK, Wren PA and Mills RP: Interim clinical outcomes in the
Collaborative Initial Glaucoma Treatment Study comparing initial
treatment randomized to medications or surgery. Ophthalmology.
108:1943–1953. 2001. View Article : Google Scholar
|
|
11.
|
Gordon MO, Beiser JA, Brandt JD, Heuer DK,
Higginbotham EJ, Johnson CA, Keltner JL, Miller JP, Parrish RK II,
Wilson MR and Kass MA: The Ocular Hypertension Treatment Study:
baseline factors that predict the onset of primary open-angle
glaucoma. Arch Ophthalmol. 120:714–720. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12.
|
Kass MA, Heuer DK, Higginbotham EJ,
Johnson CA, Keltner JL, Miller JP, Parrish RK 2nd, Wilson MR and
Gordon MO: The Ocular Hypertension Treatment Study: a randomized
trial determines that topical ocular hypotensive medication delays
or prevents the onset of primary open-angle glaucoma. Arch
Ophthalmol. 120:701–713. 2002. View Article : Google Scholar
|
|
13.
|
Leske MC, Heijl A, Hussein M, Bengtsson B,
Hyman L and Komaroff E: Factors for glaucoma progression and the
effect of treatment: the early manifest glaucoma trial. Arch
Ophthalmol. 121:48–56. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Hernandez MR: The optic nerve head in
glaucoma: role of astrocytes in tissue remodeling. Prog Retin Eye
Res. 19:297–321. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Burgoyne CF: A biomechanical paradigm for
axonal insult within the optic nerve head in aging and glaucoma.
Exp Eye Res. 93:120–132. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Lutjen-Drecoll E: Functional morphology of
the trabecular meshwork in primate eyes. Prog Retin Eye Res.
18:91–119. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tan JC, Peters DM and Kaufman PL: Recent
developments in understanding the pathophysiology of elevated
intraocular pressure. Curr Opin Ophthalmol. 17:168–174.
2006.PubMed/NCBI
|
|
18.
|
Gabelt BT and Kaufman PL: Changes in
aqueous humor dynamics with age and glaucoma. Prog Retin Eye Res.
24:612–637. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Grant WM: Experimental aqueous perfusion
in enucleated human eyes. Arch Ophthalmol. 69:783–801. 1963.
View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Johnson M: What controls aqueous humour
outflow resistance? Exp Eye Res. 82:545–557. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Tamm ER: The trabecular meshwork outflow
pathways: Structural and functional aspects. Exp Eye Res.
88:648–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Kumar J and Epstein DL: Rho
GTPase-mediated cytoskeletal organization in Schlemm’s canal cells
play a critical role in the regulation of aqueous humor outflow
facility. J Cell Biochem. 112:600–606. 2011.PubMed/NCBI
|
|
23.
|
Ethier CR: The inner wall of Schlemm’s
canal. Exp Eye Res. 74:161–172. 2002.
|
|
24.
|
Wiederholt M, Thieme H and Stumpff F: The
regulation of trabecular meshwork and ciliary muscle contractility.
Prog Retin Eye Res. 19:271–295. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Lutjen-Drecoll E: Morphological changes in
glaucomatous eyes and the role of TGF β2 for the pathogenesis of
the disease. Exp Eye Res. 81:1–4. 2005.
|
|
26.
|
Fuchshofer R and Tamm ER: The role of
TGF-β in the pathogenesis of primary open-angle glaucoma. Cell
Tissue Res. 347:279–290. 2012.
|
|
27.
|
Bradley JM, Vranka J, Colvis CM, Conger
DM, Alexander JP, Fisk AS, Samples JR and Acott TS: Effect of
matrix metalloproteinases activity on outflow in perfused human
organ culture. Invest Ophthalmol Vis Sci. 39:2649–2658.
1998.PubMed/NCBI
|
|
28.
|
Tian B, Geiger B, Epstein DL and Kaufman
PL: Cytoskeletal involvement in the regulation of aqueous humor
outflow. Invest Ophthalmol Vis Sci. 41:619–623. 2000.PubMed/NCBI
|
|
29.
|
Tian B, Gabelt BT, Geiger B and Kaufman
PL: The role of the actomyosin system in regulating trabecular
fluid outflow. Exp Eye Res. 88:713–717. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Last JA, Pan T, Ding Y, Reilly CM, Keller
K, Acott TS, Fautsch MP, Murphy CJ and Russell P: Elastic modulus
determination of normal and glaucomatous human trabecular meshwork.
Invest Ophthalmol Vis Sci. 52:2147–2152. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Erschbamer MK, Hofstetter CP and Olson L:
RhoA, RhoB, RhoC, Rac1, Cdc42, and Tc10 mRNA levels in spinal cord,
sensory ganglia, and corticospinal tract neurons and long-lasting
specific changes following spinal cord injury. J Comp Neurol.
484:224–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Etienne-Manneville S and Hall A: Rho
GTPases in cell biology. Nature. 420:629–635. 2002. View Article : Google Scholar
|
|
33.
|
Nakagawa O, Fujisawa K, Ishizaki T, Saito
Y, Nakao K and Narumiya S: ROCK-I and ROCK-II, two isoforms of
Rho-associated coiled-coil forming protein serine/threonine kinase
in mice. FEBS Lett. 392:189–193. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Riento K and Ridley AJ: Rocks:
multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol.
4:446–456. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Leung T, Chen XQ, Manser E and Lim L: The
p160 RhoA-binding kinase ROK alpha is a member of a kinase family
and is involved in the reorganization of the cytoskeleton. Mol Cell
Biol. 16:5313–5327. 1996.PubMed/NCBI
|
|
36.
|
Coleman ML, Sahai EA, Yeo M, Bosch M,
Dewar A and Olson MF: Membrane blebbing during apoptosis results
from caspase-mediated activation of ROCK I. Nat Cell Biol.
3:339–345. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Sebbagh M, Renvoizé C, Hamelin J, Riché N,
Bertoglio J and Bréard J: Caspase-3-mediated cleavage of ROCK I
induces MLC phosphorylation and apoptotic membrane blebbing. Nat
Cell Biol. 3:346–352. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Sebbagh M, Hamelin J, Bertoglio J, Solary
E and Bréard J: Direct cleavage of ROCK II by granzyme B induces
target cell membrane blebbing in a caspase-independent manner. J
Exp Med. 201:465–471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Van Aelst L and D’Souza-Schorey C: Rho
GTPases and signaling networks. Genes Dev. 11:2295–2322.
1997.PubMed/NCBI
|
|
40.
|
Ridley AJ, Paterson HF, Johnston CL,
Diekmann D and Hall A: The small GTP-binding protein rho regulates
growth factor-induced membrane ruffling. Cell. 70:401–410. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Ridley AJ and Hall A: Signal transduction
pathways regulating Rho-mediated stress fibre formation:
requirement for a tyrosine kinase. EMBO J. 13:2600–2610.
1994.PubMed/NCBI
|
|
42.
|
Nobes C and Hall A: Regulation and
function of the Rho subfamily of small GTPases. Curr Opin Genet
Dev. 4:77–81. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Takai Y, Sasaki T, Tanaka K and Nakanishi
H: Rho as a regulator of the cytoskeleton. Trends Biochem Sci.
20:227–231. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Paterson HF, Self AJ, Garrett MD, Just I,
Aktories K and Hall A: Microinjection of recombinant p21rho induces
rapid changes in cell morphology. J Cell Biol. 111:1001–1007. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
45.
|
Hirata K, Kikuchi A, Sasaki T, Kuroda S,
Kaibuchi K, Matsuura Y, Seki H, Saida K and Takai Y: Involvement of
rho p21 in the GTP-enhanced calcium ion sensitivity of smooth
muscle contraction. J Biol Chem. 267:8719–8722. 1992.PubMed/NCBI
|
|
46.
|
Narumiya S: The small GTPase Rho: cellular
functions and signal transduction. J Biochem (Tokyo). 120:215–228.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Gong MC, Iizuka K, Nixon G, Browne JP,
Hall A, Eccleston JF, Sugai M, Kobayashi S, Somlyo AV and Somlyo
AP: Role of guanine nucleotide-binding proteins - ras-family or
trimeric proteins or both - in Ca2+ sensitization of
smooth muscle. Proc Natl Acad Sci USA. 93:1340–1345. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
48.
|
Narumiya S, Ishizaki T and Watanabe N: Rho
effectors and reorganization of actin cytoskeleton. FEBS Lett.
410:68–72. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
49.
|
Kaibuchi K, Kuroda S and Amano M:
Regulation of the cytoskeleton and cell adhesion by the Rho family
GTPases in mammalian cells. Annu Rev Biochem. 68:459–486. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
50.
|
Somlyo AP and Somlyo AV: Signal
transduction by G-proteins, rho-kinase and protein phosphatase to
smooth muscle and non-muscle myosin II. J Physiol. 522:177–185.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
51.
|
Fukata Y, Amano M and Kaibuchi K:
Rho-Rho-kinase pathway in smooth muscle contraction and
cytoskeletal reorganization of non-muscle cells. Trends Pharmacol
Sci. 22:32–39. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
52.
|
Nakayama M, Amano M, Katsumi A, Kaneko T,
Kawabata S, Takefuji M and Kaibuchi K: Rho-kinase and myosin II
activities are required for cell type and environment specific
migration. Genes Cells. 10:107–117. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53.
|
Nakajima E, Nakajima T, Minagawa Y,
Shearer TR and Azuma M: Contribution of ROCK in contraction of
trabecular meshwork: proposed mechanism for regulating aqueous
outflow in monkey and human eyes. J Pharm Sci. 94:701–708. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
54.
|
Amano M, Nakayama M and Kaibuchi K:
Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell
polarity. Cytoskeleton (Hoboken). 67:545–554. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55.
|
Tan HB, Zhong YS, Cheng Y and Shen X:
Rho/ROCK pathway and neural regeneration: a potential therapeutic
target for central nervous system and optic nerve damage. Int J
Ophthalmol. 4:652–657. 2011.PubMed/NCBI
|
|
56.
|
Asano T, Ikegaki I, Satoh S, Suzuki Y,
Shibuya M, Takayasu M and Hidaka H: Mechanism of action of a novel
antivasospasm drug, HA1077. J Pharmacol Exp Ther. 241:1033–1040.
1987.PubMed/NCBI
|
|
57.
|
Asano T, Suzuki T, Tsuchiya M, Satoh S,
Ikegaki I, Shibuya M, Suzuki Y and Hidaka H: Vasodilator actions of
HA1077 in vitro and in vivo putatively mediated by the inhibition
of protein kinase. Br J Pharmacol. 98:1091–1100. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
58.
|
Honjo M, Inatani M, Kido N, Sawamura T,
Yue BY, Honda Y and Tanihara H: A myosin light chain kinase
inhibitor, ML-9, lowers the intraocular pressure in rabbit eyes.
Exp Eye Res. 75:135–142. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59.
|
Yoneda A, Multhaupt HA and Couchman JR:
The Rho kinases I and II regulate different aspects of myosin II
activity. J Cell Biol. 170:443–453. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
60.
|
Wang Y, Zheng XR, Riddick N, Bryden M,
Baur W, Zhang X and Surks HK: ROCK isoform regulation of myosin
phosphatase and contractility in vascular smooth muscle cells. Circ
Res. 104:531–540. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
61.
|
Hartshorne DJ: Myosin phosphatase:
subunits and interactions. Acta Physiol Scand. 164:483–493. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
62.
|
Pfitzer G: Invited review: regulation of
myosin phosphorylation in smooth muscle. J Appl Physiol.
91:497–503. 2001.PubMed/NCBI
|
|
63.
|
Harnett KM and Biancani P:
Calcium-dependent and calcium-independent contractions in smooth
muscles. Am J Med. 115(Suppl 3A): 24S–30S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64.
|
Kimura K, Ito M, Amano M, Chihara K,
Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K,
Iwamatsu A and Kaibuchi K: Regulation of myosin phosphatase by rho
and rho-associated kinase (rho-kinase). Science. 273:245–248. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
65.
|
Wettschureck N and Offermanns S:
Rho/Rho-kinase mediated signaling in physiology and
pathophysiology. J Mol Med. 80:629–638. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
66.
|
Somlyo AP and Somlyo AV: Ca2+
sensitivity of smooth muscle and nonmuscle myosin II: modulated by
G proteins, kinases, and myosin phosphatase. Physiol Rev.
83:1325–1358. 2003.
|
|
67.
|
Hall A: Rho GTPases and the actin
cytoskeleton. Science. 279:509–514. 1998. View Article : Google Scholar
|
|
68.
|
Ridley AJ: Rho GTPases and cell migration.
J Cell Sci. 114:2713–2722. 2001.PubMed/NCBI
|
|
69.
|
Jaffe AB and Hall A: Rho GTPases:
biochemistry and biology. Annu Rev Cell Dev Biol. 21:247–269. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
70.
|
Spindler V, Schlegel N and Waschke J: Role
of GTPases in control of microvascular permeability. Cardiovasc
Res. 87:243–253. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
71.
|
Thieme H, Nuskovski M, Nass JU, Pleyer U,
Strauss O and Wiederholt M: Mediation of calcium-independent
contraction in trabecular meshwork through protein kinase c and
rho-A. Invest Ophthalmol Vis Sci. 41:4240–4246. 2000.PubMed/NCBI
|
|
72.
|
Honjo M, Tanihara H, Inatani M, Kido N,
Sawamura T, Yue BY, Narumiya S and Honda Y: Effects of
rho-associated protein kinase inhibitor Y-27632 on intraocular
pressure and outflow facility. Invest Ophthalmol Vis Sci.
42:137–144. 2001.PubMed/NCBI
|
|
73.
|
Rao PV, Deng PF, Kumar J and Epstein DL:
Modulation of aqueous humor outflow facility by the Rho
kinase-specific inhibitor Y-27632. Invest Ophthalmol Vis Sci.
42:1029–1037. 2001.PubMed/NCBI
|
|
74.
|
Goldhagen B, Proia AD, Epstein DL and Rao
PV: Elevated levels of RhoA in the optic nerve head of human eyes
with glaucoma. J Glaucoma. 21:530–538. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
75.
|
Epstein DL, Rowlette LL and Roberts BC:
Acto-myosin drug effects and aqueous outflow function. Invest
Ophthalmol Vis Sci. 40:74–81. 1999.PubMed/NCBI
|
|
76.
|
Rao PV, Deng P, Sasaki Y and Epstein DL:
Regulation of myosin light chain phosphorylation in the trabecular
meshwork: role in aqueous humour outflow facility. Exp Eye Res.
80:197–206. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
77.
|
Geiger B, Yehuda-Levenberg S and
Bershadsky AD: Molecular interactions in the submembrane plaque of
cell-cell and cell-matrix adhesions. Acta Anat (Basel). 154:46–62.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
78.
|
Rosenthal R, Choritz L, Schlott S,
Bechrakis NE, Jaroszewski J, Wiederholt M and Thieme H: Effects of
ML-7 and Y-27632 on carbachol- and endothelin-1-induced contraction
of bovine trabecular meshwork. Exp Eye Res. 80:837–845. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
79.
|
Renieri G, Choritz L, Rosenthal R,
Meissner S, Pfeiffer N and Thieme H: Effects of endothelin-1 on
calcium-independent contraction of bovine trabecular meshwork.
Graefes Arch Clin Exp Ophthalmol. 246:1107–1115. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80.
|
Lu Z, Overby DR, Scott PA, Freddo TF and
Gong H: The mechanism of increasing outflow facility by rho-kinase
inhibition with Y-27632 in bovine eyes. Exp Eye Res. 86:271–281.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
81.
|
Johnstone MA: The aqueous outflow system
as a mechanical pump: evidence from examination of tissue and
aqueous movement in human and non-human primates. J Glaucoma.
13:421–438. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
82.
|
Rao VP and Epstein DL: Rho GTPase/Rho
kinase inhibition as a novel target for the treatment of glaucoma.
BioDrugs. 21:167–177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
83.
|
WuDunn D: Mechanobiology of trabecular
meshwork cells. Exp Eye Res. 88:718–723. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
84.
|
Wiederholt M, Bielka S, Schweig F,
Lutjen-Drecoll E and Lepple-Wienhues A: Regulation of outflow rate
and resistance in the perfused anterior segment of the bovine eye.
Exp Eye Res. 61:223–234. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
85.
|
Honjo M, Inatani M, Kido N, Sawamura T,
Yue BY, Honda Y and Tanihara H: Effects of protein kinase
inhibitor, HA1077, on intraocular pressure and outflow facility in
rabbit eyes. Arch Ophthalmol. 119:1171–1178. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
86.
|
Tokushige H, Inatani M, Nemoto S, Sakaki
H, Katayama K, Uehata M and Tanihara H: Effects of topical
administration of Y-39983, a selective rho-associated protein
kinase inhibitor, on ocular tissues in rabbits and monkeys. Invest
Ophthalmol Vis Sci. 48:3216–3222. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
87.
|
Tanihara H, Inatani M, Honjo M, Tokushige
H, Azuma J and Araie M: Intraocular pressure-lowering effects and
safety of topical administration of a selective ROCK inhibitor,
SNJ-1656, in healthy volunteers. Arch Ophthalmol. 126:309–315.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
88.
|
Fukunaga T, Ikesugi K, Nishio M, Sugimoto
M, Sasoh M, Hidaka H and Uji Y: The effect of the Rho-associated
protein kinase inhibitor, HA-1077, in the rabbit ocular
hypertension model induced by water loading. Curr Eye Res.
34:42–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
89.
|
Gottanka J, Chan D, Eichhorn M,
Lutjen-Drecoll E and Ethier CR: Effects of TGF-β2 in perfused human
eyes. Invest Ophthalmol Vis Sci. 45:153–158. 2004.
|
|
90.
|
Mettu PS, Deng PF, Misra UK, Gawdi G,
Epstein DL and Rao PV: Role of lysophospholipid growth factors in
the modulation of aqueous humor outflow facility. Invest Ophthalmol
Vis Sci. 45:2263–2271. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
91.
|
Zhang M, Maddala R and Rao PV: Novel
molecular insights into RhoA GTPase-induced resistance to aqueous
humor outflow through the trabecular meshwork. Am J Physiol Cell
Physiol. 295:C1057–C1070. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
92.
|
Waki M, Yoshida Y, Oka T and Azuma M:
Reduction of intraocular pressure by topical administration of an
inhibitor of the Rho-associated protein kinase. Curr Eye Res.
22:470–474. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
93.
|
Wiederholt M and Stumpff H: The trabecular
meshwork and aqueous humor reabsorption. Curr Top Membr.
45:163–202. 1998. View Article : Google Scholar
|
|
94.
|
Uehata M, Ishizaki T, Satoh H, Ono T,
Kawahara T, Morishita T, Tamakawa H, Yamagami K, Inui J, Maekawa M
and Narumiya S: Calcium sensitization of smooth muscle mediated by
a Rho-associated protein kinase in hypertension. Nature.
389:990–994. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
95.
|
Iizuka K, Yoshii A, Samizo K, Tsukagoshi
H, Ishizuka T, Dobashi K, Nakazawa T and Mori M: A major role for
the rho-associated coiled coil forming protein kinase in
G-protein-mediated Ca2+ sensitization through inhibition
of myosin phosphatase in rabbit trachea. Br J Pharmacol.
128:925–933. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
96.
|
Lütjen-Drecoll E, Futa R and Rohen JW:
Ultrahistochemical studies on tangential sections of the trabecular
meshwork in normal and glaucomatous eyes. Invest Ophthalmol Vis
Sci. 21:563–573. 1981.PubMed/NCBI
|
|
97.
|
Yue BY: The extracellular matrix and its
modulation in the trabecular meshwork. Surv Ophthalmol. 40:379–390.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
98.
|
Tamm ER and Fuchshofer R: What increases
outflow resistance in primary open-angle glaucoma? Surv Ophthalmol.
52(Suppl 2): S101–S104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
99.
|
Acott TS and Kelley MJ: Extracellular
matrix in the trabecular meshwork. Exp Eye Res. 86:543–561. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
100.
|
Keller KE, Aga M, Bradley JM, Kelley MJ
and Acott TS: Extracellular matrix turnover and outflow resistance.
Exp Eye Res. 88:676–682. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101.
|
Pattabiraman PP and Rao PV: Mechanistic
basis of Rho GTPase-induced extracellular matrix synthesis in
trabecular meshwork cells. Am J Physiol Cell Physiol. 298:C749–763.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
102.
|
Nakamura Y, Hirano S, Suzuki K, Seki K,
Sagara T and Nishida T: Signaling mechanism of TGF-β1-induced
collagen contraction mediated by bovine trabecular meshwork cells.
Invest Ophthalmol Vis Sci. 43:3465–3472. 2002.
|
|
103.
|
Fleenor DL, Shepard AR, Hellberg PE,
Jacobson N, Pang IH and Clark AF: TGFβ2-induced changes in human
trabecular meshwork: implications for intraocular pressure. Invest
Ophthalmol Vis Sci. 47:226–234. 2006.
|
|
104.
|
Iyer P, Maddala R, Pattabiraman PP and Rao
PV: Connective tissue growth factor-mediated upregulation of
neuromedin U expression in trabecular meshwork cells and its role
in homeostasis of aqueous humor outflow. Invest Ophthalmol Vis Sci.
53:4952–4962. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
105.
|
Bershadsky AD, Balaban NQ and Geiger B:
Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol.
19:677–695. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
106.
|
Kaunas R, Nguyen P, Usami S and Chien S:
Cooperative effects of Rho and mechanical stretch on stress fiber
organization. Proc Natl Acad Sci USA. 102:15895–15900. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
107.
|
Shikata Y, Rios A, Kawkitinarong K,
DePaola N, Garcia JG and Birukov KG: Differential effects of shear
stress and cyclic stretch on focal adhesion remodeling,
site-specific FAK phosphorylation, and small GTPases in human lung
endothelial cells. Exp Cell Res. 304:40–49. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
108.
|
Ingber DE: Cellular mechanotransduction:
putting all the pieces together again. FASEB J. 20:811–827. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
109.
|
Tzima E: Role of small GTPases in
endothelial cytoskeletal dynamics and the shear stress response.
Circ Res. 98:176–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
110.
|
Schlunck G, Han H, Wecker T, Kampik D,
Meyer-ter-Vehn T and Grehn F: Substrate rigidity modulates cell
matrix interactions and protein expression in human trabecular
meshwork cells. Invest Ophthalmol Vis Sci. 49:262–269. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
111.
|
Kameda T, Inoue T, Inatani M, Fujimoto T,
Honjo M, Kasaoka N, Inoue-Mochita M, Yoshimura N and Tanihara H:
The effect of Rho-associated protein kinase inhibitor on monkey
Schlemm’s canal endothelial cells. Invest Ophthalmol Vis Sci.
53:3092–3103. 2012.PubMed/NCBI
|
|
112.
|
Epstein DL, Freddo TF, Bassett-Chu S,
Chung M and Karageuzian L: Influence of ethacrynic acid on outflow
facility in the monkey and calf eye. Invest Ophthalmol Vis Sci.
28:2067–2075. 1987.PubMed/NCBI
|
|
113.
|
Ethier CR, Read AT and Chan DW: Effects of
latrunculin-B on outflow facility and trabecular meshwork structure
in human eyes. Invest Ophthalmol Vis Sci. 47:1991–1998. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
114.
|
Inoue T, Pattabiraman PP, Epstein DL and
Rao PV: Effects of chemical inhibition of N-WASP, a critical
regulator of actin polymerization on aqueous humor outflow through
the conventional pathway. Exp Eye Res. 90:360–367. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115.
|
Bill A and Svedbergh B: Scanning electron
microscopic studies of the trabecular meshwork and the canal of
Schlemm - an attempt to localize the main resistance to outflow of
aqueous humor in man. Acta Ophthalmol (Copenh). 50:295–320. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
116.
|
Liu Q, Wu K, Qiu X, Yang Y, Lin X and Yu
M: siRNA silencing of gene expression in trabecular meshwork: RhoA
siRNA reduces IOP in mice. Curr Mol Med. 12:1015–1027. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
117.
|
Tian B and Kaufman PL: Effects of the Rho
kinase inhibitor Y-27632 and the phosphatase inhibitor calyculinA
on outflow facility in monkeys. Exp Eye Res. 80:215–225. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
118.
|
Yu M, Chen X, Wang N, Cai S, Li N, Qiu J,
Brandt CR, Kaufman PL and Liu X: H-1152 effects on intraocular
pressure and trabecular meshwork morphology of rat eyes. J Ocul
Pharmacol Ther. 24:373–379. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
119.
|
Nishio M, Fukunaga T, Sugimoto M, Ikesugi
K, Sumi K, Hidaka H and Uji Y: The effect of the H-1152P, a potent
Rho-associated coiled coil-formed protein kinase inhibitor, in
rabbit normal and ocular hypertensive eyes. Curr Eye Res.
34:282–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
120.
|
Whitlock NA, Harrison B, Mixon T, Yu XQ,
Wilson A, Gerhardt B, Eberhart DE, Abuin A and Rice DS: Decreased
intraocular pressure in mice following either pharmacological or
genetic inhibition of ROCK. J Ocul Pharmacol Ther. 25:187–194.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
121.
|
Lama PJ and Fechtner RD: Antifibrotics and
wound healing in glaucoma surgery. Surv Ophthalmol. 48:314–346.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
122.
|
Yoon PS and Singh K: Update on
antifibrotic use in glaucoma surgery, including use in
trabeculectomy and glaucoma drainage implants and combined cataract
and glaucoma surgery. Curr Opin Ophthalmol. 15:141–146. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
123.
|
Migdal C, Gregory W and Hitchings R:
Long-term functional outcome after early surgery compared with
laser and medicine in open-angle glaucoma. Ophthalmology.
101:1651–1656. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
124.
|
Khaw PT, Occleston NL, Schultz G, Grierson
I, Sherwood MB and Larkin G: Activation and suppression of
fibroblast function. Eye. 8:188–195. 1994. View Article : Google Scholar
|
|
125.
|
Occleston NL, Daniels JT, Tarnuzzer RW,
Sethi KK, Alexander RA, Bhattacharya SS, Schultz GS and Khaw PT:
Single exposures to antiproliferatives: long-term effects on ocular
fibroblast wound-healing behavior. Invest Ophthalmol Vis Sci.
38:1998–2007. 1997.PubMed/NCBI
|
|
126.
|
Tomasek JJ, Gabbiani G, Hinz B, Chaponnier
C and Brown RA: Myofibroblasts and mechano-regulation of connective
tissue remodelling. Nat Rev Mol Cell Biol. 3:349–363. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
127.
|
Hinz B, Mastrangelo D, Iselin CE,
Chaponnier C and Gabbiani G: Mechanical tension controls
granulation tissue contractile activity and myofibroblast
differentiation. Am J Pathol. 159:1009–1020. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
128.
|
Desmouliere A, Chaponnier C and Gabbiani
G: Tissue repair, contraction, and the myofibroblast. Wound Repair
Regen. 13:7–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
129.
|
Meyer-Ter-Vehn T, Gebhardt S, Sebald W,
Buttmann M, Grehn F, Schlunck G and Knaus P: p38 inhibitors prevent
TGF-β-induced myofibroblast transdifferentiation in human tenon
fibroblasts. Invest Ophthalmol Vis Sci. 47:1500–1509. 2006.
|
|
130.
|
Ehrlich HP and Rajaratnam JB: Cell
locomotion forces versus cell contraction forces for collagen
lattice contraction: an in vitro model of wound contraction. Tissue
Cell. 22:407–417. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
131.
|
Desmouliere A, Redard M, Darby I and
Gabbiani G: Apoptosis mediates the decrease in cellularity during
the transition between granulation tissue and scar. Am J Pathol.
146:56–66. 1995.PubMed/NCBI
|
|
132.
|
Darby IA and Hewitson TD: Fibroblast
differentiation in wound healing and fibrosis. Int Rev Cytol.
257:143–179. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
133.
|
Meyer-ter-Vehn T, Sieprath S, Katzenberger
B, Gebhardt S, Grehn F and Schlunck G: Contractility as a
prerequisite for TGF-β-induced myofibroblast transdifferentiation
in human tenon fibroblasts. Invest Ophthalmol Vis Sci.
47:4895–4904. 2006.
|
|
134.
|
Miller MH, Grierson I, Unger WI and
Hitchings RA: Wound healing in an animal model of glaucoma
fistulizing surgery in the rabbit. Ophthalmic Surg. 20:350–357.
1989.PubMed/NCBI
|
|
135.
|
Desmouliere A, Geinoz A, Gabbiani F and
Gabbiani G: Transforming growth factor-beta 1 induces alpha-smooth
muscle actin expression in granulation tissue myofibroblasts and in
quiescent and growing cultured fibroblasts. J Cell Biol.
122:103–111. 1993. View Article : Google Scholar
|
|
136.
|
Watsky MA: Lysophosphatidic acid, serum,
and hyposmolarity activate Cl− currents in corneal keratocytes. Am
J Physiol. 269:C1385–C1393. 1995.PubMed/NCBI
|
|
137.
|
Cordeiro MF, Reichel MB, Gay JA,
D’Esposita F, Alexander RA and Khaw PT: Transforming growth
factor-beta1, -beta2, and -beta3 in vivo: effects on normal and
mitomycin C-modulated conjunctival scarring. Invest Ophthalmol Vis
Sci. 40:1975–1982. 1999.PubMed/NCBI
|
|
138.
|
Cordeiro MF, Chang L, Lim KS, Daniels JT,
Pleass RD, Siriwardena D and Khaw PT: Modulating conjunctival wound
healing. Eye. 14:536–547. 2000. View Article : Google Scholar
|
|
139.
|
Wong TT, Mead AL and Khaw PT: Matrix
metalloproteinase inhibition modulates postoperative scarring after
experimental glaucoma filtration surgery. Invest Ophthalmol Vis
Sci. 44:1097–1103. 2003. View Article : Google Scholar
|
|
140.
|
Itoh K, Yoshioka K, Akedo H, Uehata M,
Ishizaki T and Narumiya S: An essential part for Rho-associated
kinase in the transcellular invasion of tumor cells. Nat Med.
5:221–225. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
141.
|
Harvey SA, Anderson SC and SundarRaj N:
Downstream effects of ROCK signaling in cultured human corneal
stromal cells: microarray analysis of gene expression. Invest
Ophthalmol Vis Sci. 45:2168–2176. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
142.
|
Yoshizaki H, Ohba Y, Parrini MC,
Dulyaninova NG, Bresnick AR, Mochizuki N and Matsuda M: Cell
type-specific regulation of RhoA activity during cytokinesis. J
Biol Chem. 279:44756–44762. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
143.
|
Tomasek JJ, Vaughan MB, Kropp BP, Gabbiani
G, Martin MD, Haaksma CJ and Hinz B: Contraction of myofibroblasts
in granulation tissue is dependent on Rho/Rho kinase/myosin light
chain phosphatase activity. Wound Repair Regen. 14:313–320. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
144.
|
Parizi M, Howard EW and Tomasek JJ:
Regulation of LPA-promoted myofibroblast contraction: role of Rho,
myosin light chain kinase, and myosin light chain phosphatase. Exp
Cell Res. 254:210–220. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
145.
|
Tangkijvanich P, Melton AC, Santiskulvong
C and Yee HF Jr: Rho and p38 MAP kinase signaling pathways mediate
LPA-stimulated hepatic myofibroblast migration. J Biomed Sci.
10:352–358. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
146.
|
Honjo M, Tanihara H, Kameda T, Kawaji T,
Yoshimura N and Araie M: Potential role of Rho-associated protein
kinase inhibitor Y-27632 in glaucoma filtration surgery. Invest
Ophthalmol Vis Sci. 48:5549–5557. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
147.
|
Lukas TJ, Miao H, Chen L, Riordan SM, Li
W, Crabb AM, Wise A, Du P, Lin SM and Hernandez MR: Susceptibility
to glaucoma: differential comparison of the astrocyte transcriptome
from glaucomatous African American and Caucasian American donors.
Genome Biol. 9:R1112008. View Article : Google Scholar : PubMed/NCBI
|
|
148.
|
Hathaway DR, March KL, Lash JA, Adam LP
and Wilensky RL: Vascular smooth muscle. A review of the molecular
basis of contractility. Circulation. 83:382–390. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
149.
|
Savineau JP and Marthan R: Modulation of
the calcium sensitivity of the smooth muscle contractile apparatus:
molecular mechanisms, pharmacological and pathophysiological
implications. Fundam Clin Pharmacol. 11:289–299. 1997. View Article : Google Scholar
|
|
150.
|
Mita M, Yanagihara H, Hishinuma S, Saito M
and Walsh MP: Membrane depolarization-induced contraction of rat
caudal arterial smooth muscle involves Rho-associated kinase.
Biochem J. 364:431–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
151.
|
Sward K, Mita M, Wilson DP, Deng JT,
Susnjar M and Walsh MP: The role of RhoA and Rho-associated kinase
in vascular smooth muscle contraction. Curr Hypertens Rep. 5:66–72.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
152.
|
Kandabashi T, Shimokawa H, Miyata K,
Kunihiro I, Kawano Y, Fukata Y, Higo T, Egashira K, Takahashi S,
Kaibuchi K and Takeshita A: Inhibition of myosin phosphatase by
upregulated rho-kinase plays a key role for coronary artery spasm
in a porcine model with interleukin-1beta. Circulation.
101:1319–1323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
153.
|
Sato M, Tani E, Fujikawa H and Kaibuchi K:
Involvement of Rho-kinase-mediated phosphorylation of myosin light
chain in enhancement of cerebral vasospasm. Circ Res. 87:195–200.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
154.
|
Iizuka K, Shimizu Y, Tsukagoshi H, Yoshii
A, Harada T, Dobashi K, Murozono T, Nakazawa T and Mori M:
Evaluation of Y-27632, a rho-kinase inhibitor, as a bronchodilator
in guinea pigs. Eur J Pharmacol. 406:273–279. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
155.
|
Chitaley K, Wingard CJ, Clinton Webb R,
Branam H, Stopper VS, Lewis RW and Mills TM: Antagonism of
Rho-kinase stimulates rat penile erection via a nitric
oxide-independent pathway. Nat Med. 7:119–122. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
156.
|
Takahashi R, Nishimura J, Hirano K, Seki
N, Naito S and Kanaide H: Ca2+ sensitization in
contraction of human bladder smooth muscle. J Urol. 172:748–752.
2004.
|
|
157.
|
Schubert R, Kalentchuk VU and Krien U: Rho
kinase inhibition partly weakens myogenic reactivity in rat small
arteries by changing calcium sensitivity. Am J Physiol Heart Circ
Physiol. 283:H2288–H2295. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
158.
|
Cavarape A, Bauer J, Bartoli E, Endlich K
and Parekh N: Effects of angiotensin II, arginine vasopressin and
tromboxane A2 in renal vascular bed: role of rho-kinase. Nephrol
Dial Transplant. 18:1764–1769. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
159.
|
Cavarape A, Endlich N, Assaloni R, Bartoli
E, Steinhausen M, Parekh N and Endlich K: Rho-kinase inhibition
blunts renal vasoconstriction induced by distinct signaling
pathways in vivo. J Am Soc Nephrol. 14:37–45. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
160.
|
Randriamboavonjy V, Busse R and Fleming I:
20-HETE-induced contraction of small coronary arteries depends on
the activation of Rho-kinase. Hypertension. 41:801–806. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
161.
|
Watabe H, Abe S and Yoshitomi T: Effects
of Rho-associated protein kinase inhibitors Y-27632 and Y-39983 on
isolated rabbit ciliary arteries. Jpn J Ophthalmol. 55:411–417.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
162.
|
Tokushige H: ROCK inhibitor and glaucoma.
Bio Clinica. 17:1191–1194. 2002.
|
|
163.
|
Sugiyama T, Shibata M, Kajiura S, Okuno T,
Tonari M, Oku H and Ikeda T: Effects of fasudil, a Rho-associated
protein kinase inhibitor, on optic nerve head blood flow in
rabbits. Invest Ophthalmol Vis Sci. 52:64–69. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
164.
|
Okamura N, Saito M, Mori A, Sakamoto K,
Kametaka S, Nakahara T and Ishii K: Vasodilator effects of fasudil,
a Rho-kinase inhibitor, on retinal arterioles in stroke-prone
spontaneously hypertensive rats. J Ocul Pharmacol Ther. 23:207–212.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
165.
|
Wolfrum S, Dendorfer A, Rikitake Y,
Stalker TJ, Gong Y, Scalia R, Dominiak P and Liao JK: Inhibition of
Rho-kinase leads to rapid activation of phosphatidylinositol
3-kinase/protein kinase Akt and cardiovascular protection.
Arterioscler Thromb Vasc Biol. 24:1842–1847. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
166.
|
Anderson DR: Introductory comments on
blood flow auto-regulation in the optic nerve head and vascular
risk factors in glaucoma. Surv Ophthalmol. 43:S5–S9. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
167.
|
Chung HS, Harris A, Evans DW, Kagemann L,
Garzozi HJ and Martin B: Vascular aspects in the pathophysiology of
glaucomatous optic neuropathy. Surv Ophthalmol. 43:S43–S50. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
168.
|
Flammer J, Orgul S, Costa VP, Orzalesi N,
Krieglstein GK, Serra LM, Renard JP and Stefansson E: The impact of
ocular blood flow in glaucoma. Prog Retin Eye Res. 21:359–393.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
169.
|
Ben Simon GJ, Moroz I, Goldenfeld M and
Melamed S: Scanning laser Doppler flowmetry of nonperfused regions
of the optic nerve head in patients with glaucoma. Ophthalmic Surg
Lasers Imaging. 34:245–250. 2003.PubMed/NCBI
|
|
170.
|
Hu Y, Cui Q and Harvey AR: Interactive
effects of C3, cyclic AMP and ciliaryneurotrophic factor on adult
retinal ganglion cell survival and axonal regeneration. Mol Cell
Neurosci. 34:88–98. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
171.
|
Kitaoka Y, Kitaoka Y, Kumai T, Lam TT,
Kuribayashi K, Isenoumi K, Munemasa Y, Motoki M, Kobayashi S and
Ueno S: Involvement of RhoA and possible neuroprotective effect of
fasudil, a Rho kinase inhibitor, in NMDA-induced neurotoxicity in
the rat retina. Brain Res. 1018:111–118. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
172.
|
Bertrand J, Di Polo A and McKerracher L:
Enhanced survival and regeneration of axotomized retinal neurons by
repeated delivery of cell-permeable C3-like Rho antagonists.
Neurobiol Dis. 25:65–72. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
173.
|
Sheikh AM, Nagai A, Ryu JK, McLarnon JG,
Kim SU and Masuda J: Lysophosphatidylcholine induces glial cell
activation: role of rho kinase. Glia. 57:898–907. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
174.
|
Buchi ER: Cell death in the rat retina
after a pressure-induced ischaemia-reperfusion insult: an electron
microscopic study. I Ganglion cell layer and inner nuclear layer.
Exp Eye Res. 55:605–613. 1992. View Article : Google Scholar
|
|
175.
|
Rosenbaum DM, Rosenbaum PS, Gupta A,
Michaelson MD, Hall DH and Kessler JA: Retinal ischemia leads to
apoptosis which is ameliorated by aurintricarboxylic acid. Vision
Res. 37:3445–3451. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
176.
|
Hirooka K, Miyamoto O, Jinming P, Du Y,
Itano T, Baba T, Tokuda M and Shiraga F: Neuroprotective effects of
D-allose against retinal ischemia-reperfusion injury. Invest
Ophthalmol Vis Sci. 47:1653–1657. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
177.
|
Millán J and Ridley AJ: Free in PMC Rho
GTPases and leucocyte-induced endothelial remodelling. Biochem J.
385:329–337. 2005.PubMed/NCBI
|
|
178.
|
Wittchen ES, van Buul JD, Burridge K and
Worthylake RA: Trading spaces: Rap, Rac, and Rho as architects of
transendothelial migration. Curr Opin Hematol. 12:14–21. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
179.
|
Song H and Gao D: Fasudil, a
Rho-associated protein kinase inhibitor, attenuates retinal
ischemia and reperfusion injury in rats. Int J Mol Med. 28:193–198.
2011.PubMed/NCBI
|
|
180.
|
Tsujikawa A, Ogura Y, Hiroshiba N,
Miyamoto K, Kiryu J, Tojo SJ, Miyasaka M and Honda Y: Retinal
ischemia-reperfusion injury attenuated by blocking of adhesion
molecules of vascular endothelium. Invest Ophthalmol Vis Sci.
40:1183–1190. 1999.PubMed/NCBI
|
|
181.
|
Hirata A, Inatani M, Inomata Y, Yonemura
N, Kawaji T, Honjo M and Tanihara H: Y-27632, a Rho-associated
protein kinase inhibitor, attenuates neuronal cell death after
transient retinal ischemia. Graefes Arch Clin Exp Ophthalmol.
246:51–59. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
182.
|
Lingor P, Tönges L, Pieper N, Bermel C,
Barski E, Planchamp V and Bähr M: ROCK inhibition and CNTF interact
on intrinsic signalling pathways and differentially regulate
survival and regeneration in retinal ganglion cells. Brain.
131:250–263. 2008.PubMed/NCBI
|
|
183.
|
Tura A, Schuettauf F, Monnier PP,
Bartz-Schmidt KU and Henke-Fahle S: Efficacy of Rho-kinase
inhibition in promoting cell survival and reducing reactive gliosis
in the rodent retina. Invest Ophthalmol Vis Sci. 50:452–461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
184.
|
Fournier AE, GrandPre T, Gould G, Wang X
and Strittmatter SM: Nogo and the Nogo-66 receptor. Prog Brain Res.
137:361–369. 2002. View Article : Google Scholar
|
|
185.
|
Hunt D, Coffin RS and Anderson PN: The
Nogo receptor, its ligands and axonal regeneration in the spinal
cord; a review. J Neurocytol. 31:93–120. 2002. View Article : Google Scholar
|
|
186.
|
McKerracher L and Winton MJ: Nogo on the
go. Neuron. 36:345–348. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
187.
|
Sandvig A, Berry M, Barrett LB, Butt A and
Logan A: Myelin-, reactive glia-, and scar-derived CNS axon growth
inhibitors: expression, receptor signaling, and correlation with
axon regeneration. Glia. 46:225–251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
188.
|
Fawcett JW: Overcoming inhibition in the
damaged spinal cord. J Neurotrauma. 23:371–383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
189.
|
Du J, Fu C and Sretavan DW: Eph/ephrin
signaling as a potential therapeutic target after central nervous
system injury. Curr Pharm Des. 13:2507–2518. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
190.
|
Fabes J, Anderson P, Brennan C and Bolsove
S: Regeneration-enhancing effects of EphA4 blocking peptide
following corticospinal tract injury in adult rat spinal cord. Eur
J Neurosci. 26:2496–2505. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
191.
|
Hou ST, Jiang SX and Smith RA: Permissive
and repulsive cues and signalling pathways of axonal outgrowth and
regeneration. Int Rev Cell Molec Biol. 267:125–181. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
192.
|
Berry M, Ahmed Z, Lorber B, Douglas M and
Logan A: Regeneration of axons in the visual system. Restor Neurol
Neurosci. 26:147–174. 2008.PubMed/NCBI
|
|
193.
|
Bertrand J, Winton MJ, Rodriguez-Hernandez
N, Campenot RB and McKerracher L: Application of Rho antagonist to
neuronal cell bodies promotes neurite growth in compartmented
cultures and regeneration of retinal ganglion cell axons in the
optic nerve of adult rats. J Neurosci. 25:1113–1121. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
194.
|
Sagawa H, Terasaki H, Nakamura M, Ichikawa
M, Yata T, Tokita Y and Watanabe M: A novel ROCK inhibitor,
Y-39983, promotes regeneration of crushed axons of retinal ganglion
cells into the optic nerve of adult cats. Exp Neurol. 205:230–240.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
195.
|
Monnier PP, Sierra A, Schwab JM,
Henke-Fahle S and Mueller BK: The Rho/ROCK pathway mediates neurite
growth-inhibitory activity associated with the chondroitin sulfate
proteoglycans of the CNS glial scar. Mol Cell Neurosci. 22:319–330.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
196.
|
Lingor P, Teusch N, Schwarz K, Mueller R,
Mack H, Bahr M and Mueller BK: Inhibition of Rho kinase (ROCK)
increases neurite outgrowth on chondroitin sulphate proteoglycan in
vitro and axonal regeneration in the adult optic nerve in vivo. J
Neurochem. 103:181–189. 2007.PubMed/NCBI
|
|
197.
|
Ahmed Z, Berry M and Logan A: ROCK
inhibition promotes adult retinal ganglion cell neurite outgrowth
only in the presence of growth promoting factors. Mol Cell
Neurosci. 42:128–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
198.
|
Watanabe M: Regeneration of optic nerve
fibers of adult mammals. Dev Growth Differ. 52:567–576. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
199.
|
Tan H, Zhong Y, Shen X, Cheng Y, Jiao Q
and Deng L: Erythropoietin promotes axonal regeneration after optic
nerve crush in vivo by inhibition of RhoA/ROCK signaling pathway.
Neuropharmacology. 63:1182–1190. 2012. View Article : Google Scholar : PubMed/NCBI
|