Introduction
Multiple myeloma (MM) is characterized by clonal
expansion of malignant plasma cells in the bone marrow. Although
novel therapeutic agents and stem cell transplantation have
improved the survival of MM patients (1), MM remains an incurable disease.
Cancer stem cells are often considered to contribute
to relapse and drug resistance in various cancers (2). Matsui et al (3) reported that myeloma stem cells are
enriched in the CD138-negative population. During normal B-cell
development, abundant CD138 (also known as syndecan-1: SDC1)
expression is highly specific for terminally differentiated plasma
cells in the bone marrow (4).
Since CD138 expression is also a hallmark of malignant plasma cells
(myeloma cells), it has been used for myeloma cell purification
(5) and is considered to be a
target for treatment (6). While
the majority of myeloma cells express CD138, decreased expression
of CD138 is occasionally found in clinical practice (7–9).
Although the association between CD138 expression and myeloma stem
cells remains a matter of debate (10), several reports have shown that
CD138-low or -negative myeloma cells may contribute to drug
resistance or relapse of the disease (9,11,12).
Therefore, analysis of CD138 downregulation in myeloma cells is
required for a better understanding of myeloma biology.
Previous reports have indicated that the bone marrow
microenvironment may contribute to CD138 downregulation (13–16).
Among various factors in the tumor microenvironment, hypoxia is one
of the important factors associated with tumor progression, poor
clinical outcomes, dedifferentiation, and formation of cancer stem
cell niches in solid tumors (17).
Based on recent findings showing a correlation of MM at the
advanced stage with hypoxic conditions in the microenvironment
within the bone marrow (18), we
hypothesized that CD138 expression may be influenced by
hypoxia.
In the present study, we compared the changes in
CD138 and various transcription factor expressions in myeloma cells
under hypoxic or normoxic conditions. We also attempted to revert
CD138 expression in cells under hypoxia by treatment with all-trans
retinoic acid (ATRA). The influence of ATRA on the sensitivity to
bortezomib under hypoxic conditions was also examined.
Materials and methods
Cell culture
Human myeloma cell lines, KMS-12BM (19) and RPMI 8226 (20), were obtained from the Health
Science Research Resources Bank (Osaka, Japan) and maintained in
RPMI-1640 medium supplemented with 10% heat-inactivated fetal
bovine serum at 37°C under 5% CO2. The two myeloma cell
lines were cultured under normoxic (21% O2) and hypoxic
(1% O2) conditions for up to 30 days, with fresh medium
provided every 3 days. Experiments under hypoxic conditions were
performed in a Personal CO2 Multigas Incubator (ASTEC,
Fukuoka, Japan).
Flow cytometric analysis of surface
antigens
MM cell lines cultured under normoxic and hypoxic
conditions were stained with the following fluorescently-labeled
antibodies: FITCCD138 (clone MI15), FITC-CD38 (clone HIT2), PE-CD44
(clone 515), PE-CD45 (clone HI30), FITC-CD49d (clone gf10) (BD
Biosciences, Franklin Lakes, NJ, USA); PE-CD54 (clone HCD54),
PE-CXCR4 (clone 12G5), PE-MDR-1 (clone UIC2), APC-ABCG2 (clone 5D3)
(Biolegend, San Diego, CA, USA); FITC-CD19 (clone HD37), FITC-CD20
(clone B-Ly1) (Dako, Glostrup, Denmark); and Alexa 647-CS1 (clone
162) (AbD Serotec, Oxford, UK). Density gradient centrifugation
using Ficoll-Paque Plus (GE Healthcare, Uppsala, Sweden), the
forward/side scatter profile and 7-amino-actinomycin D (7-AAD) (BD
Biosciences) labeling were used for exclusion of non-viable cells.
Flow cytometric anal ysis was performed using a FACSCalibur or
FACSVerse flow cytometer (Becton-Dickinson, San Jose, CA, USA).
Adhesion to type-1 collagen
MM cells were plated in quadruplicate at a
concentration of 5×105 cells/ml on type-1
collagen-coated 96-well plates (Becton-Dickinson) and incubated for
1 h at 37°C. After the incubation, the cells were washed twice with
PBS and incubated with the WST-8 reagent (Dojindo, Kumamoto,
Japan). The ratios of adherent cells to total applied cells were
quantified by the light absorbance of each well at 450 nm using a
VMax absorbance microplate reader (Molecular Devices, Sunnyvale,
CA, USA).
cDNA synthesis and reverse
transcription-polymerase chain reaction (RT-PCR)
RNA was extracted from the MM cell lines using
TRIzol reagent (Invitrogen, Carlsbad, CA, USA). cDNA synthesis was
performed using a SuperScript III First-Strand Synthesis System for
RT-PCR (Invitrogen) according to the manufacturer’s protocol.
The expression levels of BCL6, PAX5, Oct-4,
NANOG and SOX2 were determined by RT-PCR. β-actin
(ACTB) was used as a normalization control. The primers for
BCL6 and PAX5 were described previously (9). The primers for Oct-4, NANOG,
SOX2 and ACTB were as follows: Oct-4 (forward,
5′-AGCC CTCATTTCACCAGGCC-3′; reverse, 5′-TGGGACTCCTCCG GGTTTTG-3′);
NANOG (forward, 5′-ACTGTCTCTCCTCT TCCTTC-3′; reverse,
5′-CCTGTTTGTAGCTGAGGTTC-3′); SOX2 (forward,
5′-ACAACTCGGAGATCAGCA-3′; reverse, 5′-GCAGCGTGTACTTATCCTTC-3′);
ACTB (forward, 5′-GGACTTCGAGCAAGAGATGG-3′; reverse, 5′-AGCAC
TGTGTTGGCGTACAG-3′).
Quantitative real-time RT-PCR was performed using
Assay-on-Demand primers and TaqMan Universal PCR Master Mix Reagent
(Applied Biosystems, Foster City, NJ, USA). Samples were analyzed
using an ECO™ Real-Time PCR System (Illumina, San Diego, CA, USA).
The ΔΔCt method was used to analyze the relative changes in gene
expression as previously described (21), using ACTB as a normalization
control. The following primers and probes were used: SDC1
(Hs00896423_m1); IRF4 (Hs01056534_m1); PRDM1
(Hs00153357_m1); XBP1 (Hs00964360_m1); and ACTB
(Hs99999903_m1).
Intracellular staining of IRF4 followed
by flow cytometric analysis
The MM cell lines cultured under normoxic or hypoxic
conditions were stained with an FITC-CD138 antibody (clone MI15; BD
Biosciences), fixed and permeabilized using a FOXP3 Staining Buffer
Set (eBioscience, San Diego, CA, USA), and then stained
intracellularly with an Alexa 647-IRF4 antibody (clone 3E4;
eBioscience) according to the manufacturer’s protocol. Flow
cytometric analysis was performed using the FACSCalibur
(Becton-Dickinson).
Western blot analysis
Cell lysates were prepared as reported previously
(22). Quantification of total
protein was performed using a Pierce BCA Protein Assay Kit (Thermo
Scientific, Waltham, MA, USA), and equal amounts of protein were
used for analysis. The cell lysates were separated in NuPAGE
Bis-Tris precast gels (Invitrogen) and transferred to PVDF
membranes using an iBlot Dry Blotting system (Invitrogen). The
membranes were blocked with 5% non-fat dry milk for 1 h at room
temperature, followed by incubation with primary antibodies at 4°C
for 18 h. The primary antibodies against HIF-1α, HIF-2α, NANOG, and
SOX2 were purchased from Cell Signaling Technology (Beverly, MA,
USA), while those against Oct-4, RARα, and actin were obtained from
Santa Cruz Biotechnology (Santa Cruz, CA, USA). The membranes were
then incubated with horseradish peroxidase-conjugated secondary
antibodies (GE Healthcare) for 1 h at room temperature.
Antibody-bound proteins were visualized using the ECL prime western
blotting detection reagent (GE Healthcare) and an LAS-1000
bio-image analyzer (GE Healthcare).
Aldehyde dehydrogenase (ALDH)
activity
The ALDH activities of the MM cell lines cultured
under normoxic and hypoxic conditions were analyzed using Aldefluor
(Stem Cell Technologies, Vancouver, Canada). After adding activated
Aldefluor reagent to the cell cultures, half of the cells were
transferred to tubes containing an ALDH inhibitor,
diethylaminobenzaldehyde (DEAB), to confirm specificity of the
reagent. Samples were incubated at 37°C for 30 min and analyzed
using the FACSCalibur (Becton-Dickinson).
Analysis of apoptosis
The MM cell lines were incubated in the presence of
1 μM ATRA (Sigma, St. Louis, MO, USA) or 5 nM bortezomib
(Sigma) for 24 h. Apoptosis in the MM cell lines was quantified by
staining with Annexin V (MBL, Nagoya, Japan) and 7-AAD (BD
Biosciences). The samples were analyzed by flow cytometry
(FACSVerse; Becton-Dickinson).
Statistical analysis
The data were analyzed statistically by Student’s
t-test using GraphPad Prism version 5.0 (GraphPad Software, La
Jolla, CA, USA). P-values of <0.05 were considered statistically
significant.
Results
Hypoxia reduces CD138 expression in MM
cell lines
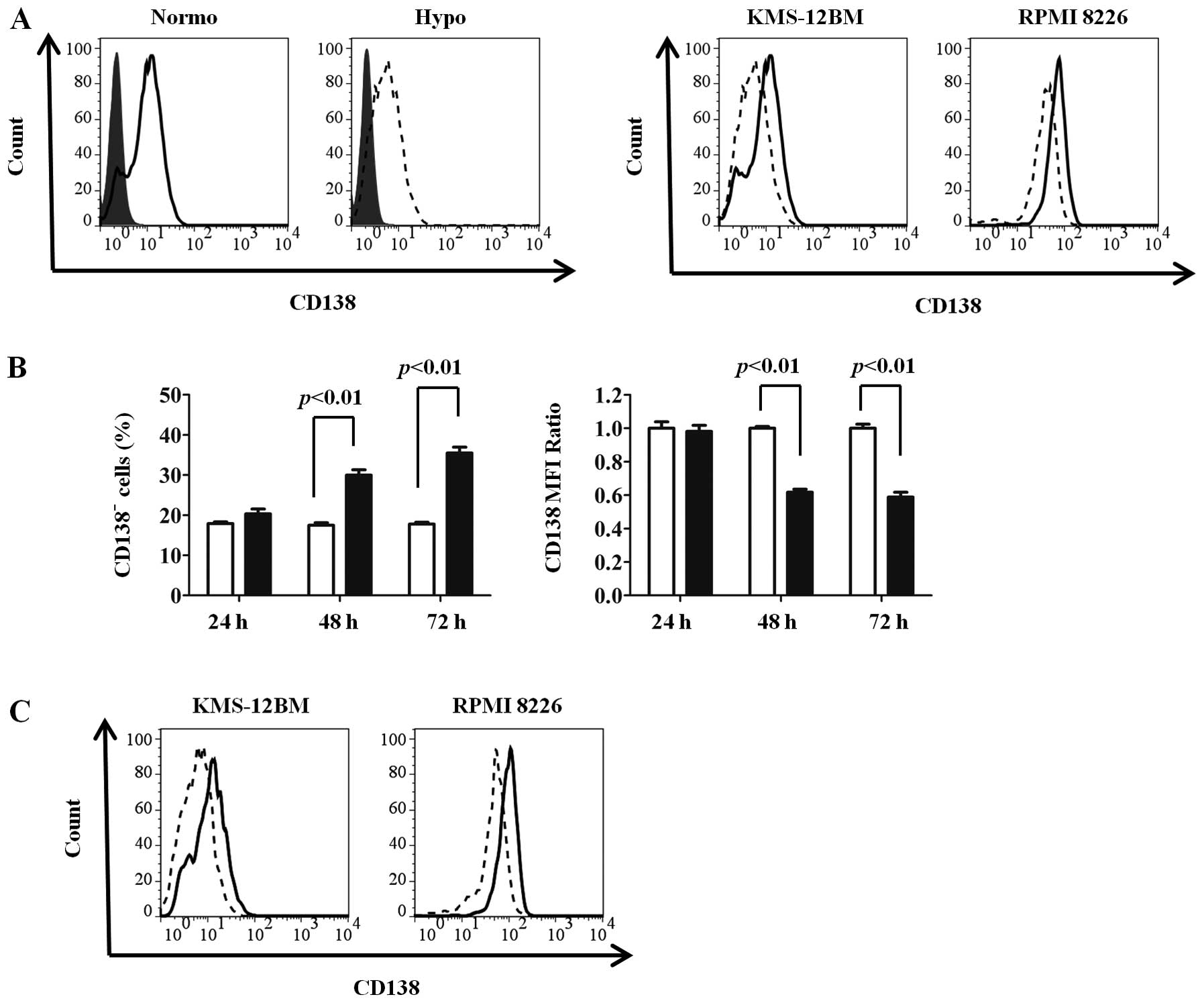
To investigate whether CD138 expression in MM cells
was influenced by oxygen levels, we cultured two MM cell lines
(KMS-12BM and RPMI 8226) under normoxic and hypoxic conditions for
up to 72 h and compared the surface CD138 expressions by flow
cytometry. Since CD138 was reported to be downregulated in
non-viable cells (23), only
viable cells were obtained by Ficoll density gradient
centrifugation followed by gating of live cells in combination with
the forward/side scatter profile and exclusion of 7-AAD-positive
cells. CD138 expression was reduced by 72 h of culture under
hypoxic conditions compared with normoxic conditions in both cell
lines (Fig. 1A). As shown in
Fig. 1B, CD138 expression was
reduced from 48 h, and subsequently proceeded in a time-dependent
manner. We then re-oxygenized the cells at 72 h after starting the
hypoxic conditions and maintained the cells under normoxic
conditions for an additional 72 h. Interestingly, we observed
recovery of the CD138 expression, indicating that the reduction in
CD138 expression under hypoxia was a reversible phenomenon
(Fig. 1C).
Changes in CD138 and other surface
antigens under long-term hypoxia
We further analyzed CD138 expression under long-term
exposure (30 days) to hypoxic or normoxic conditions. After 30 days
of incubation, CD138 expression was markedly reduced in KMS-12BM
and RPMI 8226 cells cultured under hypoxia compared with those
cultured under normoxia (Fig. 2A).
In KMS-12BM cells in particular, the positivity for CD138
expression under hypoxic conditions was reduced to only 10%.
Real-time RT-PCR showed that CD138 (SDC1) mRNA was downregulated
after long-term hypoxia, indicating that CD138 expression was
reduced at the gene transcription level (Fig. 2B).
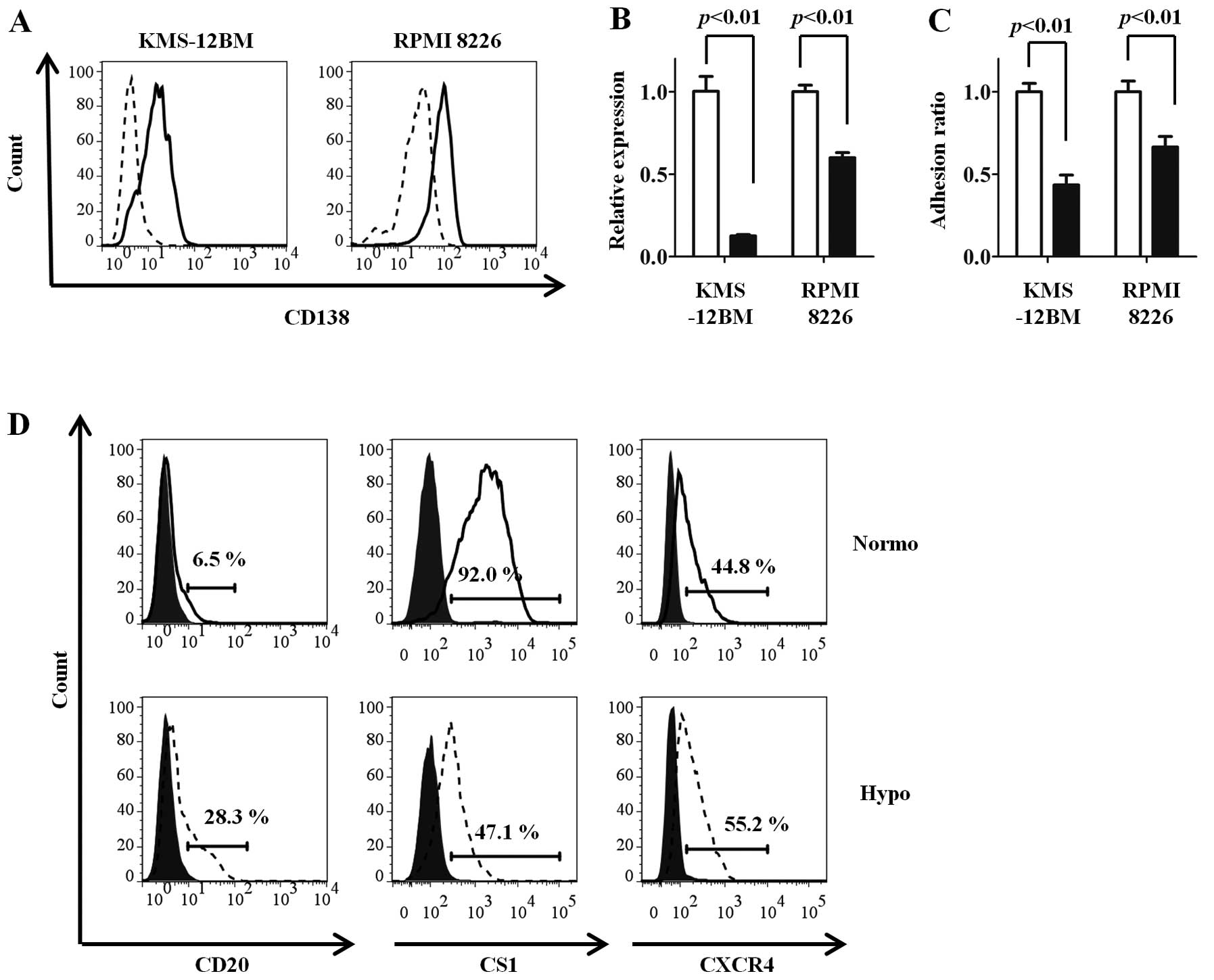
Since CD138 mediates the adhesion of MM cells to
type-1 collagen (24), which is an
important component of the bone marrow microenvironment, the
influence of oxygen concentrations on the adhesion of MM cells was
evaluated. The hypoxic MM cell lines adhered poorly to type-1
collagen, reflecting the low expression of CD138 (Fig. 2C).
The expression changes in other surface antigens
were compared between the hypoxic and normoxic MM cell lines. The
CD20 and CXCR4 expressions were increased, while the CS1 expression
was decreased, under hypoxia compared with normoxia in KMS-12BM
cells (Fig. 2D). Similar results
were observed in RPMI 8226 cells (normoxia vs. hypoxia: CD20, 1.8
vs. 14.1%; CS1, 81.3 vs. 34.6%; CXCR4, 40.2 vs. 82.1%). No changes
in expression were observed for CD19, adhesion molecules other than
CD138 (CD44, CD49d and CD54), and ATP-binding cassette transporters
(ABCG2 and MDR1) (data not shown).
Hypoxic MM cell lines have an immature
phenotype
Since CD138 (4) and
CS1 (25) expressions are highly
specific for terminally differentiated plasma cells, we
hypothesized that the hypoxic MM cell lines have a less mature
phenotype than the normoxic cell lines. To prove this hypothesis,
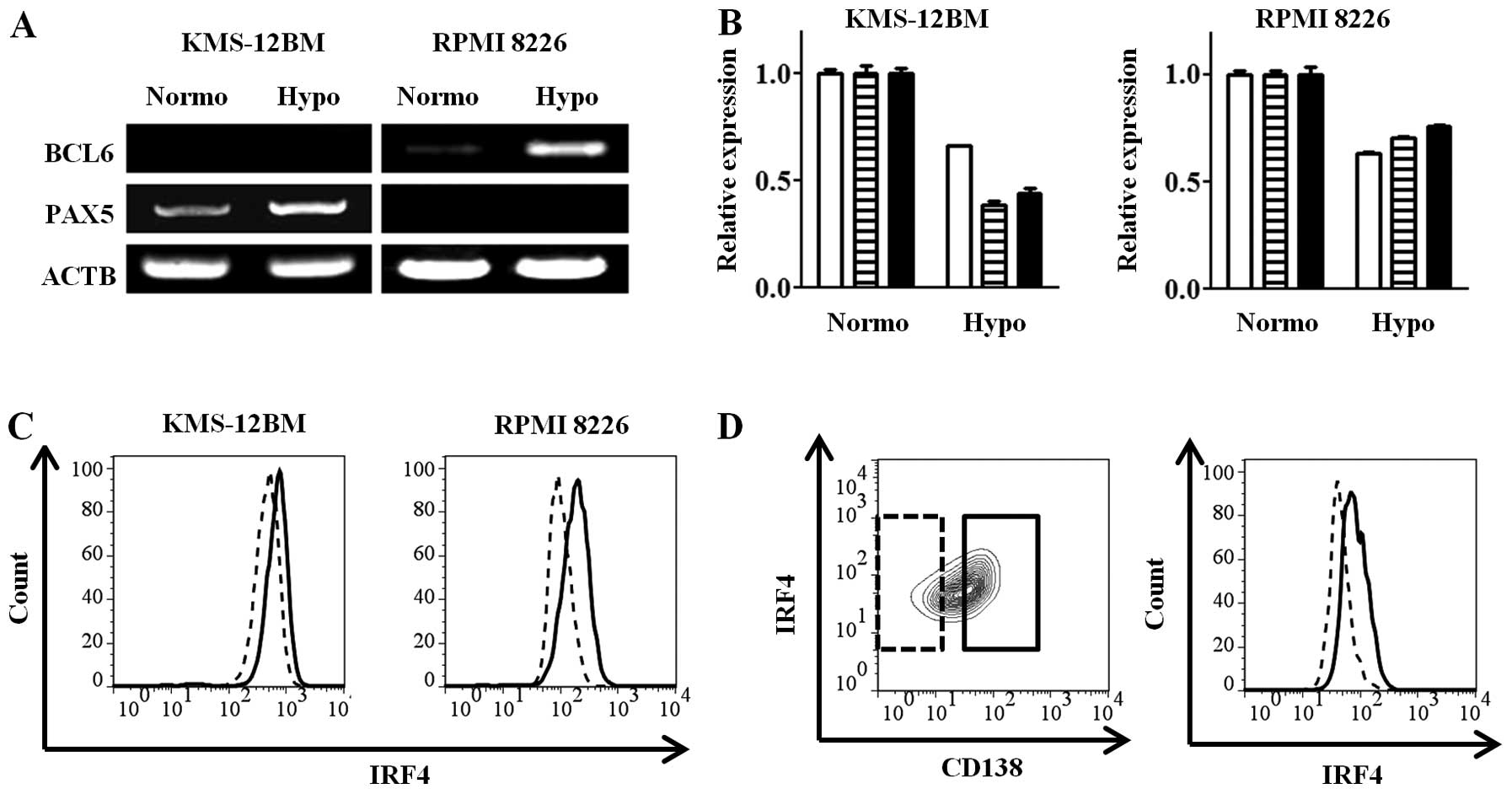
we assessed the expressions of the BCL6 and PAX5 transcription
factors, which exist in the mature B-cell state and are decreased
in mature plasma cells (26), by
RT-PCR in the MM cell lines after 30 days of culture under hypoxic
or normoxic conditions. Each of the mature B-cell transcription
factors, PAX5 and BCL6, was increased in the hypoxic
state in KMS-12BM and RPMI 8226 cells (Fig. 3A). The gene expressions of IRF4,
PRDM1 and XBP1, which are plasma cell-specific
transcription factors, were decreased in the hypoxic cells compared
with the normoxic cells (Fig. 3B).
The intracellular IRF4 protein expression, which was analyzed by
flow cytometry, was decreased in the hypoxic cells (Fig. 3C), consistent with the results for
the gene expressions. Analysis of subpopulations obtained as
CD138-high and -low cells showed high and low IRF4 expressions,
respectively (Fig. 3D). These
findings show that the hypoxic MM cell lines with low CD138
expression have an immature, so-called mature B cell-like rather
than plasma cell, transcriptional status.
Hypoxia induces a stem cell-like
transcriptional program in MM cells
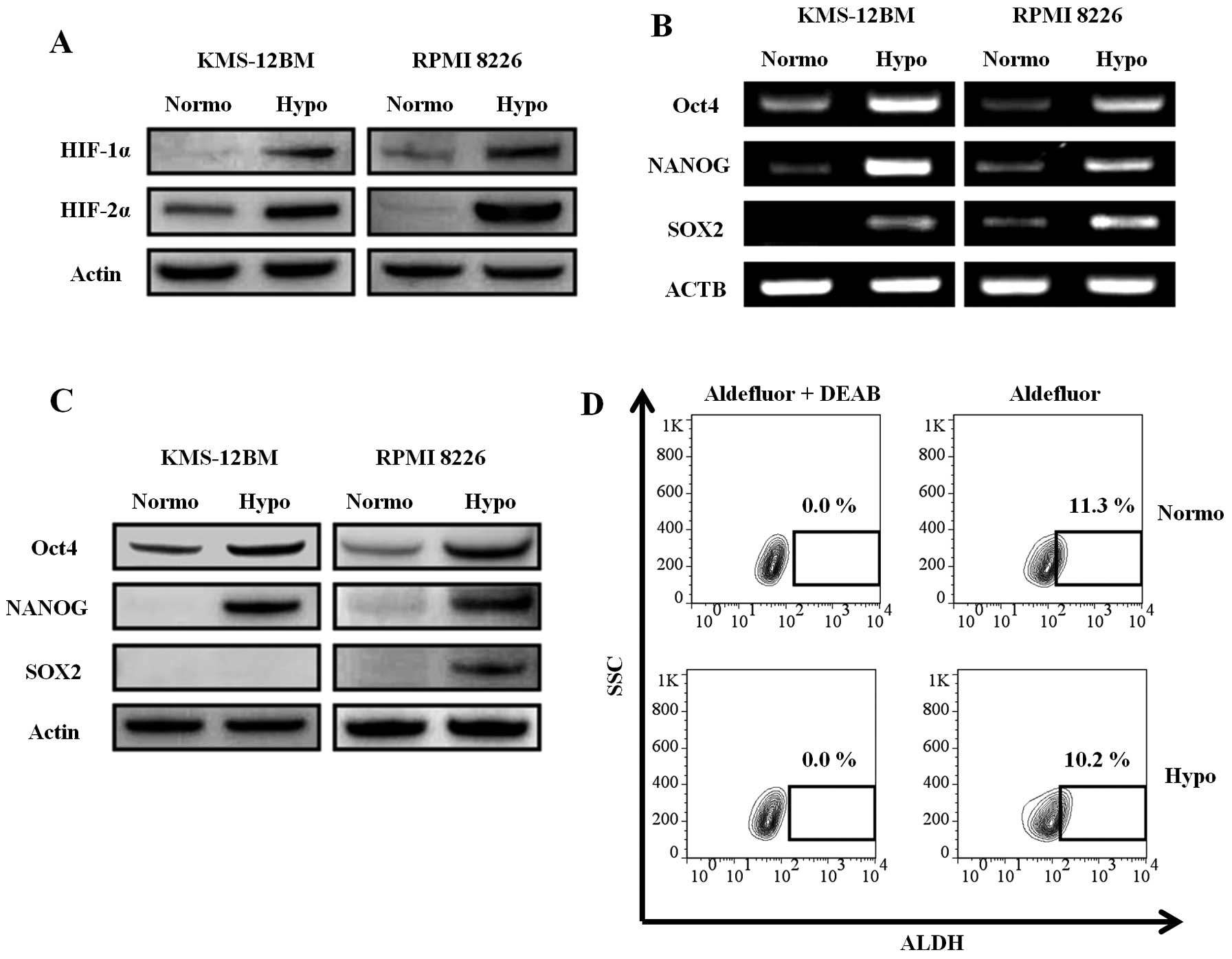
Hypoxia-inducible factors (HIFs) are key molecules
for the cellular response to hypoxia. It was reported that a
hypoxic environment and HIF activity are required not only for stem
cells, but also for cancer stem cells (27). Since HIFs induce stem cell
transcription factors in solid tumors (28), we analyzed the expression of HIFs
and stem cell transcription factors in the hypoxic MM cell lines.
HIFs, especially HIF-2α, were increased in the hypoxic MM cell
lines, as evaluated by western blotting, indicating that the cells
were actually responding to the hypoxic environment (Fig. 4A). The expression of stem cell
transcription factors (Oct-4, SOX2, and NANOG) was increased in the
hypoxic MM cell lines at both the mRNA (Fig. 4B) and protein (Fig. 4C) levels. The only exception was
the expression of SOX2 protein, which was not detected in hypoxic
KMS-12BM cells, although its mRNA expression was upregulated.
However, the ALDH activity, which is characteristic of cancer stem
cells including MM stem cells (29), was not increased in hypoxic
KMS-12BM cells (Fig. 4D) and RPMI
8226 cells (normoxia vs. hypoxia: 1.06 vs. 0.10%). The expression
of ABCG2, which is highly expressed in stem cells and associated
with side-population cells (30),
was not increased in hypoxic cells, as described earlier in this
report.
ATRA induces differentiation of hypoxic
MM cells and increase their sensitivity to bortezomib
ATRA induces cell differentiation not only of stem
cells (31), but also of MM cells
(32,33). ATRA is also known to repress the
expression of stem cell transcription factors, such as Oct-4, NANOG
and SOX2 (34,35). Based on these previous reports, we
investigated whether ATRA could induce redifferentiation of the
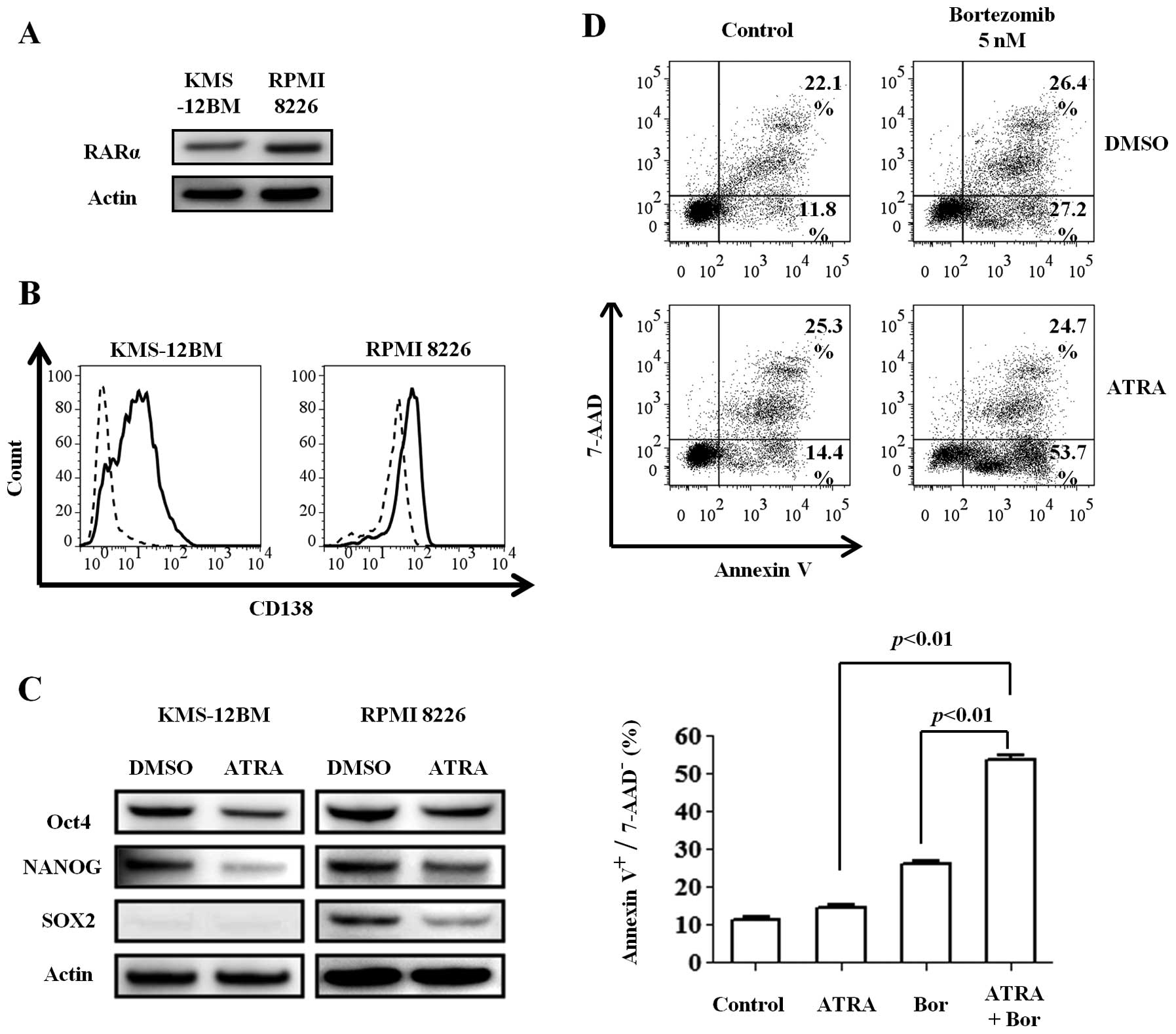
hypoxic MM cell lines. We first confirmed the expression of RARα, a
specific receptor for retinoic acid, in the MM cell lines cultured
for 30 days under hypoxic conditions (Fig. 5A). The cell lines were then
incubated with 1 μM ATRA or dimethyl sulfoxide (DMSO) for 48
h under hypoxic conditions. Surprisingly, ATRA increased the CD138
expression compared with DMSO (Fig.
5B), especially in KMS-12BM cells, while the expression of all
stem cell transcription factors was decreased (Fig. 5C). These findings indicate that
ATRA can induce the redifferentiation of immature MM cells, even
under hypoxic conditions.
Next, we investigated whether the differentiation
induced by ATRA can sensitize the immature and hypoxic cells to
bortezomib. Hypoxic KMS-12BM cells were treated with 1 μM
ATRA and 5 nM bortezomib alone or in combination for 24 h. While
ATRA alone slightly affected the viability of hypoxic KMS-12BM
cells, the combination of the two reagents increased the
cytotoxicity, especially the early apoptosis (Annexin
V+/7-AAD− cells) (Fig. 5D).
Discussion
Accumulating evidence indicates that CD138 is an
important molecule not only for the identification of plasma cells,
but also for distinguishing myeloma stem cells. CD138-negative
cells have recently been proposed as myeloma stem cells (3). On the other hand, Jakubikova et
al (10) showed that the cells
in the side population analyzed by flow cytometry, which are
considered to be cancer stem cells, are CD138-positive cells with
clonogenic potential, while Hosen et al (36) reported that both CD138-positive and
-negative lesions have the capacity to propagate MM in vivo.
Although it remains unclear whether CD138-negative myeloma cells
are ‘myeloma stem cells’, several reports have shown that CD138-low
or -negative cells may contribute to drug resistance or relapse of
the disease (9,11,12).
Therefore, these previous findings allow us to consider that the
expression of CD138 in myeloma cells is not uniformly high.
Analyses of the mechanisms underlying the regulation of CD138
expression in myeloma cells may lead to a better understanding of
myeloma biology.
In the present study, we found that a reduced oxygen
level contributes to CD138 downregulation in MM cells. We observed
a reduction in the CD138 level under hypoxia in a time-dependent
manner that lasted for at least 30 days. The recovery of CD138
expression by re-oxygenation indicates that the oxygen level plays
a major role in MM cell regulation of CD138 expression.
When the expression of CD138 was decreased, the
adhesion of the MM cell lines to type-1 collagen was also
decreased, indicating a correlation between CD138 expression and
adhesion of MM cells to the extracellular matrix. Indeed, several
reports have shown that CD138 downregulation may contribute to a
more metastatic potential not only in solid tumors (37,38),
but also in MM (12,39). It can be hypothesized that hypoxia
may promote the metastasis of myeloma cells partly through CD138
downregulation. CXCR4, which is induced under hypoxia and
contributes to migration and homing of MM cells (18), was upregulated in hypoxic MM cells.
This finding may also be associated with tumor progression under
hypoxia.
Hypoxic MM cells had lower CD138 and CS1 expressions
and higher CD20 expression than normoxic cells. Since the
expressions of CD138 (4) and CS1
(25) are highly specific for
terminally differentiated plasma cells and previous reports showed
an immature transcriptional profile in MM cells with low expression
of CD138 (9,11,16),
we hypothesized that the hypoxic MM cell lines may have a less
mature phenotype than the normoxic cell lines. Expression analyses
of transcription factors associated with plasma cells (IRF4, PRDM1
and XBP1) and B cells (BCL6 and PAX5) revealed that the hypoxic MM
cells had a relatively immature (B cell-like) transcriptional
profile compared with normoxic cells. This indicates that the
oxygen levels influence not only the surface antigens, but also the
transcription factor expressions in MM cells. Although hypoxic
cells acquired a B cell-like phenotype, CD19 expression was
negative. Since CD19 expression can distinguish MM cells from B
cells and normal plasma cells (40), this indicates that hypoxia delivers
an immature phenotype to MM cells, but they are not identical to
normal mature B cells and can be distinguished as malignant plasma
cells in terms of CD19 negativity.
HIFs (HIF-1α and HIF-2α) mediate the cellular
response to hypoxia and activate transcription factors that control
stem cell self-renewal (27). It
was reported that HIFs are positive in myeloma cells in the bone
marrow (41,42), suggesting that myeloma cells are
also hypoxic in vivo. Our analyses of stem cell
transcription factors revealed that hypoxia induces the expressions
of stem cell transcription factors at both the mRNA and protein
levels. Previous reports showing stem cell marker expression in
CD138-negative myeloma cells (29,43)
support our findings. It was also reported that Oct4 and SOX2
expression is essential for maintenance of the side-population
fraction in MM (44). These
findings indicate that hypoxia induces a stem cell-like phenotype
in MM cells. However, we did not observe any increases in ALDH
activity or ABCG2 expression, which are associated with cancer stem
cell function. Taken together, hypoxia induces stem cell-like
features in MM cells, although further analyses are needed to
conclude that MM cells under hypoxic conditions are cancer stem
cells.
It is known that MM patients with SOX2 expression
have a worse overall survival than patients without SOX2 expression
(45), and this report is
compatible with our previous finding that MM patients with low
CD138 expression showed a poor prognosis (9). MM cells under hypoxic conditions may
become less sensitive to anticancer agents, thereby contributing to
a poor prognosis through the acquisition of drug resistance.
Indeed, ATRA was able to sensitize MM cells to bortezomib under
hypoxic conditions by inducing a more differentiated phenotype.
These findings allow us to propose a new therapeutic approach
against CD138-low myeloma cells with drug resistance by combining
ATRA with other anti-myeloma reagents such as bortezomib.
Taken together, the present data suggest that
hypoxia reduces CD138 expression and provides stem cell-like
features to myeloma cells. Further analyses of the mechanisms that
regulate CD138 expression and related biological processes
including cell adhesion or drug sensitivity should contribute not
only to a better understanding of the disease, but also to an
improvement of the prognosis of myeloma.
Acknowledgements
This study was supported by a grant
from the Amyloidosis Research Committee for Research on Intractable
Diseases from the Ministry of Health, Labour and Welfare,
Japan.
References
|
1.
|
Kumar SK, Rajkumar SV, Dispenzieri A, et
al: Improved survival in multiple myeloma and the impact of novel
therapies. Blood. 111:2516–2520. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Clarke MF, Dick JE, Dirks PB, et al:
Cancer stem cells - perspectives on current status and future
directions: AACR Workshop on cancer stem cells. Cancer Res.
66:9339–9344. 2006. View Article : Google Scholar
|
|
3.
|
Matsui W, Huff CA, Wang Q, et al:
Characterization of clonogenic multiple myeloma cells. Blood.
103:2332–2336. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Medina F, Segundo C, Campos-Caro A,
Gonzalez-Garcia I and Brieva JA: The heterogeneity shown by human
plasma cells from tonsil, blood, and bone marrow reveals graded
stages of increasing maturity, but local profiles of adhesion
molecule expression. Blood. 99:2154–2161. 2002. View Article : Google Scholar
|
|
5.
|
Wijdenes J, Vooijs WC, Clement C, et al: A
plasmocyte selective monoclonal antibody (B-B4) recognizes
syndecan-1. Br J Haematol. 94:318–323. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Ikeda H, Hideshima T, Fulciniti M, et al:
The monoclonal antibody nBT062 conjugated to cytotoxic
Maytansinoids has selective cytotoxicity against CD138-positive
multiple myeloma cells in vitro and in vivo. Clin Cancer Res.
15:4028–4037. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7.
|
Witzig TE, Kimlinger T, Stenson M and
Therneau T: Syndecan-1 expression on malignant cells from the blood
and marrow of patients with plasma cell proliferative disorders and
B-cell chronic lymphocytic leukemia. Leuk Lymphoma. 31:167–175.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
8.
|
Reid S, Yang S, Brown R, et al:
Characterisation and relevance of CD138-negative plasma cells in
plasma cell myeloma. Int J Lab Hematol. 32:e190–e196. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Kawano Y, Fujiwara S, Wada N, et al:
Multiple myeloma cells expressing low levels of CD138 have an
immature phenotype and reduced sensitivity to lenalidomide. Int J
Oncol. 41:876–884. 2012.PubMed/NCBI
|
|
10.
|
Jakubikova J, Adamia S, Kost-Alimova M, et
al: Lenalidomide targets clonogenic side population in multiple
myeloma: pathophysiologic and clinical implications. Blood.
117:4409–4419. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
Van Valckenborgh E, Matsui W, Agarwal P,
et al: Tumor-initiating capacity of CD138− and
CD138+ tumor cells in the 5T33 multiple myeloma model.
Leukemia. 26:1436–1439. 2012.PubMed/NCBI
|
|
12.
|
Chaidos A, Barnes CP, Cowan G, et al:
Clinical drug resistance linked to interconvertible phenotypic and
functional states of tumor-propagating cells in multiple myeloma.
Blood. 121:318–328. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Yaccoby S: The phenotypic plasticity of
myeloma plasma cells as expressed by dedifferentiation into an
immature, resilient, and apoptosis-resistant phenotype. Clin Cancer
Res. 11:7599–7606. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Zlei M, Egert S, Wider D, Ihorst G, Wasch
R and Engelhardt M: Characterization of in vitro growth of multiple
myeloma cells. Exp Hematol. 35:1550–1561. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15.
|
Dezorella N, Pevsner-Fischer M, Deutsch V,
et al: Mesenchymal stromal cells revert multiple myeloma cells to
less differentiated phenotype by the combined activities of
adhesive interactions and interleukin-6. Exp Cell Res.
315:1904–1913. 2009. View Article : Google Scholar
|
|
16.
|
Fuhler GM, Baanstra M, Chesik D, et al:
Bone marrow stromal cell interaction reduces syndecan-1 expression
and induces kinomic changes in myeloma cells. Exp Cell Res.
316:1816–1828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Axelson H, Fredlund E, Ovenberger M,
Landberg G and Pahlman S: Hypoxia-induced dedifferentiation of
tumor cells - a mechanism behind heterogeneity and aggressiveness
of solid tumors. Semin Cell Dev Biol. 16:554–563. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18.
|
Azab AK, Hu J, Quang P, et al: Hypoxia
promotes dissemination of multiple myeloma through acquisition of
epithelial to mesenchymal transition-like features. Blood.
119:5782–5794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Ohtsuki T, Yawata Y, Wada H, Sugihara T,
Mori M and Namba M: Two human myeloma cell lines, amylase-producing
KMS-12-PE and amylase-non-producing KMS-12-BM, were established
from a patient, having the same chromosome marker,
t(11;14)(q13;q32). Br J Haematol. 73:199–204. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Matsuoka Y, Moore GE, Yagi Y and Pressman
D: Production of free light chains of immunoglobulin by a
hematopoietic cell line derived from a patient with multiple
myeloma. Proc Soc Exp Biol Med. 125:1246–1250. 1967. View Article : Google Scholar : PubMed/NCBI
|
|
21.
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
|
|
22.
|
Fujiwara S, Kawano Y, Yuki H, et al: PDK1
inhibition is a novel therapeutic target in multiple myeloma. Br J
Cancer. 108:170–178. 2013.PubMed/NCBI
|
|
23.
|
Jourdan M, Ferlin M, Legouffe E, et al:
The myeloma cell antigen syndecan-1 is lost by apoptotic myeloma
cells. Br J Haematol. 100:637–646. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Ridley RC, Xiao H, Hata H, Woodliff J,
Epstein J and Sanderson RD: Expression of syndecan regulates human
myeloma plasma cell adhesion to type I collagen. Blood. 81:767–774.
1993.PubMed/NCBI
|
|
25.
|
Shaffer AL, Emre NC, Lamy L, et al: IRF4
addiction in multiple myeloma. Nature. 454:226–231. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
Shapiro-Shelef M and Calame K: Regulation
of plasma-cell development. Nat Rev Immunol. 5:230–242. 2005.
View Article : Google Scholar
|
|
27.
|
Keith B and Simon MC: Hypoxia-inducible
factors, stem cells, and cancer. Cell. 129:465–472. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Mathieu J, Zhang Z, Zhou W, et al: HIF
induces human embryonic stem cell markers in cancer cells. Cancer
Res. 71:4640–4652. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29.
|
Brennan SK, Wang Q, Tressler R, et al:
Telomerase inhibition targets clonogenic multiple myeloma cells
through telomere length-dependent and independent mechanisms. PLoS
One. 5:2010. View Article : Google Scholar
|
|
30.
|
Zhou S, Schuetz JD, Bunting KD, et al: The
ABC transporter Bcrp1/ABCG2 is expressed in a wide variety of stem
cells and is a molecular determinant of the side-population
phenotype. Nat Med. 7:1028–1034. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Gudas LJ and Wagner JA: Retinoids regulate
stem cell differentiation. J Cell Physiol. 226:322–330. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Huang H, Wu D, Fu J, et al: All-trans
retinoic acid can intensify the growth inhibition and
differentiation induction effect of rosiglitazone on multiple
myeloma cells. Eur J Haematol. 83:191–202. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Gu JL, Li J, Zhou ZH, et al:
Differentiation induction enhances bortezomib efficacy and
overcomes drug resistance in multiple myeloma. Biochem Biophys Res
Commun. 420:644–650. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34.
|
Akamatsu W, DeVeale B, Okano H, Cooney AJ
and van der Kooy D: Suppression of Oct4 by germ cell nuclear factor
restricts pluripotency and promotes neural stem cell development in
the early neural lineage. J Neurosci. 29:2113–2124. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Gu P, LeMenuet D, Chung AC, Mancini M,
Wheeler DA and Cooney AJ: Orphan nuclear receptor GCNF is required
for the repression of pluripotency genes during retinoic
acid-induced embryonic stem cell differentiation. Mol Cell Biol.
25:8507–8519. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Hosen N, Matsuoka Y, Kishida S, et al:
CD138-negative clonogenic cells are plasma cells but not B cells in
some multiple myeloma patients. Leukemia. 26:2135–2141. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37.
|
Matsumoto A, Ono M, Fujimoto Y, Gallo RL,
Bernfield M and Kohgo Y: Reduced expression of syndecan-1 in human
hepatocellular carcinoma with high metastatic potential. Int J
Cancer. 74:482–491. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Ishikawa T and Kramer RH: Sdc1 negatively
modulates carcinoma cell motility and invasion. Exp Cell Res.
316:951–965. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Purushothaman A, Uyama T, Kobayashi F, et
al: Heparanase-enhanced shedding of syndecan-1 by myeloma cells
promotes endothelial invasion and angiogenesis. Blood.
115:2449–2457. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40.
|
Harada H, Kawano MM, Huang N, et al:
Phenotypic difference of normal plasma cells from mature myeloma
cells. Blood. 81:2658–2663. 1993.PubMed/NCBI
|
|
41.
|
Giatromanolaki A, Bai M, Margaritis D, et
al: Hypoxia and activated VEGF/receptor pathway in multiple
myeloma. Anticancer Res. 30:2831–2836. 2010.PubMed/NCBI
|
|
42.
|
Martin SK, Diamond P, Williams SA, et al:
Hypoxia-inducible factor-2 is a novel regulator of aberrant CXCL12
expression in multiple myeloma plasma cells. Haematologica.
95:776–784. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Spisek R, Kukreja A, Chen LC, et al:
Frequent and specific immunity to the embryonal stem
cell-associated antigen SOX2 in patients with monoclonal
gammopathy. J Exp Med. 204:831–840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Ikegame A, Ozaki S, Tsuji D, et al: Small
molecule antibody targeting HLA class I inhibits myeloma cancer
stem cells by repressing pluripotency-associated transcription
factors. Leukemia. 26:2124–2134. 2012. View Article : Google Scholar
|
|
45.
|
Schoenhals M, Kassambara A, De Vos J, Hose
D, Moreaux J and Klein B: Embryonic stem cell markers expression in
cancers. Biochem Biophys Res Commun. 383:157–162. 2009. View Article : Google Scholar : PubMed/NCBI
|