Introduction
The term cancer stem cells (CSCs) was defined as the
small percentage of cells within a solid tumor capable of
unregulated self-renewal, leading to continued tumor growth, as
well as of the generation of partially differentiated progenitor
cells (1–4). Neuroblastoma (NB) is the most common
extracranial solid tumor in children and accounts for 8–10% of
childhood cancers. Most children with high-risk NB have poor
prognosis because of its ability to regress spontaneously,
transform, or therapy-resistant relapse (5). The membrane of CSCs highly expressed
ATP-binding cassette (ABC) family of membrane transport proteins
and multidrug resistance protein (MDRP), which can transport drugs
and toxic substances outside the cell, and are not sensitive to
conventional chemotherapy drugs, which is the main cause of
chemotherapy failure (6). CSCs are
deemed to be the source of tumor recurrence and metastasis. Only a
tumor entirely eliminated of CSCs, can be cured completely. The CSC
theory provides a new perspective for cancer research, and has
gradually become a hot topic and a mainstream trend (7). The isolation and purification of CSCs
is the foundation for targeted cure by the combine application of
small molecule drugs and chemotherapeutic drugs.
XAV939 is a kind of small molecule tankyrase (TNKS)
inhibitor and synthetized using a chemical genetics approach. Huang
et al (8) and Chen et
al (9) have verified that
XAV939 could inhibit the proliferation of colon cancer cells by
blocking Wnt signaling through binding to TNKS catalytic
poly-ADP-ribose polymerase (PARP) domain. Tankyrase 1 (TNKS1) is a
member of the TNKS family and upregulated in a variety of cancers,
including multiple myeloma, plasma cell leukemia, high-grade
non-Hodgkin’s lymphomas, breast cancer, colon cancer and bladder
cancer (10–16). These studies suggested that TNKS1
played a role in tumor progression. Our previous studies proved
that TNKS1 was overexpressed in NB cell lines and XAV939 could
induce apoptosis of NB cells partly by inhibiting Wnt/β-catenin
signaling through TNKS1 (17).
However, it has not been reported whether XAV939 also has effect on
stemness of NB CSCs, and the involved mechanism that would
contribute to targeted therapy.
In the present study, we isolated, enriched and
identified NB CSCs from SH-SY5Y cells. Then we repressed TNKS1 by
XAV939 treatment or RNAi method, and demonstrated the inhibition
effect on the stemness and migration ability of NB CSCs. We
speculate that XAV939 is able to inhibit the stemness and migration
of NB via repression of TNKS1, and TNKS1 might be a potential
target for eliminating NB CSCs.
Materials and methods
Cell culture and TNKS1 inhibitor
Human NB SH-SY5Y cells were obtained from the
American Type Culture Collection (ATCC; Rockville, MD, USA). Cells
were cultured in Dulbecco’s modified Eagle’s medium-F12 (DMEM-F12;
Hyclone), with 10% fetal bovine serum (FBS; Gibco), 100 U/ml
penicillin, and 100 μg/ml streptomycin (Sigma Chemical Co.,
St. Louis, MO, USA) and were grown in a 5% CO2 incubator
at 37°C. The TNKS1 inhibitor XAV939 was purchased from
Sigma-Aldrich.
The comparison of isolation methods
for human NB CSCs
For side population (SP) method, 106
cells were collected and incubated in pre-warmed DMEM-F12/2% FBS
containing freshly added Hoechst 33342 (5 μg/ml final
concentration) for 90 min at 37°C with intermittent mixing. In
control group, cells were incubated with the Hoechst dye in the
presence of verapamil (50 μM). At the end of incubation,
cells were placed on ice at once and resuspended in cold
DMEM-F12/2% FBS until fluorescence-activated cell sorting (FACS)
analysis. Before analysis, cells were stained with PI (2
μg/ml). The Hoechst dye was excited with the UV laser at
351–364 nm and its fluorescence measured with a 515 nm SP filter
(Hoechst blue) and a 608 EFLP optical filter (Hoechst red). The
emission wavelengths were separated by a 540 DSP filter. For
surface marker method, 107 SH-SY5Y cells were collected and
suspended in FcR blocking reagent (Miltenyi Biotec) with 0.5% FBS
in PBS and added with mouse anti-human CD133/2(293C3)-PE monoclonal
antibody which was diluted at 1:11. In addition, mixed well and
refrigerated for 10 min in the dark (4–8°C). Then cells were washed
by adding 1–2 ml buffer per 107 cells and centrifuged at
300 × g for 10 min, supernatant was aspirated completely and the
cell pellet resuspended in DMEM-F12 with 2% FBS for isolating
CD133+ and CD133− cells, respectively, by
flow cytometry. CD133+ cells were considered as NB CSCs.
Mouse IgG2b-phycoerythrin was used as an isotype control and was
set up for each sample.
The enrichment of NB CSCs by etoposide
treatment
The SH-SY5Y cells were plated at a density of
105/ml in 6-well plates. Etoposide (V 4629,
Sigma-Aldrich, dissolved in PBS) of 20, 40 and 60 μM were
added two days after cell seeding respectively, maintained for 24
h, and removed by substituting the culture medium with fresh medium
without drug. Untreated (control) cells were also subjected to
change of medium at the same incubation times. Then the ratios of
CSCs were analyzed by flow cytometry and the group of highest ratio
was chosen. CSCs were sorted and cultured with DMEM/F12 medium
contained 40 ng/ml bFGF, 20 ng/ml EGF as well as 2% B27. This kind
of medium contributes to prevent the differentiation of CSCs and
maintain its characteristic.
Electron microscopy
The isolated CD133+ and CD133−
cells were cultured with different condition respectively. Some of
them were cultured on coverslip for the observation by scanning
electron microscope (SEM). When cells were at 60–70% confluence,
the culture medium was discarded and cells were fixed in cold
mixture of osmium tetroxide and glutaraldehyde for 1–3 h. Then
samples were dehydrated in graded ethanol series, replaced with
isoamyl acetate, dried at critical point, coated with gold on
surface, and observed by JSM-T300 SEM. Another portion of cells
were cultured in 6-well plates. The monolayer cells were collected
and fixed with 2.5% glutaraldehyde for 2 h. The cells were fixed
with 1% osmium tetroxide for 2–3 h, dehydrated with graded ethanol,
replaced with acetone, pelleted and embedded in 3% agar. The
agarized pellet was cut into 1-mm slices and immersed in 3% uranyl
acetate-lead citrate for double staining, and viewed with a
transmission electron microscope (TEM, JEM1200EX).
XAV939 treatment and RNAi
The isolated NB CSCs were cultured and proliferated
for XAV939 treatment and RNAi. XAV939 was dissolved in dimethyl
sulfoxide (DMSO, Sigma-Aldrich) and the final concentration was 1
μM based on our previous study results (16). The control group was treated with
DMSO only. The TNKS1 gene was knocked down by TNKS1-shRNA (shRNA
group) while cells in control group were transfected with
lentivirus-mediated scrambled-shRNA (SCR group). The XAV939
treatment, viral transfection and control groups were maintained
for 72 h. Then all groups were subjected to quantitative real-time
RT-PCR (qRT-PCR) analysis, western blot analysis,
immunofluorescence and migration assay.
QRT-PCR analysis
Total RNA was extracted using the RNeasy Mini kit
(Qiagen, Valencia, CA, USA) and quantitated using NanoDrop 1000
(NanoDrop, Wilmington, DE, USA). Then the RNA was reverse
transcribed into cDNA using PrimeScript® RT reagent kit
with gDNA Eraser (Takara). QRT-PCR was performed using
SYBR® Premix Ex Taq™ II (Takara) on ABI 7500 Real-Time
PCR system (Applied Biosystems). Sequences of the primers for CD133
were 5′-AGT GGCATCGTGCAAACCTG 3 (forward) and 5′-CTCCGAAT
CCATTCGACGATA-3′ (reverse). Sequences of the primers for β-actin
were 5′-TGGCACCCAGCACAATGAA-3′ (forward) and
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′ (reverse). The PCR amplification
was done at: 95°C for 30 sec; 95°C for 5 sec, 60°C for 34 sec for
40 cycles. The relative changes in gene expression data were
analyzed by the 2-ΔΔCT method. β-actin was used as an internal
control. Triplicates were run for each sample in three independent
experiments.
Western blot analysis
The NB CSCs were lysed with RIPA buffer and protein
concentration was determined by the Bradford method. Equal amounts
of protein (40 μg) were used for western blot analysis with
primary antibodies to anti-CD133 (Bioss, bs-0209R) and anti-β-actin
(Santa Cruz, sc-1616-R). Specific antibody binding was detected by
horse-radish peroxidase-conjugated goat anti-rabbit antibodies and
visualized with ECL reagent (Santa Cruz) according to the
manufacturer’s protocol. Antibody to β-actin was used to evaluate
protein loading in each lane.
Immunofluorescence
The NB CSCs were fixed with 4% paraformaldehyde for
10 min at room temperature, and permeabilized with 0.5% Triton
X-100 for 15 min. After blocking with 1% BSA for 30 min, cells were
incubated with the primary antibody (CD133, Bioss, 1:200) for 2 h
at 37°C, then incubated with secondary fluorescein conjugated
antibody (Bioss, 1:500) for 1 h at 37°C in the dark. To ensure
specificity of results, negative controls with no primary antibody
or no secondary antibody were used. The coverslips were incubated
with Hoechst 33342 (5 μg/ml for 10 min) for nuclear
counterstaining, mounted with 95% glycerol (Sigma-Aldrich), and
observed by fluorescence microscope (Olympus IX71). Five random
fields were selected and positive cells were counted.
Migration assay
To evaluate migratory properties of NB CSCs treated
with XAV939 or RNAi, migration assay was used. Cells
(1×105) were suspended in 100 μl culture medium
without FBS and loaded onto the top of Transwell chambers (24-well
plate) equipped with 8.0 μm pore-size polycarbonate
membranes (Corning). Culture medium supplemented with 10% FBS was
used as chemotactic stimuli in the bottom chambers. After 24 h of
incubation, cells on the upper surface of the filter were
mechanically removed with a cotton swab, and those which migrated
underneath the surface were fixed with 90% ethanol and stained with
0.1% crystal violet dye. Photographs were taken using an inverted
phase contrast microscope (Olympus CK40) and the number of cells
was counted.
Statistical analysis
The results obtained from three independent
experiments performed in triplicate are presented as mean ± SD.
One-way ANOVA was performed for comparison between each group.
P-values of <0.05 were considered as statistically
significant.
Results
The comparison of two methods
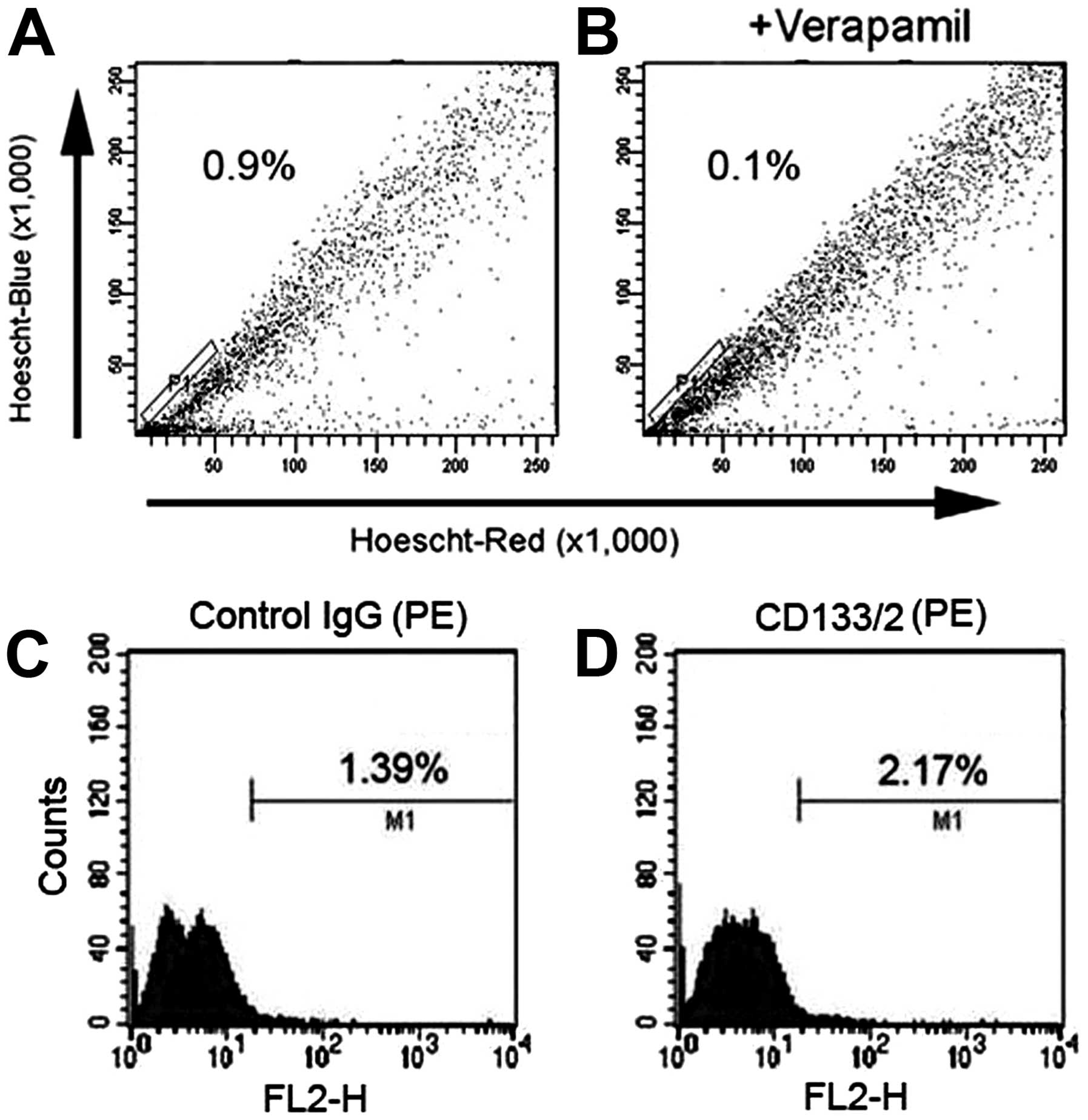
The SP and non-SP (NSP) cells were sorted from the
SH-SY5Y cell line. The P1 gate containing the SP cells accounted
for 0.9±0.06% of the total cells (Fig.
1A). The percentage of SP cells decreased to 0.1% after
treatment with verapamil, indicating that this population consisted
of SP cells (Fig. 1B). However,
the SP cell population is not obvious in flow chart and with poor
specificity. Moreover, Hoechst 33342 is harmful to cells. So the SP
cells after sorting had high death rate and poor culture result.
SH-SY5Y cells were also analyzed by FACS using the characterization
of CD133 expression on NB CSCs surface. The results showed that the
ratio of control IgG group was 1.39% (Fig. 1C), while that of CD133 antibody
group was 2.17% (Fig. 1D).
Therefore the ratio of CD133+ cells in SH-SY5Y cell line
was 0.78%.
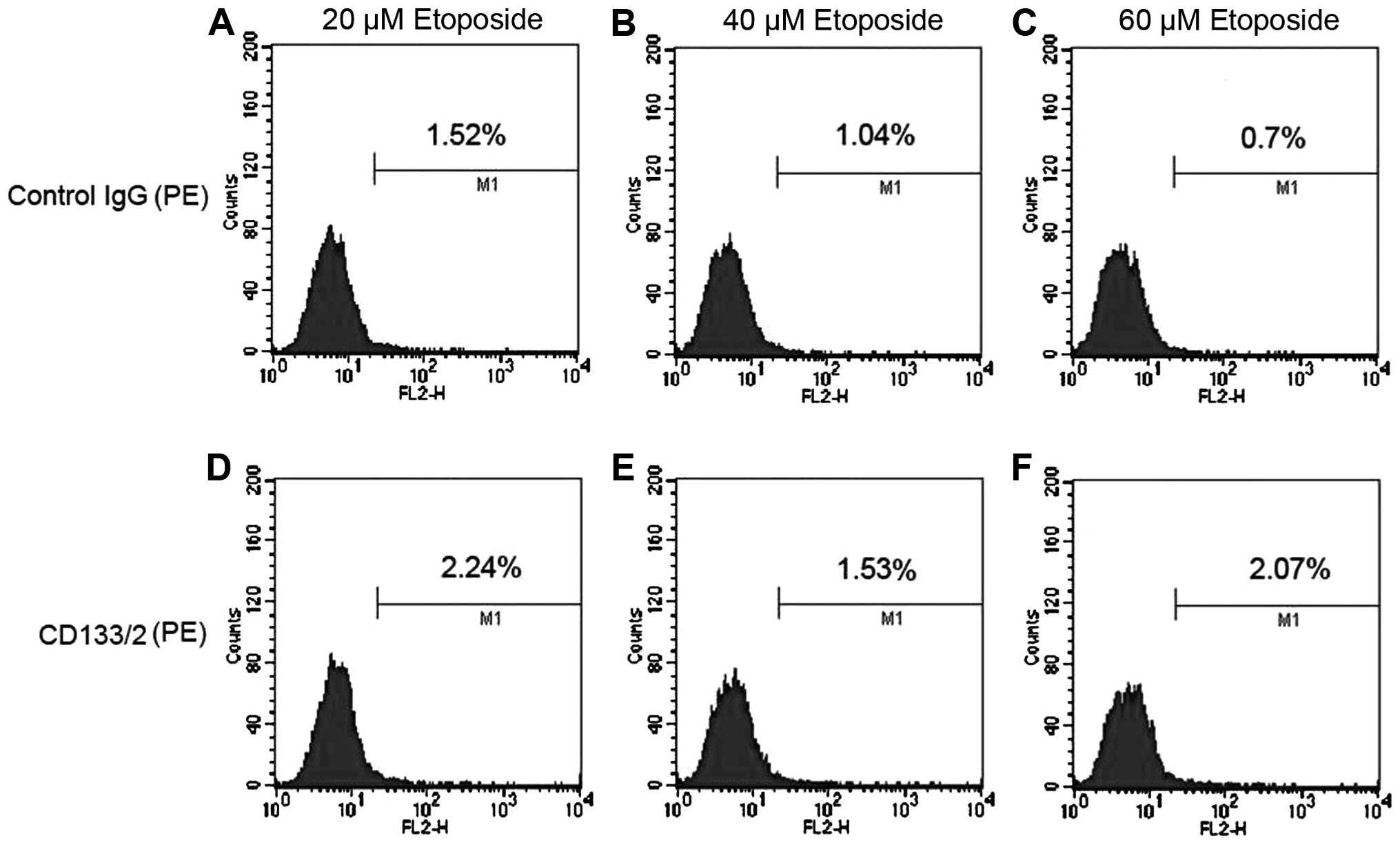
Enriched NB CSCs by etoposide
CD133 are commonly used as cell surface markers
representing the CSC subpopulation in NB (18). CSCs are resistant to conventional
chemotherapy, and therefore we further investigate the enrichment
of NB CSCs in SH-SY5Y cells using different concentrations of
etoposide and sorted NB CSCs with the cell surface marker of CD133
(Fig. 2). After treatment with
etoposide of 20, 40 and 60 μM, the flow cytometry analysis
showed that the ratio of NB CSCs was 0.72, 0.49 and 1.37%,
respectively. The ratio of NB CSCs in 60 μM etoposide
treatment group increased to nearly 2-fold of non-treatment group
(Figs. 1 and 2), and was the highest among all
groups.
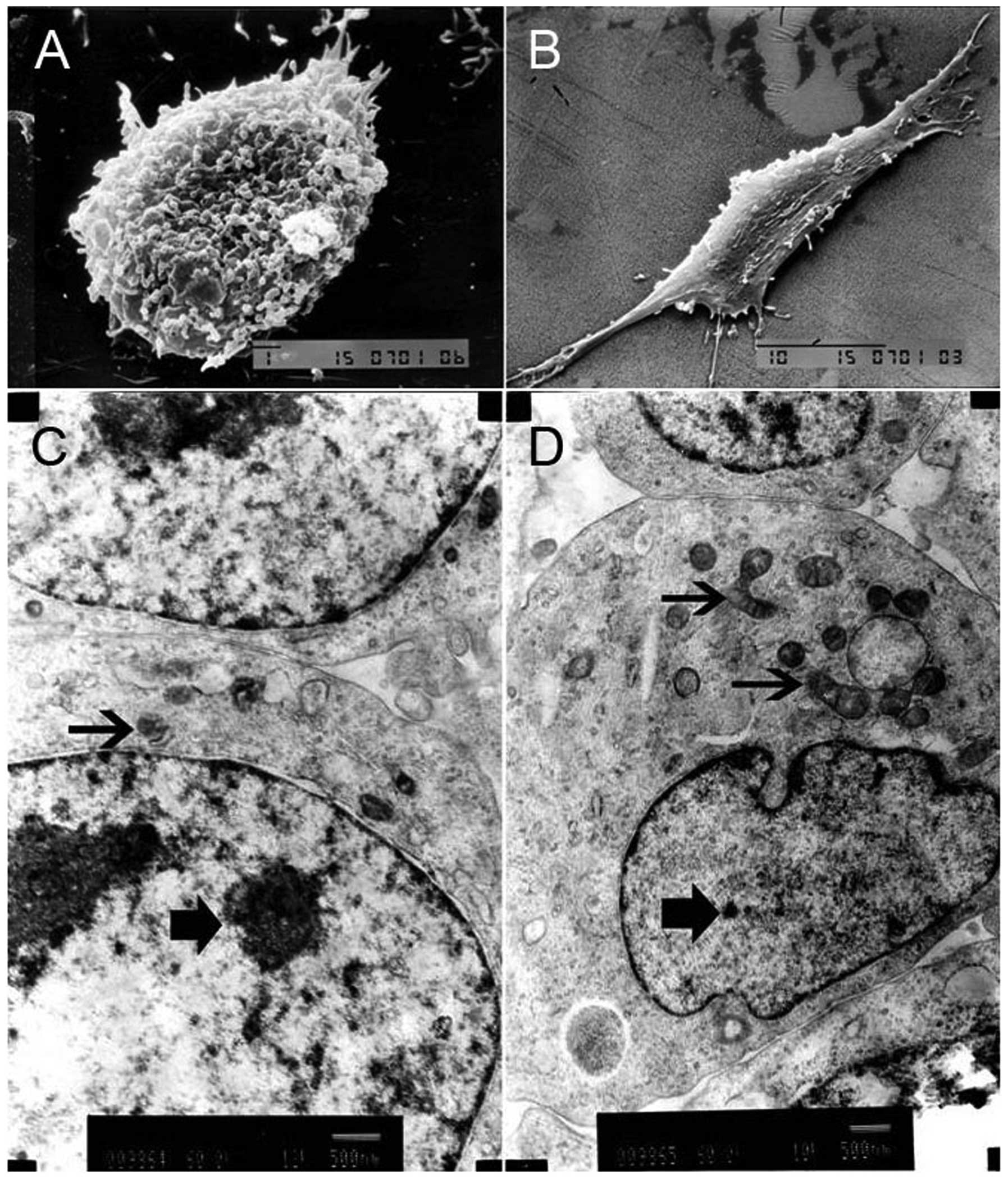
The ultrastructure of NB CSCs
Under a SEM, NB CSCs were rounded or oval with rich
and trivial cell processes on the surface, which gathered into a
ball growth, indicating that it was in an undifferentiated state
(Fig. 3A). However, the non-NB
CSCs were elongated and spindle-like with poor but obvious cell
processes (Fig. 3B). TEM showed
the nuclear-cytoplasmic ratio of NB CSCs was higher than that of
non-NB CSCs, and the NB CSCs had fewer cytoplasmic organelles than
the non-NB CSCs (Fig. 3C and D).
The undeveloped cytoplasmic organelles, such as rough endoplasmic
reticulum or Golgi apparatus, indicated that the NB CSCs were in
immature or juvenile stage.
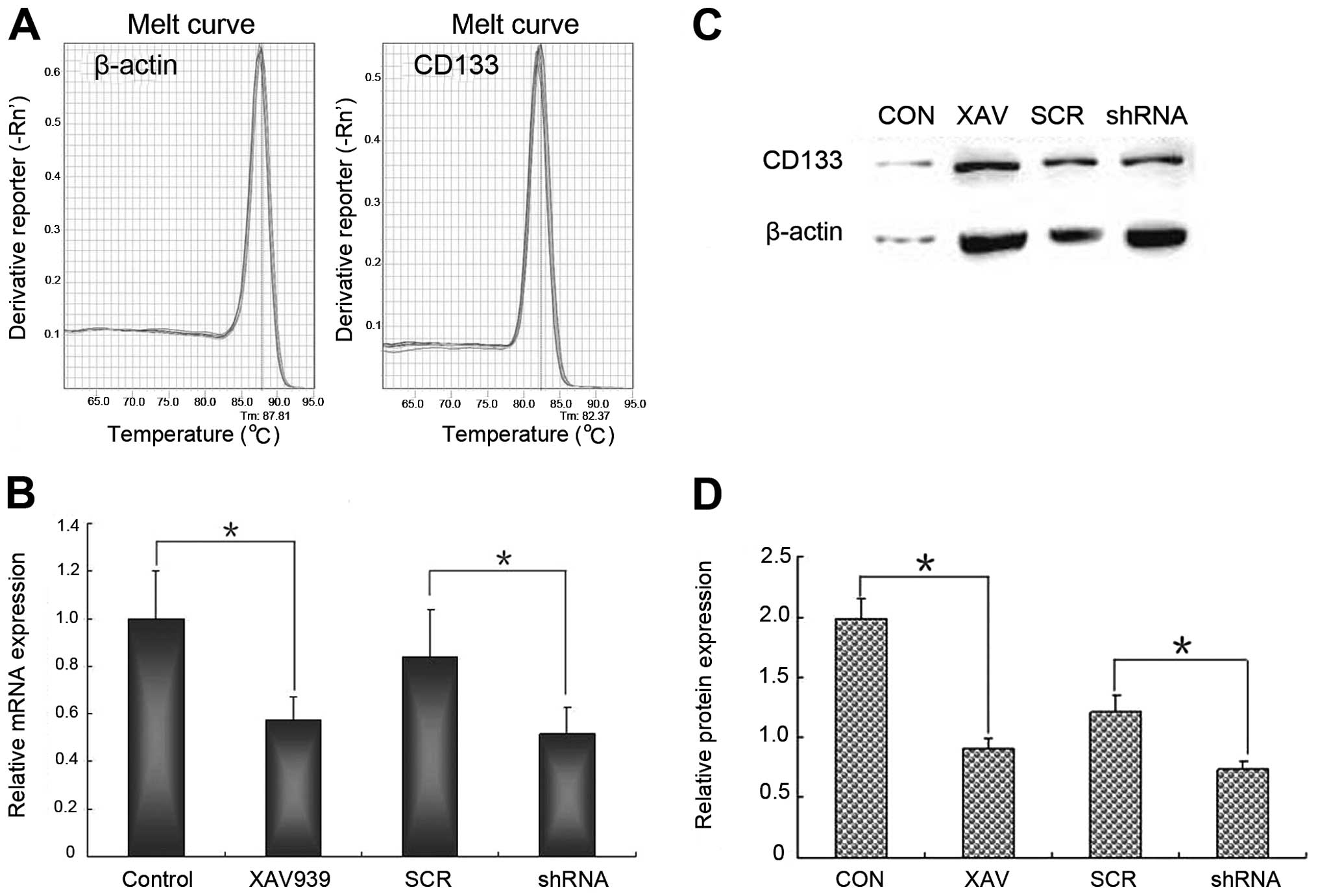
XAV939 treatment or RNAi-TNKS1 inhibits
the expression of CD133 mRNA and protein
QRT-PCR and western blot analysis were used
respectively to validate the stemness change of NB CSCs after
XAV939 treatment or RNAi-TNKS1. The results of qRT-PCR showed that
the melting curve of β-actin and CD133 was a standard single peak,
indicating specific amplification products (Fig. 4A). After XAV939 treatment or
RNAi-TNKS1, the relative expression of CD133 mRNA was 0.57±0.09 and
0.52±0.11, respectively, which were both lower than those in
control and SCR group (Fig. 4B,
P<0.05). The relative expression of CD133 protein was
significantly lower in XAV939 treatment group and RNAi-TNKS1 group
compared with control groups (Fig. 4C
and D, P<0.05). The results indicated that both XAV939
treatment and RNAi-TNKS1 repressed the stemness of CSCs to some
extent.
XAV939 treatment and RNAi-TNKS1 reduce
the subcellular localization of CD133 protein
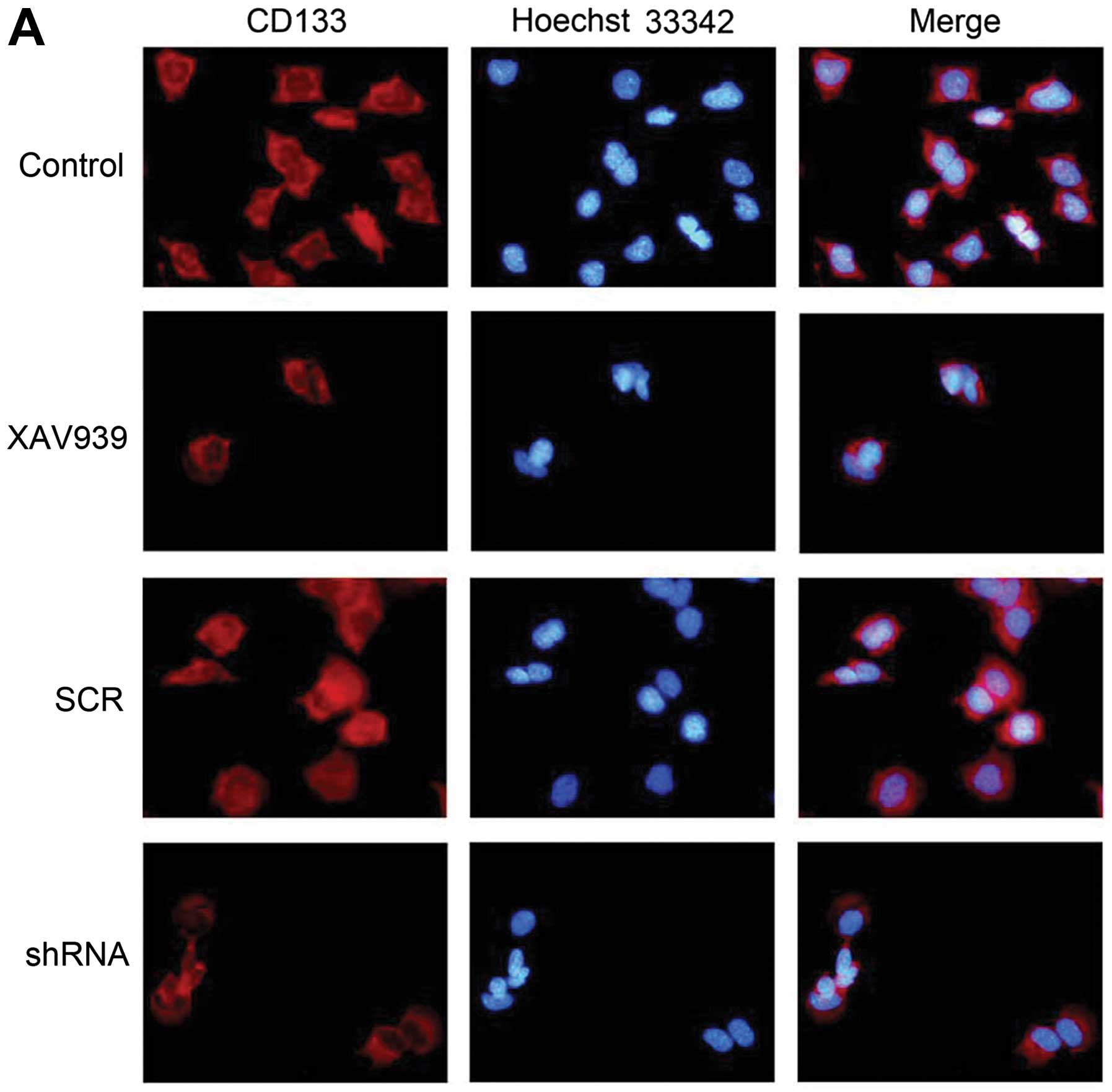
The immunofluorescence results showed that CD133
protein was expressed in the cytoplasm and membrane of NB CSCs, and
presented red fluorescence, while the nucleus of cells presented
blue fluorescence (Fig. 5A). After
XAV939 treatment or RNAi-TNKS1, the number of NB CSCs reduced and
some signs of apoptosis appeared, including the condensation and
bright stained of nuclear chromatin. The fluorescence intensity
were analyzed with image software and presented in a histogram
(Fig. 5B). The mean fluorescence
intensity of CD133 protein in control, XAV939 treatment, SCR and
shRNA group was 1±0.10, 0.21±0.06, 0.97±0.09 and 0.41±0.08,
respectively. Compared with the respective control groups, XAV939
treatment and RNAi-TNKS1 significantly decreased the expression of
CD133 protein (P<0.01). The change of mean fluorescence
intensity of Hoechst 33342 showed similar tendency to that of
CD133. The results indicated that XAV939 treatment and RNAi-TNKS1
reduced the expression of stemness of NB CSCs.
XAV939 treatment and RNAi-TNKS1 reduce
the migration of NB CSCs
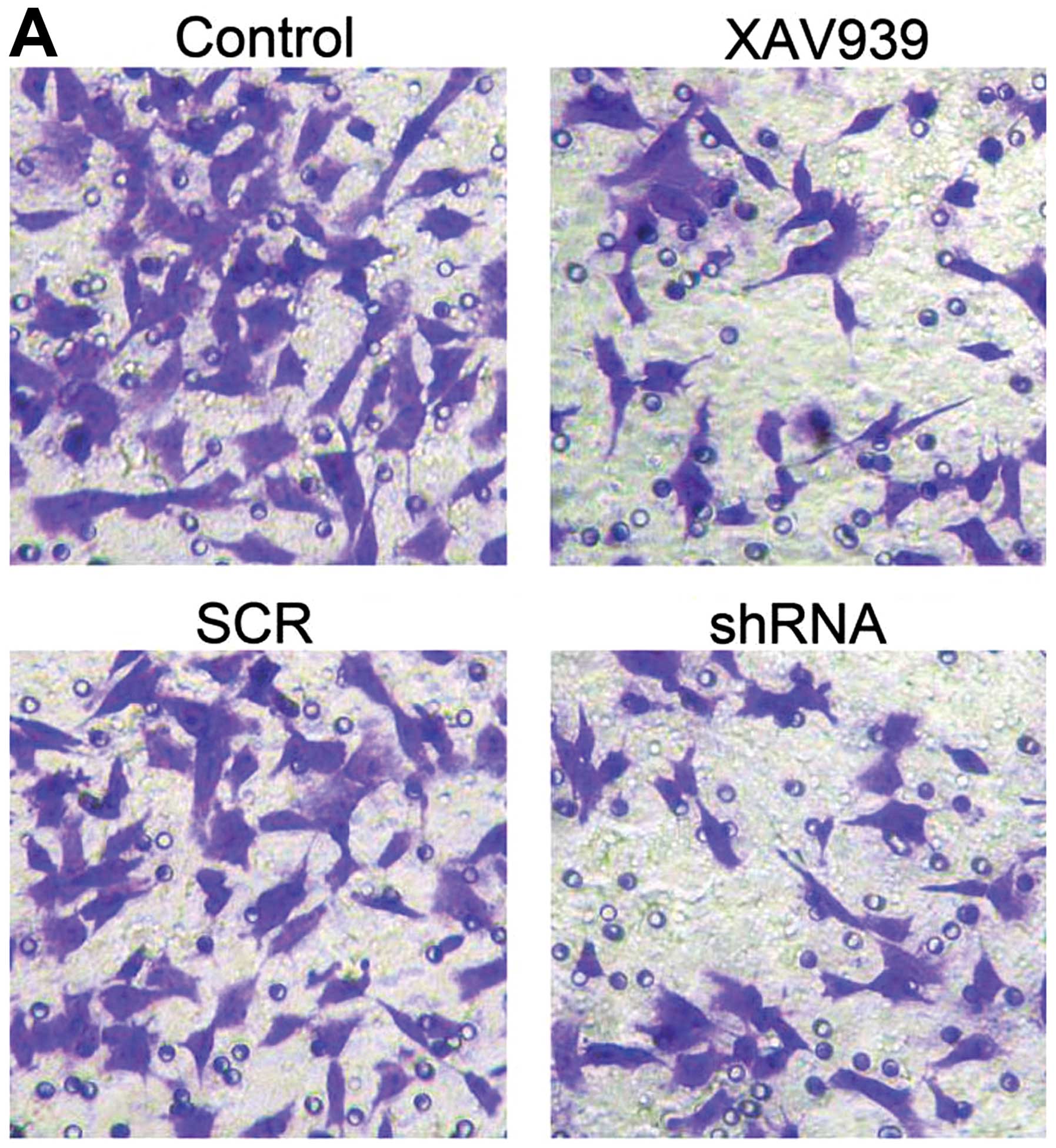
In this study, 9 high power field of view were
selected randomly, and cells migrated to the bottom of the wells
were counted. As shown in Fig. 6A,
the mean number of the migrated cells in control, XAV939 treatment,
SCR and shRNA group was 62.0±6.0, 36.7±4.5, 63.1±2.0 and 40.1±1.9,
respectively (Fig. 6B, P<0.05).
The results demonstrated that XAV939 treatment and RNAi-TNKS1 both
decreased the migration of NB CSCs.
Discussion
CSCs may play an important role in curing cancer
because CSCs have special biological characteristics, such as
self-renewal, drug resistance and tumor forming (19–22).
Traditional treatments can not effectively kill CSCs, and tumor
will recur after treatment. Therefore, the study of CSCs will
provide a new theoretical basis for curing cancer. The isolating of
CSCs is the basis and difficulty for further research because of
its very small proportion in cell lines or tumor tissues, which
accounts for 0.01–2% of the total number of cells. There are some
methods for isolating CSCs at present, such as SP method, surface
marker method, and floating sphere formation method (23–29).
It has also been reported that SH-SY5Y cells expressed CD133, but
did not form tumor spheres (30),
which was consistent with the result of our preliminary experiments
(data not shown). Therefore, we compared the advantage and
disadvantage of SP method and surface marker method for isolating
NB CSCs. The results demonstrated that SP method had universal
applicability and poor specificity, which was consistent with a
report of that not all SP cells had the characteristic of CSCs
(31). Besides, the fluorescent
dye Hoechst 33342 could bind with DNA through the membrane and had
toxic effects. Thus SP method is suitable for cells without
significant surface markers. In contrast, surface marker method had
higher specificity. Studies showed that tumor spheres obtained from
solid tumor or cell lines were positively stained with CD133, and
CD133 was considered to be the most reliable marker of brain CSCs
by far (32). Our study used the
cell surface marker CD133 method for isolating NB CSCs also in
follow-up studies.
The NB CSCs usually are enriched first because their
small proportion in SH-SY5Y cells. There are some methods for the
enrichment of CSCs, such as culturing in serum-free medium (SFM)
with specific growth factors and cytokines, chemotherapy drug
screening and long-term hypoxia (1% O2, 30 d)
intervention (30,33–35).
Since SH-SY5Y cells do not have the ability to form tumor spheres
(30) and hypoxia method takes
more time, we used etoposide treatment to enrich NB CSCs. Then
isolated NB CSCs were cultured with SFM to maintain the stemness
properties. The method is simple and low cost, and the ratio of NB
CSCs increased to nearly 2-fold. The isolated NB CSCs were further
identified with an electron microspe, and the results indicated
that NB CSCs were in immature or juvenile stage, which was in line
with the characteristics of stem cells (Fig. 3).
CD133, also known as AC133, is encoded by the PROMI
gene. A notable feature of CDl33 is that its expression will
downregulate quickly with cell differentiation (29), and make it a unique molecular
marker for isolation and identification of NB CSCs (28,29).
It has been reported that the higher the degree of malignancy, the
higher the proportion of CD133+ tumor cells are
accounted for (36). In our
previous studies, we have demonstrated that XAV939 promoted cell
apoptosis in NB cell lines in part by inhibiting the Wnt/β-catenin
signaling pathway (17). Since
CSCs have similar signaling pathways with normal stem cells, such
as Notch, and Wnt, which are closely related to the self-renewal
ability or stemness of CSCs (37),
studies have also shown that abnormal activation of the Wnt
signaling pathway can induce the transformation from stem cells to
tumor cells (38), and this
signaling pathway plays an important role in maintaining the
characteristics of CSCs (39–42).
Kim et al found that the increased expression of Wnt
inhibitory factor promoted the apoptosis of tumor cells, reduced
colony formation rate and significantly inhibited tumor growth
(43). The encoding gene of CD133
contains TCF/LEF binding region, which suggested that Wnt/β-catenin
signaling pathway might associate with the expression of CD133
(44) and CD133 might be a
downstream target gene of this pathway (45). Therefore, we speculated that XAV939
might inhibit the stemness of NB CSCs by attenuating Wnt/β-catenin
pathway and expression of CD133 via repression of TNKS1. Targeting
the key genes conferring stemness to NB CSCs can efficiently
eliminate NB CSCs, and thus may be considered to provide a new
approach to cancer therapy. In our research, the stemness and
migration ability were inhibited markedly by the repression of
TNKS1 gene. It is foreseeable that the combination of multiple
targets as a potential anticancer project will be further studied
in this direction (46).
Upon treatment with XAV939, we observed a
significant reduction in the NB CSCs marker expression. In
addition, when we knocked down the TNKS1 gene, the subpopulation
and migration of NB CSCs were reduced, suggesting a pivotal role of
TNKS1 in the maintainence and movement of NB CSCs. Moreover, our
present and previous studies showed that inhibited TNKS1 was
correlated with increased apoptosis in NB cells, increased
chemosensitivity and reduced invasiveness. Although TNKS1 may be
necessary in kidney and lung development, its overexpression in
many kinds of cancers is notable, thus make it an attractive target
for certain cancer therapies (47,48).
In conclusion, we found that surface marker CD133
was more suitable than SP method for isolating CSCs from NB cell
line, and 60 μM etoposide could be used to enrich NB CSCs.
The ultrastructure of CSCs showed its juvenescence or stemness
state. The small molecule drug XAV939 or RNAi-TNKS1 appeared to
inhibit the stemness and migration of NB CSCs via repression of
TNKS1 at different aspects evidenced by the decreased expression of
NB CSCs marker CD133 and decreased migration. The findings in the
present study provide a basis for the multiple-targeted therapy of
NB CSCs by small molecule drugs, and need to be verified in
vivo.
Acknowledgements
This study was supported by National
Natural Science Foundation of China (30772215). The authors are
grateful to the Experimental Technology Center, Department of
Developmental Biology, Department of Pharmacology and Department of
Pathophysiology in China Medical University. We deeply thank all
the people who helped us.
References
|
1.
|
Hamburger AW and Salmon SE: Primary
bioassay of human tumor stem cells. Science. 197:461–463. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
2.
|
Reya T, Morrison SJ, Clarke MF and
Weissman IL: Stem cells, cancer, and cancer stem cells. Nature.
414:105–111. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
3.
|
Huntly BJP and Gilliland DG: Leukaemia
stem cells and the evolution of cancer-stem-cell research. Nat Rev
Cancer. 5:311–321. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
4.
|
Ross RA and Spengler BA: Human
neuroblastoma stem cells. Semin Cancer Biol. 17:241–247. 2007.
View Article : Google Scholar
|
|
5.
|
Bilir A, Erguven M, Yazihan N, Aktas E,
Oktem G and Sabanci A: Enhancement of vinorelbine-induced
cytotoxicity and apoptosis by clomipramine and lithium chloride in
human neuroblastoma cancer cell line SH-SY5Y. J Neurooncol.
100:385–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6.
|
Abbott BL: ABCG2 (BCRP): a cytoprotectant
in normal and malignant stem cells. Clin Adv Hematol Oncol.
4:63–72. 2006.PubMed/NCBI
|
|
7.
|
Nan JN, Hu XG and Li HX: Research progress
in tumor stem cells: literature retrieval results based on
international database. Chin J Tiss Eng Res. 516:1085–1093.
2012.
|
|
8.
|
Huang SM, Mishina YM, Liu S, et al:
Tankyrase inhibition stabilizes axin and antagonizes Wnt signaling.
Nature. 461:614–620. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9.
|
Chen B, Dodge ME, Tang W, et al: Small
molecule-mediated disruption of Wnt-dependent signaling in tissue
regeneration and cancer. Nat Chem Biol. 5:100–107. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10.
|
Xu D, Zheng C, Bergenbrant S, Holm G,
Björkholm M, Yi Q and Gruber A: Telomerase activity in plasma cell
dyscrasias. Br J Cancer. 84:621–625. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11.
|
MacNamara B, Wang W, Chen Z, et al:
Telomerase activity in relation to pro- and anti-apoptotic protein
expression in high grade non-Hodgkin’s lymphomas. Haematologica.
86:386–393. 2001.PubMed/NCBI
|
|
12.
|
Klapper W, Krams M, Qian W, Janssen D and
Parwaresch R: Telomerase activity in B-cell non-Hodgkin lymphomas
is regulated by hTERT transcription and correlated with
telomere-binding protein expression but uncoupled from
proliferation. Br J Cancer. 89:713–719. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13.
|
Gelmini S, Poggesi M, Distante V, et al:
Tankyrase, a positive regulator of telomere elongation, is over
expressed in human breast cancer. Cancer Lett. 216:81–87. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14.
|
Gelmini S, Poggesi M, Pinzani P, et al:
Distribution of tankyrase-1 mRNA expression in colon cancer and its
prospective correlation with progression stage. Oncol Rep.
16:1261–1266. 2006.
|
|
15.
|
Gelmini S, Quattrone S, Malentacchi F, et
al: Tankyrase-1 mRNA expression in bladder cancer and paired urine
sediment: preliminary experience. Clin Chem Lab Med. 45:862–866.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16.
|
Shervington A, Patel R, Lu C, et al:
Telomerase subunits expression variation between biopsy samples and
cell lines derived from malignant glioma. Brain Res. 1134:45–52.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17.
|
Tian XH, Hou WJ, Fang Y, et al: XAV939, a
tankyrase 1 inhibitior, promotes cell apoptosis in neuroblastoma
cell lines by inhibiting Wnt/β-catenin signaling pathway. J Exp
Clin Cancer Res. 32:100–109. 2013.PubMed/NCBI
|
|
18.
|
Vangipuram SD, Buck SA and Lyman WD: Wnt
pathway activity confers chemoresistance to cancer stem-like cells
in a neuroblastoma cell line. Tumour Biol. 33:2173–2183. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19.
|
Al-Hajj M and Clarke MF: Self renewal and
solid tumor stem cells. Oncogene. 23:7274–7282. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20.
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar
|
|
21.
|
Lou H and Dean M: Targeted therapy for
cancer stem cells: the patched pathway and ABC transporters.
Oncogene. 26:1357–1360. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22.
|
Hadnagy A, Gaboury L, Beaulieu R and
Balicki D: SP analysis may be used to identify cancer stem cell
populations. Exp Cell Res. 312:3701–3710. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23.
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24.
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
25.
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26.
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumour
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
27.
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
et al: Identification and expansion of human
colon-cancer-initiating cells. Nature. 445:111–115. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
28.
|
Singh SK, Clarke ID, Terasaki M, et al:
Identification of a cancer stem cell in human brain tumors. Cancer
Res. 63:5821–5828. 2003.PubMed/NCBI
|
|
29.
|
Singh SK, Hawkins C, Clarke ID, et al:
Identification of human brain tumor initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30.
|
Mahller YY, Williams JP, Baird WH, et al:
Neuroblastoma cell lines contain pluripotent tumor initiating cells
that are susceptible to a targeted oncolytic virus. PLoS One.
4:e42352009. View Article : Google Scholar : PubMed/NCBI
|
|
31.
|
Hemmati HD, Nakano I, Lazareff JA, et al:
Cancerous stem cells can arise from pediatric brain tumors. Proc
Natl Acad Sci USA. 100:15178–15183. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32.
|
Na YR, Seok SH, Kim DJ, et al: Isolation
and characterization of spheroid cells from human malignannt
melanoma cell line WM-266-4. Tumor Biol. 30:300–309. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33.
|
Di Fiore R, Santulli A, Ferrante RD, et
al: Identification and expansion of human osteosarcoma-cancer-stem
cells by long-term 3-aminobenzamide treatment. J Cell Physiol.
219:301–313. 2009.PubMed/NCBI
|
|
34.
|
Das B, Tsuchida R, Malkin D, Koren G,
Baruchel S and Yeger H: Hypoxia enhances tumor stemness by
increasing the invasive and tumorigenic side population fraction.
Stem Cells. 26:1818–1830. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35.
|
Marzi I, D’Amico M, Biagiotti T, et al:
Purging of the neuroblastoma stem cell compartment and tumor
regression on exposure to hypoxia or cytotoxic treatment. Cancer
Res. 67:2402–2407. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36.
|
Deng YW, Fang JS, Li MC, et al:
Correlation research between cancer stem cells and the pathological
grades of neuroepithelial tumors. J Cent South Uniu (Med Sci).
31:45–51. 2006.PubMed/NCBI
|
|
37.
|
Abbott A: Cancer: the root of the problem.
Nature. 442:742–743. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
38.
|
Lindvall C, Bu W, Williams BO and Li Y:
Wnt signaling, stem cells, and the cellular origin of breast
cancer. Stem Cell Rev. 3:157–168. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39.
|
Fu JK, Wang Z and Wei S: Alterations and
its mechanisms of Wnt signal pathway in human high-matastatatic
large cell lung cancer cell line L9981 by transfecting with Nm23-H1
gene. Chin J Lung Cancer. 12:477–479. 2009.
|
|
40.
|
Nguyen DX, Chiang AC, Zhang XH, et al:
WNT/TCF signaling through LEF1 and HOXB9 mediates lung
adenocarcinoma metastasis. Cell. 138:51–62. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41.
|
Akiri G, Cherian MM, Vijayakumar S, Liu G,
Bafico A and Aaronson SA: Wnt pathway aberrations including
autocrine Wnt activation occur at high frequency in human
non-small-cell lung carcinoma. Oncogene. 28:2163–2172. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
42.
|
Sun JZ, Yang XX, Hu NY, Li X, Li FX and Li
M: Genetic variants in MMP9 and TGF2 contribute to susceptibility
to lung cancer. Chin J Cancer. 23:183–187. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43.
|
Kim J, You L, Xu Z, et al: Wnt inhibitory
factor inhibits lung cancer cell growth. J Thorac Cardiovasc Surg.
133:733–737. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44.
|
Katoh Y and Katoh M: Comparative genomics
on PROM1 gene encoding stem cell marker CD133. Int J Mol Med.
19:967–970. 2007.PubMed/NCBI
|
|
45.
|
Horst D, Kriegl L, Engel J, Jung A and
Kirchner T: CD133 and nuclear beta-catenin: the marker combination
to detect high risk cases of low stage colorectal cancer. Eur J
Cancer. 45:2034–2040. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46.
|
Ruck P, Xiao JC, Pietsch T, Von Schweinitz
D and Kaiserling E: Hepatic stem-like cells in hepatoblastoma:
expression of cytokeratin 7, albumin and oval cell associated
antigens detected by OV-1 and OV-6. Histopathology. 31:324–329.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
47.
|
Karner CM, Merkel CE, Dodge M, Ma Z, Lu J,
Chen C, Lum L and Carroll TJ: Tankyrase is necessary for canonical
Wnt signaling during kidney development. Dev Dyn. 239:2014–2023.
2010. View Article : Google Scholar
|
|
48.
|
Chung SS, Giehl N, Wu Y and Vadgama JV:
STAT3 activation in HER2-overexpressing breast cancer promotes
epithelialmesenchymal transition and cancer stem cell traits. Int J
Oncol. 44:403–411. 2014.PubMed/NCBI
|