Introduction
The human T-cell lymphotropic virus type I (HTLV1)
is a retrovirus estimated to have infected 5–10 million people
worldwide, resulting adult T-cell leukemia (ATL) in ≤5% of the
cases (1,2). Aggressive ATL can be associated with
the frequent involvement of the gastrointestinal tract as well as
meningeal or cerebral infiltration by neoplastic lymphocytes. The
extravasation of ATL cells from the blood stream to these secondary
organs is mediated by the expression of matrix metalloproteinase
(MMP) -2 and -9 that will, subsequently, degrade the subendothelial
basement membrane, hence aiding metastasis (3). In fact, these extracellular proteases
have been reported to promote the proliferation, angiogenesis and
metastatic potential of tumor cells. As such, a number of promising
strategies have been devised to repress the expression or enzymatic
activity of MMPs; however, their implementation in clinical trials
revealed meager performance (4).
Therefore, it is imperative to test alternative approaches that can
effectively target these metalloproteinases in cancer in general
and ATL specifically.
Experimental and clinical studies have revealed the
effectiveness of a specific nutrient synergy mixture composed of
ascorbic acid (AA), lysine, proline, arginine,
epigallocatechingallate (EGCG) and other micronutrient, also known
as SNS, in targeting crucial physiological mechanisms involved in
cancer progression and metastasis (5–7).
This natural assortment of nutrients, has exhibited synergistic
anticancer properties in a large number of cancer cell lines, in
part, by inhibiting MMP-2 and MMP-9 secretion (8–13).
With respect to ATL, we have previously documented that SNS induces
an antiproliferative and pro-apoptotic effect in HTLV1 infected
malignant T-cells (14,15) and inhibits the transcriptional
expression and activity of MMP-9 in these cells (Harakeh at
al, unpublished data). However, to our knowledge, there has not
been any study on the effect of SNS on MMP-2 expression and
activity in ATL.
AA, a major constituent of SNS, has been integrated
into the formulation based primarily on its own established
cytotoxic and anti-metastatic actions against a number of cancer
cell lines (16), including HTLV1
infected malignant T-cells (17)
and inhibited MMP-9 expression and activity (unpublished data).
Indeed, high dose ascorbate has been indicated to impede cancer
growth via the prevention of cancer cell invasion (18) which is supported by the fact that
AA is required for collagen synthesis that increases the stability
of the extracellular matrix (ECM) (19). In addition to its anticancer
effects, AA has been reported to harbor an effective activity
against both RNA and DNA viruses (20). EGCG, which is the one of the most
abundant catechins that are responsible for the antioxidant
properties of green tea, is another major constituent of SNS.
Previous study has indicated that it possesses pro-apoptotic and
anti-proliferative effects on ATL cancer cells (21), and also acts to inhibit the
invasiveness of cells by negating the activity of MMP-9 (22).
In light of the above, and since metastasis is the
chief cause of death of patients suffering from malignant tumors
(23) and the existing standard
treatment against aggressive ATL continues to be inadequate
(24), the aim of this study was
to investigate the respective differential effects of the nutrient
mixture SNS and two of its main components AA and EGCG on the
activity of MMP-2 as well as its expression at the transcriptional
and translational levels in both HTLV-I-infected and non-infected
malignant T-cells, so as to determine the potency of each of these
nutrients on the invasive potential of ATL cells.
Materials and methods
Cell lines
Two HTLV-1-positive (HuT-102 and C91-PL) and
-negative (CEM and Jurkat) ATL cell lines were used. The cells were
maintained in RPMI-1640 media with 25 mM of Hepes supplemented with
10% fetal bovine serum with 100 μg/ml of streptomycin and 100 U/ml
of penicillin in 37°C and 5% CO2. Cells were split every
two days at a cell:media ratio of 1:4.
Compounds
SNS
The specific nutrient synergic mixture was obtained
from Dr. Rath Research Institute and was dissolved in RPMI-1640
media in stock solutions of 33.3 mg/ml. The solution of 1 mg/ml of
SNY contains 900 μM of ascorbate, 1.1 mM of lysine, 1.1 mM of
proline, 500 μM of arginine, 250 μM of N-acetylcysteine, 150 μM of
EGCG, 85 μM of selenium, 7 μM of copper and 4 μM of manganese and 4
μM calcium.
EGCG
EGCG was obtained from Sigma (St. Louis, MO, USA) in
powder form. EGCG (50 mg) was dissolved in 5 ml of RPMI-1640 medium
and pH of solution was maintained at 7.0.
Ascorbic acid
Ascorbic acid was obtained from Sigma, in powder
form and was dissolved in RPMI-1640 medium as to prepare a 10 mg/ml
stock solution. The pH was adjusted to 7.0.
All stock solutions were filter sterilized using a
0.22-μm filter, aliquoted into Eppendorf tubes and stored at −20°C
until the day of the experiment. For the experiments, aliquots were
thawed on the day of the experiment and used for one experiment
only.
Zymography for MMP-2 activity
Cells were plated at density of 1×105
cells/ml of RPMI-1640 media with 10% FBS in 25 cm2
flasks. They were treated with various concentrations of the test
compound for three days and then starved and treated with various
amounts of the test compound for 24 h. The cells were then
centrifuged and the supernatant collected and concentrated 10-fold.
Appropriate amounts of the supernatant were loaded on 10%
acrylamide gels with 10% gelatin and run at 90 V and zymography was
conducted as described previously (22).
ELISA for MMP-2 secretion
MMP-2 released from cells were determined by ELISA
using MMP-2 detection kits (Roche, Mannheim, Germany). Supernatant
from cells grown under different conditions was diluted with
appropriate amounts of assay buffer supplied with the kit and
experiments were performed according to the manufacturer’s
instructions.
Western blotting for detection of the
MMP-2 translational level
Proteins were extracted from cells treated with
various concentrations of the test compound and kept at −70°C. The
proteins were quantified and then appropriate amounts of proteins
were run on 10% acrylamide gel at 90 V and then blotted onto a
polyvinyl difluoride (PVDF) membrane electrically at 30 V
overnight. The membrane was probed with primary antibodies for
MMP-2 (Santa Cruz Biotechnology). A secondary antibody linked to
horseradish peroxidase specific to the primary was used and then
the reaction was initiated using a chemiluminescence system. Bands
were visualized on an X-ray film developed using Xomat. Equal
loading was ensured using an antibody specific for GAPDH (Santa
Cruz Biotechnology).
RT-PCR for MMP-2 transcriptional level
detection
After treatment with different test compounds, cells
were collected and stored at −70°C. Total RNA was extracted from
the cells using NucleoSpin RNA II kit (Macherey, Nagel). Then, 2 μg
of mRNA were reverse transcribed into first strand cDNA using
RT-PCR kit; Reddy Mix Version (Abgene, Promega). PCR was conducted
in 50 μl volume using oligonucleotide primers for MMP-2 (S:
5′-GTGCTGAAGGACACACTAAAGAAGA-3′; AS:
5′-TTGCCATCCTTCTCAAAGTTGTAGG-3′) and ribosomal protein (S:
5′-GTTCACCAAGGAGGACCTCA-3′; AS: 5′-CAC-ATTAGGCAGAGGTGTCT-3′) as
described previously (22).
Results
Effects of the test compounds on MMP-2
activity
In order to check the effect of EGCG, AA and SNS
treatment on cellular invasion potential, zymography was conducted
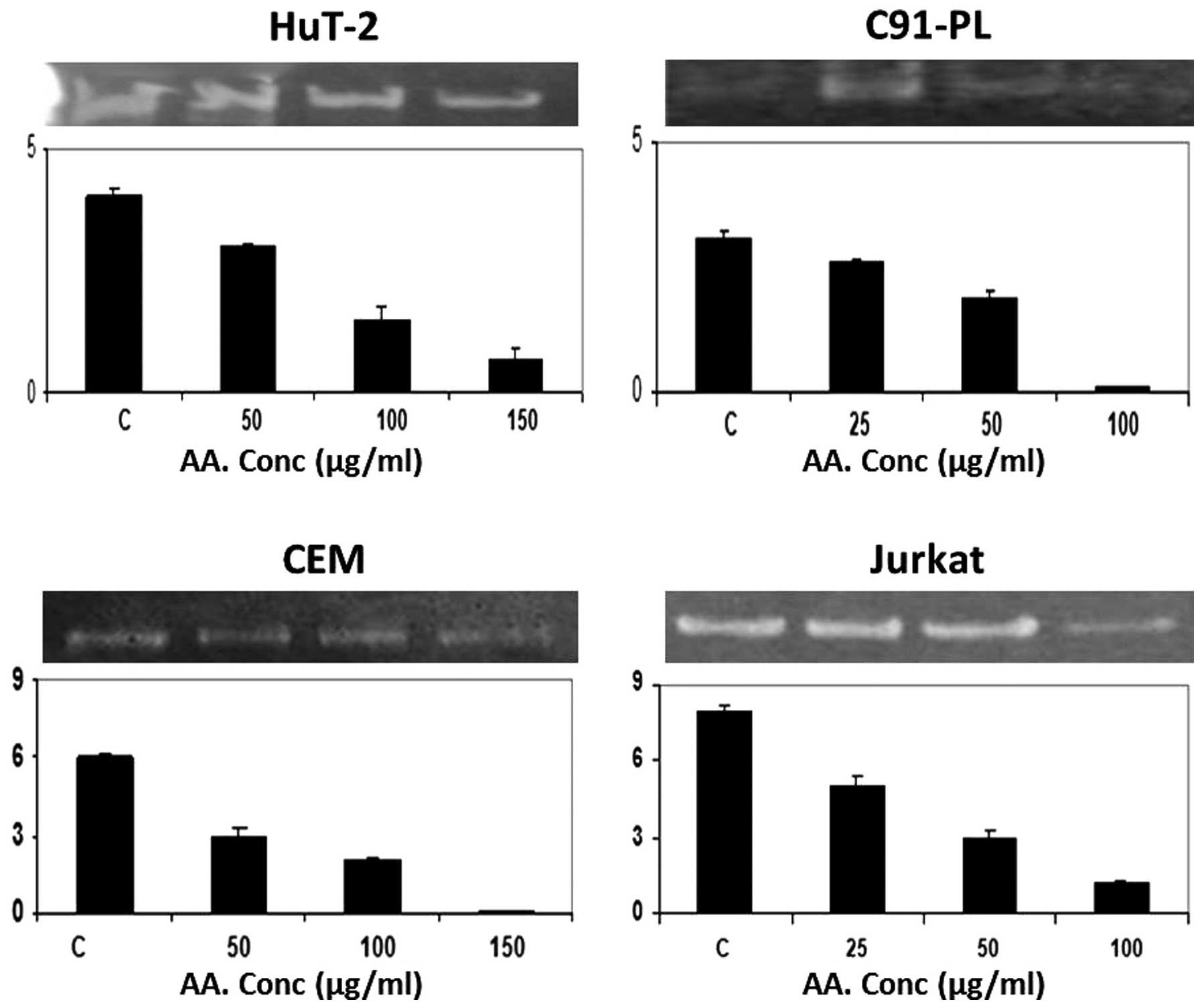
by measuring the gelatinolytic activity of MMP-2. Starting with AA,
it had an inhibitory effect on this activity only at the maximum
concentrations tested, 150 μM for the Hut-102 and CEM cell lines
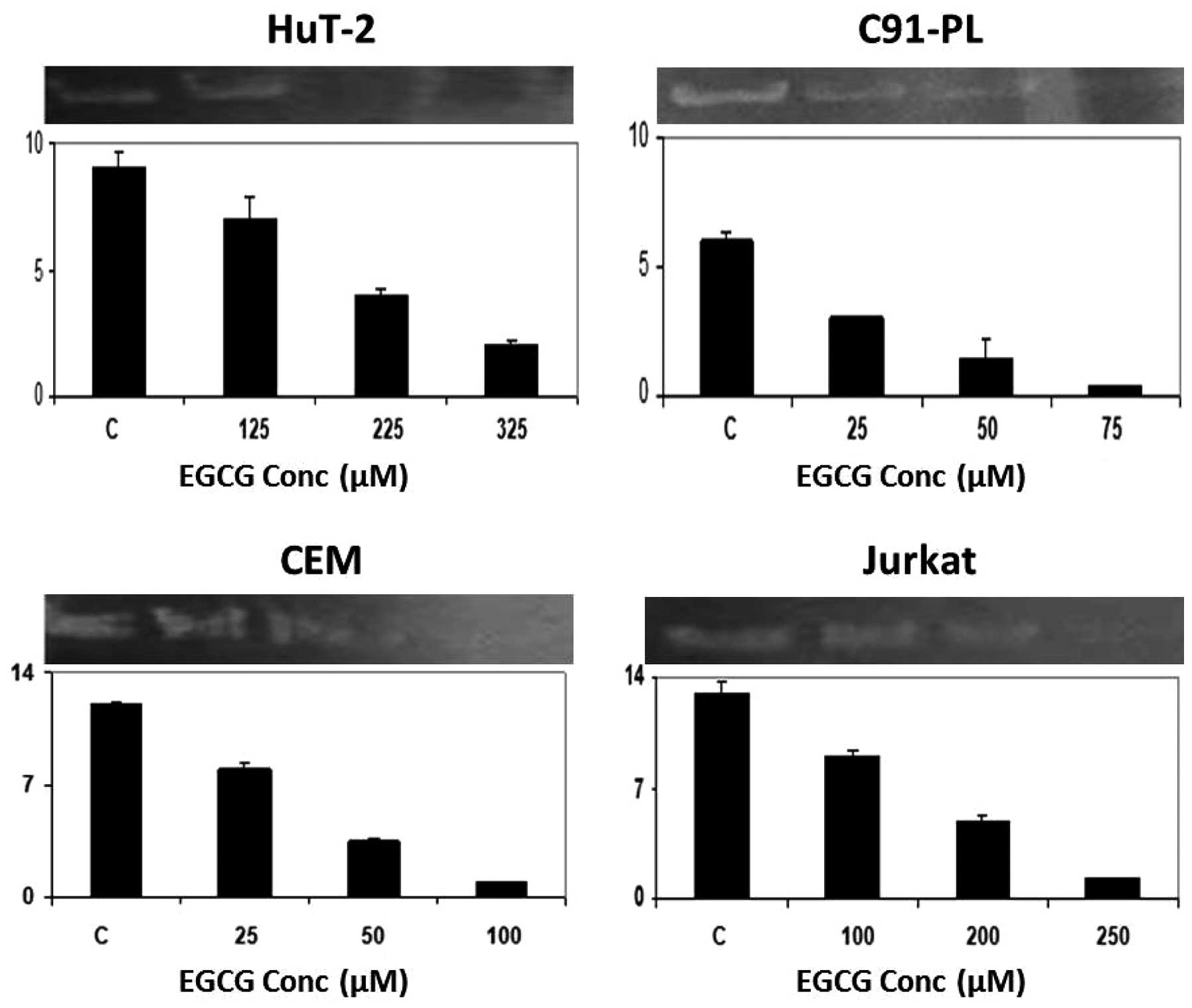
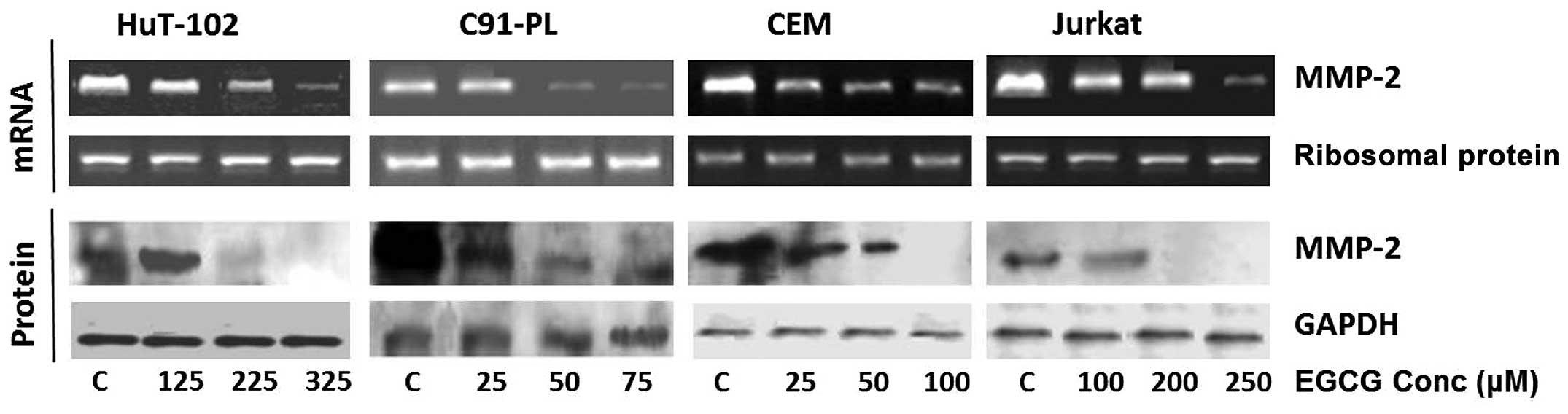
and 100 μM for the C91-PL and Jurkat cells (Fig. 1). On the other hand, EGCG induced a
dose-dependent reduction in the activity of MMP-2 in all cell
lines, which culminated in its total inhibition at 225 μM for
Hut-102; while total inhibition occurred at maximum concentrations
for the C91-PL (75 μM), CEM (100 μM) and Jurkat (250 μM) cell lines
(Fig. 3). Finally, with respect to
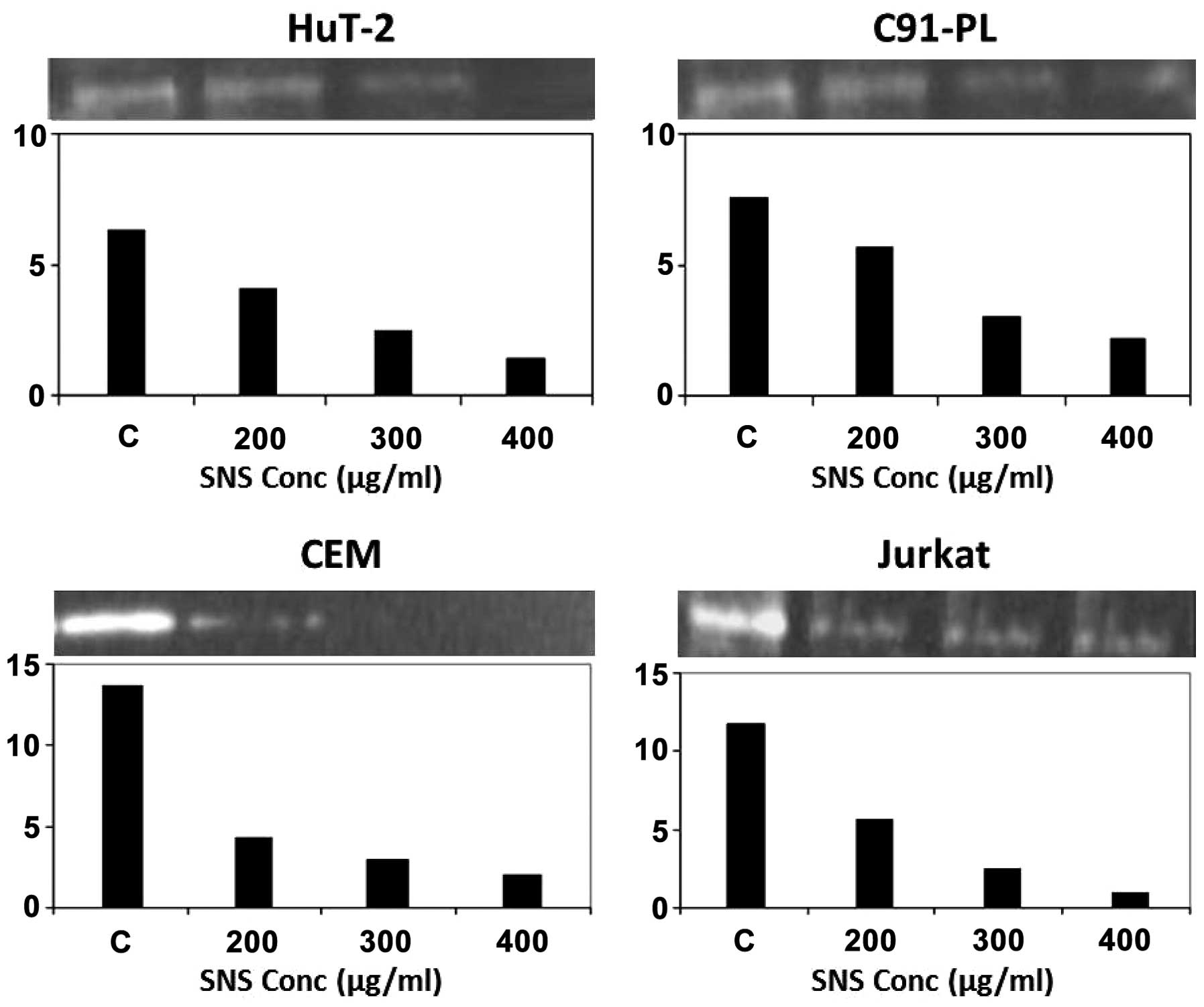
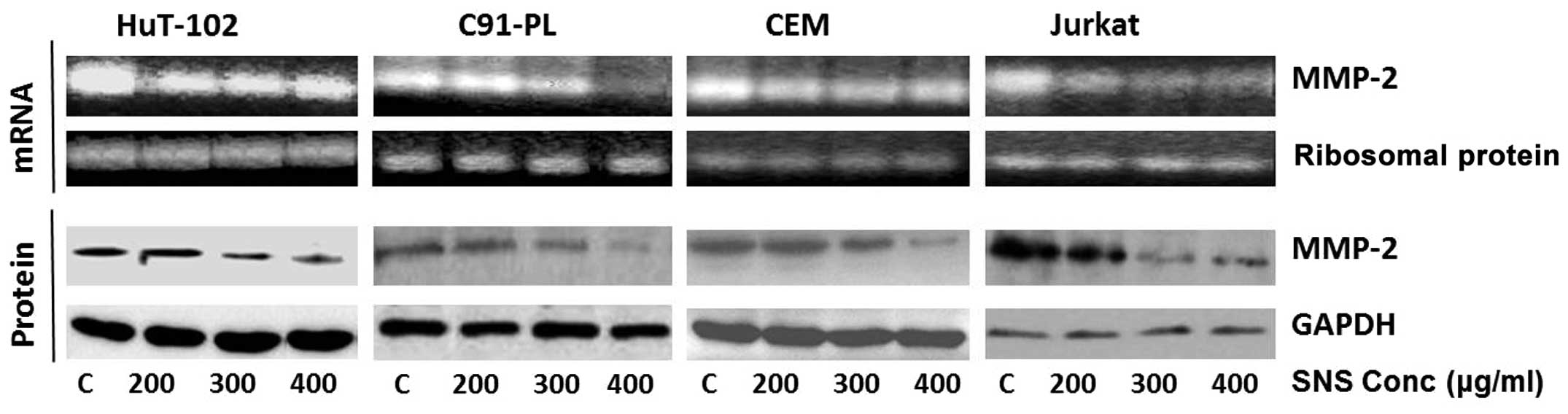
the effect of SNS, it induced total inhibition of MMP-2 activity in
the CEM cells at 300 μM, while promoting a dose-dependent decrease
in this activity in the remaining three cell lines which culminated
in total inhibition only in the Hut-102 cells at the maximum
concentration used (400 μM) (Fig.
5).
Effects of the test compounds on MMP-2
secretion
To further confirm the effect of the test compounds
on MMP-2 secretion quantitatively, ELISA was performed on the
supernatant of cells grown on serum-free media. As illustrated, the
inhibitory effects of SNS, EGCG and AA on the secretion of MMP-2
seem to be, in general, more pronounced in the non-infected
malignant T-cells (CEM and Jurkat) than the infected malignant
T-cells (HuT-102 and C91-PL) (Figs.
1, 3 and 5, histograms). The highest fold decrease
induced by AA was in the CEM cells (Fig. 1) while that for EGCG (Fig. 3) and SNS (Fig. 5) was in the Jurkat cells.
Nonetheless, both HuT-102 and C91-PL cells exhibited a ~4-fold
decrease in the level of secreted MMP-2 after a 96-h treatment with
EGCG (Fig. 3) and SNS (Fig. 5), respectively. Moreover, AA was
the most potent compound in the latter cell line (Fig. 1).
Effect of test compounds on the
transcriptional level of MMP-2
For evaluating the effect of each of EGCG, AA, and
SNS on the transcriptional level of MMP-2, RT-PCR was conducted on
the mRNA isolated from treated cells (Figs. 2, 4 and 6).
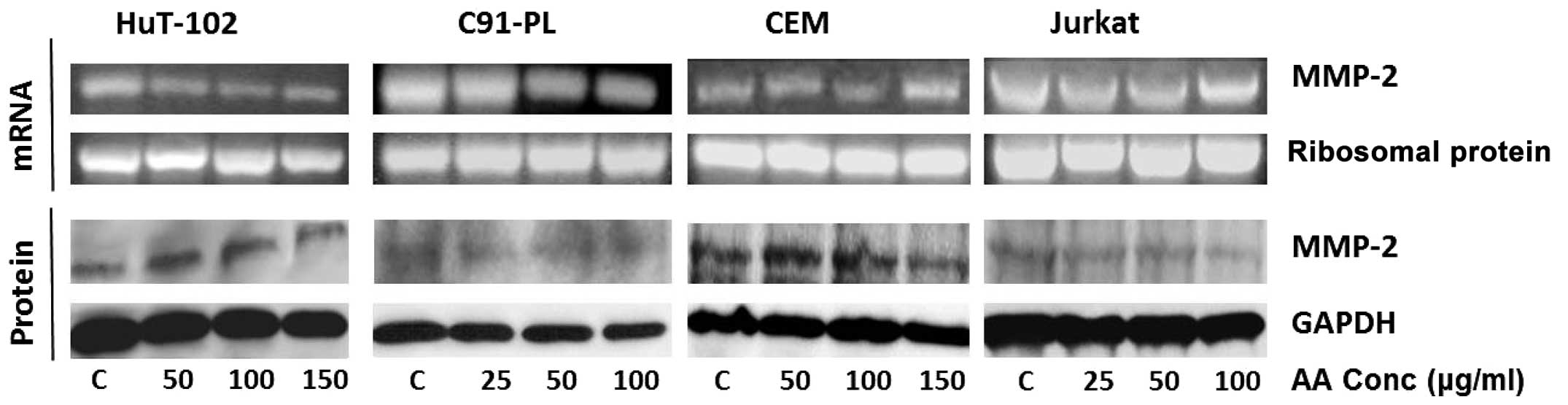
As can be seen, AA had no effect on MMP-2 mRNA levels in any of the
tested cell lines (Fig. 2), while
EGCG reduced the mRNA levels of MMP-2 in all four cell lines in a
dose-dependent manner culminating in almost total inhibition at
maximum concentrations in the HTLV1 infected cells (325 μM for the
Hut-102 cells and 75 μM for the C91-PL cells) (Fig. 4). As for SNS, it caused a reduction
in the activity at the lowest concentration used (200 μM) in the
CEM and Hut-102 cell lines that was not altered by the increase in
tested concentration, while inducing a dose-dependent decrease that
ended with almost total inhibition in MMP-2 mRNA levels in the
C91-PL and Jurkat cells at the maximum concentration (400 μM)
(Fig. 6). Equal loading was
ensured using ribosomal protein.
Effect of test compounds on translational
levels of MMP-2 and MMP-9
In order to investigate the effect of the test
compounds. The translational levels of MMP-2 were measured by
western blotting (Figs. 2,
4 and 6). AA did not show any significant effect
on MMP-2 protein level of the HTLV1-positive cells (HuT-102 and
C91-PL), however, it acted at maximum concentrations to reduce
MMP-2 levels in the HTLV1-negative cells (150 μM for CEM and 100 μM
for Jurkat) (Fig. 2). On the other
hand, the MMP-2 protein levels were totally inhibited by EGCG
starting from 225 and 200 μM in the Hut-102 and Jurkat cells
respectively, while decreasing dose-dependently inhibition in the
other two cell lines that concluded with total loss of MMP-2
protein at maximum concentrations (75 μM for the C91-PL and 250 μM
for the CEM) (Fig. 4). Finally,
SNS reduced the protein levels of MMP-2 in all four cell lines in a
dose-dependent manner culminating in almost total inhibition at
maximum concentration (400 μM) in the C91-PL and CEM cell lines
(Fig. 6).
Discussion
The majority of cancer-related deaths come as a
result of the development of secondary tumors at distant sites
which occurs upon the dissemination of the metastatic tumor cells
(25). This process relies on the
crossing of the endothelial basement membrane which is promoted by
the overexpression of MMPs, especially MMP-2 and MMP-9 (4). In patients suffering from ATL cell
infiltration, a significant increase in plasma MMP-9 was observed
(26). Moreover, MMP-2 expression
has been shown to be correlated with multi-organ extramedullary
infiltration in adult acute lymphoblastic leukemia (ALL) (27). These MMPs have been dubbed as
potential prognostic and diagnostic biomarkers in various cancer
types and stages and have therefore been targeted by a wide variety
of inhibitors, only to be met with failure in clinical trials
(4). We have previously reported
that each of AA, EGCG and SNS induce apoptosis and cell cycle
arrest in vitro in both HTLV-1 infected and non-infected
malignant T-lymphocyte cell lines (14,15,17,21)
and dose-dependently inhibit MMP-9 expression and activity in
HTLV-1-positive cells (22)
(Harakeh et al, unpublished data). In the current study, we
further investigated the potential of these natural compounds to
inhibit MMP-2 activity as well as its mRNA and protein expression
levels in four malignant T-lymphocytic cell lines, of which two
were infected with HTLV-1.
According to our results, it seems that while each
of AA, EGCG and SNS significantly and dose-dependently inhibited
the level of secreted MMP-2, their inhibitory effects on MMP-2
activity, transcriptional and translational levels as they pertain
to the different malignant T-cell lines used, were quite
different.
AA is unique among other vitamins in that it is
implicated in ECM formation and thus it increases ECM strength and
can block cancer spread (28). In
addition to its effects on HIV replication (29–33),
and its anticancer effects (17),
AA has shown inhibitory effects on matrix metalloproteinases
(34,35). In our study, ascorbic acid was only
effective against the MMP-2 gelatinolytic activity at the highest
dose applied in each of the four malignant cell lines and this
effect was independent of transcription and, at least in the case
of the HTLV-1-negative cell lines, might be related to the AA
inhibitory effect on translation. This is in accordance with
previous research which showed that a derivative of AA,
phospho-ascorbyl palmitate, had an antimetastatic effect on
fibrosarcoma and melanoma cell lines by inhibiting the production
and enzymatic activity of matrix metalloproteinases (MMP-2 and
MMP-9) (34). It was also shown by
Nagao et al that it takes repeated supplementation of
L-ascorbic acid to inhibit tumor invasion by inhibiting the
production of MMPs and cell motility (35), which would explain the high dose of
AA required to induce an effect in our tested cell lines. While the
mechanism behind MMP-2 expression is mostly unknown (36), the functional activity of MMPs is
known to be detained by tissue inhibitors of metalloproteinases
(TIMPs) and to be impacted by reactive oxygen species (ROS), where
excess production of ROS, in association with the myeloperoxidase
enzyme, would eventually inactivate MMPs (37). Ascorbate has been shown to promote
H2O2-mediated cell death (38,39)
and a combination therapy of AA and vitamin K significantly
decreased both the activity and protein expression of MMP-2 and
MMP-9 while increasing the protein expression of TIMP-1 and TIMP-2
(40). As such, the high dose of
AA possibly decreased the enzymatic activity of MMP-2 through the
production of large amounts of ROS or via an inductive effect on
TIMPs.
EGCG seemed to strongly inhibit the MMP-2 activity
as well as its mRNA and protein expression levels that culminated
with their total inhibition at various concentrations in the four
cell lines. Moreover, it is quite apparent that EGCG was more
potent, on every level, than AA which only resulted in significant
inhibition at the highest tested concentrations. The inhibitory
effect of EGCG on the activity of MMP-2 was previously documented
to occur in multiple cancers (41–45).
One study found green tea polyphenols to be more effective than
other MMP inhibitors in counteracting the activity of MMP-2 and
MMP-9 in glioblastomas and pituitary tumors (46). MMP-2 is involved in various
physiological processes via its degrading action on both
extracellular and non-extracellular matrix components which endows
it with a potential to cause tissue damage. The MMP-2 activity is
tightly regulated at multiple levels including transcription,
compartmentalization, pro-enzyme activation, and inhibition of the
active enzyme by extracellular inhibitors (47). Unlike most MMPs, proMMP-2 is
activated at the cell surface via a distinctive multistep mechanism
that involves MT-MMPs, which act as physiological activators of
MMP-2; however like other MMPs, its activity is tightly modulated
by TIMPs (48). EGCG was shown to
obstruct the activity of MMP-2 and its activation from the proMMP-2
zymogen form in various human brain tumors (46). The inhibitory effect of this green
tea extract on the activity of MMP-2 can be via its direct binding
to this protease or through its indirect effect on MT1-MMP and TIMP
(49,50). The production of MMP-2 in cancer
cells has been shown to be regulated by both p38, JNK and PI3K
(51). Treatment with EGCG
decreased MMP-2 mRNA expression through the abrogation of the
signaling in the highly invasive CL1-5 lung cancer cells (52), DU145 human prostate carcinoma cells
(53), pancreatic carcinoma
(54) and human bladder cancer
cells (55).
Finally, with respect to SNS, it showed a variable
profile since it dose-dependently inhibited MMP-2 gelatinolytic
activity and protein expression levels in all four cell lines, its
inhibitory effect on the mRNA in the Hut-102 and CEM cell lines was
slight and unaltered by increasing the dose of SNS while being
dose-dependent in the C91-PL and Jurkat cells. Therefore, the
effect of SNS was independent of infectivity with HTLV-1 and was
solely dependent on the cell line in question. Moreover, SNS was
more effective than either AA or EGCG on the inhibition of MMP-2.
In agreement with our results, SNS has been reported to inhibit the
expression of MMP-2 and MMP-9 in a number of cancer cell lines
(8–13). The inhibition of MMP transcription,
translation and activity by SNS may be due to the synergistic
effect of EGCG and AA as well as some of its other constituents
[magnesium, copper, N-acetylcysteine (NAC) and selenium]. In fact,
the supplementation of cultured rat cardiac fibroblasts with a
range of extracellular magnesium concentrations resulted in a
dose-dependent decrease in MMP-2 production (56) while copper deficiency in acute lung
injury was associated with increased pulmonary MMP-2 and MMP-9
activity (57). Moreover, the
treatment of intestinal myofibroblasts isolated from patients
suffering from Crohn’s disease with NAC decreased MMP-2 secretion
and restored its activity to physiological value (58). NAC was also shown to decrease the
expression and activity of MMP-2 in hepatic fibrosis of bile duct
ligated rats while normalizing the expression of TIMP-2 (59). Similarly, monomethyl selenium
inhibited MMP-2 expression in vascular endothelial cells (60) and selenium downregulated the gene
expression of MMP-2 in human malignant brain tumor cells in
vitro (61). Note that the
same specific nutrient synergy exhibited anti-angiogenic,
anti-metastatic, and anti-invasion roles both in vitro and
in vivo and was proven to be non-toxic even at very high
doses (7).
The present results show for the first time the
variability in the effects of AA, EGCG and SNS on MMP-2 activity as
well as its mRNA and protein expression levels in HTLV-1-infected
and non-infected malignant T-cells. It seems that while AA was able
to modulate the activity to a certain extent, on the protein
expression levels of MMP-2, it was outdone by both EGCG and SNS
which quite potently inhibited MMP-2 at every level. Therefore,
these nutrients should be considered as a supplementary, economical
and safe approach in the treatment of ATL.
References
|
1
|
Cook LB, Melamed A, Niederer H, Valganon
M, Laydon D, Foroni L, Taylor GP, Matsuoka M and Bangham CR: The
role of HTLV-1 clonality, proviral structure and genomic
integration site in adult T cell leukemia/lymphoma. Blood.
123:3925–3931. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Araujo TH, Barreto FK, Luiz Carlos A
Júnior and Miranda AC: Inferences about the global scenario of
human T-cell lymphotropic virus type 1 infection using data mining
of viral sequences. Mem Inst Oswaldo Cruz. 0:21–24. 2014.PubMed/NCBI
|
|
3
|
Bazarbachi A, Abou Merhi R, Gessain A,
Talhouk R, El-Khoury H, Nasr R, Gout O, Sulahian R, Homaidan F, de
Thé H, Hermine O and El-Sabban ME: Human T-cell lymphotropic virus
type I-infected cells extravasate through the endothelial barrier
by a local angiogenesis-like mechanism. Cancer Res. 64:2039–2046.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Gialeli C, Theocharis AD and Karamanos NK:
Roles of matrix metalloproteinases in cancer progression and their
pharmacological targeting. FEBS J. 278:16–27. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roomi MW, Kalinovsky T, Roomi NM, Cha J,
Rath M and Niedzwiecki A: In vitro and in vivo
effects of a nutrient mixture on breast cancer progression. Int J
Oncol. 44:1933–1944. 2014.
|
|
6
|
Roomi MW, Kalinovsky T, Roomi NW,
Niedzwiecki A and Rath M: Suppression of metastasis of
intratesticular inoculation of B16FO melanoma cells by a novel
nutrient mixture in male athymic nude mice. Exp Ther Med.
4:775–780. 2012.PubMed/NCBI
|
|
7
|
Niedzwiecki A, Roomi MW, Kalinovsky T and
Rath M: Micronutrient synergy - a new tool in effective control of
metastasis and other key mechanisms of cancer. Cancer Metastasis
Rev. 29:529–542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Roomi MW, Kalinovsky T, Niedzwiecki A and
Rath M: Modulation of uPA, MMPs and their inhibitors by a novel
nutrient mixture in human glioblastoma cell lines. Int J Oncol.
45:887–894. 2014.PubMed/NCBI
|
|
9
|
Roomi MW, Kalinovsky T, Rath M and
Niedzwiecki A: Effect of a nutrient mixture on matrix
metalloproteinase-9 dimers in various human cancer cell lines. Int
J Oncol. 44:986–992. 2014.PubMed/NCBI
|
|
10
|
Roomi MW, Kalinovsky T, Niedzwiecki A and
Rath M: Modulation of u-PA, MMPs and their inhibitors by a novel
nutrient mixture in pediatric human sarcoma cell lines. Int J
Oncol. 43:1027–1035. 2013.
|
|
11
|
Roomi MW, Kalinovsky T, Niedzwiecki A and
Rath M: Modulation of u-PA, MMPs and their inhibitors by a novel
nutrient mixture in adult human sarcoma cell lines. Int J Oncol.
43:39–49. 2013.
|
|
12
|
Roomi MW, Kalinovsky T, Niedzwiecki A and
Rath M: Modulation of u-PA, MMPs and their inhibitors by a novel
nutrient mixture in human lung cancer and mesothelioma cell lines.
Int J Oncol. 42:1883–1889. 2013.
|
|
13
|
Roomi MW, Monterrey JC, Kalinovsky T, Rath
M and Niedzwiecki A: Inhibition of invasion and MMPs by a nutrient
mixture in human cancer cell lines: a correlation study. Exp Oncol.
32:243–248. 2010.PubMed/NCBI
|
|
14
|
Harakeh S, Abdel-Massih RM, Gil PR,
Sperling RA, Meinhardt A, Niedwiecki A, Rath M, Parak WJ and
Baydoun E: The effect of PEG-coated gold nanoparticles on the
anti-proliferative potential of Specific Nutrient Synergy.
Nanotoxicology. 4:177–185. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harakeh S, Diab-Assaf M, Niedzwiecki A,
Khalife J, Abu-El-Ardat K and Rath M: Apoptosis induction by Epican
Forte in HTLV-1 positive and negative malignant T-cells. Leuk Res.
30:869–881. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Cha J, Roomi MW, Ivanov V, Kalinovsky T,
Niedzwiecki A and Rath M: Ascorbate supplementation inhibits growth
and metastasis of B16FO melanoma and 4T1 breast cancer cells in
vitamin C-deficient mice. Int J Oncol. 42:55–64. 2013.PubMed/NCBI
|
|
17
|
Harakeh S, Diab-Assaf M, Khalife JC,
Abu-El-Ardat KA, Baydoun E, Niedzwiecki A, El-Sabban ME and Rath M:
Ascorbic acid induces apoptosis in adult T-cell leukemia.
Anticancer Res. 27:289–298. 2007.PubMed/NCBI
|
|
18
|
Cameron E, Pauling L and Leibovitz B:
Ascorbic acid and cancer: a review. Cancer Res. 39:663–681.
1979.
|
|
19
|
Li Y and Schellhorn HE: New developments
and novel therapeutic perspectives for vitamin C. J Nutr.
137:2171–2184. 2007.PubMed/NCBI
|
|
20
|
Jariwalla RJ and Harakeh S: Antiviral and
immunomodulatory activities of ascorbic acid. Subcell Biochem.
25:213–231. 1996.PubMed/NCBI
|
|
21
|
Harakeh S, Abu-El-Ardat K, Diab-Assaf M,
Niedzwiecki A, El-Sabban M and Rath M: Epigallocatechin-3-gallate
induces apoptosis and cell cycle arrest in HTLV-1-positive and
-negative leukemia cells. Med Oncol. 25:30–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harakeh S, Diab-Assaf M, Azar R, Hassan
HM, Tayeb S, Abou-El-Ardat K, Damanhouri GA, Qadri I, Abuzenadah A,
Chaudhary A, Kumosani T, Niedzwiecki A, Rath M, Yacoub H, Azhar E
and Barbour E: Epigallocatechin-3-gallate inhibits tax-dependent
activation of nuclear factor kappa B and of matrix
metalloproteinase 9 in human T-cell lymphotropic virus-1 positive
leukemia cells. Asian Pac J Cancer Prev. 15:1219–1225. 2014.
View Article : Google Scholar
|
|
23
|
Wu KC, Yang ST, Hsia TC, Yang JS, Chiou
SM, Lu CC, Wu RS and Chung JG: Suppression of cell invasion and
migration by propofol are involved in down-regulating matrix
metalloproteinase-2 and p38 MAPK signaling in A549 human lung
adenocarcinoma epithelial cells. Anticancer Res. 32:4833–4842.
2012.PubMed/NCBI
|
|
24
|
Uozumi K: Treatment of adult T-cell
leukemia. J Clin Exp Hematop. 50:9–25. 2010. View Article : Google Scholar
|
|
25
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nakachi S, Nakazato T, Ishikawa C, Kimura
R, Mann DA, Senba M, Masuzaki H and Mori N: Human T-cell leukemia
virus type 1 Tax transactivates the matrix metalloproteinase 7 gene
via JunD/AP-1 signaling. Biochim Biophys Acta. 1813:731–741. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Devy J, Ouchani F, Oudot C, Helesbeux JJ,
Vanquelef E, Salesse S, Rabenoelina F, Al-Khara S, Letinois I,
Duval O, Martiny L and Charpentier E: The anti-invasive activity of
synthetic alkaloid ethoxyfagaronine on L1210 leukemia cells is
mediated by down-regulation of plasminogen activators and MT1-MMP
expression and activity. Invest New Drugs. 29:730–741. 2011.
View Article : Google Scholar
|
|
28
|
Rath M and Pauling L: Plasmin-induced
proteolysis and the role of apoprotein(a), lysine and synthetic
analogs. Orthomol Med. 7:17–23. 1992.
|
|
29
|
Harakeh S, Jariwalla RJ and Pauling L:
Suppression of human immunodeficiency virus replication by
ascorbate in chronically and acutely infected cells. Proc Natl Acad
Sci USA. 87:7245–7249. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Harakeh S and Jariwalla RJ: Comparative
study of the anti-HIV activities of ascorbate and thiol-containing
reducing agents in chronically HIV-infected cells. Am J Clin Nutr.
54:S1231–S1235. 1991.PubMed/NCBI
|
|
31
|
Harakeh S, Niedzwiecki A and Jariwalla RJ:
Mechanistic aspects of ascorbate inhibition of human
immunodeficiency virus. Chem Biol Interact. 91:207–215. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harakeh S and Jariwalla RJ: Ascorbate
effect on cytokine stimulation of HIV production. Nutrition.
11:684–687. 1995.PubMed/NCBI
|
|
33
|
Harakeh S and Jariwalla RJ: NF-kappa
B-independent suppression of HIV expression by ascorbic acid. AIDS
Res Hum Retroviruses. 13:235–239. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Liu JW, Nagao N, Kageyama K and Miwa N:
Antimetastatic and anti-invasive ability of phospho-ascorbyl
palmitate through intracellular ascorbate enrichment and the
resultant antioxidant action. Oncol Res. 11:479–487. 1999.
|
|
35
|
Nagao N, Nakayama T, Etoh T, Saiki I and
Miwa N: Tumor invasion is inhibited by phosphorylated ascorbate via
enrichment of intracellular vitamin C and decreasing of oxidative
stress. Cancer Res Clin Oncol. 126:511–518. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lovaas JD, Zhu L, Chiao CY, Byles V,
Faller DV and Dai Y: SIRT1 enhances matrix metalloproteinase-2
expression and tumor cell invasion in prostate cancer cells.
Prostate. 73:522–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fu X, Kassim SY, Parks WC and Heinecke JW:
Hypochlorous acid generated by myeloperoxidase modifies adjacent
tryptophan and glycine residues in the catalytic domain of matrix
metalloproteinase-7 (matrilysin): an oxidative mechanism for
restraining proteolytic activity during inflammation. J Biol Chem.
278:28403–28409. 2003. View Article : Google Scholar
|
|
38
|
Du J, Martin SM, Levine M, Wagner BA,
Buettner GR, Wang SH, Taghiyev AF, Du C, Knudson CM and Cullen JJ:
Mechanisms of ascorbate-induced cytotoxicity in pancreatic cancer.
Clin Cancer Res. 16:509–520. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Frömberg A, Gutsch D, Schulze D,
Vollbracht C, Weiss G, Czubayko F and Aigner A: Ascorbate exerts
antiproliferative effects through cell cycle inhibition and
sensitizes tumor cells towards cytostatic drugs. Cancer Chemother
Pharmacol. 67:1157–1166. 2011.
|
|
40
|
Taper HS, Jamison JM, Gilloteaux J,
Summers JL and Calderon PB: Inhibition of the development of
metastases by dietary vitamin C:K3 combination. Life Sci.
75:955–967. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Maeda-Yamamoto M, Suzuki N, Sawai Y,
Miyase T, Sano M, Hashimoto-Ohta A and Isemura M: Association of
suppression of extracellular signal-regulated kinase
phosphorylation by epigallocatechin gallate with the reduction of
matrix metalloproteinase activities in human fibrosarcoma HT1080
cells. J Agric Food Chem. 51:1858–1863. 2003. View Article : Google Scholar
|
|
42
|
Lu L, Liu HM and Tang WX: Effect of
epigallocatechin-3-gallate on the invasiveness of hepatocarcinoma
cells in vitro. Zhonghua Gan Zang Bing Za Zhi. 15:825–827.
2007.PubMed/NCBI
|
|
43
|
Sen T, Moulik S, Dutta A, Choudhury PR,
Banerji A, Das S, Roy M and Chatterjee A: Multifunctional effect of
epigallocatechin-3-gallate (EGCG) in downregulation of gelatinase-A
(MMP-2) in human breast cancer cell line MCF-7. Life Sci.
84:194–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen PN, Chu SC, Kuo WH, Chou MY, Lin JK
and Hsieh YS: Epigallocatechin-3 gallate inhibits invasion,
epithelial-mesenchymal transition, and tumor growth in oral cancer
cells. J Agric Food Chem. 59:836–844. 2011.PubMed/NCBI
|
|
45
|
Li H, Li Z, Xu YM, Wu Y, Yu KK, Zhang C,
Ji YH, Ding G and Chen FX: Epigallocatechin-3-gallate induces
apoptosis, inhibits proliferation and decreases invasion of glioma
cell. Neurosci Bull. 30:67–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Demeule M, Brossard M, Pagé M, Gingras D
and Béliveau R: Matrix metalloproteinase inhibition by green tea
catechins. Biochim Biophys Acta. 1478:51–60. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Koo BH, Kim YH, Han JH and Kim DS:
Dimerization of matrix metalloproteinase-2 (MMP-2): functional
implication in MMP-2 activation. J Biol Chem. 287:22643–22653.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Mannello F and Medda V: Nuclear
localization of matrix metalloproteinases. Prog Histochem Cytochem.
47:27–58. 2012. View Article : Google Scholar
|
|
49
|
Annabi B, Lachambre MP, Bousquet-Gagnon N,
Pagé M, Gingras D and Béliveau R: Green tea polyphenol
(−)-epigallocatechin 3-gallate inhibits MMP-2 secretion and
MT1-MMP-driven migration in glioblastoma cells. Biochim Biophys
Acta. 1542:209–220. 2002.
|
|
50
|
Farabegoli F, Papi A and Orlandi M:
(−)-Epigallocatechin-3-gallate down-regulates EGFR, MMP-2, MMP-9
and EMMPRIN and inhibits the invasion of MCF-7 tamoxifen-resistant
cells. Biosci Rep. 31:99–108. 2011.
|
|
51
|
Peng CC, Hsieh CL, Wang HE, Chung JY, Chen
KC and Peng RY: Ferulic acid is nephrodamaging while gallic acid is
renal protective in long term treatment of chronic kidney disease.
Clin Nutr. 31:405–414. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Deng YT and Lin JK: EGCG inhibits the
invasion of highly invasive CL1-5 lung cancer cells through
suppressing MMP-2 expression via JNK signaling and induces G2/M
arrest. J Agric Food Chem. 59:13318–13327. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vayalil PK and Katiyar SK: Treatment of
epigallocatechin-3-gallate inhibits matrix metalloproteinases-2 and
-9 via inhibition of activation of mitogen-activated protein
kinases, c-jun and NF-kappaB in human prostate carcinoma DU-145
cells. Prostate. 59:33–42. 2004. View Article : Google Scholar
|
|
54
|
Shankar S, Marsh L and Srivastava RK: EGCG
inhibits growth of human pancreatic tumors orthotopically implanted
in Balb C nude mice through modulation of FKHRL1/FOXO3a and
neuropilin. Mol Cell Biochem. 372:83–94. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Qin J, Wang Y, Bai Y, Yang K, Mao Q, Lin
Y, Kong D, Zheng X and Xie L: Epigallocatechin-3-gallate inhibits
bladder cancer cell invasion via suppression of NF-κB-mediated
matrix metalloproteinase-9 expression. Mol Med Rep. 6:1040–1044.
2012.PubMed/NCBI
|
|
56
|
Yue H, Uzui H, Lee JD, Shimizu H and Ueda
T: Effects of magnesium on matrix metalloproteinase-2 production in
cultured rat cardiac fibroblasts. Basic Res Cardiol. 99:257–263.
2004.
|
|
57
|
Lentsch AB, Kato A, Saari JT and Schuschke
DA: Augmented metalloproteinase activity and acute lung injury in
copper-deficient rats. Am J Physiol Lung Cell Mol Physiol.
281:L387–L393. 2001.PubMed/NCBI
|
|
58
|
Romagnoli C, Marcucci T, Picariello L,
Tonelli F, Vincenzini MT and Iantomasi T: Role of N-acetylcysteine
and GSH redox system on total and active MMP-2 in intestinal
myofibroblasts of Crohn’s disease patients. Int J Colorectal Dis.
28:915–924. 2013.PubMed/NCBI
|
|
59
|
Rezaei A, Ardestani SK, Forouzandeh M,
Tavangar SM, Khorramizadeh MR, Payabvash S, Nezami BG, Jahanshiri
Z, Tavakoli Z, Shariftabrizi A and Dehpour AR: The effects of
N-acetylcysteine on the expression of matrix metalloproteinase-2
and tissue inhibitor of matrix metalloproteinase-2 in hepatic
fibrosis in bile duct ligated rats. Hepatol Res. 38:1252–1263.
2008.PubMed/NCBI
|
|
60
|
Jiang C, Ganther H and Lu J: Monomethyl
selenium - specific inhibition of MMP-2 and VEGF expression:
implications for angiogenic switch regulation. Mol Carcinog.
29:236–250. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Rooprai HK, Kyriazis I, Nuttall RK,
Edwards DR, Zicha D, Aubyn D, Davies D, Gullan R and Pilkington GJ:
Inhibition of invasion and induction of apoptosis by selenium in
human malignant brain tumour cells in vitro. Int J Oncol.
30:1263–1271. 2007.PubMed/NCBI
|