Introduction
Chemotherapy is the ultimate therapeutic tool for
metastatic and hematological malignancies. Also, postoperative
chemotherapy is indispensable after surgical treatment. However,
multidrug resistance (MDR) remains a major impediment for
chemotherapy, accounting for >90% treatment failure in clinic
(1). Genovariation, epigenetic
changes and microenvironmental changes display the complexity of
MDR mechanisms. These include: overexpression of the ATP-binding
cassette (ABC) superfamily transporters; reduction of drug intake
or increase drug metabolism; change of drug targets; activation of
DNA repair mechanisms, and suppression of apoptosis pathways
(2). Among them, the most dominant
mechanism is the overexpression of ABC transporters. Three
prominent ABC transporters (ABCB1, ABCC1 and ABCG2) are common in
MDR tumors (3). Of these, ABCB1, a
170-kDa transmembrane glycoprotein encoded by the MDR1 gene,
has been most extensively identified in MDR cancer cells. ABCB1 has
a broad spectrum of substrates that are chemotherapeutics,
including doxorubicin (DOX), vinblastine (VCR), paclitaxel,
etoposide, and bisantrene (4).
Currently, it is believed that inhibition of the ABC
drug transporters is the most feasible strategy to overcome MDR.
Taken a panoramic view of the three generations of ABCB1
modulators, the clinical limitations come from the poor
specificity, less potency and unpredictable toxicity (5,6).
Therefore, there is an urgent need to develop more specific, potent
and relatively non-toxic modulators. Natural products such as
plants, fungi and marine organisms possess a great diversity of
compounds and are ideal source of ABCB1 modulators. Of those, the
extraction-separation of traditional Chinese medicine (TCM) with
traditional efficacy is one of the most important approaches to
discover new biological active components. For instance,
β-D-glucopyranoside has been developed as TCM monomer (7). Artemisinin is used as an effective
antimalarial agent worldwide (8).
A large number of TCM monomers and their derivatives which are
ABCB1 modulators have been found. Clitocine, an alkaloid from
Clitocybe inversa, reversed ABCB1-associated MDR in
HepG2/ADM by downregulation of NF-κB and ABCB1 (9). Our group is devoted to find ABCB1
modulators from TCM, and has found that 23-hydroxybetulinic acid
derivatives BBA (10), DABB, DHBB
(11) and B5H7 (12) could significantly reverse
ABCB1-mediated MDR cells via inhibition of the function of ABCB1.
In addition, we found that acerinol, isolated from Cimicifuga
acerina, acts as a competitive inhibitor for ABCB1 to sensitize
drug resistant cells HepG2/ADM and MCF-7/ADR to DOX, VCR and
paclitaxel (13).
Ganoderma lucidum (Leyss. ex Fr.) Karst, a
notable Chinese medicine, has been used as a folk remedy for health
improvement. It has long been used for prevention and treatment of
multiple diseases, such as cancer, neurasthenia, hepatopathy,
aging, and diabetics (14). In the
past two decades, over one hundred of triterpenoids in the fungus
have been extracted and identified from the fruiting bodies,
cultured mycelia, and spores of Ganoderma lucidum, which
have been considered to be responsible for the biological
activities of Ganoderma lucidum (15). Accumulated data show that these
triterpenoids exhibit a broad spectrum of antitumor properties. For
instance, Ganoderma lucidum extracts containing
triterpenoids (GLCTs) exhibited anti-proliferative activity in
several hematological cell lines such as HL-60, K562 and Nalm-6 via
induction of cell cycle arrest and apoptosis (16). Moreover, GLCTs activated multiple
signaling pathways to reduce secretion of vascular endothelial
growth factor (VEGF) and suppress the activities or expression of
matrix metalloproteinase (MMP) relative proteins and urokinase
plaminogen activator (uPA), thereby inhibiting the invasion and
angiogenesis of tumors in vitro and in vivo (17). Ganoderic acid DM, a lanostane-type
triterpenoid, exhibited activity of inhibition of tumor
proliferation and metastasis. Further mechanistic investigations
indicated that Ganoderic acid DM had a hormonal-like function
modulating estrogen receptor (ER) and androgen receptor (AR),
subsequently inducing DNA damage, apoptosis and G1 phase cell cycle
arrest in MCF-7 cells (18).
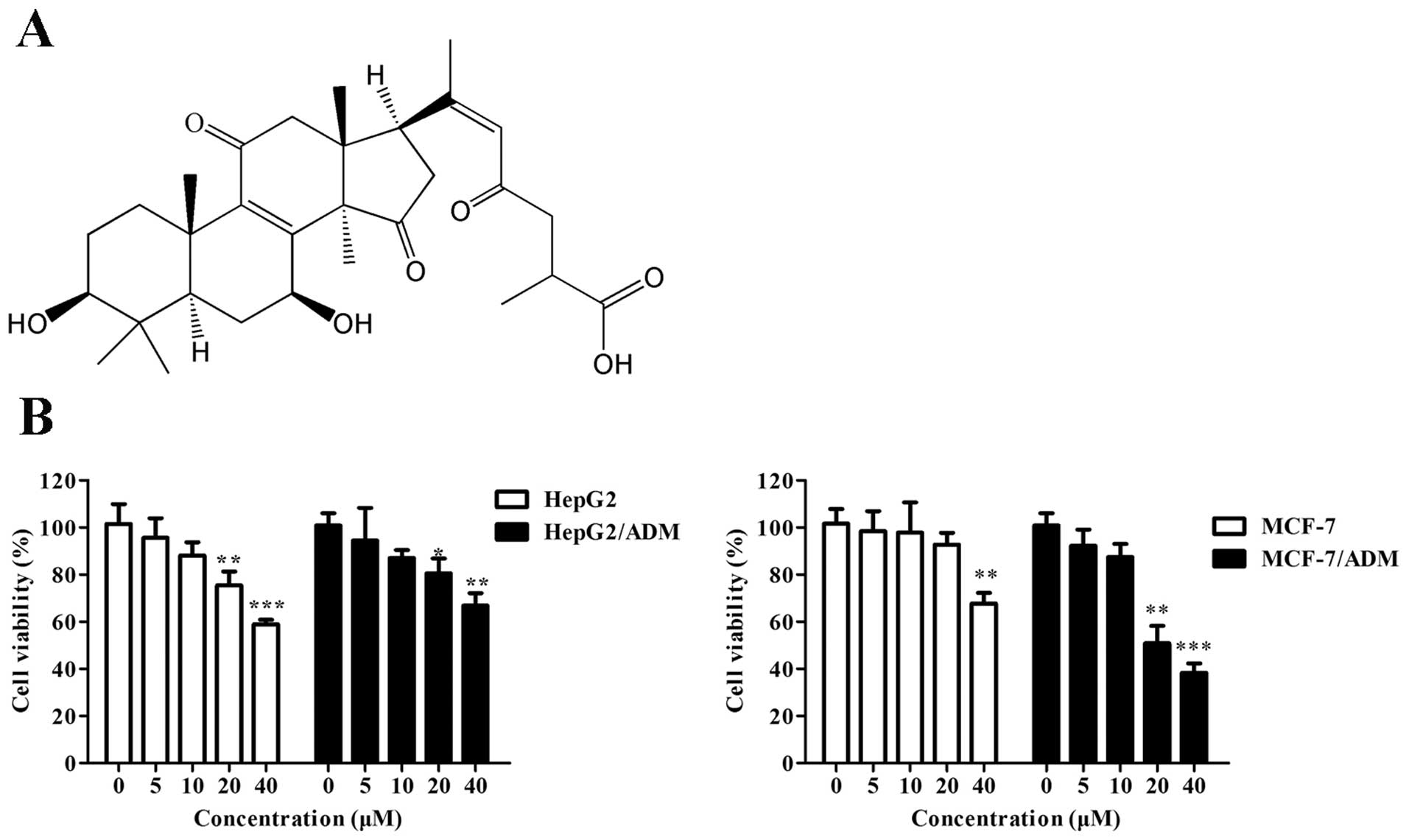
Ganoderenic acid B (GAB, Fig. 1A), a lanostane-type triterpene
isolated from Ganoderma lucidum (19), exhibited potent antineoplastic
activity in p388, BEL-7402, SGC-7901 and HeLa cells in
vitro, with IC50 values of 13.6, 18.6, 20.4 and 10
μM, respectively (14). However,
the cellular and molecular mechanisms behind its anticancer action
need to be further illuminated. So far, there is no report on its
MDR reversal activity. In this report, we demonstrate that GAB is
able to sensitize ABCB1-overexpressing MDR cells HepG2/ADM, and we
reveal the mechanisms of action for its MDR reversal activity.
Materials and methods
Materials
GAB was isolated from the fruit bodies of
Ganoderma lucidum and the purity is >95% determined by
HPLC. Doxorubicin (DOX), rhodamine-123 (Rhm-123), cisplatin,
3-(4,5-dimethylthiazol-2-yl)-2,5-dipheyltetrazolium bromide (MTT)
and paclitaxel were obtained from Sigma-Aldrich (St. Louis, MO,
USA). Recombinant human ABCB1 membranes, Pgp-Glo™ assay systems and
CYP3A4 P450-Glo™ assay kit were acquired from Promega (Madison, WI,
USA). Vincristine (VCR) was a product from Perivinkle
Pharmaceutical (Haikou, Hainan, China). ABCB1 siRNA kit was
obtained from GenePharma (Pudong, Shanghai, China). Verapamil (VRP)
and mouse anti-ABCB1 antibody were purchased from Merck Calbiochem
(Darmstadt, Hessen, Germany). Monoclonal anti-β-actin antibody was
acquired from Multisciences (Hangzhou, Zhejiang, China). Other
chemical reagents were purchased from Sigma-Aldrich.
Cell lines and cell culture
Human hepatocellular carcinoma cell line (HepG2) and
drug-resistant cell line (HepG2/ADM) were kindly provided by
Professor Kwok-Pui Fung (The Chinese University of Hong Kong, Hong
Kong, China). ABCB1-overexpressing HepG2/ADM was established from
HepG2 by progressive induction of DOX as described previously
(20). Human breast cancer cell
line MCF-7 and DOX-induced drug resistant cell line MCF-7/ADR was
provided generously by Professor Li-Wu Fu (Sun Yat-Sen University,
China) (21). All cell lines were
maintained in RPMI-1640 containing 10% FBS and 1% penicillin
streptomycin at 37°C in a humidified incubator with 5%
CO2. In order to keep the drug resistant feature,
HepG2/ADM and MCF-7/ADR cells were maintained in the culture medium
containing 1.2 μM DOX. Cells in logarithmic phase were collected to
use in cellular experiments.
Cell viability assay
The viability of cells treated with various
concentrations of GAB was evaluated by MTT assay. Cells in
logarithmic phase were plated into 96-well plates at a density of
5×103 per well and maintained for 24 h. Cells were
incubated in the medium containing various concentrations of GAB
for 72 h. MTT solution (20 μl) (5 mg/ml in PBS) was added and
cultured for another 4 h. The optical density of formazan at 595 nm
was recorded by a microplate reader (DTX880, Beckman, USA). The
viability of cells treated with dimethyl sulfoxide (DMSO) was
defined as 100%, and the concentrations at which >90% cells were
viable, were considered as non-toxic and used in the MDR reversal
assay.
MDR reversal assay
Cells were treated with GAB (5 and 10 μM) or VRP (10
μM) combined with different concentrations of DOX, VCR, paclitaxel
and cisplatin in 96-well plates. Cell viability was detected using
MTT assay as described above. The half maximal inhibitory
concentrations (IC50) were calculated using GraphPad
Prism 5.0 software according to their survival curves. The reversal
folds of GAB and VRP were calculated by dividing the
IC50 value of DOX, VCR, paclitaxel and cisplatin in the
absence or presence of GAB or VRP.
Rhm-123 accumulation assay
Intracellular Rhm-123 accumulation assay was
evaluated as previously described (10). Briefly, cells (1×104 per
well) were seeded into 96-well black clear-bottom plates for 24 h.
Cells were pre-incubated with GAB (5 and 10 μM) or VRP (10 μM) for
4 h at 37°C. After that Rhm-123 (5 μM) was added and cultured for
another 2 h. After cells were washed three times with ice-cold PBS,
cellular Rhm-123 fluorescence was detected by a microplate reader
(DTX880, Beckman) or a fluorescence microscopy (Axio Imager A2,
Zeiss, Germany).
Rhm-123 efflux assay
Cells (1×104 per well) were plated into
96-well black clear-bottom plates. After overnight attachment,
cells were pre-treated with GAB (10 μM) or VRP (10 μM) for 2 h and
cultured with Rhm-123 (5 μM) for another 2 h at 37°C, then washed 3
times with PBS at various time-points (0, 15, 30 and 60 min).
Finally, the retention of intracellular Rhm-123 was evaluated by
DTX 880 Multimode Detector. The fluorescence of Rhm-123 in cells at
0 min was considered to be 100%.
ABCB1 siRNA interference
HepG2/ADM cells (3×105 per well) were
plated in 6-well plates. When they were 60–80% confluent, cells
were incubated with the medium of Opti-MEM with Lipofectamine
reagent, 100 nM ABCB1 siRNA or scrambled control siRNA for 6 h at
37°C. Cells were then further incubated for 48 h, and cellular
ABCB1 level was detected by western blot analysis as described
below. To determine whether reversal effect of GAB were associated
with ABCB1, the siRNA-transfected cells was exposed to VCR in the
presence or absence of GAB and VRP for 48 h and cell viability was
determined by MTT assay as described above.
Western blot analysis
Total protein was extracted from HepG2/ADM and
MCF-7/ADR cells after treated with GAB (5 and 10 μM) or VRP (10 μM)
for 72 h. The concentrations of total cellular protein were
quantified using BCA protein assay kit. Proteins were separated by
10% SDS-PAGE and then transferred into PVDF membranes. After being
blocked with 5% non-fat milk in TBS-T buffer for 1 h at room
temperature, the membranes were immunoblotted with primary antibody
(1:1,000) at 4°C overnight and probed with secondary antibody
(1:1,000) for 1 h at room temperature. The bands were enhanced
using enhanced chemiluminescence solutions and imaged by X-ray film
processor (Kodak X-102, Kodak, USA).
ABCB1 ATPase activity assay
To investigate the influence of GAB on ABCB1 ATPase
activity, ABCB1-Glo™ ATPase assay kit was used following the
instructions of the manufacturer. Total ATPase inhibitor
Na3VO4 (0.25 mM), ABCB1 ATPase stimulator VRP
(0.5 mM) and various concentrations of GAB (2.5, 5, 10 and 20 μM)
in assay buffer were prepared and then added to opaque flat bottom
96-well plates, then recombinant human ABCB1 membranes were added
and treated for 5 min at 37°C. Ten μl of MgATP (25 mM) was added to
each well. After incubation for 40 min at 37°C, the reaction was
stopped by adding 50 μl of ATP detection buffer and then incubated
at room temperature for 20 min. The luminescence with positive
proportion to ATP was tested by a microplate reader (DTX880,
Beckman).
CYP3A4 activity assay
CYP3A4 P450-Glo assay kit was used to detect the
influence of GAB on CYP3A4 according to the manufacturer’s
instructions. Briefly, GBA (20 and 40 μM) or ketoconazole (20 μM)
diluted 4-fold by luciferin-free water were added into opaque flat
bottom 96-well plates and co-treated with 4× CYP3A4 reaction
mixture (0.5 M potassium phosphate, 12 μM luciferin-IPA and 0.008
pM recombinant human CYP3A4 membranes) for 10 min at 37°C. 2× NADPH
regeneration system (10% solution A and 2% solution B) was used to
start reactions. The luminescent signal was detected after the
reactions were stopped by luciferin detection reagent with
esterase.
Docking analysis
Sybyl 8.0 in Surflex-dock module, a parented docking
engine to explore binding modes and sites of ligands and proteins,
was used to simulate the docking of GAB and ABCB1. VRP was defined
as reference standard. The charge of ABCB1 structure was calculated
in MMFF94 protein structure-module without energy optimization.
Three-dimensional structures of GAB and VRP with energy
optimization were carried out using Tripos molecular mechanics
force field. In the docking process, the preferable docking
conformation and the best docking score using an empirical scoring
function, were guidelines in the docking of GAB and VRP to ABCB1
binding site residues. GAB and VRP were docked into the idealized
active sites of ABCB1 and further binding energy was
calculated.
Statistical analysis
All experiments were performed at least three times,
and results are shown as means ± SD. Data were analyzed using
Graphpad Prism 4.0 with Student’s t-test. P<0.05 was considered
to be significant.
Results
Effect of GAB on cell viability
To examine the reversal capacity of GAB under
non-toxic concentrations on ABCB1-mediated multidrug resistance of
HepG2/ADM and MCF-7/ADR cancer cells, the cytotoxicity of GBA
towards all cell lines was determined firstly. The relative cell
viability of HepG2, HepG2/ADM, MCF-7 and MCF-7/ADR treated with or
without GAB is shown in Fig. 1B.
After treated with GBA less than the concentration of 10 μM for 72
h, the cell viability was >90% in all four cell lines, whereas
GAB at the concentration >20 μM was toxic. Thus, the
concentrations of 10 and 5 μM were considered to be the optimal
concentrations for GAB in the following MDR reversal
experiments.
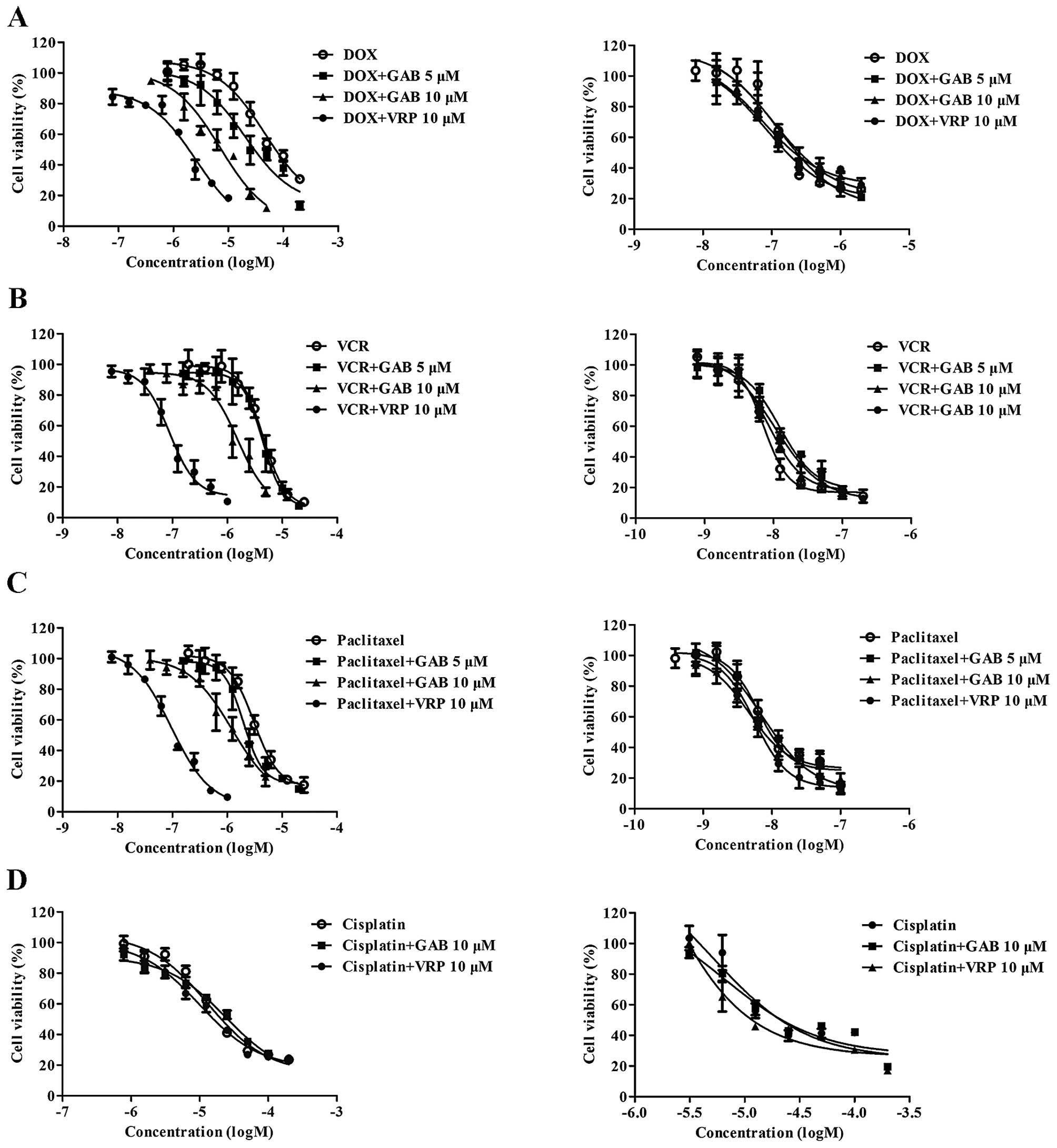
GAB sensitizes ABCB1-overexpressing MDR
cells to DOX, VCR and paclitaxel
To evaluate the reversal activity of GAB in
HepG2/ADM, MCF-7/ADR and their parental sensitive cell lines,
IC50 values of DOX, VCR, paclitaxel and cisplatin in the
presence or absence of GAB were determined by MTT assay. Reversal
activity comparison experiments were conducted using the same
concentration of VRP (10 μM) as a positive control, which is a
known ABCB1 inhibitor (22). As
shown in Fig. 2 and Table I, while HepG2 cells were sensitive
to DOX, VCR and paclitaxel, HepG2/ADM cells exhibited significant
resistance characteristics to those traditional antitumor drugs
with resistance-fold of 451, 531 and 503, respectively. GAB at
concentrations of 10 and 5 μM remarkably reversed the resistance of
HepG2/ADM cells to DOX in a concentration-dependent manner, the
IC50 values were decreased to 6.0446±0.1176 and
23.0050±1.7477 μM. Similarly, the increased sensitivity of
HepG2/ADM to VCR and paclitaxel was found when cells were
co-treated with GAB at 10 μM. However, GAB at lower concentration
(5 μM) could not significantly reverse HepG2/ADM to VCR and
paclitaxel. GAB was still able to reverse resistance of MCF-7/ADR
cells to DOX, producing 3.36- and 1.90-fold reversal activity,
respectively, at concentrations of 10 and 5 μM. In comparison, the
MDR reversal effect of GBA in HepG2/ADM and MCF-7/ADR at 10 μM was
a little weaker than VRP. In their parental sensitive cell lines
HepG2 and MCF-7, the cytotoxicity of DOX, VCR and paclitaxel showed
no significant difference in the presence or absence of GAB,
similarly to VRP. Cisplatin, a water-soluble chemotherapeutic drug,
which can not be transported by ABCB1, was not sensitive to
HepG2/ADM and HepG2 cells, which was not affected by GAB. The above
data suggest that GAB significantly sensitizes ABCB1 substrates to
ABCB1-overexpressing MDR cells, and its reversal effect is related
to ABCB1.
 | Table IReversal effect of GAB on HepG2/ADM
cells and their parent cells. |
Table I
Reversal effect of GAB on HepG2/ADM
cells and their parent cells.
| IC50 ±
SDa (μM) (fold-reversal) |
|---|
|
|
|---|
| HepG2/ADM | HepG2 |
|---|
| DOX | 77.4565±4.2154
(1.00) | 0.1715±0.0116
(1.00) |
| + GAB 5 μM | 23.0050±1.7477
(3.37) | 0.1595±0.0106
(0.93) |
| + GAB 10 μM | 6.0446±0.1176
(12.81) | 0.2024±0.0215
(1.18) |
| +VRPb 10 μM | 1.4247±0.3601
(54.36) | 0.1727±0.0243
(1.01) |
| VCR | 5.2046±0.6377
(1.00) | 0.0098±0.0012
(1.00) |
| + GAB 5 μM | 4.4045±0.2575
(1.18) | 0.0111±0.0005
(0.88) |
| + GAB 10 μM | 1.1627±0.0265
(4.48) | 0.0106±0.009
(0.92) |
| +VRPb 10 μM | 0.1084±0.0066
(48.03) | 0.0103±0.0011
(0.94) |
| Paclitaxel | 4.0754±0.2169
(1.00) | 0.0081±0.0009
(1.00) |
| + GAB 5 μM | 2.2603±0.0831
(1.81) | 0.0097±0.0016
(0.83) |
| + GAB 10 μM | 1.3232±0.0594
(3.08) | 0.0071±0.0006
(1.13) |
| +VRPb 10 μM | 0.0942±0.0121
(42.23) | 0.0072±0.0007
(1.13) |
| Cisplatin | 18.5201±2.7032
(1.00) | 15.5270±2.3874
(1.00) |
| + GAB 10 μM | 18.9788±1.3646
(0.98) | 15.0938±1.7880
(1.03) |
| +VRPb 10 μM | 16.4769±2.8430
(1.12) | 18.1916±1.2672
(0.85) |
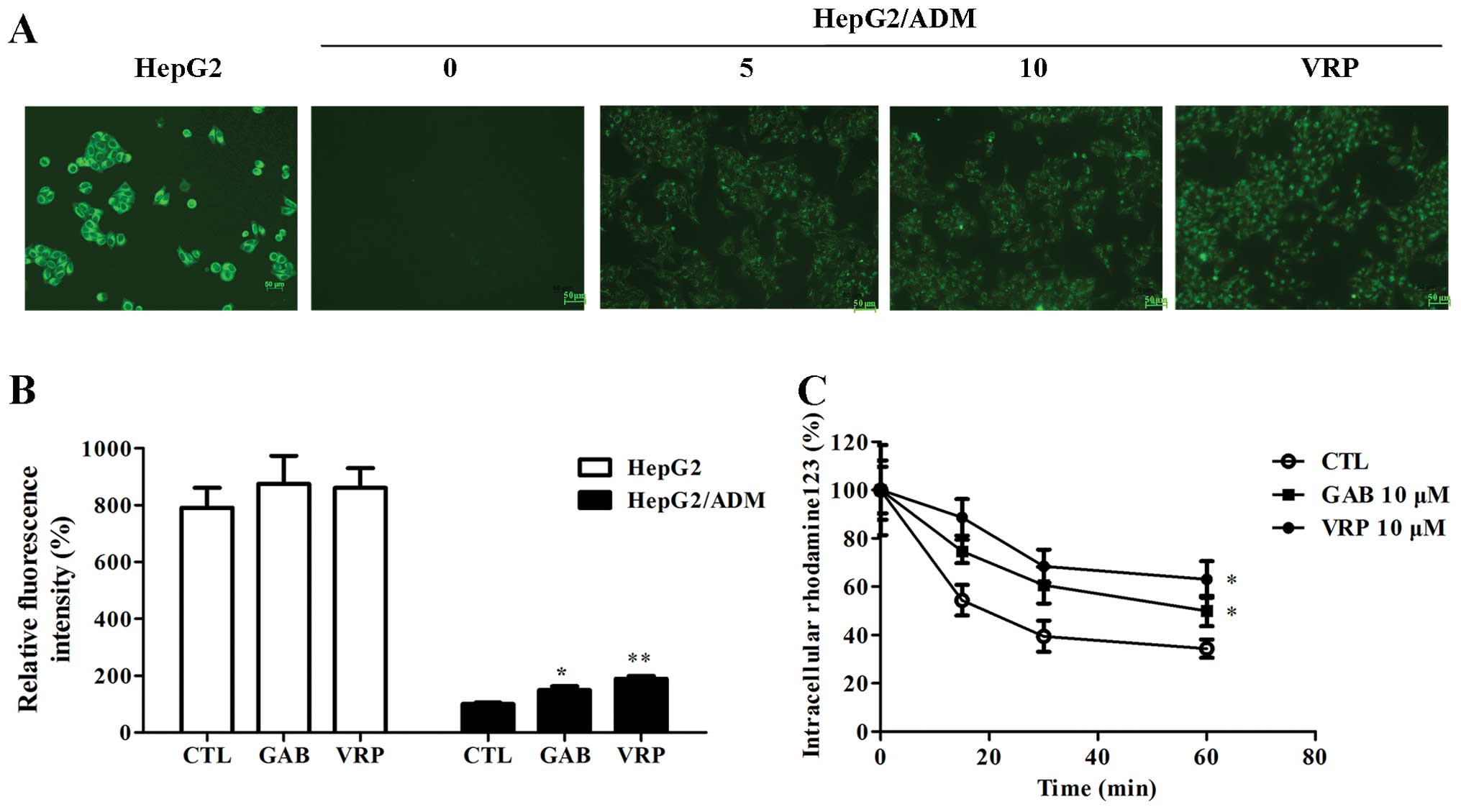
GAB stimulates the accumulation of
Rhm-123 and attenuates Rhm-123 efflux velocity in HepG2/ADM
cells
MDR reversal assay suggests that GAB is able to
sensitize ABCB1-overexpressing cells to ABCB1 substrates. To
understand the underlining mechanism of this effect, we examine the
accumulation of an ABCB1 specific fluorescent substrate, Rhm-123,
in HepG2/ADM and HepG2 cells. It was found that GAB treatment
increased the intracellular content of Rhm-123, similarly to VRP
(Fig. 3A). Similarly to the trend
shown in Fig. 3B, the fluorescence
intensity was much higher in HepG2 cells than that in HepG2/ADM
cells. HepG2/ADM cells could strongly pump out intracellular
Rhm-123 to medium, displaying weak fluorescence in cells. GAB at a
concentration of 10 μM obviously induced intracellular accumulation
of Rhm-123 in HepG2/ADM, with a relative Rhm-123 level ≤148%
compared with GAB untreated cells. However, GAB can not alter the
accumulation of Rhm-123 in HepG2 cells. In addition, GAB inhibited
Rhm-123 efflux velocity in HepG2/ADM, while Rhm-123 level in
GAB-untreated cells was rapidly transported to extracellular medium
(Fig. 3C). Consistent with the
results in reversal activity assay, the effect of GAB on
stimulation of accumulation of Rhm-123 and inhibition of its efflux
was weaker than that of VRP. These data suggest that GAB may
modulate the drug transport of ABCB1, thereby leading to the
enhancement of the intracellular Rhm-123 accumulation and
inhibition of Rhm-123 efflux.
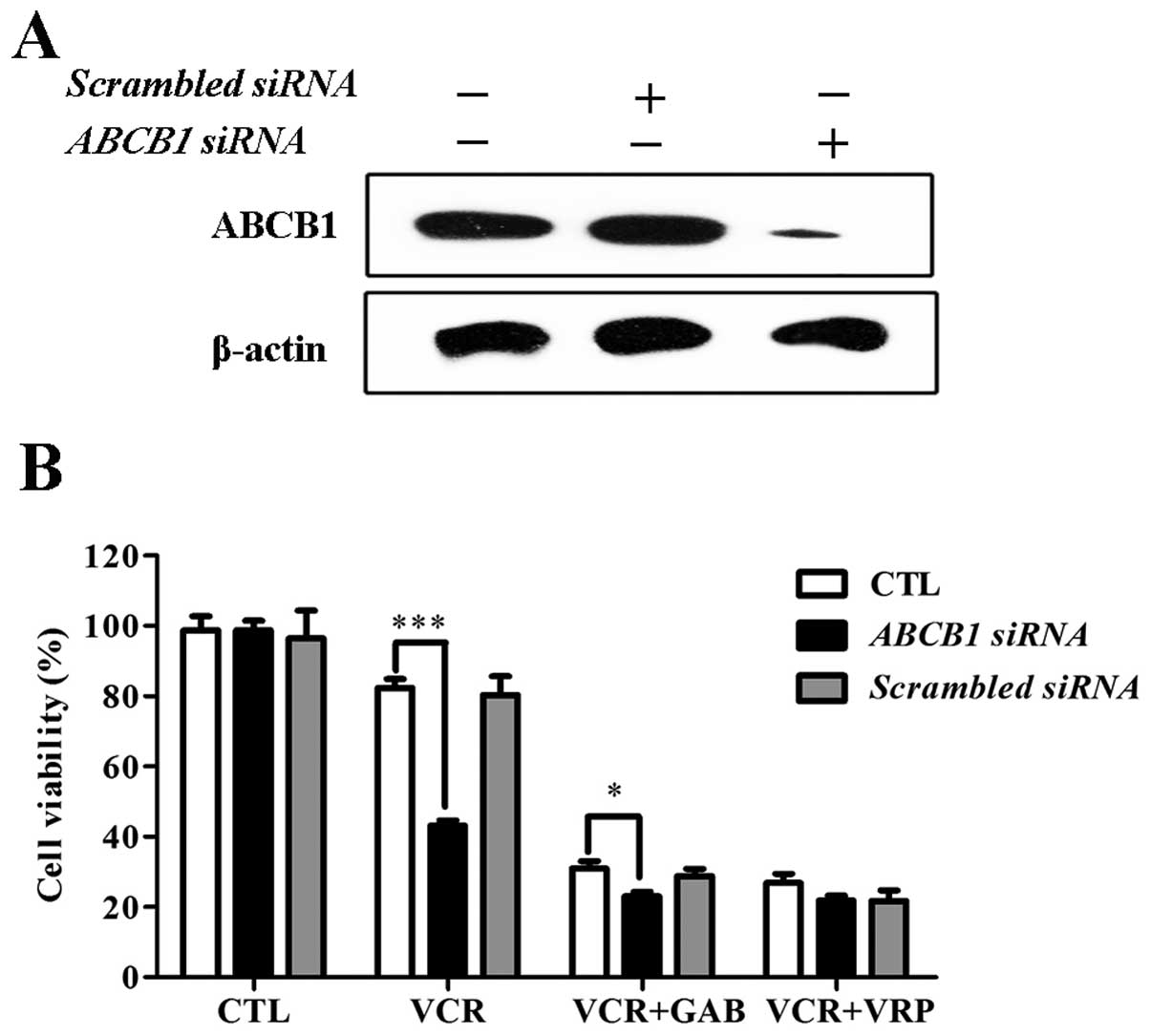
GAB reverses ABCB1-mediated MDR to VCR is
dependent on ABCB1
The results above indicated that the reversal effect
of GAB may relate to influence of ABCB1 transporter. To further
clarify whether the MDR reversal activity of GAB was dependent on
ABCB1, ABCB1 gene was silenced by specific siRNA. As shown in
Fig. 4A, ABCB1 protein level
decreased significantly in HepG2/ADM cells after ABCB1 siRNA
transfection. Scrambled control siRNA had no effect on the
expression of ABCB1. As shown in Fig.
4B, ABCB1 siRNA-transfected HepG2/ADM cells showed attenuated
reversal effect to VCR in the presence of GAB or VRP, indicating
that GAB reverses ABCB1-mediated MDR depending on ABCB1.
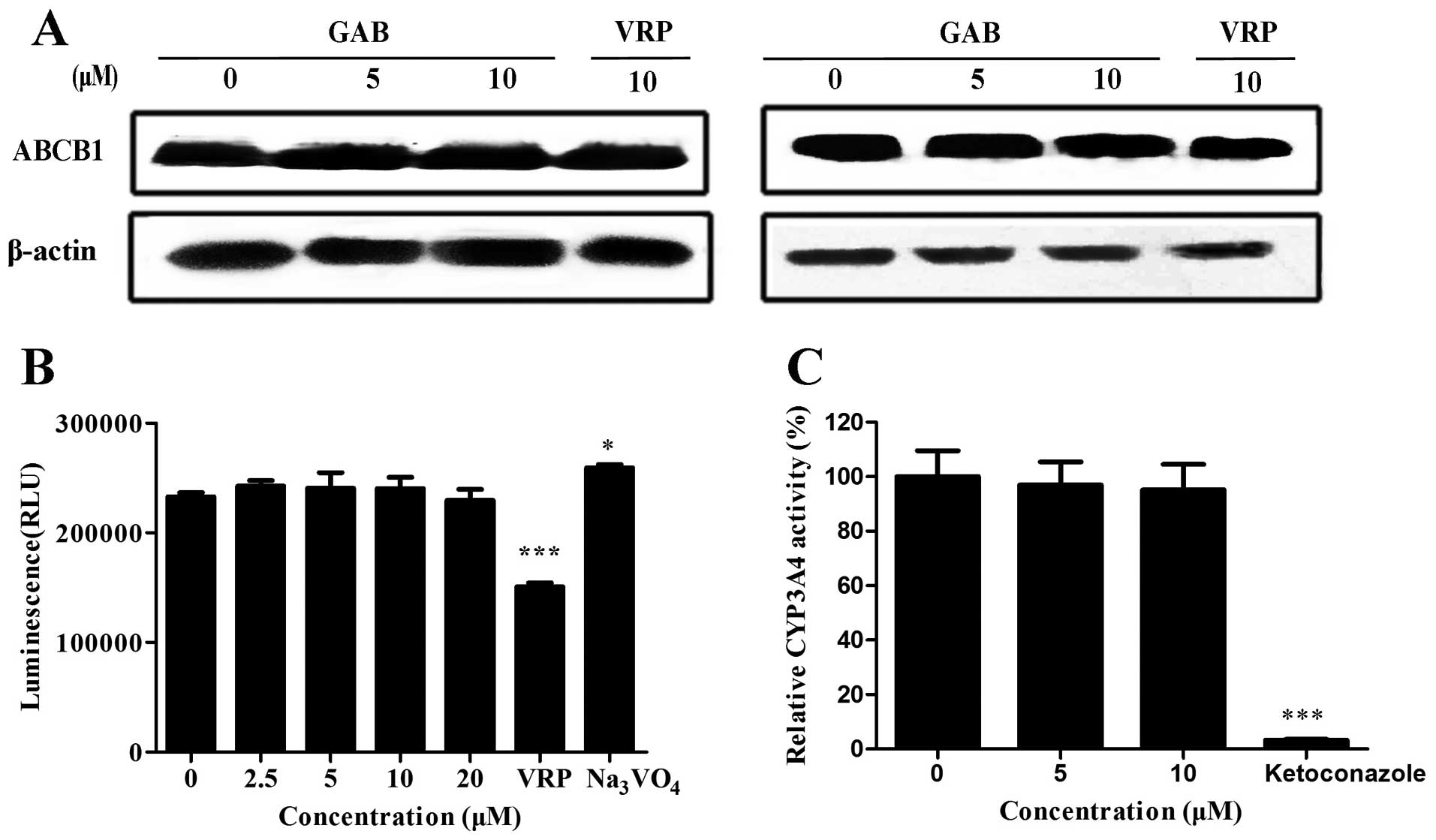
GAB can not alter ABCB1 expression
Downregulation of expression and inhibition of
function of ABCB1 are main reversal mechanisms of ABCB1 modulators.
To further investigate the MDR reversal mechanisms of GAB, ABCB1
expression level in HepG2/ADM and MCF-7/ADR cells were measured by
western blot analysis. As shown in Fig. 5A, the expression level of ABCB1 was
not altered after GAB or VRP treatment. Our results indicate that
GAB reverses ABCB1-mediated MDR without downregulation of ABCB1,
further suggesting that GAB may influence the function of ABCB1 to
reverse MDR.
GAB does not affect ABCB1 ATPase
activity
Because GAB does not alter the expression of ABCB1,
GAB is likely to inhibit its transport function. Two ATP hydrolysis
events are needed to change the conformation when ABCB1 transports
the substrate out of the cells (23). ABCB1 substrates stimulate the
ATPase activity in nucleotide-binding domains (NBD). Thus, the
ABCB1 ATPase activity assay may contribute to reflect the action
mode of GAB. As shown in Fig. 5B,
ABCB1 substrate VRP was able to stimulate the activity of ABCB1,
whereas ATP consumption was completely inhibited by
Na3VO4. GAB at the reversal concentrations
(10 and 5 μM) did not alter the ATPase activity of ABCB1. Moreover,
the influence of ABCB1 ATPase was not observed even at higher or
lower concentrations (20 and 2.5 μM). These data illustrate that
GAB does not impact the activity of ABCB1 ATPase, and is not a
competitive inhibitor for ABCB1.
GAB does not alter CYP3A4 activity
CYP3A4, one of the members of P450 family, is an
important metabolic enzyme for anticancer drugs. Its broad spectrum
substrate overlaps with ABCB1 modulators. A relative non-toxic
ABCB1 modulator has the characteristic of no effect on CYP3A4
activity. We tested whether GAB inhibits the activity of CYP3A4.
Ketoconazole was used as a positive control. GAB, even up to a
concentration of 10 μM, did not inhibit CYP3A4 activity. Inversely,
ketoconazole almost completely inhibited its activity (Fig. 5C). Taken together, these results
indicate that GAB is not a CYP3A4 inhibitor.
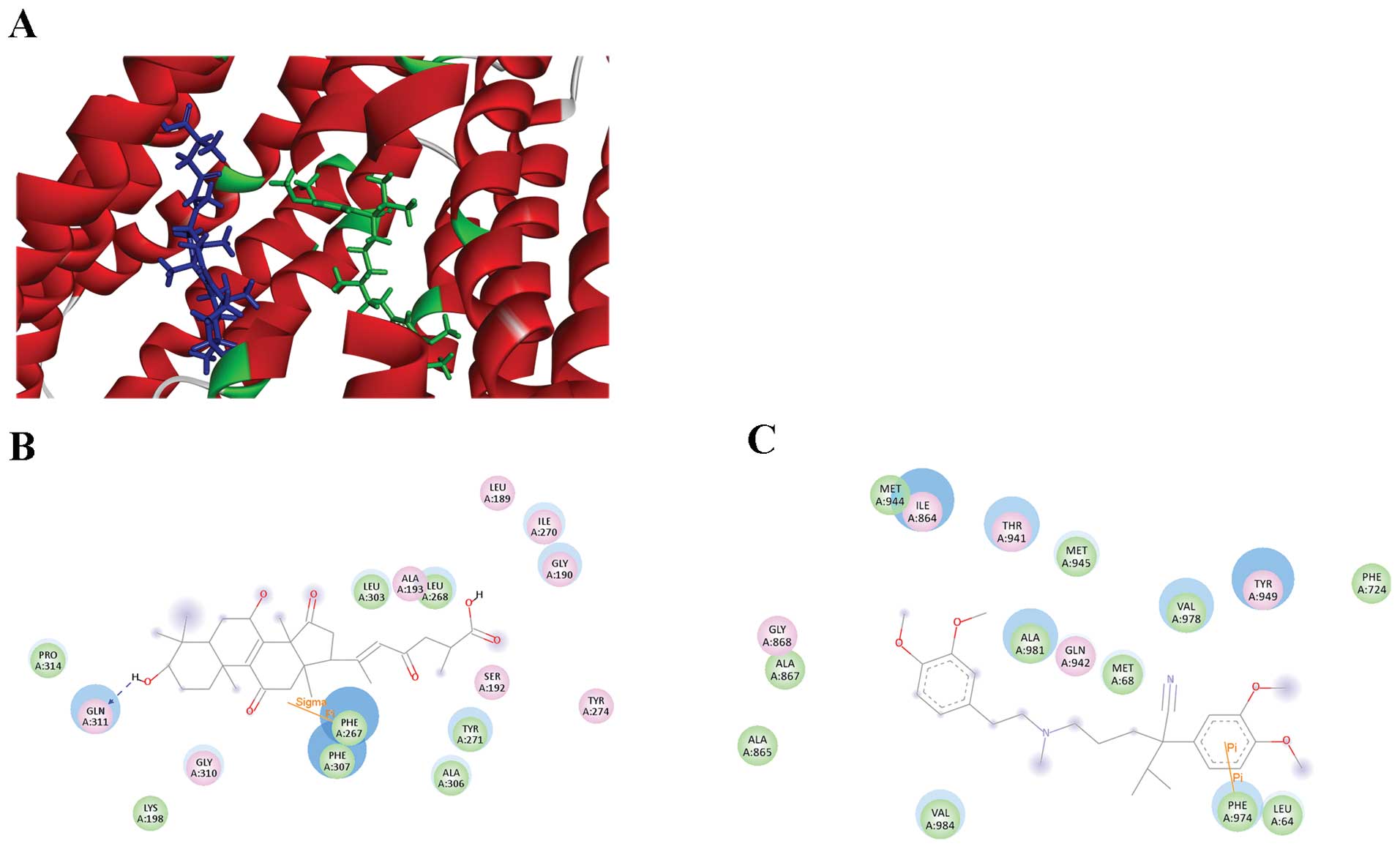
Molecular docking model of GAB binding to
ABCB1
To further explore the interaction mode and binding
sites of GAB with ABCB1, GAB and VRP were docking toward ABCB1. As
shown in Fig. 6A, GAB (blue) could
bind to various conformation of ABCB1 transmembrane domain (TMD),
far away from the VRP (green) binding position located to substrate
binding region. ABCB1 residues and GAB binding sites are shown in
Fig. 6B, GAB was surrounded by
residues of LEU189, GLY190, SER192, ALA193, LYS198, PHE267, LEU268,
ILE270, TYR271, TYR274, LEU303, ALA306, PHE307, GLY310, GLN311 and
PRO314. The residual amino acids around VRP were LEU64, MET68,
PHE724, ILE864, ALA865, ALA867, GLY868, THR941, GLN942, MET944,
MET945, TYR949, PHE974, VAL978, ALA981 and VAL984, which were
matched with previous reports (Fig.
6C) (23). Among these
residues, none was around the GAB. This result further confirmed
that GAB binding site to ABCB1 was different with the site of
VRP.
Discussion
Chemotherapy is thought to be the most effective
treatment for disseminated cancer. Long-term continuous
chemotherapy in clinic predisposes individuals to the development
of acquired or intrinsic MDR. Overexpression of ABCB1
(P-glycoprotein, MDR1), which can pump out intracellular drugs to
lead to reduce therapeutic dose in tumor cells, has been confirmed
to be correlated with chemotherapeutic resistance and poor
prognosis (24). In search for the
third generation of ABCB1 modulators, steps have been taken to
develop targeted, more specific modulators, with high selectivity
and great potency. ABCB1 inhibitors or modulators obtained from
natural sources usually have low toxicity and are well tolerated by
humans. TCMs provide some natural products with novel structure,
diverse skeleton and promising biological activities, that are
suitable to be developed into potent, selective and low-toxic ABCB1
inhibitors (25). Ganoderma
lucidum are precious fungi with good nutrition and medicinal
properties, and have a long-historical utilization in China for
promoting health and longevity. Some biologically active components
of Ganoderma lucidum were found to have antitumor activity.
Ethanol extracts from Ganoderma lucidum, inclusive of
nucleosides, triterpenoids and sterols, showed significant
antiproliferative activities on myriad tumor cell lines (15). Polysaccharides from Ganoderma
lucidum have been reported to be able to attenuate DOX
resistance to K562/ADM cells by inhibiting ABCB1 expression
(26). The present study is the
first to report that ganoderenic acid B (GAB), a lanostane type
triterpene isolated from Ganoderma lucidum, markedly
increase the cytotoxicity of DOX, VCR and paclitaxel to reverse
ABCB1-mediated MDR cells by disrupting the function of ABCB1.
Previously, we reported that drug resistant cancer
cells HepG2/ADM and MCF-7/ADR developed by long-term treatment of
DOX, overexpress the ABCB1 transporter. These cells showed marked
resistance to multiple chemotherapeutics, such as DOX, VCR and
paclitaxel (10). In the present
study, we found that GAB strongly enhanced the sensitivity of
HepG2/ADM to several ABCB1 substrates such as DOX, VCR and
paclitaxel (Fig. 2 and Table I). GAB was able to sensitize
MCF-7/ADR cells to DOX (Table
II). However, GAB had no such effect on their parental
sensitive cells HepG2 and MCF-7 (Fig.
2 and Tables I and II). Moreover, GAB did not alter the
sensitivity of HepG2/ADM and MCF-7/ADR cells to cisplatin which is
a non-ABCB1 substrate, indicating that the reversal efficacy of GAB
is related to ABCB1-mediated drug resistance. Indeed, the
speculation was strongly supported by the fact that GAB could make
the retention of ABCB1 specific substrate Rhm-123 in HepG2/ADM
cells, and this fluorescence accumulation study was consistent with
the cytotoxic results. Simultaneously, inhibitive effect of GAB on
the Rhm-123 efflux was also detected. RNA interference could
decrease the expression level of ABCB1 in HepG2/ADM, following
attenuation of the reversal effect of GAB in transfected HepG2/ADM.
The results suggested ABCB1 is required in the reversal effect of
GAB in MDR cells. Future studies on reversal effect of GAB will
involve evaluation of MDR reversal effect in HepG2/ADM xenograft
models.
 | Table IIReversal effect of GAB in MCF-7/ADR
cells and their parent cells. |
Table II
Reversal effect of GAB in MCF-7/ADR
cells and their parent cells.
| IC50 ±
SDa (μM) (fold-reversal) |
|---|
|
|
|---|
| MCF-7/ADR | MCF-7 |
|---|
| DOX | 40.0992±5.8447
(1.00) | 0.4472±0.0441
(1.00) |
| + GAB 5 μM | 21.0516±1.0390
(1.90) | 0.5301±0.0354
(0.85) |
| + GAB 10 μM | 11.9206±0.7707
(3.36) | 0.4854±0.0412
(0.92) |
| +VRPb 10 μM | 2.5183±0.3560
(15.92) | 0.4535±0.0378
(0.98) |
Some active ingredients derived from the medical
plants, such as icaritin, astragaloside II/IV and oroxylinA
(27), could potentiate the
cytotoxicity of anticancer drugs to MDR cells by downregulation of
ABCB1 mRNA and protein levels, leading to the changes in MDR
phenotype. Different from above active components, GAB did not
alter the mRNA and protein expression levels of ABCB1 even after
treatment for 72 h in HepG2/ADM and MCF-7/ADR cells (Fig. 5), suggesting that GAB may influence
the drug transport function instead of expression of ABCB1.
Structure of ABCB1 contains two ATP-binding sites (NBD) and two ATP
hydrolysis events which are changed in the conformation when ABCB1
transports a substrate (23).
ABCB1 substrates can stimulate the ATPase activity in NBD regions
to pump them to the outer membrane. Some tyrosine kinase inhibitors
such as erlotinib (28), apatinib
(29) and lapatinib (30), inhibit ABCB1 transport function by
stimulating ABCB1 ATPase activity. On the contrary, some of ABCB1
modulators, including 23-hydroxybetulinic acid derivatives DABB and
DHBB, could inhibit the activity of ABCB1 ATPase thereby leading to
dysfunction of ABCB1 (11).
However, GAB neither stimulate nor inhibit the activity of ABCB1
ATPase (Fig. 5), ruling out the
possibility that GAB binds to substrate-binding pocket to act as a
competitive inhibitor of ABCB1 or that it binds to ATP-binding
pocket to act as a ABCB1 ATPase inhibitor. The investigation on
XR9576 binding to ABCB1 suggested the interaction between XR9576
and ABCB1 substrates vinblastine and paclitaxel in a
non-competitive manner, indicating that vinblastine and paclitaxel
could only fractionally displace [3H]-XR9576 binding to
ABCB1 (31). The longer term
investigation of GAB showed that the reversal activity of GAB was
retained after removal, suggesting its high binding affinity for
ABCB1 and GAB was different from VRP reported as a typical
substrate of ABCB1. The data showed that GAB does not appear to be
a substrate for ABCB1, but may bind to ABCB1 in a non-competitive
or uncompetitive manner. This hypothesis was probed further by the
binding sites and style between GAB and ABCB1 in docking analysis.
The most suitable docking conformation between GAB and ABCB1 has
the best docking score far away from the binding sites of the
substrate pocket (VRP binding region). Indeed, the characterization
of the molecular interaction of GAB with ABCB1 needs to be further
conducted by analysis of the inhibition kinetics of GAB on DOX
transport by ABCB1. The properties of bilayer lipid membranes,
including lipid composition, fluidity and cholesterol content,
could influence ABCB1 function of ATP hydrolysis, drug interaction
and transport (23). The
relationship between GAB, lipid membrane and ABCB1 still remains an
open question. Future efforts should focus on investigation of the
interaction between GAB and ABCB1 to elucidate its underlying
mechanism.
CYP3A4, a major anticancer drug metabolic enzyme
in vivo, shares largely the same spectrum to substrates of
ABCB1 modulators. In the case of the second generation reversal
agent, PSC-338 inhibited CYP3A4 and induced the detention of
6-hydroxypaclitaxel in plasma during paclitaxel therapy, thus
greatly increasing the risk of adverse effects (32). Fortunately, GAB did not markedly
affect the CYP3A4 activity at reversal concentrations, indicating
that it may interact with ABCB1 but not CYP3A4. Nevertheless,
further clinical pharmacokinetics and toxicity assessment were
expected to evaluate the side effects in human body.
Collectively, we for the first time report that GAB,
a lanostane type triterpene from Ganoderma lucidum, can
reverse ABCB1-mediated MDR by effectively inhibiting the transport
function of ABCB1 and increasing the intracellular drug
accumulation in MDR cells. GAB does not affect ABCB1 expression or
ATPase in cells.
Acknowledgements
This study was supported by Science and Technology
Program of China (2012ZX09103101-053), Guangzhou City
(2011Y1-00017-11 and 2011J2200045), National Science Foundation of
China (30901847) and Guangdong Province (S2013050014183 and
2013CXZDA006), and Program for New Century Excellent Talents in
University (D.M. Zhang).
References
|
1
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multidrug resistance in cancer chemotherapeutics: mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dean M, Hamon Y and Chimini G: The human
ATP-binding cassette (ABC) transporter superfamily. J Lipid Res.
42:1007–1017. 2001.PubMed/NCBI
|
|
4
|
Breier A, Gibalova L, Seres M, Barancik M
and Sulova Z: New insight into p-glycoprotein as a drug target.
Anticancer Agents Med Chem. 13:159–170. 2013. View Article : Google Scholar
|
|
5
|
Palmeira A, Sousa E, Vasconcelos MH and
Pinto MM: Three decades of P-gp inhibitors: skimming through
several generations and scaffolds. Curr Med Chem. 19:1946–2025.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee CH: Reversing agents for ATP-binding
cassette drug transporters. Methods Mol Biol. 596:325–340. 2010.
View Article : Google Scholar
|
|
7
|
Wang YS, Liao Z, Li YA, et al: New
megastigmane diglycoside from Litsea glutinosa (Lour. ) C B Rob J
Brazil Chem Soc. 22:2234–2238. 2011. View Article : Google Scholar
|
|
8
|
Ansari MT, Saify ZS and Sultana N: Malaria
and artemisinin derivatives: an updated review. Mini-Rev Med Chem.
13:1879–1902. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun J, Yeung CA, Co NN, et al: Clitocine
reversal of P-glycoprotein associated multi-drug resistance through
downregulation of transcription factor NF-κB in R-HepG2 cell line.
PLoS One. 7:e407202012. View Article : Google Scholar
|
|
10
|
Zhang DM, Shu C, Chen JJ, et al: BBA, a
derivative of 23-hydroxybetulinic acid, potently reverses
ABCB1-mediated drug resistance in vitro and in vivo. Mol Pharm.
9:3147–3159. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang DM, Li YJ, Shu C, et al:
Bipiperidinyl derivatives of 23-hydroxybetulinic acid reverse
resistance of HepG2/ADM and MCF-7/ADR cells. Anticancer Drugs.
24:441–454. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao N, Liu DL, Li YJ, et al: B5H7, a
morpholine derivative of 23-hydroxybetulinic acid, reverses
doxorubicin resistance in HepG2/ADM. J Cancer Res Updates. 3:59–66.
2014.
|
|
13
|
Liu DL, Li YJ, Yao N, et al: Acerinol, a
cyclolanstane triterpenoid from Cimicifuga acerina, reverses
ABCB1-mediated multidrug resistance in HepG2/ADM and MCF-7/ADR
cells. Eur J Pharmacol. 733:34–44. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu GS, Guo JJ, Bao JL, et al: Anti-cancer
properties of triterpenoids isolated from Ganoderma lucidum
(Review). Expert Opin Inv Drug. 22:981–992. 2013. View Article : Google Scholar
|
|
15
|
Guan SH, Xia JM, Yang M, Wang XM, Liu X
and Guo DA: Cytotoxic lanostanoid triterpenes from Ganoderma
lucidum. J Asian Nat Prod Res. 10:695–700. 2008. View Article : Google Scholar
|
|
16
|
Wu G, Qian Z, Guo J, et al: Ganoderma
lucidum extract induces G1 cell cycle arrest, and apoptosis in
human breast cancer cells. Am J Chinese Med. 40:631–642. 2002.
View Article : Google Scholar
|
|
17
|
Wang J, Chan JY, Fong CC, Tzang CH, Fung
KP and Yang M: Transcriptional analysis of doxorubicin-induced
cytotoxicity and resistance in human hepatocellular carcinoma cell
lines. Liver Int. 129:1338–1347. 2009. View Article : Google Scholar
|
|
18
|
Wu GS, Lu JJ, Gu JJ, et al: Ganoderic acid
DM, a natural triterpenoid, induces DNA damage, G1 cell cycle
arrest and apoptosis in human breast cancer cells. Fitoterapia.
83:408–414. 2012. View Article : Google Scholar
|
|
19
|
Wang YL, Zhang XQ, Wang GC and Ye WC:
Chemical constituents of the fruiting bodies of Ganoderma lucidum.
Jiangsu Pharm Clin Res. 14:349–351. 2006.
|
|
20
|
Chan JY, Chu AC, Fung KP, et al:
Inhibition of P-glycoprotein expression and reversal of drug
resistance of human hepatoma HepG2 cells by multidrug resistance
gene (mdr1) antisense RNA. Life Sci. 67:2117–2124. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fu LW, Zhang YM, Liang YJ, Yang XP and Pan
QC: The multidrug resistance of tumor cells was reversed by
tetrandrine in vitro and in xenografts derived from human breast
adenocarcinoma MCF-7/adr cells. Eur J Cancer. 38:418–426. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Massart C, Gibassier J, Lucas C, Pourquier
P and Robert J: Expression of the MDR1 gene in five human cell
lines of medullary thyroid cancer and reversal of the resistance to
doxo-rubicine by ciclosporin A and verapamil. B Cancer. 83:39–45.
1996.
|
|
23
|
Aller SG, Yu J, Ward A, et al: Structure
of P-glycoprotein reveals a molecular basis for poly-specific drug
binding. Science. 323:1718–1722. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sharom FJ: Complex interplay between the
P-glycoprotein multidrug efflux pump and the membrane: its role in
modulating protein function. Front Oncol. 4:412014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu CP, Ohnuma S and Ambudkar SV:
Discovering natural product modulators to overcome multidrug
resistance in cancer chemotherapy. Curr Pharm Biotechnol.
12:609–620. 2011. View Article : Google Scholar
|
|
26
|
Li WD, Zhang BD, Wei R, Liu JH and Lin ZB:
Reversal effect of Ganoderma lucidum polysaccharide on multidrug
resistance in K562/ADM cell line. Acta Pharmacol Sin. 29:620–627.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang PP, Xu DJ, Huang C, Wang WP and Xu
WK: Astragaloside IV reduces the expression level of P-glycoprotein
in multidrug-resistant human hepatic cancer cell lines. Mol Med
Rep. 9:2131–2137. 2014.PubMed/NCBI
|
|
28
|
Shi Z, Peng XX, Kim IW, et al: Erlotinib
(Tarceva, OSI-774) antagonizes ATP-binding cassette subfamily B
member 1 and ATP-binding cassette subfamily G member 2-mediated
drug resistance. Cancer Res. 67:11012–11022. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mi YJ, Liang YJ, Huang HB, et al: Apatinib
(YN968D1) reverses multidrug resistance by inhibiting the efflux
function of multiple ATP-bindingcassette transporters. Cancer Res.
70:7981–7991. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Dai CL, Tiwari AK, Wu CP, et al: Lapatinib
(Tykerb, GW572016) reverses multidrug resistance in cancer cells by
inhibiting the activity of ATP-binding cassette subfamily B member
1 and G member 2. Cancer Res. 68:7905–7914. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Martin C, Berridge G, Mistry P, Higgins C,
Charlton P and Callaghan R: The molecular interaction of the high
affinity reversal agent XR9576 with P-glycoprotein. Br J Pharmacol.
128:403–411. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang MH, Figg WD, Ando Y, et al: The
P-glycoprotein antagonist PSC 833 increases the plasma
concentrations of 6alpha-hydroxypaclitaxel, a major metabolite of
paclitaxel. Clin Cancer Res. 7:1610–1617. 2001.PubMed/NCBI
|