Introduction
Butyrate is a short-chain fatty acid that plays a
key role in maintaining intestinal homeostasis. It is principally
derived from ingested dietary fiber and dairy products and is
formed in the colon as a result of fermentation by anaerobic
bacteria (1,2). Butyrate is the principal oxidative
fuel for colonic epithelial cells, unlike other cells that use
glucose and has long been regarded as a potential neutriceutical
anticarcinogen (3). Butyrate has
been shown to reduce the growth and motility of colon cancer cell
lines, as well as inhibit proliferation and induce apoptosis in
vitro (4,5). Inhibition of histone deacetylases
(HDAC) plays an important role in the anticarcinogenic properties
of butyrate (6). When present in
sufficient amounts butyrate and HDAC inhibitors reactivate the
transcription of silenced genes and increase differentiation and
apoptosis in cancer cells (6).
L-carnitine (β-hydroxy-γ-trimethylaminobutyrate) is
a non-essential amino acid synthesized from methionine and lysine
in the liver, kidney and brain. Principally derived from the diet,
L-carnitine is absorbed in the intestine and transferred to other
tissues by recognized transporters. Carnitine plays an important
role in lipid metabolism, in the β-oxidation of fatty acids, and
consequently in the production of cellular energy (7). Carnitine facilitates the transport of
long-chain fatty acids across the mitochondrial inner membrane as
acylcarnitine esters (8). Free
carnitine is available in equilibrium with acetyl L-carnitine
(ALCAR) due to the ubiquitous presence of carnitine
acetyltransferase, which also produces other short chain
acylcarnitines, including butyrylcarnitine (9).
Previous study in our lab reported that carnitine
inhibited the development of precancerous lesions and macroscopic
colonic tumors in AOM-treated C57BL/6J mice (10). We also found a positive effect of
carnitine on butyrate-induced Caco-2 colon carcinoma cell death
(11). However, further analysis
of the beneficial effects of carnitine was limited by its poor
uptake in vitro. Carnitine transport was very limited in
undifferentiated Caco-2 cells, a well established model of colon
cancer, compared to differentiated Caco-2 cells, a model of small
intestinal absorptive epithelial cells. We previously reported that
undifferentiated Caco-2 cells express negligible levels of the
carnitine transporter OCTN2 (12).
We hypothesized that using a cell line with more efficient
carnitine transport would likely increase the beneficial effects of
carnitine and ALCAR alone or in combination with butyrate.
SW480 is a human colon adenocarcinoma cell line that
has been used as an in vitro model for colorectal cancer to
study tumor markers and biochemistry of tumorigenicity (13,14).
In this study, we first identified SW480 as a colon cancer cell
line with relatively high carnitine uptake. We then determined the
effect of carnitine and ALCAR on cancer cell death and apoptosis,
and evaluated possible synergism with butyrate. We also
investigated the mechanisms underlying the beneficial effects by
examining proteins implicated in pathways important for cell
proliferation and survival.
Materials and methods
Cell culture
SW480 and Caco-2 human colon cancer cell lines were
obtained from the American Type Culture Collection (Manassas, VA,
USA). SW480 cells were grown in RPMI medium (Gibco-BRL, Grand
Island, NY, USA) containing 10% FBS. Caco-2 cells, at passage 17,
were grown in MEM supplemented with decomplemented 10% FBS,
penicillin/ streptomycin 1%, and DMEM non-essential amino acid
solution (all from Gibco-BRL). Both cell lines were grown in
75-cm2 plastic flasks (Corning Glass Works, Corning, NY,
USA) and maintained at 37°C in a 95% air - 5% CO2
atmosphere. After confluence (70–90%), cells were split by using
0.05% trypsin (0.5 nM) in EDTA (Gibco-BRL).
Carnitine uptake in colon cancer
cells
Carnitine uptake studies were carried out according
to the method of McCloud et al (15), with minor modifications as reported
in our previous study (12). SW480
cells (between passages 108–110) were plated (5×106/
well) onto 12-well plates (Corning Glass Works) in RPMI medium
containing 5% FBS. Cells were incubated in 1 ml of pre-warmed
Krebs-Ringer phosphate buffer (in mM: 123 NaCl, 4.93 KCl, 1.23
MgSO4, 0.85 CaCl2, 5 glucose, 5 glutamine,
and 20 NaH2PO4), at pH 7.4.
Methyl-L-[3H]-carnitine (Amersham Pharmacia Biotech,
Buckinghamshire, UK) was added at a concentration of 50, 100 or 5
mM. After different incubation periods, the medium was aspirated
and cells were immediately washed with ice-cold phosphate buffer
and digested at 70°C with 0.5 ml of 1 N NaOH. The reaction was
neutralized after 1 h by adding 0.5 ml of 1 N HCl. The cells were
then collected, scintillation liquid was added, and radioactivity
counted. In other series of experiments, higher concentrations of
methyl-L-[3H]-carnitine, as well as Na+, and
Cl− dependence of the transport were tested.
Furthermore, the effect of the amino acid transporter
(ATB0+) substrates on carnitine uptake, such as glycine,
lysine and tryptophan (500 μM and 1 mM), was examined. All the
experiments were conducted at 37°C.
Effect of inhibitors on carnitine
transport by SW480 cells
For this set of experiments, confluent SW480 were
pre-incubated for 30 min with 500 μM D-carnitine, pyrilamine,
tetraethyl-ammonium bromide (TEA), or valproate. These drugs were
all purchased from Sigma (St. Louis, MO, USA).
Western blot analysis of OCTN2
Rabbit polyclonal antibodies were raised against a
synthetic polypeptide from Research Genetics (Huntsville, AL, USA),
5-QWQIQSQTRMQKDGEE SPT-3, corresponding to amino acids 532–550 of
mouse OCTN2 (16). SW480 cells,
undifferentiated (subconfluent) and mature (10–15 days
post-confluence) were tested. The cells were washed, lysed and
sonicated for 20 sec in ice-cold lysis buffer (50 mM Tris, 150 mM
NaCl, 10 mM ethylenediaminetetra-acetic acid, 1% Triton X-100)
containing a mixture of protease inhibitors (Roche Diagnostics,
Indianapolis, IN, USA). After determination of protein
concentrations using the Micro BCA Protein Assay Reagent kit
(Pierce, Rockford, IL, USA), samples were added to sample buffer
[4% SDS, 20% glycerol, 200 mM DTT, 120 mM Tris (pH 6.8), and 0.002%
bromophenol blue] and denatured in a boiling water bath for 5 min.
Samples were then separated by electrophoresis on a 7.5% SDS-PAGE.
The proteins were transferred to a polyvinylidene difluoride (PVDF)
Immobilon membrane (Millipore, Bedford, MA, USA). The PVDF membrane
was then incubated in PBS buffer containing 5% skim milk.
Subsequently, the membranes were incubated overnight with OCTN2
polyclonal anti-peptide antibody (12,17),
then incubated with a secondary, goat anti-rabbit IgG, horseradish
peroxidase-linked whole antibody (Sigma). The membranes were washed
and the ELISA substrate added (Boehringer Mannheim, Laval, QC,
Canada). Molecular weights were estimated by using pre-stained
SDS-PAGE broad-range standards (Bio-Rad, Hercules, CA, USA).
Cell death and apoptosis detection
assays
SW480 cells were cultured as described above, and
once sub-confluence was reached, stimulated with various
concentrations of Na-butyrate (2 and 3 mM), alone or combined with
carnitine (5 mM) or ALCAR (5 mM) for 48 h. The cells were then
stained with propidium iodide (PI) and cell death was verified by
flow cytometry (FACScan, Becton-Dickinson, Mississauga, ON,
Canada). In parallel, the ApoStrand Enzyme-linked Immunosorbent
Assay Apoptosis Detection kit (Biomol Research Laboratories Inc.,
Plymouth, PA, USA) was employed to quantify the number of apoptotic
cells with single-strand DNA breaks.
Western blot analysis of proteins
implicated in cell cycle control and apoptosis
Western blotting was employed to monitor changes in
the levels of acetyl histone H4, p21, dephosphorylated β-catenin,
survivin, cyclin D1, PARP p89 and Bcl-XL. SW480 cells
were treated with butyrate (2 and 3 mM) and carnitine (5 mM) or
ALCAR (5 mM) for 48 h. Lysates were prepared using an ice-cold
lysis buffer containing a mixture of protease inhibitors (as
described above). After determination of protein concentrations
using the Micro BCA Protein Assay Reagent kit, equivalent samples
(40 μg) were resolved using 10 and 15% sodium dodecylsulfate
polyacrylamide gel electrophoresis and then transferred to PVDF
membrane Immobilon. Membranes were blocked in 5% non-fat milk for 1
h and then incubated with acetyl histone H4 (Upstate Biotechnology
Corp., Waltham, MA, USA), dephosphorylated β-catenin (Alexis, San
Diego, CA, USA), phosphorylated p65 (Pharmingen, San Diego, CA,
USA), p21 (Santa Cruz, CA, USA), survivin (Santa Cruz), cyclin D1
(Santa Cruz), PARP p89 (Santa Cruz), Bcl-XL (Santa
Cruz), NF-κB (Santa Cruz), and β-actin (Santa Cruz) primary
antibodies at 4°C overnight. After washing with Tris-buffered
saline/Tween-20, membranes were incubated with the corresponding
peroxidase-conjugated secondary antibody, anti-goat or anti-rabbit
immunoglobulin G, for 1 h, and then developed according to the
enhanced chemiluminescence system (Supersignal West Dura, Pierce).
The levels of these proteins were quantified with the luminescent
image analyzer LAS 4000 (Fuji Film, Tokyo, Japan) and normalized to
β-actin.
Statistical analysis
The data are presented as mean ± SE. Statistical
analysis was performed by GraphPad Prism Software (San Diego, CA,
USA). One-way ANOVA (Dunnett’s multiple comparison test) was used
for group analysis. For continuous variables, the Mann-Whitney
non-parametric test was employed. A p-value of <0.05 was
considered statistically significant.
Results
Carnitine uptake in colon cancer
cells
In order to determine whether carnitine enhances the
anticancer effect of butyrate in SW480, we first evaluated the
capacity of this cell line to transport carnitine; we then
determined the transport characteristics of carnitine into SW480
cells.
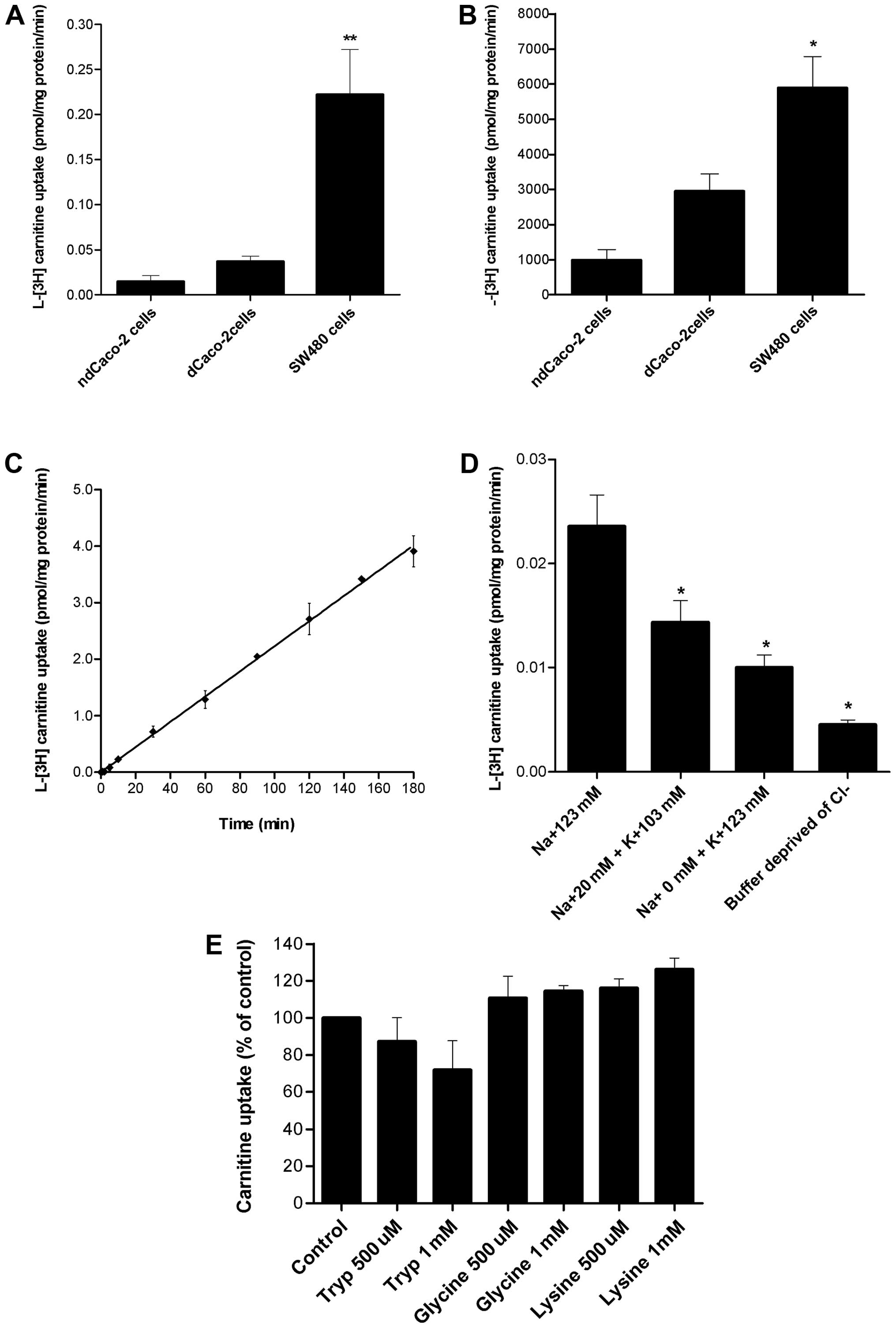
Carnitine uptake by confluent SW480 cells was
compared to that by differentiated and non-differentiated Caco-2
cells. We used a low carnitine concentration as classically
employed to carry out transport studies (12–17);
higher concentrations were tested as well. After 30-min incubation
of 100 nM of L-[3H]-carnitine, the transport by SW480
cells was 8 and 12 times higher (p<0.01) than that by
differentiated and non-differentiated Caco-2 cells (Fig. 1A). Similarly, after 30-min
incubation of 5 mM L-[3H]-carnitine, the transport by
SW480 cells was significantly higher (p<0.05) than that by
differentiated and non-differentiated Caco-2 cells (Fig. 1B).
The SW480 uptake of L-[3H]-carnitine as a
function of time was appreciable and linear with time (r=0.97),
reaching an equilibrium at ~180 min with 3.91 pmol/mg protein
(Fig. 1C).
L-[3H]-carnitine uptake by SW480 cells diminished
significantly (p<0.05) when the luminal Na+
concentration was reduced, and inhibited significantly (p<0.05)
when the Na+ was completely iso-osmotically substituted
by K+, achieved by adding KCl to the transport buffer
(Fig. 1D). These results suggest
that carnitine transport is Na+-coupled in SW480.
The ability of SW480 cells to transfer high
concentrations of carnitine (5 mM) (Fig. 1B) suggests the potential
involvement of another transporter, with lower affinity. To verify
whether carnitine is transported by ATB0,+, a
Cl−-coupled amino acid transporter, we examined the
influence of Cl− on carnitine uptake. Cl−
depleted uptake buffer was used, replacing it with sodium
gluconate. Removal of Cl− from the uptake buffer
significantly reduced (p<0.05) the transport of carnitine,
illustrating the effect of Cl− on this system (Fig. 1D), suggesting a possible role of
transport by ATB0,+. To confirm involvement of
ATB0,+, we investigated the effect of known amino acid
substrates of ATB0,+ on carnitine transport. Neither low
(500 μM) nor high concentrations (1 mM) of tryptophan, glycine or
lysine significantly inhibited carnitine transport in SW480 cells
(Fig. 1E). Taken together, these
results suggest that there is a lack of involvement of
ATB0,+ in this transport system.
We then performed kinetic studies to determine the
Km value for carnitine. At 50 nM
L-[3H]-carnitine, uptake appears to be mediated by a
membrane transporter with a high affinity for carnitine
(Km ~4.3 μM). This value, in the same range as the
reported Km of OCTN2 (18), further supports the involvement of
this transporter in carnitine uptake of by SW480 cells. An
Eadie-Hofstee plot was then examined and the curve was compatible
with a model involving 2 transporters (results not shown). At
higher concentrations of carnitine, the Km value was
~0.7 mM, suggesting that carnitine was also transported by a low
affinity transporter.
Effect of inhibitors on carnitine
transport by SW480 cells
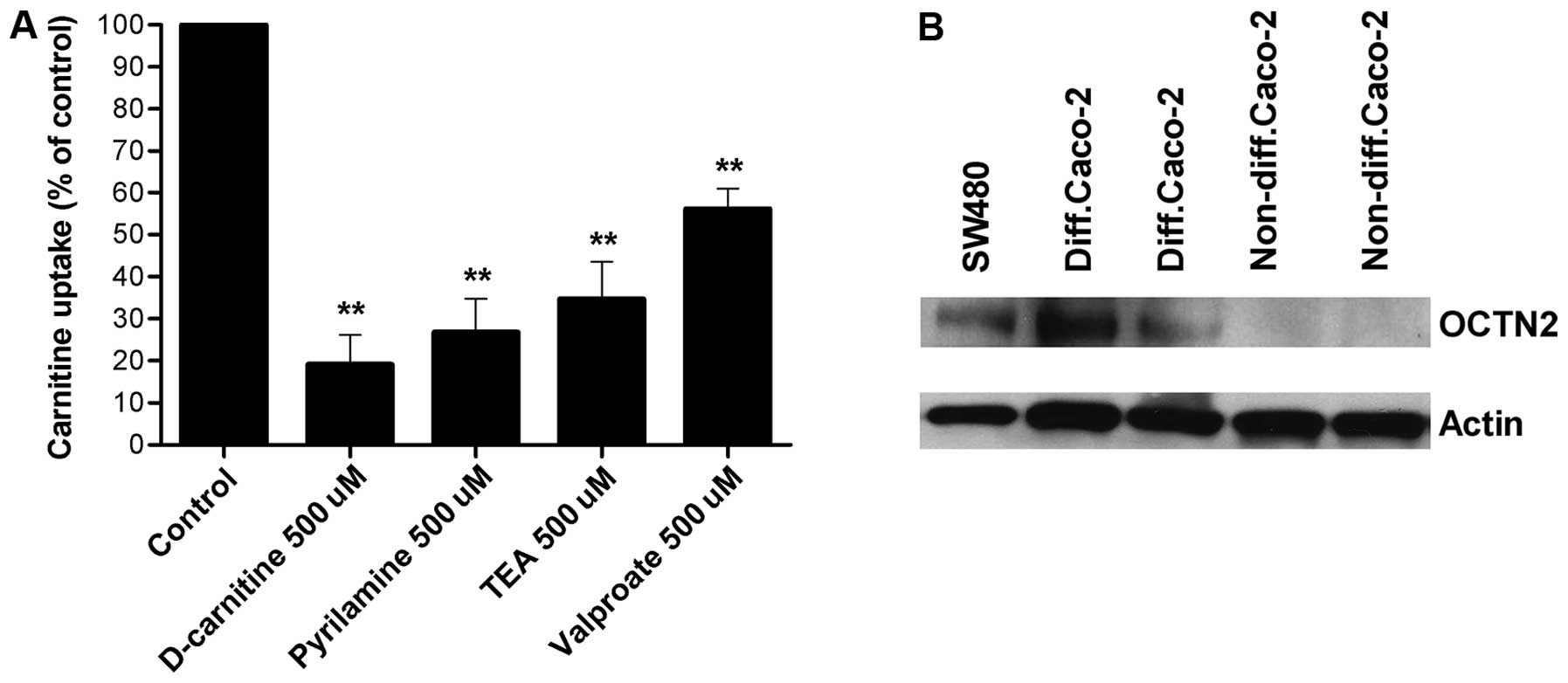
The ability of specific drugs, known to interact
with OCTN2, to inhibit carnitine transport was then investigated
(Fig. 2A). D-carnitine,
pyrilamine, TEA, and valproate all inhibited carnitine transport by
SW480 cells (inhibition 40–80%).
Western blot analysis of OCTN2
The next step was to confirm the role of OCTN2 in
carnitine uptake by western blot analysis. Cell lysates were probed
using polyclonal antibodies. Western blot analysis revealed a
protein with an apparent molecular weight of 67 kDa labeled by the
mouse OCTN2-specific antibody in SW480 cells and in differentiated
Caco-2 cells. No bands were detected in non-differentiated Caco-2
cells (Fig. 2B).
Cell death and apoptosis
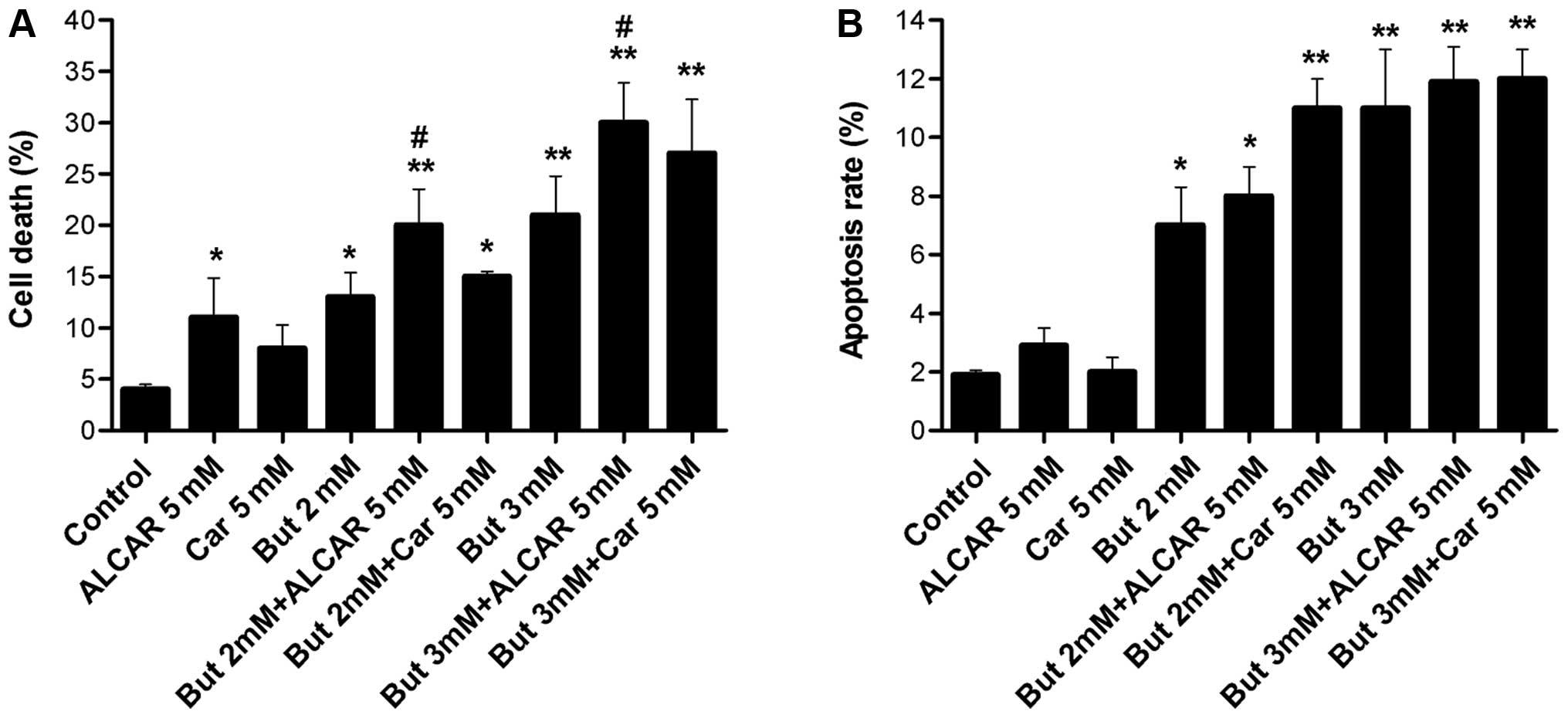
The effects of butyrate (2 or 3 mM) alone or
combined with carnitine (5 mM) or ALCAR (5 mM) on SW480 cell death
were examined after 48 h of incubation. SW480 colon cancer cell
death was significantly increased by 2 and 3 mM butyrate alone
(p<0.01, p<0.05), as well as by ALCAR alone (p<0.05). The
carnitine ester ALCAR potentiated the effects of butyrate, as cell
mortality increased (p<0.05). However, addition of carnitine did
not have a significant effect on SW480 cell death (Fig. 3A).
The results in Fig.
3A show that butyrate alone (2–3 mM) increased apoptosis
fourfold after 48 h, while carnitine and ALCAR alone (5 mM) had no
effect. The combination of butyrate and carnitine or ALCAR slightly
induced apoptotic effect after 48 h, but the difference was not
statistically different when compared to butyrate alone (Fig. 3B).
Western blot analysis of proteins
implicated in cell cycle control and apoptosis
To verify the mechanisms of the action of ALCAR on
SW480 cells, changes in proteins implicated in cell cycle
progression, apoptosis, as well as acetylation of histone proteins
were measured. Butyrate alone induced significant changes in all
the proteins studied, while carnitine and ALCAR had no significant
effect.
Most colorectal cancers have mutations of the APC
gene, allowing nuclear translocation of dephosphorylated β-catenin
and upregulation of target genes, such as survivin and cyclin
D1.
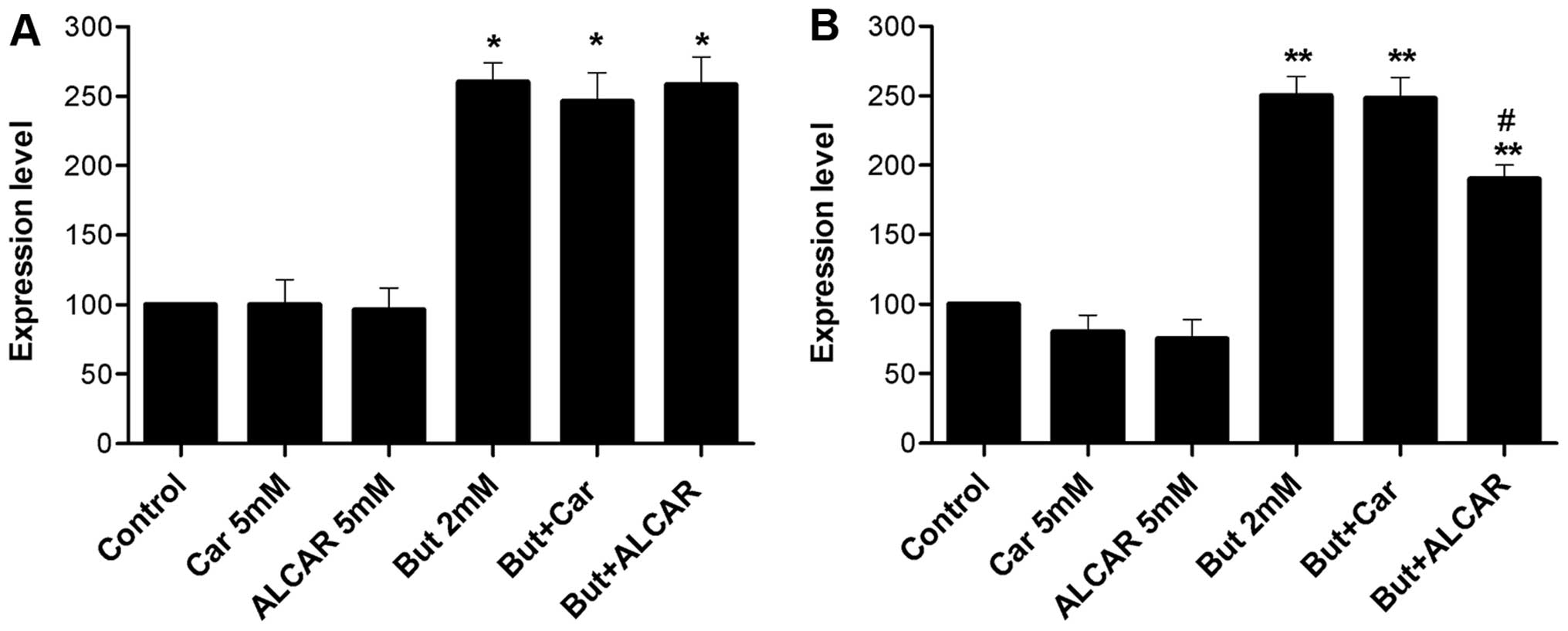
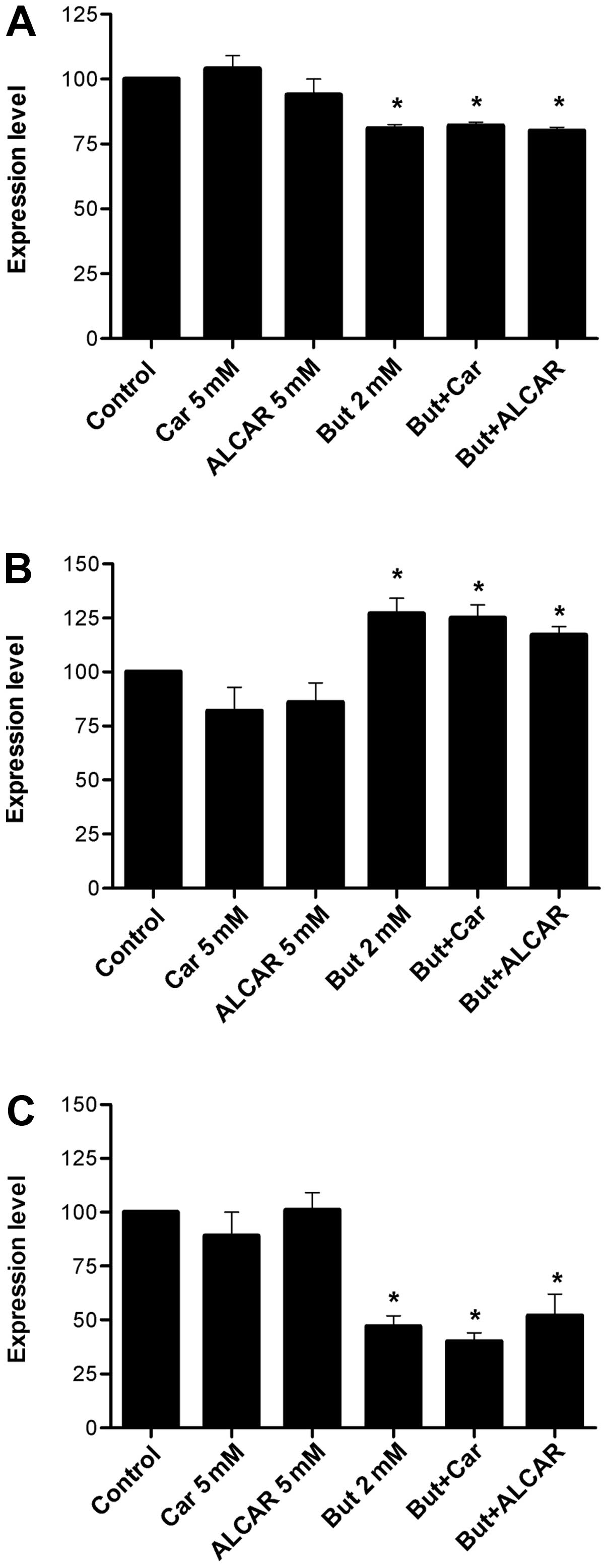
Butyrate alone downregulated dephospho-β-catenin and
increased acetylated histone H4 levels (2.6-fold, p<0.01). When
it was combined with carnitine or ALCAR, the levels of these
proteins were not affected (Figs.
4A and 5A). ALCAR alone
induced a 20% decrease in p21 (p<0.05; Fig. 4B). At butyrate concentrations of 2
mM, carnitine consistently decreased survivin levels (Fig. 5C). No change was observed in cyclin
D1 levels (Fig. 5B).
During apoptosis, caspases cleave poly(ADP-ribose)
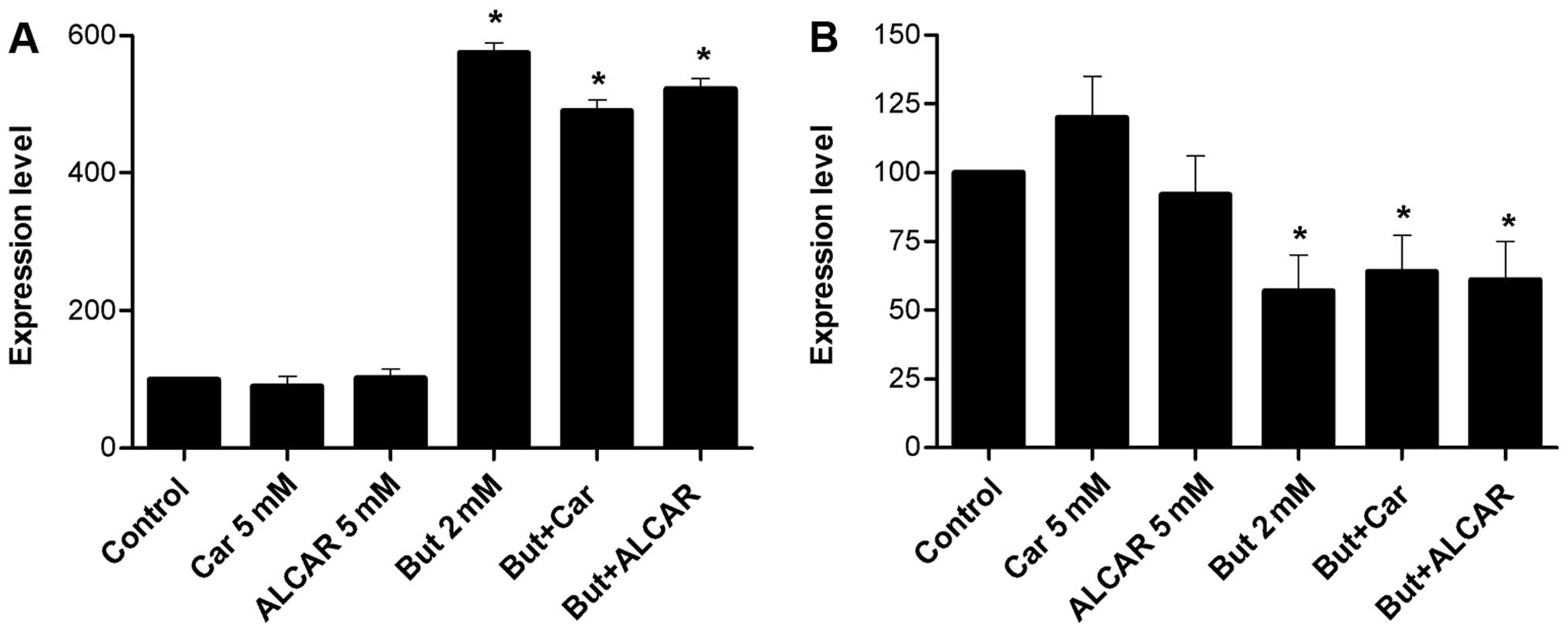
polymerase (PARP). Butyrate increased the level of cleaved PARP
(5.9-fold), whereas it was not observed in response to carnitine or
ALCAR (Fig. 6A).
Discussion
Free carnitine, ALCAR and others carnitine acyl
esters offer protection from oxidative damage (19–21)
by acetylating membrane proteins (22), removing long-chain acyl CoAs from
cell membranes (23) and by
scavenging free radicals (24).
Recent studies investigated by Huang et al, have shown that
carnitine inhibits cancer cell growth in vivo and in
vitro by increasing histone acetylation, inducing accumulation
of acetylated histones and by directly inhibiting HDAC I/II
activities (25). ALCAR, sharing
many of the same beneficial properties as carnitine, is considered
to be more advantageous as a nutriceutical to enhance the effects
of chemotherapy (26). ALCAR
provides a source of acetyl groups that could be used for the
acetylation of proteins involved in cellular response. Studies have
shown that acetyl-L-carnitine has free radical scavenging activity
protecting against hydroxyl free radicals, inhibits oxidant-induced
DNA single-strand breaks and acts as a histone hyperacetylating
agent (27–32). A study by Pisano et al
(33) reported significant
anti-metastatic activity of ALCAR in wild-type p53 tumors and a
significant synergistic effect with a histone deacetylase
inhibitor.
We previously reported that carnitine has a
beneficial impact on butyrate induced colon cancer cell death in
Caco-2 cells (5,11). The mechanisms by which carnitine
modulates butyrate-induced cancer cell death have not been
elucidated. One hypothesis is that carnitine may affect butyrate
availability and metabolism in colon cancer cells. In the course of
our experiments, we noted that carnitine transport was limited in
Caco-2 cells, a cell line that express negligible levels of OCTN2,
a key carnitine transporter (12).
One of the first aims of the present study was to identify a human
colon cancer cell line with more efficient carnitine transport, in
order to determine if the beneficial effects of carnitine and ALCAR
would be increased.
We identified SW480 cells as a colon cancer cell
line with high carnitine uptake (Fig.
1A–C) and express OCTN2 (Fig.
2B). Carnitine transport was significantly reduced (p<0.05)
when the luminal Na+ concentration was reduced or
depleted by substituting KCl for NaCl (Fig. 1D). Uptake was also inhibited
significantly (p<0.05) in Cl−-depleted buffer
(Fig. 1D). These results are
consistent with Na+-dependent carrier-mediated system
for L-carnitine in SW480 cells.
At low carnitine concentrations typically used to
study transport, [3H]-carnitine uptake by SW480 cells
was 8 and 12 times higher than that in differentiated and
non-differentiated Caco-2 cells, respectively (Fig. 1A–C). These differences were
attenuated at higher concentrations. At a carnitine concentration
of 5 mM, uptake by SW480 cells was 6 times higher than that by
non-differentiated Caco-2 cells (Fig.
1A and B). At 50 nM concentration, carnitine transport appeared
to be mediated by a high affinity membrane transporter with a
Km ~4.3 μM. This in the same range as that reported for
OCTN2 (18). Furthermore,
carnitine transport by SW480 cells was found to be
Na+-dependent (Fig.
1D). We also examined the effect of D-carnitine and certain
cationic drugs known to inhibit L-carnitine transport by OCTN2
(12,34,35).
Pyrilamine, TEA and valproate, competitively inhibit carnitine
transport (Fig. 2A).
Western blot experiments using an OCTN2-specific
antibody confirmed the presence of OCTN2 in SW480 cells (Fig. 2B). The molecular size of the
detected band is close to the estimated size (63 kDa) of OCTN2
(12,17,36).
As we reported previously (11),
differentiated Caco-2 cells (a model of small intestinal absorptive
cells) express OCTN2 (Fig. 2B),
whereas non-differentiated Caco-2 cells do not, in keeping with the
absence of transport of L-carnitine in malignant Caco-2 cells.
Recent studies (30,37,38)
have shown that OCTN2 is also involved in the transport of ALCAR.
This suggests that ALCAR uptake by SW480 will potentially enhance
the synergistic effect of butyrate on these cancer cells.
An Eadie-Hofstee plot was compatible with a model
involving 2 transporters in SW480 cells. At higher doses, carnitine
was transported by a low affinity (~0.7 mM) transporter. Uptake at
5 mM carnitine was also Na+-dependent; as well as
Cl-dependent, implicating the ATB0,+ transporter
(39,40). At low concentration, carnitine
transport in SW480 cells was not inhibited by tryptophane, glycine
or lysine, amino acid substrates of ATB0,+. Studies at
high concentration were physiologically irrelevant due to the high
concentrations (>100-fold excess) required to inhibit carnitine
transport. There are no suitable commercially available antibodies
specific for ATB0,+ to carry out western blot analyses.
SW480 cells were found to express low levels of ATB0,+
by RT-PCR (41). Nevertheless, the
transport characteristics observed are not sufficient to eliminate
members of the OCTN family, and identify ATB0,+ as the
transporter involved in carnitine transport at high
concentrations.
Once we identified the relatively high carnitine
uptake by SW480, we proceeded with experiments to determine the
effect of carnitine and ALCAR alone or with butyrate, on inducing
apoptosis and cell death in these colon cancer cells. Butyrate
alone (2 and 3 mM) reduced cell growth (p<0.05 and p<0.01).
ALCAR but not carnitine significantly increased SW480 cell death
(10.2 vs. 4.6%, p<0.05). Cells treated with a combination of
butyrate and ALCAR significantly increased the mortality of cancer
cells (p<0.05, Fig. 3A).
We next examined if the same combined treatment
induced apoptosis. Butyrate alone induced apoptosis in SW480 cells.
Carnitine and ALCAR increased butyrate-induced apoptosis by 8–14%,
although the difference failed to reach statistical significance.
ALCAR (10 mM) was shown to enhance the p53 activation and sensitize
several cancer cells lines to cisplatin. However, it was not
effective in sensitizing PC-3 and the colon cancer cells SW620 and
HT-29, in which p53 is null or mutated (33). Our study is therefore the first one
to show that ALCAR can enhance cell death induced by an
anticarcinogenic compound in cancer cells with mutated p53. The
results concerning apoptosis (Fig.
3B) and PARP suggest the involvement of caspase-independent
pathways in this antitumor effect.
Epigenic alterations of histone and non-histone
proteins are a central event in the initiation and progression of
cancer. Histone deacetylase inhibitors such as butyrate can
reactivate the transcription of silenced genes and restore normal
cellular growth and differentiation (6). We therefore studied the effect of
butyrate, carnitine and ALCAR on histone acetylation. Butyrate
increased histone 4 acetylation while carnitine or ALCAR had no
effect. When used in combination with butyrate, carnitine and ALCAR
did not induce changes compared to butyrate alone. Thus, despite
the fact that uptake of ALCAR can supply the cell with acetyl
groups (42), acetylation of
histones was not increased (Fig.
4A). This may be due to the existence of compartmented pools of
acetyl inside the cells and limited access to the nucleus.
Alternatively, the efficiency of this transfer that relies on the
activity of nuclear carnitine/acylcarnitine translocase might be
lower in cancer cells. Another explanation might be that there is
already a good supply of intracellular acetyl groups that is
minimally affected by exogenous carnitine.
We undertook the study of key proteins implicated in
cell cycle progression and apoptosis (p21, cyclin D1, survivin,
Bcl-XL). The p21 protein is a cyclin-dependent kinase
inhibitor that mediates cell cycle progression through G1 phase
arrest. Expression of p21 coincides with hyperacetylation of
histones H3 and H4 in the promoter region. We showed that butyrate
induced p21 expression, consistent with other studies (43,44).
Although the role of p21 on cell cycle progression is well
established, controversy exists whether p21 is pro- or
anti-apoptotic. p21 is an important downstream effector of
p53-signaling. SW480 cells contain a mutated form of p53,
presumably without pro-apoptotic potential, but with some
transcriptional activity such as the ability to induce p21
(45). Paradoxically, p21 has been
shown to promote apoptosis as well as to protect tumor cells from
apoptosis, particularly when DNA-damaging agents are used. Enhanced
p21 expression was associated with increased apoptosis and
protection in colon carcinogenesis in rats provided fish
oil-supplemented diets. In contrast enhanced p21 led to higher
levels of aberrant crypt formation in rats provided corn-oil
supplemented diet (43). We
observed that ALCAR decreased butyrate-induced p21 upregulation
(Fig. 4B).
We furthermore analyzed the capacity of
carnitine/ALCAR to alter β-catenin expression in SW480 cells. Most
colorectal cancers have mutations of the adenomatous polyposis coli
(APC) gene that stabilize β-catenin, allowing nuclear translocation
and upregulation of β-catenin target genes, notably survivin,
cyclin D1 and c-myc (46).
Phosphorylation directs β-catenin towards degradation and
non-phosphorylated β-catenin translocates to the nucleus. Levels of
dephosphorylated β-catenin were assessed following treatment of
SW480 with butyrate. Butyrate downregulated dephospho-β-catenin by
20%, while carnitine and ALCAR had no appreciable effect (Fig. 5A).
Cyclin D1 is a downstream molecule of the βcatenin
pathway that plays an important role in cell cycle progression as
it drives the G1/S phase transition (47). Cyclin D1 levels were 27% increased
by butyrate (Fig. 5B). This
increase is distinctive to SW480 cells, as cyclin D1 level is
decreased by butyrate in Caco-2 and HT-29 colon cancer cells
(48). Cyclin D1 expression was
decreased by 19–20% when SW480 cells were treated with carnitine
and ALCAR. Combined treatment with butyrate resulted in levels
similar to butyrate treatment alone. Therefore, although single
agents have opposite effects on cyclin D1, the butyrate effect
predominates when the compounds were provided together. Increased
cyclin D1 upon butyrate treatment appears to contradict its effect
on the Wnt/β-catenin pathway. Similar induction of cyclin D1
despite inhibition of proliferation in SW480 cells was already
observed. It was postulated that combined c-Myc reduction and p21
induction had more determinant effect than cyclin D1 on cell cycle
progression (45).
Survivin is an anti-apoptotic protein frequently
upregulated in cancer cells. Survivin levels were markedly
decreased by butyrate (50% reduction) using a lower dose of
butyrate (2 mM), as a 3-mM dose decreased survivin by >80% (not
shown). These data are congruent with other findings indicating the
growth-inhibitory action of butyrate by decreasing survivin
expression in Caco-2 cells and by increase caspase-3 activity,
cleavage of PARP and caspase-8 (49). When cells were treated by
carnitine, a trend towards decreased survivin levels (88% of basal
levels) was observed. The trend was maintained when carnitine was
added to butyrate (55% reduction vs. 50%). However, these changes
did not reach statistical significance due to variability between
repeat experiments and the low overall magnitude (Fig. 5B).
Poly(ADP-ribose) polymerase (PARP) is cleaved by
caspases during apoptosis and is therefore a marker of
caspase-dependent apoptosis (49).
As expected, PARP cleavage was increased by butyrate (5.9-fold).
This was not modified by carnitine or ALCAR (Fig. 6A). Bcl-XL is an
anti-apoptotic protein that is often upregulated in cancer cells.
Levels of Bcl-XL were decreased by butyrate, whereas
carnitine and ALCAR had no effect on its expression (Fig. 6B).
In conclusion, our findings suggest that butyrate
and acetyl-carnitine are potentially beneficial anticarcinogenic
nutrients that inhibit colon cancer cell survival. The combination
of both nutrients may have superior anticarcinogenic properties
than the single agents alone. Recently, the efficacy of carnitine
and acylcarnitine to slow down the growth of colon cancer was
reported in a murine model using the 1,2,-dimethylhydrazine
(DMH)-induced colon carcinogenesis model (50). Further research in this area is
needed to determine whether carnitines may be useful in the human
setting.
Acknowledgements
This study was supported by a research grant (IQ,
EGS) co-funded by the Dairy Farmers of Canada and the National
Science and Engineering Council of Canada (NSERC). Funding for
equipment was provided by the Canadian Foundation for Innovation
(EGS). Funding support for salary was awarded by a Canada Research
Chair in Immune Mediated Gastrointestinal Disorders (EGS), the
Bruce Kaufman Chair at McGill (EGS) and the J.A. de Sève Research
Chair in Nutrition (EL).
References
|
1
|
Hamer HM, Jonkers D, Venema K, Vanhoutvin
S, Troost FJ and Brummer RJ: The role of butyrate on colonic
function. Aliment Pharmacol Ther. 27:104–119. 2008. View Article : Google Scholar
|
|
2
|
Macfarlane GT and Macfarlane S:
Fermentation in the human large intestine: its physiologic
consequences and the potential contribution of prebiotics. J Clin
Gastroenterol. 45(Suppl): S120–S127. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Clarke JM, Young GP, Topping DL, Bird AR,
Cobiac L, Scherer BL, Winkler JG and Lockett TJ: Butyrate delivered
by butyrylated starch increases distal colonic epithelial apoptosis
in carcinogen-treated rats. Carcinogenesis. 33:197–202. 2012.
View Article : Google Scholar :
|
|
4
|
Wang HG, Huang XD, Shen P, Li LR, Xue HT
and Ji GZ: Anticancer effects of sodium butyrate on hepatocellular
carcinoma cells in vitro. Int J Mol Med. 31:967–974.
2013.PubMed/NCBI
|
|
5
|
Ruemmele FM, Dionne S, Qureshi I, Sarma
DS, Levy E and Seidman EG: Butyrate mediates Caco-2 cell apoptosis
via upregulation of pro-apoptotic BAK and inducing caspase-3
mediated cleavage of poly-(ADP-ribose) polymerase (PARP). Cell
Death Differ. 6:729–735. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berni Canani R, Di Costanzo M and Leone L:
The epigenetic effects of butyrate: potential therapeutic
implications for clinical practice. Clin Epigenetics. 4:42012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Reuter SE and Evans AM: Carnitine and
acylcarnitines: pharmacokinetic, pharmacological and clinical
aspects. Clin Pharmacokinet. 51:553–572. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steiber A, Kerner J and Hoppel CL:
Carnitine: a nutritional, biosynthetic, and functional perspective.
Mol Aspects Med. 25:455–473. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hoppel C: The role of carnitine in normal
and altered fatty acid metabolism. Am J Kidney Dis. 41(Suppl 4):
S4–S12. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dionne S, Elimrani I, Roy MJ, Qureshi IA,
Sarma DR, Levy E and Seidman EG: Studies on the chemopreventive
effect of carnitine on tumorigenesis in vivo, using two
experimental murine models of colon cancer. Nutr Cancer.
64:1279–1287. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Roy MJ, Dionne S, Marx G, Qureshi I, Sarma
D, Levy E and Seidman EG: In vitro studies on the inhibition of
colon cancer by butyrate and carnitine. Nutrition. 25:1193–1201.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Elimrani I, Lahjouji K, Seidman E, Roy MJ,
Mitchell GA and Qureshi I: Expression and localization of organic
cation/carnitine transporter OCTN2 in Caco-2 cells. Am J Physiol
Gastrointest Liver Physiol. 284:G863–G871. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu MH and Zhang GY: Effect of indomethacin
on cell cycle proteins in colon cancer cell lines. World J
Gastroenterol. 11:1693–1696. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao L, Liu L, Wang S, Zhang YF, Yu L and
Ding YQ: Differential proteomic analysis of human colorectal
carcinoma cell lines metastasis-associated proteins. J Cancer Res
Clin Oncol. 133:771–782. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCloud E, Ma TY, Grant KE, Mathis RK and
Said HM: Uptake of L-carnitine by a human intestinal epithelial
cell line, Caco-2. Gastroenterology. 111:1534–1540. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Koizumi A, Nozaki J, Ohura T, Kayo T, Wada
Y, Nezu J, Ohashi R, Tamai I, Shoji Y, Takada G, et al: Genetic
epidemiology of the carnitine transporter OCTN2 gene in a Japanese
population and phenotypic characterization in Japanese pedigrees
with primary systemic carnitine deficiency. Hum Mol Genet.
8:2247–2254. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lahjouji K, Elimrani I, Lafond J, Leduc L,
Qureshi IA and Mitchell GA: L-Carnitine transport in human
placental brush-border membranes is mediated by the
sodium-dependent organic cation transporter OCTN2. Am J Physiol
Cell Physiol. 287:C263–C269. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohashi R, Tamai I, Nezu Ji J, Nikaido H,
Hashimoto N, Oku A, Sai Y, Shimane M and Tsuji A: Molecular and
physiological evidence for multifunctionality of carnitine/organic
cation transporter OCTN2. Mol Pharmacol. 59:358–366.
2001.PubMed/NCBI
|
|
19
|
Virmani A and Binienda Z: Role of
carnitine esters in brain neuropathology. Mol Aspects Med.
25:533–549. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang B, Nishikawa M, Nishiguchi S and
Inoue M: L-carnitine inhibits hepatocarcinogenesis via protection
of mitochondria. Int J Cancer. 113:719–729. 2005. View Article : Google Scholar
|
|
21
|
Zhang R, Zhang H, Zhang Z, Wang T, Niu J,
Cui D and Xu S: Neuroprotective effects of pre-treatment with
l-carnitine and acetyl-L-carnitine on ischemic injury in vivo and
in vitro. Int J Mol Sci. 13:2078–2090. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pettegrew JW, Levine J and McClure RJ:
Acetyl-L-carnitine physical-chemical, metabolic, and therapeutic
properties: relevance for its mode of action in Alzheimer’s disease
and geriatric depression. Mol Psychiatry. 5:616–632. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Branca D, Toninello A, Scutari G, Florian
M, Siliprandi N, Vincenti E and Giron GP: Involvement of long-chain
acyl CoA in the antagonistic effects of halothane and L-carnitine
on mitochondrial energy-linked processes. Biochem Biophys Res
Commun. 139:303–307. 1968. View Article : Google Scholar
|
|
24
|
Gulcin I: Antioxidant and antiradical
activities of L-carnitine. Life Sci. 78:803–811. 2006. View Article : Google Scholar
|
|
25
|
Huang H, Liu N, Guo H, Liao S, Li X, Yang
C, Liu S, Song W, Liu C, Guan L, et al: L-carnitine is an
endogenous HDAC inhibitor selectively inhibiting cancer cell growth
in vivo and in vitro. PLoS One. 7:e490622012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pisano C, Pratesi G, Laccabue D, Zunino F,
Lo Giudice P, Bellucci A, Pacifici L, Camerini B, Vesci L,
Castorina M, et al: Paclitaxel and cisplatin-induced neurotoxicity:
a protective role of acetyl-L-carnitine. Clin Cancer Res.
9:5756–5767. 2003.PubMed/NCBI
|
|
27
|
Pascale E, Battiloro E, Cimino Reale G,
Pietrobono R, Pomponi MG, Chiurazzi P, Nicolai R, Calvani M, Neri G
and D’Ambrosio E: Modulation of methylation in the FMR1 promoter
region after long term treatment with L-carnitine and
acetyl-L-carnitine. J Med Genet. 40:e762003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rosca MG, Lemieux H and Hoppel CL:
Mitochondria in the elderly: is acetylcarnitine a rejuvenator? Adv
Drug Deliv Rev. 61:1332–1342. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tabolacci E, Pietrobono R, Moscato U,
Oostra BA, Chiurazzi P and Neri G: Differential epigenetic
modifications in the FMR1 gene of the fragile X syndrome after
reactivating pharmacological treatments. Eur J Hum Genet.
13:641–648. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Loots DT, Mienie LJ, Bergh JJ and Van der
Schyf CJ: AcetylL-carnitine prevents total body hydroxyl free
radical and uric acid production induced by
1-methyl-4-phenyl-1,2,3,6-tetrahy-dropyridine (MPTP) in the rat.
Life Sci. 75:1243–1253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Boerrigter ME, Franceschi C,
Arrigoni-Martelli E, Wei JY and Vijg J: The effect of L-carnitine
and acetyl-L-carnitine on the disappearance of DNA single-strand
breaks in human peripheral blood lymphocytes. Carcinogenesis.
14:2131–2136. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schinetti ML, Rossini D, Greco R and
Bertelli A: Protective action of acetylcarnitine on NADPH-induced
lipid peroxidation of cardiac microsomes. Drugs Exp Clin Res.
13:509–515. 1987.PubMed/NCBI
|
|
33
|
Pisano C, Vesci L, Milazzo FM, Guglielmi
MB, Fodera R, Barbarino M, D’Incalci M, Zucchetti M, Petrangolini
G, Tortoreto M, et al: Metabolic approach to the enhancement of
antitumor effect of chemotherapy: a key role of acetyl-L-carnitine.
Clin Cancer Res. 16:3944–3953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ohashi R, Tamai I, Yabuuchi H, Nezu JI,
Oku A, Sai Y, Shimane M and Tsuji A: Na(+)-dependent carnitine
transport by organic cation transporter (OCTN2): its
pharmacological and toxicological relevance. J Pharmacol Exp Ther.
291:7787–84. 1999.
|
|
35
|
Glube N, Closs E and Langguth P:
OCTN2-mediated carnitine uptake in a newly discovered human
proximal tubule cell line (Caki-1). Mol Pharm. 4:160–168. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lahjouji K, Mitchell GA and Qureshi IA:
Carnitine transport by organic cation transporters and systemic
carnitine deficiency. Mol Genet Metab. 73:2872–2897. 2001.
View Article : Google Scholar
|
|
37
|
Cano MM, Calonge ML and Ilundain AA:
Expression of OCTN2 and OCTN3 in the apical membrane of rat renal
cortex and medulla. J Cell Physiol. 223:451–459. 2010.PubMed/NCBI
|
|
38
|
Tachikawa M, Takeda Y, Tomi M and Hosoya
K: Involvement of OCTN2 in the transport of acetyl-L-carnitine
across the inner blood-retinal barrier. Invest Ophthalmol Vis Sci.
51:430–436. 2010. View Article : Google Scholar
|
|
39
|
Nakanishi T, Hatanaka T, Huang W, Prasad
PD, Leibach FH, Ganapathy ME and Ganapathy V: Na+- and
Cl−-coupled active transport of carnitine by the amino
acid transporter ATB(0,+) from mouse colon expressed in HRPE cells
and Xenopus oocytes. J Physiol. 532:297–304. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nakanishi T, Kekuda R, Fei YJ, Hatanaka T,
Sugawara M, Martindale RG, Leibach FH, Prasad PD and Ganapathy V:
Cloning and functional characterization of a new subtype of the
amino acid transport system N. Am J Physiol Cell Physiol.
281:C1757–C1768. 2001.PubMed/NCBI
|
|
41
|
Karunakaran S, Umapathy NS, Thangaraju M,
Hatanaka T, Itagaki S, Munn DH, Prasad PD and Ganapathy V:
Interaction of tryptophan derivatives with SLC6A14
(ATB0,+) reveals the potential of the transporter as a
drug target for cancer chemotherapy. Biochem J. 414:343–355. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Madiraju P, Pande SV, Prentki M and
Madiraju SR: Mitochondrial acetylcarnitine provides acetyl groups
for nuclear histone acetylation. Epigenetics. 4:399–403. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu S, Dong TS, Dalal SR, Wu F, Bissonnette
M, Kwon JH, Chang EB, et al: The microbe-derived short chain fatty
acid butyrate targets miRNA-dependent p21 gene expression in human
colon cancer. PLoS One. 6:e162212011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Crim KC, Sanders LM, Hong MY, Taddeo SS,
Turner ND, Chapkin RS and Lupton JR: Upregulation of
p21Waf1/Cip1 expression in vivo by butyrate
administration can be chemoprotective or chemopromotive depending
on the lipid component of the diet. Carcinogenesis. 29:1415–1420.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hartman J, Edvardsson K, Lindberg K, Zhao
C, Williams C, Strom A and Gustafsson JA: Tumor repressive
functions of estrogen receptor beta in SW480 colon cancer cells.
Cancer Res. 69:6100–6106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bottomly D, Kyler SL, McWeeney SK and
Yochum GS: Identification of {beta}-catenin binding regions in
colon cancer cells using ChIP-Seq. Nucleic Acids Res. 5735–5745.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ravichandran K, Velmurugan B, Gu M, Singh
RP and Agarwal R: Inhibitory effect of silibinin against
azoxymethane-induced colon tumorigenesis in A/J mice. Clin Cancer
Res. 16:4595–4606. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Nepelska M, Cultrone A, Béguet-Crespel F,
Le Roux K, Doré J, Arulampalam V and Blottière HM: Butyrate
produced by commensal bacteria potentiates phorbol esters induced
AP-1 response in human intestinal epithelial cells. PLoS One.
7:e528692012. View Article : Google Scholar
|
|
49
|
Schwab M, Reynders V, Steinhilber D and
Stein J: Combined treatment of Caco-2 cells with butyrate and
mesalazine inhibits cell proliferation and reduces survivin protein
level. Cancer Lett. 273:98–106. 2009. View Article : Google Scholar
|
|
50
|
Roscilli G, Marra E, Mori F, Di Napoli A,
Mancini R, Serlupi-Crescenzi O, Virmani A, Aurisicchio L and
Ciliberto G: Carnitines slow down tumor development of colon cancer
in the DMH-chemical carcinogenesis mouse model. J Cell Biochem.
114:1665–1673. 2013. View Article : Google Scholar : PubMed/NCBI
|