Introduction
Epithelial ovarian cancer is the most lethal
gynecological cancer (1).
Cytoreductive surgery with chemotherapy is the standard of care for
ovarian cancer (2). However,
20–40% of patients do not respond to first-line chemotherapy
(3). Furthermore, a large
proportion of patients will have a relapse of the disease within 5
years (1), especially those in
advanced stage. Unfortunately, recurrence is typically less
responsive to current chemotherapeutic strategies (1).
Angiogenesis plays an important role in the growth
and progression of solid tumors (4). Tumor angiogenesis is characterized by
the formation of new irregular blood vessels from a preexisting
vascular network (5). Tumor
vasculature usually has poor blood flow and high vascular
permeability, which may lead to decreased efficiency of cytotoxic
chemotherapy and increased potential for metastasis (6). Angiogenesis can be regulated by many
signaling molecules and growth factors, including vascular
endothelial growth factor (VEGF). VEGF is a crucial factor in
modulating multiple vascular steps. VEGF expression can be
upregulated by hypoxia-inducible factor 1 (HIF-1). HIF-1 is a
basic-loop helix PER-ARNT-SIM transcription factor consisting of
two subunits, HIF-1α and HIF-1β. Overexpression of HIF-1α has been
demonstrated in >70% of human cancers and metastases compared to
adjacent normal tissue (7).
Stabilization and upregulation of HIF-1α promotes the expression of
VEGF by binding to HIF-responsive elements in promoters. Therefore,
anti-angiogenic agents targeting HIF-1α and VEGF are highlighted
for anticancer treatment.
Black tea is one of the most popular beverages
worldwide. Basic procedures of making black tea include withering,
rolling, fermentation, and drying. During the fermentation process,
green tea polyphenols are polymerized and oxidized to form
oligomeric flavanols, such as theaflavins, thearubigin and other
oligomers (8). Thus black tea has
low tea catechin content (9).
Theaflavins account for 2–6% of the dry weight of solids in brewed
black tea (9). Theaflavin-3,
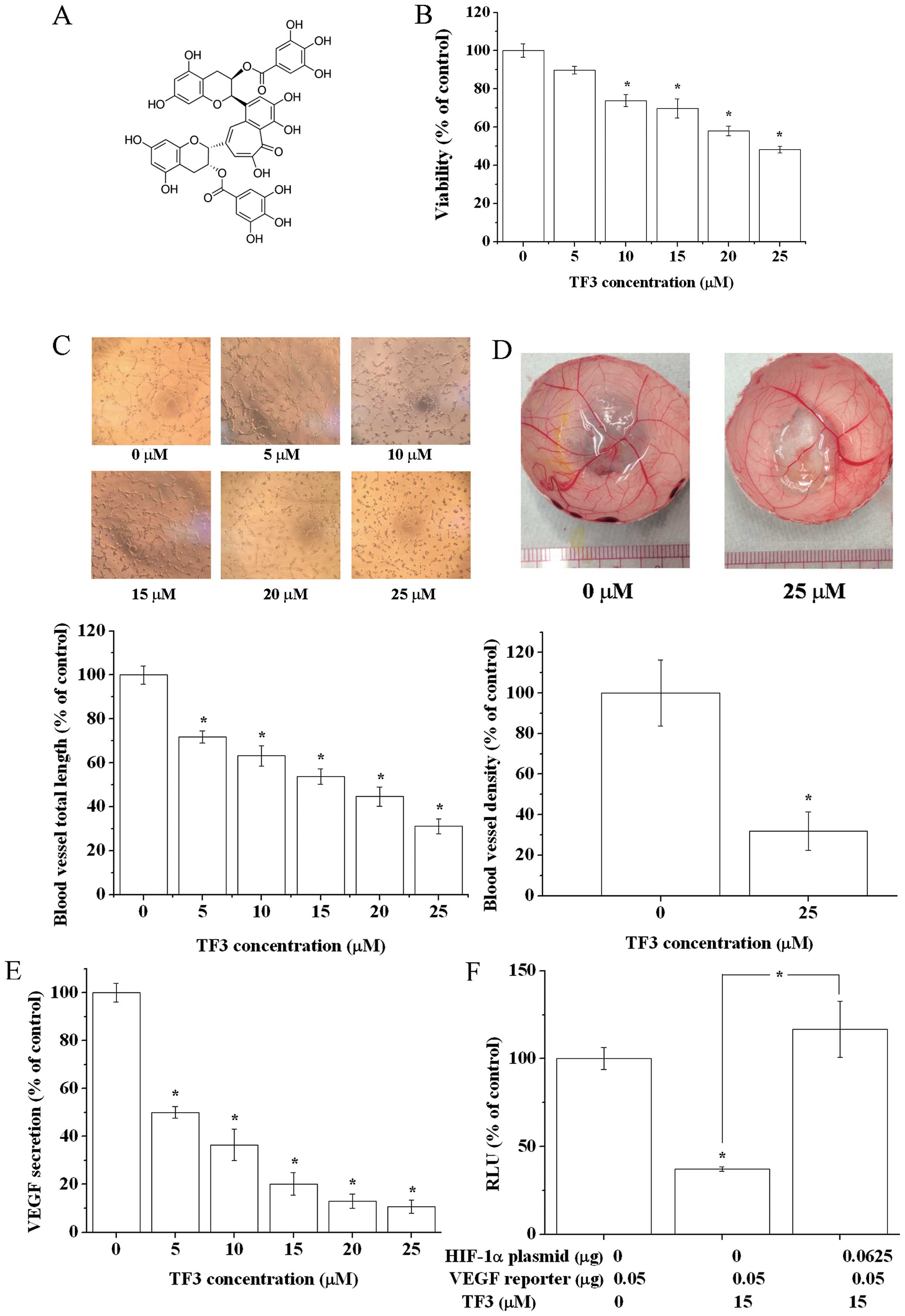
3′-digallate (TF3) (Fig. 1A) is
one of the four main theaflavins in black tea, which is produced by
the oxidative dimerization of epicatechin gallate (ECG) and
(−)-epigallocatechin-3-gallate (EGCG). TF3 is a potent anticancer
agent. It showed inhibitory effects on the growth of various human
cancer cells (10). It induced
apoptosis and cell cycle arrest in cancer cells by generating
reactive oxygen species and/or modulating signaling pathways
(8,11,12).
Recently, theaflavins were observed to exhibit anti-angiogenic
activities. TF3 inhibited tube formation in cocultured endothelial
cells with fibroblasts (13). In
human prostate cancer-bearing athymic nude mice, theaflavins
significantly diminished the expression of VEGF in tumors (14). Nevertheless, the mechanisms remain
unclear.
In the present study, the antitumor-induced
angiogenic effect and mechanisms of TF3 were investigated. TF3
impaired human ovarian carcinoma OVCAR-3 cell-induced angiogenesis.
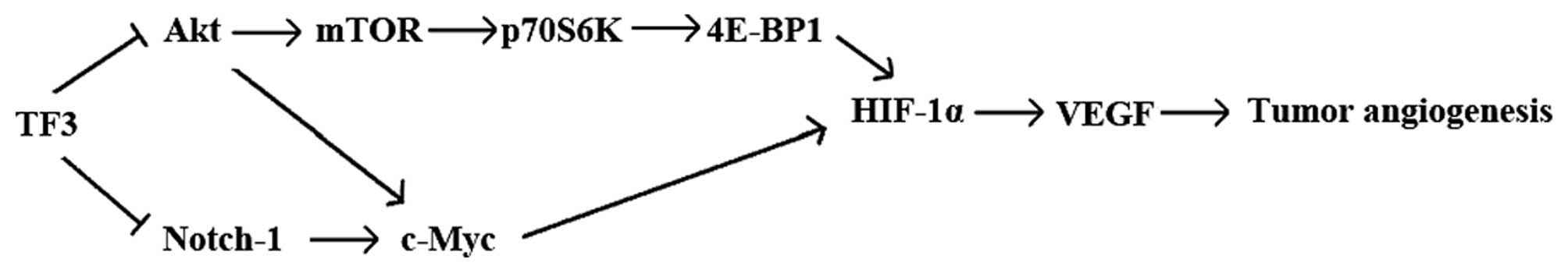
Part of the underlying mechanisms was TF3 targeted
Akt/mTOR/p70S6K/4E-BP1 pathway, Akt/c-Myc pathway and Notch-1/c-Myc
pathway to regulating HIF-1α and VEGF expression in OVCAR-3 cells.
Our study suggests that TF3 can be regarded as a candidate
anti-angiogenic agent for the adjunctive therapy of cancer.
Materials and methods
Cell cultures and reagents
Human ovarian carcinoma cell line OVCAR-3, a kind
gift from Dr Bing-Hua Jiang, Thomas Jefferson University, was
cultured in RPMI-1640 medium (Sigma, St. Louis, MO, USA)
incorporating 10% fetal bovine serum (FBS) (Invitrogen, Grand
Island, NY, USA). Human umbilical vein endothelial cells (HUVECs),
purchased from American Type Culture Collection (ATCC, Manassas,
VA, USA), were maintained in F-12K medium (ATCC) supplemented with
10% FBS (Invitrogen) and Endothelial Cell Growth Kit-VEGF (ATCC).
Cells were grown in a humidified incubator containing 5%
CO2 at 37ºC.
Reagents
TF3 monomer (purity: 92.4%) was isolated and
purified using previously established method (15). Wortmannin and
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester (DAPT) were purchased from Sigma and Santa Cruz Biotechnology
(Santa Cruz, CA, USA), respectively. Antibodies against phospho-Akt
(Ser473) (p-Akt), Akt, phospho-p70S6 kinase (Thr421/Ser424)
(p-p70S6K), p70S6K, eukaryotic initiation factor 4E-binding
protein-1 (4E-BP1), HIF-1α, Notch-1, c-Jun N-terminal kinases
(JNK), p38 and phosphor-Forkhead Box O1 (Thr24) (p-FoxO1) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
Antibodies against phosphor-mammalian target of rapamycin (p-mTOR)
(Ser2448), mTOR were purchased from R&D Systems (Minneapolis,
MN, USA). Antibodies against phosphor-4E-BP1 (Ser65/Thr70)
(p-4E-BP1), c-Myc, phosphor-extracellular signal-regulated kinases
1/2 (Thr202/Tyr204) (p-ERK1/2), ERK1/2 and GAPDH were purchased
from Santa Cruz Biotechnology Inc. (Santa Cruz). Plasmids (myrAkt
delta4-129, pcDNA3-Flag mTOR wt, pWZL Neo Myr Flag RPS6KB1, pET14b
PHAS-I, HA-HIF1alpha-pcDNA3, 3XFlagNICD1, pMXs-hc-MYC,
ODD-Luciferase-pcDNA3, VEGF promoter, cMyc promoter (TBE1/2-wt),
and pCBFRE-luc) were purchased from Addgene (Cambridge, MA,
USA).
Cell viability assay
OVCAR-3 cells were seeded into 96-well plates at a
density of 2×104 per well in medium with 10% FBS. After
overnight growth, cells were treated with different concentrations
of TF3 for 24 h. Cell viability was measured using CellTiter
96® Aqueous One Solution Cell Proliferation assay
(Promega, Madison, WI, USA), according to the manufacturer's
instructions. Cell viability was expressed as a percentage compared
to control cells (vehicle treatment).
HUVEC tube formation assay
Growth factor-reduced Matrigel (50 μl) (BD
Biosciences, San Jose, CA, USA) was added into each well of a
96-well plate and polymerized for 30 min at 37ºC. HUVECs
(1.5×104/well) in 100 μl conditioned medium (90 μl F12-K
medium+10 μl cell culture supernatant of vehicle or TF3-treated
OVCAR-3 cells) were seeded into each growth factor-reduced
Matrigel-coated well, incubated at 37ºC in 5% CO2 for 6
h, and photographed under a microscope. Tube length was quantified
using the NIH ImageJ software (NIH, Bethesda, MD, USA). Tube length
was expressed as a percentage compared to the control group.
Chick chorioallantoic membrane (CAM)
assay
Specific pathogen-free fertile chicken eggs (Charles
River Laboratories, North Franklin, CT, USA) were incubated at
37.5ºC and slowly turned by an automatic egg turner (G.Q.F.
Manufacturing Co., Savannah, GA, USA). On the 8th day of
development of fertilized chicken eggs, a 1-cm diameter window was
opened in the shell of each egg to expose the CAM. On the 9th day,
106 OVCAR-3 cells in 20 μl FBS-free RPMI-1640 medium
were mixed with 80 μl Matrigel (BD Biosciences), supplemented with
TF3 at a final concentration of 25 μmol/l (μM) or equivalent
vehicle, and implanted onto the CAM. After incubating for another 5
days, tumor implants and blood vessels were photographed and
counted for branching blood vessels. Blood vessel density was
normalized to the control group.
Enzyme linked immunosorbent assays
OVCAR-3 cells were seeded into 96-well plates at a
density of 2×104 per well, incubated overnight and
treated with different concentrations of TF3 for 24 h. Cell culture
supernatants were collected. The concentration of human VEGF
protein was determined by a human VEGF Duo-set ELISA kit (R&D)
according to the manufacturer's instructions.
Western blot analysis
OVCAR-3 cells were treated for 24 h with various
concentrations of TF3 in 60 mm dishes, and then lysed in 100 μl
mammalian protein extraction reagent supplemented with Halt™
Protease and Phosphatase Inhibitor Single-Use Cocktail (Life
Technologies, Grand Island, NY, USA). The concentration of protein
was measured using a BCA Protein assay kit (Thermo, Waltham, MA,
USA). Equal amounts of protein were prepared and separated by
SDS-PAGE and transferred onto the nitrocellulose membranes with a
Mini-Protean 3 system (Bio-Rad, Hercules, CA, USA). The membrane
was blocked with 5% non-fat milk in Tris-buffer saline containing
0.1% Tween-20 for 1 h at room temperature and incubated with
indicated primary antibodies overnight at 4ºC followed by
horseradish peroxidase-conjugated secondary antibody for 2 h at
37ºC. Detection was performed by SuperSignal West Dura Extended
Duration Substrate (Life Technologies) and ChemiDoc™ MP System
(Bio-Rad). Protein bands were quantified with the NIH ImageJ
software (NIH), normalized by corresponding GAPDH for analysis.
Transient transfection and luciferase
reporter assay
OVCAR-3 cells were seeded in 96-well plates at a
density of 2×104 per well and transfected with plasmids
(including a targeted gene plasmid, a corresponding luciferase
reporter plasmid and β-galactosidase control vector) using
jetPrime™ DNA and siRNA Transfection reagent (VWR
International, West Chester, PA, USA). Four hours later, medium was
replaced by FBS-free RPMI-1640 medium containing TF3 or equivalent
vehicle. Cells were incubated for another 24 h. Luciferase assays
and β-galactosidase assay were performed using One-Glo™ luciferase
assay system and β-galactosidase enzyme assay system (Promega),
respectively. Luciferase activity was expressed relative to
β-galactosidase activity.
Statistical analysis
The data are expressed as mean ± standard error of
mean (SEM) from at least three independent experiments. The results
were analyzed with SPSS Version 18.0 for Windows (SPSS, Chicago,
IL, USA) using one-way analysis of variance (ANOVA) and post hoc
test (2-sided Dunnett's test) to test both overall differences and
specific differences between each treatment and control. P-values
<0.05 were considered statistically significant.
Results
TF3 reduces tumor-induced angiogenesis in
vitro and in vivo by downregulating VEGF and HIF-1α
To evaluate the cytotoxicity of TF3 on OVCAR-3
cells, CellTiter 96® Aqueous One Solution Cell
Proliferation assay was performed (Fig. 1B). TF3 treatment for 24 h
dose-dependently inhibited the viability of OVCAR-3 cells, ranging
from 100 to 48.3%. The anti-proliferative activity of TF3 is weak
at low concentrations. The viability of OVCAR-3 cells was >70%
when treated with concentrations of 15 μM and below of TF3.
Black tea extracts, which contains theaflavins, has
been reported to inhibit tumor angiogenesis. To assess whether TF3
repressed angiogenesis induced by OVCAR-3 cells, HUVEC tube
formation assay was carried out. HUVECs were cultured in F12-K
medium supplemented with cell culture supernatant of vehicle or
TF3-treated OVCAR-3 cells. Mature, interconnected capillary-like
networks were observed in the control group. However, the tube-like
structures found in TF3-treated groups were less organized and
leaky. Tube length was determined to quantify tube formation. The
data showed TF3 interrupted OVCAR-3 cell-induced HUVEC tube
formation in a dose-dependent manner (Fig. 1C).
To examine the anti-angiogenic potential of TF3
in vivo, CAM assay was conducted. Highly vascularized
structure was found in the control group. TF3 treatment markedly
reduced blood vessel density. These results confirmed that TF3
inhibited cancer cells induced angiogenesis in vivo
(Fig. 1D).
VEGF is an important growth factor involved in tumor
vascular development and maintenance. We examined the effect of TF3
on VEGF secretion using a VEGF ELISA kit. The protein level of VEGF
in TF3-treated OVCAR-3 cell culture supernatant was much lower
compared with that in the control group. TF3 had an excellent
activity on diminishing the secretion of VEGF (Fig. 1E), indicating TF3 inhibited tumor
angiogenesis by targeting VEGF.
HIF-1α has a direct regulatory impact on the
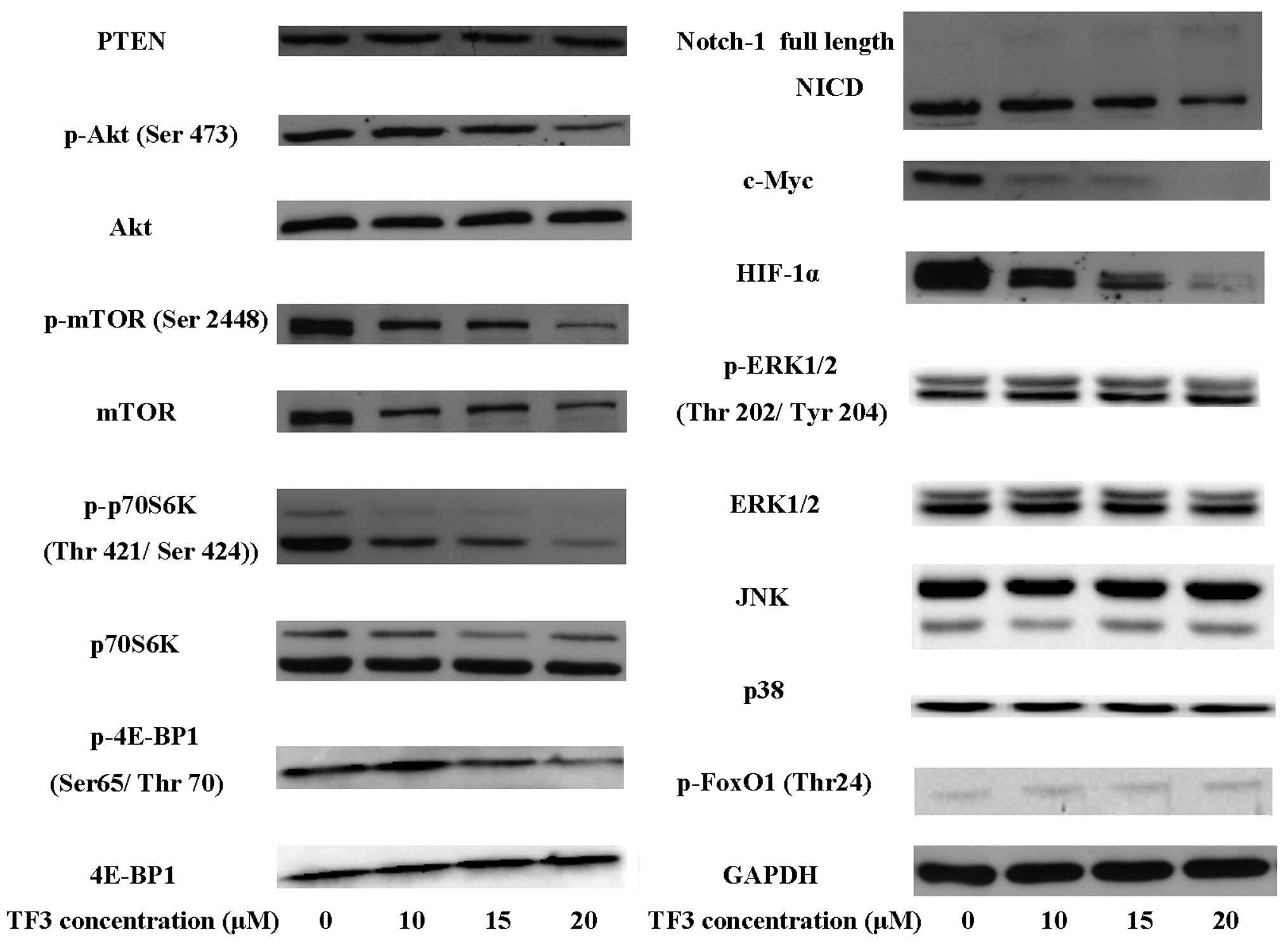
expression of VEGF. Western blot analysis revealed that TF3
significantly decreased the protein level of HIF-1α in OVCAR-3
cells (Fig. 2). The data of
luciferase reporter assay implied TF3 strongly eliminated the
transcriptional activity of VEGF promoter. However, this inhibitory
effect was abrogated by overexpression of HIF-1α (Fig. 1F). It hinted TF3 downregulated VEGF
by repressing HIF-1α expression in OVCAR-3 cells.
TF3 inhibits HIF-1α and VEGF via
Akt/mTOR/p70S6K/4E-BP1 pathway
Previous studies demonstrated that
PI3K/Akt signaling was required for VEGF expression
through HIF-1 in response to growth factor stimulation and oncogene
activation (16).
mTOR/p70S6K/RPS6/4E-BP1 signaling pathway also played an important
role in suppressing HIF-1α and VEGF expression (17). To explore whether TF3 decreased
HIF-1α and VEGF expression via Akt pathway, the protein levels of
PTEN, p-Akt, Akt, p-mTOR, mTOR, p-p70S6K, p70S6K, p-4E-BP1 and
4E-BP1 were detected by western blot analysis. As shown in Fig. 2, TF3 significantly lowered the
protein levels of p-Akt, p-mTOR, p-p70S6K and p-4E-BP1. Whereas,
the expression of PTEN, a negative regulator of the Akt pathway,
was not affected by TF3 treatment. The result indicated that TF3
inactivated Akt pathway through inhibiting the phosphorylation of
Akt, mTOR, p70S6K and 4E-BP1 in OVCAR-3 cells.
When treated with TF3 and wortmannin, a selective
Akt pathway inhibitor, an additive inhibitory effect on Akt pathway
was observed in OVCAR-3 cells. The protein levels of p-Akt, p-mTOR,
p-p70S6K, p-4E-BP1, HIF-1α and VEGF in TF3 and wortmannin
co-treated cells were much more reduced compared with that in TF3
or wortmannin alone treated cells (Fig. 3A and C). Consistent with this, the
result of luciferase reporter assay validated the transcriptional
activity of VEGF promoter was lower than that in TF3 or wortmannin
alone treated cells (Fig. 3B). On
the contrary, transfected OCVAR-3 cells with a plasmid expressing
constitutively active Akt made the cells less responsive to TF3
treatment. TF3-mediated decrease in the protein levels of p-Akt,
p-mTOR, p-p70S6K, p-4E-BP1, HIF-1α and VEGF was partially
attenuated in Akt-overexpressing OVCAR-3 cells (Fig. 3D and G). Besides, transfected cells
with plasmid expressing Akt, mTOR, p70S6K or 4E-BP1 reversed
TF3-induced transcription inhibition of VEGF promoter and HIF-1α
promoter (Fig. 3E and F). These
data provided evidence that TF3 reduced HIF-1α and VEGF via
Akt/mTOR/p70S6K/4E-BP1 pathway in OVCAR-3 cells.
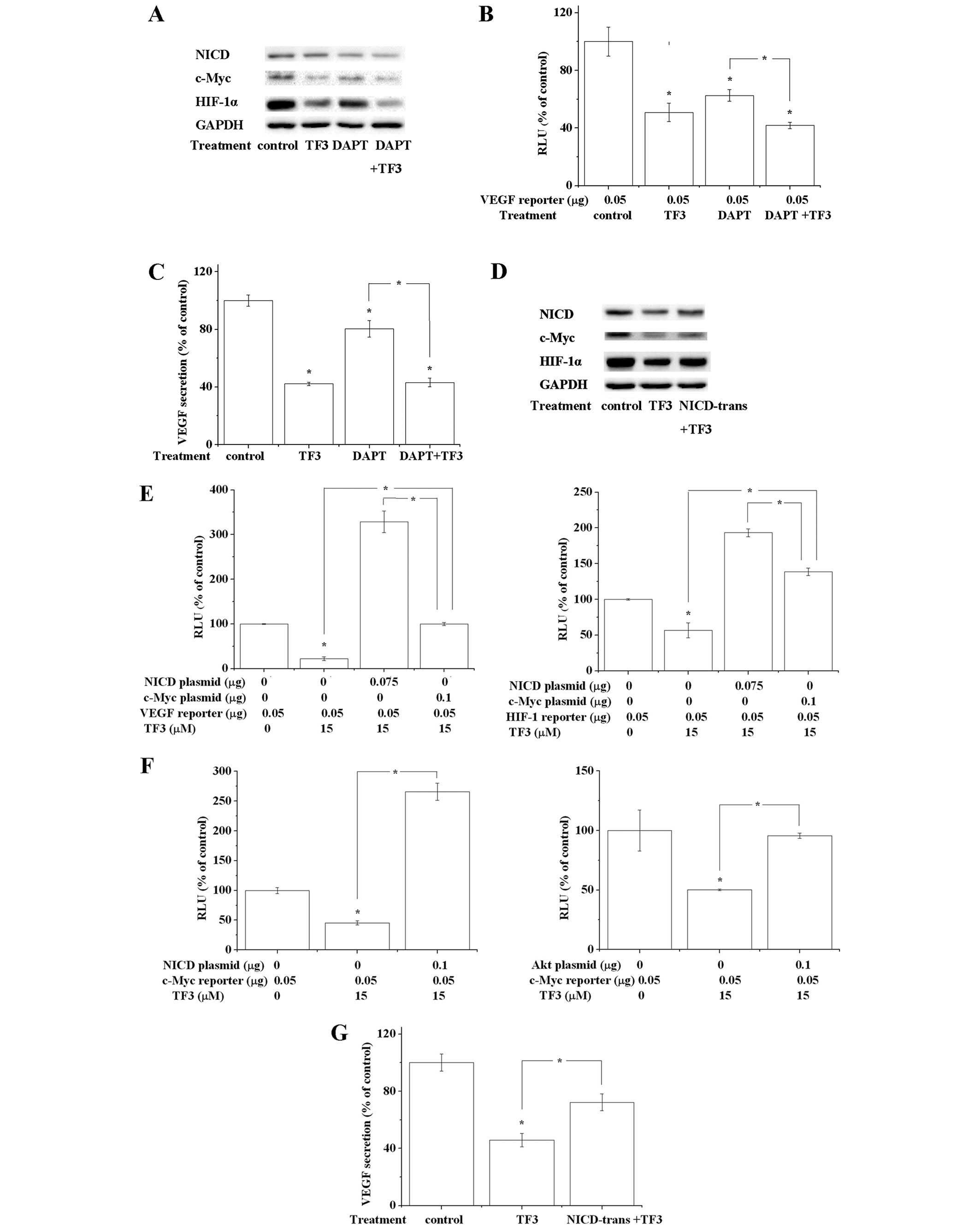
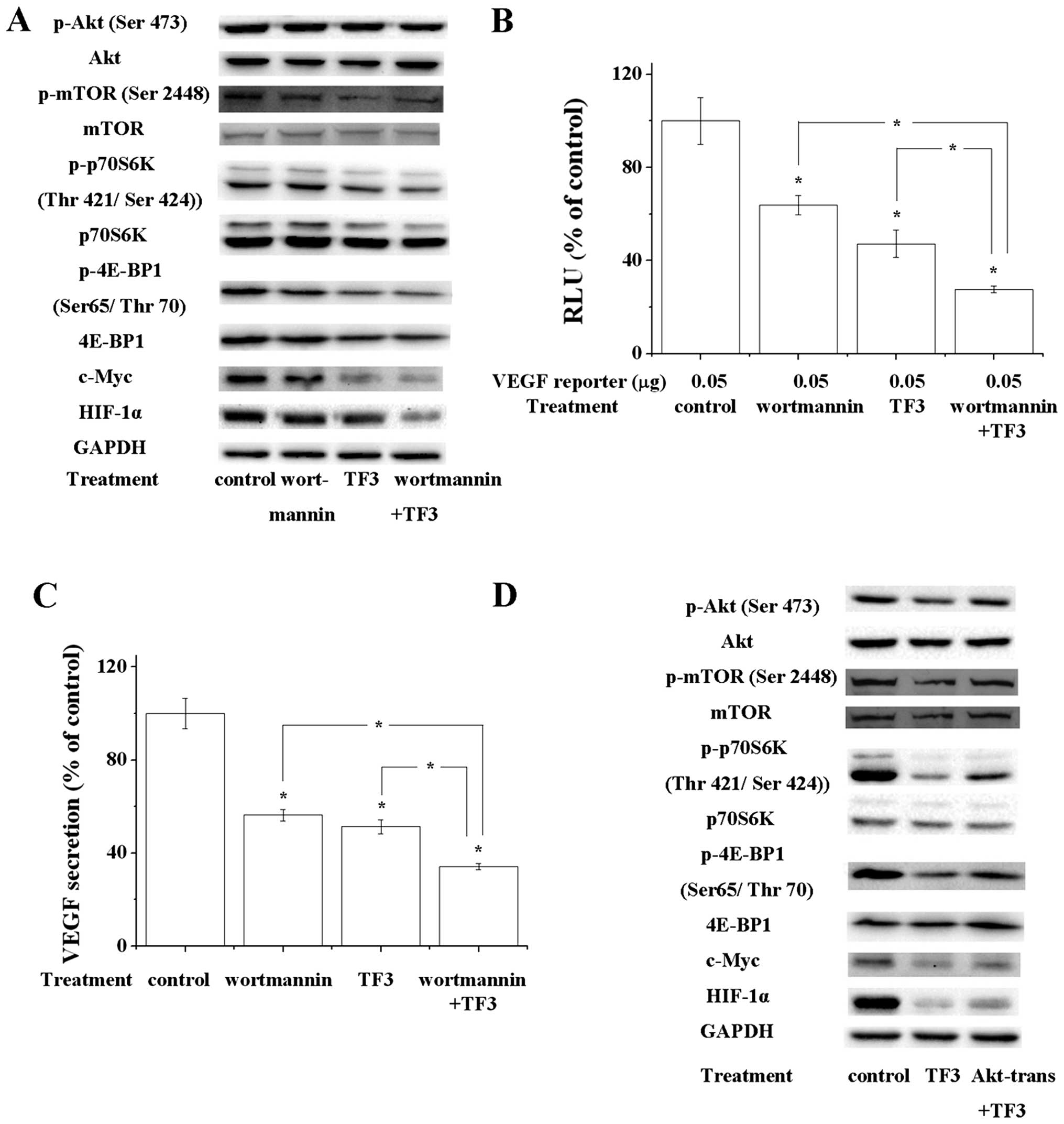 | Figure 3Akt/mTOR/p70S6k/4E-BP1 pathway and
Akt/c-Myc pathway are involved in TF3-induced inhibition of HIF-1α
and VEGF. (A) Western blot analysis showed that 100 nM wortmannin,
10 μM TF3 and 100 nM wortmannin+10 μM TF3 decreased the
phosphorylation of Akt, mTOR, p70S6K and 4E-BP1, and expression of
c-Myc and HIF-1α. TF3+wortmannin exhibited the strongest effect
among them. GAPDH served as the loading control. (B) Luciferase
reporter assay and (C) VEGF ELISA showed 100 nM wortmannin, 10 μM
TF3 and 100 nM wortmannin+10 μM TF3 suppressed the transcriptional
activity of VEGF promoter and VEGF secretion, respectively.
TF3+wortmannin elicited strongest effect among them. (D)
Overexpression of active Akt attenuated the 15 μM TF3-induced
decrease of phosphorylation of Akt, mTOR, p70S6K and 4E-BP1, and
expression of c-Myc and HIF-1α. Overexpression of Akt, mTOR, p70S6K
or 4E-BP1 attenuated TF3-induced inhibition of transcriptional
activity of VEGF promoter (E) and HIF-1α promoter (F). (G)
Overexpression of active Akt reversed the 15 μM TF3-induced
reduction of VEGF secretion. The data are presented as the mean ±
standard error of mean. *P<0.05 compared with control
or between specific groups. |
TF3 suppresses HIF-1α and VEGF via
Akt/c-myc and Notch-1/c-myc pathway
c-Myc is a major human oncogene which exerts many
biological functions. It has been elucidated that c-Myc enhances
cancer cell-mediated angiogenesis through the regulation of HIF-1α
(18). c-Myc expression can be
regulated in an Akt-dependent manner at the level of transcription
(19). c-Myc expression also can
be affected by Notch-1. Notch-1 upregulates c-Myc transcription by
directly binding to its promoter (20). To investigate whether Akt/c-Myc and
Notch-1/c-Myc participated in tumor angiogenesis and the influence
of TF3 on these pathways, western blot analysis and luciferase
reporter assay were carried out. According to the results, TF3
significantly suppressed the expression of c-Myc, HIF-1α and VEGF,
the cleavage of Notch-1, and the phosphorylation of Akt (Figs. 1E and 2). TF3 and wortmannin co-treatment
enhanced the effect of TF3 on decreasing the protein levels of
c-Myc, HIF-1α and VEGF (Fig. 3A and
C), while overexpression of active Akt rescued it (Fig. 3D and G). Similarly, exposure to the
combination of TF3 and DAPT, a Notch inhibitor, caused a stronger
repression of c-Myc and HIF-1α than DAPT or TF3 alone (Fig. 4A). Whereas, overexpression of
Notch-1 intracellular domain (NICD) had an adverse effect on the
TF3-triggered inhibition of c-Myc, HIF-1α and VEGF expression
(Fig. 4D and G). Luciferase
reporter assay confirmed that wortmannin, DAPT and TF3 declined the
transcriptional activity of VEGF promoter (Figs. 3B and 4B). On the contrary, overexpression of
active Akt or NICD abrogated TF3-induced transcription inhibition
of c-Myc promoter, HIF-1α promoter and VEGF promoter (Figs. 3E and F, and 4E and F). Transfected cells with plasmid
expressing c-Myc eliminated TF3-induced transcription inhibition of
HIF-1α and VEGF (Fig. 4E). Taken
together, Akt/c-Myc/HIF-1α/VEGF pathway and
Notch-1/c-Myc/HIF-1α/VEGF pathway are involved in the antitumor
angiogenic mechanisms of TF3.
TF3 does not affect HIF-1α and VEGF via
the MAPK pathways
MAPK pathways play essential roles in cell
proliferation and differentiation. Activation of MAPK signaling
pathways has been reported to participate in tumorigenesis,
metastasis, and angiogenesis of multiple human malignancies
(21). To investigate whether TF3
had an influence on MAPK pathways, western blot assay was carried
out. In this study, TF3 showed no sign of affecting the protein
levels of p-ERK1/2, ERK1/2, JNK, p38 and p-FoxO1 (Fig. 5), suggesting MAPK pathways were not
relevant to the anti-tumor angiogenic activity of TF3.
Discussion
Ovarian cancer is one of the most common cancers in
women. Due to the fact that current chemotherapies do not work on a
proportion of patients and may cause severe side effect, it is
critical to identify alternative strategies. Some nutrients were
found to have anticancer activities with little side effect. A
previous study presented that black tea consumption was associated
with a linear decline in ovarian cancer risk (22). TF3 is produced in black tea
manufacturing process. It has multiple biological activities,
including the anti-oxidant, anti-bacterial, anti-obese, and
anticancer effects. TF3 inhibits proliferation and/or induce
apoptosis of various cancer cells (11,12).
In this study, we tested the cytotoxicity of TF3 on OVCAR-3 cells
and found TF3 inhibited cell proliferation, especially at high
concentrations. This is consistent with the former research that
theaflavins exhibited anti-proliferative and apoptotic activity in
human teratocarcinoma of ovarian origin (23). Although previous studies and the
current study proved TF3 exerted an anti-proliferative effect of
cancer cells, the working concentrations were much higher than the
attainable concentrations of TF3 in plasma and tissues (24).
Previous studies showed that some food-derived
natural compounds elicited growth inhibitory activity at higher
concentrations while anti-angiogenic activity at relatively lower
concentrations (25,26). Tumor angiogenesis is related with
poor prognosis in ovarian carcinoma (27). Cancer cells can secrete
pro-angiogenic factors, including VEGF and basic fibroblast growth
factor, to stimulate normal endothelial cell growth through
paracrine mechanisms (28). VEGF
regulates the key steps of angiogenic process, particularly
endothelial cell proliferation, survival, permeability, and
migration (29). TF3 has been
demonstrated to inhibit gene expression of VEGF in A549 lung cancer
cells (30). However, the impact
of TF3 on ovarian cancer-induced angiogenesis has not been reported
yet. To explore whether TF3 hampered tumor induced angiogenesis, we
tested the anti-angiogenic activity of TF3 in the HUVEC model and
CAM model, and the VEGF protein content in the cell culture
supernatant of TF3-treated OVCAR-3 cells. In this study, TF3 was
verified to block OVCAR-3 cell-triggered HUVEC tube formation and
blood vessel development in the CAM model. TF3 started to inhibit
HUVEC tube formation at 5 μM. About half of OVCAR-3 cell-induced
HUVEC tube formation was blocked after 15 μM TF3 treatment. The
result of VEGF ELISA was in accordance with that of tube formation
assay. TF3 strongly reduced the VEGF secretion of OVCAR-3 cells.
Treatment with 5 μM TF3 caused an ~50% decline of VEGF secretion.
The data implied that TF3 might exert its antitumor angiogenic
function by downregulating VEGF. It also provided evidence
supporting a multifunction of TF3 on OVCAR-3 cells between
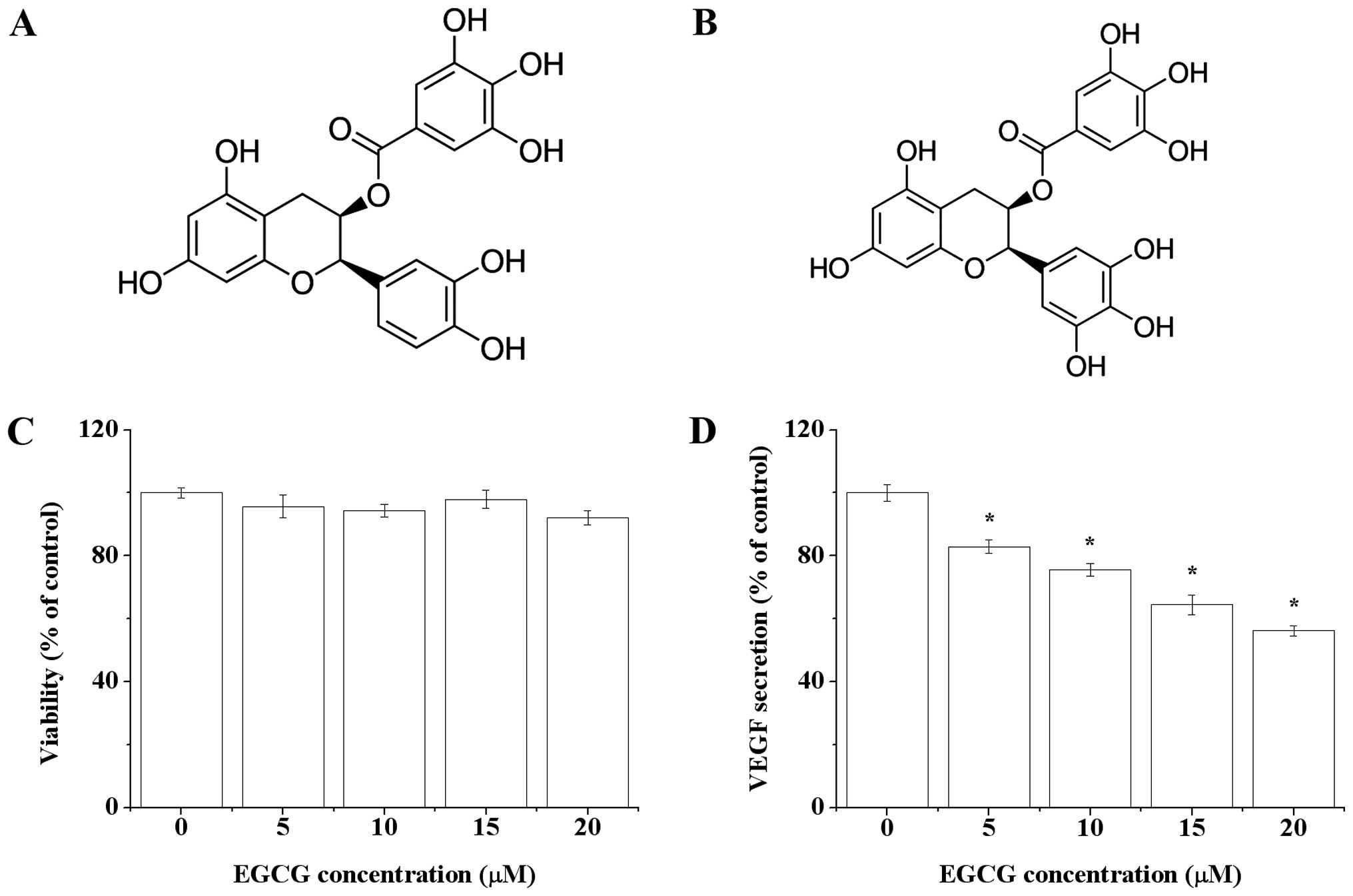
different concentrations. In the present study, we also measured
the effect of EGCG, a precursor of TF3, on VEGF secretion of
OVCAR-3 cells. EGCG was regarded as one of the most potent
bioactives in green tea and has been proven to inhibit tumor
angiogenesis (31). Compared with
EGCG, the working concentration of TF3 was much lower (Fig. 5D). One of the possible reasons
might be that TF3 was more stable than EGCG in aqueous system (pH
7.4) (32). Structural differences
might also be responsible for this (Figs. 1A and 5B). The activity of tea polyphenols is
related with the number and position of hydroxyl groups in
molecules (33). TF3 is produced
by the oxidative dimerization of ECG and EGCG. Therefore, TF3
contains more hydroxyl groups than ECG or EGCG alone.
HIF-1α is a subunit of the heterodimeric
transcription factor HIF-1, which regulates the transcription of
>60 genes, including VEGF. Dysregulation and overexpression of
HIF-1α are heavily implicated in cancer biology, specifically in
areas of vascularization and angiogenesis. Constitutively elevated
levels of HIF-1α protein were detected under normal conditions in
OVCAR-3 cells (16). We observed
TF3 remarkably decreased the expression of HIF-1α in OVCAR-3 cells.
Luciferase reporter assay suggested TF3 inhibited VEGF by
suppressing HIF-1α. EGCG has been reported to suppress breast tumor
angiogenesis and growth via inhibiting the activation of HIF-1α and
expression of VEGF (34). Similar
functions of EGCG were also found in human cervical carcinoma and
hepatoma cells (35). It suggested
that HIF-1α/VEGF might be key therapeutic targets for tea
polyphenols.
Multiple signaling pathways are involved in the
regulation of HIF-1α/VEGF. One of them is the Akt signaling
pathway. Akt signaling pathway has been considered to participate
in angiogenesis in the neoplastic and non-neoplastic process
(36). Growth factors, cytokines,
and other signaling molecules stimulate HIF-1 protein synthesis via
activation of Akt pathway (16).
The activation of Akt pathway starts from the phosphorylation of
Akt. Then, mTOR can be phosphorylated and activated by Akt.
Subsequently, activated mTOR regulates the phosphorylation and
activation of p70S6K and 4E-BP1, two known downstream targets of
mTOR (37). Active p70S6K and
4E-BP1 enhance the translation of mRNAs that bear a 5′ terminal
oligopyrimidine tract, which encodes proteins related with the
translational apparatus like ribosomal proteins, elongation factors
and the poly A-binding protein (38). In the present study, we proved TF3
markedly decreased the phosphorylation of Akt, mTOR, p70S6K, and
4E-BP1, reduced the expression of HIF-1α and VEGF. This effect was
potentiated by the PI3K/Akt inhibitor wortmannin and
impaired by overexpression of active Akt. This is the first report
that Akt/mTOR/p70S6K/4E-BP1/HIF-1α/VEGF pathway was involved in the
anti-angiogenic effect of TF3. Recently, some natural compounds
have been proven to inhibit HIF-1α and VEGF expression by targeting
Akt signaling pathway. Quercetin, a flavonol found in many fruits
and vegetables, inhibited angiogenesis mediated human prostate
tumor growth by targeting VEGFR-2 regulated Akt/mTOR/p70S6K
signaling pathways (39).
Silibinin, a natural flavonoid extracted from the milk thistle
seeds, suppressed HIF-1α and mTOR/p70S6K/4E-BP1 signaling pathway
in human cervical and hepatoma cancer cells (17). Tanshinone IIA, a derivative of
phenanthrene-quinone from the dried root of Salvia
miltiorrhiza, regulated breast cancer growth and tumor
angiogenesis via mTOR/p70S6K/RPS6/4E-BP1-mediated repression of
HIF-1α and VEGF expression (37).
Our study was in agreement with the previous studies and suggested
that Akt/mTOR/p70S6K/4E-BP1/HIF-1α/VEGF pathway was an important
target for plant-derived nutrients to inhibit tumor
angiogenesis.
c-Myc is a transcription factor essential for
vasculogenesis and angiogenesis during development and tumor
progression (40). Targeting
c-Myc/HIF-1α pathway inhibits cancer growth and angiogenesis
(41).
Akt pathway has an influence on c-Myc (19). LY294002, a PI3K/Akt
inhibitor, reduced c-Myc expression and regulated the degradation
of c-Myc via affecting the phosphorylation status of Thr58
(19). In this study, western blot
analysis revealed that c-Myc expression and phosphorylation of Akt
was greatly inhibited by TF3. Hence, we hypothesized that
Akt/c-Myc/HIF-1α pathway might be related to the anti-tumor
angiogenic effect of TF3. To confirm this hypothesis, western blot
and luciferase reporter assay were performed. Both TF3 and
wortmannin inhibited the activation of Akt, expression of c-Myc,
HIF-1α and VEGF. TF3 and wortmannin combination led to a more
effective suppression. Conversely, transfected cells with plasmid
expressing active Akt enhanced TF3-resistance of OVCAR-3 cells.
These results indicated that TF3 inhibited HIF-1α and VEGF via
Akt/c-Myc pathway. Akt/c-Myc pathway has been discovered to have an
impact on cellular metabolism, cell proliferation and the cell
cycle. Akt or c-Myc alone has been proven to regulate angiogenesis
via HIF-1α/VEGF pathway. In this study, Akt and c-Myc was
demonstrated to be relevant in stimulating tumor angiogenesis. Akt
regulated the pro-angiogenic effect of c-Myc through modulating the
expression of c-Myc. Thus, we discovered a novel target pathway
responsible for the anti-angiogenic activity of TF3 in OVCAR-3
cells.
Notch-1 is important upstream of c-Myc. It modulates
the expression of c-Myc by directly binding to its promoter.
Notch-1 is a type I transmembrane receptor. When binding to its
ligands, metalloproteinase-mediated and γ-secretase-mediated
cleavage is induced. Subsequently, NICD is released from plasma
membrane, translocated into the nucleus, and binds CBF-1/suppressor
of hairless/Lag-1, mastermind-like-1, and p300/CBP, to form a
transcriptional coactivator (42).
Notch-1 is a regulator of tumor angiogenesis (43). Previous studies showed
overexpression of Notch-1 promoted cell growth and tumor
angiogenesis in myeloma (44).
Whereas, Notch-1 antibody presents an adverse effect on tumor
growth and angiogenesis (45).
β-elemene, a sesquiterpene found in a variety of plants, has been
demonstrated to attenuate angiogenesis capacity of CD44+
gastric cancer stem-like cells by interfering with the expression
of Notch-1 (46). Notch-1
signaling is active in ovarian cancer (47). In the present study, we observed
TF3 inhibited the cleavage of Notch-1. To verify whether there was
a link between Notch-1/c-Myc pathway and HIF-1α/VEGF pathway, DAPT,
a γ-secretase inhibitor was used. According to the results, DAPT
lowered the expression of NICD, c-Myc, HIF-1α and VEGF. It hinted
that HIF-1α and VEGF were regulated by Notch-1/c-Myc pathway in
OVCAR-3 cells. Further experiments revealed that TF3 promoted the
repressive effect of DAPT on Notch-1/c-Myc pathway. On the
contrary, overexpression of NICD hampered TF3-trigged inhibition of
these proteins. Based on the above results, we concluded that TF3
downregulated HIF-1α and VEGF partially by suppressing
Notch-1/c-Myc pathway. The protein level of NICD in TF3 and/or DAPT
treated cells was control group>TF3 group>DAPT
group>TF3+DAPT group, while the protein levels of c-Myc and
HIF-1α were control group>DAPT group>TF3 group>TF3+DAPT
group. This inconsistence could be explained by TF3 suppressing
c-Myc/HIF-1α pathway not only via Notch-1, but also other targets
(for example, Akt pathway). Besides, the combination of TF3 and
DAPT failed to cause an additive effect on decreasing VEGF
secretion, implying Notch-1/c-Myc pathway might only play a
subordinate role in the anti-angiogenic function of TF3.
MAPK pathways regulate many cellular activities,
including angiogenesis. MAPKs are activated by the dual
phosphorylation of neighboring threonine and tyrosine residues in
response to various extracellular stimuli (48). ERK, JNK and p38 are three major
groups of MAPKs. ERKs are often activated by growth signals, while
JNK and p38 are often initiated by stress-responsive signaling
(48). FoxO1 is a forkhead
transcription factor which specifically regulates a nonredundant,
but overlapping set of angiogenesis- and vascular
remodeling-related genes (49).
FoxO1 contains 15 consensus phosphorylation sites for the MAPK
family. It has been demonstrated that ERK and p38 directly
regulated the transcriptional activity of FoxO1 via phosphorylation
(50). Similarly, JNK-dependent
phosphorylation of FoxO proteins results in nuclear accumulation
and enhanced transcriptional activity (51). In the former studies, EGCG
inhibited tumor angiogenesis through inactivating ERK1/2 in human
colon carcinoma cells (52) and
human pancreatic tumors (53).
EGCG increased the activity of p38, but not ERK 1/2 in OVCAR-3
cells (54). In HUVECs, inhibition
of PI3K/AKT and MEK/ERK pathways enhanced the
anti-angiogenic effects of EGCG through activation of FoxO
transcription factors (48).
However, we did not observe any effect of TF3 on ERK1/2, JNK or p38
in this case. This result implicated that although TF3 and EGCG
shared some anti-angiogenic targets, they had their specific
targets. This might be related to their molecular structures. TF3
possesses a benzotropolone skeleton that is formed from
co-oxidation of EGCG and ECG, one with a vic-trihydroxy moiety and
the other with an ortho-dihydroxy structure (9). The benzotropolone moiety has been
proven to be important in the antioxidant activity (55), anti-inflammatory activity (56), anti-peptide transport activity and
activating AMP-activated protein kinase (57). Thus, we speculated that
benzotropolone moiety might also be vital for its anticancer
activity. Quantitative structure-activity relationship analysis
should be carried out to investigate this assumption.
In conclusion, this study presented that TF3 had the
ability to inhibit ovarian cancer cell-induced angiogenesis in
vitro and in vivo. TF3 exerted this effect through
suppression of HIF-1α and VEGF. One of the mechanisms is that TF3
inhibited Akt/mTOR/p70S6K/4E-BP1 pathway. Other mechanisms included
TF3-mediated inactivation of Akt/c-Myc and Notch-1/c-Myc pathways.
MAPK pathways were not involved. This study provides novel
perspectives and potential targets for the anticancer activity of
TF3 (Fig. 6). Further studies in
animal models and human trials are needed to evaluate the antitumor
angiogenic activity of TF3.
Acknowledgements
The authors acknowledge financial support from the
West Virginia Higher Education Policy Commission/Division of
Science Research. This study was also supported by NIH grants
P20RR016477 from the National Center for Research Resources and
P20GM103434 from the National Institute for General Medical
Sciences (NIGMS) awarded to the West Virginia IDeA Network of
Biomedical Research Excellence.
Abbreviations:
|
CAM
|
chick chorioallantoic membrane
|
|
DAPT
|
N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl
ester
|
|
4E-BP1
|
eukaryotic initiation factor
4E-binding protein-1
|
|
FBS
|
fetal bovine serum
|
|
HUVEC
|
human umbilical vein endothelial
cell
|
|
HIF-1α
|
hypoxia-inducible factor 1α
|
|
JNK
|
c-Jun N-terminal kinases
|
|
MAPK
|
mitogen-activated protein kinase
|
|
p70S6K
|
p70S6 kinase
|
|
mTOR
|
mammalian target of rapamycin
|
|
RLU
|
relative luminescence units
|
|
SEM
|
standard error of mean
|
|
NICD
|
Notch-1 intracellular domain
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Mizuno T, Suzuki N, Makino H, Furui T,
Morii E, Aoki H, Kunisada T, Yano M, Kuji S, Hirashima Y, et al:
Cancer stem-like cells of ovarian clear cell carcinoma are enriched
in the ALDH-high population associated with an accelerated
scavenging system in reactive oxygen species. Gynecol Oncol.
137:299–305. 2015. View Article : Google Scholar
|
|
2
|
Barakat RR, Markman M and Randall M:
Principles and Practice of Gynecologic Oncology. Wolters Kluwer
Health/Lippincott Williams & Wilkins; Philadelphia, PA:
2009
|
|
3
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Folkman J, Watson K, Ingber D and Hanahan
D: Induction of angiogenesis during the transition from hyperplasia
to neoplasia. Nature. 339:58–61. 1989. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giri S, Karakoti A, Graham RP, Maguire JL,
Reilly CM, Seal S, Rattan R and Shridhar V: Nanoceria: A rare-earth
nanoparticle as a novel anti-angiogenic therapeutic agent in
ovarian cancer. PLoS One. 8:e545782013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Burger RA: Overview of anti-angiogenic
agents in development for ovarian cancer. Gynecol Oncol.
121:230–238. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhong H, De Marzo AM, Laughner E, Lim M,
Hilton DA, Zagzag D, Buechler P, Isaacs WB, Semenza GL and Simons
JW: Overexpression of hypoxia-inducible factor 1alpha in common
human cancers and their metastases. Cancer Res. 59:5830–5835.
1999.PubMed/NCBI
|
|
8
|
Liang YC, Chen YC, Lin YL, Lin-Shiau SY,
Ho CT and Lin JK: Suppression of extracellular signals and cell
proliferation by the black tea polyphenol,
theaflavin-3,3′-digallate. Carcinogenesis. 20:733–736. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sajilata MG, Bajaj PR and Singhai RS: Tea
polyphenols as nutraceuticals. Compr Rev Food Sci Food Saf.
7:229–254. 2008. View Article : Google Scholar
|
|
10
|
Tu YY, Tang AB and Watanabe N: The
theaflavin monomers inhibit the cancer cells growth in vitro. Acta
Biochim Biophys Sin (Shanghai). 36:508–512. 2004. View Article : Google Scholar
|
|
11
|
Schuck AG, Ausubel MB, Zuckerbraun HL and
Babich H: Theaflavin-3,3′-digallate, a component of black tea: an
inducer of oxidative stress and apoptosis. Toxicol In Vitro.
22:598–609. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gao Y, Li W, Jia L, Li B, Chen YC and Tu
Y: Enhancement of (−)-epigallocatechin-3-gallate and
theaflavin-3-3′-digallate induced apoptosis by ascorbic acid in
human lung adenocarcinoma SPC-A-1 cells and esophageal carcinoma
Eca-109 cells via MAPK pathways. Biochem Biophys Res Commun.
438:370–374. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kobayashi S, Iwai S, Tsujiyama K,
Kurahashi C, Udaka Y, Sanbe T, Suzaki H and Oguchi K:
Theaflavin-3,3′-digallate inhibits tube formation in cocultured
endothelial cells with fibroblasts. Showa Univ J Med Sci. 19:59–72.
2007. View Article : Google Scholar
|
|
14
|
Siddiqui IA, Zaman N, Aziz MH, Reagan-Shaw
SR, Sarfaraz S, Adhami VM, Ahmad N, Raisuddin S and Mukhtar H:
Inhibition of CWR22Rnu1 tumor growth and PSA secretion in athymic
nude mice by green and black teas. Carcinogenesis. 27:833–839.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu Y, Jin YX, Wu YY and Tu YY: Isolation
and purification of four individual theaflavins using
semi-preparative high performance liquid chromatography. J Liquid
Chromatogr Relat Technol. 33:1791–1801. 2010. View Article : Google Scholar
|
|
16
|
Fang J, Xia C, Cao Z, Zheng JZ, Reed E and
Jiang BH: Apigenin inhibits VEGF and HIF-1 expression via
PI3K/AKT/p70S6K1 and HDM2/p53 pathways. FASEB J. 19:342–353. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
García-Maceira P and Mateo J: Silibinin
inhibits hypoxia-inducible factor-1alpha and mTOR/p70S6K/4E-BP1
signalling pathway in human cervical and hepatoma cancer cells:
Implications for anticancer therapy. Oncogene. 28:313–324. 2009.
View Article : Google Scholar
|
|
18
|
Chen C, Cai S, Wang G, Cao X, Yang X, Luo
X, Feng Y and Hu J: c-Myc enhances colon cancer cell-mediated
angiogenesis through the regulation of HIF-1α. Biochem Biophys Res
Commun. 430:505–511. 2013. View Article : Google Scholar
|
|
19
|
Asano T, Yao Y, Zhu J, Li D, Abbruzzese JL
and Reddy SA: The PI 3-kinase/Akt signaling pathway is activated
due to aberrant Pten expression and targets transcription factors
NF-kappaB and c-Myc in pancreatic cancer cells. Oncogene.
23:8571–8580. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weng AP, Millholland JM, Yashiro-Ohtani Y,
Arcangeli ML, Lau A, Wai C, Del Bianco C, Rodriguez CG, Sai H,
Tobias J, et al: c-Myc is an important direct target of Notch1 in
T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev.
20:2096–2109. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang D, Ding Y, Luo WM, Bender S, Qian
CN, Kort E, Zhang ZF, VandenBeldt K, Duesbery NS, Resau JH, et al:
Inhibition of MAPK kinase signaling pathways suppressed renal cell
carcinoma growth and angiogenesis in vivo. Cancer Res. 68:81–88.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Baker JA, Boakye K, McCann SE, Beehler GP,
Rodabaugh KJ, Villella JA and Moysich KB: Consumption of black tea
or coffee and risk of ovarian cancer. Int J Gynecol Cancer.
17:50–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Banerjee P, Banerjee S and Mazumder S:
Effect of theaflavin, A black tea extract on ovarian cancer cell
line. Bombay Hosp J. 53:341–348. 2011.
|
|
24
|
Maity S, Ukil A, Vedasiromoni JR and Das
PK: Biodistribution and pharmacokinetics of
theaflavin-3,3′-digallate, the major antioxidant of black tea, in
mice. Int J Pharmacol. 2:240–246. 2006. View Article : Google Scholar
|
|
25
|
Huang H, Chen AY, Rojanasakul Y, Ye X,
Rankin GO and Chen YC: Dietary compounds galangin and myricetin
suppress ovarian cancer cell angiogenesis. J Funct Foods.
15:464–475. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen J, Chen AY, Huang H, Ye X, Rollyson
WD, Perry HE, Brown KC, Rojanasakul Y, Rankin GO, Dasgupta P, et
al: The flavonoid nobiletin inhibits tumor growth and angiogenesis
of ovarian cancers via the Akt pathway. Int J Oncol. 46:2629–2638.
2015.PubMed/NCBI
|
|
27
|
Schoell WM, Pieber D, Reich O, Lahousen M,
Janicek M, Guecer F and Winter R: Tumor angiogenesis as a
prognostic factor in ovarian carcinoma: Quantification of
endothelial immunoreactivity by image analysis. Cancer.
80:2257–2262. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ciardiello F, Caputo R, Bianco R, Damiano
V, Fontanini G, Cuccato S, De Placido S, Bianco AR and Tortora G:
Inhibition of growth factor production and angiogenesis in human
cancer cells by ZD1839 (Iressa), a selective epidermal growth
factor receptor tyrosine kinase inhibitor. Clin Cancer Res.
7:1459–1465. 2001.PubMed/NCBI
|
|
29
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu LQ, Liu J and Ma CJ: Effect of
theaflavin diagallate on anti-tumor proliferation and VEGF gene
expression in lung adenocancer A549 cells. Cent S Pharm. 9:23–26.
2011.
|
|
31
|
Jung YD and Ellis LM: Inhibition of tumour
invasion and angiogenesis by epigallocatechin gallate (EGCG), a
major component of green tea. Int J Exp Pathol. 82:309–316. 2001.
View Article : Google Scholar
|
|
32
|
Su YL, Leung LK, Huang Y and Chen ZY:
Stability of tea theaflavins and catechins. Food Chem. 83:189–195.
2003. View Article : Google Scholar
|
|
33
|
Yang ZY, Tu YY, Xia HL, Jie GL, Chen XM
and He PM: Suppression of free-radicals and protection against
H2O2-induced oxidative damage in HPF-1 cell
by oxidized phenolic compounds present in black tea. Food Chem.
105:1349–1356. 2007. View Article : Google Scholar
|
|
34
|
Gu JW, Makey KL, Tucker KB, Chinchar E,
Mao X, Pei I, Thomas EY and Miele L: EGCG, a major green tea
catechin suppresses breast tumor angiogenesis and growth via
inhibiting the activation of HIF-1α and NFκB, and VEGF expression.
Vasc Cell. 5:92013. View Article : Google Scholar
|
|
35
|
Zhang Q, Tang X, Lu Q, Zhang Z, Rao J and
Le AD: Green tea extract and (−)-epigallocatechin-3-gallate inhibit
hypoxia- and serum-induced HIF-1alpha protein accumulation and VEGF
expression in human cervical carcinoma and hepatoma cells. Mol
Cancer Ther. 5:1227–1238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li W, Tan D, Zhang Z, Liang JJ and Brown
RE: Activation of Akt-mTOR-p70S6K pathway in angiogenesis in
hepatocellular carcinoma. Oncol Rep. 20:713–719. 2008.PubMed/NCBI
|
|
37
|
Li G, Shan C, Liu L, Zhou T, Zhou J, Hu X,
Chen Y, Cui H and Gao N: Tanshinone IIA inhibits HIF-1α and VEGF
expression in breast cancer cells via mTOR/p70S6K/RPS6/4E-BP1
signaling pathway. PLoS One. 10:e01174402015. View Article : Google Scholar
|
|
38
|
Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li
D, Lai L and Jiang BH: MiR-145 directly targets p70S6K1 in cancer
cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res.
40:761–774. 2012. View Article : Google Scholar :
|
|
39
|
Pratheeshkumar P, Budhraja A, Son YO, Wang
X, Zhang Z, Ding S, Wang L, Hitron A, Lee JC, Xu M, et al:
Quercetin inhibits angiogenesis mediated human prostate tumor
growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling
pathways. PLoS One. 7:e475162012. View Article : Google Scholar
|
|
40
|
Baudino TA, McKay C, Pendeville-Samain H,
Nilsson JA, Maclean KH, White EL, Davis AC, Ihle JN and Cleveland
JL: c-Myc is essential for vasculogenesis and angiogenesis during
development and tumor progression. Genes Dev. 16:2530–2543. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lee JG and Wu R: Erlotinib-cisplatin
combination inhibits growth and angiogenesis through c-MYC and
HIF-1α in EGFR-mutated lung cancer in vitro and in vivo. Neoplasia.
17:190–200. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meng RD, Shelton CC, Li YM, Qin LX,
Notterman D, Paty PB and Schwartz GK: gamma-Secretase inhibitors
abrogate oxaliplatin-induced activation of the Notch-1 signaling
pathway in colon cancer cells resulting in enhanced
chemosensitivity. Cancer Res. 69:573–582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Garcia A and Kandel JJ: Notch: A key
regulator of tumor angiogenesis and metastasis. Histol Histopathol.
27:151–156. 2012.
|
|
44
|
Guo D, Li C, Teng Q, Sun Z, Li Y and Zhang
C: Notch1 over-expression promotes cell growth and tumor
angiogenesis in myeloma. Neoplasma. 60:33–40. 2013. View Article : Google Scholar
|
|
45
|
Proia T, Jiang F, Bell A, Nicoletti R,
Kong L, Kreuter K, Poling L, Winston WM, Flaherty M, Weiler S, et
al: 23814, an inhibitory antibody of ligand-mediated Notch1
activation, modulates angiogenesis and inhibits tumor growth
without gastrointestinal toxicity. Mol Cancer Ther. 14:1858–1867.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yan B, Zhou Y, Feng S, et al:
beta-elemene-attenuated tumor angiogenesis by targeting Notch-1 in
gastric cancer stem-like cells. J Evid Based Complementary Altern
Med. 2013:2684682013.
|
|
47
|
Rose SL, Kunnimalaiyaan M, Drenzek J and
Seiler N: Notch 1 signaling is active in ovarian cancer. Gynecol
Oncol. 117:130–133. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shankar S, Chen Q and Srivastava RK:
Inhibition of PI3K/AKT and MEK/ERK pathways act synergistically to
enhance anti-angiogenic effects of EGCG through activation of FOXO
transcription factor. J Mol Signal. 3:72008. View Article : Google Scholar
|
|
49
|
Potente M, Urbich C, Sasaki K, Hofmann WK,
Heeschen C, Aicher A, Kollipara R, DePinho RA, Zeiher AM and
Dimmeler S: Involvement of Foxo transcription factors in
angiogenesis and postnatal neovascularization. J Clin Invest.
115:2382–2392. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Asada S, Daitoku H, Matsuzaki H, Saito T,
Sudo T, Mukai H, Iwashita S, Kako K, Kishi T, Kasuya Y, et al:
Mitogen-activated protein kinases, Erk and p38, phosphorylate and
regulate Foxo1. Cell Signal. 19:519–527. 2007. View Article : Google Scholar
|
|
51
|
Lam EW, Francis RE and Petkovic M: FOXO
transcription factors: Key regulators of cell fate. Biochem Soc
Trans. 34:722–726. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Jung YD, Kim MS, Shin BA, Chay KO, Ahn BW,
Liu W, Bucana CD, Gallick GE and Ellis LM: EGCG, a major component
of green tea, inhibits tumour growth by inhibiting VEGF induction
in human colon carcinoma cells. Br J Cancer. 84:844–850. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Shankar S, Marsh L and Srivastava RK: EGCG
inhibits growth of human pancreatic tumors orthotopically implanted
in Balb C nude mice through modulation of FKHRL1/FOXO3a and
neuropilin. Mol Cell Biochem. 372:83–94. 2013. View Article : Google Scholar
|
|
54
|
Wang F, Chang Z, Fan Q and Wang L:
Epigallocatechin-3-gallate inhibits the proliferation and migration
of human ovarian carcinoma cells by modulating p38 kinase and
matrix metal-loproteinase-2. Mol Med Rep. 9:1085–1089.
2014.PubMed/NCBI
|
|
55
|
Jhoo JW, Lo CY, Li S, Sang S, Ang CY,
Heinze TM and Ho CT: Stability of black tea polyphenol, theaflavin,
and identification of theanaphthoquinone as its major radical
reaction product. J Agric Food Chem. 53:6146–6150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sang S, Lambert JD, Tian S, Hong J, Hou Z,
Ryu JH, Stark RE, Rosen RT, Huang MT, Yang CS, et al: Enzymatic
synthesis of tea theaflavin derivatives and their anti-inflammatory
and cytotoxic activities. Bioorg Med Chem. 12:459–467. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Park HY, Kunitake Y and Matsui T:
Benzotropolone moiety in theaflavins is responsible for inhibiting
peptide-transport and activating AMP-activated protein kinase in
Caco-2 cells. Funct Foods Health Dis. 3:111–121. 2013.
|