Introduction
Triple-negative breast cancer (TNBC) is a highly
aggressive subcategory of breast cancer that currently lacks
well-defined molecular targets for effective targeted therapies.
Disease relapse, metastasis and drug resistance render standard
chemotherapy ineffective in the treatment of TNBC. The acquisition
of metastatic phenotypes by mammary tumors has been linked to the
alterations in integrin expression (1,2).
High level expressions of many integrins, including α5β1, α6 and
αvβ3 have been correlated with tumor progression (3,4).
β3 integrin is frequently overexpressed in tumor
cells, including lung cancer, melanoma, glioblastoma and breast
cancer cells (5,6). Previous studies coupled β3 integrin
to epithelial-mesenchymal transition (EMT) and metastasis; β3
integrin inhibition is a therapeutic target to treat TNBC,
attenuates TGF-β-mediated EMT and invasion, and inhibits
3-dimensional organoid growth (7).
β3 integrin-mediated adhesion can trigger the activation of
numerous signaling intermediates, such as FAK, Src, ILK, PI3K and
MAPK (8,9). Evidence that ERK signaling promotes
cell proliferation, cell survival and metastasis and that this
pathway is aberrantly activated in breast cancer at an overwhelming
frequency support current efforts to identify inhibition strategies
for this pathway. Thus, finding out whether alterations in β3
integrin affect ERK signaling in tumor cells is crucial to improve
strategies for treating or preventing metastatic disease.
MiRNAs are powerful regulators of gene expression in
cancer cell invasion and metastasis that downregulate gene
expression at the post-transcriptional level (10–12).
miR-30a has been identified as one of the crucial regulators for
development and progression of breast and prostate cancers by
directly targeting MTDH and ERG, respectively (13,14).
However, the exact function and underlying mechanisms of miR-30a in
the progression of breast cancer still warrant further
investigation. In the present study, we focused on the functional
analysis of miR-30a-5p, a member of the miR-30 family that is
reportedly downregulated in cancer cells.
Our data suggested that the activation of signaling
cascades downstream from β3 integrin involved the ERK/Est-1
pathway. Results also showed that miR-30a-5p suppressed the
proliferation and invasion of breast cancer cells in vitro
by directly targeting the β3 integrin and suspended β3
integrin-Erk-Ets-1 loop. Thus, a tumor suppressor role of
miR-30a-5p in breast cancer was suggested.
Materials and methods
Patient samples
Breast cancer specimens were obtained from 156
patients at the Weifang Medical University Affiliated Hospital
after surgical resection. Twenty para-cancerous tissues were
allocated into the negative control group. No patient in the
present study received chemotherapy or radiation therapy prior to
surgery. This study was approved by the Institutional Review Board
of Weifang Medical University Hospital and informed consent was
obtained from each patient. All fresh samples were stored at
−80ºC.
Immunohistochemistry, immunofluorescence
and cytoskel-etal staining
Labeled streptavidin biotin method was used for
immunohistochemistry. After deparaffinization and rehydration,
primary antibodies were added for overnight storage at 4ºC, and
slides were incubated with biotin-labeled secondary antibodies.
Finally, the slides were incubated with HRP-streptavidin for 15
min. After DAB staining, the results were graded for intensity (0,
1, 2 and 3 for negative, weak, moderate and strong, respectively).
The percentage of positive cells, i.e., 0 and 1 (1–24%), 2
(25–49%), 3 (50–74%) and 4 (75–100%), was determined. Discrepancies
were resolved by consensus. The grades were multiplied to determine
the scores. Tumor scores were defined by using the following rules:
low (score, 0–4) and high (score ≥5). For immunofluorescence, cells
were grown on coverslips, fixed in 4% paraformaldehyde, and
incubated in a blocking buffer (1% BSA, 0.25% Triton X-100 in PBS,
pH 7.4). The cells were then probed with primary antibody and
fluorescein-conjugated goat anti-mouse IgG (Beyotime Institute of
Biotechnology, Haimen, China). The cells were counterstained with
DAPI to label the cell nuclei. Cell cytoskeleton was stained with
FITC-phalloidin. The cells were seeded into 24-well culture plates,
washed with PBS, fixed with 4% paraformaldehyde and incubated with
0.2% Triton X-100. After blocking with 1% bovine serum albumin,
cells were incubated with CY3-phalloidin. Images were captured by
confocal fluorescent microscopy.
Cell lines and culture conditions
Breast cancer cell lines MCF-7, MDA-MB-231 and
MDA-MB-468 were obtained from the American Type Culture Collection
(ATCC; Manassas, VA, USA) and were routinely cultured in Dulbecco's
modified Eagle's medium (DMEM; Gibco Laboratories, Grand Island,
NY, USA) supplemented with 10% FBS (Tianjin Hao Yang Biological
Manufacture Co., Ltd., Tianjin, China). All cells were cultured at
37ºC and 5% CO2.
Plasmid construction and
transfection/infection
The sequences of miR-30a-5p mimic and mock were
synthesized according to the method of Baraniskin et al
(15) and were ligated into the
restriction sites of pCDH-CMV-MCS-EF1-Puro vectors. Lentiviruses
were produced by transfecting human embryonic kidney 293T with a
3-plasmid system according to manual instructions. miR-30a-5p
inhibitor was synthesized by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). For β3 integrin overexpression, the cDNA of β3
integrin was cloned into the pcDNA3.1, as previously described, and
transfected into human breast cancer cells using Lipofectimine
according to the manufacturer's instructions (16). Total RNA and protein were collected
for 2 days post-transfection or viral infection assay.
Quantitative real-time PCR analysis
Total RNA was isolated using TRIzol (Invitrogen,
Carlsbad, CA, USA), and complementary DNA was synthesized using
reverse transcriptase (Sangon Biotech Co., Ltd., Shanghai, China).
Real-time quantitative PCR reactions were performed using
SYBR-Green (Takara Bio, Dalian, China). To analyze mature
miR-30a-5p, quantitative PCR (RT-qPCR) was performed using the
miScript PCR System (Qiagen, Hilden, Germany). The mRNA levels of
β3 integrin, E-cadherin, vimentin, and Zeb1 were quantified by
qRT-PCR using QuantiTect SYBR-Green PCR kit (Vazyme Biotech Co.,
Ltd., Nanjing, China). Primers used are described in Table I. Changes in expression were
calculated using the ΔΔCt method. We calculated the median
expression value from signal values (log2). Patients
were labeled based on higher or lower β3 integrin expression
compared with the median value, as follows: individuals with low β3
integrin expression (< median) and those with high β3 integrin
expression (≥ median).
 | Table IThe primers or oligonucleotides. |
Table I
The primers or oligonucleotides.
| Genes | Primers or
oligonucleotides |
|---|
| mir-30a-5p
mimic | Sense:
5′-ccggcttccagtcgaggatgtttacactcgagtgtaaacatcctcgactggaagtttttg-3′
Antisense:
5′-aattcaaaaacttccagtcgaggatgtttacactcgagtgtaaacatcctcgactggaag-3′ |
| mir-30a-5p scramble
sequence | Sense:
5′-ctagaggagctccaccgcggtggcatcgatggagctccaccgcggtggcatggtac-3′
Antisense:
5′-catgccaccgcggtggagctccatcgatgccaccgcggtggagctcct-3′ |
| E-cadherin | Forward:
5′-accattaacaggaacacagg-3′
Reverse: 5′-cagtcactttcagtgtggtg-3′ |
| Vimentin | Forward:
5′-gacctctacgaggaggagat-3′
Reverse: 5′-tccaccaccctgttgctgta-3′ |
| Zeb1 | Forward:
5′-agcagtgaaagagaagggaatgc-3′
Reverse: 5′-ggtcctcttcaggtgcctcag-3′ |
| β3 integrin | Forward:
5′-ctgtatccagccgggctcctatg-3′
Reverse: 5′-gccccggtacgtgatattggtgaa-3′ |
| mir-30a-5p | Forward:
5′-gccgctgtaaacatcctacact-3′
Reverse: 5′-gtgcagggtccgaggt-3′ |
| U6 | Forward:
5′-ctcgcttcggcagcaca-3′
Reverse: 5′-aacgcttcacgaatttgcgt-3′ |
| β-actin | Forward:
5′-cctgtacgccaacacagtgc-3′
Reverse: 5′-atactcctgcttgctgatcc-3′ |
Cell proliferation assay and flow
cytometric analysis
Cell proliferation was measured via
methyl-thiazolyltetrazolium (MTT) assay. Cells were seeded at a
density of 5×103/well into 96-well plates and cultured
for 24, 48, 72 and 96 h. The cells were then incubated with 20 μl
MTT (5 mg/ml) for 4 h at 37ºC, and 150 μl dimethyl sulfoxide was
added to solubilize the crystals for 10 min at room temperature
(RT). The optical density was measured at 540 nm. For cell cycle
analysis, the adhered cells were collected by trypsinization at 48
h after transfection. The cells were incubated with propidium
iodide (0.05 mg/ml; Sigma) and RNase A (0.1 mg/ml; Sigma) for 30
min at RT in the dark and analyzed by using BD FACSCalibur flow
cytometer and CellQuest software.
Adhesion, wound healing and invasion
assays
Cells (0.5×106 cells/well) were added to
each well with 5% CO2 and incu-bated for 4 h at 37ºC.
After washing, the attached cells were fixed with 70% ethanol
followed by staining with 0.1% crystal violet in 20% ethanol. The
stained crystal violet was dissolved in 10% acetic acid, and the
absorbance value was measured at 597 nm. The cell matrix adhesion
index was calculated as the OD value (test-negative control)/OD
value (positive control-negative control). Each test group was
assayed in triplicate and repeated at least thrice. For the scratch
wound healing assay, cells were cultured in a serum-free medium for
24 h and wounded with pipette tips. Wound closing procedure was
observed for 48 h with images taken every 24 h. For the invasion
assay, cell invasion through a 3D extracellular matrix (ECM) was
assessed using BD Matrigel invasion chambers ((BD Biosciences,
Bedford, MA, USA) with 8.0 μm filter membranes. After 24 h, cells
invading the lower surface of the filters were fixed, stained and
counted. Percentage change during invasion was determined by
counting the number of cells that migrated to the lower surface of
the filters. Three separate microscopic fields were counted per
membrane.
β3 integrin 3′-UTR reporter analysis
The 3′-UTR of β3 integrin containing a putative
miR-30a-5p binding site was amplified and cloned into pGL3 vector
to generate the wild-type construct. An overlap extension PCR assay
was used for mutant plasmids, as previously described (17). Cells were cultured in 24-well
plates. For the transfection complex, 2 μl of 20 μM chemically
synthesized miR-30a-5p mimic, 150 ng pGL3 reporter plasmid, and 50
ng pRL-TK plasmid were mixed with Lipofectamine 2000. Luciferase
activities were measured according to the manufacturer's
instructions (Dual-Luciferase assay system; Promega).
Renilla luciferase activity was normalized to corresponding
firefly luciferase activity and was plotted as a percentage.
Western blot analysis
Cells were lysed in RIPA buffer. Proteins were
separated by using SDS-PAGE and transferred to nitrocellulose. The
blots were probed with primary antibodies and incubated with
1:5,000 secondary antibody. Signals were detected with enhanced
chemiluminescence. Images were analyzed by the Gel-Pro-Analyzer
software. The membranes were stripped and probed with monoclonal
antibody for β-actin as loading control.
Statistical analysis
SPSS version 20.0 software was used for statistical
analysis. Student's t-test, Chi-squared test and one-way ANOVA
analysis were used to determine significance. P<0.05 was
considered to indicate a statistically significant result.
Results
TNBC expresses low levels of miR-30a-5p
and high levels of β3 integrin
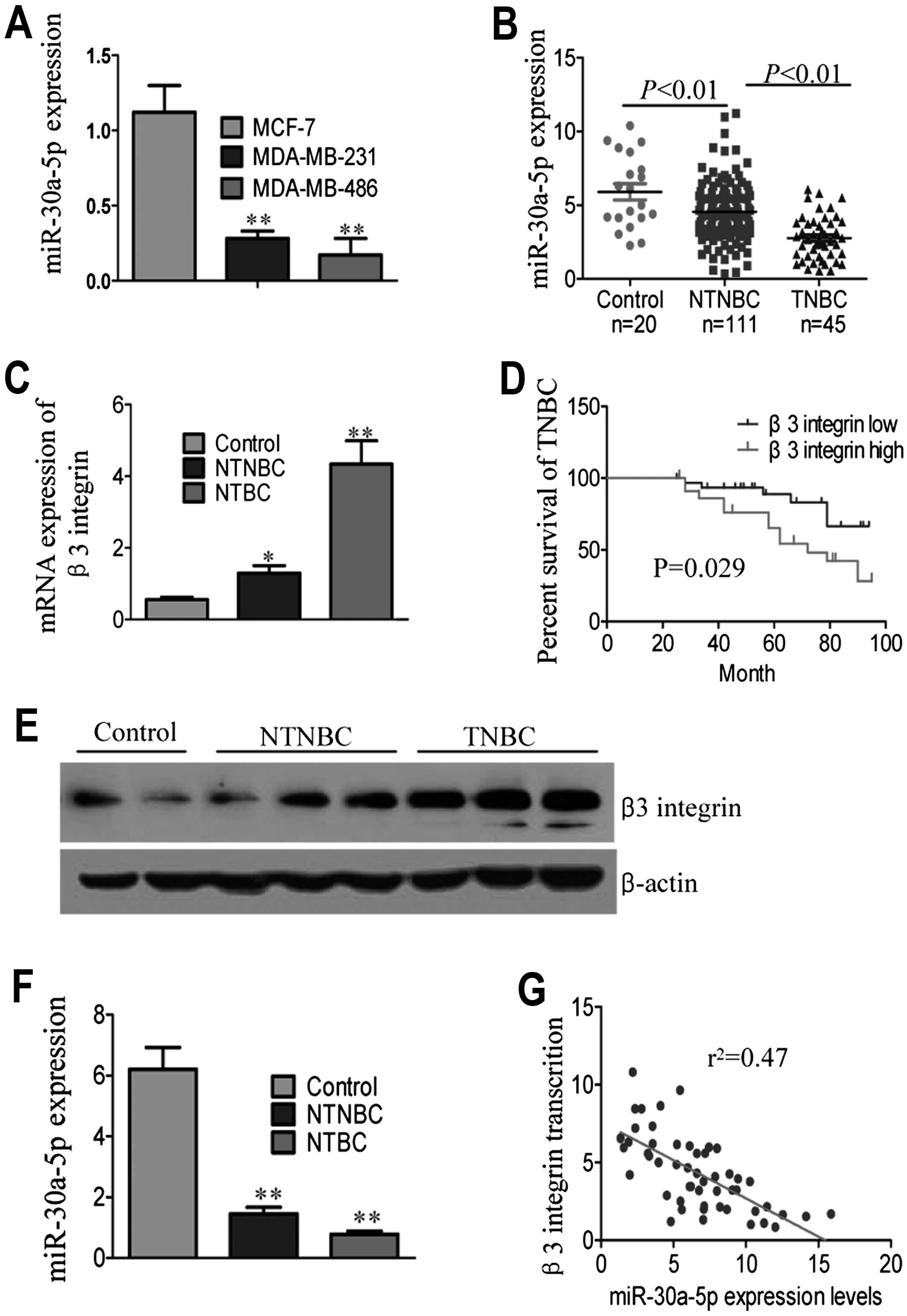
The expression levels of miR-30a-5p in TNBC breast
cancer cell lines (MDA-MB-231 and MDA-MB-486) were much lower than
in NTNBC breast cancer cell lines (MCF-7), as shown in Fig. 1A. We compared miR-30a-5p expression
levels in para-cancerous tissues of breast cancer and breast cancer
patients. Breast cancer tissues had reduced miR-30a-5p transcript
levels compared with para-cancerous tissues (Table II for patient characteristics of
all donors). Significant differences were observed when comparing
TNBC with NTNBC tissues (Fig. 1B
and Table II). Further analysis
showed that miR-30a-5p expression strongly correlated with
histological grade and survival status (Table II).
 | Table IIClinicopathological characteristics
and miR-30a-5p expression in breast cancer. |
Table II
Clinicopathological characteristics
and miR-30a-5p expression in breast cancer.
| Clinicopathological
variables | Cases (%) | log2
(fold of repression) (mean ± SD) | P-value |
|---|
| Age (years) |
| ≤45 | 72 (46.1) | 4.17±0.47 | 0.268a |
| >45 | 84 (53.9) | 4.02±0.51 | |
| Molecular-based
classification | | | 0.006b |
| TNBC | 45 (28.8) | 2.47±0.39 | |
| NTNBC | 111 (71.2) | 4.51±0.59 | |
| Tumor size
(cm) | | | 0.173b |
| ≤2 | 66 (42.3) | 4.37±0.53 | |
| 2–5 | 79 (50.6) | 4.05±0.65 | |
| >5 | 11 (7.1) | 3.71±0.24 | |
| Histological
grade | | | 0.003b |
| I | 48 (30.4) | 4.47±0.32 | |
| II | 83 (52.5) | 4.16±0.53 | |
| III | 27 (17.1) | 2.19±0.22 | |
| Clinical stage | | | 0.047b |
| I, II | 113 (72.4) | 4.52±0.42 | |
| III | 36 (23.1) | 4.18±0.36 | |
| IV | 7 (4.5) | 3.89±0.32 | |
| Positive lymph
nodes | | | 0.001b |
| 0 | 64 (41.0) | 4.83±0.32 | |
| 1–3 | 51 (32.7) | 4.31±0.64 | |
| ≥4 | 41 (26.3) | 4.19±0.22 | |
| Survival | | | 0.003b |
| Alive | 114 (73.1) | 5.49±0.52 | |
| Deceased | 42 (26.9) | 2.86±0.36 | |
The β3 integrin expression in TNBC patients was
compared with that in NTNBC patients. Notably, β3 integrin mRNA
expression was significantly higher in TNBC patients compared with
NTNBC patients and para-cancerous tissues (Fig. 1C) (P<0.001). Kaplan-Meier
analysis was performed by using the log-rank test to calculate the
effect of β3 integrin mRNA expression on TNBC patient survival.
High β3 inte-grin expression was markedly associated with reduced
overall survival in TNBC patient subgroups (Fig. 1D). Western blot analyses of protein
extracts revealed a significantly higher relative β3 integrin
expression in TNBC patients (Fig.
1E). Given that miRNAs exploit their inhibitory activity at the
post-transcriptional level and the reduced miR-30a-5p expression in
the TNBC patients, we subsequently verified the expression of
miR-30a-5p and β3 integrin in breast tumor tissues. As shown in
Fig. 1F, miR-30a-5p expression was
lower in TNBC than NTNBC samples. An inverse correlation was found
between mRNA expression of miR-30a-5p and β3 integrin in TNBC
patients (Fig. 1G,
r2=0.47, P<0.05; Pearson's correlation). These
differences in mRNA and protein expression of β3 integrin in breast
cancer patients suggested probable post-transcriptional regulation
by miR-30a-5p.
Knockdown of β3 integrin results in the
alteration of EMT markers
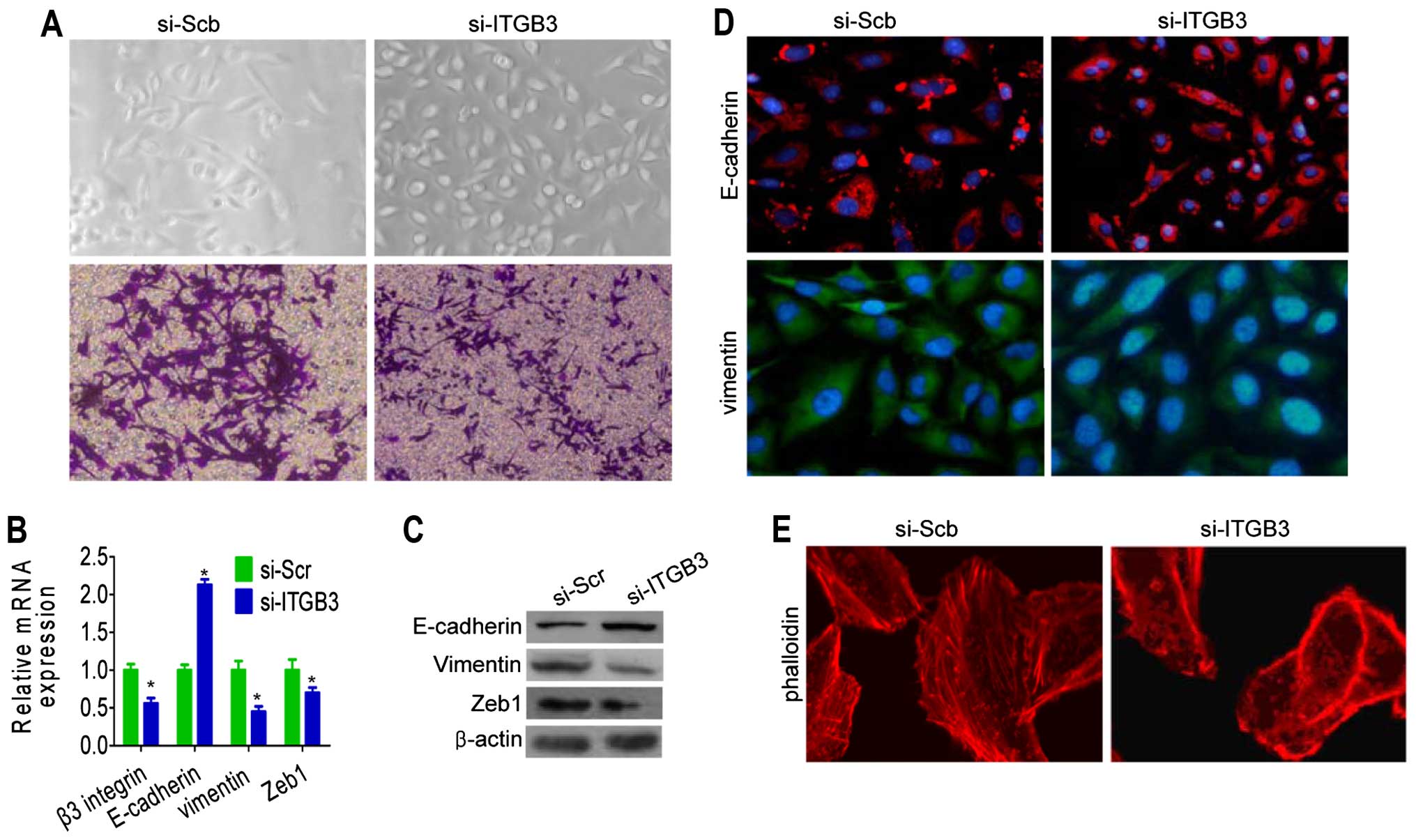
To investigate the role of β3 integrin knockdown on
the reversal of EMT phenotype of breast cancer cells,
siRNA-targeting β3 integrin was transfected into MDA-MB-231 cells.
After 14 days of transfection, the morphology of β3
integrin-silenced MDA-MB-231 was partially changed from elongated
fibroblastoid to epithelial cobblestone-like appearance, with the
cells appearing to grow in close contact with each other (Fig. 2A). The silencing of β3 integrin in
the MDA-MB-231 cells resulted in the elevation of epithelial marker
E-cadherin and the downregulation of mesenchymal markers, including
Zeb1 and vimentin, at mRNA and protein levels (Fig. 2B and C).
We next investigated whether molecular alterations
were present in E-cadherin and vimentin protein by immuno-staining.
Silencing of β3 integrin showed that E-cadherin protein was
significantly upregulated compared with the levels detected in
control cells (Fig. 2D). To
determine the role of β3 integrin in actin cytoskeletal
reorganization, cells with silenced β3 integrin were stained for
F-actin and vinculin and showed morphological changes, including
formation of protrusions and destruction of actin filaments
(Fig. 2E). These results suggest
that the β3 integrin is critical for the acquisition of EMT
characteristics and that inhibition of β3 integrin was able to
reverse the EMT phenotype of breast cancer cells.
miR-30a-5p directly targets the β3
integrin 3′ untranslated region (3′-UTR)
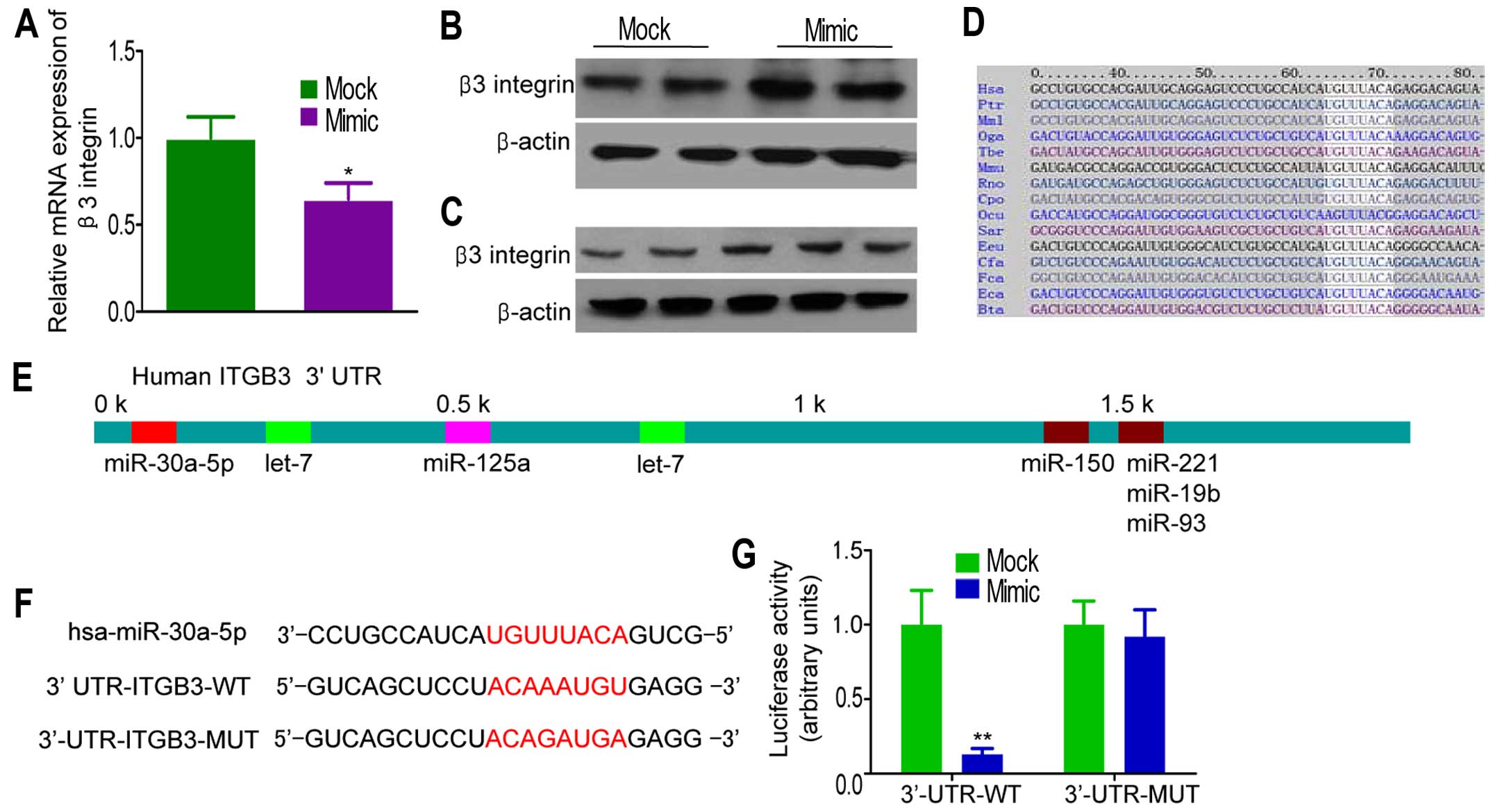
To investigate the direct effects of miR-30a-5p on
β3 integrin expression in breast cancer cell lines, we performed
miR-30a-5p overexpression experiments in MDA-MB-231 cells.
Infection of miR-30a-5p mimic into MDA-MB-231 cells increased
miR-30a-5p levels (data not shown). Ectopic overexpression of
miR-30a-5p resulted in a significantly decrease in β3 integrin mRNA
levels, as determined by qRT-PCR (Fig.
3A). This suppression was also found at the protein level, as
observed by western blot analysis (Fig. 3B). On the contrary, miR-30a-5p
inhibitor restored β3 integrin expression in a dose-dependent
manner (Fig. 3C). Online programs
(TargetScan, miRBase and PicTar) revealed that a region in the
65–72 β3 integrin 3′-UTR had a perfect complementary matching
region in the seed sequence of miR-30a-5p (Fig. 3D and E). To confirm that the
silencing of β3 integrin expression is consequent to miR-30a-5p
targeting of the 3′-UTR in β3 integrin transcript, the complete
3′-UTR of β3 integrin and corresponding mutant counterparts were
cloned into pGL3 firefly luciferase-containing vector (Fig. 3F). HEK-293T cells were
cotransfected with the firefly luciferase-containing vector,
Renilla luciferase-containing vector, and pre-miR-30a-5p.
The results revealed a strong repression of luciferase activity
after transfection with wild-type 3′-UTR of β3 integrin, but not in
cells with mutant 3′-UTR (Fig.
3G).
miR-30a-5p reduces the adhesion capacity
of TNBC cells
Ectopic miR-30a-5p also inhibited the proliferation
rate of MDA-MB-231 and MDA-MB-486 cells by inducing cell cycle
arrest at the G0/G1 phase, as shown by the MTT assay and flow
cytometry (data not shown). To eliminate the potential cofounding
effect of cell proliferation on cell migration and invasion, the
Transwell experiment and scratch assay were conducted in the
presence of mitomycin C to subsequently arrest cell
proliferation.
β3 integrin signaling is associated with many
cellular functions. Integrin-mediated interactions with the
extra-cellular matrix (ECM) are required for attachment,
cytoskeletal organization, mechanosensing, migration,
proliferation, differentiation and survival of cells. To test
whether miR-30a-5p functionally behaves as a tumor suppressor by
targeting β3 integrin, we stably overexpressed miR-30a-5p in
MDA-MB-231 and MDA-MB-486 using a lentiviral vector.
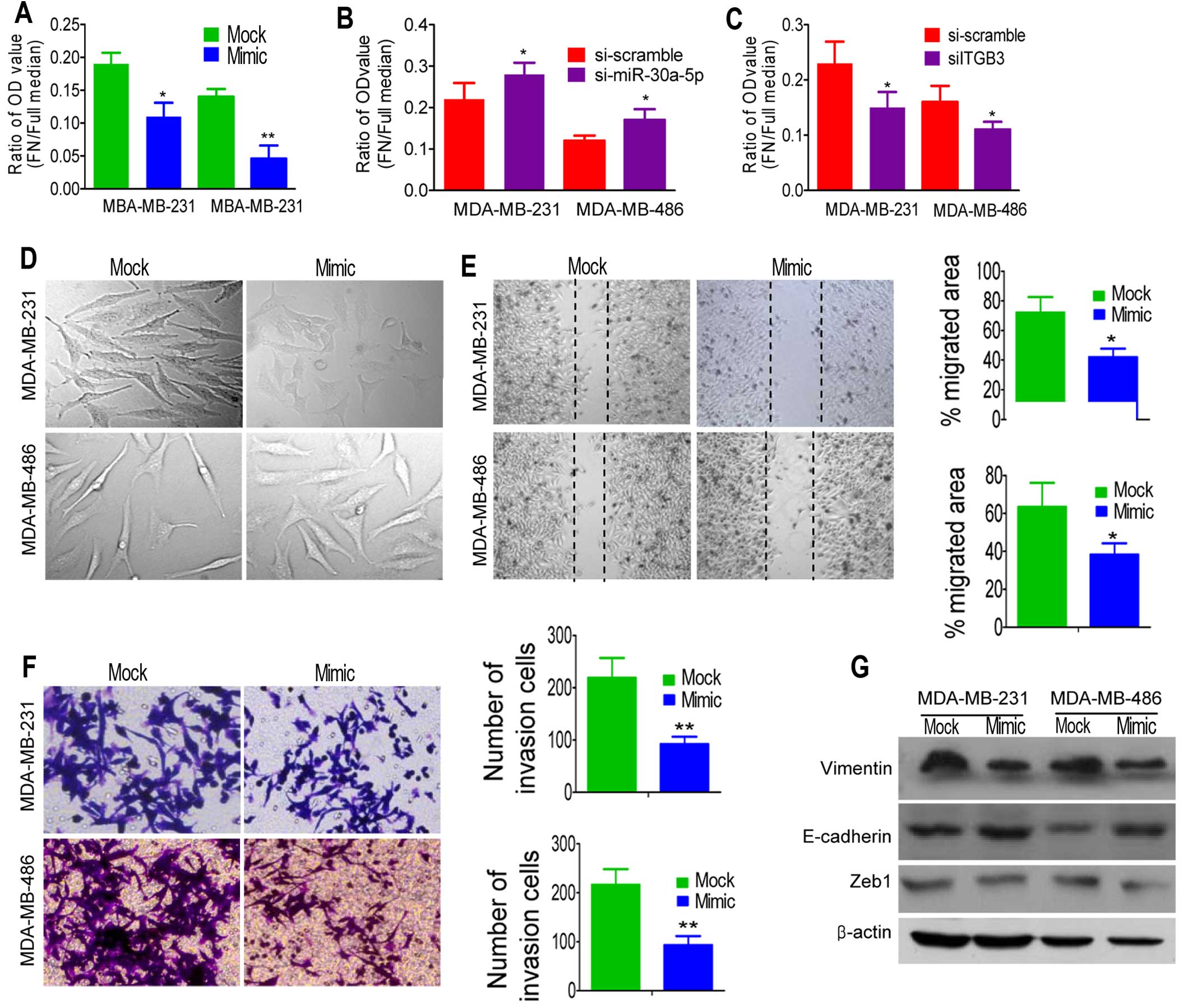
We focused on the effect of miR-30a-5p on the
adhesion of TNBC cells by planting cells in plates coated with
fibronectin, which could bind to a β3 integrin receptor expressed
on the surface of tumor cells (18). As shown in Fig. 4A, the adhesion ability of
MDA-MB-231 and MDA-MB-486 cells with miR-30a-5p overexpression
showed significantly reduced adherence compared with the control
cells. On the contrary, the adhesion ability of cells was lower
when miR-30a-5p was inhibited using siRNA than when scramble siRNA
was used (Fig. 4B). Moreover,
reduction occurrence after transfection with siRNA directed against
β3 integrin (siITGB3) elucidated the significance of reduced β3
integrin in overexpressed miR-30a-5p cells (Fig. 4C).
Ectopic expression of miR-30a-5p
suppresses cell migration and invasion in vitro
To test whether miR-30a-5p overexpression suppressed
tumor migration and invasion, we first examined the morphological
changes in miR-30a-5p-overexpressed cells. As shown in Fig. 4D, MDA-MB-231 cells overexpressing
miR-30a-5p exhibited epithelial morphology. Cells overexpressing
miR-30a-5p showed a non-aggressive appearance and depressed
adhesion of cell to fibronectin. Thus, we hypothesized that
miR-30a-5p could suppress the migration and invasive behavior of
TNBC cancer cells. Then, wound scratch and Transwell assay were
employed to detect the migration and invasion after miR-30a-5p
manipulation, respectively. We found that miR-30a-5p could
significantly suppress migration (Fig.
4E) and invasion (Fig. 4F) in
TNBC cells lines. To further investigate whether the inhibitory
effect of miR-30a-5p on migration and invasion was mediated by
mesenchymal to epithelia transition (MET), we examined the
expression of several MET markers. As expected, miR-30a-5p
overexpression increased the expression level of E-cadherin and
decreased the expression levels of vimentin and Zeb1 (Fig. 4G). These observations suggested
that ectopic expression of miR-30a-5p was able to impede migration
and invasion mediated by MET in vitro.
miR-30a-5p interrupts the β3
integrin-Erk-Ets-1 network in triple-negative breast cancer
Integrins elicit a series of transduction events
that regulate cell cycle progression and apoptosis in a process
known as ‘outside-in’ signaling. The ‘outside-in’ β3
integrin-mediated signaling proceeds primarily via the Erk1/2 MAPK
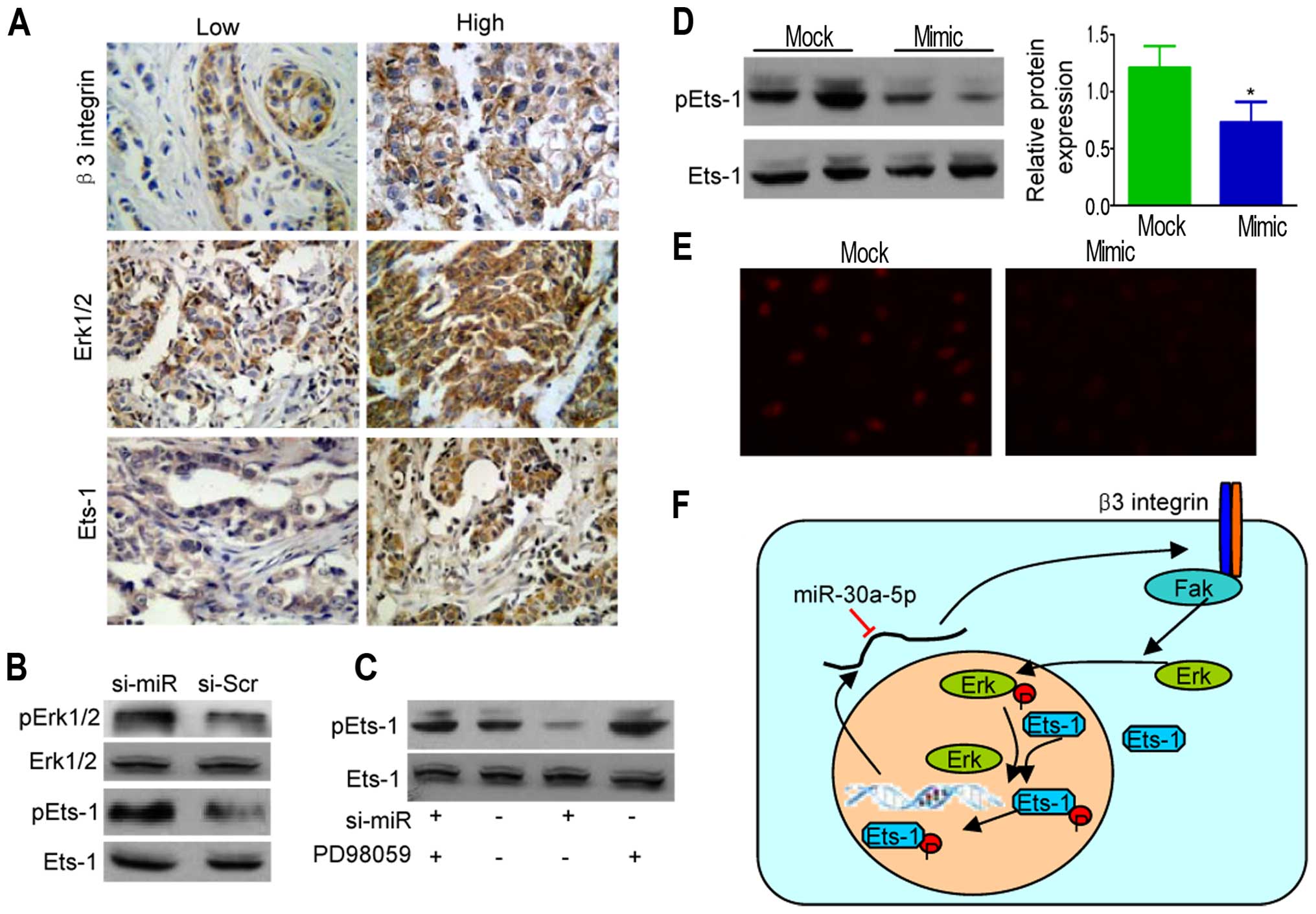
pathway in MDA-MB-231 breast cancer cells. Immunohistochemical
staining of β3 integrin, Erk1/2, and Ets-1 in breast cancer and
normal tissues are shown in Fig.
5A. Results indicated that expression of β3 and Erk1/2
(P<0.05) and β6 and Ets-1 (P<0.01) were positively
correlated, as shown in Table
III.
 | Table IIIExpression correlation between β6
integrin and Erk1/2 or transcriptional factor Ets-1. |
Table III
Expression correlation between β6
integrin and Erk1/2 or transcriptional factor Ets-1.
| β6 integrin
expression | Erk1/2
expression | Ets-1
expression |
|---|
|
|
|---|
| Negative or
low | High | P-value | Negative or
low | High | P-value |
|---|
| Negative or
low | 55 | 41 | <0.05 | 63 | 38 | <0.01 |
| High | 22 | 38 | | 22 | 33 | |
To further confirm the association between
Erk1/2-EtS phosphorylation and miR-30a-5p inhibition, we collected
extracts from si-miR-30a-5p MDA-MB-231 cells. Western blot analysis
of the extracts showed a specific enhancement of pErk1/2 (Fig. 5B). As the activities of Ets-1 were
enhanced by Erk1/2-mediated phosphorylation at threonine 38 for
Ets-1 (26), we further examined
the phosphorylation levels of Ets-1, showing Erk1/2 activation
correlated well with the elevated phosphorylation levels of pEts-1,
whereas PD98059 (Erk pathway inhibitor) markedly lowered pEts-1
levels (Fig. 5C). However, Ets-1
phosphorylation was also enhanced (Fig. 5D) with obvious nuclear
translocation in ectopic miR-30a-5p-expressing MDA-MB-231 cells
(Fig. 5E). Similar correlations
between miR-30a-5p overexpression and reduced Erk1/2-Ets
phosphorylation were found in MDA-MB-486 cells (data not shown).
Thus, β3 integrin induced ERK1/2 phosphorylation, thereby
triggering the activation of Ets-1 transcription factors leading to
β3 integrin upregulation, whereas miR-30a-5p interdicted the
positive feedback loop (Fig.
5F).
Discussion
TNBC has a high incidence of early relapse and
metastasis and contributes to poor clinical outcomes. Treatment
options are limited for TNBC because endocrinotherapy and targeted
therapy, which aim directly at human epidermal growth factor
receptor-2, are ineffective. Interactions between cells and ECM
convey micro-environmental cues that influence cell proliferation,
differentiation, adhesion and migration (19,20).
Thus, targeting cell-ECM interactions can become a potential
component of an oncologist's therapeutic arsenal as a novel therapy
for TNBC. Integrins, a family of cell adhesion molecules, are
involved in a wide range of cell-ECM and cell-cell interactions.
Basement membrane proteins interact with mammary epithelial cells
via integrins and transmembrane proteoglycans and syndecan, which
all couple to the cytoskeleton and assemble signaling platforms to
control cell function (21).
Integrin and associated intracellular signaling effector expression
levels and/or activity are modified in TNBCs, thereby suggesting
that the adhesion machinery has a role in malignant transformation
and tumor progression (7,22).
Integrin adhesion receptors modulate cell functions,
including cell proliferation; hypodermic injection of breast cancer
cells stably transfected with β1 integrin into athymic nude mice
resulted in decreased size and weight of subcutaneous xenograft
tumors (23). Moreover, β1
integrins support outgrowth of metastatic colonies in the lung. In
the present study, we demonstrated that ectopic expression of β3
integrin attenuated the proliferation and induced cell cycle arrest
at G0/G1 phase in breast cancer cells. This confirms and extends
earlier reports where outgrowth of breast cancer cells arrest also
showed the role of β3 integrin signaling in the modulation of cell
proliferation of breast cancer (24).
β3 integrin has been detected in tumor tissues from
patients with melanoma, breast cancer and its expression is
particularly pronounced in metastatic tissue (7,25,26).
TNBC is a highly aggressive subgroup of breast cancer and currently
lacks definite molecular targets for effective targeted therapies.
Among the 156 tissue specimens, 57 (36.5%) were positive for β3
expression. A significant statistical difference existed between
TNBC and NTNBC groups. Previous studies exploited β3 integrin as a
therapeutic target for treating TNBC by delivering siRNA (7,8).
Breast cancer cell adhesion and spread are triggered by the contact
between β3 integrin and fibrinogen. Blocking β3 integrin with
antibodies and siRNA leads to significantly lower adhesion of
MDA-MB-231 cells to fibronectin (27). EMT is a crucial procedure in tumor
metastasis; therefore, prevention of EMT represents a very
promising therapeutic strategy to prevent tumor metastasis
(28,29). In the present study, reduced
expression of β3 integrin could cause morphological changes in
MDA-MB-231 cells, i.e., from fibroblastoid cells to epithelial-like
cells with weak invasive capacity. Silencing of β3 integrin
promoted MDA-MB-231 cell migration due to the change in the amount
of organized actin cytoskeleton. This finding is significative
because targeting TNBC by β3 integrin might provide a valuable tool
in developing new therapeutic avenues against metastasis.
MiRNAs negatively regulate EMT-related genes at the
post-transcriptional level and play critical roles in cancer
metastasis. In the present study, we reported that miR-30a-5p is
associated with the regulation of EMT. Aberrant expression of miRNA
has been implicated in the deregulation of integrin expression and
activity. MiRNA-130b suppresses migration and invasion of
colorectal cancer cells through downregula-tion of β1 integrin
(30). We found that
overexpression of miR-30a-5p in breast cancer cells significantly
suppressed β3 integrin in vitro, whereas, miR-30a-5p was
inversely correlated with β3 integrin expression in breast cancer
tissues. Computational prediction by using the TargetScan software
revealed an evolutionarily conserved region in the β3 integrin
3′-UTR mRNA, which has a perfect complementary matching region to
the seed sequence of the miR-30a-5p. Luciferase activity assay
indicated that miR-30a-5p could bind to the 3′-UTR sequence of β3
integrin mRNA, whereas β3 integrin is a direct target of
miR-30a-5p.
Many cellular responses to soluble growth factors,
such as EGF, PDGF, LPA and thrombin, depend on the adhesion of
cells to substrates via integrins (31). The integrin family represents major
receptors that mediate adhension to the ECM and trigger critical
intracellular signaling pathways involved in the invasion and
migration (32). Previous studies
show that the presence of a positive feedback loop between β3
integrin and Ets-1 (33,34). Thus, β3 integrin might be a novel
therapeutic target. Results from our study indicated that the
expression level of β3 and Ets-1 was associated with
differentiation, TNM stage, and breast cancer classification.
Correlation analysis showed that the expression of β3 and Ets-1
were positively correlated. To verify the potential impact of
ectopic miR-30a-5p on phosphorylation Ets-1, the activation of the
ERK/Est-1 was determined. Overexpression of miR-30a-5p displayed
reduced phosphorylation of Erk1/2 and Est-1, and weakened the
nuclear localization of Ets-1 in MDA-MB-231 cells. Furthermore,
inhibition of Erk attenuated the silence-mediated upregulation of
pEts-1 in miR-30a-5p.
In conclusion, β3 integrin contributed to cancer
progression in TNBCs by regulating Erk1/2 and Est-1, which drive
malignant cell behavior. This regulation can be exploited in
therapeutic strategies to inhibit cancer progression. The present
study showed that miR-30a-5p, as a tumor suppressor, is a regulator
of β3 integrin replication in breast cancer. This validates the
concept of targeting the cross link of β3 integrin/Erk/Ets-1
network as a novel and promising modality to treat TNBCs.
Acknowledgements
The present study was funded by grants from the
National Nature Scientific Foundation of China (30901779 and
81471048) and the Natural Science Foundation of Shandong Province
(ZR2015HL064).
References
|
1
|
Taylor MA, Davuluri G, Parvani JG,
Schiemann BJ, Wendt MK, Plow EF, Schiemann WP and Sossey-Alaoui K:
Upregulated WAVE3 expression is essential for TGF-β-mediated EMT
and metastasis of triple-negative breast cancer cells. Breast
Cancer Res Treat. 142:341–353. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Barcus CE, Keely PJ, Eliceiri KW and
Schuler LA: Stiff collagen matrices increase tumorigenic prolactin
signaling in breast cancer cells. J Biol Chem. 288:12722–12732.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang Y, Shenouda S, Baranwal S, Rathinam
R, Jain P, Bao L, Hazari S, Dash S and Alahari SK: Integrin
subunits alpha5 and alpha6 regulate cell cycle by modulating the
chk1 and Rb/E2F pathways to affect breast cancer metastasis. Mol
Cancer. 10:842011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Felding-Habermann B, O'Toole TE, Smith JW,
Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil
SJ, Saven A, et al: Integrin activation controls metastasis in
human breast cancer. Proc Natl Acad Sci USA. 98:1853–1858. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salvo E, Garasa S, Dotor J, Morales X,
Peláez R, Altevogt P and Rouzaut A: Combined targeting of TGF-β1
and integrin β3 impairs lymph node metastasis in a mouse model of
non-small-cell lung cancer. Mol Cancer. 13:1122014. View Article : Google Scholar
|
|
6
|
Li N, Zhang JP, Guo S, Min J, Liu LL, Su
HC, Feng YM and Zhang HL: Down-regulation of β3-integrin inhibits
bone metastasis of small cell lung cancer. Mol Biol Rep.
39:3029–3035. 2012. View Article : Google Scholar
|
|
7
|
Parvani JG, Gujrati MD, Mack MA, Schiemann
WP and Lu ZR: Silencing β3 integrin by targeted ECO/siRNA
nanoparticles inhibits EMT and metastasis of triple-negative breast
cancer. Cancer Res. 75:2316–2325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Huveneers S and Danen EH: Adhesion
signaling - crosstalk between integrins, Src and Rho. J Cell Sci.
122:1059–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raymond K, Faraldo MM, Deugnier MA and
Glukhova MA: Integrins in mammary development. Semin Cell Dev Biol.
23:599–605. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harquail J, Benzina S and Robichaud GA:
MicroRNAs and breast cancer malignancy: an overview of
miRNA-regulated cancer processes leading to metastasis. Cancer
biomarkers: section. Dis Markers. 11:269–280. 2012.
|
|
11
|
de Krijger I, Mekenkamp LJ, Punt CJ and
Nagtegaal ID: MicroRNAs in colorectal cancer metastasis. J Pathol.
224:438–447. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Shi M and Guo N: MicroRNA expression and
its implications for the diagnosis and therapeutic strategies of
breast cancer. Cancer Treat Rev. 35:328–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang N, Wang X, Huo Q, Sun M, Cai C, Liu
Z, Hu G and Yang Q: MicroRNA-30a suppresses breast tumor growth and
metastasis by targeting metadherin. Oncogene. 33:3119–3128. 2014.
View Article : Google Scholar
|
|
14
|
Kao CJ, Martiniez A, Shi XB, Yang J, Evans
CP, Dobi A, deVere White RW and Kung HJ: miR-30 as a tumor
suppressor connects EGF/Src signal to ERG and EMT. Oncogene.
33:2495–2503. 2014. View Article : Google Scholar
|
|
15
|
Baraniskin A, Birkenkamp-Demtroder K,
Maghnouj A, Zöllner H, Munding J, Klein-Scory S, Reinacher-Schick
A, Schwarte-Waldhoff I, Schmiegel W and Hahn SA: MiR-30a-5p
suppresses tumor growth in colon carcinoma by targeting DTL.
Carcinogenesis. 33:732–739. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu G, Chong RA, Yang Q, Wei Y, Blanco MA,
Li F, Reiss M, Au JL, Haffty BG and Kang Y: MTDH activation by 8q22
genomic gain promotes chemoresistance and metastasis of
poor-prognosis breast cancer. Cancer Cell. 15:9–20. 2009.
View Article : Google Scholar :
|
|
17
|
Heckman KL and Pease LR: Gene splicing and
mutagenesis by PCR-driven overlap extension. Nat Protoc. 2:924–932.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Knowles LM, Gurski LA, Engel C, Gnarra JR,
Maranchie JK and Pilch J: Integrin αvβ3 and fibronectin upregulate
Slug in cancer cells to promote clot invasion and metastasis.
Cancer Res. 73:6175–6184. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hynes RO: Integrins: Bidirectional,
allosteric signaling machines. Cell. 110:673–687. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hynes RO: Targeted mutations in cell
adhesion genes: What have we learned from them? Dev Biol.
180:402–412. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Morgan MR, Humphries MJ and Bass MD:
Synergistic control of cell adhesion by integrins and syndecans.
Nat Rev Mol Cell Biol. 8:957–969. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gehler S, Ponik SM, Riching KM and Keely
PJ: Bi-directional signaling: Extracellular matrix and integrin
regulation of breast tumor progression. Crit Rev Eukaryot Gene
Expr. 23:139–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Truong HH, Xiong J, Ghotra VP, Nirmala E,
Haazen L, Le Dévédec SE, Balcioğlu HE, He S, Snaar-Jagalska BE,
Vreugdenhil E, et al: β1 integrin inhibition elicits a
prometastatic switch through the TGFβ-miR-200-ZEB network in
E-cadherin-positive triple-negative breast cancer. Sci Signal.
7:ra152014. View Article : Google Scholar
|
|
24
|
Sumimoto S, Muramatsu R, Fujii S and
Yamashita T: Vascular endothelial cells promote cortical neurite
outgrowth via an integrin β3-dependent mechanism. Biochem Biophys
Res Commun. 450:593–597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nasulewicz-Goldeman A, Uszczyńska B,
Szczaurska-Nowak K and Wietrzyk J: siRNA-mediated silencing of
integrin β3 expression inhibits the metastatic potential of B16
melanoma cells. Oncol Rep. 28:1567–1573. 2012.PubMed/NCBI
|
|
26
|
Li A, Guo Q, Kim C, Hu W and Ye F:
Integrin alphaII b tail distal of GFFKR participates in inside-out
alphaII b beta3 activation. J Thromb Haemost. 12:1145–1155. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Beauvais DM and Rapraeger AC:
Syndecan-1-mediated cell spreading requires signaling by
alphavbeta3 integrins in human breast carcinoma cells. Exp Cell
Res. 286:219–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kudo-Saito C, Shirako H, Takeuchi T and
Kawakami Y: Cancer metastasis is accelerated through
immunosuppression during Snail-induced EMT of cancer cells. Cancer
Cell. 15:195–206. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Spaderna S, Schmalhofer O, Hlubek F, Berx
G, Eger A, Merkel S, Jung A, Kirchner T and Brabletz T: A
transient, EMT-linked loss of basement membranes indicates
metastasis and poor survival in colorectal cancer.
Gastroenterology. 131:830–840. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Augoff K, Das M, Bialkowska K, McCue B,
Plow EF and Sossey-Alaoui K: miR-31 is a broad regulator of
β1-integrin expression and function in cancer cells. Mol Cancer
Res. 9:1500–1508. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Frijns E, Sachs N, Kreft M, Wilhelmsen K
and Sonnenberg A: EGF-induced MAPK signaling inhibits hemidesmosome
formation through phosphorylation of the integrin {beta}4. J Biol
Chem. 285:37650–37662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei Y, Tang CH, Kim Y, Robillard L, Zhang
F, Kugler MC and Chapman HA: Urokinase receptors are required for
alpha 5 beta 1 integrin-mediated signaling in tumor cells. J Biol
Chem. 282:3929–3939. 2007. View Article : Google Scholar
|
|
33
|
Sato T and Miwa A: Ets-1 and integrin
beta3 for lung metastasis from colorectal cancer. APMIS.
110:347–353. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rothhammer T, Hahne JC, Florin A, Poser I,
Soncin F, Wernert N and Bosserhoff AK: The Ets-1 transcription
factor is involved in the development and invasion of malignant
melanoma. Cell Mol Life Sci. 61:118–128. 2004. View Article : Google Scholar : PubMed/NCBI
|