Introduction
Ovarian cancer is one of the most common
gynecological malignant tumor, although the incidence is lower than
that of cervical and uterine cancer, the mortality is the highest
(1). The initial debulking surgery
is still the main method to cure ovarian cancer, but it often recur
after surgery. Although there are some progress in both surgical
approach and chemotherapeutic regimen, there is no substantial
breakthrough in the treatment of ovarian cancer. Because many
ovarian cancer symptoms do not appear in the early stage, it is not
easy to be diagnosed. More than 70% of patients are in an advanced
stage when diagnosed, for whom the 5-year survival is only 20%
(2–4). Currently, cis-diaminodichloroplatinum
(DDP) is still chosen as a feasible first-line chemotherapeutic
drug for clinical treatment of ovarian cancer, and its antitumor
effect has been recorded. However, DDP has significant side effects
on patients with renal toxicity, and ototoxicity. Therefore, it is
necessary to find an alternative therapeutic drug with high
efficiency and low toxicity for ovarian cancer patients.
In recent years, the bioactive peptides derived from
milk proteins were found to have anticancer function, especially
for gynecological cancer (5–7).
Although these bioactive peptides were not as effective as
chemotherapy drugs such as DDP in the treatment of cancer, they
have no toxicity, or low toxicity to normal cells (untransformed
cells). Thus they have a broad prospect and potential for the
development as anticancer drugs (8,9).
The hexapeptide (Pro-Gly-Pro-Ile-Pro-Asn, PGPIPN,
63–68 residues of bovine β-casein), also known as immune
hexapeptide or immunomodulating peptide, was originally isolated
from hydrolysate of bovine β-casein (10–14).
Our previous studies showed that PGPIPN significantly decreased the
growth of xenografted human ovarian tumor in vivo, and
induced human ovarian cancer cell apoptosis (9). However, there is no report on whether
this peptide could resist the invasion and migration of human
ovarian cancer cells or not. We studied the effect and mechanism of
PGPIPN in inhibiting the invasion and metastasis of human ovarian
cancer cells in vitro through both the cell line
SKOV3 and primary ovarian cancer cells from fresh
primary ovarian tumor tissues of 37 ovarian cancer patients who
underwent initial debulking surgery. The findings in this study
provide the proof of concept for using PGPIPN as a potential
therapeutic agent for the treatment of ovarian cancer invasion and
metastasis. This study provides the foundation for further in
vivo experiments (animal model) and clinical trials.
Materials and methods
Reagents
PGPIPN was provided by Shanghai Sangon Biological
Engineering Technology (Shanghai, China), and the purity was
confirmed by RP-HPLC to be >99.5%. RPMI-1640 culture medium
(RPMI: Roswell Park Memorial Institute) was purchased from Gibco
BRL Co., Ltd., USA. Fetal bovine serum (FBS) was purchased from
Sijiqing Co., Ltd. (Hangzhou, China). DDP was purchased from Qilu
Pharmaceutical Co., Ltd. (Jinan, China). Matrigel (artificial
reconstituted basement membrane) was purchased from
Becton-Dickinson Co. (Franklin Lakes, NJ, USA). Transwell chamber
with 8-μm pore was purchased from Coster Company (Santa Fe Springs,
CA, USA).
Mouse monoclonal antibodies of MTA1, NM23H and
β-actin were purchased from Santa Cruz Biotechnology, Inc. (Santa
Cruz, CA, USA). The horseradish peroxidase conjugated secondary
antibody (goat anti-mouse IgG) and Super Signal West Pico Trial kit
(ECL chromogenic reagent kit) were purchased from Pierce, USA. BCA
kit for protein quantitative assay was purchased from Shanghai
Sangon Biological Engineering Technology. The other common
biochemical reagents were purchased from Shanghai Biotechnology
Co., Ltd.
Cell cultures
Human ovarian cancer cell line SKOV3
(ovarian serous papillary cystadenocarcinoma) was originally
purchased from ATCC (American Type Culture Collection, USA) and
preserved by the Biochemistry and Molecular Biology Laboratory of
Anhui Medical University. Human ovarian cancer cell line
SKOV3 was cultured in RPMI-1640 medium with 10% FBS in
5% CO2 at 37ºC, and then digested with 0.4% trypsin
after growing to the logarithm for the next experiment.
For primary ovarian cancer cell culture, fresh
primary ovarian tumor tissues, which were assessed and classified
as serous ovarian adenocarcinoma (III–IV grade) according to WHO
criteria, were obtained from 37 patients with ovarian cancer at
initial debulking surgery between January 2014 and March 2015 at
the first affiliated hospital of Anhui Medical University. The
patients had not received adjuvant therapies such as chemotherapy
or radiotherapy before surgery. All the patients signed a written
consent documenting donation of their tissue for research purpose
according to the Declaration of Helsinki before tissue deposition.
The fresh ovarian cancer tissues were washed with PBS 2–3 times to
remove the blood, surrounding fats, necrosis tissues and non-tumor
tissues, then cut into small pieces ~1 mm3 and again
rinsed with PBS two times. The pieces of tumor tissues were
digested with 0.4% trypsin in sterile centrifuge tube at 37ºC for
30 min, shaken once every 5 min. To obtain the single suspension
cells, the above digested tissues were filtered with 100 μm cell
strainer. After centrifuged at 1,000 rpm for 5 min, the cell pellet
was re-suspended in RPMI-1640 medium containing 0.1 mg/l epidermal
growth factor (EGF), 0.1 mg/l insulin-like growth factor (IGF) and
0.1 mg/l β-estradiol with 10% FBS, which were subsequently cultured
in 5% CO2 at 37ºC. Once cells reached 70–80% confluence,
cell culture medium was drained from the flask, and cells were
digested with 0.25% collagenase II until ~1/3 of the cells fell to
the bottom of the dish as evaluated using a microscope. Due to
their initial shedding, most fibroblasts were eliminated by
collagenase digestion. The remaining cells were continually
cultured in 5% CO2 at 37ºC. A portion of these cells
were placed on the cell slide and identified by using
anti-cytokeratin 7-FITC (FITC: fluorescein isothiocyanate, a green
fluorescent dye) for cytokeratin 7 of ovarian cancer cells and
Hoechst 33258 (blue fluorescent dye for the nuclei) to assay their
purity.
Transwell assay of celI invasion
The Transwell assay was conducted to study cell
invasion. The cells in the logarithmic growth were pretreated with
PGPIPN at 0 (as control), 1×10−5, 1×10−4,
1×10−3 and 1×10−2 g/l respectively for 30
min. In addition, DDP at 1×10−4 g/l was added in the
same plate as positive control. Then the above cells at a density
of 3×105 cells/ml in sextuplicate were respectively
seeded in the upper insert (8-μm pore) using 200 μl RPMI-1640
medium without FBS, which contained the above concentrations of
PGPIPN or 1×10−4 g/l DDP. In addition, the fitted
culture dishes contained 500 μl RPMI-1640 medium with 10% FBS.
After 30, 36 and 42 h (30 and 36 h for SKOV3 cells; 36
and 42 h for primary ovarian cancer cells) respectively, the
chambers were removed from the plates, fixed, and the migrated
cells were stained with hematoxylin and eosin. For each well five
representative ×100 images were quantified. Each experiment was
performed in two independent sets. Invasion inhibition rate (%) =
(1 − invasive cell number of experimental group/that of control
group) × 100%.
Scratch assay of cell migration
Migration of cells in logarithmic growth was
examined using a scratch assay. The cells in sextuplicate were
cultured in a 12-well plate at 5×104 cells/well until a
confluent monolayer was formed. A 200-μl pipette tip was used to
scratch the wells. The wells were sequentially rinsed with PBS and
cultured in RPMI-1640 medium with 10% FBS. The wells were treated
with 0 (as control), 1×10−4, 1×10−3 and
1×10−2 g/l PGPIPN respectively, and DDP at
1×10−4 g/l was added in the same plate as positive
control. The images of the scratch test were taken by microscopy.
The cell migration was measured by Image J software (version 1.48,
National Institutes of Health, USA) with contrasting quantification
of pixels in the area of scratch at 0, 12 and 24 h. Each experiment
was performed in two independent sets. Migration inhibition rate
(%) = (1 − experimental group pixels/control group pixels) ×
100%.
Colony formation assay
The cells in logarithmic phase were digested by
trypsin, resuspended, and counted. The cells in sextuplicate were
seeded in 6-well plates at 1,000 cells/well (500 μl), in which
RPMI-1640 medium with 10% FBS contained 0 (as control),
1×10−5, 1×10−4, 1×10−3 and
1×10−2 g/l PGPIPN respectively, and DDP at
1×10−4 g/l was added in the same plate as positive
control. The fresh media were replaced once every 2 days. Cells
were cultured in 5% CO2 at 37ºC for 12 days, the numbers
of colonies were counted. A colony containing >30 cells was
defined as positive, and counted. The inhibition rate of colony
formation was calculated as follows. Each experiment was performed
in two independent sets. Colony inhibition rate (%) = (1 − colony
number of experimental group/that of control group) × 100%.
Real-time PCR
An optimized RT-PCR protocol was employed to analyze
the mRNA levels of NM23H1 (metastasis-associated gene-1) and
MTA1 (metastatic tumor antigen 1). β-actin was used as a
housekeeping gene. According to primer sequences of NM23H1,
MTA1 and β-actin genes retrieved from Primer-Bank,
primers were designed with Primer 5.0 software, which were
synthesized by Shanghai Sangon Biological Engineering Technology.
These primer sequences are as follows: NM23H1 forward,
CTTTAGGGATCGTCTTTCAAGG; reverse, TGCCAACTTGTATGCAGAAGTC 399 bp;
MTA1 forward, TGGCAGATAAAGGAGAGATTCG; reverse,
TGTCGTAGATGTTCTTGTGGAGA 312 bp; β-actin forward,
TGACGTGGACATCCGCAAAG; reverse, CTGGAAGGTGGACAGCGAGG 205 bp.
SKOV3 cells or primary ovarian cancer
cells were harvested after PGPIPN treatment (sextuplicate) at
different doses for 48 h, respectively. The total RNAs in cells
were extracted according to the TRIzol kit manufacturer's
instructions, and the purity and concentration were determined by
ultraviolet spectrophotometry. The reverse transcription reaction
conditions were 42ºC, 15 min and 95ºC, 5 min. After the reaction,
the reverse-transcribed cDNAs were diluted with RNase-free water to
a final volume of 60 μl and preserved at 80ºC. Real-time PCR adopts
Takara SYBR Green as real-time PCR Master Mix in ABI7500
fluorescent real-time PCR instrument. The reaction conditions were
as follows: 95ºC×30 sec (1 cycle); 95ºC×5 sec, 60ºC×34 sec (40
cycles). The ABI SDS software (Applied Biosystems) was used to
determine a critical threshold (Ct), which was defined
as the cycle number where the linear phase for each sample crossed
the threshold level. The mRNAs of target gene expression were
denoted by ΔCt (ΔCt = target gene
Ct − β-actin Ct value). Finally, the relative
mRNA expression of all samples were calculated using the
2−ΔΔCt method (15).
All reactions were performed in triplicate, and a mixture lacking a
complementary DNA template (NTC) was used as the negative
control.
Western blotting
The cells were harvested after PGPIPN treatment
(sextuplicate) at different doses for 48 h, respectively. Proteins
from the above cells were separated by SDS-PAGE and transferred to
PVDF membrane based on the method described by Green and Sambrook
(16). After blocking with 5%
(w/v) dry skim milk, membranes were incubated with primary
antibodies (mouse monoclonal NM23H1, MTA1 and β-actin antibodies,
1:1,000) and then incubated with horseradish peroxidase conjugated
secondary antibody (goat anti-mouse IgG, 1:8,000). The proteins
were detected with the enhanced chemiluminescence (ECL) system
followed by exposure to X-ray film. β-actin was used as a loading
control. Digital images were captured by Gel Doc™ gel documentation
system (Bio-Rad, USA) and intensities were quantified using
Quantity-One software version 4.62 (Bio-Rad).
Statistical analysis
All statistical analyses were performed with SPSS
version 16.0. The effect of the treatment on each parameter was
analyzed by one-way analysis of variance (ANOVA). Results are shown
by mean ± SD, and P<0.05 was considered to be statistically
significant.
Results
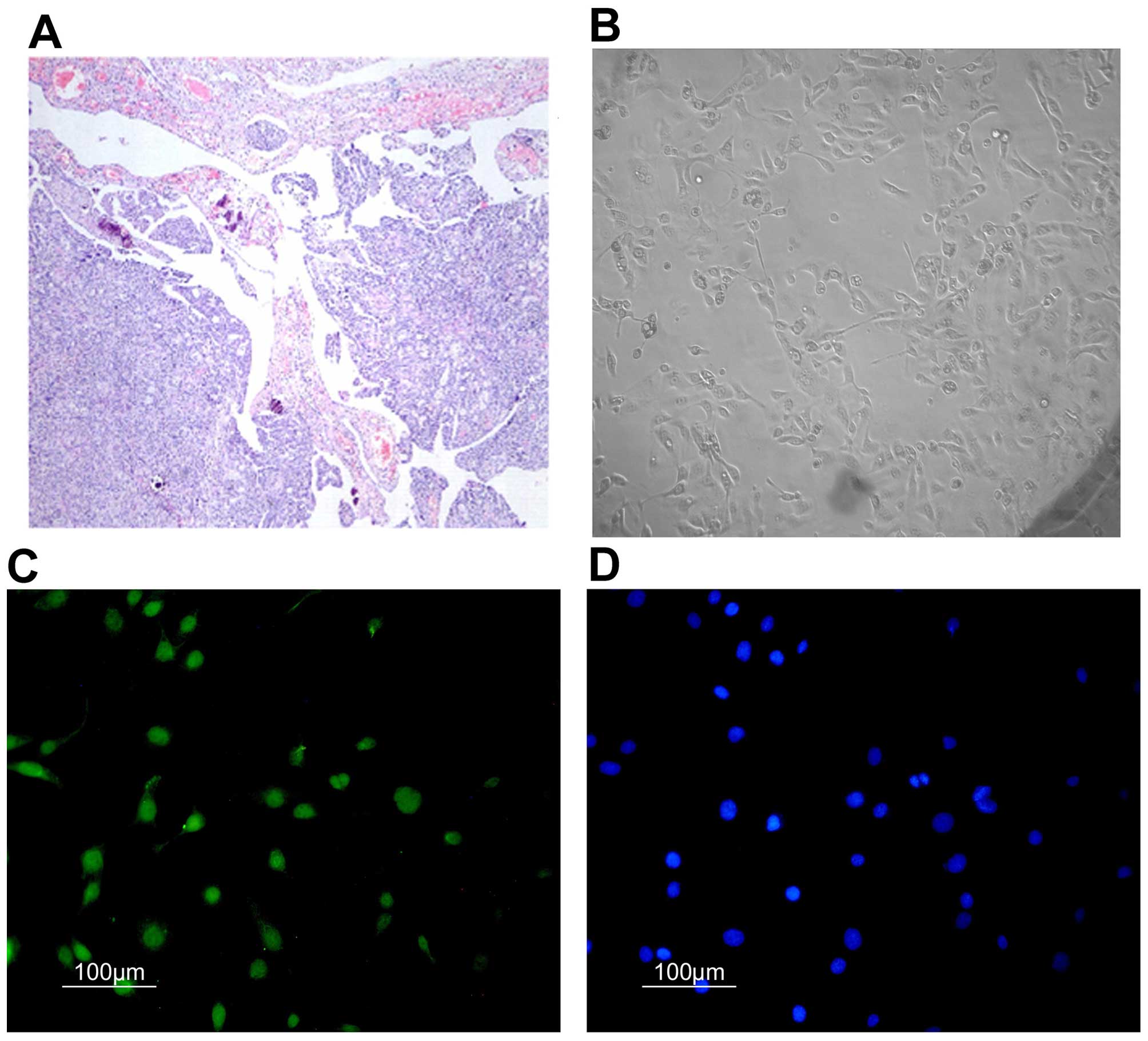
Culture and identification of primary
cells
The primary ovarian cancer cells from fresh ovarian
tumor tissues, which were assessed and classified as serous ovarian
adenocarcinoma (III–IV grade) according to WHO criteria (Fig. 1A), were cultured (Fig. 1B). By immunofluorescence through
cytokeratin 7 (CK7), the purities of these primary ovarian cancer
cells were identified using anti-cytokeratin 7-FITC and Hoechst
33258 (Fig. 1C and D), their
average purity reached 84.5%.
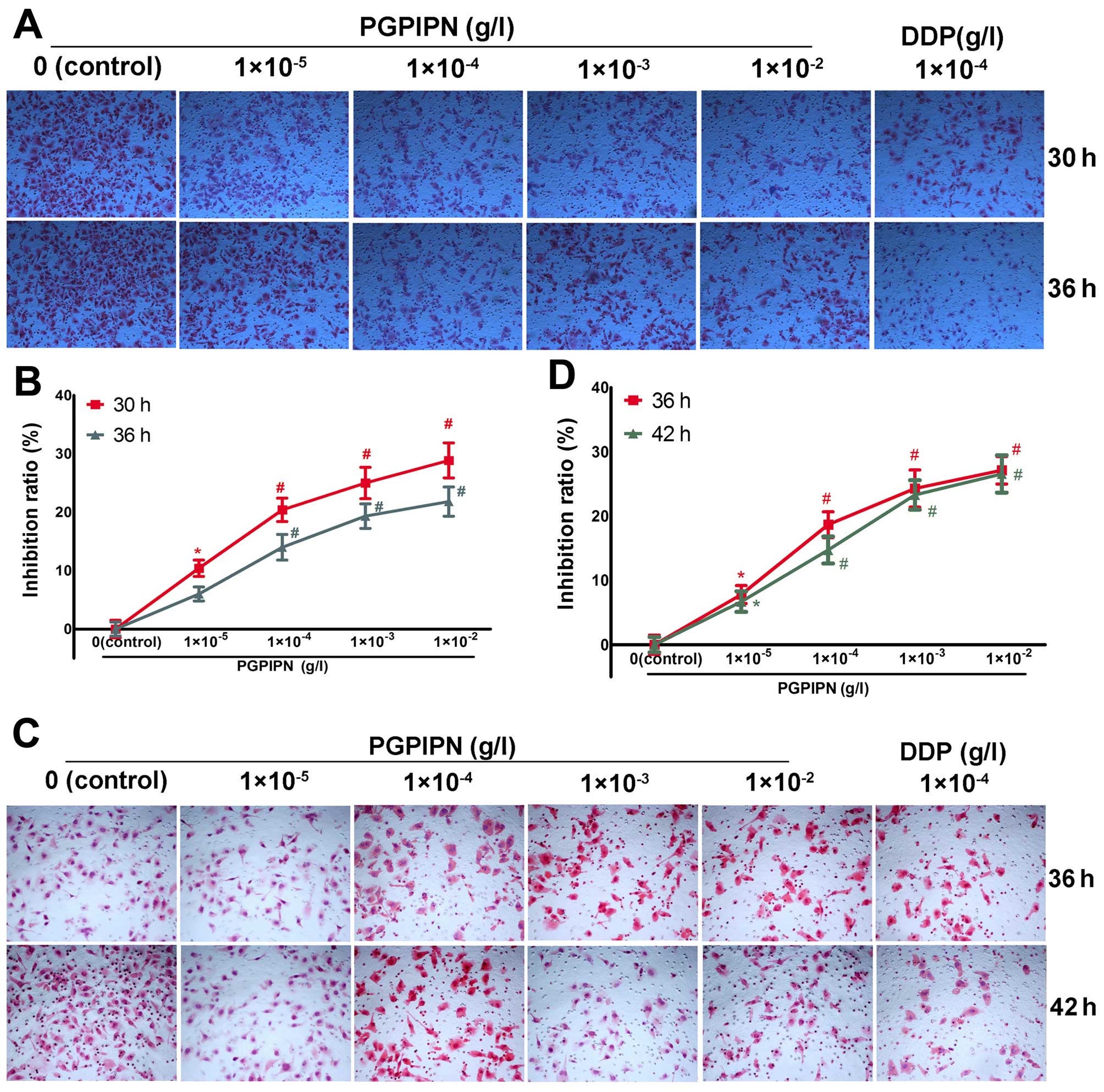
PGPIPN inhibited the invasion and
migration of ovarian cancer cells in vitro
The effect of PGPIPN on the invasive ability of
ovarian cancer cells was assayed by Transwell. PGPIPN significantly
decreased the invasive ability of the SKOV3 cells
(Fig. 2A and B) and primary
ovarian cancer cells (Fig. 2C and
D), in which the maximum inhibition ratios were (28.86±3.01)%
in SKOV3 line cells and (27.14±2.14)% in primary ovarian
cancer cells. The inhibition effect of PGPIPN showed a
dose-dependent manner (Fig. 2B and
D). However, the inhibitory effect of the peptide was less than
that of clinical anticancer drug-DDP. The invasion inhibition of
DDP at 1×10−4 g/l already exceeded 40% on both ovarian
cancer SKOV3 cells and the primary ovarian cancer cells
at 30–42 h.
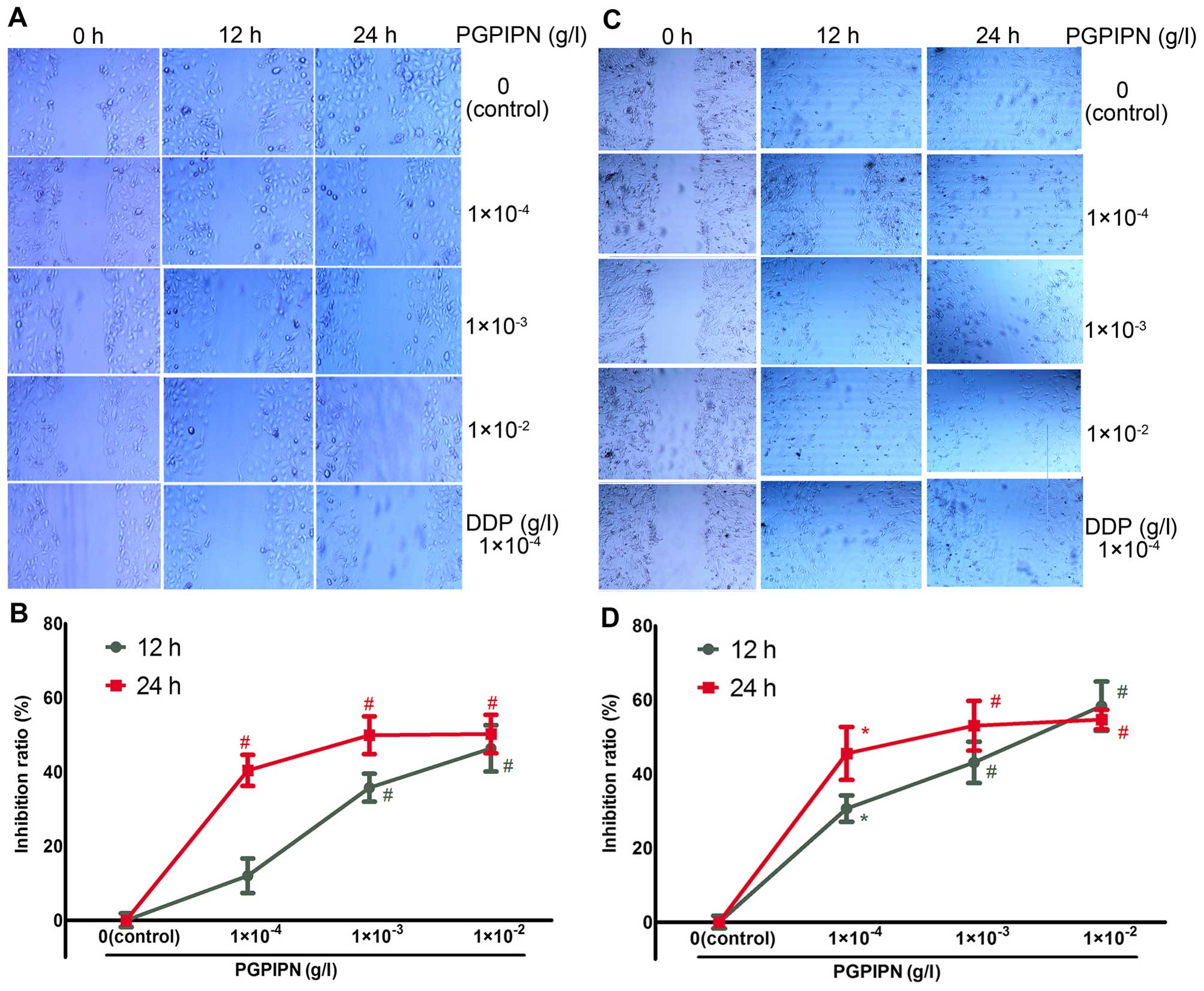
Cell scratch assay was used to detect the effect of
PGPIPN on human ovarian cancer cell migration. PGPIPN was found to
attenuate cell migrations of both SKOV3 (Fig. 3A and B) and the primary ovarian
cancer cells (Fig. 3C and D).
PGPIPN significantly attenuated gap closure after 12 and 24 h
(Fig. 3). Hence, PGPIPN can
significantly inhibit the migration of ovarian cancer cells, which
was dose-dependent within the dose range of
1×10−4–1×10−3 g/l (Fig. 3). The effect of the peptide on
primary ovarian cancer cells is much better than that of the
SKOV3 cells. The inhibitory effects of the peptide to
human ovarian cancer cells were similar to that of DDP at 12–24
h.
PGPIPN inhibits colony formation of
ovarian cancer SKOV3 cells
We examined the colony formation capacity of
SKOV3 cells treated with different concentrations of
PGPIPN. The cells were allowed to grow for 12 days to form
colonies. PGPIPN can significantly inhibit cell colony formation of
ovarian cancer SKOV3 cells (Fig. 4). Our results revealed that PGPIPN
could significantly suppress the colony formation capacity of
ovarian cancer cells. However, the inhibition effect was slightly
less than that of the same concentration of DDP, and the inhibition
rate of 1×10−4 g/l DDP was 39.78±7.8%.
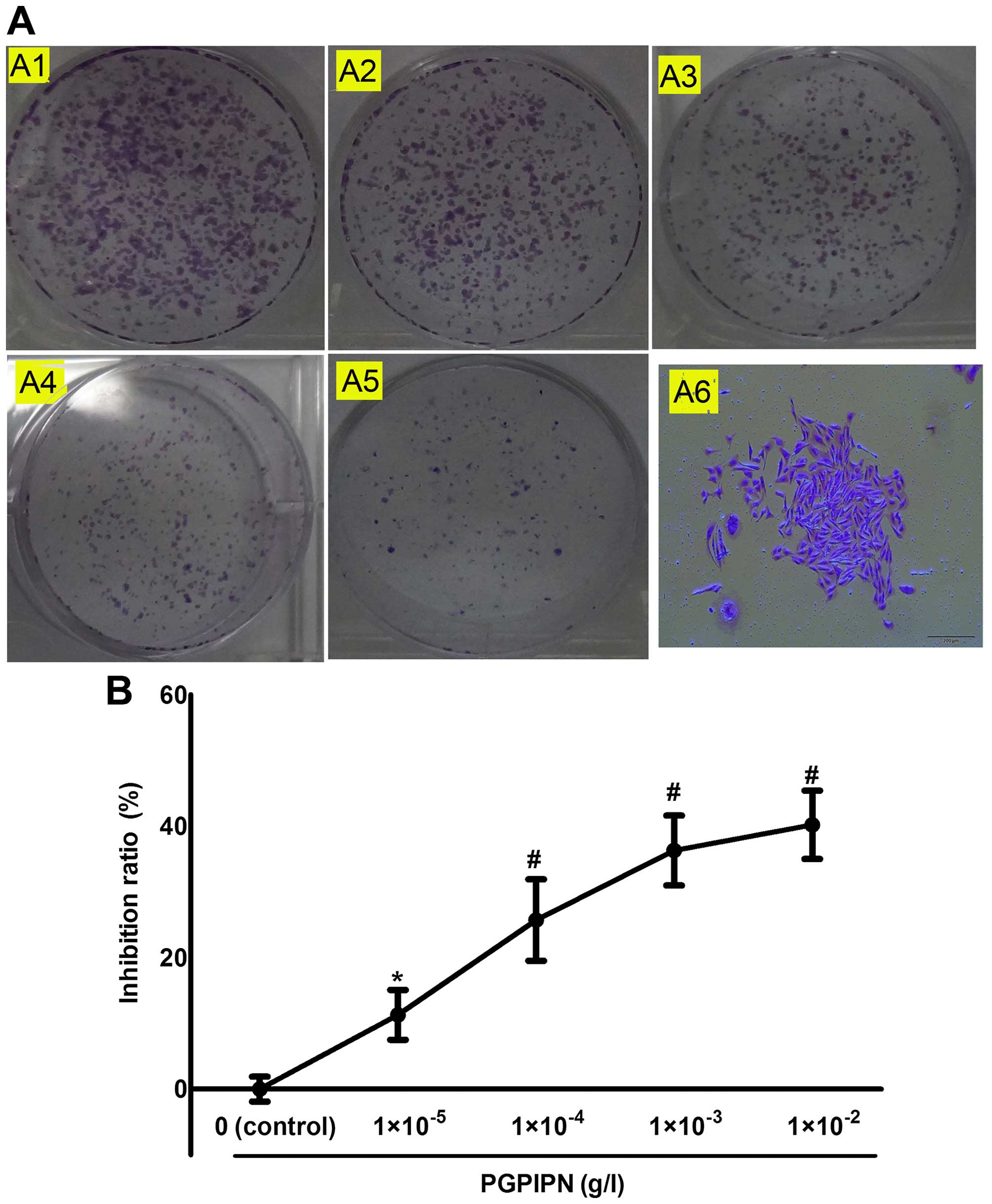 | Figure 4PGPIPN inhibits human ovarian cancer
SKOV3 cell colony formation. (A) The colony formation of
SKOV3 cells in 6-well plates under drug treatment; A1,
A2, A3, A4 and A5 show the colony formation capacity of
SKOV3 cell treated with 0 (as control),
1×10−5, 1×10−4, 1×10−3 and
1×10−2 g/l PGPIPN respectively, and A6 a
SKOV3 colony (crystal violet stain, ×100). (B) Histogram
of inhibition ratio of human ovarian cancer SKOV3 cells
treated with PGPIPN at different concentrations. A colony
containing >30 cells was defined as positive, and counted. The
data are shown as means ± SD, *P<0.05,
#P<0.01 compared with control (the vehicle
group). |
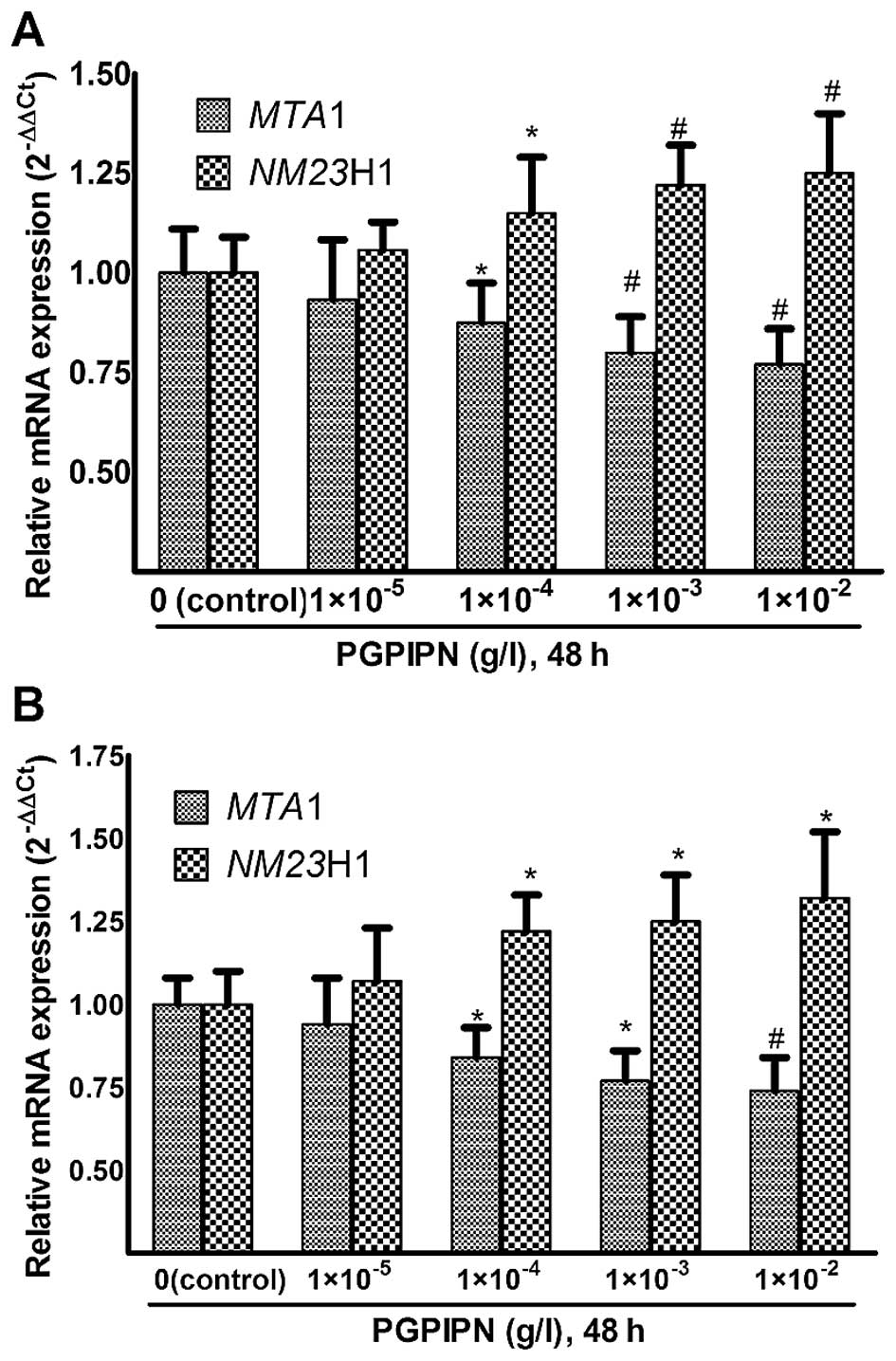
PGPIPN regulates the mRNAs of NM23H1 and
MTA1 genes related with invasion and migration of tumor cells
Real-time PCR experiments were performed using
MTA1 and NM23H1-specific primers to assess their
relative mRNA expression (2−ΔΔCt) in human ovarian
cancer cells treated with PGPIPN for 48 h (Fig. 5). The changes of MTA1 and
NM23H1 mRNAs in SKOV3 cells are displayed in
Fig. 5A. PGPIPN significantly
decreased the mRNA level of MTA1 gene and increased the mRNA
level of NM23H1 gene in contrast. The effect of PGPIPN on
mRNA expression levels of MTA1 and NM23H1 genes were
dose-dependent.
The PGPIPN also reduced mRNA levels of MTA1
and NM23H1 genes in primary ovarian cancer cells similarly
to SKOV3 cells (Fig.
5B). Compared with the cell line SKOV3, the effects
of PGPIPN on primary ovarian cancer cells were more obvious.
PGPIPN affected levels of MTA1 and NM23H1
proteins related with invasion and migration of tumor cells
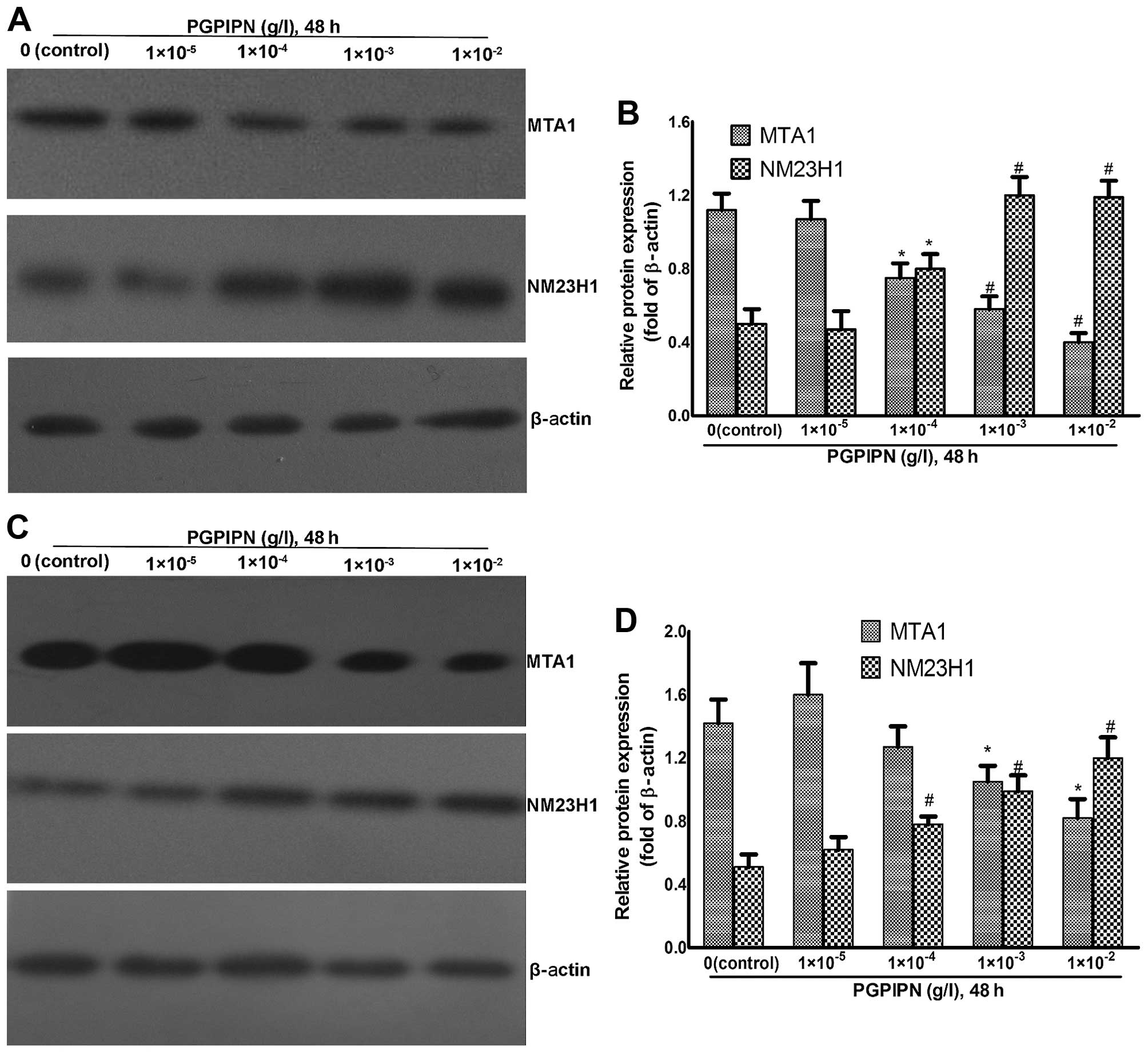
Western blotting was used to analyze MTA1 and NM23H1
protein levels of human ovarian cancer cells treated with PGPIPN at
different concentrations for 48 h (Fig. 6). NM23H1 in SKOV3 cells
was elevated in PGPIPN-treated groups compared to control group,
while MTA1 protein gradually decreased with increasing drug
concentration (Fig. 6A and B).
Notably, PGPIPN-mediated effects on protein levels were significant
at the concentration of ≥1×10−4 g/l (P<0.05 or
P<0.01) (Fig. 6B).
PGPIPN also affected NM23H1 and MTA1 protein levels
in primary ovarian cancer cells after treatment for 48 h, which was
similar to that of SKOV3 cells (Fig. 6C and D).
Discussion
In recent years, studies have shown that many
peptides significantly inhibited invasion and metastasis of tumors
and were considered potential therapeutic agents for the treatment
of malignant tumors, and some were applied in clinical treatment.
For example, Chi et al (17) reported that CAAT/enhancer binding
peptide delta (CEBPD) can reduced and inhibited resistance,
invasion and metastasis of malignant tumor cells. Shan et al
(18) reported the cyclic
arginyl-glycyl-aspartic acid (cRGD) peptide had been explored as an
αvβ3 integrin receptor-specific targeting moiety for the targeted
delivery of nanoparticle-loaded therapeutics. The cRGD peptide has
been used as prevention and treatment of breast cancer devopment,
invasion and metastasis. In the past 20 years, studies (19,20)
have shown that many peptides derived from milk can inhibit tumor
metastasis and showed great potential for the treatment of cancer
or as adjuvant therapy, some of which have already been applied in
clinical treatment. For example, HAMLET (human α-lactalbumin made
lethal to tumor cells) and lactoferricin (antibacterial peptide
from lactoferrin, Lfcin) have already been applied in clinic as an
immunotherapeutic agent for the treatment of cancer (cancer
immunotherapies). Lactoferricin, colostrum and other special
milk-derived peptides have already been used as nutritional and
protective agents for clinical treatment and chemotherapy of cancer
(19). Bonuccelli et al
(21) reported that the milk
protein α-casein could effectively inhibit the growth and
metastasis of breast cancer tumors by activating the STAT1 signal
pathway. The milk-derived peptides have almost no side effects;
some short peptides also possess anti-enzymatic hydrolysis and are
easily absorbed. Thus, these peptides can be taken orally and also
be used as an adjuvant therapy for tumors.
The hexapeptide (PGPIPN) used in this study is
derived from the 63–68 amino acid sequence of bovine β-casein. The
results of the study showed that PGPIPN could significantly inhibit
the invasion and metastasis of both human ovarian cancer
SKOV3 cells and the primary ovarian cancer cells from
fresh human ovarian cancer tissues in vitro, displaying
dose-dependency. However, the inhibition effects of PGPIPN were
less than that of DDP (a conventional anticancer drug) as the
positive control. However, our early studies (9) showed that DDP not only significantly
inhibited ovarian cancer cell proliferation, but also had a strong
side effect on untransformed normal cells. We showed that PGPIPN
had no, or slight side effects on untransformed normal cells by MTT
assay of human normal hepatic cell line LO2, murine embryo
fibroblast cells (MEFs) and para-carcinoma tissues of human ovarian
cancer.
Tumor metastasis involves many processes, a key step
is how to move through biological barriers in the dynamic process
of invasion and metastasis of tumor cells (22). Among them, extracellular matrixc
(ECM) is a natural barrier for tumor invasion and metastasis, and
the degradation of ECM is a key step in tumor metastasis. Tumor
cells have the characteristics of migration, secretion of various
hydrolytic enzymes, adhesion closely linked with ECM. In addition,
the adhesion of tumor cells is related to transmembrane
glycoproteins on the surface of the cells. These adhesion molecules
can combine with the same or different adhesion molecules and
trigger biological effects.
In the study of invasion and metastasis of malignant
tumor cells, MTA1 and NM23H1 proteins have attracted much
attention. Many studies showed that the levels of MTA1 and NM23H1
proteins were closely related to invasion and metastasis of tumor
cells. MTA1 gene was first isolated as the associated gene
of metastasis of breast cancer from rat breast cancer cell line by
Pencil et al (23) using
phage difference hybridization technique in 1993. Toh et al
(24) determined the nucleotide
sequence of the gene and the amino acid sequence of the protein
products, named as metastasis-associated 1 (MTA1). MTA1 protein
regulated a series of proteins related with invasion and metastasis
by signal transduction and gene expression, which can affect the
invasion and metastasis of cancer cells. The expression of MTA1
protein was closely related to the staging and the great omentum
metastasis of ovarian cancer. The MTA1 protein may have the
function of the Src homology 3 (SH3) binding motif participating in
regulating signal transduction pathway and cytoskeleton proteins.
Moreover, MTA1 may act as a transcription factor (TF) to regulate
gene expression. Therefore, MTA1 played an important role in
regulating proteins related to invasion and metastasis through
affecting signal transduction and gene expression (25,26).
The NM23H1 has been widely investigated as a tumor
metastasis suppressor gene. The NM23H1 gene was found
expressed at low levels in many malignant tumors, whose expression
was negatively correlated with metastasis of malignant tumors.
NM23H1 could inhibit the metastasis of malignant tumors by
participating in trans-membrane information transfer affecting the
related protein synthesis and in polymerizing/depolymerizing
microtubules affecting cytoskeletal state. Finally, NM23H1
decreased the activity and the adhesion of the malignant tumor
cells (27–29).
We applied real-time PCR and western blotting to
determine the changes of expression of MTA1 and
NM23H1 in ovarian cancer cells. Our results showed that
PGPIPN may inhibit the invasion and metastasis of ovarian cancer
cells by regulating the expression of MTA1 and NM23H1
in ovarian cancer cells and the related signal pathway. PGPIPN as a
signal molecule may combine with target molecules of ovarian cancer
cells to trigger the signaling pathway affecting the expression of
MTA1 and NM23H1 genes, thus the invasion and
metastasis of tumor cells was reduced. However, the target molecule
of PGPIPN in ovarian cancer cells is not yet determined. We
investigated how PGPIPN effects the expression of MTA1 and
NM23H1 genes and the signal pathway. According to our
preliminary experiment, this peptide can bind to the cell membrane
of ovarian cancer cells (data not shown). Recent studies indicated
that bioactive peptides derived from bovine milk proteins are
capable of binding and affecting cells. For example, Kreider et
al reported the milk peptide mixture from normal cow's milk
inhibits the tyrosine kinase activity of epidermal growth factor
receptor (EGFR), vascular endothelial growth factor receptor 2
(VEGFR2) and insulin receptor (IR), respectively (30). Fiedorowicz et al reported
the bioactive peptides from bovine caseins could bind the μ-opioid
receptor on cytomembrane to influence the proliferation and
cytokine secretion of human peripheral blood mononuclear cells
(PBMCs) (31). To clarify the
receptor or ligand of this peptide further studies are required.
However, our study confirmed that PGPIPN can significantly inhibit
the invasion and metastasis of human ovarian cancer cells in
vitro, and PGPIPN shows promise as a new therapeutic drug for
adjuvant therapy of human ovarian cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (81472448), the National Natural
Science Foundation of China (30872992), and the Province Natural
Science Foundation of Anhui (1508085MH196). We would like to thank
Liyu Cao and Shijie Yan in the First Affiliated Hospital of Anhui
Medical University, for help in collecting, assessing and
classifying fresh primary ovarian tumor tissue from patients with
ovarian cancer, and sampling and pathological examining fresh
primary normal ovarian tissues from patients with uterine fibromas
at initial debulking surgery in the First Affiliated Hospital of
Anhui Medical University.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hennessy BT, Coleman RL and Markman M:
Ovarian cancer. Lancet. 374:1371–1382. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vergote I, Tropé CG, Amant F, Kristensen
GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ,
Panici PB, et al; European Organization for Research and Treatment
of Cancer-Gynaecological Cancer Group; NCIC Clinical Trials Group.
Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV
ovarian cancer. N Engl J Med. 363:943–953. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Baldi A, Ioannis P, Chiara P, Eleonora F,
Roubini C and Vittorio D: Biological effects of milk proteins and
their peptides with emphasis on those related to the
gastrointestinal ecosystem. J Dairy Res. 72(S1): 66–72. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Walhout AJ, Sordella R, Lu X, Hartley JL,
Temple GF, Brasch MA, Thierry-Mieg N and Vidal M: Protein
interaction mapping in C. elegans using proteins involved in vulval
development. Science. 287:116–122. 2000. View Article : Google Scholar
|
|
7
|
Zhou J, Chen J, Mokotoff M and Ball ED:
Targeting gastrin-releasing peptide receptors for cancer treatment.
Anticancer Drugs. 15:921–927. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mader JS and Hoskin DW: Cationic
antimicrobial peptides as novel cytotoxic agents for cancer
treatment. Expert Opin Investig Drugs. 15:933–946. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang W, Gu F, Wei C, Tang Y, Zheng X, Ren
M and Qin Y: PGPIPN, a therapeutic hexapeptide, suppressed human
ovarian cancer growth by targeting BCL2. PLoS One. 8:e607012013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fiat AM, Migliore-Samour D, Jollès P,
Drouet L, Bal dit Sollier C and Caen J: Biologically active
peptides from milk proteins with emphasis on two examples
concerning antithrombotic and immunomodulating activities. J Dairy
Sci. 76:301–310. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ganjam LS, Thornton WH Jr, Marshall RT and
MacDonald RS: Antiproliferative effects of yogurt fractions
obtained by membrane dialysis on cultured mammalian intestinal
cells. J Dairy Sci. 80:2325–2329. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kayser H and Meisel H: Stimulation of
human peripheral blood lymphocytes by bioactive peptides derived
from bovine milk proteins. FEBS Lett. 383:18–20. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meisel H: Biochemical properties of
regulatory peptides derived from milk proteins. Biopolymers.
43:119–128. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meisel H and FitzGerald RJ: Biofunctional
peptides from milk proteins: Mineral binding and cytomodulatory
effects. Curr Pharm Des. 9:1289–1295. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
16
|
Green MR and Sambrook J: Molecular
Cloning: A Laboratory Manual. 4th edition. Cold Spring Habor
Laboratory Press; New York, NY: 2012
|
|
17
|
Chi JY, Hsiao YW, Li CF, Lo YC, Lin ZY,
Hong JY, Liu YM, Han X, Wang SM, Chen BK, et al: Targeting
chemotherapy-induced PTX3 in tumor stroma to prevent the
progression of drug-resistant cancers. Oncotarget. 6:23987–24001.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shan D, Li J, Cai P, Prasad P, Liu F,
Rauth AM and Wu XY: RGD-conjugated solid lipid nanoparticles
inhibit adhesion and invasion of αvβ3 integrin-overexpressing
breast cancer cells. Drug Deliv Transl Res. 5:15–26. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen HY, Mollstedt O, Tsai MH and Kreider
RB: Potential clinical applications of multi-functional milk
proteins and peptides in cancer management. Curr Med Chem.
21:2424–2437. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nongonierma AB and FitzGerald RJ:
Bioactive properties of milk proteins in humans: A review.
Peptides. 73:20–34. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bonuccelli G, Castello-Cros R, Capozza F,
Martinez-Outschoorn UE, Lin Z, Tsirigos A, Xuanmao J,
Whitaker-Menezes D, Howell A, Lisanti MP, et al: The milk protein
α-casein functions as a tumor suppressor via activation of STAT1
signaling, effectively preventing breast cancer tumor growth and
metastasis. Cell Cycle. 11:3972–3982. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Terasaki-Fukuzawa Y, Kijima H, Suto A,
Takeshita T, Iezumi K, Sato S, Yoshida H, Sato T, Shimbori M and
Shiina Y: Decreased nm23 expression, but not Ki-67 labeling index,
is significantly correlated with lymph node metastasis of breast
invasive ductal carcinoma. Int J Mol Med. 9:25–29. 2002.
|
|
23
|
Pencil SD, Toh Y and Nicolson GL:
Candidate metastasis-associated genes of the rat 13762NF mammary
adenocarcinoma. Breast Cancer Res Treat. 25:165–174. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Toh Y, Pencil SD and Nicolson GL: A novel
candidate metastasis-associated gene, mta1, differentially
expressed in highly metastatic mammary adenocarcinoma cell lines.
cDNA cloning, expression, and protein analyses. J Biol Chem.
269:22958–22963. 1994.PubMed/NCBI
|
|
25
|
Toh Y, Pencil SD and Nicolson GL: Analysis
of the complete sequence of the novel metastasis-associated
candidate gene, mta1, differentially expressed in mammary
adenocarcinoma and breast cancer cell lines. Gene. 159:97–104.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Toh Y, Oki E, Oda S, Tokunaga E, Ohno S,
Maehara Y, Nicolson GL and Sugimachi K: Overexpression of the MTA1
gene in gastrointestinal carcinomas: Correlation with invasion and
metastasis. Int J Cancer. 74:459–463. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Futamura M, Nishimori H, Shiratsuchi T,
Saji S, Nakamura Y and Tokino T: Molecular cloning, mapping, and
characterization of a novel human gene, MTA1-L1, showing homology
to a metastasis-associated gene, MTA1. J Hum Genet. 44:52–56. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Mao H and Fu X: Expression of nm23
in breast cancer: Correlation with distant metastasis and
prognosis. Zhonghua Zhong Liu Za Zhi. 23:224–227. 2001.(In
Chinese).
|
|
29
|
Huang G, Song Y and He G: mRNA expression
and mutation of MTA1 and nm23H1 genes in ovarian carcinoma in
relation to lymph node metastasis. Zhonghua Zhong Liu Za Zhi.
23:31–34. 2001.(In Chinese).
|
|
30
|
Kreider RB, Iosia M, Cooke M, Hudson G,
Rasmussen C, Chen H, Mollstedt O and Tsai MH: Bioactive properties
and clinical safety of a novel milk protein peptide. Nutr J.
10:992011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fiedorowicz E, Jarmołowska B, Iwan M,
Kostyra E, Obuchowicz R and Obuchowicz M: The influence of μ-opioid
receptor agonist and antagonist peptides on peripheral blood
mononuclear cells (PBMCs). Peptides. 32:707–712. 2011. View Article : Google Scholar
|