Introduction
Lung cancer is the second most common cancer threat
to human health and life in the world and its incidence is growing
in many countries (1). Non-small
cell lung cancers (NSCLCs) accounts for almost 80% of all lung
cancer cases (2). In recent years,
lung cancer is still a great challenge in clinical treatment.
Surgery resection is only applicable for the patients with early
stage. Due to lack of characteristic clinical manifestations, a
majority of lung cancer patients are diagnosed with serious
symptoms in the late stages. Moreover, chemo-resistance to many
common chemo-agents is emerged in the treatment of lung cancer
(3). Therefore, an effective and
safe strategy is required for the diagnosis and therapy of lung
cancer.
Tetramethylpyrazine (TMP) is one of the active
compounds extracted from the Chinese medicinal plant Ligusticum
chuanxiong (4). It has been
widely used as an active ingredient in the clinical treatment of
neurovascular and cardiovascular diseases (4,5). The
soluble salts tetramethylpyrazine hydrochloride (TMPH) and TMP
phosphate, administrated by injection or oral tablets, have been
widely used in clinical treatment (6). The underlying mechanisms involve
inhibition of platelet aggregation, suppression of apoptosis, and
scavenging peroxyl radicals and superoxide and hydroxyl radicals
(1,7). A substantial amount of evidence has
revealed that TMP has various biological activities, such as
antioxidant activity, antitumor activity including glioma,
osteosarcoma, hepatocyte carcinoma, gastric, breast and lung cancer
(4,8–13).
However, its effectiveness of lung cancer and the molecular
mechanisms related to tumor growth are still far from completely
known.
The development and metastasis of lung cancer cells
are always related to dysregulation of cell proliferation and
abnormal tumor microenvironment. The vascular niche acts as a major
compartment of tumor microenvironment and has an important role in
the initiation, progression and metastasis of tumors. Endothelial
cells (ECs) are composed of the majority of vascular cells. The
interaction between cancer cells and endothelial cells is
associated with the process of angiogenesis within the tumor
microenvironment (14).
Angiogenesis, the process of new capillary blood vessel formation,
is considered to be crucial for growth, maintenance, and metastasis
of solid tumor. The strategy of blocking angiogenesis is an
effective approach to inhibit tumor growth in lung cancer. It has
been investigated that COX-2 is related to the regulation of cancer
angiogenesis, which has an effect on the prognosis of patients with
non-small cell lung cancer (15).
In recent studies, TMP has been found to inhibit lung cancer cell
proliferation via suppressing cell cycle progression and a
significant inhibition of cancer cell invasion and metastasis via
COX-2 pathway (1). Moreover, it
was reported that the inhibition of neovascularization and fibrosis
of microcirculation was mediated by TMP via its regulation of the
SDF-1/CXCR4 pathway. These results suggest that TMP may be used as
a promising candidate for tumor suppression via angiogenesis.
However, the molecular mechanism underlying the TMP functional
roles in human lung carcinoma needs to be further investigated.
Thus, in this study, we investigated the effect of TMP on
angiogenesis and tumor growth of lung cancer.
Materials and methods
Chemicals and reagents
Tetramethylpyrazine (TMP), L-glutamine, penicillin,
streptomycin, noggin, 3-
(4,5-dimethylthiazol-2-yl)-2,5-dephenyltetrazolium bromide (MTT),
and epidermal growth factor (EGF) were purchased from Sigma-Aldrich
(St. Louis, MO, USA). MCDB131, DMEM culture medium and fetal bovine
serum were purchased from Gibco (New York, NY, USA). Horseradish
peroxidase (HRP) conjugated anti-rabbit and anti-mouse secondary
antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA).
Cell lines and cell culture
Human microvascular endothelial cell line (HMEC-1)
was purchased from American Type Culture Collection (Manassas, VA,
USA). The cells were maintained in MCDB131 medium, supplemented
with 10% fetal bovine serum, 2 mM L-glutamine, 10 ng/ml EGF and
antibiotics (100 μg/ml of penicillin, and 100 μg/ml of
streptomycin). A549 human NSCLC cell line was obtained from Cell
Bank in the Type Culture Collection Center in Chinese Academy of
Sciences (Shanghai, China). A549 cell line was cultured in DMEM
medium supplemented with 10% fetal bovine serum and antibiotics.
All the cells were maintained in a humidified atmosphere of 5%
CO2 at 37°C. In all experiments, exponentially growing
cells were used.
MTT assay of cell growth
HMEC-1 cell growth was measured by the
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
(MTT) following the manufacturer's instructions. Briefly, cells
were incubated in a 96-well plate at a density of 5×103
cells/well with 100 μl culture medium. The cells were incubated for
24 h at 37°C with 5% CO2 in the humidified incubator.
The next day, cells were treated with 0, 0.2, 0.4 and 0.8 mg/ml of
TMP for 24, 48 and 72 h, respectively. At the end of detection
time-point, 20 μl of MTT solution (5 mg/ml) was added into each
well and incubated for 4 h at 37°C with 5% CO2 in the
humidified incubator. Then the supernatant was discarded and 200 μl
DMSO was added, followed by incubating for 5 min on the oscillator.
Finally, the optical density (OD) value was measured at a
wavelength of 490 nm using a microplate reader. The OD values of
treatment groups were normalized to that of control group.
Wound healing assay
HMEC-1 cells (5×105 cells/well) were
seeded into a 6-well plate. After incubation for 24 h, a yellow tip
was used to create a wound in each well. The cells were incubated
with vehicle (control) or new MCDB131 medium containing 0.2, 0.4
and 0.8 mg/ml of TMP after rinsing with PBS three times. After
incubation for 12 h, three randomly selected regions were
photographed under a microscope. The distance of the wound was then
estimated quantitatively using Image-Pro Plus software. Migration
rate was calculated as follows: migration rate (%) =
(D0h-D12h)/D0h × 100%.
Migration assay
The migration assay was further carried out on a
Transwell assay using cell culture chambers (Corning, Cambridge,
MA, USA). HMEC-1 cells (2×105 cells/well) were cultured
in the inner chamber in the vehicle (control) or MDCB 131 medium
containing 0.2, 0.4 and 0.8 mg/ml of TMP. The outer chamber was
maintained the same medium with 15% FBS. After incubated at 37°C
with in a humidified 5% CO2 atmosphere for 12 h, the
cells in the upper chamber and on the Matrigel were removed
mechanically with a cotton swab. The migrated cells adherent to the
outer surface of the membrane were fixed with 90% ethanol and
stained using 0.1% crystal violet, then washed with distilled water
till the water was colorless. Images of migrated cells were
photographed by microscopy.
Tube formation assay
The effect of TMP on angiogenesis in vitro
was measured using the HMEC-1 cells capillary-like tube formation
assay. Briefly, a 96-well plate was pre-coated with 50 μl Matrigel
per well and solidified at 37°C for 30 min. HMEC-1 cells
(5×104 cells/well) and samples (TMP of 0, 0.2, 0.4 and
0.8 mg/ml) were added into each well correspondingly, and incubated
for another 12 h. The enclosed capillary networks of tube formation
were recorded by an Olympus IX51 digital camera (Tokyo Japan).
RT-PCR
After TMP treatment for 24 h, total RNA was
extracted using a TRIzol method according to the manufacture's
protocol. Subsequently, first-strand cDNA was synthesized from 1 μg
of total RNA using avian myeloblastosis virus reverse transcriptase
(Takara Biotechnology, Dalian, China). The PCR primers and the
corresponding conditions for BMP2, BMP receptor 1A (BMPR1A), BMP
receptor 1B (BMPR1B), BMP receptor II (BMPRII), Smad 4, Id-1, and
18S rRNA were based on previous reports (16,17).
Western blotting
Total proteins were extracted and further subjected
to concentration determination using the bicinchoninic acid (BCA)
protein assay reagent kit (Beyotime Biotechnology, China). Then the
total cellular protein extraction was separated using SDS-PAGE gel
electrophoresis and transferred to a nitrocellulose membrane
(Millipore, USA). After blocking with TBST containing 5% non-fat
milk for 20 min, the membrane was treated with antibodies against
Id-1 (Santa Cruz Biotechnology), phospho-Smad1/5/8
Ser463/465, Smad4 (Cell Signaling Technology, Shanghai,
China), and β-actin (Santa Cruz Biotechnology) overnight at 4°C.
Protein bands were detected by incubation with horseradish
peroxidase (HRP)-conjugated secondary antibodies for 1 h, and
visualized with SuperSignal Chemiluminescent HRP substrates (Thermo
Fisher Scientific, Shanghai, China).
A549 tumor xenografts in nude mice
Male BALB/c nude mice, aged 4–6 weeks, were used in
this study and purchased from the Shanghai Laboratory Animal
Center. All of the experiments were approved by the Experimental
Animal Research Committee of our affiliation. A549 cancer cells
(1×106 cells/0.2 ml serum-free DMEM medium) were
inoculated into the right thigh of nude mice. As the tumors reached
an average volume of about 100 mm3, the mice were
randomized into control and treatment groups with 10 mice each
group: a) vehicle; b) TMP (40 mg/kg); c) TMP (80 mg/ kg). TMP was
injected intraperitoneally (i.p.) into the mice in the treatment
group daily, and mice in control group were subjected to same
volume of physiological saline daily. The diet and mental status of
the mice were examined every day, and the tumor was measured with a
caliper every two days. The tumor volume (in mm3) was
calculated as follows: volume = (width)2 × length/2.
Immunohistochemistry (IHC)
At the end of the experiment, the mice were
sacrificed. Then, the xenograft tissues were excised, and fixed in
4% paraformaldehyde. Next, the tissues were embedded in paraffin,
and sectioned for immunohistochemical analysis. The staining was
carried out on a section (5 μm) of xenograft tissues according to
standard methods. We incubated the section in an appropriate
dilution of primary antibody against CD31 (Proteintech, Wuhan,
China), phosphorylated Smad1/5/8, or Id-1 (1:100) overnight at 4°C,
and subsequently stained them with HRP-conjugated secondary
antibody (Santa Cruz Biotechnology). The sections were then
visualized with 3,3′-diaminobenzidine and counterstaining with
hematoxylin.
Statistical analysis
The results are presented as mean ± standard
deviation (SD). Statistical analyses were performed using Student's
t-test for comparison of two groups or one-way analysis of variance
(ANOVA) for multiple comparisons by Prism software (GraphPad
Software). P<0.05 was considered statistically significant
(*p<0.05, **p<0.01,
***p<0.001).
Results
TMP inhibits HMEC-1 cell proliferation,
migration and tube formation
Angiogenesis involves multiple steps in endothelial
cells, including proliferation, migration, and capillary tube
formation. First, we used an MTT assay to study the effect of TMP
on HMEC-1 cell proliferation. We observed that TMP exhibited a
significant inhibition of HMEC-1 cell proliferation in a time- and
dose-dependent manner (Fig. 1A).
Moreover, we used a wound healing assay and a Transwell migration
assay to evaluate the effect of TMP on the endothelial cell
migration. As shown in Fig. 1B and
E, after incubated with TMP for 12 h, the cell migration was
inhibited significantly compared with the control. This effect of
TMP was also observed using the transwell model (Fig. 1C). TMP showed a significant
inhibition of cell migration in a dose-dependent manner using both
of these methods. Finally, an in vitro Matrigel model was
employed to study its effect on tube formation of HMEC-1 cells. The
results showed that TMP treatment at 0.4 mg/ml and 0.8 mg/ml
disrupted the enclosed capillary structures (Fig. 1D). The numbers of capillary
networks was significantly decreased after treated with TMP
(Fig. 1F). Taken together, the
above results showed that TMP inhibited HMEC-1 cell proliferation,
migration, and capillary structure formation in vitro.
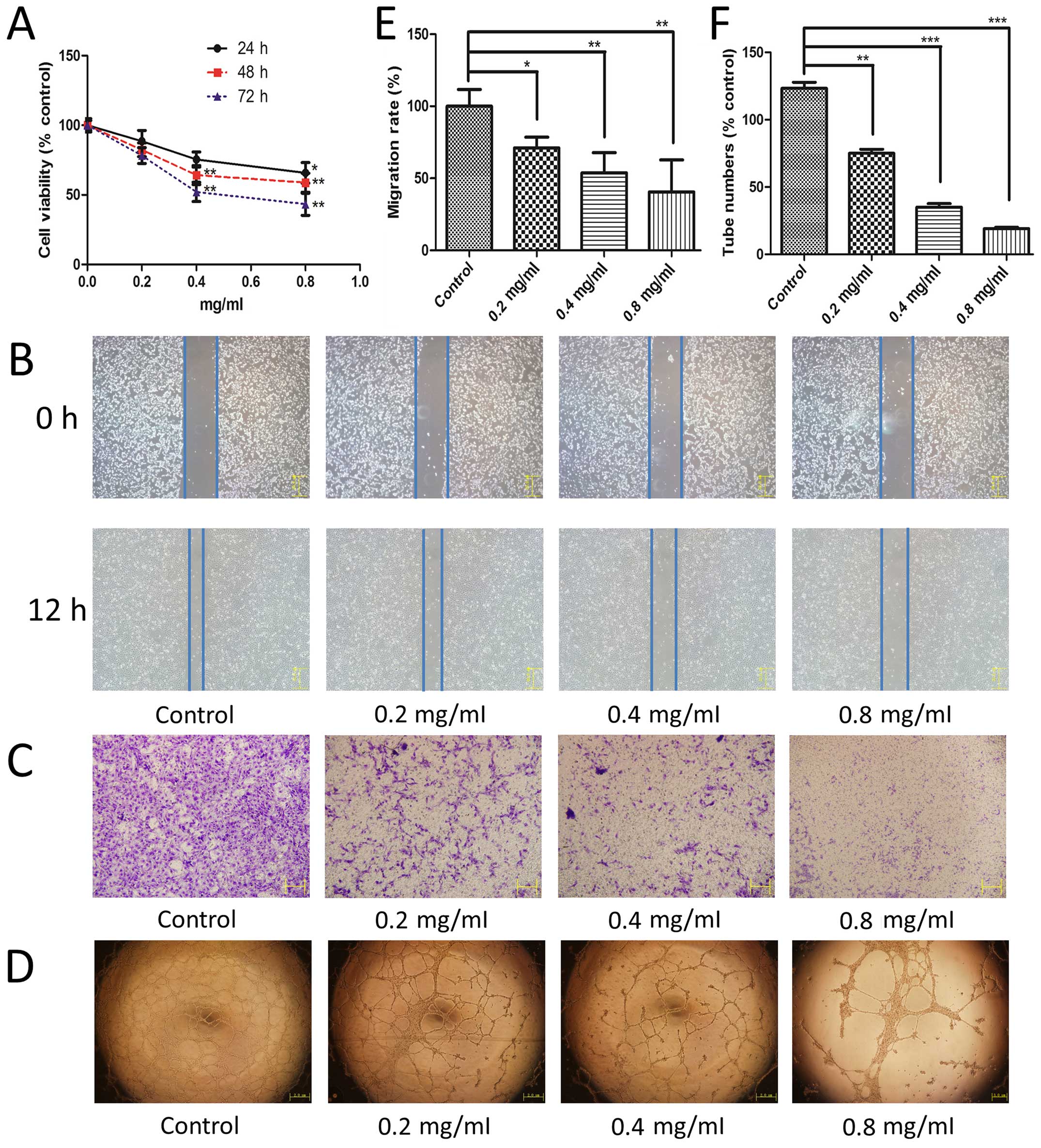 | Figure 1The effect of TMP on HMEC-1 cell
proliferation, migration, and capillary structure. (A) After 24 h
of incubation, HMEC-1 cells were treated with 0, 0.2, 0.4 and 0.8
mg/ml of TMP for 24, 48 and 72 h, respectively. Cell viability was
measured by the MTT assay (black solid line for 24 h; red dashed
line for 48 h; blue dotted line for 72 h). TMP inhibited the
migration of HMEC-1 cells in the wound healing assay (B) and a
Transwell assay (C). (D) TMP disrupted the tube formation of HMEC-1
cells on Matrigel. HMEC-1 cells were treated with vehicle as the
control and 0.2, 0.4 and 0.8 mg/ml TMP for 12 h. (E) Migration rate
was calculated from cell migrated distances in the wound healing
assay. (F) Capillary tube numbers are shown based on the inhibition
of tube formation. Data are presented as mean ± standard deviation
(SD). Statistical analyses were performed using Student's t-test
for comparison of two groups. Control stands for vehicle group.
*p<0.05 vs. group of control, **p<0.01
vs. group of control, ***p<0.001 vs. group of
control. |
TMP inhibits BMP/Smad/Id-1 signaling in
HMEC-1 cells
As previously reported, the activation of
BMP/Smad/Id-1 signaling promoted angiogenesis by upregulation of
Id-1 (18,19). In order to investigate whether the
inhibition of angiogenesis by TMP was due to the blocking of
BMP/Smad/Id-1 signaling in HMEC-1 cells, we evaluated the effect of
TMP (0.4 mg/ml) on the signaling pathway. As shown in Fig. 2A, an increased expression of BMP2
was observed in the prior stage of TMP treatment, whereas, a
slightly decreased expression of BMP2 was emerged after treatment
for 9 h. Additionally, a significant reduction of BMP2 receptor
BMPR1B, and BMPRII was observed, while, the receptor BMPR1A did not
alter obviously. Furthermore, TMP showed little effect on the
expression of Smad4. However, the expression of Id-1 decreased
significantly after TMP treatment. The results indicated that TMP
modulated the BMP2 expression, and blocked its receptors (BMPR1A
and BMPRII), further downregulating Id-1 expression. In order to
confirm the role of Smad molecules in the modulation of Id-1, we
further investigated the effect of TMP on the phosphorylation of
Smad1/5/8 and Id-1 expression. It was observed (Fig. 2B) that TMP showed downregulation of
the phosphorylation of Smad1/5/8, but no significant inhibition on
Smad4. Moreover, the Id-1 expression was significantly decreased
after treated with TMP (0.4 mg/ ml). Actually, the effect of TMP
blocking BMP/Smad/Id-1 signaling was similar to that of noggin, an
endogenous BMP2 antagonist (Fig.
2B). Together, these results suggested that TMP may inhibit
angiogenesis via blocking the BMP/Smad/ Id-1 signaling in HMEC-1
cells.
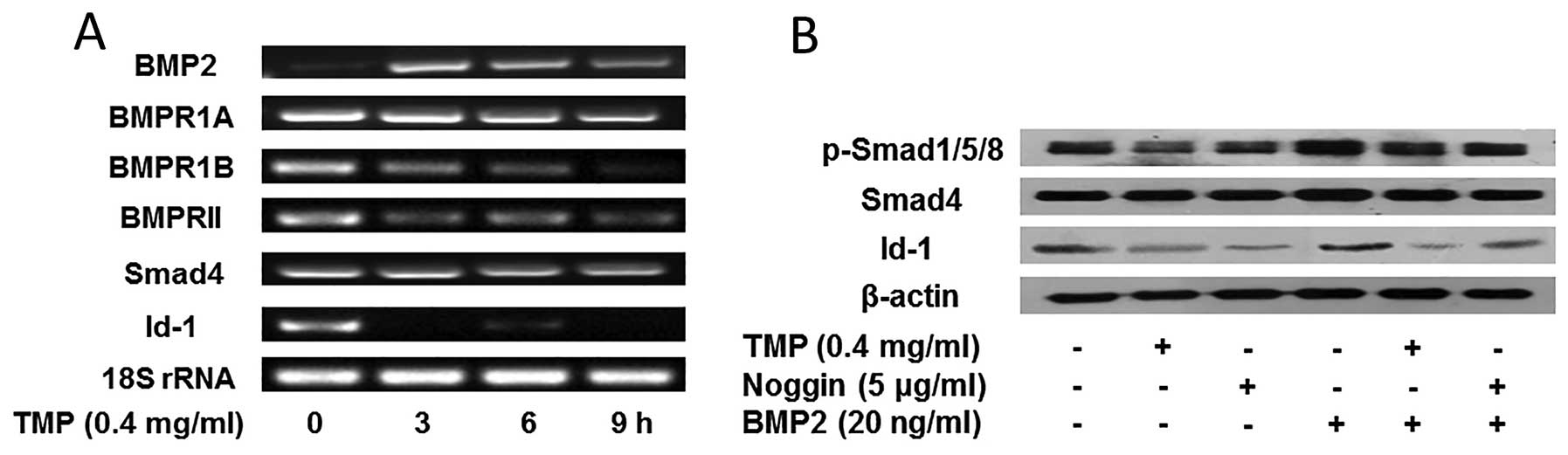 | Figure 2Effect of TMP on BMP/Smad/Id-1
signaling pathway. (A) The expression of BMP2, BMP receptors
(BMPR1A, BMPR1B, and BMPRII), Smad 4, Id-1, and 18S rRNA was
analyzed by RT-PCR. (B) TMP or noggin inhibited the expression of
phosphorylated Smad1/5/8, and Id-1 induced by BMP2 treatment in
HMEC-1 cells. The cells were pretreated with vehicle, 0.4 mg/ml of
TMP, and 5 μg/ml of noggin for 24 h, and then with 20 ng/ml of BMP2
or vehicle for another 4 h. The above mentioned proteins were
analyzed by western blotting. β-actin was used as the control. |
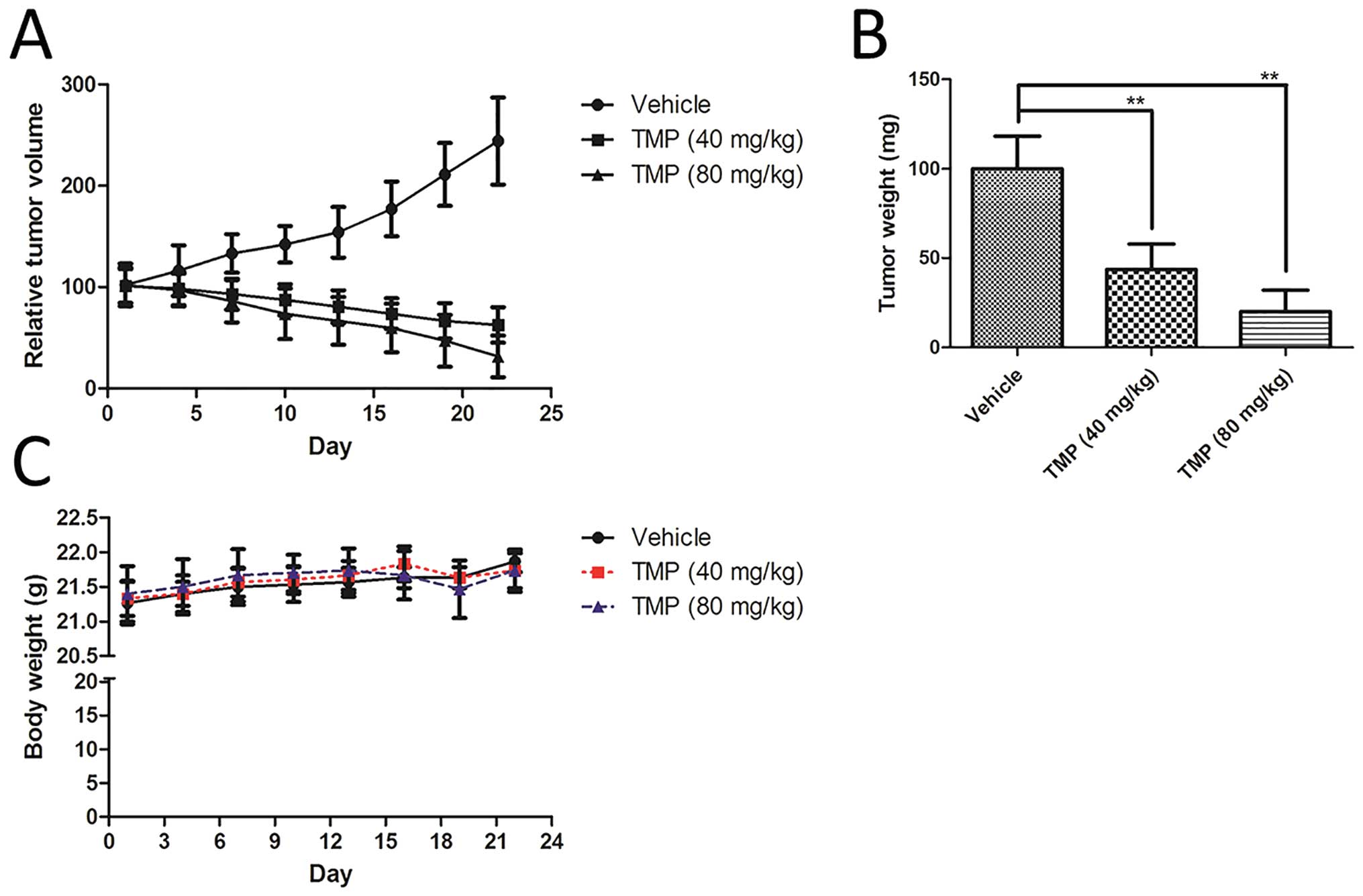
TMP inhibits the growth of A549 xenograft
nude mice
To further evaluate the anti-angiogenic activity of
TMP in vivo, we used an A549 lung cancer cell line xenograft
tumor model. As shown in Fig. 3A,
a low and high dose of TMP (40, and 80 mg/kg/day) was
intraperitoneally administered into the mice. After 22-day
treatment, TMP significantly inhibited the growth of A549 xenograft
in nude mice, compared to the vehicle group (Fig. 3A). In addition, the tumor weight of
TMP-treated groups was remarkably decreased compared to the
vehicle-treated group (Fig. 3B).
However, the body weight of the mice did not show any significant
change in any of the A549 xenograft nude mice during the
experiments (Fig. 3C).
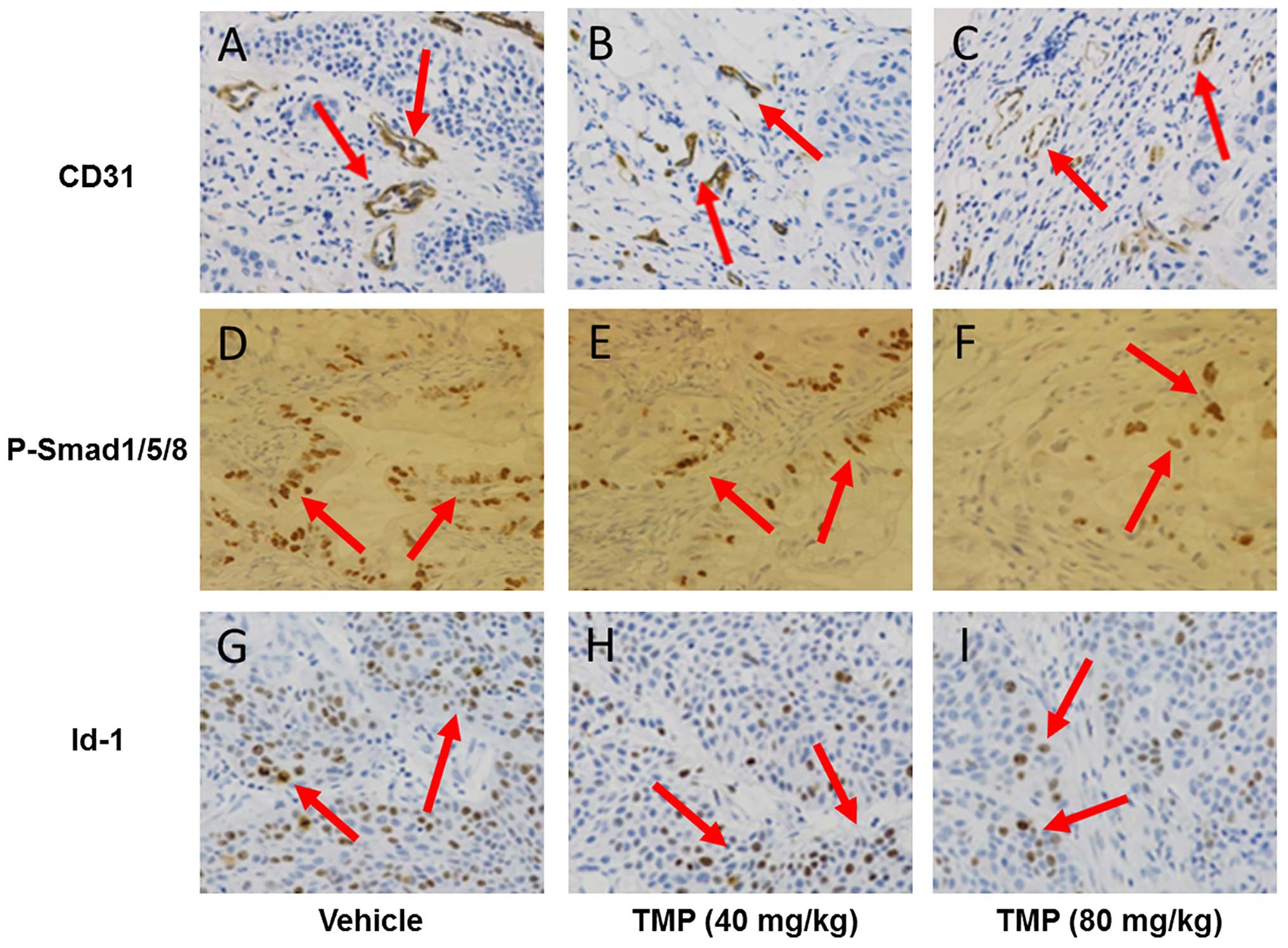
TMP inhibits BMP/Smad/Id-1 signaling in
A549 xenograft nude mice
In order to confirm the in vivo
anti-angiogenic activity of TMP via BMP/Smad/Id-1 signaling, we
used an immunohistochemical assay to detect the expression of CD31,
phosphorylation of Smad1/5/8, and Id-1 expression in the A549 tumor
tissues. CD31 is a positive biomarker related to microvessel
density in endothelial cells. As shown in Fig. 4A–C, the CD31 expression of TMP
treatment (40 and 80 mg/kg/day) was significantly reduced in the
tumor tissues compared to the vehicle-treated tumor tissues. The
result suggested that TMP also had an anti-angiogenic activity
in vivo. Interestingly, we also observed downregulation of
phosphorylated Smad1/5/8 expression in the TMP-treated tumor
tissues (Fig. 4D–F). Moreover, the
expression of Id-1 in the tumor tissues was also decreased after
TMP treatment (Fig. 4G–I). These
results indicated that suppression of A549 xenograft in nude mice
may be attributed to the impairment of angiogenesis via blocking
the BMP/Smad/Id-1 signaling.
Discussion
In this study, we demonstrated that
tetramethylpyrazine (TMP) could potently inhibit HMEC-1 cell
proliferation and migration in vitro, and capillary
structures of HMEC-1 cells using Matrigel model. Most importantly,
the inhibition of angiogenesis was found to be associated with the
blockade of BMP/Smad/Id-1 signaling, suggesting that the BMP/Smad/
Id-1 signaling was a potential target of TMP.
The blood vessel circulatory system fuels the
neighboring tissues with nutrients and oxygen. A proper formation
of blood vessels is necessary for cell and tissue growth. Thus, the
disruption of tube formation has been used as an effective strategy
to inhibit tumor growth (20).
Angiogenesis involves multiple steps, including vasodilation,
basement membrane degradation, endothelial cell migration,
endothelial cell proliferation, and capillary tube formation
(21). As described in the
previous study, TMP significantly decreases migration and tube
formation in human umbilical endothelial cell line ECV304 (6). Our results demonstrated that TMP
remarkably inhibited human microvascular endothelial cell (HMEC-1)
proliferation, migration, and capillary structure formation
compared with the control (Fig.
1). In addition, the inhibition of angiogenesis by TMP was
dose- and time-dependent.
BMPs are secreted extracellular signaling ligands of
the transforming growth factor β (TGF-β) family, including four
groups: i) the BMP2 and -4 subgroup; ii) the growth and
differentiation factor (GDF) 5, -6, and -7 group; iii) the BMP5,
-6, -7, and -8 group; and iv) the BMP9 and -10 group (20). Originally, the BMPs were discovered
due to their ability to induce bone and cartilage formation
(22). Subsequently, BMPs were
also found to have an important role in the development of blood
vessels. Accumulating evidence has demonstrated that BMPs are
essential for angiogenic processes by binding to their receptors on
the cells, such as endothelial and smooth muscle cells (22). It is reported that BMP2 can enhance
angiogenesis in melanoma (23).
The angiogenic effect is exerted via the signaling triggered by the
BMP type I receptor (BMP1A, and BMP1B) and BMPRII. Most
importantly, it has been demonstrated that BMPRII is required for
maintenance of vascular integrity (24). The result obtained in this study
demonstrated upregulated BMP2 expression, and downregulated BMPR1B
and BMPRII expression after TMP treatment. Interestingly, the
expression of BMP2 was upregulated after 3 h of treatment with TMP.
However, BMP2 was subsequently decreased slightly after 9 h of the
treatment. We hypothesize that TMP compete with BMP2 to bind with
BMPs receptors. Thus, BMPR1B and BMPRII expression are
downregulated in the early stage of TMP treatment, corresponding to
the upregulation of BMP2 expression. Whereas, with the
downregulation of BMP receptors (BMPR1B and BMPRII), the expression
of BMP2 was further decreased slightly. Actually, this hypothesis
still needs to be confirmed by future study.
BMP signal binding to type I and II transmembrane
serine/ threonine kinase type receptors further triggers
phosphorylation of the receptor-regulated Smads, such as Smad1, -5,
and -8, and initiation of the canonical or Smad-mediated pathway
(20). The other Smads involved in
the BMP signaling include the common-mediator Smad4 and the
inhibitory Smad6, and -7 (25,26).
Once activated, the phosphorylated Smad1/5/8 forms a hetero complex
with Smad4, and translocates into the nucleus, where they interact
with different co-activators, repressors, then positively or
negatively regulate transcription of target genes, such as the
genes for Id-1 (inhibitor of differentiation-1 or inhibitor
of DNA binding-1), inhibitory Smads (Smad6 and -7), and
Serpine1 (plasminogen activator inhibitor-1) (27). Id-1 activated by BMPs is crucial
for the stimulation of endothelial cells (28). In addition, loss of Id-1 has been
demonstrated to result in the downregulation of proangiogenic genes
in tumor endothelial cells (29).
It suggests that Id-1 has an important role in tumor metastasis via
promoting angiogenesis (30). The
expression of Smad4 did not show any significant changes in our
data (Fig. 2A). On the contrary,
the phosphorylation of Smad1/5/8 was downregulated significantly
after treated with TMP at 0.4 mg/ml (Fig. 2B). Most importantly, the expression
of Id-1 decreased significantly induced by the treatment of TMP
(Fig. 2), whereas, the effect of
TMP on the BMP/Smad/ Id-1 signaling was similar to that of BMP2
antagonist noggin (Fig. 2B).
In addition, Id-1 has a pivotal role in cell
proliferation, differentiation, invasion, and metastasis (31,32).
Further, TMP-exerted anti-A549 activity in vivo was
confirmed by the A549 xenografts in nude mice. The inhibitory
effect of TMP on the growth of A549 lung cancer cells in nude mice
was attributed to the inhibition of tumor growth (Fig. 3A and B). Moreover, inhibition of
angiogenesis in tumor tissues was also demonstrated. CD31, a
well-established biomarker in endothelial cells, is appropriate for
evaluating angiogenesis (33–35).
Compared with the control, the CD31 expression was significantly
reduced in the TMP-treated mice (Fig.
4A–C), which suggested that the angiogenesis was also inhibited
by TMP in the A549 tumor xenograft model. The in vivo result
was in accordance with that of in vitro result (Fig. 1). Furthermore, the expression of
phosphorylated Smad1/5/8, and Id-1 in the TMP-treated tumor tissues
was lower than that of vehicle-treated tissues. Thus, TMP was
confirmed to inhibit BMP/ Smad/Id-1 signaling in vivo.
In conclusion, in this study, we illustrated that
TMP was a potent angiogenesis inhibitor. This anti-angiogenic
activity was correlated with the blockade of BMP/Smad/Id-1
signaling. In addition, TMP presented a significant inhibition of
the nude mouse tumor xenograft model of A549 human lung cancer
cells. Hence, TMP may be a potential drug candidate for human lung
cancer therapy.
Acknowledgements
This study was supported by Medical Science Research
Project of Hebei Province Health Department (grant no.
ZD20140221).
References
|
1
|
Zheng CY, Xiao W, Zhu MX, Pan XJ, Yang ZH
and Zhou SY: Inhibition of cyclooxygenase-2 by tetramethylpyrazine
and its effects on A549 cell invasion and metastasis. Int J Oncol.
40:2029–2037. 2012.PubMed/NCBI
|
|
2
|
Yan JH, Zhao CL, Ding LB and Zhou X: FOXD3
suppresses tumor growth and angiogenesis in non-small cell lung
cancer. Biochem Biophys Res Commun. 466:111–116. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhengfu H, Hu Z, Huiwen M, Zhijun L,
Jiaojie Z, Xiaoyi Y and Xiujun C: 1-o-acetylbritannilactone (ABL)
inhibits angiogenesis and lung cancer cell growth through
regulating VEGF-Src-FAK signaling. Biochem Biophys Res Commun.
464:422–427. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yi B, Liu D, He M, Li Q, Liu T and Shao J:
Role of the ROS/ AMPK signaling pathway in
tetramethylpyrazine-induced apoptosis in gastric cancer cells.
Oncol Lett. 6:583–589. 2013.PubMed/NCBI
|
|
5
|
Zheng Z, Li Z, Chen S, Pan J and Ma X:
Tetramethylpyrazine attenuates TNF-α-induced iNOS expression in
human endothelial cells: Involvement of Syk-mediated activation of
PI3K-IKK-IκB signaling pathways. Exp Cell Res. 319:2145–2151. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cai X, Chen Z, Pan X, Xia L, Chen P, Yang
Y, Hu H, Zhang J, Li K, Ge J, et al: Inhibition of angiogenesis,
fibrosis and thrombosis by tetramethylpyrazine: Mechanisms
contributing to the SDF-1/CXCR4 axis. PLoS One. 9:e881762014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang X-Y, Ma Z-C, Wang Y-G, Tan HL, Xiao
CR, Liang QD, Tang XL, Cheng Y and Gao Y: Tetramethylpyrazine
protects lymphocytes from radiation-induced apoptosis through
nuclear factor-κB. Chin J Nat Med. 12:730–737. 2014.PubMed/NCBI
|
|
8
|
Cao J, Miao Q, Miao S, Bi L, Zhang S, Yang
Q, Zhou X, Zhang M, Xie Y, Zhang J, et al: Tetramethylpyrazine
(TMP) exerts antitumor effects by inducing apoptosis and autophagy
in hepatocellular carcinoma. Int Immunopharmacol. 26:212–220. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Z, Pan X, Georgakilas AG, Chen P, Hu
H, Yang Y, Tian S, Xia L, Zhang J, Cai X, et al:
Tetramethylpyrazine (TMP) protects cerebral neurocytes and inhibits
glioma by down regulating chemokine receptor CXCR4 expression.
Cancer Lett. 336:281–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan Y, Zhao J, Cao C, Jia Z, Zhou N, Han
S, Wang Y, Xu Y, Zhao J, Yan Y, et al: Tetramethylpyrazine promotes
SH-SY5Y cell differentiation into neurons through epigenetic
regulation of Topoisomerase IIβ. Neuroscience. 278:179–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim M, Kim S-O, Lee M, Lee JH, Jung WS,
Moon SK, Kim YS, Cho KH, Ko CN and Lee EH: Tetramethylpyrazine, a
natural alkaloid, attenuates pro-inflammatory mediators induced by
amyloid β and interferon-γ in rat brain microglia. Eur J Pharmacol.
740:504–511. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang Y, Liu X, Zuo T, Liu Y and Zhang JH:
Tetramethylpyrazine reverses multidrug resistance in breast cancer
cells through regulating the expression and function of
P-glycoprotein. Med Oncol. 29:534–538. 2012. View Article : Google Scholar
|
|
13
|
Yan YX, Zhao JX, Han S, Zhou NJ, Jia ZQ,
Yao SJ, Cao CL, Wang YL, Xu YN, Zhao J, et al: Tetramethylpyrazine
induces SH-SY5Y cell differentiation toward the neuronal phenotype
through activation of the PI3K/Akt/Sp1/TopoIIβ pathway. Eur J Cell
Biol. 94:626–641. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Du H, Shi H, Chen D, Zhou Y and Che G:
Cross-talk between endothelial and tumor cells via basic fibroblast
growth factor and vascular endothelial growth factor signaling
promotes lung cancer growth and angiogenesis. Oncol Lett.
9:1089–1094. 2015.PubMed/NCBI
|
|
15
|
Li Y, Li S, Sun D, Song L and Liu X:
Expression of 15-hydroxy-prostaglandin dehydrogenase and
cyclooxygenase-2 in non-small cell lung cancer: Correlations with
angiogenesis and prognosis. Oncol Lett. 8:1589–1594.
2014.PubMed/NCBI
|
|
16
|
Cejalvo T, Sacedón R, Hernández-López C,
Diez B, Gutierrez-Frías C, Valencia J, Zapata AG, Varas A and
Vicente A: Bone morphogenetic protein-2/4 signalling pathway
components are expressed in the human thymus and inhibit early
T-cell development. Immunology. 121:94–104. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ling MT, Wang X, Tsao SW and Wong YC:
Down-regulation of Id-1 expression is associated with TGF beta
1-induced growth arrest in prostate epithelial cells. Biochim
Biophys Acta. 1570:145–152. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Qiu H, Yang B, Pei ZC, Zhang Z and Ding K:
WSS25 inhibits growth of xenografted hepatocellular cancer cells in
nude mice by disrupting angiogenesis via blocking bone
morphogenetic protein (BMP)/Smad/Id1 signaling. J Biol Chem.
285:32638–32646. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song X, Liu S, Qu X, Hu Y, Zhang X, Wang T
and Wei F: BMP2 and VEGF promote angiogenesis but retard terminal
differentiation of osteoblasts in bone regeneration by
up-regulating Id1. Acta Biochim Biophys Sin (Shanghai). 43:796–804.
2011. View Article : Google Scholar
|
|
20
|
Beets K, Huylebroeck D, Moya IM, Umans L
and Zwijsen A: Robustness in angiogenesis: Notch and BMP shaping
waves. Trends Genet. 29:140–149. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bao P, Kodra A, Tomic-Canic M, Golinko MS,
Ehrlich HP and Brem H: The role of vascular endothelial growth
factor in wound healing. J Surg Res. 153:347–358. 2009. View Article : Google Scholar :
|
|
22
|
Wang S, Cai R, Ma J, Liu T, Ke X, Lu H and
Fu J: The natural compound codonolactone impairs tumor induced
angiogenesis by downregulating BMP signaling in endothelial cells.
Phytomedicine. 22:1017–1026. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rothhammer T, Bataille F, Spruss T,
Eissner G and Bosserhoff AK: Functional implication of BMP4
expression on angiogenesis in malignant melanoma. Oncogene.
26:4158–4170. 2007. View Article : Google Scholar
|
|
24
|
Liu D, Wang J, Kinzel B, Müeller M, Mao X,
Valdez R, Liu Y and Li E: Dosage-dependent requirement of BMP type
II receptor for maintenance of vascular integrity. Blood.
110:1502–1510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu MY and Hill CS: TGF-beta superfamily
signaling in embryonic development and homeostasis. Dev Cell.
16:329–343. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Conidi A, Cazzola S, Beets K, Coddens K,
Collart C, Cornelis F, Cox L, Joke D, Dobreva MP, Dries R, et al:
Few Smad proteins and many Smad-interacting proteins yield multiple
functions and action modes in TGFβ/BMP signaling in vivo. Cytokine
Growth Factor Rev. 22:287–300. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morikawa M, Koinuma D, Miyazono K and
Heldin CH: Genome-wide mechanisms of Smad binding. Oncogene.
32:1609–1615. 2013. View Article : Google Scholar :
|
|
28
|
Valdimarsdottir G, Goumans MJ, Rosendahl
A, Brugman M, Itoh S, Lebrin F, Sideras P and ten Dijke P:
Stimulation of Id1 expression by bone morphogenetic protein is
sufficient and necessary for bone morphogenetic protein-induced
activation of endothelial cells. Circulation. 106:2263–2270. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fong S, Debs RJ and Desprez PY: Id genes
and proteins as promising targets in cancer therapy. Trends Mol
Med. 10:387–392. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ling MT, Wang X, Zhang X and Wong YC: The
multiple roles of Id-1 in cancer progression. Differentiation.
74:481–487. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fong S, Itahana Y, Sumida T, Singh J,
Coppe JP, Liu Y, Richards PC, Bennington JL, Lee NM, Debs RJ, et
al: Id-1 as a molecular target in therapy for breast cancer cell
invasion and metastasis. Proc Natl Acad Sci USA. 100:13543–13548.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Xu K, Ling MT, Wong YC, Feng HC,
Nicholls J and Tsao SW: Evidence of increased Id-1 expression and
its role in cell proliferation in nasopharyngeal carcinoma cells.
Mol Carcinog. 35:42–49. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Biswas S, Charlesworth PJ, Turner GD, Leek
R, Thamboo PT, Campo L, Turley H, Dildey P, Protheroe A, Cranston
D, et al: CD31 angiogenesis and combined expression of HIF-1α and
HIF-2α are prognostic in primary clear-cell renal cell carcinoma
(CC-RCC), but HIFα transcriptional products are not: Implications
for antiangiogenic trials and HIFα biomarker studies in primary
CC-RCC. Carcinogenesis. 33:1717–1725. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Miyata Y, Sagara Y, Watanabe S, Asai A,
Matsuo T, Ohba K, Hayashi T and Sakai H: CD105 is a more
appropriate marker for evaluating angiogenesis in urothelial cancer
of the upper urinary tract than CD31 or CD34. Virchows Arch.
463:673–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SJ, Kim JS, Papadopoulos J, Wook Kim
S, Maya M, Zhang F, He J, Fan D, Langley R and Fidler IJ:
Circulating monocytes expressing CD31: Implications for acute and
chronic angiogenesis. Am J Pathol. 174:1972–1980. 2009. View Article : Google Scholar : PubMed/NCBI
|