Introduction
Breast cancer is fatal among women (522.000 deaths
in 2012) and one of the most frequently diagnosed cancers in 140 of
184 countries worldwide. Its clinical classification is based on
the assessment of the type of expression of estrogen receptors
(ERs), progesterone receptors (PRs), or human epidermal growth
factor receptor 2 (HER2) (1).
Triple-negative breast cancer (TNBC), which represents about 15% of
cases, does not express any of these receptors, and, thus, it is
more difficult to treat with current therapies, also because it is
more likely to metastasize determining a poorer prognosis (1). Chemotherapy is routinely used for
cancer treatment although its success is quite limited, due to
severe side-effects caused by drug resistance, targeting of healthy
cells, and metabolic stress (2).
Doxorubicin (Dox) is one of the most effective agents in the
treatment of breast cancer patients (3), even if it shows severe side-effects
in the form of typhlitis, cardiotoxicity, nephrotoxicity,
hepatotoxicity and other toxicities (4,5). In
general, Dox increases the oxidative stress, which kills cancer
cells and induces an inflammatory microenvironment, with a
generalized unwanted cellular toxicity (6); thus, Dox alone is not a preferable
drug. A myriad of plant products have shown very promising
anticancer properties in vitro, but they have yet to be
evaluated in humans (7). However,
some phytochemicals have shown a chemopreventive effect and the
ability to sensitize cancer cells toward Dox (8). For example, recently it has been
demonstrated that the combination of resveratrol and Dox in breast
cancer is able to synergize their effects and to induce apoptosis,
by downregulation of inflammatory factors, NF-κB, COX-2, and
autophagy (LC3B, Beclin-1) (5).
Even, ferulic acid showed a protective effect against
cardiotoxicity induced by Dox in tumor-bearing mice as assessed by
the level reduction of serum marker enzymes like CK and LDH and
transaminases and activating lipid peroxidation (9). Furthermore, Dox-antioxidant compound,
obtained by mixing Dox with ferulic acid, was found to be less
toxic than Dox in cardiomyocytes (10). In the last two decades, flaxseed
(FS) has been a focal point of interest in the field of nutrition
and disease research due to the potential health benefits
associated with its biologically active components (11). It has been shown that FS and/or its
oil inhibit the formation of colon, breast, skin, and lung tumors
reduce blood vessel cell formation in female rats, thus suggesting
a protective effect against breast, colon and ovarian cancer
(12). Since no detailed
information was reported on the effect of the phenols in FS oil on
breast cancer, we have characterized and assessed its potential
effects on two human breast cancer cell lines, MCF-7 and MDA-MB231.
Our results highlight that the main components of the phenolic
extract from FS oil, named PEFSO, were ferulic, vanillic and
p-hydroxybenzoic acids. They were very effective only on MCF-7 cell
line by inducing an increase of apoptosis and lipid peroxidation,
cell cycle G0/G1 phase modification, and a related activation of
the H2AX signaling pathway and of some pro-oxidant genes, when
compared to untreated cells. The aim of the present study is to
verify that PEFSO has a possible synergic effect with Dox.
Therefore, we have analyzed the effects of their combination on
ER-positive MCF-7 and receptor-negative MDA-MB231 human breast
cancer cell lines in terms of cytotoxicity, apoptosis induction,
cell cycle modification, mitochondrial membrane depolarization and
gene expression involved in extrinsic/death receptor and
mitochondrial/intrinsic apoptotic pathway.
Materials and methods
Cell culture and treatment
Two human breast cancer cell lines, estrogen
receptor-positive MCF-7 (HTB-22, adenocarcinoma) and estrogen
receptor-negative MDA-MB231 (HTB-26, adenocarcinoma) (both from
Lonza, Verviers, Belgium) were grown as described elsewhere
(13). Subsequently the cells were
treated with Dox (Ebewe Pharma, Unterach, Austria) and PEFSO for 48
h. For the MDA-MB231 cells, both compounds were dissolved in
RPMI-1640 supplemented with 1% FBS at concentrations of 0.09, 0.18,
0.38, 0.75, 1.5, 3, and 6 µM for Dox and 4.03, 8.06, 16.13,
32.25, 64.5, 129, and 258 µg/ml for PEFSO. For MCF-7 cells,
two compounds were dissolved in DMEM supplemented with 1% FBS at
concentrations of 0.08, 0.15, 0.3, 0.6, 1.2, 2.4, and 4.8 µM
for Dox and 3.93, 7.88, 15.75, 31.5, 63, 126, and 252 µg/ml
for PEFSO. The concentrations used are similar to those of our
previous studies (13,14). The phenolic extract from FS oil was
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich) at a
concentration of 100 mM. In cell cultures the DMSO concentration
remained always <0.1%, a dose that did not exert toxic effects
(15). Subsequently according to
the results, combination treatment (co-treatment) with Dox and
PEFSO was carried out at IC50 doses obtained for 48
h.
Sulforhodamine B (SRB) assay
The cell survival/proliferation was measured in
96-well plates by a spectrophotometric dye incorporation assay
using SRB after 48 h of treatment with PEFSO, Dox and PEFSO + Dox.
Cells were fixed with trichloroacetic acid (Sigma-Aldrich, St.
Louis, MO, USA) for 1 h and after stained for 30 min with 0.4%
(w/v) SRB (Sigma-Aldrich) dissolved in 1% acetic acid. The number
of viable cells was directly proportional to the protein bound-dye
formation which was then solubilized with 10 mM Tris base solution
pH 10.5 and measured by fluorometric assay ELISA at 540 nm
(microplate reader; Bio-Rad, Hercules, CA, USA). All experiments
were performed in duplicate and repeated three times. Cellular
viability was estimated as % compared to untreated cells. The
IC50 was assessed from the dose-response curves.
Drug combination studies
Drug combination studies were based on
concentration-effect curves generated as a plot of the fraction of
unaffected (surviving) cells vs. the drug concentration after 48 h
of treatment. Briefly, the two molecules were tested for 48 h in
combination equiactive doses (cytotoxic ratio, 50:50). Synergism,
additivity, and antagonism were quantified by determining the
combination index (CI) calculated by the Chou-Talalay equation and
with the software CalcuSyn (Biosoft, Cambridge, UK) (16). Assuming 0.9 as the cut-off value,
CI<0.9; CI, 0.9–1; or CI>1 indicates synergistic, additive,
or antagonistic effects, respectively. The dose reduction index
(DRI) represents the measure of how much the dose of each substance
in a synergistic combination may be reduced at a given effect level
compared with the dose of each drug alone. The linear correlation
coefficient (r) of the median-effect plot is considered a
conformity measure of the data according to the mass-action law
principle when the experimental measurement is assumed to be
accurate. An r-value equal to 1 indicates perfect conformity while
a poor value may be the result of biological variability or
experimental deviations. All our experiment r-values were between
0.91 and 0.98 indicating a good data conformity.
Apoptosis assay
The cells (1×106) were labeled with
Annexin V and Dead Cell Assay kit according to the manufacturer's
instructions (Merck Millipore, Darmstadt, Germany) after they were
harvested and washed twice with ice-cold PBS. This test is based on
the phosphatidylserine (PS) detection on the apoptotic cell
surface, using fluorescently labeled Annexin V in combination with
the dead cell marker, 7-amino-actinomycin D (7-AAD). The apoptotic
ratio was calculated by identifying four populations: i) Annexin V
(−) and dead cell marker (−), the viable cells; ⅱ) Annexin V (+)
and dead cell marker (−), the early apoptotic cells; ⅲ) Annexin V
(+) and dead cell marker (+), the late apoptotic cells; and ⅳ)
Annexin V (−) and dead cell marker (+), the cells died through
non-apoptotic pathway. The samples were counted and analyzed by the
Muse™ Cell Analyzer and a software provided by Merck Millipore,
respectively.
Cell cycle analysis
Cell cycle analysis was performed utilizing Muse™
Cell Analyzer following the manufacturer's instructions. After
treatments, cells were washed with PBS, centrifuged and after
removal of the supernatant, 1 ml of ice-cold 70% ethanol was added
to the cell pellet. The samples were capped and frozen at −20°C for
at least 3 h prior to staining. Ethanol-fixed cells were
centrifuged and the pellet was re-suspended in PBS. After a further
centrifugation, the supernatant was removed and discarded and cell
pellet was re-suspended in 200 µl of Muse™ Cell Cycle
Reagent containing propidium iodide (PI) and RNase A in a
proprietary formulation. PI discriminates cells at different stages
of the cell cycle, based on the differential DNA content in the
presence of RNase to increase the specificity of DNA staining. The
cells were incubated for 30 min at room temperature, in the dark.
After staining, the cells were processed for cell cycle
analysis.
RNA preparation and quantitative reverse
transcription polymerase chain reaction (RT-qPCR)
RNA isolation and cDNA preparation were performed as
previously described by Sorice et al (13). The reverse transcribed products
were used to perform qPCR in order to evaluate the expression level
of transcripts of selected genes. Sequences for mRNAs from the
nucleotide data bank (National Center for Biotechnology
Information, Bethesda, MD, USA) were used to design primer pairs
for RT-qPCR (Primer Express Software; Applied Biosystems, Foster
City, CA, USA). Oligonucleotides were obtained from Sigma-Aldrich.
The efficiency of each primer pair was calculated according to the
standard curve method using the equation E = 10−1/slope.
Five serial dilutions were set up to determine Ct values and
reaction efficiencies for all primer pairs. Standard curves were
generated for each oligonucleotide pair using Ct values vs. the
logarithm of each dilution factor. RT-qPCR assays were run on the
7900HT Fast Real-Time PCR System (Applied Biosystems).
The primer sequences are provided in Table I. Starting with 2 µg of
total RNA, we have prepared a 20-fold dilution of the resulting
cDNA to achieve the concentration equivalent of starting with 100
ng of RNA (Life Technologies/Invitrogen), according to the
manufacturer's instructions. A total of 10 ng of cDNA was amplified
in a total volume of 25 µl containing 1X SYBR-Green PCR
Master Mix (Applied Biosystems) and 300 nM of forward and reverse
primers. The thermal profile conditions were as follows: 5 min of
denaturation at 95°C followed by 44 cycles at 95°C for 30 sec and
60°C for 1 min. We have added one cycle for melting curve analysis
at 95°C for 15 sec, 60°C for 15 sec and 95°C for 15 sec to verify
the presence of a single product. Melting curve analysis was
carried out after amplification to verify the validity of the
amplicon. Each assay included a no-template control for each primer
pair. To capture intra-assay variability, all RT-qPCR reactions
were carried out in triplicate. For all RT-qPCR experiments, the
data from each cDNA sample were normalized using β-actin mRNA as
endogenous level (17). Sample ΔCt
values were calculated as the difference between the means of gene
markers Ct and housekeeping assay Ct from the same sample. The
1-fold expression level was chosen as the threshold for
significance of target genes. Statistical analyses (paired
Student's t-tests) were performed using Prism software (GraphPad
Software, Inc., La Jolla, CA, USA).
 | Table IPrimer sequences of the genes used in
this study. |
Table I
Primer sequences of the genes used in
this study.
| Gene name | Primer sequence
(5′→3′) |
|---|
| p38 MAPK | GCC CAA GCC CTT GCA
CAT (18) |
| TGG TGG CAC AAA GCT
GAT GAC (21) |
| p53 | CTG GCC CCT GTC ATC
TTC TG (20) |
| CCG TCA TGT GCT GTG
ACT GC (20) |
| Bax | GGA CGA ACT GGA CAG
TAA CAT GG (23) |
| GCA AAG TAG AAA AGG
GCG ACA AC (23) |
| Caspase-3 | CAG TGG AGG CCG ACT
TCT TG (20) |
| TGG CAC AAA GCG ACT
GGA T (19) |
| Caspase-8 | GGA TGG CCA CTG TGA
ATA ACT G (22) |
| TCG AGG ACA TCG CTC
TCT CA (20) |
| β-actin | TCT GGC ACC ACA CCT
TCT ACA ATG (24) |
| AGC ACA GCC TGG ATA
GCA ACG (21) |
Mitochondrial membrane
depolarization
Measurement of changes in the mitochondrial membrane
potential (ΔΨm) was performed with the Muse MitoPotential Assay
kit™ (EMD Millipore). The assay utilizes the MitoPotential Dye, a
cationic, lipophilic dye to detect changes in the ΔΨm and 7-AAD as
an indicator of cell death. High membrane potential drives the
accumulation of MitoPotential Dye within inner membrane of intact
mitochondria resulting in high fluorescence, while cells with
depolarized mitochondria demonstrate a decrease in fluorescence.
Therefore, this flow cytometry-based assay differentiates four
populations of cells: live cells with depolarized mitochondrial
membrane, MitoPotential−/7-AAD−; live cells
with intact mitochondrial membrane,
MitoPotential+/7-AAD−; dead cells with
depolarized mitochondrial membrane,
MitoPotential+/7-AAD+; and dead cells with
intact mitochondrial membrane,
MitoPotential−/7-AAD+. After treated with
different concentrations of drugs, the cells were incubated with
the fluorescent dyes and the percentage of depolarized cells
(depolarized live + depolarized dead) were determined by Muse Cell
Analyzer. We measured with Muse MitoPotential Assay two important
cell health parameters: change in mitochondrial potential and cell
death. The software provides percentages of live, depolarized,
depolarized/death and death cells. Briefly cells, after the
treatment with PEFSO alone or in combination with Dox, were
harvested and the cell pellet was suspended in assay buffer.
MitoPotential Dye working solution was added and the cell
suspension incubated at 37°C for 20 min. After the addition of Muse
MitoPotential 7-AAD dye and incubation for 5 min, changes in ΔΨm
and in cellular plasma membrane permeability were assessed using
the fluorescence intensities of both analyzed dyes by Muse Cell
Analyzer, flow cytometry.
Results
Cytotoxicity assay
The cytotoxic effects of Dox and its combination
with PEFSO were evaluated on MCF-7 and MDA-MB231 cell lines by SRB
assay to identify the concentrations at which the 50% of cell
growth was inhibited. As reported in our recent report (13), after the treatment with PEFSO alone
the MCF-7 and MDA-MB231 cells reached an inhibition corresponding
to an IC50 of 63 and 64.5 µg/ml, respectively. In
the case of Dox treatment the two cell lines reached their
respective IC50 values at concentrations of 1.2 and 1.5
µM for MCF-7 and MDA-MB231 cells, respectively, when
compared to non-treated cells (Fig.
1). Subsequently, on the basis of the median value obtained
from the effect analysis of Dox and PEFSO alone in calculating CIs,
we explored the anti-proliferative effects of Dox and PEFSO
combinations by testing equipotent doses of the two agents (ratio,
50:50). In this way we have verified that the MCF-7 and MDA-MB231
cells reached an IC50 inhibition comparable to those of
stimulations with 15.75 µg/ml (PEFSO) and 0.3 µM
(Dox) and with 24 µg/ml (PEFSO) and 0.49 µM (Dox),
respectively. A strong synergistic effect with low CIs (CIs<0.9)
was demonstrated when simultaneous equipotent combination doses
were used for both cell lines (Fig.
1). Therefore, after combined treatment we have achieved a dose
reduction of 19.46-fold for Dox and 7.74-fold for PEFSO in MCF-7
cells at IC50 values (DRI50) as well as of 3.10-fold and
4.62-fold for Dox and PEFSO in MDA-MB231 cells, respectively, when
compared to concentrations of two compounds taken individually
(Table II).
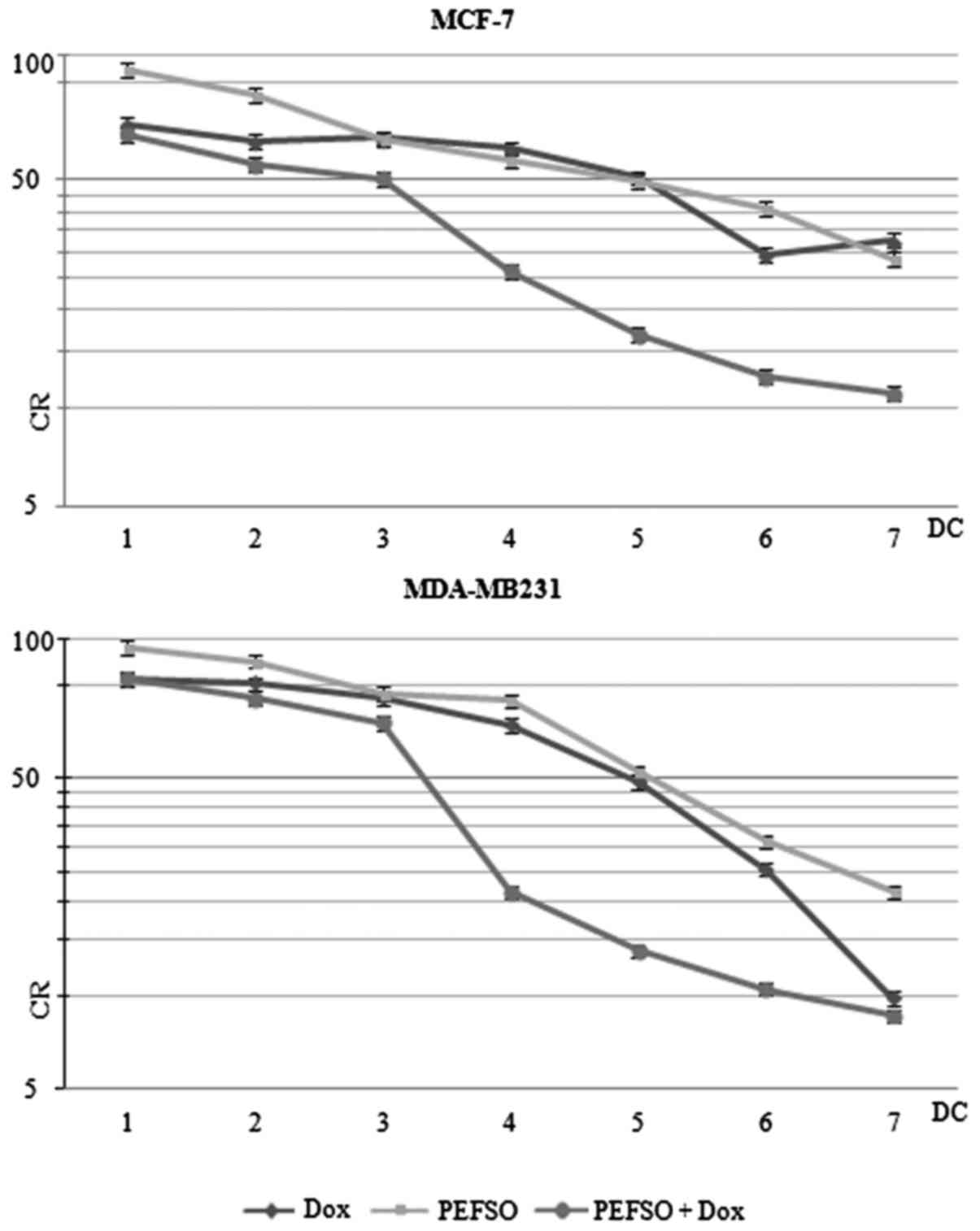 | Figure 1SRB assays. MCF-7 and MDA-MB231 CR
curves after treatment with Dox, PEFSO and their combination for 48
h. On the x-axis the different DCs are shown: 1 (Dox: 0.08
µM; PEFSO: 3.93 µg/ml), 2 (Dox: 0.15 µM;
PEFSO: 7.88 µg/ml), 3 (Dox: 0.3 µM; PEFSO: 15.75
µg/ml), 4 (Dox: 0.6 µM; PEFSO: 31.5 µg/ml), 5
(Dox: 1.2 µM; PEFSO: 63 µg/ml), 6 (Dox: 2.4
µM; PEFSO: 126 µg/ml) and 7 (Dox: 4.8 µM;
PEFSO: 252 µg/ml) for MCF-7 cells; and 1 (Dox: 0.09
µM; PEFSO: 4.03 µg/ml), 2 (Dox: 0.18 µM;
PEFSO:8.06 µg/ml), 3 (Dox: 0.38 µM; PEFSO: 16.13
µg/ml), 4 (Dox: 0.75 µM; PEFSO: 32.25 µg/ml),
5 (Dox: 1.5 µM; PEFSO: 64.5 µg/ml), 6 (Dox: 3
µM; PEFSO: 129 µg/ml) and 7 (Dox: 6 µM; PEFSO:
258 µg/ml) for MDA-MB231 cells. On the y-axis: CR. SRB,
sulforhodamine; CR, cell growth rate; Dox, doxorubicin; DCs, drug
concentrations. |
 | Table IIPEFSO and Dox co-treatment induced a
synergistic anti-proliferative effect compared to the treatment
with drugs administered individually as demonstrated by median drug
effect analysis calculating the CI and the DRI with CalcuSyn
software. |
Table II
PEFSO and Dox co-treatment induced a
synergistic anti-proliferative effect compared to the treatment
with drugs administered individually as demonstrated by median drug
effect analysis calculating the CI and the DRI with CalcuSyn
software.
| Cell lines | Treatment | CI50 (± SD) | r (± SD) | DRI at
IC50 (± SD)
|
|---|
| Dox | PEFSO |
|---|
| MCF-7 | PEFSO + Dox | 0.2 (±0.01) | 0.94 (±0.03) | 19.46 (±0.05) | 7.74 (±0.06) |
| MDA-MB231 | PEFSO + Dox | 0.5 (±0.01) | 0.97 (±0.02) | 3.10 (±0.08) | 4.62 (±0.05) |
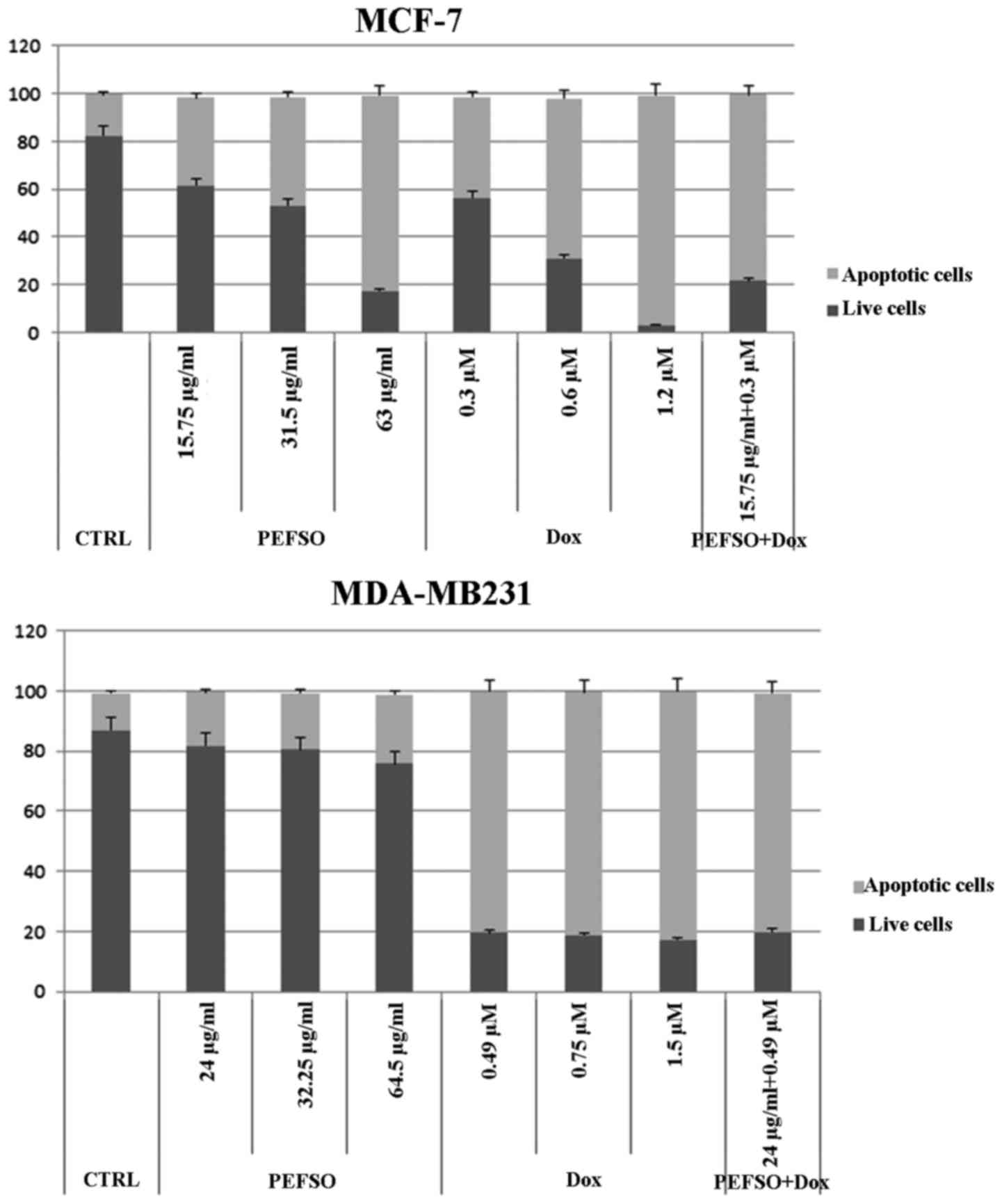
Apoptosis studies
Our previous data showed that the treatment with 63
and 64.5 µg/ml of PEFSO alone in MCF-7 and MDA-MB231 cells
resulted in induction of apoptotic death equal to 82.05% (±0.02)
and 22.95% (±0.04), respectively (13). Therefore, we investigated also the
Dox ability and its combination with PEFSO to induce apoptosis in
the two cell lines. Fig. 2 shows
that the treatments with only Dox at IC50 concentrations
induced an apoptotic death equal to 96.36% (±0.08) and 82.08%
(±0.06), in MCF-7 and MDA-MB231 cells, respectively. The treatment
with PEFSO + Dox concentrations corresponding at IC50,
like 0.3 µM (Dox) + 15.75 µg/ml (PEFSO) for MCF-7
cells and 0.49 µM (Dox) + 24 µg/ml (PEFSO) for
MDA-MB231 cells, resulted in induction of apoptotic death equal to
77.90% (±0.01) and 79.31% (±0.05), in MCF-7 and MDA-MB231 cells,
respectively. This confirmed the specific synergistic effect of
this combination by evidencing that the advantage, which is
achieved with a combined formulation, is due to the fact that the
dose of the chemotherapeutic agent is significantly reduced, from
1.2 to 0.3 µM for MCF-7 cells and from 1.5 to 0.49 µM
for MDA-MB231 cells.
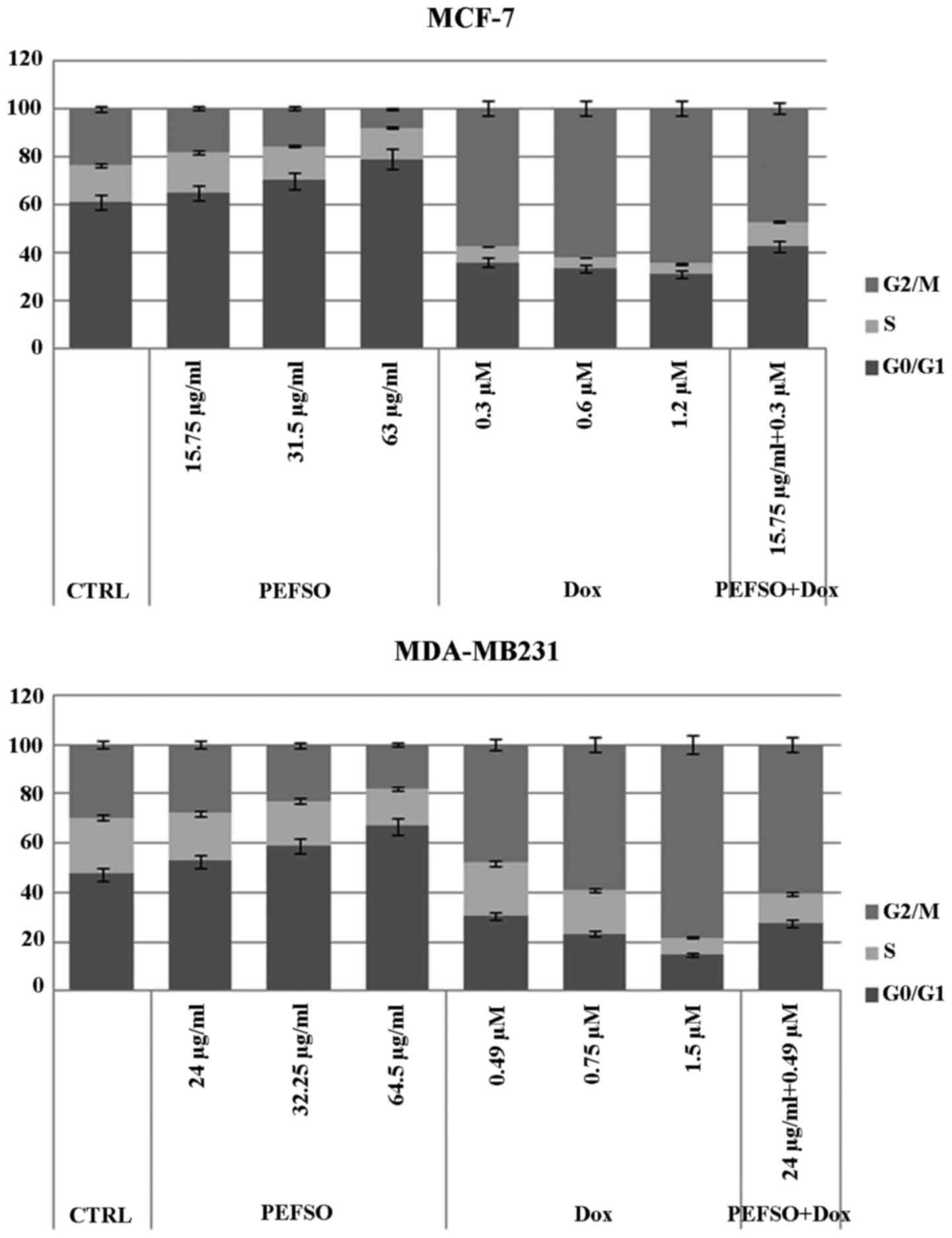
Cell cycle assay
Considering that the two compounds affected the cell
proliferation inducing death in both cell lines, we analyzed their
effects also on the cell cycle distribution after 48 h of treatment
at the same concentrations used for the apoptosis assay. In our
recent report, we observed in both cell lines a dose-dependent
increase of the percentage of cells in G0/G1 phase as well as a
decrease in G2/M and a slight decrease in S phase, when treated
with PEFSO and compared to the control (13). After treatment by Dox, we have a
decrease of G0/G1 and S phases and an increase of G2/M phase in
both cell lines (Fig. 3). While
after treatment with PEFSO + Dox at concentrations corresponding to
IC50, we had an increase of G2/M phase similar to that
of Dox alone. However, even if the data after the co-treatment are
not comparable to those obtained using Dox and PEFSO alone, it is
important to underline that the concentrations used during the
co-treatment were lower than IC50 values obtained by
dose-response assays with Dox and PEFSO alone, and that the effects
after Dox + PEFSO are certainly influenced from two different
action mechanisms due to the two molecules.
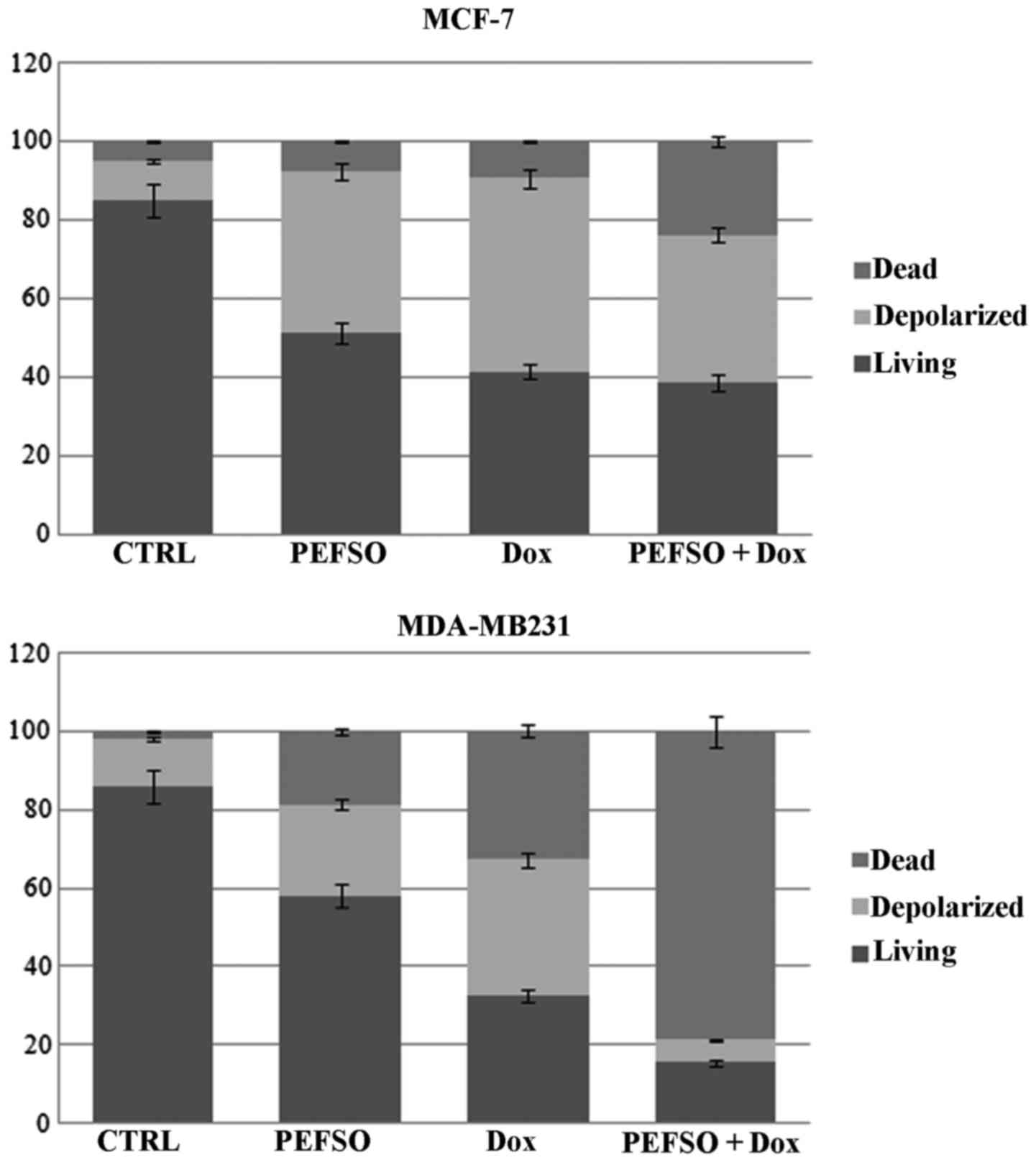
Mitochondrial membrane
depolarization
We evaluated, on both cell lines, the effects on
depolarization of the mitochondrial membranes (loss of ΔΨm) by Muse
system when treated with the two compounds alone or in combination.
Loss of the mitochondrial inner transmembrane potential is a
reliable indicator of mitochondrial dysfunction and cellular
health. This effect is often observed to be associated with the
early stages of apoptosis (18).
We observed in MCF-7 cell line an increase of the mean percentage
of cellular depolarization in presence of IC50
concentrations for PEFSO and Dox, respectively, when compared to
untreated. Their combination at two concentrations below the
IC50 values produced a nearly similar depolarization in
respect to each compound alone. Furthermore, we noted an increase
of death cells by means of co-treatment that we had not observed
during the treatment with the individual compounds (Fig. 4). A significant change in the ΔΨm
was also evident in MDA-MB231 cells compared to the control. We
observed also an increase of cell death when treated with PEFSO and
Dox alone, compared to the control. Interestingly, cells co-treated
with two concentrations below the IC50 values (15.75 and
24 µg/ml for PEFSO, 0.3 and 0.49 µM for Dox in MCF-7
and MDA-MB231 cells, respectively) showed a decrease of
depolarization and a significant increase of cell death (Fig. 4). This is evidence that the
co-treatment is able to activate two different death pathways in
the two cancer cell lines.
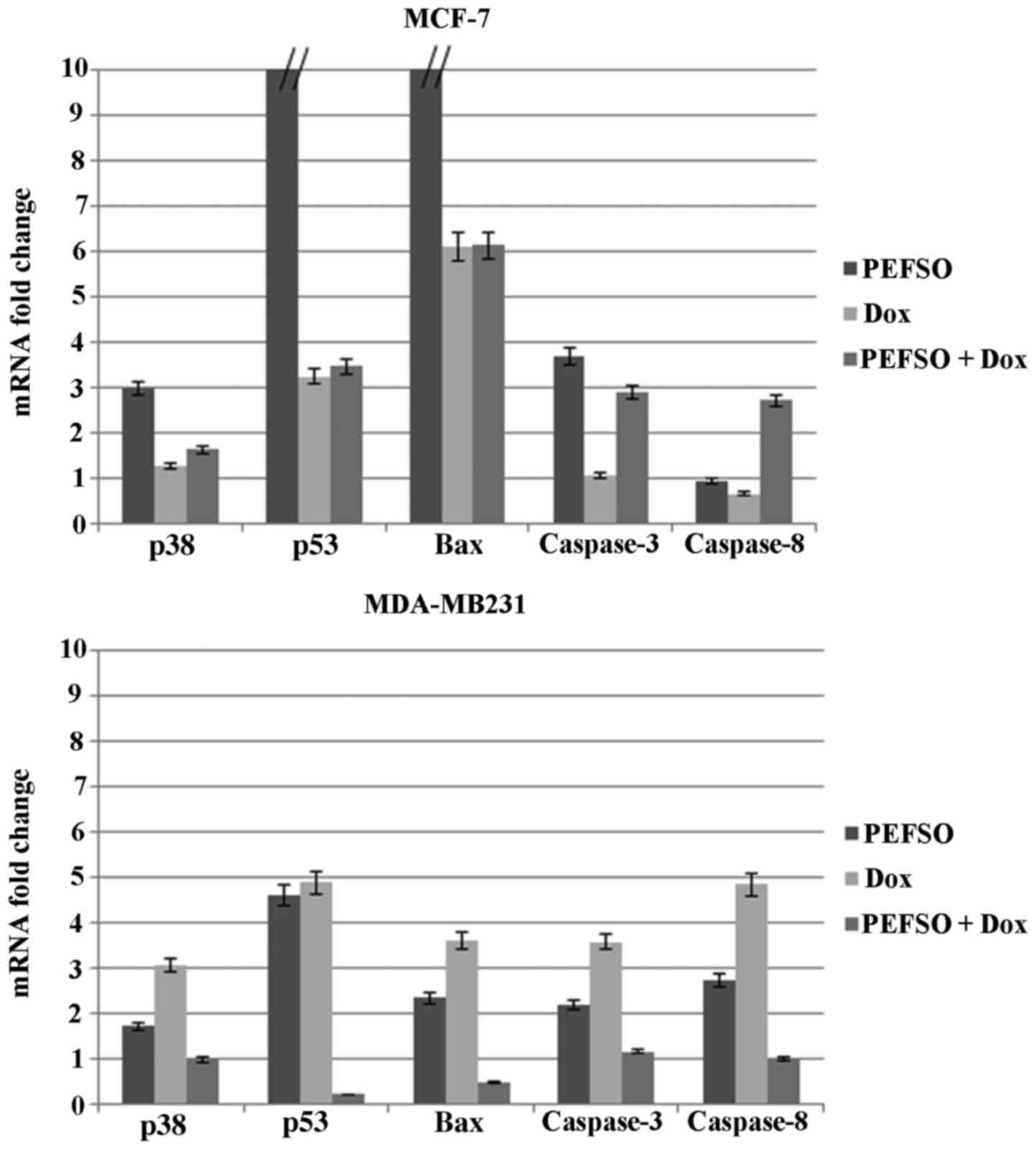
RT-qPCR analysis
To further elucidate the molecular mechanism through
which PEFSO and Dox and their combination were able to induce
apoptosis in breast cancer cells, we have examined mRNA expression
of certain genes involved in the intrinsic mitochondrial pathway
such as p53, Bax, p38, and caspase-3 as well the extrinsic death
receptor pathway such as those of caspase-3 and -8, by focusing
mainly on the activation mechanism of caspase-3 (Table I). RT-qPCR was used to detect the
mRNA expression after separate treatment with Dox or PEFSO at their
IC50 concentrations as well as at two lower
IC50 concentrations combined. mRNA expression change was
normalized on β-actin mRNA expression (Fig. 5). Results showed that the mRNA
expression of p53, Bax, p38, and caspase-3 genes increased
significantly after treatment for 48 h with Dox, PEFSO and their
combination in MCF-7 cell line. On the contrary, no increase of
caspase-8 gene expression was highlighted in this cell line after
treatment with Dox and PEFSO taken individually, while an increase
of its expression is noticed when the two compounds are combined.
Caspase-8 is involved in an extrinsic apoptotic pathway activated
by a death receptor. Taken together, these observations indicated
that Dox and PEFSO induced only the intrinsic apoptotic pathway
while their combination induces apoptosis by both intrinsic and
extrinsic pathways. In MDA-MB231 cell line the two compounds, PEFSO
and Dox, caused a significant increase of p53, Bax, caspase-3, p38
and caspase-8 expression indicating an activation of both apoptotic
pathways. Their combination did not show any significant increase
of p53, Bax and p38 expression levels, while caspase-3 and -8
expression levels were activated. Therefore, we supposed that Dox
and PEFSO combination in MDA-MB231 cells induced only activation of
genes involved in the extrinsic apoptotic pathway, whereas when
used individually they activate both pathways.
Discussion
Dox is an anthrax-cyclin antibiotic, which remains
an important agent in many chemotherapy regimens (3). Although Dox is currently considered
to be one of the most effective agents in the treatment of human
breast cancer, its chemotherapeutic use is associated with severe
side-effects to non-tumor tissues, such as the heart, liver, and
kidney, thus greatly limiting its clinical application (19). In recent years, FS have attracted
considerable interest for their potential health benefits,
including the prevention of chronic non-communicable diseases, the
cardiovascular disease reduction, atherosclerosis, diabetes,
cancer, arthritis, osteoporosis, and neurological disorders
(11,20,21).
Different studies have also reported that the FS components are
effective in reducing breast cancer risk and tumor growth, or in
interacting beneficially with breast cancer drugs (22). In particular, in our recent study
we also characterized the phenolic components extracted from FS oil
(PEFSO) and analyzed their anticancer effect on two human breast
cancer cell lines, MCF-7 and MDA-MB231, and on the human
non-cancerous breast cell line, MCF-10A (13). Therefore, in this study we
investigated the effect of the combination of PEFSO with Dox in
order to define its ability to reduce the doses of this
chemotherapeutic agent also decreasing its side-effects.
Hence considering that in our study (13) the healthy breast cells, MCF-10A,
retained a quite constant viability with increasing concentrations
of PEFSO and that, hence, this extract was not able to induce
modulation of apoptosis and cell cycle on MCF-10A, we decided to
test the effects of the combination between Dox and PEFSO only on
breast cancer cell lines.
Firstly we observed that Dox in combination with
PEFSO had anti-proliferative effects reaching IC50 at
concentrations equal to 0.3 and 0.49 µM in MCF-7 and
MDA-MB231 cell lines, respectively. These IC50
combination values are lower than the concentration of Dox alone,
1.2 and 1.5 µM in MCF-7 and MDA-MB231, respectively, which
is able to induce an anti-proliferative effect also in comparison
with data already reported in literature where Dox concentrations
(ranging from 0.1 to 10 µM) decreased the viability of MCF-7
cells in a time- and concentration-dependent manner (23). This supports our view that this
natural compound in combination with Dox, a conventional breast
cancer chemotherapeutic agent, is useful to enhance the drug
antitumor activity reducing its active concentration and the
adverse toxic effect. In particular, we found that the strongly
synergistic combination of the two compounds in both cell lines, as
evaluated by means of CalcuSyn software, induced an increase of
apoptosis and a modulation of cell cycle through a decrease of
G0/G1 and S phases and an increase of G2/M phase similarly to Dox,
when individually used; in fact, Dox was able to arrest the MCF-7
and T47D breast cancer cell lines at G2/M phase (24). Different studies also have
indicated that Dox-induced apoptosis is associated with two
distinct apoptotic pathways, i.e., the extrinsic and mitochondrial
or intrinsic pathways (19). The
extrinsic pathway involved the death receptors and ligand
interaction such as FasL/Fas and then activated caspase-8 (25). It is reported that caspase-8 levels
increased in MCF-7 cell line after a 48- and 72-h incubation with
0.1 and 1 µM concentrations of Dox (23). Moreover, upregulation of
pro-caspase-8 was found upon treatment with Dox in colon carcinomas
cells (26). The mitochondrial or
intrinsic pathway is the major mechanism of Dox-induced apoptosis,
in which the central process involves the change of permeability of
the outer mitochondrial membrane with the subsequent release of
several pro-apoptotic factors into the cytosol (23). Furthermore, Dox causes apoptosis of
bone marrow-derived mesenchymal stem cells (BMSCs) through ROS
increase and the loss of ΔΨm, as well as the activation of p38,
p53, Bax and caspase-3 genes, which consequently trigger apoptosis
and dysfunction of cells (27).
According to data reported in literature, we decided to investigate
certain apoptosis-associated genes, which might contribute to
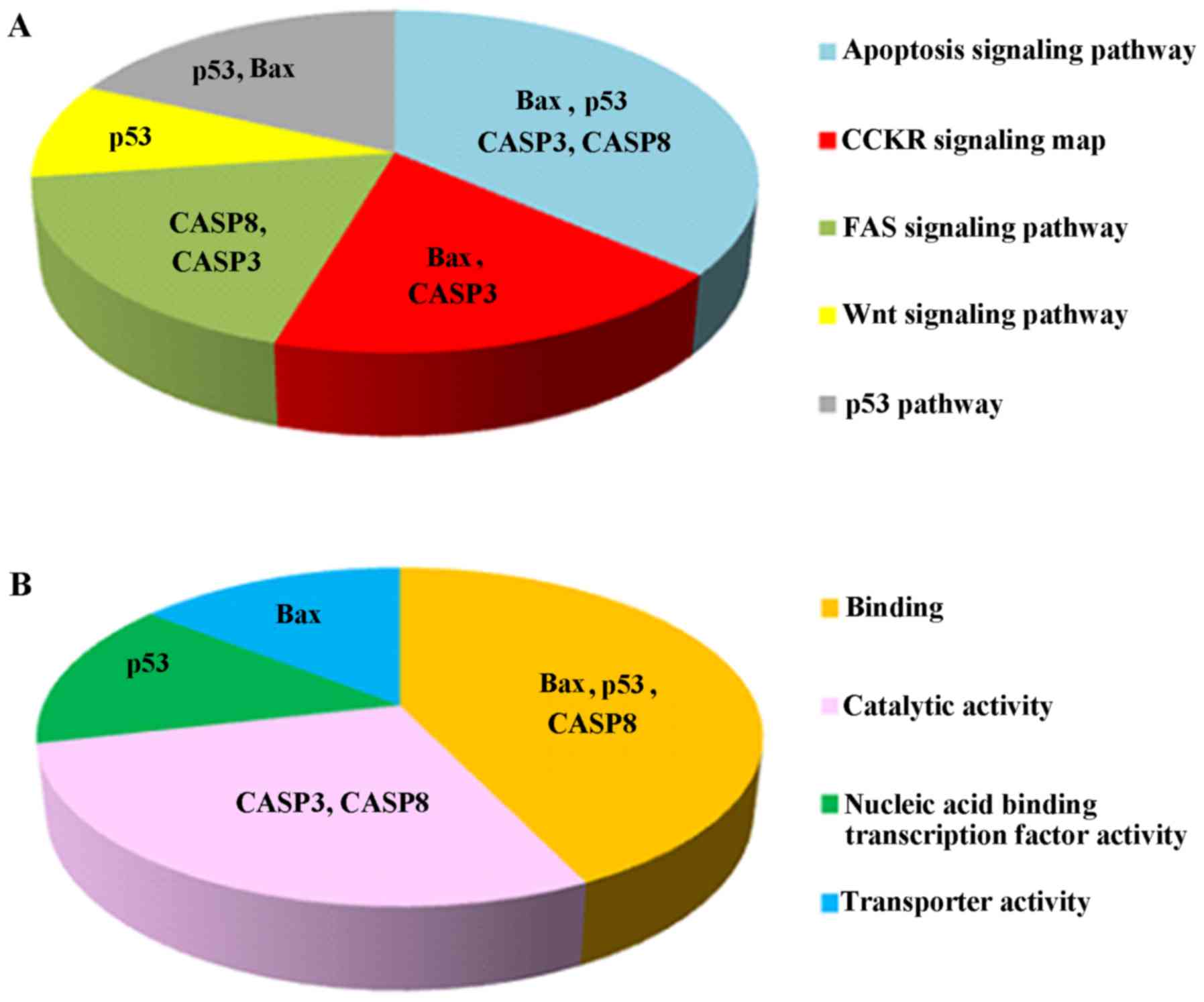
Dox-induced apoptosis and to its combination with PEFSO. Fig. 6 shows the functional and pathway
analysis performed by PANTHER program (28) on the chosen genes showing their
involvement also in other signaling pathways underlining their
important role in cancer. First, we analyzed the p53 gene
expression that can directly trigger the permeability of the outer
mitochondrial membrane through activation of pro-apoptotic proteins
such as Bax (26). High expression
of Bax gene in MCF-7 cells incubated with Dox demonstrated that
there are Bax enhancer effects followed by induction of the
intrinsic apoptotic pathway (23).
Indeed, we found activation of p53 and Bax in MCF-7 and MDA-MB231
cell lines treated with Dox and PEFSO alone. Their combination
activates these genes only in MCF-7 cells, not in MDA-MB231
cells.
Moreover, a recent study has shown that the
mitogen-activated protein kinase (MAPKs) signaling is able to
regulate apoptosis-associated pathways in tumor cells (29,30)
where p38 protein is involved in intrinsic pathway and appears to
have a pro-apoptotic effect activating a variety of cellular stress
and dysfunctions of mithocondria and caspase activation in cell
apoptosis (31). In our study Dox
and PEFSO induce p38 MAPK increase in both cell lines but their
combination has effect on p38 activation only in MCF-7 cells.
However, regarding caspases it is important to underline that the
cell death induction of both extrinsic and intrinsic apoptotic
pathways is associated with caspase activation, where caspase-8,
which can directly activate caspase-3 (32), is activated mainly in the extrinsic
apoptotic pathway (23). Both
pathways converged on caspase-3, and later on other enzymes lead to
final events of apoptosis (33),
for this reason our aim was mainly to evaluate its role in the
activation of the intrinsic and extrinsic pathway of apoptosis and
how it was activated.
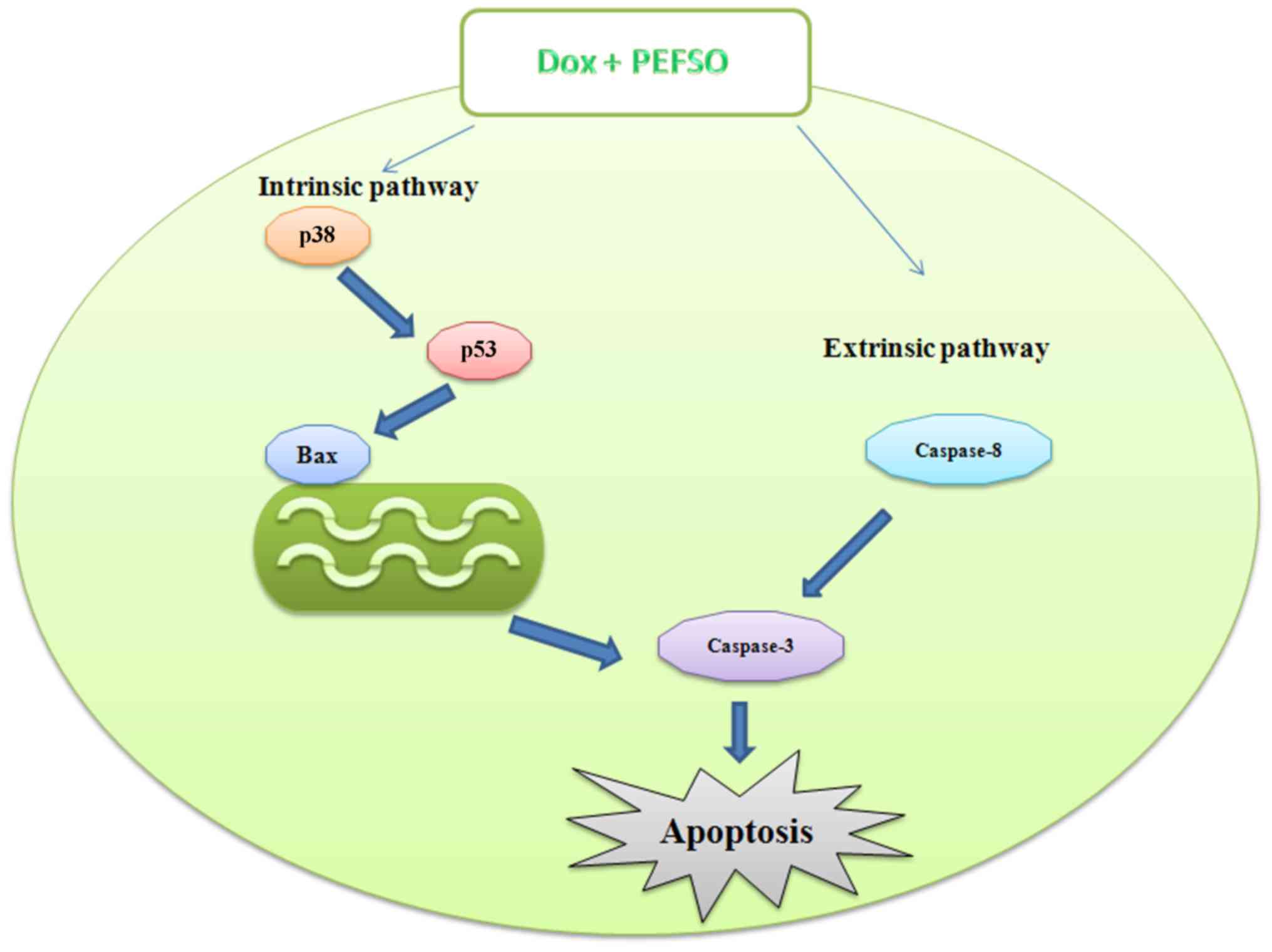
Overall, in our study we found that: i) caspase-3
levels increased after a 48-h incubation with Dox and PEFSO alone
and after their combination in both breast cell lines but; ⅱ)
caspase-8 levels did not increase in MCF-7 cells after treatment
with the two compounds, only when the cells were stimulated with
Dox + PEFSO alone; and ⅲ) in MDA-MB231 cells, caspase-8 increased
always in both individual treatment and in co-treatment. Hence,
these data demonstrate that in MCF-7 cell line Dox + PEFSO induce
an apoptotic intrinsic pathway by p53, Bax p38 and caspase-3
activation as well as an apoptotic extrinsic pathway by caspase-8
activation. In MDA-MB231 cells, the cellular death may be mediated
through both extrinsic and intrinsic apoptotic pathways when the
compounds are used individually, but their combination activated
only the extrinsic pathway (Fig.
7). These results have been confirmed by the assessment of ΔΨm
by Muse system. Indeed, in the MDA-MB231 co-treatment we did not
find any mitochondrial membrane depolarization but an increase of
dead cells. This did not happen when the two compounds were used
individually. However, several studies have also shown that in the
extrinsic pathway, there is a caspase-8 activation, which bypasses
mitochondria and leads directly to caspase-3 activation, followed
by apoptosis (33,34). In MCF-7 cells we observed
depolarization of the mitochondrial membrane when the compounds
were used individually as well as when they were combined.
Therefore, on the basis of our results, the combined
use of the natural product, PEFSO, with the conventional
chemotherapy drug, Dox, could be proficiently used to decrease the
Dox effective dose and, hence, most likely also its
side-effects.
Acknowledgments
We are grateful to Dr Maria Grazia Volpe (Istituto
di Scienze dell'Alimentazione, CNR, Avellino, Italy) for the
preparation of PEFSO extract.
References
|
1
|
Guerriero E, Sorice A, Capone F,
Napolitano V, Colonna G, Storti G, Castello G and Costantini S:
Vitamin C effect on mitoxantrone-induced cytotoxicity in human
breast cancer cell lines. PLos One. 9:e1152872014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Iwamoto T: Clinical application of drug
delivery systems in cancer chemotherapy: Review of the efficacy and
side effects of approved drugs. Biol Pharm Bull. 36:715–718. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Smith L, Watson MB, O'Kane SL, Drew PJ,
Lind MJ and Cawkwell L: The analysis of doxorubicin resistance in
human breast cancer cells using antibody microarrays. Mol Cancer
Ther. 5:2115–2120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rashid S, Ali N, Nafees S, Ahmad ST,
Arjumand W, Hasan SK and Sultana S: Alleviation of
doxorubicin-induced nephrotoxicity and hepatotoxicity by chrysin in
Wistar rats. Toxicol Mech Methods. 23:337–345. 2013. View Article : Google Scholar
|
|
5
|
Rai G, Mishra S, Suman S and Shukla Y:
Resveratrol improves the anticancer effects of doxorubicin in vitro
and in vivo models: A mechanistic insight. Phytomedicine.
23:233–242. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thakur JS, Chauhan CG, Diwana VK, Chauhan
DC and Thakur A: Extravasational side effects of cytotoxic drugs: A
preventable catastrophe. Indian J Plast Surg. 41:145–150. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Desai AG, Qazi GN, Ganju RK, El-Tamer M,
Singh J, Saxena AK, Bedi YS, Taneja SC and Bhat HK: Medicinal
plants and cancer chemoprevention. Curr Drug Metab. 9:581–591.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Vinod BS, Maliekal TT and Anto RJ:
Phytochemicals as chemosensitizers: From molecular mechanism to
clinical significance. Antioxid Redox Signal. 18:1307–1348. 2013.
View Article : Google Scholar
|
|
9
|
Divakaran SA and Nai CKK: Amelioration of
doxorubicin induced cardiotoxicity in tumor bearing mice by ferulic
acid: A mechanistic study at cellular and biochemical level. Int J
Tumor Ther. 1:6–13. 2012. View Article : Google Scholar
|
|
10
|
Chegaev K, Riganti C, Rolando B, Lazzarato
L, Gazzano E, Guglielmo S, Ghigo D, Fruttero R and Gasco A:
Doxorubicin-antioxidant co-drugs. Bioorg Med Chem Lett.
23:5307–5310. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goyal A, Sharma V, Upadhyay N, Gill S and
Sihag M: Flax and flaxseed oil: An ancient medicine & modern
functional food. J Food Sci Technol. 51:1633–1653. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Truan JS, Chen JM and Thompson LU:
Comparative effects of sesame seed lignan and flaxseed lignan in
reducing the growth of human breast tumors (MCF-7) at high levels
of circulating estrogen in athymic mice. Nutr Cancer. 64:65–71.
2012. View Article : Google Scholar
|
|
13
|
Sorice A, Guerriero E, Volpe MG, Capone F,
La Cara F, Ciliberto G, Colonna G and Costantini S: Differential
response of two human breast cancer cell lines to the phenolic
extract from flaxseed oil. Molecules. 21:3192016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Capone F, Guerriero E, Sorice A, Colonna
G, Storti G, Pagliuca J, Castello G and Costantini S: Synergistic
antitumor effect of doxorubicin and tacrolimus (FK506) on
hepatocellular carcinoma cell lines. Sci World J. 2014:4503902014.
View Article : Google Scholar
|
|
15
|
Tirosh O, Sen CK, Roy S, Kobayashi MS and
Packer L: Neuroprotective effects of alpha-lipoic acid and its
positively charged amide analogue. Free Radic Biol Med.
26:1418–1426. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou TC: Theoretical basis, experimental
design, and computerized simulation of synergism and antagonism in
drug combination studies. Pharmacol Rev. 58:621–681. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Porichi O, Nikolaidou ME, Apostolaki A,
Tserkezoglou A, Arnogiannaki N, Kassanos D, Margaritis L and
Panotopoulou E: BCL-2, BAX and P53 expression profiles in
endometrial carcinoma as studied by real-time PCR and
immunohistochemistry. Anticancer Res. 29:3977–3982. 2009.PubMed/NCBI
|
|
18
|
Stefanowicz-Hajduk J, Bartoszewski R,
Bartoszewska S, Kochan K, Adamska A, Kosiński I and Ochocka JR:
Pennogenyl saponins from Paris quadrifolia L. induce extrinsic and
intrinsic pathway of apoptosis in human cervical cancer HeLa cells.
PLoS One. 10:e01359932015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang G, Zhang J, Liu L, Sharma S and Dong
Q: Quercetin potentiates doxorubicin mediated antitumor effects
against liver cancer through p53/Bcl-xl. PloS One. 7:e517642012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maggio M, Artoni A, Lauretani F, Borghi L,
Nouvenne A, Valenti G and Ceda GP: The impact of omega-3 fatty
acids on osteoporosis. Curr Pharm Des. 15:4157–4164. 2009.
View Article : Google Scholar
|
|
21
|
Rodriguez-Leyva D, Dupasquier CM,
McCullough R and Pierce GN: The cardiovascular effects of flaxseed
and its omega-3 fatty acid, α-linolenic acid. Can J Cardiol.
26:489–496. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mason JK, Fu M, Chen J and Thompson LU:
Flaxseed oil enhances the effectiveness of trastuzumab in reducing
the growth of HER2-overexpressing human breast tumors (BT-474). J
Nutr Biochem. 26:16–23. 2015. View Article : Google Scholar
|
|
23
|
Sharifi S, Barar J, Hejazi MS and Samadi
N: Doxorubicin changes Bax/Bcl-xL ratio, caspase-8 and 9 in breast
cancer cells. Adv Pharm Bull. 5:351–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meiyanto E, Fitriasari A, Hermawan A,
Junedi S and Susidarti RA: The improvement of doxorubicin activity
on breast cancer cell lines by tangeretin through cell cycle
modulation. Orient Pharm Exp Med. 11:183–190. 2011. View Article : Google Scholar
|
|
25
|
Cheng YY, Yang JS, Tsai SC, Liaw CC, Chung
JG, Huang LJ, Lee KH, Lu CC, Chien HC, Tsuzuki M, et al: The newly
synthesized
2-(3-hydroxy-5-methoxyphenyl)-6,7-methylene-dioxyquinolin-4-one
triggers cell apoptosis through induction of oxidative stress and
upregulation of the p38 MAPK signaling pathway in HL-60 human
leukemia cells. Oncol Rep. 28:1482–1490. 2012.PubMed/NCBI
|
|
26
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang F, Chen H, Liu Y, Yin K, Wang Y, Li
X, Wang G, Wang S, Tan X, Xu C, et al: Doxorubicin caused apoptosis
of mesenchymal stem cells via p38, JNK and p53 pathway. Cell
Physiol Biochem. 32:1072–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mi H, Lazareva-Ulitsky B, Loo R, Kejariwal
A, Vandergriff J, Rabkin S, Guo N, Muruganujan A, Doremieux O,
Campbell MJ, et al: The PANTHER database of protein families,
subfamilies, functions and pathways. Nucleic Acids Res.
33:D284–D288. 2005. View Article : Google Scholar :
|
|
29
|
Ortiz MA, Lopez-Hernandez FJ, Bayon Y,
Pfahl M and Piedrafita FJ: Retinoid-related molecules induce
cytochrome c release and apoptosis through activation of c-Jun
NH(2)-terminal kinase/p38 mitogen-activated protein kinases. Cancer
Res. 61:8504–8512. 2001.PubMed/NCBI
|
|
30
|
Chuang SM, Wang IC and Yang JL: Roles of
JNK, p38 and ERK mitogen-activated protein kinases in the growth
inhibition and apoptosis induced by cadmium. Carcinogenesis.
21:1423–1432. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ding H, Gabali AM, Jenson SD, Lim MS and
Elenitoba-Johnson KS: P38 mitogen activated protein kinase
expression and regulation by interleukin-4 in human B cell
non-Hodgkin lymphomas. J Hematop. 2:195–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Parrish AB, Freel CD and Kornbluth S:
Cellular mechanisms controlling caspase activation and function.
Cold Spring Harb Perspect Biol. 5:a0086722013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Merhi F, Tang R, Piedfer M, Mathieu J,
Bombarda I, Zaher M, Kolb JP, Billard C and Bauvois B: Hyperforin
inhibits Akt1 kinase activity and promotes caspase-mediated
apoptosis involving Bad and Noxa activation in human myeloid tumor
cells. PloS One. 6:e259632011. View Article : Google Scholar : PubMed/NCBI
|