Introduction
More than forty years ago, Mizejewski et al
(1–4), Tsukada et al (5) and others (6–8)
demonstrated in mouse, rat, and human cultured cell systems in
vitro and mouse and rat experiments in vivo, that
treatment with anti-α-fetoprotein (AFP) serum or its purified
antibody had a growth inhibitory effect on AFP-producing tumor
cells, such as hepatocellular carcinoma and yolk sac tumor. Ohkawa
et al focused on the finding that the administered anti-AFP
antibody led to remarkable inhibition of sugar uptake by the
neoplastic cells (8), suggesting
that one of the main mechanisms of the antitumor effect of the
antibody was impeding the transportation of nourishment across the
cell membrane. However, most of the mechanisms of action of the
antibody-derived antitumor effect remained obscure, because the
main research focus shifted to the application of the specific
antibodies as carriers of anticancer drugs or toxins in drug
delivery systems (9–17). In addition, antibody-mediated
immuno-radioimaging experiments aimed at tumor diagnosis and/or
therapeutic modalities using the antibody directed against tumor
markers coupled to radioisotopes was promulgated (7,18–23)
and the mechanism of action underlying the antitumor effect of
anti-AFP antibody was overlooked.
The possibility that AFP is a cell growth factor has
been discussed recently (24–31),
and its effect in promoting cell proliferation has been reported in
a number of cell types. There have also been many reports that the
cell growth-promoting effects of intracellular AFP are based on the
close relationship with the phosphoinositide 3-kinase (PI3K)/AKT
(known as protein kinase B) signaling pathway (30–36).
Interactions of intracytoplasmic membrane-bound AFP with several
kinds of intracellular functional and essential proteins have been
also reviewed (31). These
findings suggest that AFP must be a new member of the intracellular
molecule family of the PI3K/AKT signaling pathway. It has been
reported that AFP in the cell strongly interacts with the
phosphatase and tensin homolog deleted on chromosome 10 (PTEN),
which is a tumor suppressor molecule that strictly regulates
PI3K/AKT signaling (30–36), and the interaction leads to a
functional decline of PTEN. As a result, the PI3K/AKT signaling
pathway might be activated as a tumor survival system. Conversely,
experiments on the suppression of AFP protein expression using the
RNA interference technology or microRNA network demonstrated that a
severe decrease in AFP within the cell liberated intracellular PTEN
molecules from the cytoplasmic AFP molecules, and led to
restoration of the phosphatase-dependent and/or -independent
activities with a regulatory function to the PI3K/AKT signaling
pathway (32–34). Innovative research with a
breakthrough result was recently reported and entailed adding a
novel antibody with single-chain variable fragment (scFv) to AFP,
thereby blocking AFP and inhibiting hepatoma cell growth via the
PI3K/AKT/PTEN signaling pathway (37). However, possible inhibitory
mechanism of action of anti-AFP antibody against tumor cell growth
did remain obscure in the report.
In the present experiments, to determine the
antitumor efficacy of the antibody, the effect of antibody against
human AFP on the AFP denaturation and/or degradation caused by
antigen-antibody immune reactions, as well as on the biological
behavior of PTEN molecules and on the PI3K/AKT signaling pathway
were investigated using the AFP-producing human hepatocellular
carcinoma cell line, FLC7. FLC7 cells are able to grow in
chemically defined synthetic medium without any addition of peptide
growth factors or animal proteins. Use of this cell line in the
study is convenient and essential in order to determine the effects
on cells of an antibody under a culture environment in which the
trace effects of known and unknown growth-related materials are
absent. This study was conducted to elucidate part of the mechanism
underlying the antitumor effect of anti-AFP antibody.
Materials and methods
Cell culture
The FLC7 cell line (previously known as JHH-7),
initially established from Japanese patient with hepatocellular
carcinoma (38), has been adapted
to grow under serum-free condition with differentiated liver
functions. In brief, FLC7 cells were initially cultivated in
ASF104N medium (Ajinomoto Healthy Supply Co., Inc., Tokyo, Japan),
which is a chemically defined serum-free medium containing only
recombinant human transferrin and insulin as growth factors. Then
the media were replaced with chemically defined completely
synthetic media (IS-RPMI, RPMI-1640, 5 mM HEPES, 3×10−8
M Na2SeO3, 1×10−8 M
NH4VO3, 3×10−9 M
(NH4)6Mo7O24-4H2O,
1×10−7 M FeSO4-7H2O,
3×10−9 M linoleic acid, 3×10−9 M oleic acid
and 1.55×10−2 M NaHCO3) without any addition
of peptides or animal materials (39) and then the cells were continuously
cultivated under the conventional culture conditions in a
CO2 incubator. The spent culture media of IS-RPMI (CM)
of FLC7 cells culturing were stocked as supplement for sub-culture.
FLC7 cells were routinely plated and sub-cultured with 50%
CM-containing IS-RPMI and the media were replaced with fresh
IS-RPMI after 2 days of culture.
Cytotoxicity
FLC7 cells (5×104) were initially
cultured in 24-well culture plates (Greiner Bio-One, Tokyo, Japan)
with 1 ml of IS-RPMI containing 0.5% of ASF104N, instead of CM in
which AFP and other growth factor related various materials with
known and unknown types were probably contained. After 12 h of
culture, the cells, attached to the plates, were twice washed with
2 ml of warmed IS-RPMI and then media were replaced completely with
1 ml of fresh warmed IS-RPMI, which contained various
concentrations of rabbit immunoglobulin (Ig) G of anti-human AFP
antibody or rabbit pre-immune (normal) IgG. The cells were cultured
continuously for 96 h with each IgG. After the incubation, viable
cells were determined with the colorimetric assay using WST-8 (Cell
Counting kit-8, Dojindo Lab., Kumamoto, Japan) and the results were
expressed by the following equation: survival rate (%) = 100 ×
(absorbance at 450 nm of the each IgG-exposed cells) / (absorbance
at 450 nm of the non-treated control cells). Additionally another
type of cytotoxic assay was also carried out using each rabbit
anti-human AFP antibody IgG or rabbit normal IgG at the
concentration of the 50% growth inhibitory concentration value
(IC50), determined with the above results. In brief, to
determine the IC50 in this assay, the cell viability
assessed by WST-8 was expressed as the fraction of surviving cells
in anti-human AFP antibody IgG-treated cells relative to those in
the normal IgG-treated controls, instead of IgG non-treated cells
and the IC50 was calculated. At the concentration of
IC50, the cells were cultured for indicated periods of
time in the IS-RPMI. Results were calculated as mean ± SD of
triplicate determinations of two independent experiments.
Cell lysate
Either rabbit anti-human AFP antibody IgG- or rabbit
normal IgG-treated cultured cells with various time intervals and
concentrations were rapidly washed twice with 2 ml of ice-cold
Dulbecco's PBS (−) in wells, then added 200 μl of ice-cold
cell lysis buffer (50 mM Tris-HCl, pH 7.5, 0.15 M NaCl, 1 mM EDTA,
0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethyl sulphonyl
fluoride, 1% Triton X-100, 1% deoxycholate, 1 mM
Na3VO4, 1 mM NaF, 0.02% NaN3 and
protease inhibitor cocktail and phosphatase inhibitor cocktail 2
and 3 (Sigma-Aldrich Japan, Tokyo, Japan). After incubation for 20
min on ice, collected lysates were centrifuged at 23,000 × g for 20
min at 4°C. The supernatants were kept frozen at −80°C until use.
The protein concentration was measured by the DC protein assay kit
(Bio-Rad, Richmond, CA, USA). Bovine serum albumin (BSA) was used
as the standard.
AFP-enzyme-linked immunosorbent assay
(ELISA)
AFP concentrations in the cultured media and the
cell lysates were determined using the sandwich ELISA system (AFP
ELISA kit; Abnova, Taipei, Taiwan), according to the manufacturer's
instructions. Intracellular AFP concentrations were expressed as
(ng)/(mg of cell lysate). Data were expressed as mean ± SD of two
independent experiments.
SDS polyacrylamide gel
electrophoresis/immunoblotting (SDS-PAGE/WB)
After SDS-PAGE/WB, the polyvinylidene fluoride
filters (Abcam, Tokyo, Japan) were blocked and then incubated with
the corresponding primary antibodies, followed by horseradish
peroxidase (HRP)-labeled second antibodies directed against rabbit,
mouse, or goat IgG (Abcam), as described previously (40). Enhanced chemiluminescence (ECL)
signals (ImmunoStar, Wako Pure Chemical Industries, Ltd., Tokyo,
Japan) were detected by a cooled CCD camera system (ATTO
light-capture II type AE-6981; ATTO Co., Tokyo, Japan). Primary
antibodies used were AKT, AKT phosphorylated at serine (S) 473
(pAKT), p53 protein (P53) phosphorylated at S20 (pP53S20), MDM2
phosphorylated at S166 (pMDM2) (Cell Signaling Technology Inc.,
Tokyo, Japan), extracellular signal regulated kinase 1/2 (ERK1/2),
PTEN, β-catenin (BD Biosciences, Tokyo, Japan), ERK1/2
diphosphorylated at T183 and Y185 (pERK1/2), β-actin, α-tubulin
(Sigma-Aldrich Japan), PTEN phosphorylated at S380 (pPTEN),
glycogen synthase kinase 3β phosphorylated at S9 (pGSK3β), glucose
transporter isoform 1 (GLUT1), P53, P53 phosphorylated at S392
(pP53S392), P53 phosphorylated at S46 (pP53S46) and AFP (Abcam).
Densitometric analysis of bands was performed using ImageJ software
(1.50i, NIH). Data were expressed in arbitrary units as average of
at least three independent experiments. The data of densitometric
analyses from all bands were corrected by densities of each
α-tubulin band, which was used as a loading control, and this
provided the exact changes of the protein levels after antibody
treatment. The data were calculated according to the following
formula: the relative intensity = (the band intensity of the
indicated day) / (the band intensity at day 7 of rabbit normal
IgG-treated cells, instead of IgG non-treated control cells at day
0 or day 7).
Dot blot analysis
Cell lysates prepared from cells treated with either
rabbit normal IgG or anti-human AFP antibody IgG for 7 days at 50.0
μg/ml were spotted (each 1 μl of 10, 5, 2.5 and 1
μg/μl) on nitrocellulose filters (Bio-Rad), dried and
blocked. For detection of rabbit IgG, such as antibody administered
and probably internalized in the cells, HRP-labeled goat
anti-rabbit IgG was employed. For detection of intracellular AFP,
the same above filters were used after procedure of de-probing the
antibodies with signal stripping buffer (Restore™ PLUS Western Blot
Stripping Buffer; Thermo Fisher Scientific Inc., Yokohama, Japan).
After blocking, the filters were incubated with goat anti-human AFP
antibody (Abcam) followed by HRP-labeled donkey anti-goat IgG
(Abcam). Signals of each spot were obtained by ECL capture (ATTO
light-capture II). Densitometric data were expressed as mean ± SD
of three independent experiments.
Immunofluorescence cytology of
subcellular localization of GLUT1
FLC7 cells were cultured with media containing
either rabbit normal IgG or rabbit anti-human AFP antibody IgG, at
75 μg/ml concentration using a glass bottom culture dish
(Iwaki Glass Base Dish, Asahi Glass Co., Ltd., Tokyo, Japan). After
5 days of culture, the cells were gently rinsed twice with cold
Dulbecco's PBS (−), fixed with 3.3% formaldehyde in Dulbecco's PBS
(−) for 10 min at 4°C. After rinsing with Dulbecco's PBS (−), the
cells were permeabilized with 0.1% Triton X-100 in Dulbecco's PBS
(−) for 10 min at 4°C and then blocked with 5% BSA and donkey
normal IgG (5 mg/ml) in Dulbecco's PBS (−) containing 0.1% Tween-20
(TPBS) for 4 h. The cells were incubated with goat anti-GLUT 1
antibody (C-20; Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
diluted with 5% BSA in TPBS for 17 h at 4°C. After gentle washing,
the cells were further incubated with Alexa Fluor® 488-labeled
donkey anti-goat IgG (Abcam) in 5% BSA containing TPBS for 1 h at
37°C. After rinsing with Dulbecco's PBS (−), immunofluorescence
images were captured using a Keyence BZ-8000 microscope (Keyence
Corp., Inc., Osaka, Japan).
Reagents
For investigation on cell growth inhibition tests,
rabbit polyclonal anti-human AFP antibody IgG (#PA-012) was
obtained from Nippon Bio-Test Lab. Inc. (Tokyo, Japan). Rabbit
normal IgG was purchased from Sigma-Aldrich Japan. For cell
treatments, both IgG solutions were extensively dialyzed against
Dulbecco's PBS (−) followed by IS-RPMI at 4°C. The solutions were
filter-sterilized before use. BSA and donkey normal serum were from
Sigma-Aldrich Japan. Donkey normal IgG fraction was prepared from
its normal serum by the salting out method using 33% ammonium
sulfate, followed by dialyzed extensively against 0.9% NaCl at 4°C.
Protein concentration was measured by the DC protein assay kit
(Bio-Rad) using the bovine serum IgG as the standard. RPMI-1640
medium was from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan). All
other chemicals were of reagent grade.
Statistical analysis
Values of p<0.05 were considered statistically
significant using Fisher's exact test.
Results and Discussion
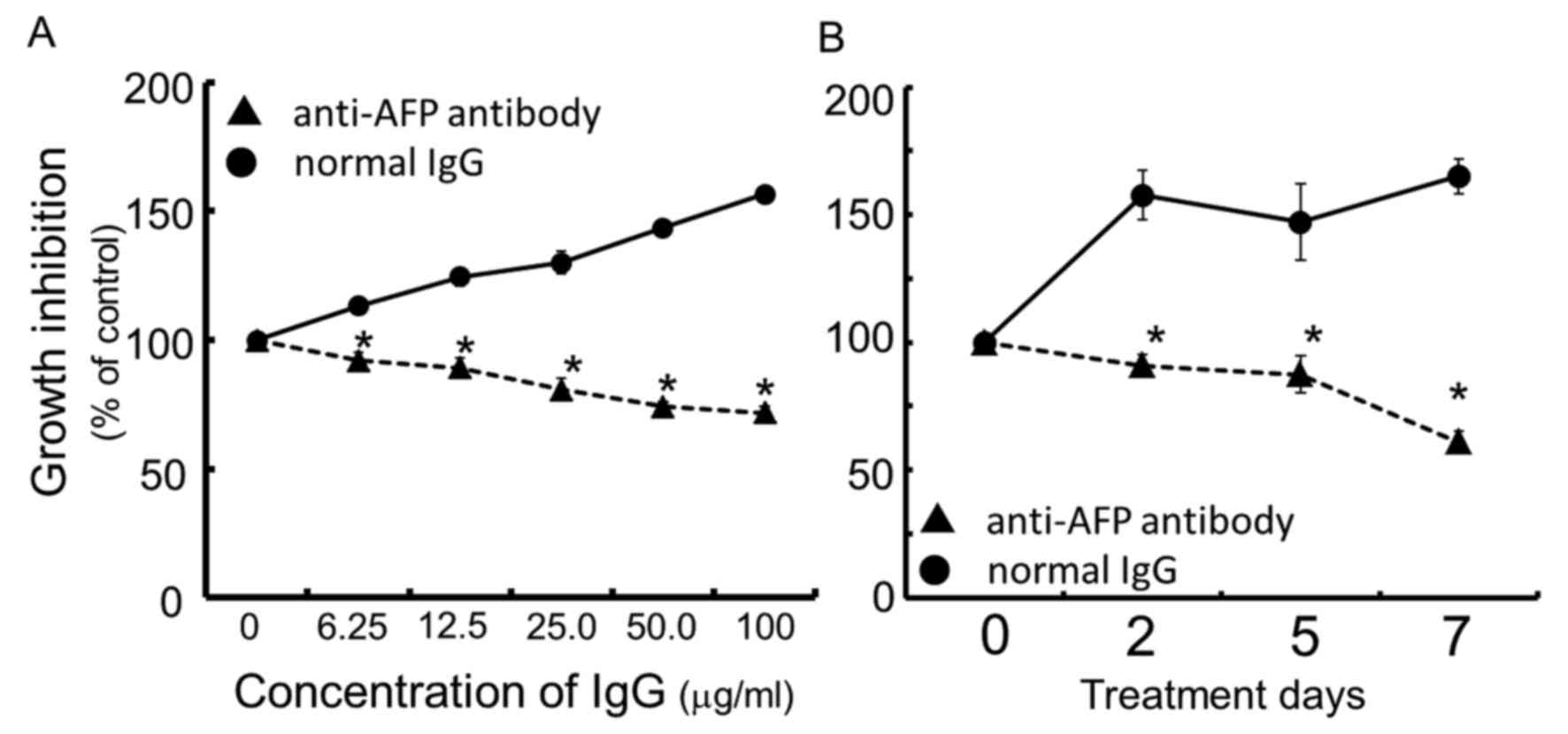
Growth inhibition by anti-AFP
antibody
Continuous exposure of the cells for 96 h to rabbit
anti-human AFP antibody dose-dependently inhibited cell growth of
the human hepatocellular carcinoma FLC7, as compared with the
normal IgG control (Fig. 1A). The
concentration of IgG of anti-human AFP antibody at IC50
was calculated to be ~50.0 μg/ml. As a result of treating
cells for up to 7 days with rabbit anti-human AFP antibody at the
concentration of 50.0 μg/ml, the antibody exhibited
significant and time-dependent growth inhibition compared to the
rabbit normal IgG used as the control (Fig. 1B).
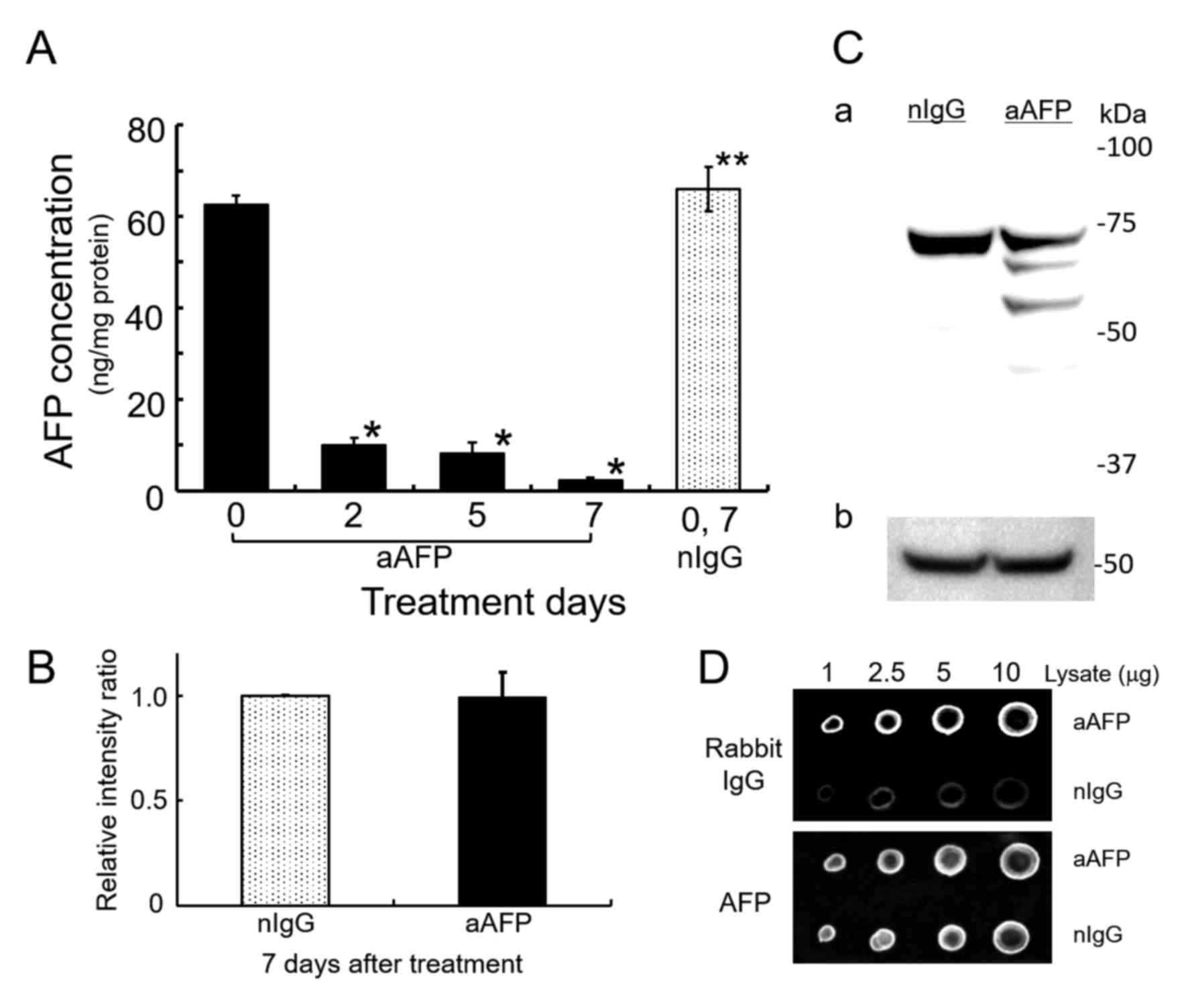
Possibility of the formation of immune
complexes
ELISA-determination of AFP showed that addition of
anti-human AFP antibody decreased the AFP concentration in the
medium below the detection limit (data not shown). At the same
time, anti-human AFP antibody significantly reduced intracellular
AFP levels up to 3.67–15.7% of the AFP concentration in rabbit
normal IgG-treated control cells (Fig.
2A). Interestingly, however, SDS-PAGE/WB analysis of cell
lysates using a goat antibody against human AFP and an HRP-labeled
donkey anti-goat IgG antibody without cross-reactions to rabbit IgG
revealed that the amount of AFP in the anti-AFP antibody-treated
cells did not decrease substantially compared with that in
IgG-treated control cells (Fig. 2B and
C). Additionally, by anti-AFP antibody treatment of the cells,
three detectable immunoreactive bands were found and they might be
degradation products of the AFP molecules. Typical result is shown
in Fig. 2C. Dot blot analyses
demonstrated that the presence of anti-rabbit IgG specific
immunoreactive substances, presumably derived from administered
rabbit anti-AFP antibody to the cells, was sufficiently detectable
with higher significance compared to normal IgG-administered cells
(Fig. 2D and Table I). By contrast, the AFP reactive
densitometric signals in both cell lysates were nearly the same. In
each concentration of cell lysates applied on nitrocellulose
filters, rabbit IgG derived signals were ~2.4–3.4 times higher in
the anti-AFP antibody-treated cell lysates as compared to normal
IgG-treated corresponding cell lysates (Table I). In these results, treatment of
the cells with anti-AFP antibody might have led to the presence of
free antibody due to an excess of antibody and the presence of
immune complex (IC) generated from the administered antibody and
AFP in the cell. When intracellular AFP is measured by an
immunochemical technique such as ELISA, it can be predicted that
both the excess antibody and the IC competitively inhibit the assay
system. Analysis of the amount of intracellular AFP molecules by
two different methods showed an obvious discrepancy between the
results of each method, pointing to the generation and accumulation
of IC in the cells. These results suggest that the amount of AFP
was not reduced in antibody-treated cells, but rather, the reaction
products of AFP and polyclonal anti-AFP antibody administered, the
so-called ICs, had accumulated. Generation and accumulation of ICs
consisting of multimeric aggregates of antibody and AFP is expected
to inhibit AFP function without a large fluctuation in the amount
of intracellular AFP. In other words, a large amount of AFP exists
in the cytoplasm, but it is possible that its functions have been
lost. As shown for the first time in this experiment the existence
of intracellular IC derived from the antibody added extracellularly
is sufficiently suspected.
 | Table IIncreased densitometric intensities
of intracellular rabbit IgG immunoreactivity in cell lysates
treated with rabbit anti-AFP antibody IgG (dot blot analysis). |
Table I
Increased densitometric intensities
of intracellular rabbit IgG immunoreactivity in cell lysates
treated with rabbit anti-AFP antibody IgG (dot blot analysis).
| Antibody to | Cell lysate
(µg) | Integrated density
|
|---|
aAFP treated cells
| nIgG treated cells
|
|---|
| Mean | SD | Mean | SD |
|---|
| Rabbit IgG | 10 | 205936a | 34735.3 | 67785.7 | 28598.6 |
| 5 | 142118a | 31881.8 | 51623.7 | 17121.1 |
| 2.5 | 101126a | 13536.7 | 44261.0 | 20816.0 |
| 1 | 63133.7a | 30589.2 | 18683.3 | 7903.6 |
| AFP | 10 | 408798 | 51751.8 | 341240 | 58997.5 |
| 5 | 315853 | 52217.1 | 291631 | 49265.0 |
| 2.5 | 237805 | 31213.6 | 221156 | 50002.1 |
| 1 | 145434 | 49046.1 | 123246 | 40601.6 |
Intracellular uptake of IgG molecules such as
antibodies is known to occur by pinocytosis, macropinocytosis or
endocytosis across the cell membrane. However, the mechanism for
maintaining the function of antibody internalized in the cytoplasm
is not well known (41–43). In recent years, McEwan et al
and Watkinson et al and their co-workers proposed the
concept of intracellular antibody immunity (44,45)
and many investigators reported methods for screening
cell-internalizing antibodies, which possess high
cell-internalizing activity, from polyclonal antibodies (46,47).
It is possible that the cell-internalized antibody exerts its own
unique function in the cell. Accordingly, there are many unknown
factors to elucidate in this interesting field.
It is well -known that the cell growth promoting
effect of AFP is exerted by interaction with many other molecules
(32–37). Furthermore, in principle,
intermolecular interaction is based on the three-dimensional
structure of related molecules. As a result of antibody binding
followed by IC generation, it is highly possible that AFP molecules
with a conformational change may lose their ability to interact
with other related molecules and become so-called non-functional
AFP. Even though abundant non-functional AFP is present in the
cell, AFP functions such as growth promotion must be suppressed.
Conversely, it is reasonable to infer that the intracellular AFP
concentration measured by ELISA may correspond to the amount of
AFP, which is not affected by IC generation and remains functional.
Based on these results, it can be deduced that most of the AFP
molecules detected by SDS - PAGE/WB in antibody-treated cells are
non-functional AFP, and the cellular environment resulting from
anti-AFP antibody treatment in this study was very similar to the
environment of intracellular AFP elimination caused by experiments
on RNA interference and microRNA technology, as reported previously
(32–34,37).
Therefore, we confirmed the importance of further analyzing the
changes in some major molecules interacting with the AFP molecule
in the cell induced by anti-AFP antibody treatment.
Additionally in this experiment, the amount of AFP
in the antibody-treated cells fluctuated slightly (Fig. 2B). The possible cause of this
variation is considered to be the result of intracellular
degradation of AFP derived from ICs, based on the anti-AFP antibody
reactive bands in the low molecular weight region recognized by
SDS-PAGE/WB analysis of the cell lysate treated with anti-AFP
antibody (Fig. 2C). It is also
another possibility on AFP-fragmentation that anti-AFP antibody in
the cytoplasm binds to fragments of AFP molecule on messenger RNA
being translated at the ribosomes, as reported in the purification
experiment for messenger RNA of AFP by Miura et al (48). If this phenomenon occurs in the
cell, it can be predicted that AFP protein synthesis would be
inhibited and the protein fragments with incomplete length would be
generated.
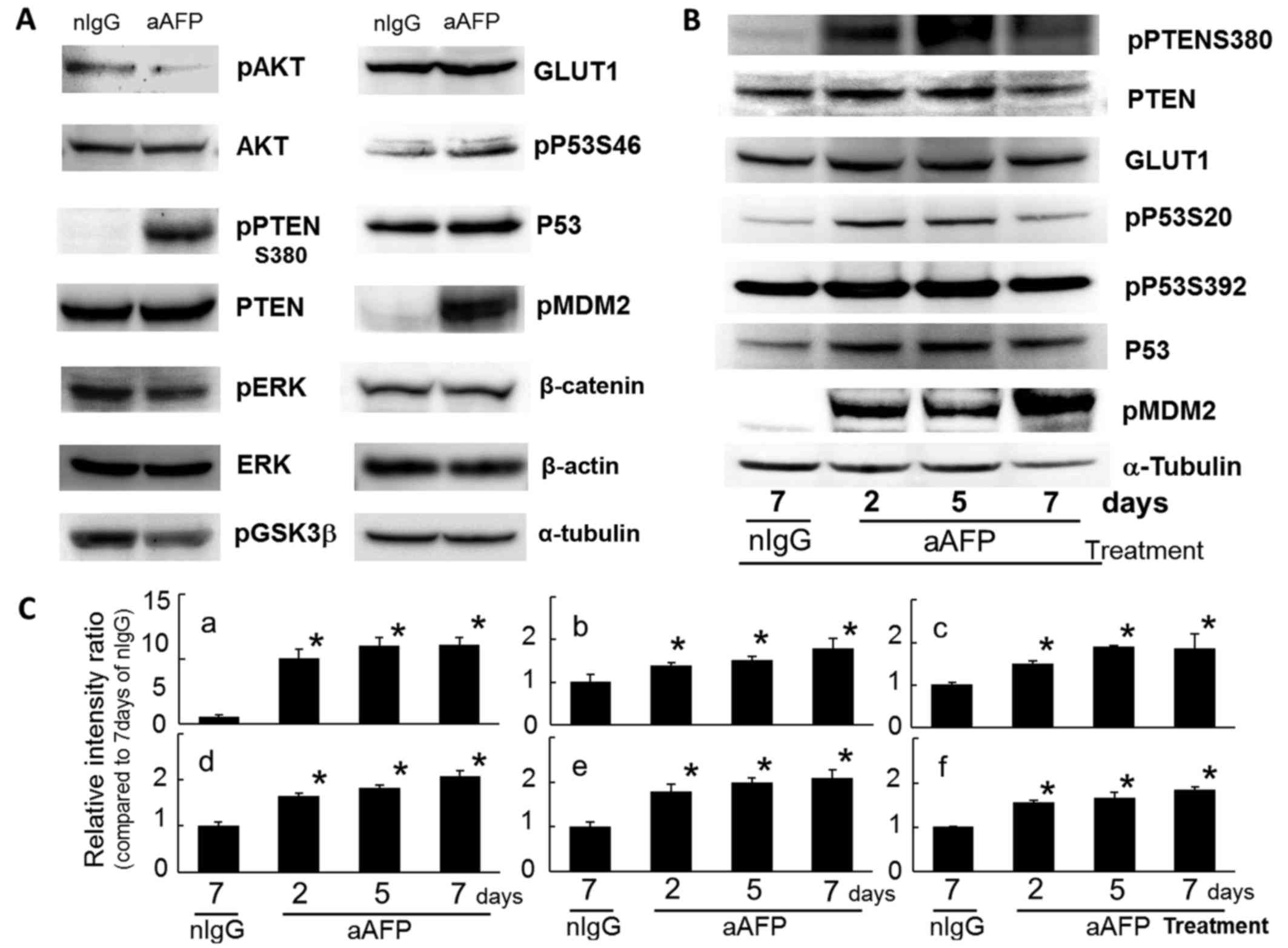
Anti-AFP antibody-induced suppression of
the P13K/AKT pathway via PTEN stabilization
In this study, phosphorylation on S473 of the AKT
molecule which is the protagonist of the PI3K/AKT signaling pathway
was reduced by anti-human AFP antibody treatment (Fig. 3A). This result indicated that the
PI3K/AKT signaling pathway was suppressed in anti-AFP
antibody-treated FLC7 cells. It has been reported that in
AFP-producing tumor cells, intracellular AFP molecules bind and
interact closely with PTEN molecules, thereby suppressing the PTEN
functions (both phosphatase-dependent and -independent activities)
and consequently upregulating the PI3K/AKT signaling pathway
(30,32–35).
Interestingly, the phosphorylated PTEN molecules at S380 were
increased markedly by antibody treatment, with an extremely slight
but significant variation in the intracellular protein level of
PTEN (Fig. 3). At the same time,
phosphorylation of ERK1/2 (T183 and Y185) was also reduced,
suggesting an inhibition state of the MAP kinase signaling systems
in the cells (Fig. 3A). There have
been some reports that each PTEN molecule, which localizes in the
nucleus or cytoplasm, exhibited different effects on various
signaling pathways (49–53). According to these reports, PTEN
localizing in the cytoplasm decreases the level of phosphorylated
AKT, upregulates P27kip1 and is required for apoptosis,
whereas PTEN localizing in the nucleus downregulates phosphorylated
MAP kinase and cyclin D1 and is crucial for cell cycle arrest,
respectively. A recent study demonstrated that the
anti-proliferative effect of synthetic anti-AFP scFv antibody on
hepatic tumor cells was based on induction of G1 cell cycle arrest
and apoptosis, probably related to the PI3K/AKT/PTEN signaling
network (37), but a possible
mechanism of action of anti-AFP scFv on cell-growth inhibition was
not discussed in their report. As a result, the PTEN molecule can
be expected to participate in the mechanism underlying the
antitumor effect manifested by anti-AFP antibody.
PTEN was modified through strong phosphorylation at
S380 of the carboxyl-terminal region (C-terminal tail domain) on
the second day and thereafter due to antibody treatment.
Phosphorylation of the S380, S385, T382, and T383 residues, but not
T366, in the C-terminal region of PTEN molecules is known to not
only increase the stability of the PTEN molecule but also decrease
its phosphatase activity (54–60).
The C-terminal tail domain of PTEN has been shown to be important
in regulating the stability and half-life of the molecules.
Specifically, it is known that phosphorylation of the S380 residue
is critical for PTEN protein stability (61–64).
Phosphorylation on S380 of PTEN at an early stage after antibody
administration indicated that molecular stability was improved,
but, on the other hand, excessive phosphatase activity was somewhat
inhibited. It is thought that this phenomenon may be related to
maintaining the molecular numbers of PTEN and to sustaining the
potential for PTEN to control the PI3K/AKT signaling pathway.
However, further investigations are necessary to clarify the true
action mechanism(s) of each PTEN molecule with phosphorylation at
various target residues, because neither change in PI3K enzyme
activity nor exact contents of
phosphatidylinositol-3,4,5-triphosphate was determined in this
study.
As a result of a decrease in phosphorylated AKT
(S473), phosphorylation on S9 of the GSK3β molecule, which is one
of the target molecules of AKT (65), was relatively reduced (Fig. 3A). Conversely, enhancement of
kinase activity of GSK3β is probable. Participation of GSK3β in
PTEN-phosphorylation is already well recognized (54,55,58,65,66),
and it is thought that an increase in kinase activities of GSK3β
might enhance phosphorylation at T366 on the target PTEN molecules
(58). T366 phosphorylation
promotes destabilization and degradation of PTEN molecules as
reported previously (58,67). However, in the present study, PTEN
protein levels did show gradual and significant increase during
treatment for up to 7 days (Fig. 3B
and C). Due to the failure of molecular co-localization and
interaction between AFP and PTEN occurring in the cytoplasm of the
AFP-producing tumor cells, acute and severe reduction of the
intracellular functional, immunoreactive AFP concentration due to
IC generation caused by the administration of anti-AFP antibody
resulted in a massive release of free and probably functional PTEN
molecules from AFP in the cytoplasm. Moreover, intracellular
protein levels of PTEN after antibody administration in this
experiment were elevated slightly but significantly as shown in
Fig. 3B and C. This finding
suggests that selective phosphorylation on S380 of the free PTEN
molecules induced molecular stabilization with the suppression of
enzyme activity, as a mechanism to maintain potential control of
the PI3K/AKT signaling pathway without degrading the rapidly
increased level of PTEN molecules in the cell. To the contrary,
this reaction should maintain the cancer cell property of
preferential growth as a cancer cell inactivates excess PTEN
molecules, which act as a major suppressive regulator in the
PI3K/AKT signaling pathway and are, therefore, an impediment to
proliferation reactions of the cancer cells. This mechanism is a
self-defense reaction that usually occurs in cancer cells in order
to survive. This is an interesting phenomenon in cancer biology and
requires further elucidation. It has been reported that
phosphorylation at S380 of the PTEN molecule at the C-terminal
tail, does not involve casein kinase 2 and GSK3β (58–60),
but a result of the interaction of other proteins, such as protein
interacting with carboxyl terminus-1 (PICT-1) (63,64).
Okahara et al suggested that PICT-1 is a PTEN-interacting
protein that promotes the phosphorylation and stability of PTEN,
and can regulate the phosphorylation of PTEN at S380 (63,64).
The authors concluded that the binding of PTEN to PICT-1 governs
its turnover via phosphorylation of the C-terminal region. Their
result and the findings of our present study provide insight into
unknown other molecular mechanism(s) by which PTEN turnover is
controlled.
Upregulation of P53 functions by anti-AFP
antibody treatment
Protein levels of P53 were slightly and
significantly increased during antibody treatment (Fig. 3), because PTEN increased P53
stability and function via interaction of both molecules, which
bind the C2 domain of PTEN to the C-terminus of P53 (68,69).
Therefore, in cells lacking PTEN, P53 levels are significantly
reduced owing to decreased stability as reported previously
(68). Expression of wild-type or
phosphatase-dead forms of PTEN with a recombinant construct also
increases P53 stability in MDM2 (a ubiquitin ligase for
P53)-independent manner (68).
Phosphorylation reactions of P53 on S20 and on S392 were
significantly enhanced after antibody treatment (Fig. 3), suggesting stabilization and
activation of the molecule. Post translational modifications of the
P53 molecule, such as phosphorylation, acetylation, and
ubiquitination, have been recognized to regulate the stability and
functions of P53 proteins (69–74).
It is well-known that DNA damage-induced phosphorylation at S20 of
P53 leads to a reduced interaction between P53 and its negative
regulator, MDM2. As a result, S20 phosphorylation impairs the
ability of MDM2 to bind P53, promoting both accumulation and
functional activation of P53 in response to DNA damage (72). MDM2 plays a central role in
regulating the stability of P53 and inhibits P53 accumulation by
targeting it for ubiquitination and proteasomal degradation
(75–77). Phosphorylated P53 at S392 is also
essential for promoting its tetramerization, stability, and
functional activity as well as for S20-phosphorylation reactions
(70,71). These findings indicate that the
regular functions of P53 recovered after antibody treatment.
Phosphorylation of P53 at S46, which regulates the ability of P53
to induce apoptosis (73), also
increased slightly, suggesting the triggering of apoptotic
signaling (Fig. 3A).
It is of interest that an extreme and significant
increase in phosphorylated MDM2 on S166 (pMDM2) was observed in
antibody-treated tumor cells within 2 days after the beginning of
antibody administration (Fig. 3).
As known previously (76,77), the presence of pMDM2 indicates the
enhancement of E3 ligase activity to promote ubiquitin-dependent
degradation of P53. Additionally, phosphorylation at S166 and at
S186 of MDM2 has been shown to depend on AKT enzyme activity
(76,77). In the present study, antibody
administration elicited a decrease in intracellular AFP, releasing
PTEN from AFP, followed by stabilization of the molecular functions
of free PTEN. The resultant free but functional PTEN was able to
suppress the PI3K/AKT signaling system, which also serves as a
survival pathway in cancer cells. PTEN also promoted stabilization
and expression of the biological functions of the P53 molecule that
interacts with the PTEN molecule. Under such circumstances, it can
be deduced that the accumulation and abnormal increase of pMDM2
(S166) in liver cancer cells are needed in order to maintain a
steady state of the cancer cells and enhance the degradation of
P53, the tumor suppressor molecule, even when AKT enzyme activity
is inhibited. There was no difference in the expression level of
β-catenin protein (Fig. 3A), a
major protein of the Wnt/β-catenin pathway, which is one of the
important cell survival regulatory pathways involving the
PI3K/AKT/GSK3β axis (66).
Induction of subcellular translocation of
GLUT1 by anti-AFP antibody
In the present study, protein levels of a universal
molecule in the glucose transporter species, GLUT1, showed a
significant increase immediately after antibody treatment and
continued for 7 days (Fig. 3). The
increase in GLUT1 protein levels observed early during antibody
treatment clearly pointed to a deficiency of and need for glucose
in the tumor cells. It is known that a proliferating tumor cell
vigorously consumes sugar, especially glucose, irrespective of the
presence or absence of oxygen. The fact that the PTEN function in
FLC7 cells with a high level of AFP production was strongly
suppressed by the AFP is compatible with the above-mentioned
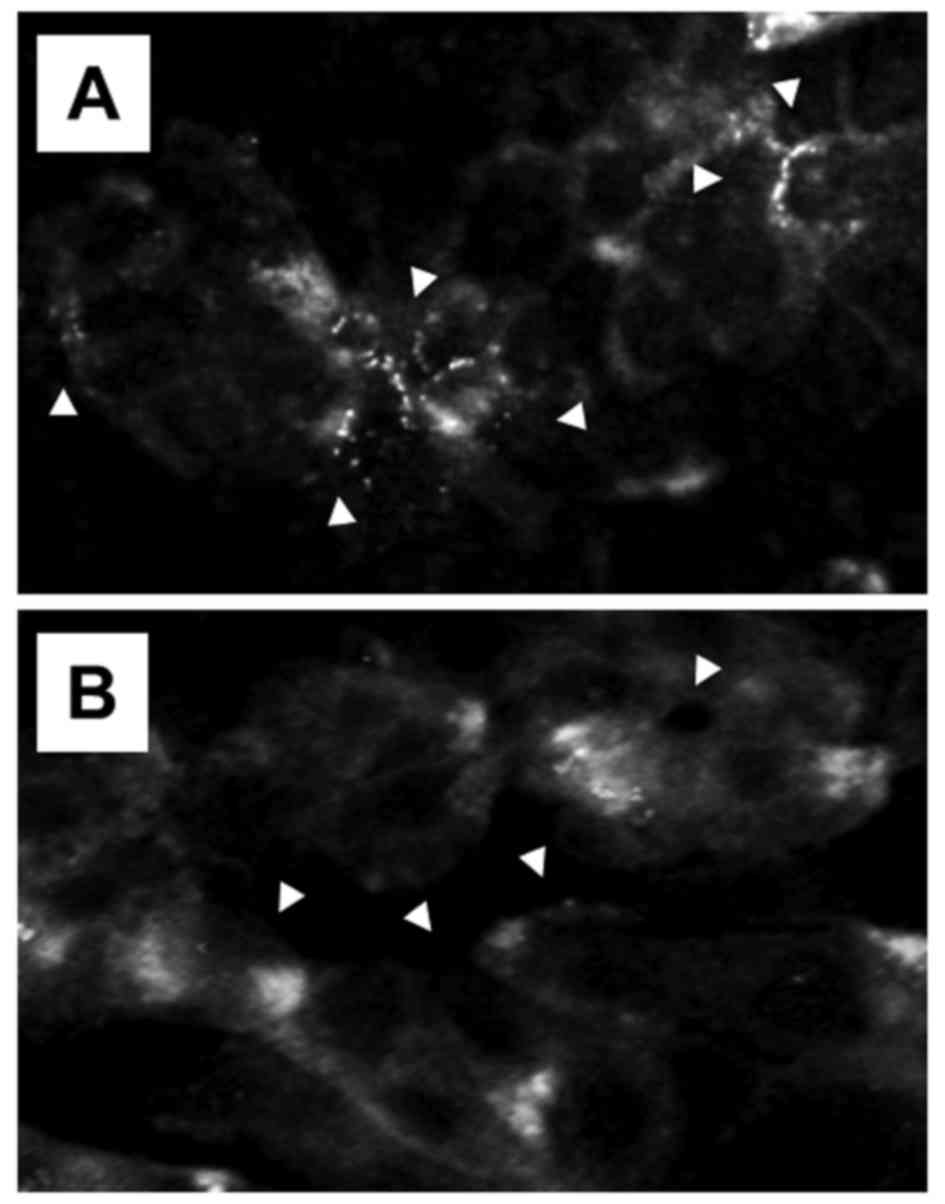
results. Under the condition of malfunction of PTEN,
immunofluorescence cytology revealed a change in the subcellular
localization of GLUT1 molecules (Fig.
4). In control cells with malfunction of PTEN, treated with
rabbit normal IgG, many GLUT1 molecules were localized patchily on
the surface of the cell membrane. In contrast, in cells treated
with rabbit anti-AFP antibody IgG, the distribution of the weak
fluorescence indicating GLUT1 was found throughout the cytoplasm,
and localization of GLUT1 on the cell surface membranes was
reduced. Morani et al reported that in genetically
manipulated human thyroid cancer cell lines, the loss of PTEN
expression was associated with increased expression of GLUT1 on the
cell surface plasma membrane and enhanced the translocation of
GLUT1 proteins onto the surface of plasma membrane from the
cytoplasm (78). They concluded
that the PTEN protein regulated plasma membrane expression of GLUT1
and that the loss of function of PTEN increased the probability of
cancer detection by 18F-fluorodeoxyglucose positron
emission tomography or other glucose-based imaging diagnosis in a
clinical setting. This result was similar to the reported finding
that the plasma membrane translocation of GLUT1 was dependent on
the PI3K/AKT signaling pathway (79–81).
Immunocytohistologic studies have also shown that enhanced glucose
uptake in cancer cells correlates with overexpression of GLUTs,
especially of GLUT1 (78,81). Our results fully supported the
previous findings that the restored PTEN molecules liberated from
AFP closely participated in the process of intracellular
distribution of GLUT1. To clarify the relationship between PTEN
function and the translocation of GLUT1, it may be important to
investigate other unknown function(s) of the PTEN molecule involved
in regulating subcellular migration of the target molecules. As
reported previously in our study (8), the inhibition of glucose uptake by
the treatment of AFP-producing rat hepatoma AH66 cells with horse
anti-rat AFP antibody occurred within a few hours after the
treatment. There was no remarkable change in the Km
value in the kinetic analysis of the uptake, while a decrease in
the Vmax was observed indicating that non-competitive
inhibition was the main cause of glucose uptake failure. At that
time, it was assumed that no qualitative change in the transport
carrier occurred, but there was a quantitative change and a
decrease in the number of unknown sugar transporters. Currently,
one of the effective treatments of hepatocellular carcinoma in the
clinical setting is inhibition of angiogenesis in liver cancer
tissue to induce malnutrition, such as deficiency of sugar, other
nutrients and oxygen (82–85). The induction of such malnutrition
in cancer cells in the present study by administration of anti-AFP
antibody is a significant finding.
There have been several reports that small but
detectable amounts of autoantibodies against human AFP were
produced by an unknown factor in vivo, including in the
presence of cancer (86–90). Generation of autoantibodies to
carcinoembryonic antigen (CEA) has been also reported in several
kinds of cancers and the expression of these anti-CEA
autoantibodies depleted the serum CEA antigen levels (91–94).
It can be postulated that by a similar unknown mechanism, excess
amounts of autoantibodies raised against AFP in the serum or in the
cells are generated, and then bind to AFP, thereby decreasing the
AFP concentration. However, there are no reports yet of any
findings related to AFP that might compare with the rapid deletion
of serum CEA due to increased expression of anti-CEA autoantibody.
The phenomenon of anti-AFP antibody eliminating abundant functional
AFP from the cytoplasm as well as from the extracellular matrices,
with severe accumulation of ICs as a result of some unknown
phenomenon cannot be expected for liver cancer cells. Liver cells,
parenchymal and non-parenchymal interstitial cells, as well as
liver cancer cells exhibit a confusing biological reaction in
response to the cellular environment resulting from anti-AFP
antibody treatment.
As shown in this study, the administration of
anti-AFP antibody had an inhibitory effect on cell growth via
suppression of the PI3K/AKT signaling pathway. Because of a
decrease in intracellular functional AFP resulting from the
generation of ICs consisting of AFP and anti-AFP antibody, the
production and accumulation of ICs derived from the AFP-anti AFP
antibody reaction exerted the same cell growth inhibitory effect as
inhibition of intracellular AFP expression. Unfortunately, the
cytotoxicity and antitumor efficacy of the specific antibody alone
were weaker in comparison to so-called anticancer agents. However,
a more recent report indicated that intracellular AFP expression is
closely related to hepatocarcinogenesis, as well as liver cancer
progression (95). For such
reasons, elucidating the mechanism of action of cytotoxicity of
anti-AFP antibody on AFP-producing tumor cells serves as a sound
basis not only for use in combination with other effective liver
cancer therapy but also for developing strategic measures for liver
cancer prevention.
Acknowledgments
This study was supported in part by MEXT-Supported
Program for the Strategic Research Foundation at Private
Universities (2011–2015) from the Ministry of Education, Culture,
Sports, Science and Technology.
References
|
1
|
Mizejewski GJ and Allen RP:
Immunotherapeutic suppression in transplantable solid tumours.
Nature. 250:50–52. 1974. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mizejewski GJ, Young SR and Allen RP: α
fetoprotein: Effect of heterologous antiserum on hepatoma cells in
vitro. J Natl Cancer Inst. 54:1361–1367. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mizejewski GJ and Allen RP: α-fetoprotein:
Studies of tumor-associated antigen cytotoxicity in mouse hepatoma
BW7756. Clin Immunol Immunopathol. 11:307–317. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mizejewski GJ and Dillon WR:
Immunobiologic studies in hepatoma-bearing mice passively immunized
to α-fetoprotein. Arch Immunol Ther Exp (Warsz). 27:655–662.
1979.
|
|
5
|
Tsukada Y, Mikuni M, Watabe H, Nishi S and
Hirai H: Effect of anti-alpha-fetoprotein serum on some cultured
tumor cells. Int J Cancer. 13:187–195. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wepsic HT, Tsukada Y, Takeichi N, Nishi S
and Hirai H: Effect of horse antibody to rat alpha-fetoprotein upon
the growth of AH-66 in Donryu rats. Int J Cancer. 25:655–661. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koji T, Ishii N, Munehisa T, Kusumoto Y,
Nakamura S, Tamenishi A, Hara A, Kobayashi K, Tsukada Y, Nishi S,
et al: Localization of radioiodinated antibody to alpha-fetoprotein
in hepatoma transplanted in rats and a case report of
alpha-fetoprotein antibody treatment of a hepatoma patient. Cancer
Res. 40:3013–3015. 1980.PubMed/NCBI
|
|
8
|
Ohkawa K, Tsukada Y, Hibi N and Hirai H:
The inhibitory effects of horse anti-rat AFP antiserum on the
uptake of 2-deoxy-D-glucose by AFP-producing rat hepatoma cells.
Int J Cancer. 33:497–502. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsukada Y, Bischof WK, Hibi N, Hirai H,
Hurwitz E and Sela M: Effect of a conjugate of daunomycin and
antibodies to rat alpha-fetoprotein on the growth of
alpha-fetoprotein-producing tumor cells. Proc Natl Acad Sci USA.
79:621–625. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsukada Y, Kato Y, Umemoto N, Takeda Y,
Hara T and Hirai H: An anti-alpha-fetoprotein antibody-daunorubicin
conjugate with a novel poly-L-glutamic acid derivative as
intermediate drug carrier. J Natl Cancer Inst. 73:721–729.
1984.PubMed/NCBI
|
|
11
|
Tsukada Y, Hurwitz E, Kashi R, Sela M,
Hibi N, Hara A and Hirai H: Chemotherapy by intravenous
administration of conjugates of daunomycin with monoclonal and
conventional anti-rat alpha-fetoprotein antibodies. Proc Natl Acad
Sci USA. 79:7896–7899. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tsukada Y, Hurwitz E, Kashi R, Sela M,
Hibi N, Hara A and Hirai H: Effect of a conjugate of daunomycin and
purified polyclonal or monoclonal antibodies to rat
alpha-fetoprotein on the growth of alpha-fetoprotein-producing
tumor cells. Ann NY Acad Sci. 417:262–269. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kato Y, Tsukada Y, Hara T and Hirai H:
Enhanced antitumor activity of mitomycin C conjugated with
anti-alpha-fetoprotein antibody by a novel method of conjugation. J
Appl Biochem. 5:313–319. 1983.PubMed/NCBI
|
|
14
|
Tsukada Y, Ohkawa K and Hibi N:
Suppression of human alpha-foetoprotein-producing hepatocellular
carcinoma growth in nude mice by an anti alpha-foetoprotein
antibody-daunorubicin conjugate with a poly-L-glutamic acid
derivative as intermediate drug carrier. Br J Cancer. 52:111–116.
1985. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ohkawa K, Hibi N and Tsukada Y: Evaluation
of a conjugate of purified antibodies against human
AFP-dextran-daunorubicin to human AFP-producing yolk sac tumor cell
lines. Cancer Immunol Immunother. 22:81–86. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tsukada Y, Ohkawa K and Hibi N:
Therapeutic effect of treatment with polyclonal or monoclonal
antibodies to alpha-fetoprotein that have been conjugated to
daunomycin via a dextran bridge: Studies with an
alpha-fetoprotein-producing rat hepatoma tumor model. Cancer Res.
47:4293–4295. 1987.PubMed/NCBI
|
|
17
|
Ohkawa K, Tsukada Y, Hibi N, Umemoto N and
Hara T: Selective in vitro and in vivo growth inhibition against
human yolk sac tumor cell lines by purified antibody against human
alpha-fetoprotein conjugated with mitomycin C via human serum
albumin. Cancer Immunol Immunother. 23:81–86. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim EE, DeLand FH, Nelson MO, Bennett S,
Simmons G, Alpert E and Goldenberg DM: Radioimmunodetection of
cancer with radiolabeled antibodies to alpha-fetoprotein. Cancer
Res. 40:3008–3012. 1980.PubMed/NCBI
|
|
19
|
Kim EE, Deland FH, Casper S, Corgan RL,
Primus FJ and Goldenberg DM: Radioimmunodetection of colorectal
cancer. Cancer. 45(Suppl): 1243–1247. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Uriel J, Villacampa MJ, Moro R, Naval J
and Failly-Crépin C: Uptake of radiolabeled a-fetoprotein by mouse
mammary carcinomas and its usefulness in tumor scintigraphy. Cancer
Res. 44:5314–5319. 1984.PubMed/NCBI
|
|
21
|
Goldenberg DM: Cancer imaging with CEA
antibodies: Historical and current perspectives. Int J Biol
Markers. 7:183–188. 1992.PubMed/NCBI
|
|
22
|
Behr TM, Liersch T, Greiner-Bechert L,
Griesinger F, Béhé M, Markus PM, Gratz S, Angerstein C, Brittinger
G, Becker H, et al: Radioimmunotherapy of small-volume disease of
metastatic colorectal cancer. Cancer. 94(Suppl): 1373–1381. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aarts F, Boerman OC, Sharkey RM, Hendriks
T, Chang CH, McBride WJ, Bleichrodt RP, Oyen WJ and Goldenberg DM:
Pretargeted radioimmunoscintigraphy in patients with primary
colorectal cancer using a bispecific anticarcinoembryonic antigen
CEA X anti-di-diethylenetriaminepentaacetic acid F(ab′)2 antibody.
Cancer. 116(Suppl): 1111–1117. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mizejewski GJ: Biological role of
alpha-fetoprotein in cancer: Prospects for anticancer therapy.
Expert Rev Anticancer Ther. 2:709–735. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li MS, Li PF, Yang FY, He SP, Du GG and Li
G: The intracellular mechanism of alpha-fetoprotein promoting the
proliferation of NIH 3T3 cells. Cell Res. 12:151–156. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li MS, Li PF, He SP, Du GG and Li G: The
promoting molecular mechanism of alpha-fetoprotein on the growth of
human hepatoma Bel7402 cell line. World J Gastroenterol. 8:469–475.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li P, Wang SS, Liu H, Li N, McNutt MA, Li
G and Ding HG: Elevated serum alpha fetoprotein levels promote
pathological progression of hepatocellular carcinoma. World J
Gastroenterol. 17:4563–4571. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Moro R, Gulyaeva-Tcherkassova J and
Stieber P: Increased alpha-fetoprotein receptor in the serum of
patients with early-stage breast cancer. Curr Oncol. 19:e1–e8.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S, Jiang W, Chen X, Zhang C, Li H,
Hou W, Liu Z, McNutt MA, Lu F and Li G: Alpha-fetoprotein acts as a
novel signal molecule and mediates transcription of Fn14 in human
hepatocellular carcinoma. J Hepatol. 57:322–329. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhu M, Lin B, Zhou P and Li M: Molecular
analysis of AFP and HSA interactions with PTEN potein. BioMed Res
Int. 2015:2569162015. View Article : Google Scholar
|
|
31
|
Mizejewski GJ: Nonsecreted cytoplasmic
alpha-fetoprotein: A newly discovered role in intracellular
signaling and regulation. An update and commentary. Tumour Biol.
36:9857–9864. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li M, Li H, Li C, Wang S, Jiang W, Liu Z,
Zhou S, Liu X, McNutt MA and Li G: Alpha-fetoprotein: A new member
of intracellular signal molecules in regulation of the PI3K/AKT
signaling in human hepatoma cell lines. Int J Cancer. 128:524–532.
2011. View Article : Google Scholar
|
|
33
|
Gao R, Cai C, Gan J, Yang X, Shuang Z, Liu
M, Li S and Tang H: miR-1236 down-regulates alpha-fetoprotein, thus
causing PTEN accumulation, which inhibits the PI3K/Akt pathway and
malignant phenotype in hepatoma cells. Oncotarget. 6:6014–6028.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu M, Guo J, Li W, Lu Y, Fu S, Xie X, Xia
H, Dong X, Chen Y, Quan M, et al: Hepatitis B virus X protein
induces expression of alpha-fetoprotein and activates PI3K/mTOR
signaling pathway in liver cells. Oncotarget. 6:12196–12208. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Su R, Nan H, Guo H, Ruan Z, Jiang L, Song
Y and Nan K: Associations of components of PTEN/AKT/mTOR pathway
with cancer stem cell markers and prognostic value of these
biomarkers in hepatocellular carcinoma. Hepatol Res. 46:1380–1391.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhu M, Guo J, Xia H, Li W, Lu Y, Dong X,
Chen Y, Xie X, Fu S and Li M: Alpha-fetoprotein activates AKT/mTOR
signaling to promote CXCR4 expression and migration of hepatoma
cells. Oncoscience. 2:59–70. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ji X, Shen Y, Sun H and Gao X: A novel
anti-alpha-fetoprotein single-chain variable fragment displays
anti-tumor effects in HepG2 cells as a single agent or in
combination with paclitaxel. Tumour Biol. 37:10085–10096. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Matsumoto M, Matsuura T, Aoki K, Maehashi
H, Iwamoto T, Ohkawa K, Yoshida K, Yanaga K and Takada K: An
efficient system for secretory production of fibrinogen using a
hepatocellular carcinoma cell line. Hepatol Res. 45:315–325. 2015.
View Article : Google Scholar
|
|
39
|
Nakabayashi H, Taketa K, Miyano K, Yamane
T and Sato J: Growth of human hepatoma cells lines with
differentiated functions in chemically defined medium. Cancer Res.
42:3858–3863. 1982.PubMed/NCBI
|
|
40
|
Ohkawa K, Tsukada Y, Murae M, Kimura E,
Takada K, Abe T, Terashima Y and Mitani K: Serum levels and
biochemical characteristics of human ovarian carcinoma-associated
antigen defined by murine monoclonal antibody, CF511. Br J Cancer.
60:953–960. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baumann H and Doyle D: Metabolic fate of
cell surface glycoproteins during immunoglobulin-induced
internalization. Cell. 21:897–907. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Press OW, Hansen JA, Farr A and Martin PJ:
Endocytosis and degradation of murine anti-human CD3 monoclonal
antibodies by normal and malignant T-lymphocytes. Cancer Res.
48:2249–2257. 1988.PubMed/NCBI
|
|
43
|
Kyriakos RJ, Shih LB, Ong GL, Patel K,
Goldenberg DM and Mattes MJ: The fate of antibodies bound to the
surface of tumor cells in vitro. Cancer Res. 52:835–842.
1992.PubMed/NCBI
|
|
44
|
McEwan WA, Tam JC, Watkinson RE, Bidgood
SR, Mallery DL and James LC: Intracellular antibody-bound pathogens
stimulate immune signaling via the Fc receptor TRIM21. Nat Immunol.
14:327–336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Watkinson RE, McEwan WA and James LC:
Intracellular antibody immunity. J Clin Immunol. 34(Suppl 1):
S30–S34. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yoshikawa M, Mukai Y, Okada Y, Tsumori Y,
Tsunoda S, Tsutsumi Y, Aird WC, Yoshioka Y, Okada N, Doi T, et al:
Robo4 is an effective tumor endothelial marker for antibody-drug
conjugates based on the rapid isolation of the anti-Robo4
cell-internalizing antibody. Blood. 121:2804–2813. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ha KD, Bidlingmaier SM, Su Y, Lee NK and
Liu B: Identification of novel macropinocytosing human antibodies
by phage display and high-content analysis. Methods Enzymol.
585:91–110. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Miura K, Law SW, Nishi S and Tamaoki T:
Isolation of alphafetoprotein messenger RNA from mouse yolk sac. J
Biol Chem. 254:5515–5521. 1979.PubMed/NCBI
|
|
49
|
Zhou BP, Liao Y, Xia W, Spohn B, Lee MH
and Hung MC: Cytoplasmic localization of p21Cip1/WAF1 by
Akt-induced phosphorylation in HER-2/neu-overexpressing cells. Nat
Cell Biol. 3:245–252. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chung JH and Eng C: Nuclear-cytoplasmic
partitioning of phosphatase and tensin homologue deleted on
chromosome 10 (PTEN) differentially regulates the cell cycle and
apoptosis. Cancer Res. 65:8096–8100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chung JH, Ginn-Pease ME and Eng C:
Phosphatase and tensin homologue deleted on chromosome 10 (PTEN)
has nuclear localization signal-like sequences for nuclear import
mediated by major vault protein. Cancer Res. 65:4108–4116. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chung JH, Ostrowski MC, Romigh T,
Minaguchi T, Waite KA and Eng C: The ERK1/2 pathway modulates
nuclear PTEN-mediated cell cycle arrest by cyclin D1
transcriptional regulation. Hum Mol Genet. 15:2553–2559. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Gil A, Andrés-Pons A, Fernández E,
Valiente M, Torres J, Cervera J and Pulido R: Nuclear localization
of PTEN by a Ran-dependent mechanism enhances apoptosis:
Involvement of an N-terminal nuclear localization domain and
multiple nuclear exclusion motifs. Mol Biol Cell. 17:4002–4013.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Al-Khouri AM, Ma Y, Togo SH, Williams S
and Mustelin T: Cooperative phosphorylation of the tumor suppressor
phosphatase and tensin homologue (PTEN) by casein kinases and
glycogen synthase kinase 3beta. J Biol Chem. 280:35195–35202. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Tamguney T and Stokoe D: New insights into
PTEN. J Cell Sci. 120:4071–4079. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Georgescu MM, Kirsch KH, Akagi T, Shishido
T and Hanafusa H: The tumor-suppressor activity of PTEN is
regulated by its carboxyl-terminal region. Proc Natl Acad Sci USA.
96:10182–10187. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Tolkacheva T and Chan AM: Inhibition of
H-Ras transformation by the PTEN/MMAC1/TEP1 tumor suppressor gene.
Oncogene. 19:680–689. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Maccario H, Perera NM, Davidson L, Downes
CP and Leslie NR: PTEN is destabilized by phosphorylation on
Thr366. Biochem J. 405:439–444. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Torres J and Pulido R: The tumor
suppressor PTEN is phosphorylated by the protein kinase CK2 at its
C terminus. Implications for PTEN stability to proteasome-mediated
degradation. J Biol Chem. 276:993–998. 2001. View Article : Google Scholar
|
|
60
|
Milella M, Falcone I, Conciatori F, Cesta
Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S,
Cognetti F, et al: PTEN: Multiple functions in human malignant
tumors. Front Oncol. 5:242015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Vazquez F, Ramaswamy S, Nakamura N and
Sellers WR: Phosphorylation of the PTEN tail regulates protein
stability and function. Mol Cell Biol. 20:5010–5018. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Birle D, Bottini N, Williams S, Huynh H,
deBelle I, Adamson E and Mustelin T: Negative feedback regulation
of the tumor suppressor PTEN by phosphoinositide-induced serine
phosphorylation. J Immunol. 169:286–291. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Okahara F, Ikawa H, Kanaho Y and Maehama
T: Regulation of PTEN phosphorylation and stability by a tumor
suppressor candidate protein. J Biol Chem. 279:45300–45303. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Okahara F, Itoh K, Nakagawara A, Murakami
M, Kanaho Y and Maehama T: Critical role of PICT-1, a tumor
suppressor candidate, in phosphatidylinositol 3,4,5-trisphosphate
signals and tumorigenic transformation. Mol Biol Cell.
17:4888–4895. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Doble BW and Woodgett JR: GSK-3: Tricks of
the trade for a multi-tasking kinase. J Cell Sci. 116:1175–1186.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Saini MK and Sanyal SN: PTEN regulates
apoptotic cell death through PI3-K/Akt/GSK3p signaling pathway in
DMH induced early colon carcinogenesis in rat. Exp Mol Pathol.
93:135–146. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Tibarewal P, Zilidis G, Spinelli L,
Schurch N, Maccario H, Gray A, Perera NM, Davidson L, Barton GJ and
Leslie NR: PTEN protein phosphatase activity correlates with
control of gene expression and invasion, a tumor-suppressing
phenotype, but not with AKT activity. Sci Signal. 5:ra182012.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Freeman DJ, Li AG, Wei G, Li HH, Kertesz
N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, et
al: PTEN tumor suppressor regulates p53 protein levels and activity
through phosphatase-dependent and -independent mechanisms. Cancer
Cell. 3:117–130. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Li AG, Piluso LG, Cai X, Wei G, Sellers WR
and Liu X: Mechanistic insights into maintenance of high p53
acetylation by PTEN. Mol Cell. 23:575–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Hupp TR, Meek DW, Midgley CA and Lane DP:
Regulation of the specific DNA binding function of p53. Cell.
71:875–886. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sakaguchi K, Sakamoto H, Lewis MS,
Anderson CW, Erickson JW, Appella E and Xie D: Phosphorylation of
serine 392 stabilizes the tetramer formation of tumor suppressor
protein p53. Biochemistry. 36:10117–10124. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Shieh SY, Taya Y and Prives C: DNA
damage-inducible phosphorylation of p53 at N-terminal sites
including a novel site, Ser20, requires tetramerization. EMBO J.
18:1815–1823. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Oda K, Arakawa H, Tanaka T, Matsuda K,
Tanikawa C, Mori T, Nishimori H, Tamai K, Tokino T, Nakamura Y, et
al: p53AIP1, a potential mediator of p53-dependent apoptosis, and
its regulation by Ser-46-phosphorylated p53. Cell. 102:849–862.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Hirao A, Kong YY, Matsuoka S, Wakeham A,
Ruland J, Yoshida H, Liu D, Elledge SJ and Mak TW: DNA
damage-induced activation of p53 by the checkpoint kinase Chk2.
Science. 287:1824–1827. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Mayo LD and Donner DB: A
phosphatidylinositol 3-kinase/Akt pathway promotes translocation of
Mdm2 from the cytoplasm to the nucleus. Proc Natl Acad Sci USA.
98:11598–11603. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhou BP, Liao Y, Xia W, Zou Y, Spohn B and
Hung MC: HER-2/neu induces p53 ubiquitination via Akt-mediated MDM2
phosphorylation. Nat Cell Biol. 3:973–982. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Morani F, Phadngam S, Follo C, Titone R,
Aimaretti G, Galetto A, Alabiso O and Isidoro C: PTEN regulates
plasma membrane expression of glucose transporter 1 and glucose
uptake in thyroid cancer cells. J Mol Endocrinol. 53:247–258. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Samih N, Hovsepian S, Aouani A, Lombardo D
and Fayet G: Glut-1 translocation in FRTL-5 thyroid cells: Role of
phosphatidylinositol 3-kinase and N-glycosylation. Endocrinology.
141:4146–4155. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Hajduch E, Litherland GJ and Hundal HS:
Protein kinase B (PKB/Akt) - a key regulator of glucose transport?
FEBS Lett. 492:199–203. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Ciampi R, Vivaldi A, Romei C, Del Guerra
A, Salvadori P, Cosci B, Pinchera A and Elisei R: Expression
analysis of facilitative glucose transporters (GLUTs) in human
thyroid carcinoma cell lines and primary tumors. Mol Cell
Endocrinol. 291:57–62. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang CH, Wey KC, Mo LR, Chang KK, Lin RC
and Kuo JJ: Current trends and recent advances in diagnosis,
therapy, and prevention of hepatocellular carcinoma. Asian Pac J
Cancer Prev. 16:3595–3604. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Taketomi A: Clinical trials of
antiangiogenic therapy for hepatocellular carcinoma. Int J Clin
Oncol. 21:213–218. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Dhir M, Melin AA, Douaiher J, Lin C, Zhen
WK, Hussain SM, Geschwind JF, Doyle MB, Abou-Alfa GK and Are C: A
review and update of treatment options and controversies in the
management of hepatocellular carcinoma. Ann Surg. 263:1112–1125.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lin J, Wu L, Bai X, Xie Y, Wang A, Zhang
H, Yang X, Wan X, Lu X, Sang X, et al: Combination treatment
including targeted therapy for advanced hepatocellular carcinoma.
Oncotarget. 7:71036–71051. 2016.PubMed/NCBI
|
|
86
|
Nakata K, Muro T, Furukawa R, Kono K,
Kusumoto Y, Ishii N, Munehisa T, Koji T and Nagataki S: Presence of
immunoglobulin G in human sera binding to alphafetoprotein. Oncodev
Biol Med. 4:C101–C104. 1983.PubMed/NCBI
|
|
87
|
Asano T, Yamada N, Ochiai T, Sato H and
Fukao T: Presence of anti-AFP-antibody producing B cells in
peripheral blood lymphocyte of hepatocellular carcinoma patient.
Nihon Shokakibyo Gakkai Zasshi. 81:2781984.In Japanese.
|
|
88
|
Sassi F, Ayed K, el Gaied A and Dellagi K:
Presence of antialphafetoprotein immunoglobulin G in serum of a
patient with hepatocellular carcinoma. Gastroenterol Clin Biol.
15:661–662. 1991.in French.
|
|
89
|
Liu H, Zhang J, Wang S, Pang Z, Wang Z,
Zhou W and Wu M: Screening of autoantibodies as potential
biomarkers for hepatocellular carcinoma by using T7 phase display
system. Cancer Epidemiol. 36:82–88. 2012. View Article : Google Scholar
|
|
90
|
Negm OH, Hamed MR, Schoen RE, Whelan RL,
Steele RJ, Scholefield J, Dilnot EM, Shantha Kumara HM, Robertson
JF and Sewell HF: Human blood autoantibodies in the detection of
colorectal cancer. PLoS One. 11:e01569712016. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ura Y, Ochi Y, Hamazu M, Ishida M,
Nakajima K and Watanabe T: Studies on circulating antibody against
carcinoembryonic antigen (CEA) and CEA-like antigen in cancer
patients. Cancer Lett. 25:283–295. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Konstadoulakis MM, Syrigos KN,
Albanopoulos C, Mayers G and Golematis B: The presence of
anti-carcinoembryonic antigen (CEA) antibodies in the sera of
patients with gastrointestinal malignancies. J Clin Immunol.
14:310–313. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Haidopoulos D, Konstadoulakis MM,
Antonakis PT, Alexiou DG, Manouras AM, Katsaragakis SM and
Androulakis GF: Circulating anti-CEA antibodies in the sera of
patients with breast cancer. Eur J Surg Oncol. 26:742–746. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Ladd J, Lu H, Taylor AD, Goodell V, Disis
ML and Jiang S: Direct detection of carcinoembryonic antigen
autoantibodies in clinical human serum samples using a surface
plasmon resonance sensor. Colloids Surf B Biointerfaces. 70:1–6.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zhu M, Li W, Lu Y, Dong X, Lin B, Chen Y,
Zhang X, Guo J and Li M: HBx drives alpha fetoprotein expression to
promote initiation of liver cancer stem cells through activating
PI3K/AKT signal pathway. Int J Cancer. 140:1346–1355. 2017.
View Article : Google Scholar
|