1. Introduction
Glioblastoma multiforme (GBM) is the most common and
deadliest form of primary brain tumor. While current standard
treatment includes a rigorous regimen of surgery, radiotherapy, and
chemotherapy with temozolomide, its effectiveness remains low. The
median survival rate for the condition is only 14.6 months and the
two-year survival rate is a mere 30% (1,2).
Temozolomide is the only FDA-approved chemotherapy for primary GBM,
highlighting the need for better drugs to improve patient outcomes.
One possible reason for this observed treatment resistance is the
heterogeneity of GBM. Studies have shown that many types of cancers
including GBM contain a subpopulation of cells known as cancer stem
cells (CSCs) (3–6). CSCs are the only cells that have the
ability to proliferate, differentiate, and generate tumors. Because
of this, they are important to recurrence and metastasis. CSCs have
also been found resistant to radiation and chemotherapy (7,8).
Investigations on the susceptibility of glioblastoma cancer stem
cells (GSCs) to temozolomide have conflicting results, with some
finding depletion and others, enrichment (9–13).
These studies have also found a variety of genetic factors that
prevent temozolomide effectiveness on GBM including the methylation
status of the O(6)-methylguanine-DNA-methyltransferase
(MGMT) promoter, p53 mutations, and ATP-binding cassette (ABC)
transporters. In order to effectively treat glioblastoma, a drug
must be identified that can target GSCs and overcome these
mechanisms of resistance.
One emerging drug for targeting GSCs is salinomycin.
Salinomycin is an ionophore with a preference for Na+
and K+ that was isolated from Streptomyces albus
(14). Salinomycin has
antimicrobial activity and has been used since the 1980s as a
coccidiostat and growth promoter (15–18).
It was not until a 2009 study by Gupta et al that
salinomycin's selectivity for CSCs was elucidated (19). In this study, 16,000 compounds were
screened for their ability to kill breast cancer stem-like cells
created by the knockout of CDH1 with shRNA. This cell population
displayed all of the primary CSC characteristics including
tumorsphere formation, chemoresistance, CSC marker expression, and
tumor seeding ability. Salinomycin was found to kill these cells
with a greater than 10-fold lower IC50 as compared to
the non-knockout population and reduce tumor seeding ability in
mice by 100-fold. Since this initial identification of salinomycin
as having CSC potency, researchers have discovered effectiveness
against numerous other types of cancer stem cells including chronic
lymphocytic leukemia, prostate cancer, colorectal cancer, and lung
adenocarcinoma (20–24). Furthermore, unlike temozolomide,
salinomycin has been shown to act independently of p53 and is able
to overcome ABC transporters (25,26).
Salinomycin's mechanism of action however remains to be clearly
identified. While some researchers have found evidence of
apoptosis, others have indicated autophagy, and still others have
identified the mechanism as controlled necrosis (25–28).
This study focuses on salinomycin's potential to treat GBM
including its ability to reduce the CSC population, its toxicity to
normal brain cells, its mechanism of action, and its potential for
combination treatment.
2. Salinomycin's effect on GSCs
There are a variety of methods to test the effect of
salinomycin on GSCs. One method is comparing the potency of
salinomycin on cells that are enriched for GSCs to those that are
not. In 2015, Chen et al grew the GL261 GBM line in both
GSC-enriching neurosphere culture and differentiation-inducing
adherent culture (29). They found
the neurosphere culture cells to be much more sensitive to
salinomycin than the adherent culture cells, suggesting an
increased toxicity in GSCs. Using similar logic, Xipell et
al determined the viability of 18 different cell lines when
treated with salinomycin (30).
They compared the IC50 values of the neurosphere
cultures and adherent cultures and found the neurosphere cultures
had significantly lower IC50 dosages.
Another method to measure stemness is by determining
the ability of cells to form neurospheres in vitro. This is
known as a clonogenicity assay and has been conducted on
salinomycin treated GBM in studies by Chen et al in 2015,
Qin et al in 2015, and Xipell et al in 2016 (27,29,30).
The ability to form neurospheres is a mark of stemness, so
inhibiting this ability is evidence of a drug that targets GSCs.
Chen et al and Qin et al both demonstrated
salinomycin's ability to decrease clonogenicity (27,29).
Furthermore, Xipell et al tested multiple salinomycin
concentrations finding a dose-dependent decrease in neurosphere
formation (30).
A final criterion that has been employed to test the
effect of salinomycin on GSCs is gene expression. A variety of
markers have been used to identify GSCs including CD133, SOX2,
Nestin, and Musashi-1 (5,31–33).
However, only one study has investigated the effect of salinomycin
on these GSC markers. Xipell et al used qRT-PCR to show that
an unspecified concentration of salinomycin resulted in decreased
express of the GSC markers Musashi, Sox2, and Nestin in one cell
line (30).
Together these three indicators provide evidence of
salinomycin's ability to kill GSCs. In order to confirm this
hypothesis though, more data are needed. GSC marker gene expression
should be repeated with additional cell lines. The effect of
salinomycin on GSC markers should also be assessed using
alternative methods such as flow cytometry and western blotting.
Most importantly, salinomycin's ability to reduce GBM tumor seeding
ability in mice should be assessed as it was for breast cancer in
Gupta et al (19).
3. Normal neural cell toxicity
A potentially significant limitation to the use of
salinomycin for killing GSCs is its toxicity to normal neural
cells. An outbreak of toxic polyneuropathy in cats occurred in the
Netherlands in 1996 as a result of cat food contaminated with
salinomycin at a level of 13–21 ppm (34). Of the estimated 100,000 exposed
cats, 823 developed acute paralysis which began in the hindlimbs
and then, for some cats, progressed to the forelimbs. Morphological
findings included loss of axons and Schwann cell swelling.
Salinomycin toxicity has also been identified in dogs, horses, and
even humans (35–37). In 2004, a 35-year-old man working
in a factory making animal feed accidentally inhaled/ingested an
estimated 1 mg/kg body weight of salinomycin. He developed nausea,
shortness of breath, and dizziness within minutes and complained of
leg weakness once arriving at the hospital. The patient
subsequently developed rhabdomyolysis and was not able to be
discharged until 40 days after exposure (37).
In 2011, Boehmerle et al investigated this
neural cell toxicity using dorsal root ganglia neurons and Schwann
cells from mice (38). They found
significant viability reductions in both cell types when treated
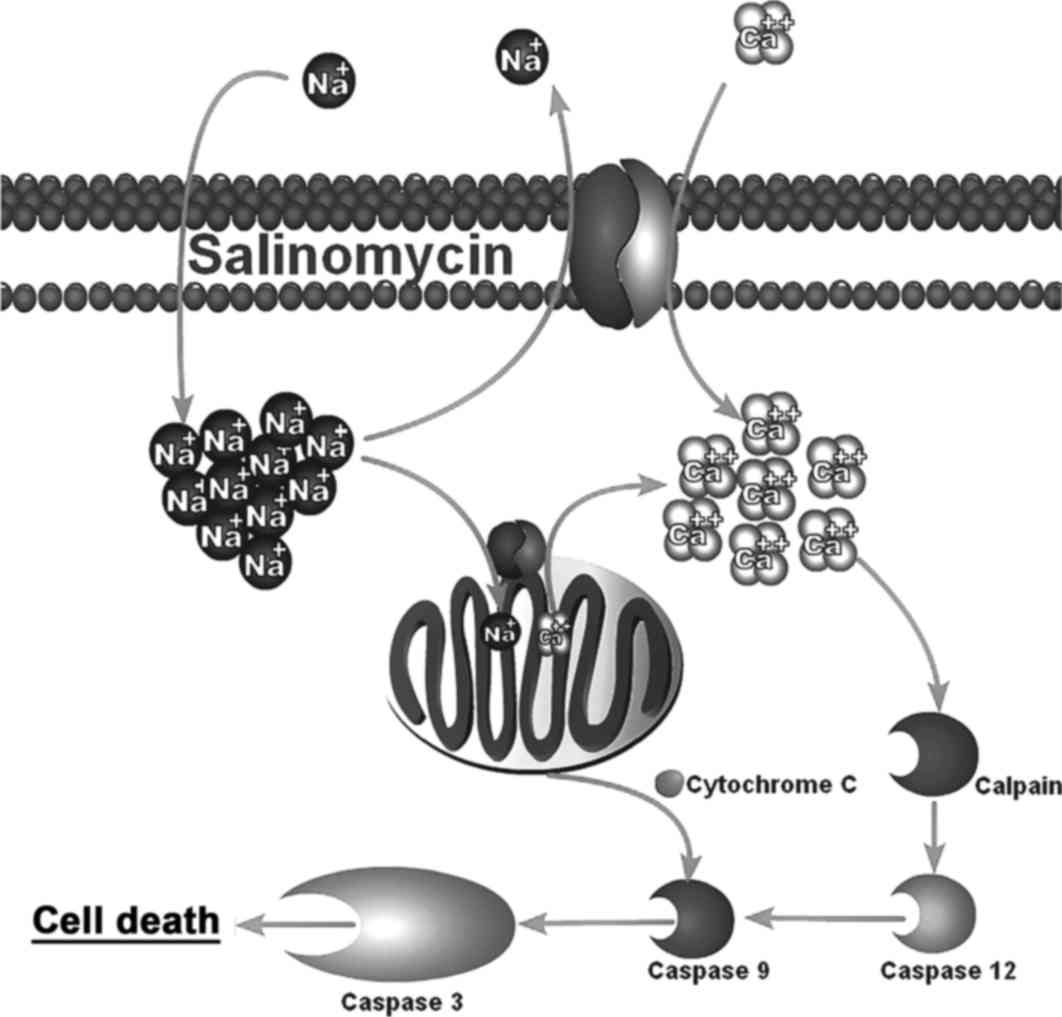
with salinomycin. They discovered this death was occurring via
apoptosis and is a result of salinomycin's action as a sodium
ionophore. Salinomycin causes an upregulation of intracellular
sodium which subsequently causes an intracellular calcium
upregulation due to reversal of the Na+/Ca2+
exchangers (39). This
intracellular calcium influx then leads to calpain and cytochrome
c-mediated apoptosis (38).
This mechanism is shown in Fig.
1.
In order for salinomycin to be utilized as a
clinically effective treatment against GBM, salinomycin must either
1) be more sensitive to calcium induced apoptosis than normal cells
or 2) act through a different mechanism that is specific to cancer
cells. In the former case, a dosage high enough to kill GSCs but
low enough to prevent neurotoxicity could be used to selectively
kill GSCs without harming normal cells. This potential selectivity
may be due to the presence of a greater concentration of
Na+/Ca2+ exchangers or a higher than normal
intracellular calcium concentration in GSCs. Calcium is a secondary
messenger in the Wnt pathway which has been found to be expressed
in GBM, providing a possible explanation (40,41).
If this is not the case, salinomycin may act through a different,
GSC-specific mechanism. Under this scenario, salinomycin could be
used as an effective treatment whether or not the salinomycin
concentration required to kill GSCs is less than the concentration
which kills normal cells. In the case where the required
salinomycin dose is low, salinomycin alone would be effective in
killing GSCs without causing normal cell toxicity. However, if the
salinomycin dose required to eliminate GSCs is similar to that
causing normal cell toxicity, salinomycin could be administered
along with a Na+/Ca2+ exchanger inhibitor.
This inhibitor would prevent salinomycin induced apoptosis of
normal neural cells while allowing salinomycin to kill GSC through
GSC-specific mechanisms. Therefore, understanding salinomycin's
mechanism of action is of great importance to understanding
salinomycin's safety and potential for clinical application.
4. Salinomycin's effect on CSC stemness
Studies on other types of cancer have demonstrated
salinomycin can overcome many characteristics that make CSCs
difficult to treat. ABC transporters export many drugs out of the
cell, thus decreasing their intracellular concentration and potency
(42,43). This allows CSCs to survive many
commonly administered chemotherapy agents. However, Fuchs et
al discovered salinomycin is able to overcome these
transporters and remain effective against leukemia stem cells
(25). Another important CSC
characteristic is invasiveness. CSCs are believed to play an
important role in metastasis, the deadliest cancer progression.
Multiple researchers have shown salinomycin decreases CSC
invasiveness and migration (44–47).
This physiologic change is associated with the FAK-ERK1/2 signaling
pathway in liver CSCs (44) and
the abolition of STAT3 and STAT1 interactions in colorectal CSCs
(45). Salinomycin has also been
shown to differentiate CSCs, transforming them from their
chemoresistant and tumorogenic state to a state that can be
eliminated by common chemotherapeutics (46,48).
While it is not known exactly how salinomycin modulates these CSC
characteristics, numerous studies have shown the drug interferes
with the Wnt, Notch, and Hedgehog signally pathways, all of which
are important for CSC maintenance (20,49–52).
5. Mechanism of action on GBM
The three primary regulated cell death pathways are
apoptosis, autophagic cell death, and necrosis (53,54).
Apoptosis can be triggered either extrinsically or intrinsically
and results in the activation of caspase-enzymes that breakdown the
cell in a controlled manner that does not negatively impact the
surrounding tissue (54–56). Autophagy on the other hand, is a
pathway that can have pro-survival or pro-death effects (54,57,58).
Autophagy can degrade organelles to provide energy for the cell,
but over activation can lead to cell death. Necrosis is thought to
be a more uncontrolled cell death which causes the release of cell
contents into the extracellular environment leading to
inflammation. However, recent studies have identified specific
necrotic pathways leading to the idea of controlled necrosis
(54,59). The effect of salinomycin on
indicators for these three pathways in GBM is discussed here and
summarized in Table I.
 | Table IEvidence for the three different cell
death pathways. |
Table I
Evidence for the three different cell
death pathways.
| Cell death
pathways | Evidence | Number of studies
| Refs. |
|---|
| Yes | No |
|---|
| Apoptosis | Annexin V/PI
Increase | 2 | 2 | (27,29,30,62) |
| Caspase-3
cleavage | 2 | 3 | (27,29,30,62,63) |
| Increased caspase-3
activity | 1 | 1 | (27,30) |
| Autophagy | p62
accumulation | 2 | 0 | (30,63) |
| LC3-II
upregulation | 2 | 0 | (30,63) |
| Programmed
necrosis | ROS increase | 3 | 0 | (27,30,63) |
| Mitochondrial PTP
opening | 3 | 0 | (27,30,62) |
Apoptosis
Evidence of salinomycin's ability to induce
apoptosis has been shown for leukemia, prostate cancer, breast
cancer, and ovarian cancer (21,22,60,61).
However, studies on GBM have not found such a consistent trend.
Common methods used to assess apoptosis include Annexin V flow
cytometry, pro-caspase-3 cleavage via western blotting, and
caspase-3/7 activity. Of the four studies that analyzed Annexin V
flow cytometry, Chen et al showed a 25% positive population
with salinomycin treatment, while the other three studies showed no
or minimal Annexin V staining (27,29,30,62).
Western blot analysis of pro-caspase-3 cleavage was detected in
Chen et al and Qin et al but not in Calzolari et
al, Booth et al, or Xipell et al (27,29,30,62,63).
Surprisingly, the amount of pro-caspase-3 cleavage detected in Chen
et al was greater for the lower salinomycin concentration,
calling into questing the validity of the data. Only Qin et
al and Xipell et al analyzed caspase-3/7 activity. While
Qin et al found an increase in activity, Xipell et al
found no difference (27,30). Though, Qin et al found the
cleavage of pro-caspase-3 and an increase in caspase-3/7 activity,
they found apoptosis was not the primary contributor to cell death
in their GBM cells. Only a small part of the cell death was
ameliorated using the apoptosis inhibitor zVADfmk (27). Together these data suggest
apoptosis may play a role but is not the main mechanism of cell
death of GBM. This is a promising conclusion for it suggests GBM
are killed using a different mechanism than normal neuron and
Schwann cells.
Autophagic cell death
The role of autophagy in salinomycin-induced GBM
death has not been investigated to as great a degree as apoptosis.
The studies that have looked into it though indicate it plays some
kind of role. Xipell et al found a larger number of acidic
vesicles in salinomycin treated cells and confirmed this finding
with transmission electron microscopy images showing an increase in
autophagosomes, autolysosomes, and lysosomes (30). Biochemically, they found an
increased conversion from LC3-I to LC3-II, a marker for
autophagosome synthesis (64).
They also, however, found increased p62 accumulation, suggesting
insufficient autolysosome degradation (30). Consistent with these findings,
Booth et al also found upregulation of LC3-II and p62 upon
treatment with salinomycin (63).
These results are similar to those obtained when GBM is treated
with the autophagy inhibitor Bafilomycin A1 (30). The combination of salinomycin and
Bafilomycin A1 amplified these effects (30). Lysosomal maturation was found to be
decreased by salinomycin as evidence by a decrease in the amount of
active cathepsin B, which requires a low lysosomal pH (30). This lack of lysosomal maturation
may be explained by the destabilization of Donnon potentials by
salinomycin as a result of its action as an ionophore for
Na+ and K+ (30). Xipell et al found ROS also
plays an interesting role in autophagy. The ROS inhibitor NAC
(N-Acetyl-cystein) reduced p62 accumulation without affecting
LC3-II upregulation in salinomycin treated cells (30). This suggests salinomycin induced
ROS may be an important cause of the aberrant autophagic
response.
Necrosis
While apoptosis and autophagy are affected to some
degree by salinomycin, the most compelling studies point to
necrosis as the predominant mechanism of salinomycin induced GBM
death. Xipell et al found salinomycin results in three of
the most common executioners of necrosis: low levels of
intracellular ATP, lysosome membrane permeability, and osmotic
swelling (30). Qin et al
showed a high salinomycin concentration causes over 40% of cells to
stain positive for PI but not Annexin V, indicating necrotic cell
death (27). Furthermore, Qin
et al found the general necrosis inhibitor Necrostatin-1 was
able to prevent most of the salinomycin-induced necrosis and
viability reduction (27). The
combination of Necrostatin-1 and the apoptosis inhibitor zVADfmk
eliminated virtually all salinomycin-induced death suggesting
necrosis and apoptosis are both active in salinomycin-induced cell
death with necrosis playing the larger role.
Though specific necrosis pathways are still being
understood, Vaseva et al identified a necrosis pathway in
2012 involving p53 opening of the mitochondrial permeability
transition pore (mPTP), known as the mitochondrial permeability
transition-driven regulated cell death pathway (MPT-driven RCD)
(53,65–67).
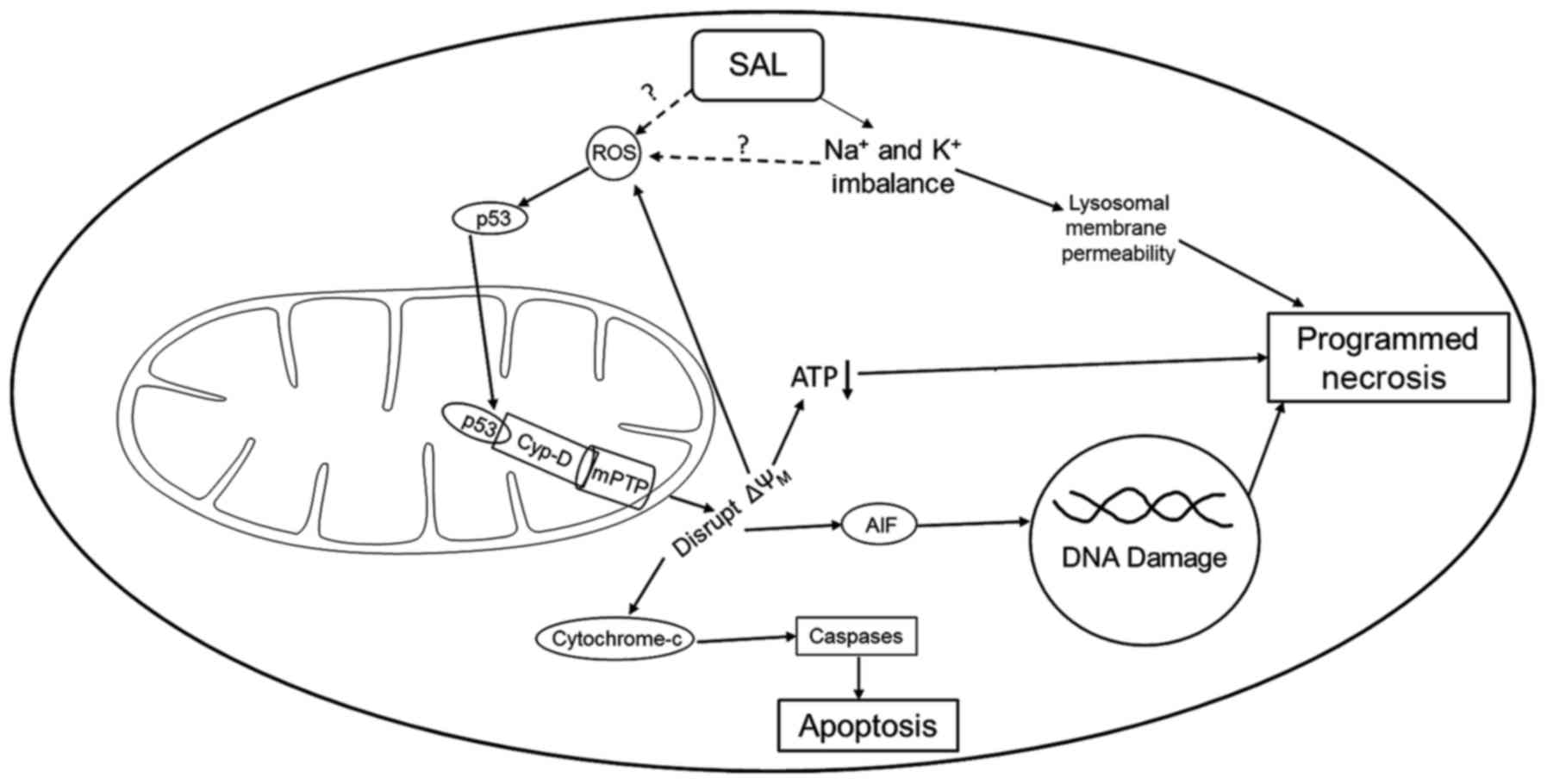
In this pathway, an increase in reactive oxygen species (ROS)
causes unphosphorylated p53 to migrate into the mitochondrial
matrix where it binds with cyclophilin D (Cyp-D, PPID) forming a
p53-CypD complex. This complex stimulates mPTP opening causing a
loss in the mitochondrial membrane potential and the release of
cytochrome c and apoptosis-inducing factor, leading to
necrosis. Three studies investigating salinomycin and GBM have
discovered that salinomycin increases ROS (27,30,63)
and three studies (two overlapping) have also found that
salinomycin causes a deterioration of the mitochondrial membrane
potential (27,30,62).
Unlike the inconsistency of the apoptosis indicators, there are no
studies that suggest salinomycin does not increase ROS or interfere
with the mitochondrial membrane potential. Qin et al
demonstrated in a step-wise manner that salinomycin-induced
necrosis proceeds through the same necrotic pathway as identified
by Vaseva et al (27,65).
By knocking out and then overexpressing Cyp-D, they showed its
necessity for salinomycin-induced necrosis. Knockdown of p53 also
prevented cell death and the formation of the p53-CypD complex
identified using western blot. ROS inhibition with
N-acetyl-L-cysteine reduced p53 translocation into the mitochondria
as well as mPTP opening, indicating the importance of ROS in this
pathway (27). The details of this
mechanism are shown in Fig. 2.
From an analysis of the current research on
salinomycin's effect against GBM, MTP-driven RCD appears to be the
primary mechanism of action with a small amount of death attributed
to apoptosis. This is a different mechanism than the
calcium-induced apoptosis that kills neurons and Schwann cells,
providing support for potential therapeutic applications in the
future (38). However, two
important questions remain: 1) how does salinomycin's structure or
action as an ionophore lead to ROS and 2) why is salinomycin
selective to GBM over normal tissue and GSCs specifically?
6. Combination therapy
Combination therapy is a method that can potentially
be used to reduce the concentration of salinomycin required. This
can help prevent the neuron and Schwann cell toxicity caused by
higher doses of the drug. Delwar et al used salinomycin in
combination with the alkylating chemotherapy agents temozolomide
(TMZ), carmustine (BCNU), and lomustine (CCNU) in a two-phase
treatment approach (68). Cells
were treated with one of the alkylating agents for three weeks and
then with a low concentration of salinomycin or vehicle control for
ten weeks. While the cells in the wells treated with vehicle
control remained alive and were able to regrow, the number of
surviving cells was drastically reduced in the wells treated with
salinomycin. Only 21% of TMZ/salinomycin treated wells contained
any live cells, suggesting combination therapy with TMZ and
salinomycin as a promising treatment for prolonging patient
survival (68).
Tumor necrosis factor-related apoptosis-inducing
ligand (TRAIL) and valproate have also been found effective against
GBM in combination with salinomycin (62,63).
TRAIL is a protein that binds to TRAIL receptor I or II in order to
induce apoptosis, usually in tumor cells (69,70).
Calzolari et al showed that combination of salinomycin and
TRAIL resulted in 5–10% GBM viability while salinomycin alone
resulted in 70% viability (62).
The combination therapy leads to apoptotic death indicated by
Annexin V/PI flow cytometry and pro-caspase-3 cleavage.
Interestingly, the apoptosis inhibitor zVAD was able to prevent the
significant viability reduction in only one of the three cell lines
examined. Salinomycin was found to upregulate TRAIL-R2 leading to
the increased potency of the combination (62). Valproate is a histone deacetylase
inhibitor commonly used to treat epilepsy and bipolar disorder
(71). When combined with
valproate, only a low salinomycin concentration was required to
induce significant cell death (63). Valproate reduced the autophagic
effects caused by salinomycin, preventing LC3-II and p62
accumulation. The combination resulted in an upregulation of ROS,
the suppression of which reduced cell death (63). Overexpression of the caspase-8
inhibitor c-FLIP-s and knockdown of the death receptor CD95 both
significantly reduced the combination's toxicity (63). The ability of TRAIL and valproate
to increase salinomycin's toxicity will allow a lower dose of
salinomycin to be used, leading to potential clinical benefits.
7. Conclusion
Glioblastoma multiforme is the most common and
deadliest form of primary brain tumor. It is heterogeneous in
nature, containing a subpopulation of cells known as GSCs which are
resistant to chemotherapy and radiation (1,2). In
order to effectively treat GBM, these cells must be eliminated.
Salinomycin has shown efficacy in treating other types of cancer
stem cells and the studies that have been conducted on GBM have
shown signs of GSC depletion including a greater sensitivity of
GSC-enriched cultures (29,30),
decreased neurosphere formation (27,29,30),
and decreased GSC markers detection via qRT-PCR (30). However, this evidence is
insufficient to prove that salinomycin targets GSCs. To confirm
this hypothesis, future research should look more extensively at
the effect of salinomycin on protein and gene expression as well as
examine the tumor-seeding ability of salinomycin-treated cells.
It is not only important that salinomycin can kill
GSCs, but also that it does not harm normal neural cells. Neural
toxicity has been shown in cats, dogs and even humans in case
studies (34,36,37).
In 2011, Boehmerle et al demonstrated that this mechanism of
death is through the calcium-induced apoptotic pathway and can be
prevented by Na+/Ca2+ exchanger (NCX)
inhibitors (38). In order for
salinomycin to still be a useful GBM treatment, GBM and GSCs
specifically must either 1) be more sensitive to calcium-induced
apoptosis or 2) be killed through a different, GSC-specific,
mechanism. This review found that salinomycin likely acts through a
GSC-specific mechanism, with most death caused by MPT-driven RCD,
with apoptosis playing a lesser role. To confirm this hypothesis,
GBM NCXs should be either inhibited or knocked out and salinomycin
toxicity determined. If salinomycin is able to still kill GSCs in
the absence of working NCXs, then combination treatment with
salinomycin and an NCX inhibitor is a promising future treatment
regimen for GBM. Salinomycin would kill GSCs specifically via
MPT-driven RCD while the NCX inhibitor would prevent salinomycin
from harming normal neurons through the calcium-induced apoptotic
pathway.
Acknowledgments
This material is based upon work supported by the
National Science Foundation under grant no. 1604677.
References
|
1
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al European Organisation for Research and Treatment of Cancer
Brain Tumor and Radiotherapy Groups; National Cancer Institute of
Canada Clinical Trials Group: Radiotherapy plus concomitant and
adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arvold ND and Reardon DA: Treatment
options and outcomes for glioblastoma in the elderly patient. Clin
Interv Aging. 9:357–367. 2014.PubMed/NCBI
|
|
3
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh SK, Clarke ID, Terasaki M, Bonn VE,
Hawkins C, Squire J and Dirks PB: Identification of a cancer stem
cell in human brain tumors. Cancer Res. 63:5821–5828.
2003.PubMed/NCBI
|
|
5
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, Henkelman RM, Cusimano MD and Dirks PB:
Identification of human brain tumour initiating cells. Nature.
432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galli R, Binda E, Orfanelli U, Cipelletti
B, Gritti A, De Vitis S, Fiocco R, Foroni C, Dimeco F and Vescovi
A: Isolation and characterization of tumorigenic, stem-like neural
precursors from human glioblastoma. Cancer Res. 64:7011–7021. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bao S, Wu Q, McLendon RE, Hao Y, Shi Q,
Hjelmeland AB, Dewhirst MW, Bigner DD and Rich JN: Glioma stem
cells promote radioresistance by preferential activation of the DNA
damage response. Nature. 444:756–760. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim Y, Joo KM, Jin J and Nam DH: Cancer
stem cells and their mechanism of chemo-radiation resistance. Int J
Stem Cells. 2:109–114. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Beier D, Röhrl S, Pillai DR, Schwarz S,
Kunz-Schughart LA, Leukel P, Proescholdt M, Brawanski A, Bogdahn U,
Trampe-Kieslich A, et al: Temozolomide preferentially depletes
cancer stem cells in glioblastoma. Cancer Res. 68:5706–5715. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mihaliak AM, Gilbert CA, Li L, Daou MC,
Moser RP, Reeves A, Cochran BH and Ross AH: Clinically relevant
doses of chemotherapy agents reversibly block formation of
glioblastoma neurospheres. Cancer Lett. 296:168–177. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ghods AJ, Irvin D, Liu G, Yuan X,
Abdulkadir IR, Tunici P, Konda B, Wachsmann-Hogiu S, Black KL and
Yu JS: Spheres isolated from 9L gliosarcoma rat cell line possess
chemoresistant and aggressive cancer stem-like cells. Stem Cells.
25:1645–1653. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eramo A, Ricci-Vitiani L, Zeuner A,
Pallini R, Lotti F, Sette G, Pilozzi E, Larocca LM, Peschle C and
De Maria R: Chemotherapy resistance of glioblastoma stem cells.
Cell Death Differ. 13:1238–1241. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Beier D, Schulz JB and Beier CP:
Chemoresistance of glioblastoma cancer stem cells - much more
complex than expected. Mol Cancer. 10:1282011. View Article : Google Scholar :
|
|
14
|
Mitani M, Yamanishi T and Miyazaki Y:
Salinomycin: A new monovalent cation ionophore. Biochem Biophys Res
Commun. 66:1231–1236. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Danforth HD, Ruff MD, Reid WM and Johnson
J: Anticoccidial activity of salinomycin in floor-pen experiments
with broilers. Poult Sci. 56:933–938. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou S, Wang F, Wong ET, Fonkem E, Hsieh
TC, Wu JM and Wu E: Salinomycin: A novel anti-cancer agent with
known anti-coccidial activities. Curr Med Chem. 20:4095–4101. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Callaway TR, Edrington TS, Rychlik JL,
Genovese KJ, Poole TL, Jung YS, Bischoff KM, Anderson RC and Nisbet
DJ: Ionophores: Their use as ruminant growth promotants and impact
on food safety. Curr Issues Intest Microbiol. 4:43–51.
2003.PubMed/NCBI
|
|
18
|
Lindemann MD, Kornegay ET, Stahly TS,
Cromwell GL, Easter RA, Kerr BJ and Lucas DM: The efficacy of
salinomycin as a growth promotant for swine from 9 to 97 kg. J Anim
Sci. 61:782–788. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gupta PB, Onder TT, Jiang G, Tao K,
Kuperwasser C, Weinberg RA and Lander ES: Identification of
selective inhibitors of cancer stem cells by high-throughput
screening. Cell. 138:645–659. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu D, Choi MY, Yu J, Castro JE, Kipps TJ
and Carson DA: Salinomycin inhibits Wnt signaling and selectively
induces apoptosis in chronic lymphocytic leukemia cells. Proc Natl
Acad Sci USA. 108:13253–13257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kim KY, Yu SN, Lee SY, Chun SS, Choi YL,
Park YM, Song CS, Chatterjee B and Ahn SC: Salinomycin-induced
apoptosis of human prostate cancer cells due to accumulated
reactive oxygen species and mitochondrial membrane depolarization.
Biochem Biophys Res Commun. 413:80–86. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dong TT, Zhou HM, Wang LL, Feng B, Lv B
and Zheng MH: Salinomycin selectively targets 'CD133+'
cell subpopulations and decreases malignant traits in colorectal
cancer lines. Ann Surg Oncol. 18:1797–1804. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y: Effects of salinomycin on cancer
stem cell in human lung adenocarcinoma A549 cells. Med Chem.
7:106–111. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kim YJ, Liu Y, Li S, Rohrs J, Zhang R,
Zhang X and Wang P: Co-eradication of breast cancer cells and
cancer stem cells by cross-linked multilamellar liposomes enhances
tumor treatment. Mol Pharm. 12:2811–2822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fuchs D, Daniel V, Sadeghi M, Opelz G and
Naujokat C: Salinomycin overcomes ABC transporter-mediated
multidrug and apoptosis resistance in human leukemia stem cell-like
KG-1a cells. Biochem Biophys Res Commun. 394:1098–1104. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fuchs D, Heinold A, Opelz G, Daniel V and
Naujokat C: Salinomycin induces apoptosis and overcomes apoptosis
resistance in human cancer cells. Biochem Biophys Res Commun.
390:743–749. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin LS, Jia PF, Zhang ZQ and Zhang SM:
ROS-p53-cyclophilin-D signaling mediates salinomycin-induced glioma
cell necrosis. J Exp Clin Cancer Res. 34:572015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Jangamreddy JR, Ghavami S, Grabarek J,
Kratz G, Wiechec E, Fredriksson BA, Rao Pariti RK, Cieślar-Pobuda
A, Panigrahi S and Łos MJ: Salinomycin induces activation of
autophagy, mitophagy and affects mitochondrial polarity:
Differences between primary and cancer cells. Biochim Biophys Acta.
1833:2057–2069. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen T, Yi L, Li F, Hu R, Hu S, Yin Y, Lan
C, Li Z, Fu C, Cao L, et al: Salinomycin inhibits the tumor growth
of glioma stem cells by selectively suppressing glioma-initiating
cells. Mol Med Rep. 11:2407–2412. 2015.
|
|
30
|
Xipell E, Gonzalez-Huarriz M, Martinez de
Irujo JJ, García-Garzón A, Lang FF, Jiang H, Fueyo J, Gomez-Manzano
C and Alonso MM: Salinomycin induced ROS results in abortive
autophagy and leads to regulated necrosis in glioblastoma.
Oncotarget. 7:30626–30641. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neradil J and Veselska R: Nestin as a
marker of cancer stem cells. Cancer Sci. 106:803–811. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song WS, Yang YP, Huang CS, Lu KH, Liu WH,
Wu WW, Lee YY, Lo WL, Lee SD, Chen YW, et al: Sox2, a stemness
gene, regulates tumor-initiating and drug-resistant properties in
CD133-positive glioblastoma stem cells. J Chin Med Assoc.
79:538–545. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lagadec C, Vlashi E, Frohnen P, Alhiyari
Y, Chan M and Pajonk F: The RNA-binding protein Musashi-1 regulates
proteasome subunit expression in breast cancer- and
glioma-initiating cells. Stem Cells. 32:135–144. 2014. View Article : Google Scholar :
|
|
34
|
van der Linde-Sipman JS, van den Ingh TS,
van nes JJ, Verhagen H, Kersten JG, Beynen AC and Plekkringa R:
Salinomycin-induced polyneuropathy in cats: Morphologic and
epidemiologic data. Vet Pathol. 36:152–156. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rollinson J, Taylor FG and Chesney J:
Salinomycin poisoning in horses. Vet Rec. 121:126–128. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Novilla MN, Owen NV and Todd GC: The
comparative toxicology of narasin in laboratory animals. Vet Hum
Toxicol. 36:318–323. 1994.PubMed/NCBI
|
|
37
|
Story P and Doube A: A case of human
poisoning by salinomycin, an agricultural antibiotic. N Z Med J.
117:U7992004.PubMed/NCBI
|
|
38
|
Boehmerle W and Endres M: Salinomycin
induces calpain and cytochrome c-mediated neuronal cell death. Cell
Death Dis. 2:e1682011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lattanzio FA Jr and Pressman BC:
Alterations in intracellular calcium activity and contractility of
isolated perfused rabbit hearts by ionophores and adrenergic
agents. Biochem Biophys Res Commun. 139:816–821. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yu JM, Jun ES and Jung JS, Suh SY, Han JY,
Kim JY, Kim KW and Jung JS: Role of Wnt5a in the proliferation of
human glioblastoma cells. Cancer Lett. 257:172–181. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
De A: Wnt/Ca2+ signaling
pathway: A brief overview. Acta Biochim Biophys Sin (Shanghai).
43:745–756. 2011. View Article : Google Scholar
|
|
42
|
An Y and Ongkeko WM: ABCG2: The key to
chemoresistance in cancer stem cells? Expert Opin Drug Metab
Toxicol. 5:1529–1542. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dean M: ABC transporters, drug resistance,
and cancer stem cells. J Mammary Gland Biol Neoplasia. 14:3–9.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sun J, Luo Q, Liu L, Yang X, Zhu S and
Song G: Salinomycin attenuates liver cancer stem cell motility by
enhancing cell stiffness and increasing F-actin formation via the
FAK-ERK1/2 signalling pathway. Toxicology. 384:1–10. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chung SS, Adekoya D, Enenmoh I, Clarke O,
Wang P, Sarkyssian M, Wu Y and Vadgama JV: Salinomycin abolished
STAT3 and STAT1 interactions and reduced telomerase activity in
colorectal cancer cells. Anticancer Res. 37:445–453. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kuo SZ, Blair KJ, Rahimy E, Kiang A,
Abhold E, Fan JB, Wang-Rodriguez J, Altuna X and Ongkeko WM:
Salinomycin induces cell death and differentiation in head and neck
squamous cell carcinoma stem cells despite activation of
epithelial-mesenchymal transition and Akt. BMC Cancer. 12:5562012.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qu H, Ma B, Yuan HF, Wang ZY, Guo SJ and
Zhang J: Effect of salinomycin on metastasis and invasion of
bladder cancer cell line T24. Asian Pac J Trop Med. 8:578–582.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Beug H: Breast cancer stem cells:
Eradication by differentiation therapy? Cell. 138:623–625. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Klose J, Eissele J, Volz C, Schmitt S,
Ritter A, Ying S, Schmidt T, Heger U, Schneider M and Ulrich A:
Salinomycin inhibits metastatic colorectal cancer growth and
interferes with Wnt/β-catenin signaling in CD133(+) human
colorectal cancer cells. BMC Cancer. 16:8962016. View Article : Google Scholar
|
|
50
|
Lu Y, Ma W, Mao J, Yu X, Hou Z, Fan S,
Song B, Wang H, Li J, Kang L, et al: Salinomycin exerts anticancer
effects on human breast carcinoma MCF-7 cancer stem cells via
modulation of Hedgehog signaling. Chem Biol Interact. 228:100–107.
2015. View Article : Google Scholar
|
|
51
|
He M, Fu Y, Yan Y, Xiao Q, Wu H, Yao W,
Zhao H, Zhao L, Jiang Q, Yu Z, et al: The Hedgehog signalling
pathway mediates drug response of MCF-7 mammosphere cells in breast
cancer patients. Clin Sci (Lond). 129:809–822. 2015. View Article : Google Scholar
|
|
52
|
Takebe N, Harris PJ, Warren RQ and Ivy SP:
Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog
pathways. Nat Rev Clin Oncol. 8:97–106. 2011. View Article : Google Scholar
|
|
53
|
Galluzzi L, Bravo-San Pedro JM, Vitale I,
Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D,
Annicchiarico-Petruzzelli M, et al: Essential versus accessory
aspects of cell death: Recommendations of the NCCD 2015. Cell Death
Differ. 22:58–73. 2015. View Article : Google Scholar
|
|
54
|
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT,
Liu B and Bao JK: Programmed cell death pathways in cancer: A
review of apoptosis, autophagy and programmed necrosis. Cell
Prolif. 45:487–498. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Kerr JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ghobrial IM, Witzig TE and Adjei AA:
Targeting apoptosis pathways in cancer therapy. CA Cancer J Clin.
55:178–194. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Kundu M and Thompson CB: Autophagy: Basic
principles and relevance to disease. Annu Rev Pathol. 3:427–455.
2008. View Article : Google Scholar
|
|
58
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Galluzzi L and Kroemer G: Necroptosis: A
specialized pathway of programmed necrosis. Cell. 135:1161–1163.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Al Dhaheri Y, Attoub S, Arafat K, Abuqamar
S, Eid A, Al Faresi N and Iratni R: Salinomycin induces apoptosis
and senescence in breast cancer: Upregulation of p21,
downregulation of survivin and histone H3 and H4 hyperacetylation.
Biochim Biophys Acta. 1830:3121–3135. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kaplan F and Teksen F: Apoptotic effects
of salinomycin on human ovarian cancer cell line (OVCAR-3). Tumour
Biol. 37:3897–3903. 2016. View Article : Google Scholar
|
|
62
|
Calzolari A, Saulle E, De Angelis ML,
Pasquini L, Boe A, Pelacchi F, Ricci-Vitiani L, Baiocchi M and
Testa U: Salinomycin potentiates the cytotoxic effects of TRAIL on
glioblastoma cell lines. PLoS One. 9:e944382014. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Booth L, Roberts JL, Conley A,
Cruickshanks N, Ridder T, Grant S, Poklepovic A and Dent P: HDAC
inhibitors enhance the lethality of low dose salinomycin in
parental and stem-like GBM cells. Cancer Biol Ther. 15:305–316.
2014. View Article : Google Scholar :
|
|
64
|
Klionsky DJ, Abdelmohsen K, Abe A, Abedin
MJ, Abeliovich H, Acevedo Arozena A, Adachi H, Adams CM, Adams PD,
Adeli K, et al: Guidelines for the use and interpretation of assays
for monitoring autophagy (3rd edition). Autophagy. 12:1–222. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Vaseva AV, Marchenko ND, Ji K, Tsirka SE,
Holzmann S and Moll UM: p53 opens the mitochondrial permeability
transition pore to trigger necrosis. Cell. 149:1536–1548. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Bernardi P: The mitochondrial permeability
transition pore: A mystery solved? Front Physiol. 4:952013.
View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Szalai G, Krishnamurthy R and Hajnóczky G:
Apoptosis driven by IP(3)-linked mitochondrial calcium signals.
EMBO J. 18:6349–6361. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Delwar ZM, Avramidis D, Siden Å, Cruz M,
Paulsson K and Sebastian Yakisich J: Low concentration of
salinomycin prevents regrowth and partially depletes human glioma
cells surviving high concentrations of alkylating agents. Clin
Cancer Drugs. 1:72–77. 2014. View Article : Google Scholar
|
|
69
|
Wiley SR, Schooley K, Smolak PJ, Din WS,
Huang CP, Nicholl JK, Sutherland GR, Smith TD, Rauch C, Smith CA,
et al: Identification and characterization of a new member of the
TNF family that induces apoptosis. Immunity. 3:673–682. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pitti RM, Marsters SA, Ruppert S, Donahue
CJ, Moore A and Ashkenazi A: Induction of apoptosis by Apo-2
ligand, a new member of the tumor necrosis factor cytokine family.
J Biol Chem. 271:12687–12690. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Michaelis M, Doerr HW and Cinatl J Jr:
Valproic acid as anti-cancer drug. Curr Pharm Des. 13:3378–3393.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Tığlı Aydın RS, Kaynak G and
Gümüşderelioğlu M: Salinomycin encapsulated nanoparticles as a
targeting vehicle for glioblastoma cells. J Biomed Mater Res A.
104:455–464. 2016. View Article : Google Scholar
|