Introduction
Cancer of the oral cavity is one of the leading
causes of cancer-associated mortality in Taiwan; patients with oral
cancer generally have a poor prognosis and experience high rates of
mortality (1,2). In Taiwan, oral cancer is the fifth
leading cause of cancer-associated mortality, according to the 2016
annual report by the Ministry of Health and Welfare (Taiwan,
R.O.C.) (3). The primary risk
factors for oral cancer are betel nut chewing, smoking, alcohol
consumption, human papillomavirus infection, and cell inflammation
(1,4). Surgery, radiation therapy, and
chemotherapeutic drugs are currently the preferred methods of
treating oral cancer (4,5). Cisplatin, carboplatin,
5-fluorouracil, paclitaxel and docetaxel are the chemotherapeutic
agents currently used to treat patients with oral cancer (6–9);
however, they do not significantly improve survival rates.
Furthermore, the development of resistance to chemotherapeutic
reagents is a serious clinical issue (10,11);
therefore, novel and safe chemotherapeutic agents are required for
the effective treatment of oral cancer.
Natural products and phytochemicals found in plants
commonly used in Traditional Chinese Medicine have been
investigated for their possible efficacy against different types of
cancer. Some have exhibited anticancer activity and low toxicity
such that they may be potential candidates for treatment (12,13).
Stilbenes are a class of natural polyphenolic compounds found in
peanuts, berries, grapes, and red wine (13). Pterostilbene
(trans-3,5-dimethoxy-4-hydroxystilbene) is a stillbene that is a
structural derivative of resveratrol and is found in blueberries,
grapes, tree wood and Pterocarpus marsupium (14,15).
The pharmacological activities of pterostilbene mean that it may be
used treat different types of diseases, including cancer, diabetes,
inflammation and dyslipidemia (13–17).
Studies have also indicated that pterostilbene may exhibit
anticancer activity against various types of cancer, including
breast (18,19), pancreatic (20,21),
lung (22–24), prostate (25,26),
colorectal (27–29), bladder (30), gastric (31) and oral cancer (32–34),
as well as hepatocellular carcinoma (35,36)
and leukemia (37–40). The results of in vitro and
in vivo studies have demonstrated that pterostilbene induces
apoptotic and/or autophagic cell death (29,32,41,42).
Pterostilbene may induce autophagy and apoptosis by modulating the
activities of protein kinase B (AKT) and mitogen-activated protein
kinase in oral cancer SAS and OECM-1 cells (32). Furthermore, pterostilbene inhibits
the migration and invasion of SAS and OECM-1 cells by suppressing
the activity and expression of matrix metalloproteinase 2 (34). However, to the best of our
knowledge, the effects of pterostilbene on cisplatin-resistant oral
cancer have not yet been evaluated. The present study aimed to
investigate the potential anti-proliferative effects of
pterostilbene and the mechanisms by which it induces cell death and
suppresses multidrug resistance protein 1 (MDR1) expression in
cisplatin-resistant human oral cancer CAR cells.
Materials and methods
Reagents
Dulbecco's modified Eagle's medium (DMEM), fetal
bovine serum (FBS), L-glutamine and penicillin/streptomycin were
purchased from HyClone; GE Healthcare Life Sciences (Logan, UT,
USA). Acridine orange (AO), Hoechst 33342, LysoTracker Red DND-99,
trypsin-EDTA, the High Capacity cDNA Reverse Transcription kit,
Pierce bicinchoninic acid (BCA) protein assay kit and SYBR-Green
PCR Master mix were sourced from Thermo Fisher Scientific, Inc.
(Waltham, MA, USA). The Immobilon-P polyvinylidene difluoride
transfer membrane and Immobilon western chemiluminescent
horseradish peroxidase (HRP) substrate were purchased from EMD
Millipore (Billerica, MA, USA). Caspase-3 (cat. no. K106-100), -8
(cat. no. K113-100), and -9 (cat. no. K119-100) colorimetric assay
kits were obtained from R&D Systems, Inc. (Minneapolis, MN,
USA). All primary antibodies, as well as anti-mouse and anti-rabbit
immunoglobulin (Ig)G HRP-linked secondary antibodies were all
procured from GeneTex International Corporation (Hsinchu, Taiwan).
Pterostilbene, 3-methyladenine (3-MA), chloroquine (CQ),
carbobenzoxyvalyl-alanyl-aspartyl fluoromethyl ketone (Z-VAD-FMK),
monodansylcadaverin (MDC) and all other chemicals and reagents were
obtained from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany),
unless otherwise specified.
Cell culture
Cisplatin-resistant human oral cancer CAR cells were
established from the human oral cancer cell line CAL 27 (American
Type Culture Collection, Manassas, VA, USA) following a previously
reported method (43,44). CAR cells were then cultured in DMEM
containing 10% FBS, 1% penicillin/streptomycin (100 μg/ml
streptomycin and 100 U/ml penicillin), 2 mM L-glutamine and 80 μM
cisplatin. Cells were maintained at 37°C in a 5% CO2/95%
air humidified incubator. Prior to administration, pterostilbene
was dissolved in dimethyl sulfoxide (DMSO) and the final
concentration of DMSO was <0.5%. Control cells were exposed to
0.5% DMSO alone.
Cell viability assay and morphological
determination
CAR cells were seeded onto 96-well culture plates at
a density of 1×104 cells/well (100 μl/well) and
subsequently incubated with 5, 10, 25, 50, 75 and 100 μM
pterostilbene for 24, 48 and 72 h. The effect of pterostilbene on
cell viability was then determined using an MTT assay, as
previously described (45,46). For the inhibition assays, cells
were pretreated with autophagy inhibitors (10 mM 3-MA and 20
μM CQ) and 15 μM Z-VAD-FMK (a pan-caspase inhibitor)
for 1 h prior to exposure to 50 or 75 μM pterostilbene for
24 or 48 h. Following the exposure of cells to pterostilbene, MTT
(5 mg/ml) was dissolved in phosphate-buffered saline, and 10
μl MTT solution was added to each well at a final
concentration of 500 μg/ml for 2 h. The blue formazan
crystals were then dissolved in DMSO (100 μl/well) by
constant shaking for 10 min. The absorbance of each well was
measured using an enzyme-linked immunosorbent assay (ELISA) plate
reader at a test wavelength of 570 nm, with a reference wavelength
of 620 nm. Half maximal inhibitory concentrations (IC50)
were calculated using the improved Karber method, using the
following formula: (lgIC50 = Xm-I[P-(3-Pm-Pn)/4], where
Xm, lg(maximum dose); I, lg(maximum dose/adjacent dose); P, sum of
the positive reaction rates; Pm, maximum positive reaction rate;
and Pn, minimum positive reaction rate (47). For the examination of cell
morphology, cells were observed without any treatments (control),
observed following treatment with pterostilbene alone, treatment
with the autophagy inhibitors (10 mM 3-MA or 20 μM CQ)
alone, and following treatment with pterostilbene and each of the
autophagy inhibitors. Cells were incubated and subsequently
observed and photographed using a phase-contrast microscope at a
magnification of ×400.
Dynamic cell confluence assay
CAR cells (1×104 cells/well) were seeded
onto a 96-well plate and treated with 0, 25, 50, 75 or 100
μM pterostilbene. The cell confluence experiment was
conducted over 48 h with data collection every 2 h and was
monitored using the IncuCyte ZOOM System instrument (Essen
BioScience, Ann Arbor, MI, USA), as previously described (48,49).
Autophagy assays and Hoechst 33342
staining
CAR cells were plated onto sterile chamber slides
onto 10-cm tissue culture dishes at a density of 1×106
cells/plate. Cells were treated with 0, 25, 50 or 75 μM
pterostilbene for 24 h following pretreatment with or without 10 mM
3-MA or 20 μM CQ for 1 h. Cells were then fixed with 4%
paraformaldehyde on ice for 15 min and were individually probed
with either 1 μg/ml AO, 100 μM MDC, 1 μg/ml
LysoTracker Red or 1 μg/ml Hoechst 33342 for 15 min at room
temperature, as previously described (50,51).
Lysosomal activity in the pterostilbene-treated cells were
determined using the Magic Red Cathepsin B assay kit
(ImmunoChemistry Technologies, LLC, Bloomington, MN, USA) following
the manufacturer's protocol. Following staining, each slide was
mounted with coverslips, and photomicrographs of acidic vesicular
organelles (AVOs), autophagic vacuoles, lysosomal biogenesis, and
nuclei were taken using a fluorescence microscope.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
CAR cells (5×106) in T75 flasks were
treated with 0, 50 and 75 μM pterostilbene for 24 h. Total
RNA was then isolated using QIAGEN RNeasy Mini kit (Qiagen, Inc.,
Valencia, CA, USA), according to the manufacturer's instructions
and cDNA synthesis was completed using the High Capacity cDNA
Reverse Transcription kit. qPCR was performed under the following
conditions: 10 min at 95°C, 40 cycles of 15 sec at 95°C and 1 min
at 60°C. qPCR was performed using 2X SYBR-Green PCR Master mix and
200 nM forward and reverse primers for light chain 3 (LC3)-II,
autophagy-related gene (Atg)12 and MDR1. GAPDH was used as a
reference gene. The sequences of the primers used were as follows:
LC3-II, forward, 5′-CCGACCGCTGTAAGGAGGTA-3′ and reverse,
5′-AGGACGGGCAGCTGCTT-3′; Atg12, forward,
5′-TGTGGCCTCAGAACAGTTGTTTA-3′ and reverse,
5′-CGCCTGAGACTTGCAGTAATGT-3′; MDR1, forward,
5′-GTGTGGTGAGTCAGGAACCTGTAT-3′ and reverse,
5′-TCTCAATCTCATCCATGGTGACA-3′; GAPDH, forward,
5′-ACACCCACTCCTCCACCTTT-3′ and reverse,
5′-TAGCCAAATTCGTTGTCATACC-3′. Each assay was run on an Applied
Biosystems 7300 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.) in triplicate, and the fold-changes of
gene expression were derived using the comparative
2−ΔΔCq method, as previously described (50,52).
Western blot analysis
CAR cells at a density of 5×106 cells per
75T flask were incubated with 0, 50 and 75 μM pterostilbene
for 24 or 48 h. Cell samples were lysed in the Trident RIPA Lysis
Buffer (cat. no. GTX400005; GeneTex) and collected as previously
described (53,54). Protein concentration was determined
using the Pierce BCA protein assay kit. Equal amounts of the
protein sample (40 μg) were prepared and subsequently 10–12%
SDS-PAGE was performed. Proteins were transferred to the
Immobilon-P polyvinylidene difluoride transfer membrane prior to
blocking with Trident Universal Protein Blocking Reagent (cat. no.
GTX30963; GeneTex) for 1 h at room temperature. The membrane was
subsequently incubated with primary antibodies against Atg5 (cat.
no. GTX113309), Atg7 (cat. no. GTX113613), Atg12 (cat. no.
GTX629815), Beclin-1 (cat. no. GTX631396), LC3 (cat. no. GTX39752;
LC3-I, 17 kDa; LC3-II, 14 kDa), Bcl-2 (cat. no. GTX100064), Bax
(cat. no. GTX109683), cytochrome c (cat. no. GTX108585),
caspase-9 (cat. no. GTX112888; active form, 19 kDa), caspase-3
(cat. no. GTX110543; active form, 19 kDa), caspase-7 (cat. no.
GTX22301; active form, 20 kDa), poly (ADP-ribose) polymerase (PARP;
cat. no. GTX100573; p85, 85 kDa), MDR1 (cat. no. GTX108370),
phosphorylated (p)-AKT (Ser473) (cat. no. GTX28932), AKT (cat. no.
GTX121937) and β-actin (cat. no. GTX109639) at 4°C overnight. All
antibodies were purchased from GeneTex and used at a dilution of at
1:1,000. Membranes were then incubated with appropriate anti-mouse
(cat. no. GTX213111-01) and anti-rabbit (cat. no. GTX213110-01) IgG
HRP-linked secondary antibodies at a dilution of 1:10,000 for 1 h
at room temperature. Blot visualization was performed using the
Immobilon Western Chemiluminescent HRP Substrate and all bands of
immunoblots were normalized to the densitometric value of β-actin.
The bands were quantified by densitometry using ImageJ software
version 1.41 (National Institutes of Health, Bethesda, MA,
USA).
Quantification of cellular apoptosis and
DNA breaks
CAR cells (1×105 cells/ml) on 12-well
plates were harvested following treatment with 0, 25, 50, 75 and
100 μM pterostilbene for 48 h and re-suspended with the
In Situ Cell Death Detection kit, Fluorescein
(Sigma-Aldrich; Merck KGaA) to perform terminal deoxynucleotidyl
transferase-mediated d-UTP nick end labeling (TUNEL), following the
manufacturer's protocol. TUNEL-positive cells were then assessed
using a BD FACSCalibur Flow Cytometer (BD Biosciences, San Jose,
CA, USA) and the data were quantified using the BD CellQuest Pro
Software version 5.1 (BD Biosciences), as previously described
(55).
Assessment of caspase-3 and -9 activity
via colorimetric assays
CAR cells (5×106 cells/75T flask) were
exposed to 0, 25, 50, 75 and 100 μM pterostilbene for 48 h.
Cell lysates were harvested and the activities of caspases-3 and -9
were then measured using the caspase-3 and -9 colorimetric assay
kits, following the manufacturer's protocol.
Statistical analysis
All results are expressed as the mean ± standard
deviation of triplicate samples. Statistical analysis was performed
using SPSS software version 16.0 (SPSS, Inc., Chicago, IL, USA).
Differences among groups were determined using one-way analysis of
variance followed by Dunnett's test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Pterostilbene induces cytotoxicity in
cisplatin-resistant human oral cancer CAR cells
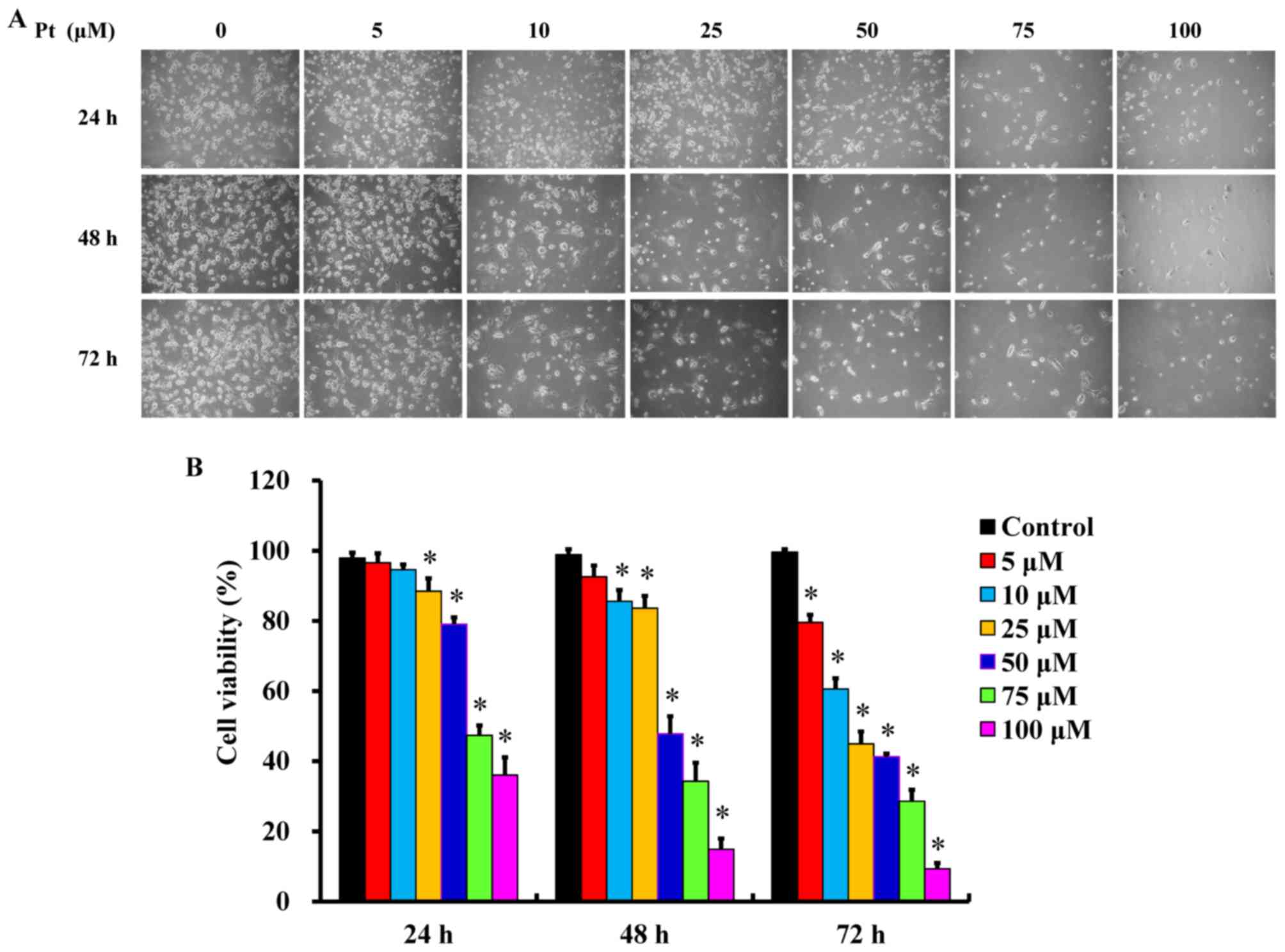
Following treatment with different concentrations
(5, 10, 25, 50, 75 and 100 μM) of pterostilbene for 24, 48
and 72 h, cells underwent apoptotic changes, including cell
shrinkage, membrane blebbing and rounding and acquire autophagic
characteristics (Fig. 1A).
Furthermore, pterostilbene treatment decreased the number of CAR
cells compared with the untreated control, as recorded by a
phase-contrast microscope. These effects occurred in a time- and
concertation-dependent manner (Fig.
1A). Furthermore, incubation with pterostilbene for 24, 48 and
72 h significantly decreased cell viability in a time- and
concentration-dependent manner (Fig.
1B). The IC50 values of pterostilbene in CAR cells
following 24, 48 and 72 h incubation were 78.26±4.33, 48.04±3.68
and 20.65±4.88 μM, respectively. Interestingly,
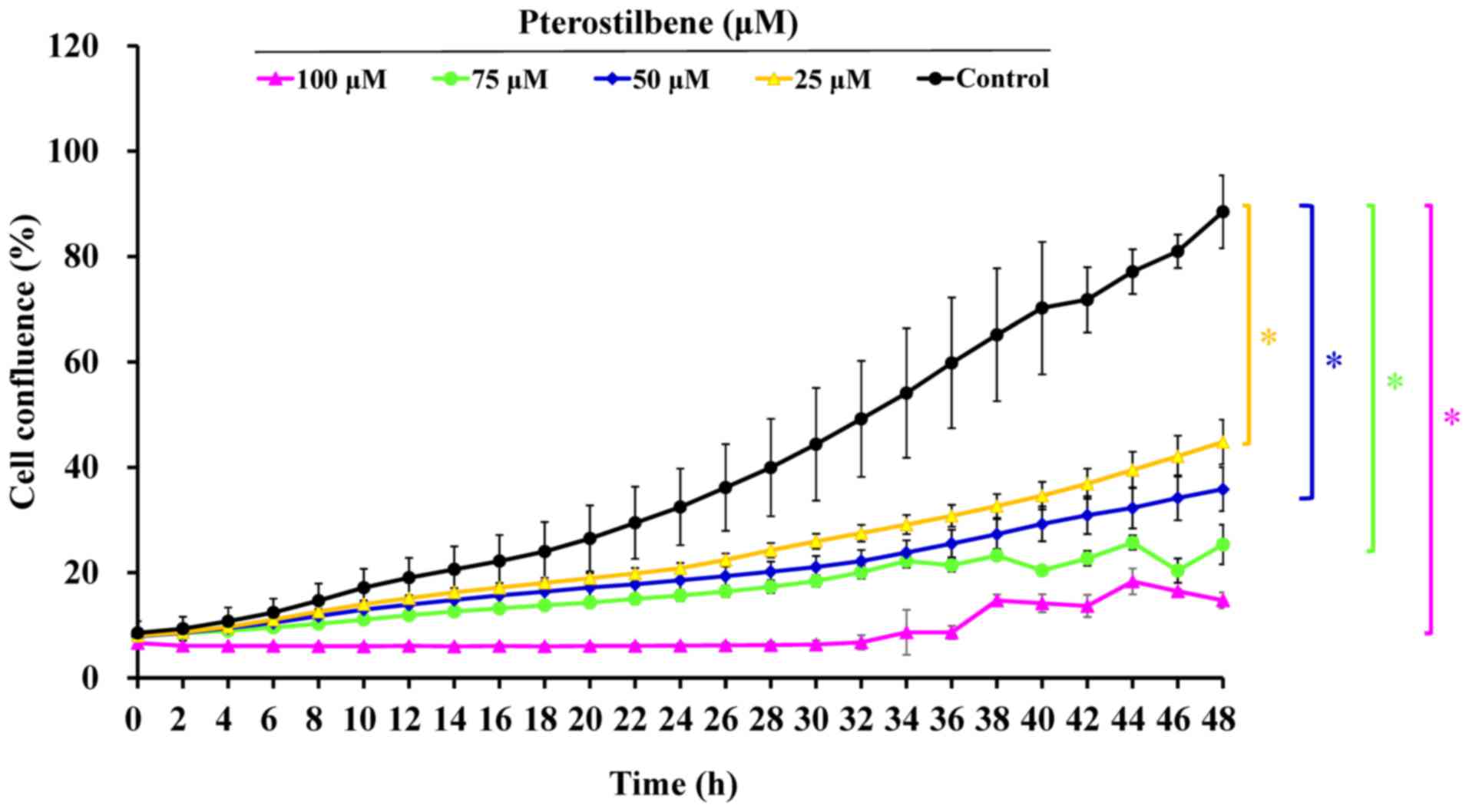
administration of 0, 25, 50, 75 and 100 μM pterostilbene
suppressed cell confluence over a 48-h period in a time- and
concentration-dependent manner (Fig.
2 and supplementary data: https://youtu.be/P7MCMTJnO00). Thus, pterostilbene may
induce CAR cell death via autophagy and apoptosis.
Pterostilbene triggers the autophagy and
apoptosis of CAR cells
To determine whether autophagic cell death is caused
by pterostilbene, AVOs, autophagosome vesicles or lysosome activity
were detected using different molecular probes, including AO, MDC,
LysoTracker Red and cathepsin B. AO and MDC staining indicated that
pterostilbene markedly increased the number of AVOs within the
cytoplasm compared with the untreated control (Fig. 3A and B). LysoTracker Red and
cathepsin B staining also indicated that treatment with
pterostilbene caused the accumulation of autophagic vacuole marker
and suppressed lysosome activity (Fig.
3C and D). In addition, increased DNA condensation occurred in
cells treated with 25, 50 and 75 μM pterostilbene for 24 h,
as indicated by Hoechst 33342 staining (Fig. 3E). These results demonstrate that
pterostilbene-elicited CAR cell death is mediated by autophagic and
apoptotic responses.
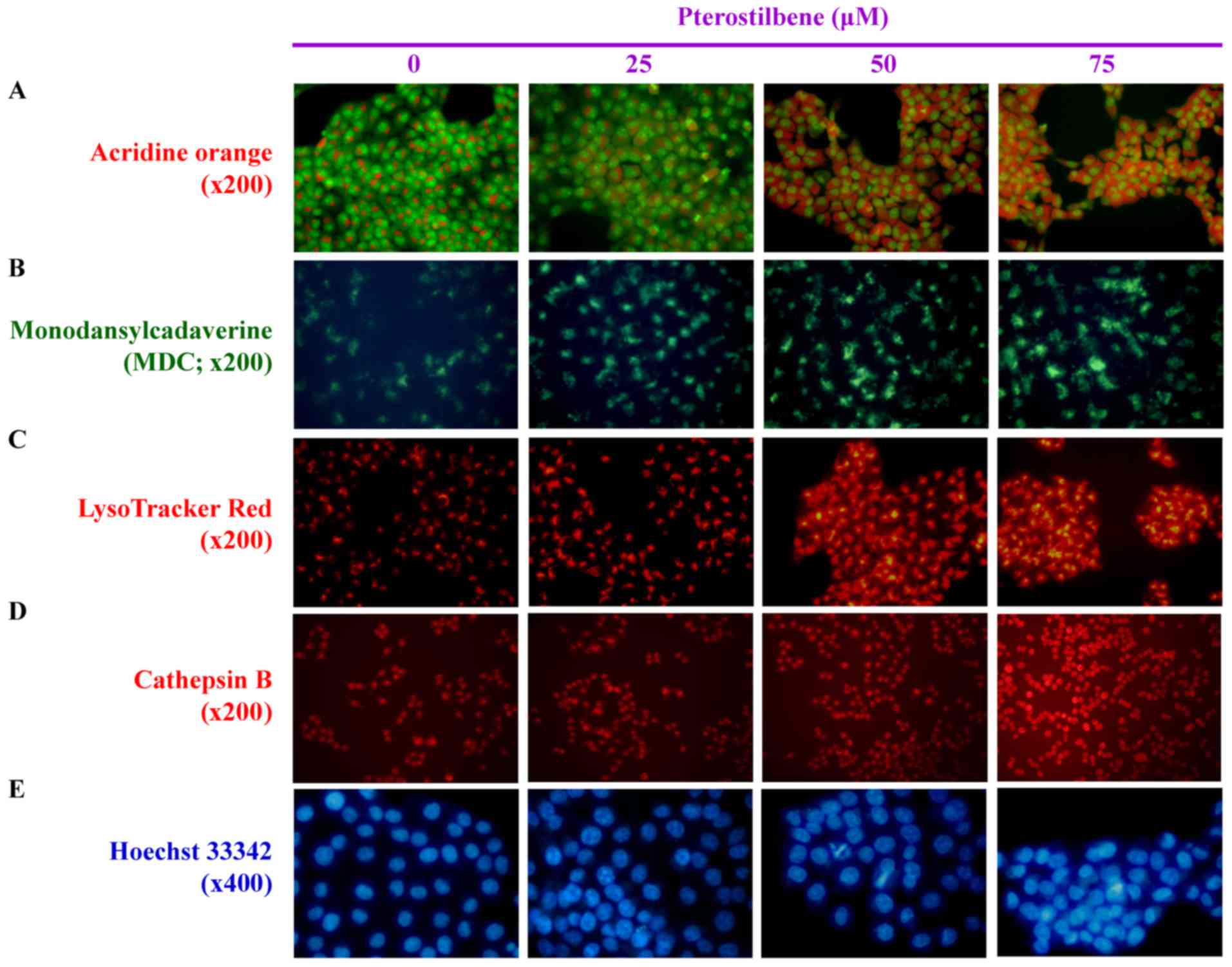 | Figure 3Effects of pterostilbene on the
autophagy and DNA condensation of CAR cells. The cells were treated
with 0, 25, 50 and 75 μM pterostilbene for 24 h and then
probed using (A) acridine orange to detect acidic vesicular
organelles, indicated by a red color (magnification, ×200). (B)
Monodansylcadaverin, an autophagolysosome marker, indicated by a
green color (magnification, ×200). (C) LysoTracker Red to determine
lysosomal function, indicated by a red color (magnification, ×200).
(D) Cathepsin B to detect lysosomal activity, indicated by a red
color (magnification, ×200). (E) Hoechst 33342 staining to observe
cell nuclei, as indicated by a blue color (magnification, ×400).
Representative images were taken from three independent
experiments. |
Pterostilbene induces the expression of
autophagy-associated genes and stimulates its signaling in CAR
cells
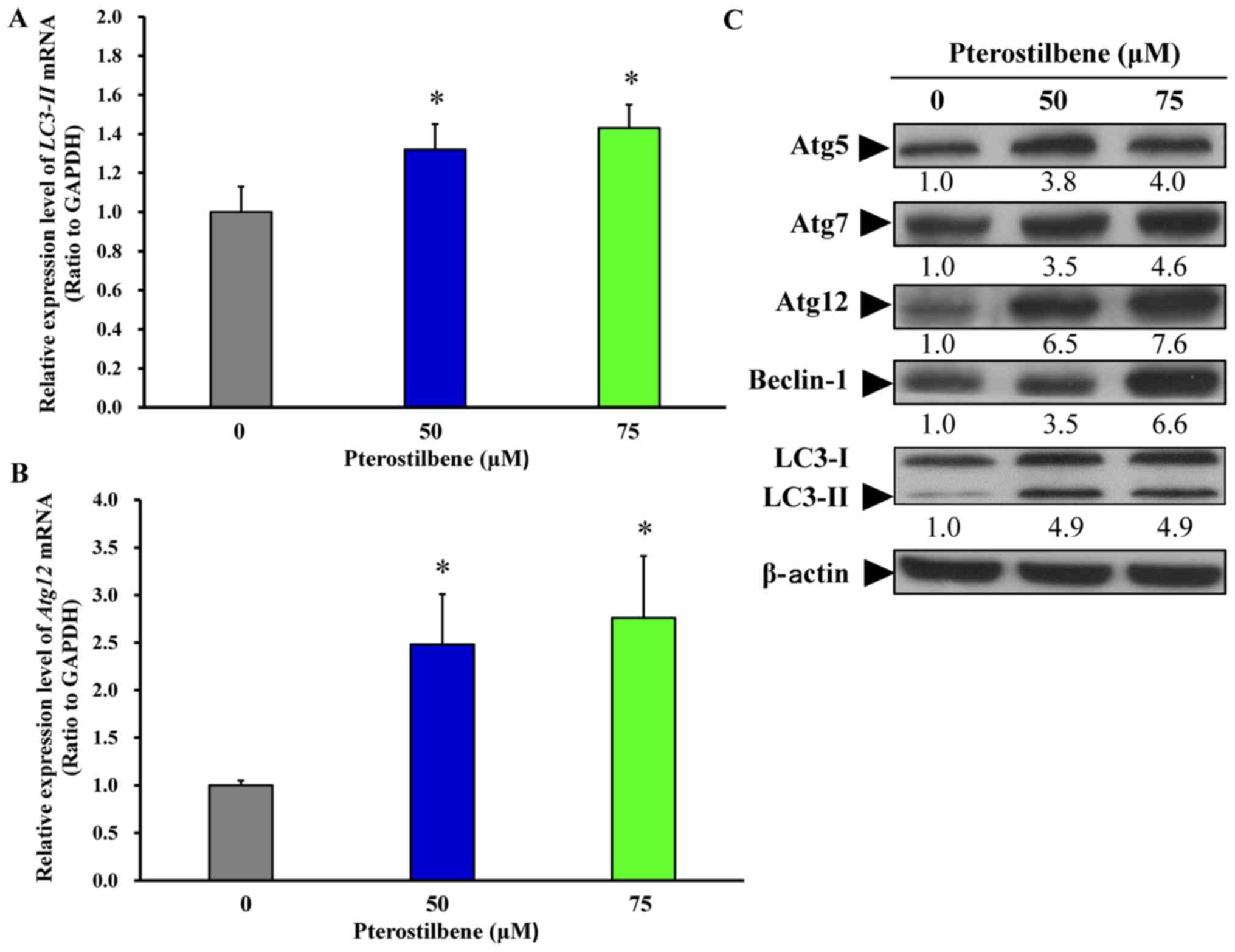
To further investigate the effect of pterostilbene
on autophagy, the gene and protein levels of key autophagic
regulators, including Atg5, Atg7, Atg12, Beclin-1 and LC3 were
assessed using RT-qPCR analysis and western blotting. Following
treatment of cells with 50 and 75 μM pterostilbene for 24 h,
there was a significant increase in the mRNA expression of
LC3-II (Fig. 4A) and
Atg12 (Fig. 4B). Treatment
with 50 and 75 μM pterostilbene also markedly increased the
protein expression of Atg5, Atg7, Atg12, Beclin-1 and LC3-II in CAR
cells. These results imply that pterostilbene provokes the
autophagy of CAR cells by increasing the expression of
Atg/Beclin-1/LC3-associated molecules.
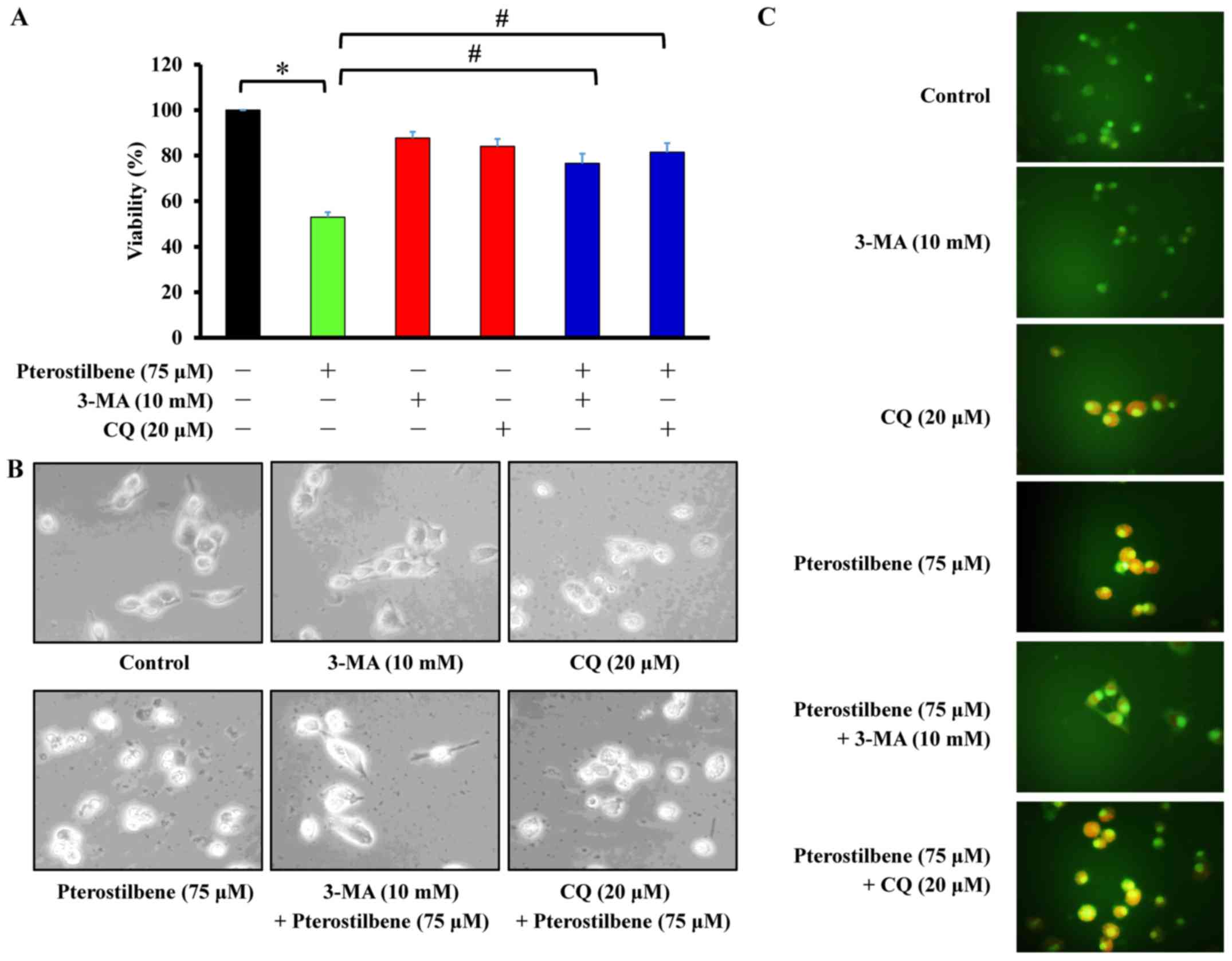
The autophagy inhibitors 3-MA and CQ
reverse the decrease in CAR cell viability induced by
pterostilbene
Cells were pretreated with 10 mM 3-MA or 20
μM CQ and subsequently exposed to 75 μM pterostilbene
for 24 h. Cell viability, autophagic characteristics and AVOs were
individually monitored using an MTT assay, microscopic examination
of cell morphology and staining with AO, followed by fluorescence
microscopy. The results demonstrated that 3-MA and CQ significantly
increased the viability of CAR cells following pterostilbene
treatment, compared with cells treated with pterostilbene alone
(Fig. 5A). Similarly, pretreatment
of CAR cells with 3-MA and CQ suppressed the development of
autophagic characteristics (Fig.
5B) and AVOs (Fig. 5C)
following exposure to pterostilbene. These results suggest that
pterostilbene-induced autophagy in CAR cells may be mediated by
phosphatidylinositol-4,5-bisphosphate 3-kinase class III
signaling.
Pterostilbene induces the apoptosis of
CAR cells via a caspase-dependent pathway
The effects of pterostilbene on cell apoptosis and
its underlying mechanism of action was subsequently assessed.
Following treatment with 25, 50, 75 and 100 μM pterostilbene
for 48 h, cells were detected for DNA breaks and direct apoptotic
responses. Pterostilbene significantly increased the number of
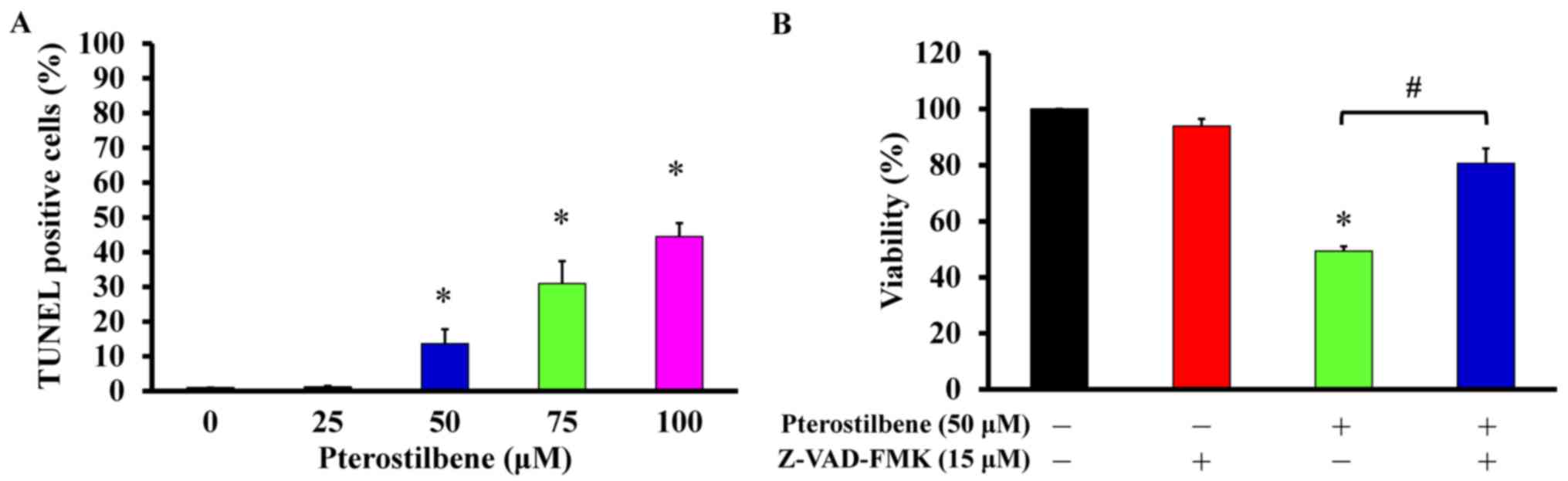
TUNEL-positive cells in a concentration-dependent manner (Fig. 6A), indicating that it induces
apoptosis. To determine if the caspase cascade contributes to this
pterostilbene-induced apoptosis, the pan-caspase inhibitor
Z-VAD-FMK was used to pretreat CAR cells prior to incubation with
50 μM pterostilbene. Z-VAD-FMK significantly reversed the
viability of cells treated with pterostilbene alone, by 32.6%
(Fig. 6B). These results indicate
that pterostilbene induces the apoptosis of CAR cells via
caspase-dependent signaling.
The mitochondria-dependent pathway
contributes to pterostilbene-induced apoptosis in CAR cells
Following the determination of the
apoptosis-inducing effect of pterostilbene on molecular signals,
its underlying mechanism of action was further investigated.
Treatment with 50, 75 and 100 μM pterostilbene for 48 h
significantly increased caspase-3 (Fig. 7A) and caspase-9 (Fig. 7B) activity in a
concentration-dependent manner, compared with untreated control
cells. Furthermore, there was no significant increase in caspase-8
activity (data not shown) following pterostilbene treatment. To
determine changes in the expression of mitochondria-modulated pro-
and anti-apoptotic proteins, levels of these proteins in
pterostilbene-treated cells were measured. The results demonstrated
that 50 and 75 μM pterostilbene upregulated the expression
of Bax, cytochrome c, the active forms of caspase-9,
caspase-3, caspase-7 and PARP, but it downregulated the expression
of Bcl-2 (Fig. 7C). These results
indicate that pterostilbene induces CAR cell death by activating
the intrinsic (caspase-9-and caspase-3-dependent) apoptotic
cascade.
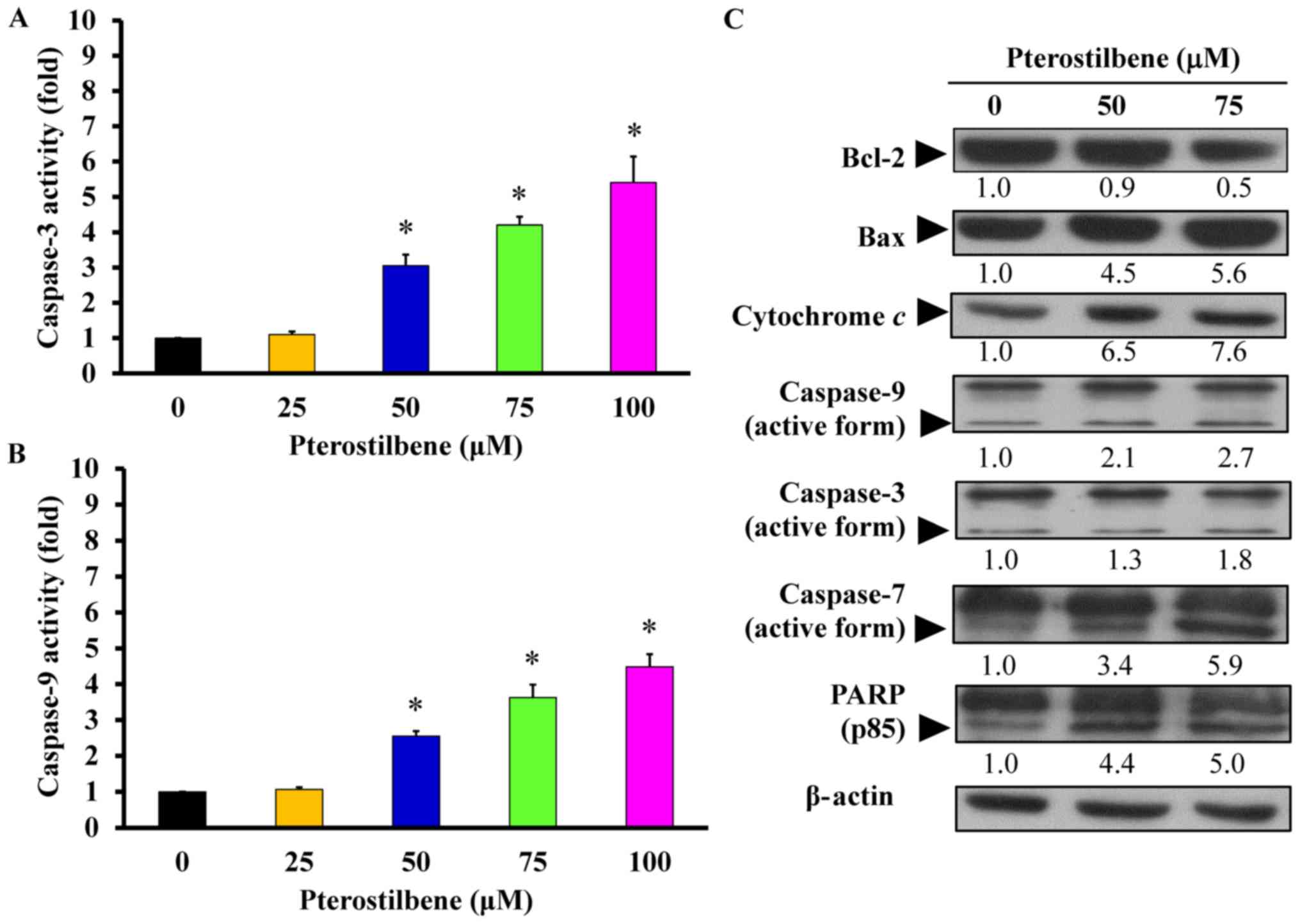 | Figure 7Effects of pterostilbene on the
mitochondria-dependent apoptotic signaling of CAR cells. Cells were
incubated with 0, 25, 50, 75 or 100 μM pterostilbene for 48
h, and whole-cell lysates were then harvested. (A) Caspase-3 and
(B) -9 activities were determined using a colorimetric assay.
Values represent the mean ± standard deviation of three independent
experiments. *P<0.05 vs. untreated control. (C) Cell
fractions were probed with anti-Bcl-2, anti-Bax, anti-cytochrome
c, anti-caspase-9, anti-caspase-3, anti-caspase-7 and
anti-PARP antibodies and assessed via western blotting.
Representative images were taken from three independent
experiments. PARP, Poly (ADP-ribose) polymerase. |
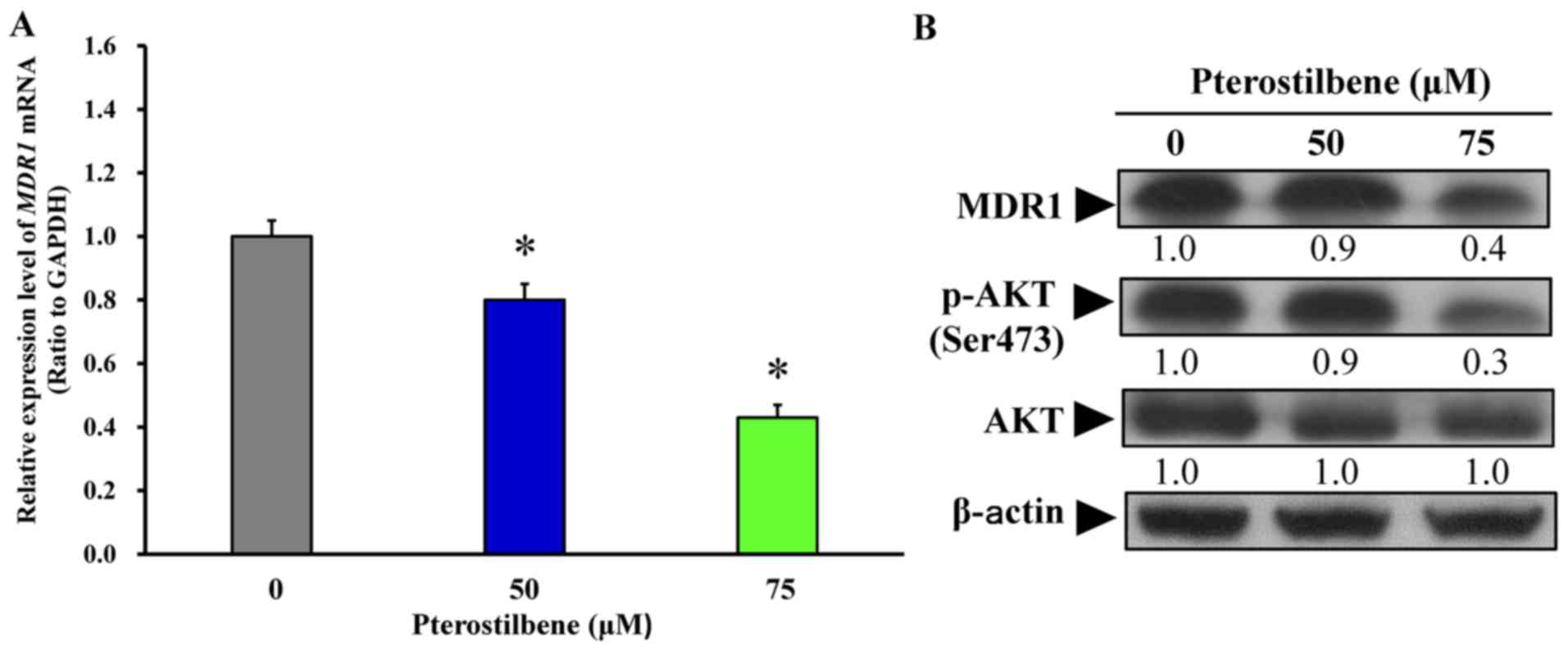
Pterostilbene suppresses the expression
of MDR1 and AKT signaling in CAR cells
Following treatment with 50 and 75 μM
pterostilbene for 24 h, the mRNA and protein expression of MDR1 was
monitored. Pterostilbene treatment significantly decreased the
expression of MDR1 mRNA (Fig. 8A)
and protein (Fig. 8B).
Furthermore, treatment with 50 and 75 μM pterostilbene
decreased the phosphorylation of AKT on the Ser473 site but had no
effect on total AKT protein expression (Fig. 8B). Collectively, these results
indicate that pterostilbene triggers the autophagy and apoptosis of
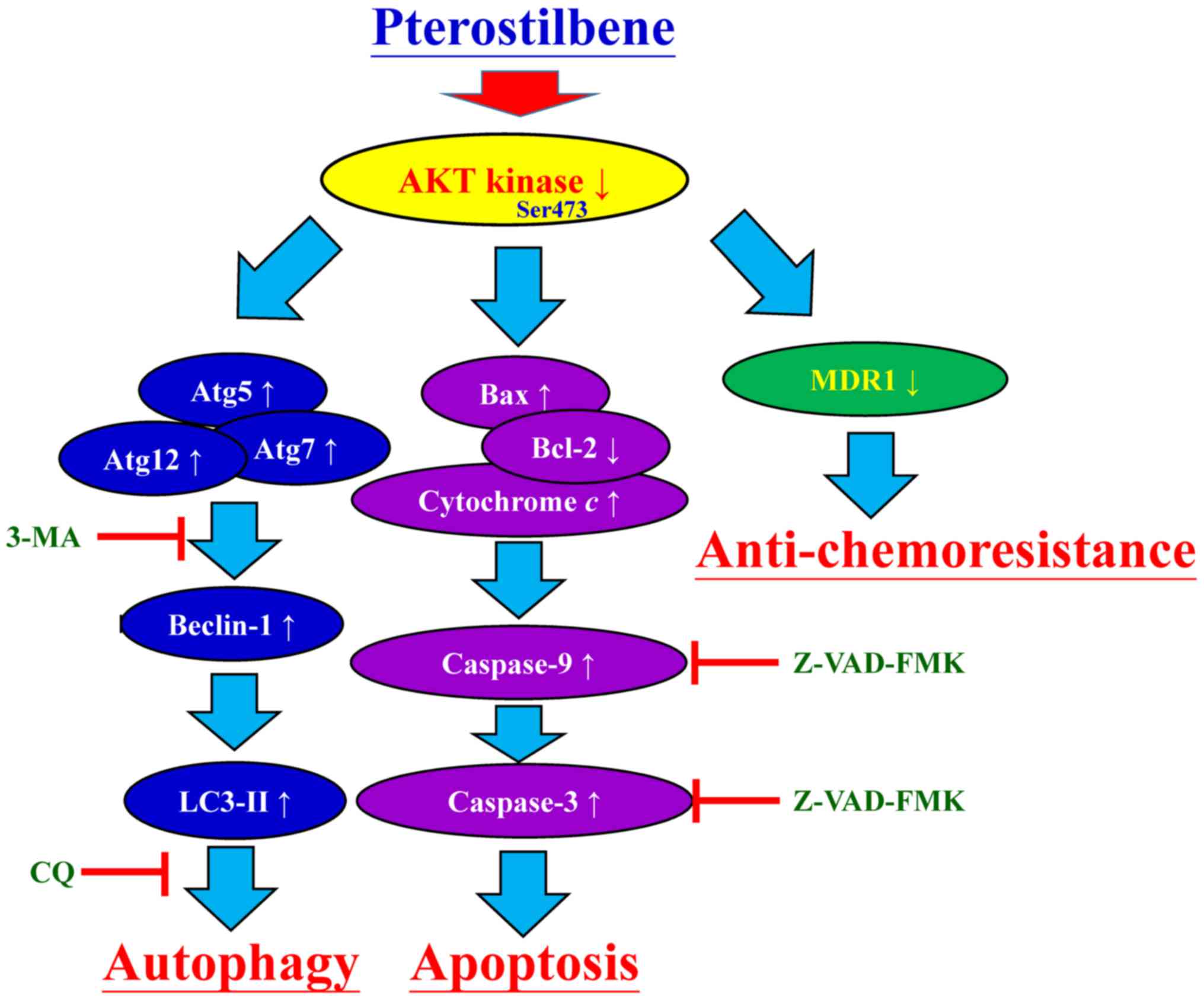
CAR cells by suppressing MDR1 expression and AKT signaling. The
proposed schematic representation of the plausible molecular
signaling induced by pterostilbene in CAR cells is summarized in
Fig. 9.
Discussion
A previous study by our group indicated that
resveratrol induces autophagy and apoptosis by modulating
AMP-activated protein kinase and AKT/mechanistic target of
rapamycin signaling in cisplatin-resistant human oral cancer CAR
cells (51). To the best of our
knowledge, the present study is the first to report that
pterostilbene triggers the autophagy and apoptosis of CAR cells, as
well as the first to indicate that pterostilbene suppresses MDR1
expression. Pterostilbene and resveratrol exhibit similar
anticancer and biological activities; however, pterostilbene is
more effective: i) Pterostilbene contains two methoxy groups and
one hydroxyl group, whereas resveratrol contains three hydroxyl
groups. Therefore, pterostilbene has more lipophilic properties
than resveratrol, as the two methoxy groups that it contains that
serve to increase oral absorption and cellular uptake (56,57).
ii) Pharmacokinetic analysis indicates that pterostilbene has 95%
bioavailability however resveratrol only has 20% when administered
orally (58,59). iii) The half-life of pterostilbene
is 93.9±22.3 min in Sprague-Dawley rats (60), while that of resveratrol is 14 min
in rabbits (61). Furthermore, the
IC50 values of resveratrol in CAR cells following 24, 48
and 72 h exposure were 95.23±3.26, 73.23±2.29 and 51.62±3.36
μM, respectively (51).
However, the present study indicated that the IC50
values of pterostilbene were 78.26±4.33, 48.04±3.68 and 20.65±4.88
μM in CAR cells following 24, 48 and 72 h incubation,
respectively. This indicates that pterostilbene exhibits greater
effects at lower concentrations than resveratrol. These results
suggest that pterostilbene exhibits greater anticancer effects than
resveratrol.
Previous studies have demonstrated that
pterostilbene is an effective antioxidant with anticancer
potential, which induces cell death and exhibits anti-metastatic
actions (16,30,32,34,37).
Furthermore, it has been demonstrated that pterostilbene triggers
apoptosis in pancreatic cancer (MIA PaCa-2 and PANC-1) (20,21),
breast cancer (MDA-MB-468, MCF-7 and MDA-MB-231) (18,19),
lung cancer (LCC, PC9, NCI-H460, SK-MES-1 and A549) (22–24,29),
osteosarcoma (SOSP-9607) (62),
prostate cancer (PC-3 and LNCaP) (25,26),
leukemia (Jurkat, Hut-78, HL60, MOLT4 and K562) (37–40),
colon cancer (Colo 205, HCT116 and HT29) (27–29),
bladder cancer (T24) (30),
hepatocellular carcinoma (HepG2) (35,36),
gastric carcinoma (AGS) (31) and
oral cancer (SAS and OECM-1) (32)
cell lines. In addition, pterostilbene exhibits autophagic effects
in various types of cancer (29,30,38,39,42).
The results of the present study demonstrated that treatment with
5–100 μM pterostilbene significantly inhibited the viability
and confluence of CAR cells. These results are in accordance with
those of a study by Ko et al (32), which demonstrated that
pterostilbene inhibits the proliferation of human oral cancer SAS
and OECM-1 cells.
In the present study, AO and MDC staining indicated
that pterostilbene stimulated the formation of autophagic vesicles
in CAR cells. LysoTracker Red staining also detected lysosome
activity following treatment with pterostilbene. LC3 expression is
a characteristic of autophagic vesicle membrane in early
autophagosome formation (63). The
results of the present study indicated that pterostilbene increased
the mRNA expression of the autophagic genes LC3-II and
Atg12 and the protein expression of the autophagy-associated
proteins Atg5, Atg7, Atg12, Beclin-1 and LC3-II in CAR cells. To
determine the autophagic effect on CAR cells, the autophagy
inhibitors 3-MA and CQ were used to determine whether pterostilbene
induces autophagy. The results demonstrated that they protected
against the pterostilbene-induced decrease in cell viability. The
results of the present study are in accordance with those of a
previous study (32), which
demonstrated that pterostilbene stimulated the formation AVOs and
autophagic vacuoles and increased the expression of LC3-II and
Beclin-1 protein in human oral cancer SAS and OECM-1 cells. It is
important to note that autophagy was detected following 24 h
treatment with pterostilbene in the present study; however, no
dramatic activation of caspase-3 was observed following exposure to
pterostilbene for 24 h. These results imply that
pterostilbene-induced autophagy occurs prior to CAR cell
apoptosis.
The present study also demonstrated that
pterostilbene induced apoptosis by performing an TUNEL assay, which
demonstrated that pterostilbene significantly increased the number
of TUNEL-positive cells. Pterostilbene-triggered apoptosis was
confirmed by the pan-caspase inhibitor Z-VAD-FMK, which reversed
the reduction of cellular viability in the pterostilbene-treated
cells. A significant increase in the activity of caspases-3 and -9
and the expression of their active forms were observed in CAR cells
following pterostilbene treatment. Hsiao et al (64) reported that pterostilbene induced
mitochondria-dependent apoptosis in human leukemia HL60 cells.
Furthermore, Schneider et al (24) demonstrated that pterostilbene
induced mitochondria-dependent apoptosis in human lung cancer
NCI-H460 and SK-MES-1 cells. The results of the present study
suggest that pterostilbene provokes caspase-dependent
mitochondria-derived apoptosis in CAR cells.
MDR1, also known as p-glycoprotein, is encoded by
the ATP Binding Cassette Subfamily B Member 1 gene. It is a subunit
of the ATP-dependent transporter and is involved in the development
of resistance of tumor cells to chemotherapeutic agents (65,66).
It has been demonstrated that the inhibition of AKT effectively
stimulates the sensitivity of chemotherapeutic agents by inhibiting
AKT-mediated MDR1 gene expression (66). Previous studies have demonstrated
that MDR1 overexpression is involved in the development of
cisplatin-resistance in CAR cells (44,50,66).
The results of the present study demonstrated that pterostilbene
inhibited MDR1 mRNA and protein expression by downregulating the
expression of phosphorylated AKT on Ser473 in CAR cells.
In conclusion, the results of the present study
support the proposition that pterostilbene-induced autophagic and
apoptotic CAR cell death may be involved in AKT-mediated MDR1
suppression. Therefore, the present study indicates that
pterostilbene may be a promising candidate as an oral anticancer
drug or an adjuvant to chemotherapeutic reagents and may be
developed as a potential therapeutic agent to treat oral cancer in
the future.
Acknowledgments
We thank Mr. Meng-Jou Liao (Tekon Scientific Corp.,
Taiwan), Mr. Chin-Chen Lin (Tekon Scientific Corp., Taiwan) and Mr.
Chang-Wei Li (AllBio Science Incorporated, Taiwan) for their
excellent technique and equipment support.
Notes
[1]
Funding
The present study was supported by China Medical
University Hospital (Taichung, Taiwan; grant no. DMR-107-123) and
partly by the Ministry of Science and Technology, Taiwan (grant no.
MOST 105-2320-B-039-033-).
[2] Availability
of data and materials
The data sets generated during the study are
available from the corresponding author on reasonable request.
[3] Authors'
contributions
HPC, CCL and JSY conceived and designed the
experiments. JHC, YNJ, JWT and HYC performed the experiments. CCL,
FJT and JSY analyzed the data. HPC, CCL and JSY wrote and modified
the paper. All authors read and approved the final manuscript.
[4] Ethics
approval and consent to participate
Not applicable.
[5] Consent for
publication
Not applicable.
[6] Competing
interests
The authors declare that they have no competing
interests.
References
|
1
|
Tsai SC, Huang SF, Chiang JH, Chen YF,
Huang CC, Tsai MH, Tsai FJ, Kao MC and Yang JS: The differential
regulation of microRNAs is associated with oral cancer. Oncol Rep.
38:1613–1620. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kawakita D, Lee YA, Li Q, Chen Y, Chen CJ,
Hsu WL, Lou PJ, Zhu C, Pan J, Shen H, et al: Impact of oral hygiene
on head and neck cancer risk in a Chinese population. Head Neck.
39:2549–2557. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ministry of Health and Welfare: Republic
of China (Taiwan). https://www.mohw.gov.tw/cp-3425-33347-2.html.
2017
|
|
4
|
Chiang SL, Velmurugan BK, Chung CM, Lin
SH, Wang ZH, Hua CH, Tsai MH, Kuo TM, Yeh KT, Chang PY, et al:
Preventive effect of celecoxib use against cancer progression and
occurrence of oral squamous cell carcinoma. Sci Rep. 7:62352017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macha MA, Rachagani S, Qazi AK, Jahan R,
Gupta S, Patel A, Seshacharyulu P, Lin C, Li S, Wang S, et al:
Afatinib radiosensitizes head and neck squamous cell carcinoma
cells by targeting cancer stem cells. Oncotarget. 8:20961–20973.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rapidis A, Sarlis N, Lefebvre JL and Kies
M: Docetaxel in the treatment of squamous cell carcinoma of the
head and neck. Ther Clin Risk Manag. 4:865–886. 2008. View Article : Google Scholar
|
|
7
|
Bauman J, Langer C, Quon H, Algazy K, Lin
A, Desai A, Mutale F and Weiss J: Induction chemotherapy with
cetuximab, carboplatin and paclitaxel for the treatment of locally
advanced squamous cell carcinoma of the head and neck. Exp Ther
Med. 5:1247–1253. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Torres-Bugarín O, Ventura-Aguilar A,
Zamora-Perez A, Gómez-Meda BC, Ramos-Ibarra ML, Morgan-Villela G,
Gutiérrez-Franco A and Zúñiga-González G: Evaluation of cisplatin +
5-FU, carboplatin + 5-FU, and ifosfamide + epirubicine regimens
using the micronuclei test and nuclear abnormalities in the buccal
mucosa. Mutat Res. 539:177–186. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Passiglia F, Listì A, Castiglia M, Perez
A, Rizzo S, Bazan V and Russo A: EGFR inhibition in NSCLC: New
findings…. and opened questions? Crit Rev Oncol Hematol.
112:126–135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Housman G, Byler S, Heerboth S, Lapinska
K, Longacre M, Snyder N and Sarkar S: Drug resistance in cancer: An
overview. Cancers (Basel). 6:1769–1792. 2014. View Article : Google Scholar
|
|
11
|
Zahreddine H and Borden KL: Mechanisms and
insights into drug resistance in cancer. Front Pharmacol. 4:282013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YY, Chen YK, Hsu YL, Chiu WC, Tsai
CH, Hu SC, Hsieh PW and Yuan SF: Synthetic β-nitrostyrene
derivative CYT-Rx20 as inhibitor of oral cancer cell proliferation
and tumor growth through glutathione suppression and reactive
oxygen species induction. Head Neck. 39:1055–1064. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
McCormack D and McFadden D: A review of
pterostilbene antioxidant activity and disease modification. Oxid
Med Cell Longev. 2013:5754822013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dvorakova M and Landa P: Anti-inflammatory
activity of natural stilbenoids: A review. Pharmacol Res.
124:126–145. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
McCormack D and McFadden D: Pterostilbene
and cancer: Current review. J Surg Res. 173:e53–e61. 2012.
View Article : Google Scholar
|
|
16
|
Xue EX, Lin JP, Zhang Y, Sheng SR, Liu HX,
Zhou YL and Xu H: Pterostilbene inhibits inflammation and ROS
production in chondrocytes by activating Nrf2 pathway. Oncotarget.
8:41988–42000. 2017.PubMed/NCBI
|
|
17
|
Bhakkiyalakshmi E, Sireesh D,
Sakthivadivel M, Sivasubramanian S, Gunasekaran P and Ramkumar KM:
Antihyperlipidemic and anti-peroxidative role of pterostilbene via
Nrf2 signaling in experimental diabetes. Eur J Pharmacol. 777:9–16.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wakimoto R, Ono M, Takeshima M, Higuchi T
and Nakano S: Differential anticancer activity of pterostilbene
against three subtypes of human breast cancer cells. Anticancer
Res. 37:6153–6159. 2017.
|
|
19
|
Nikhil K, Sharan S, Chakraborty A,
Bodipati N, Krishna Peddinti R and Roy P: Role of isothiocyanate
conjugate of pterostilbene on the inhibition of MCF-7 cell
proliferation and tumor growth in Ehrlich ascitic cell induced
tumor bearing mice. Exp Cell Res. 320:311–328. 2014. View Article : Google Scholar
|
|
20
|
Kostin SF, McDonald DE and McFadden DW:
Inhibitory effects of (-)-epigallocatechin-3-gallate and
pterostilbene on pancreatic cancer growth in vitro. J Surg Res.
177:255–262. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mannal PW, Alosi JA, Schneider JG,
McDonald DE and McFadden DW: Pterostilbene inhibits pancreatic
cancer in vitro. J Gastrointest Surg. 14:873–879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ma Z, Yang Y, Di S, Feng X, Liu D, Jiang
S, Hu W, Qin Z, Li Y, Lv J, et al: Pterostilbene exerts anticancer
activity on non-small-cell lung cancer via activating endoplasmic
reticulum stress. Sci Rep. 7:80912017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang YJ, Lin JF, Cheng LH, Chang WT, Kao
YH, Chang MM, Wang BJ and Cheng HC: Pterostilbene prevents AKT-ERK
axis-mediated polymerization of surface fibronectin on suspended
lung cancer cells independently of apoptosis and suppresses
metastasis. J Hematol Oncol. 10:722017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schneider JG, Alosi JA, McDonald DE and
McFadden DW: Pterostilbene inhibits lung cancer through induction
of apoptosis. J Surg Res. 161:18–22. 2010. View Article : Google Scholar
|
|
25
|
Nikhil K, Sharan S, Chakraborty A and Roy
P: Pterostilbene-isothiocyanate conjugate suppresses growth of
prostate cancer cells irrespective of androgen receptor status.
PLoS One. 9:e933352014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lin VC, Tsai YC, Lin JN, Fan LL, Pan MH,
Ho CT, Wu JY and Way TD: Activation of AMPK by pterostilbene
suppresses lipogenesis and cell-cycle progression in p53 positive
and negative human prostate cancer cells. J Agric Food Chem.
60:6399–6407. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sun Y, Wu X, Cai X, Song M, Zheng J, Pan
C, Qiu P, Zhang L, Zhou S, Tang Z, et al: Identification of
pinostilbene as a major colonic metabolite of pterostilbene and its
inhibitory effects on colon cancer cells. Mol Nutr Food Res.
60:1924–1932. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tolba MF and Abdel-Rahman SZ:
Pterostilbine, an active component of blueberries, sensitizes colon
cancer cells to 5-fluorouracil cytotoxicity. Sci Rep. 5:152392015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mena S, Rodríguez ML, Ponsoda X, Estrela
JM, Jäättela M and Ortega AL: Pterostilbene-induced tumor
cytotoxicity: A lysosomal membrane permeabilization-dependent
mechanism. PLoS One. 7:e445242012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen RJ, Ho CT and Wang YJ: Pterostilbene
induces autophagy and apoptosis in sensitive and chemoresistant
human bladder cancer cells. Mol Nutr Food Res. 54:1819–1832. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pan MH, Chang YH, Badmaev V, Nagabhushanam
K and Ho CT: Pterostilbene induces apoptosis and cell cycle arrest
in human gastric carcinoma cells. J Agric Food Chem. 55:7777–7785.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ko CP, Lin CW, Chen MK, Yang SF, Chiou HL
and Hsieh MJ: Pterostilbene induce autophagy on human oral cancer
cells through modulation of Akt and mitogen-activated protein
kinase pathway. Oral Oncol. 51:593–601. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bundela S, Sharma A and Bisen PS:
Potential compounds for oral cancer treatment: Resveratrol,
nimbolide, lovastatin, bortezomib, vorinostat, berberine,
pterostilbene, deguelin, andrographolide, and colchicine. PLoS One.
10:e01417192015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lin CW, Chou YE, Chiou HL, Chen MK, Yang
WE, Hsieh MJ and Yang SF: Pterostilbene suppresses oral cancer cell
invasion by inhibiting MMP-2 expression. Expert Opin Ther Targets.
18:1109–1120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Guo L, Tan K, Wang H and Zhang X:
Pterostilbene inhibits hepatocellular carcinoma through
p53/SOD2/ROS-mediated mitochondrial apoptosis. Oncol Rep.
36:3233–3240. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Lombardi G, Vannini S, Blasi F,
Marcotullio MC, Dominici L, Villarini M, Cossignani L and Moretti
M: In vitro safety/protection assessment of resveratrol and
pterostilbene in a human hepatoma cell line (HepG2). Nat Prod
Commun. 10:1403–1408. 2015.PubMed/NCBI
|
|
37
|
Chang G, Xiao W, Xu Z, Yu D, Li B, Zhang
Y, Sun X, Xie Y, Chang S, Gao L, et al: Pterostilbene induces cell
apoptosis and cell cycle arrest in T-cell leukemia/lymphoma by
suppressing the ERK1/2 pathway. BioMed Res Int. 2017:98720732017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siedlecka-Kroplewska K, Jozwik A,
Boguslawski W, Wozniak M, Zauszkiewicz-Pawlak A, Spodnik JH,
Rychlowski M and Kmiec Z: Pterostilbene induces accumulation of
autophagic vacuoles followed by cell death in HL60 human leukemia
cells. J Physiol Pharmacol. 64:545–556. 2013.PubMed/NCBI
|
|
39
|
Siedlecka-Kroplewska K, Jozwik A,
Kaszubowska L, Kowalczyk A and Boguslawski W: Pterostilbene induces
cell cycle arrest and apoptosis in MOLT4 human leukemia cells.
Folia Histochem Cytobiol. 50:574–580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Roslie H, Chan KM, Rajab NF, Velu SS,
Kadir SA, Bunyamin I, Weber JF, Thomas NF, Majeed AB, Myatt G, et
al: 3,5-Dibenzyloxy-4′-hydroxystilbene induces early caspase-9
activation during apoptosis in human K562 chronic myelogenous
leukemia cells. J Toxicol Sci. 37:13–21. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Y, Ding L, Wang X, Zhang J, Han W,
Feng L, Sun J, Jin H and Wang XJ: Pterostilbene simultaneously
induces apoptosis, cell cycle arrest and cyto-protective autophagy
in breast cancer cells. Am J Transl Res. 4:44–51. 2012.PubMed/NCBI
|
|
42
|
Chakraborty A, Bodipati N, Demonacos MK,
Peddinti R, Ghosh K and Roy P: Long term induction by pterostilbene
results in autophagy and cellular differentiation in MCF-7 cells
via ROS dependent pathway. Mol Cell Endocrinol. 355:25–40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gosepath EM, Eckstein N, Hamacher A,
Servan K, von Jonquieres G, Lage H, Györffy B, Royer HD and Kassack
MU: Acquired cisplatin resistance in the head-neck cancer cell line
Cal27 is associated with decreased DKK1 expression and can
partially be reversed by overexpression of DKK1. Int J Cancer.
123:2013–2019. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chang PY, Peng SF, Lee CY, Lu CC, Tsai SC,
Shieh TM, Wu TS, Tu MG, Chen MY and Yang JS: Curcumin-loaded
nanoparticles induce apoptotic cell death through regulation of the
function of MDR1 and reactive oxygen species in cisplatin-resistant
CAR human oral cancer cells. Int J Oncol. 43:1141–1150. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Lu CC, Huang BR, Liao PJ and Yen GC:
Ursolic acid triggers nonprogrammed death (necrosis) in human
glioblastoma multiforme DBTRG-05MG cells through MPT pore opening
and ATP decline. Mol Nutr Food Res. 58:2146–2156. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Huang WW, Chiu YJ, Fan MJ, Lu HF, Yeh HF,
Li KH, Chen PY, Chung JG and Yang JS: Kaempferol induced apoptosis
via endoplasmic reticulum stress and mitochondria-dependent pathway
in human osteosarcoma U-2 OS cells. Mol Nutr Food Res.
54:1585–1595. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sun JM, Yang LN, Xu H, Chang B, Wang HY
and Yang G: Inhibition of Aurora A promotes chemosensitivity via
inducing cell cycle arrest and apoptosis in cervical cancer cells.
Am J Cancer Res. 5:1133–1145. 2015.PubMed/NCBI
|
|
48
|
Gelles JD and Chipuk JE: Robust
high-throughput kinetic analysis of apoptosis with real-time
high-content live-cell imaging. Cell Death Dis. 7:e24932016.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lee MR, Lin C, Lu CC, Kuo SC, Tsao JW,
Juan YN, Chiu HY, Lee FY, Yang JS and Tsai FJ: YC-1 induces
G0/G1phase arrest and mitochondria-dependent apoptosis in
cisplatin-resistant human oral cancer CAR cells. Biomedicine
(Taipei). 7:122017. View Article : Google Scholar
|
|
50
|
Hsieh MT, Chen HP, Lu CC, Chiang JH, Wu
TS, Kuo DH, Huang LJ, Kuo SC and Yang JS: The novel pterostilbene
derivative ANK-199 induces autophagic cell death through regulating
PI3 kinase class III/beclin 1/Atg related proteins in cisplatin
resistant CAR human oral cancer cells. Int J Oncol. 45:782–794.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chang CH, Lee CY, Lu CC, Tsai FJ, Hsu YM,
Tsao JW, Juan YN, Chiu HY, Yang JS and Wang CC: Resveratrol-induced
autophagy and apoptosis in cisplatin-resistant human oral cancer
CAR cells: A key role of AMPK and Akt/mTOR signaling. Int J Oncol.
50:873–882. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
53
|
Chiang JH, Yang JS, Lu CC, Hour MJ, Chang
SJ, Lee TH and Chung JG: Newly synthesized quinazolinone HMJ-38
suppresses angiogenetic responses and triggers human umbilical vein
endothelial cell apoptosis through p53-modulated Fas/death receptor
signaling. Toxicol Appl Pharmacol. 269:150–162. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ma YS, Weng SW, Lin MW, Lu CC, Chiang JH,
Yang JS, Lai KC, Lin JP, Tang NY, Lin JG, et al: Antitumor effects
of emodin on LS1034 human colon cancer cells in vitro and in vivo:
Roles of apoptotic cell death and LS1034 tumor xenografts model.
Food Chem Toxicol. 50:1271–1278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lu CC, Yang JS, Chiang JH, Hour MJ, Lin
KL, Lee TH and Chung JG: Cell death caused by quinazolinone HMJ-38
challenge in oral carcinoma CAL 27 cells: Dissections of
endoplasmic reticulum stress, mitochondrial dysfunction and tumor
xenografts. Biochim Biophys Acta. 1840.2310–2320. 2014.
|
|
56
|
Tsai HY, Ho CT and Chen YK: Biological
actions and molecular effects of resveratrol, pterostilbene, and
3′-hydroxypterostilbene. J Food Drug Anal. 25:134–147. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Traversi G, Fiore M, Leone S, Basso E, Di
Muzio E, Polticelli F, Degrassi F and Cozzi R: Resveratrol and its
methoxy-derivatives as modulators of DNA damage induced by ionising
radiation. Mutagenesis. 31:433–441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lin HS, Yue BD and Ho PC: Determination of
pterostilbene in rat plasma by a simple HPLC-UV method and its
application in pre-clinical pharmacokinetic study. Biomed
Chromatogr. 23:1308–1315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lin HS and Ho PC: Preclinical
pharmacokinetic evaluation of resveratrol trimethyl ether in
sprague-dawley rats: The impacts of aqueous solubility, dose
escalation, food and repeated dosing on oral bioavailability. J
Pharm Sci. 100:4491–4500. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yeo SC, Ho PC and Lin HS: Pharmacokinetics
of pterostilbene in Sprague-Dawley rats: The impacts of aqueous
solubility, fasting, dose escalation, and dosing route on
bioavailability. Mol Nutr Food Res. 57:1015–1025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Asensi M, Medina I, Ortega A, Carretero J,
Baño MC, Obrador E and Estrela JM: Inhibition of cancer growth by
resveratrol is related to its low bioavailability. Free Radic Biol
Med. 33:387–398. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Liu Y, Wang L, Wu Y, Lv C, Li X, Cao X,
Yang M, Feng D and Luo Z: Pterostilbene exerts antitumor activity
against human osteosarcoma cells by inhibiting the JAK2/STAT3
signaling pathway. Toxicology. 304:120–131. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Yang JS, Lu CC, Kuo SC, Hsu YM, Tsai SC,
Chen SY, Chen YT, Lin YJ, Huang YC, Chen CJ, et al: Autophagy and
its link to type II diabetes mellitus. Biomedicine (Taipei).
7:82017. View Article : Google Scholar
|
|
64
|
Hsiao PC, Chou YE, Tan P, Lee WJ, Yang SF,
Chow JM, Chen HY, Lin CH, Lee LM and Chien MH: Pterostilbene
simultaneously induced G0/G1-phase arrest and MAPK-mediated
mitochondrial-derived apoptosis in human acute myeloid leukemia
cell lines. PLoS One. 9:e1053422014. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Davoudi Z, Akbarzadeh A, Rahmatiyamchi M,
Movassaghpour AA, Alipour M, Nejati-Koshki K, Sadeghi Z,
Dariushnejad H and Zarghami N: Molecular target therapy of AKT and
NF-kB signaling pathways and multidrug resistance by specific cell
penetrating inhibitor peptides in HL-60 cells. Asian Pac J Cancer
Prev. 15:4353–4358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Yuan CH, Horng CT, Lee CF, Chiang NN, Tsai
FJ, Lu CC, Chiang JH, Hsu YM, Yang JS and Chen FA: Epigallocatechin
gallate sensitizes cisplatin-resistant oral cancer CAR cell
apoptosis and autophagy through stimulating AKT/STAT3 pathway and
suppressing multidrug resistance 1 signaling. Environ Toxicol.
32:845–855. 2017. View Article : Google Scholar
|