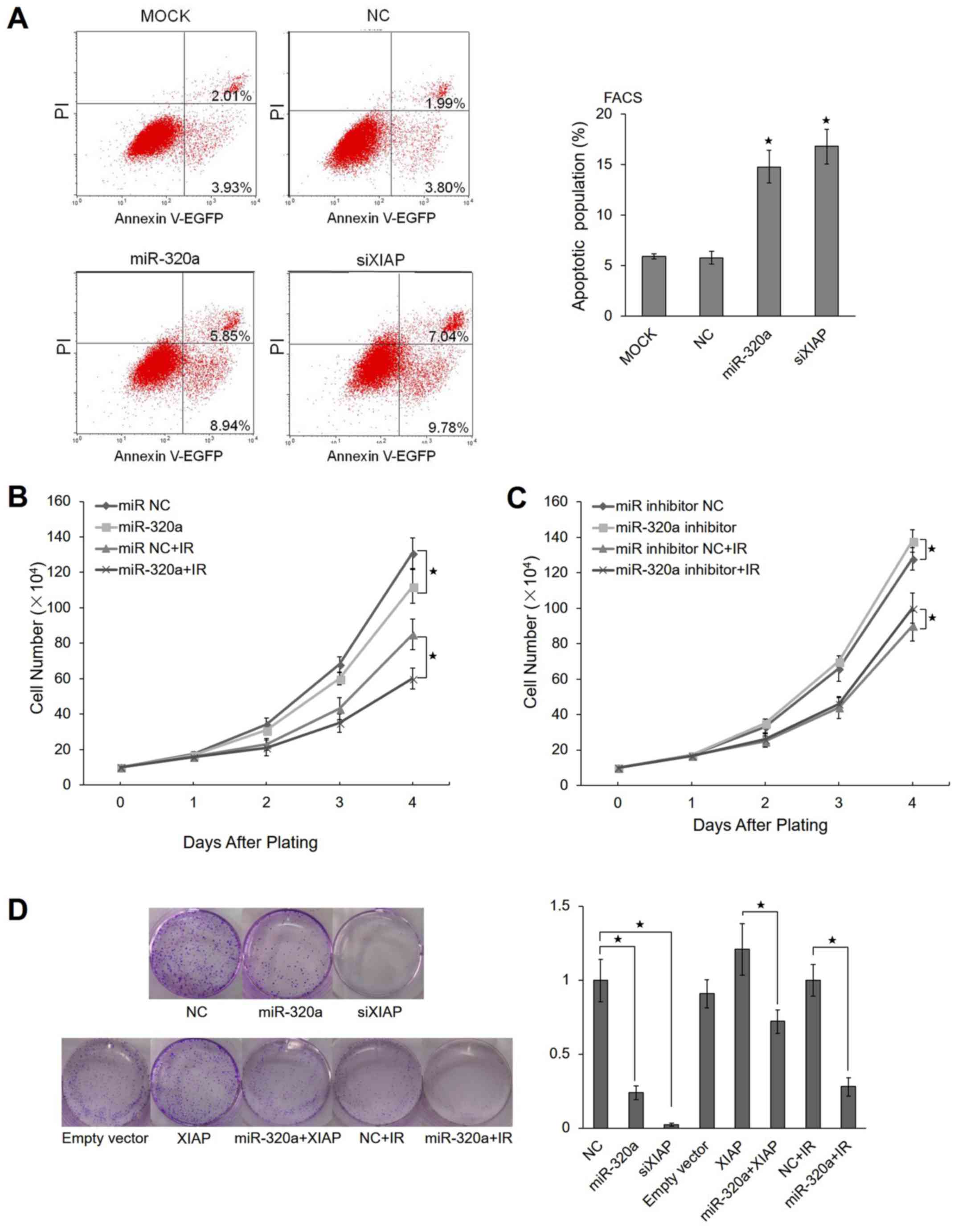
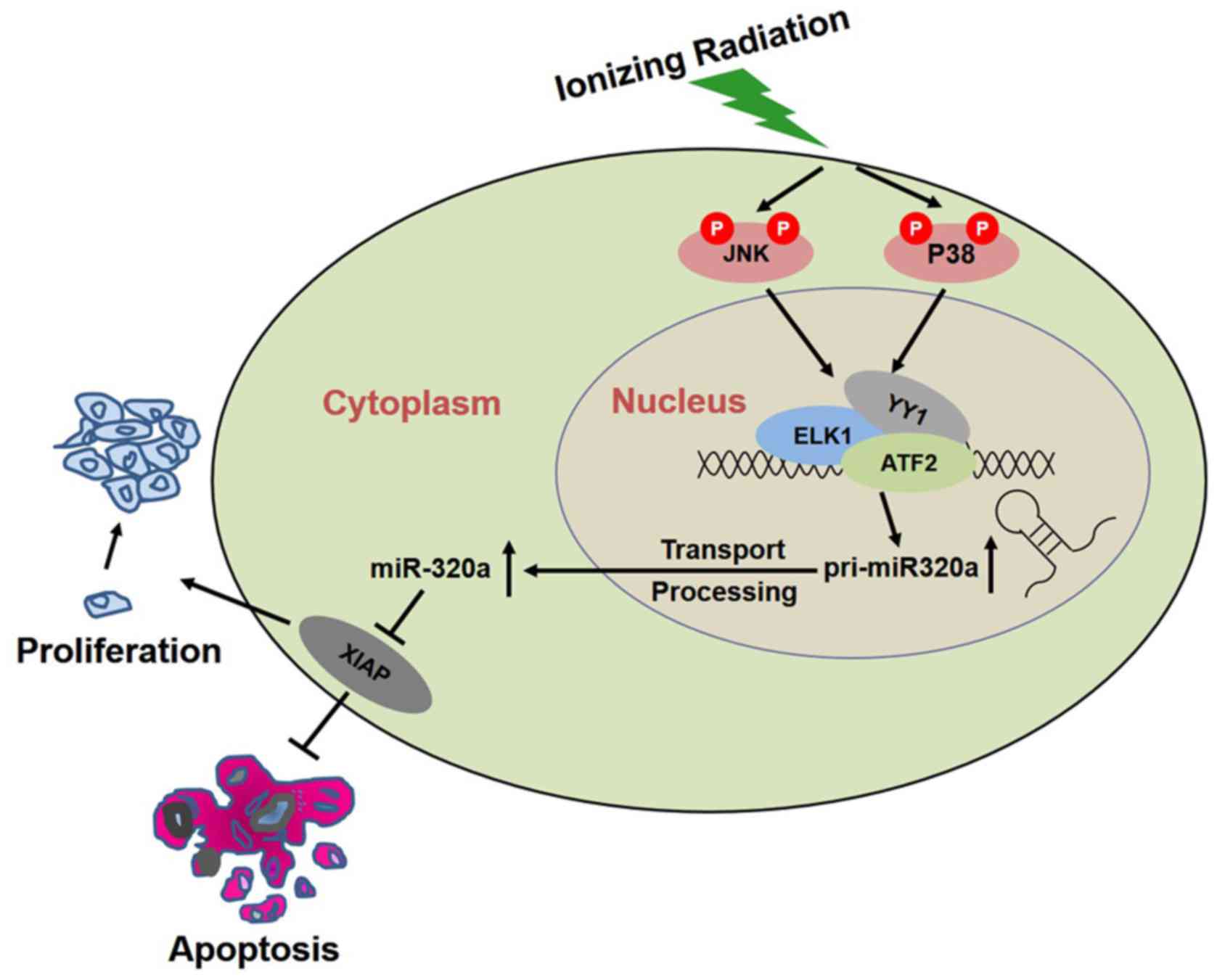
|
1
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Huang X, Ding L, Bennewith KL, Tong RT,
Welford SM, Ang KK, Story M, Le QT and Giaccia AJ:
Hypoxia-inducible mir-210 regulates normoxic gene expression
involved in tumor initiation. Mol Cell. 35:856–867. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Leung AK and Sharp PA: MicroRNA functions
in stress responses. Mol Cell. 40:205–215. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Simone NL, Soule BP, Ly D, Saleh AD,
Savage JE, Degraff W, Cook J, Harris CC, Gius D and Mitchell JB:
Ionizing radiation-induced oxidative stress alters miRNA
expression. PLoS One. 4:e63772009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wilke CM, Hess J, Klymenko SV, Chumak VV,
Zakhartseva LM, Bakhanova EV, Feuchtinger A, Walch AK,
Selmansberger M, Braselmann H, et al: Expression of miRNA-26b-5p
and its target TRPS1 is associated with radiation exposure in
post-Chernobyl breast cancer. Int J Cancer. 142:573–583. 2018.
View Article : Google Scholar
|
|
6
|
Niemoeller OM, Niyazi M, Corradini S,
Zehentmayr F, Li M, Lauber K and Belka C: MicroRNA expression
profiles in human cancer cells after ionizing radiation. Radiat
Oncol. 6:292011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yano H, Hamanaka R, Nakamura-Ota M, Zhang
JJ, Matsuo N and Yoshioka H: Regulation of type I collagen
expression by microRNA-29 following ionizing radiation. Radiat
Environ Biophys. 57:41–54. 2018. View Article : Google Scholar
|
|
8
|
Chaudhry MA, Sachdeva H and Omaruddin RA:
Radiation-induced micro-RNA modulation in glioblastoma cells
differing in DNA-repair pathways. DNA Cell Biol. 29:553–561. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
He L, He X, Lim LP, de Stanchina E, Xuan
Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al: A microRNA
component of the p53 tumour suppressor network. Nature.
447:1130–1134. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kovalchuk O, Zemp FJ, Filkowski JN,
Altamirano AM, Dickey JS, Jenkins-Baker G, Marino SA, Brenner DJ,
Bonner WM and Sedelnikova OA: microRNAome changes in bystander
three-dimensional human tissue models suggest priming of apoptotic
pathways. Carcinogenesis. 31:1882–1888. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cha HJ, Seong KM, Bae S, Jung JH, Kim CS,
Yang KH, Jin YW and An S: Identification of specific microRNAs
responding to low and high dose gamma-irradiation in the human
lymphoblast line IM9. Oncol Rep. 22:863–868. 2009.PubMed/NCBI
|
|
12
|
Sokolov M and Neumann R: Global gene
expression alterations as a crucial constituent of human cell
response to low doses of ionizing radiation exposure. Int J Mol
Sci. 17:172015. View Article : Google Scholar
|
|
13
|
Begg AC, Stewart FA and Vens C: Strategies
to improve radiotherapy with targeted drugs. Nat Rev Cancer.
11:239–253. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lal A, Pan Y, Navarro F, Dykxhoorn DM,
Moreau L, Meire E, Bentwich Z, Lieberman J and Chowdhury D:
miR-24-mediated downregulation of H2AX suppresses DNA repair in
terminally differentiated blood cells. Nat Struct Mol Biol.
16:492–498. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Mateescu B, Batista L, Cardon M, Gruosso
T, de Feraudy Y, Mariani O, Nicolas A, Meyniel JP, Cottu P,
Sastre-Garau X, et al: miR-141 and miR-200a act on ovarian
tumorigenesis by controlling oxidative stress response. Nat Med.
17:1627–1635. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Adusumilli PS, Chan MK, Hezel M, Yu Z,
Stiles BM, Chou TC, Rusch VW and Fong Y: Radiation-induced cellular
DNA damage repair response enhances viral gene therapy efficacy in
the treatment of malignant pleural mesothelioma. Ann Surg Oncol.
14:258–269. 2007. View Article : Google Scholar
|
|
17
|
Hu Z, Tie Y, Lv G, et al: Correlation of
microRNAs responding to high dose γ-irradiation with predicted
target mRNAs in HeLa cells using microarray analyses. Chin Sci
Bull. 58:4622–4629. 2013. View Article : Google Scholar
|
|
18
|
Diakos C, Zhong S, Xiao Y, Zhou M,
Vasconcelos GM, Krapf G, Yeh RF, Zheng S, Kang M, Wiencke JK, et
al: TEL-AML1 regulation of survivin and apoptosis via miRNA-494 and
miRNA-320a. Blood. 116:4885–4893. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie F, Yuan Y, Xie L, Ran P, Xiang X,
Huang Q, Qi G, Guo X, Xiao C and Zheng S: miRNA-320a inhibits tumor
proliferation and invasion by targeting c-Myc in human
hepatocellular carcinoma. OncoTargets Ther. 10:885–894. 2017.
View Article : Google Scholar
|
|
20
|
Bronisz A, Godlewski J, Wallace JA,
Merchant AS, Nowicki MO, Mathsyaraja H, Srinivasan R, Trimboli AJ,
Martin CK, Li F, et al: Reprogramming of the tumour
microenvironment by stromal PTEN-regulated miR-320. Nat Cell Biol.
14:159–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Q, Fu H, Sun F, Zhang H, Tie Y, Zhu J,
Xing R, Sun Z and Zheng X: miR-16 family induces cell cycle arrest
by regulating multiple cell cycle genes. Nucleic Acids Res.
36:5391–5404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu HJ, Zhu J, Yang M, Zhang ZY, Tie Y,
Jiang H, Sun ZX and Zheng XF: A novel method to monitor the
expression of microRNAs. Mol Biotechnol. 32:197–204. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang J, Lee EJ, Gusev Y and Schmittgen
TD: Real-time expression profiling of microRNA precursors in human
cancer cell lines. Nucleic Acids Res. 33:5394–5403. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ho SY, Wu WJ, Chiu HW, Chen YA, Ho YS, Guo
HR and Wang YJ: Arsenic trioxide and radiation enhance apoptotic
effects in HL-60 cells through increased ROS generation and
regulation of JNK and p38 MAPK signaling pathways. Chem Biol
Interact. 193:162–171. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang J and Bowden GT: Activation of p38
MAP kinase and JNK pathways by UVA irradiation. Photochem Photobiol
Sci. 11:54–61. 2012. View Article : Google Scholar
|
|
26
|
Shin C, Nam JW, Farh KK, Chiang HR,
Shkumatava A and Bartel DP: Expanding the microRNA targeting code:
Functional sites with centered pairing. Mol Cell. 38:789–802. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kashkar H: X-linked inhibitor of
apoptosis: A chemoresistance factor or a hollow promise. Clin
Cancer Res. 16:4496–4502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Owens TW, Foster FM, Valentijn A, Gilmore
AP and Streuli CH: Role for X-linked Inhibitor of apoptosis protein
upstream of mitochondrial permeabilization. J Biol Chem.
285:1081–1088. 2010. View Article : Google Scholar :
|
|
29
|
Ward JF: DNA damage produced by ionizing
radiation in mammalian cells: Identities, mechanisms of formation,
and reparability. Prog Nucleic Acid Res Mol Biol. 35:95–125. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kraemer A, Anastasov N, Angermeier M,
Winkler K, Atkinson MJ and Moertl S: MicroRNA-mediated processes
are essential for the cellular radiation response. Radiat Res.
176:575–586. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li B, Shi XB, Nori D, et al:
Down-regulation of microRNA 106b is involved in p21-mediated cell
cycle arrest in response to radiation in prostate cancer cells.
Prostate. 71:567–574. 2011. View Article : Google Scholar
|
|
32
|
Bhoumik A, Lopez-Bergami P and Ronai Z:
ATF2 on the double - activating transcription factor and DNA damage
response protein. Pigment Cell Res. 20:498–506. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Breitwieser W, Lyons S, Flenniken AM,
Ashton G, Bruder G, Willington M, Lacaud G, Kouskoff V and Jones N:
Feedback regulation of p38 activity via ATF2 is essential for
survival of embryonic liver cells. Genes Dev. 21:2069–2082. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hai T and Hartman MG: The molecular
biology and nomenclature of the activating transcription
factor/cAMP responsive element binding family of transcription
factors: Activating transcription factor proteins and homeostasis.
Gene. 273:1–11. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kanzawa T, Iwado E, Aoki H, Iwamaru A,
Hollingsworth EF, Sawaya R, Kondo S and Kondo Y: Ionizing radiation
induces apoptosis and inhibits neuronal differentiation in rat
neural stem cells via the c-Jun NH2-terminal kinase (JNK) pathway.
Oncogene. 25:3638–3648. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kumar P, Miller AI and Polverini PJ: p38
MAPK mediates gamma-irradiation-induced endothelial cell apoptosis,
and vascular endothelial growth factor protects endothelial cells
through the phosphoinositide 3-kinase-Akt-Bcl-2 pathway. J Biol
Chem. 279:43352–43360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Marais R, Wynne J and Treisman R: The SRF
accessory protein Elk-1 contains a growth factor-regulated
transcriptional activation domain. Cell. 73:381–393. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Driák D, Osterreicher J, Reháková Z,
Vilasová Z and Vávrová J: Expression of phospho-Elk-1 in rat gut
after the whole body gamma irradiation. Physiol Res. 57:753–759.
2008.
|
|
39
|
Gordon S, Akopyan G, Garban H and Bonavida
B: Transcription factor YY1: Structure, function, and therapeutic
implications in cancer biology. Oncogene. 25:1125–1142. 2006.
View Article : Google Scholar
|
|
40
|
Eckelman BP, Salvesen GS and Scott FL:
Human inhibitor of apoptosis proteins: Why XIAP is the black sheep
of the family. EMBO Rep. 7:988–994. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Datta R, Oki E, Endo K, Biedermann V, Ren
J and Kufe D: XIAP regulates DNA damage-induced apoptosis
downstream of caspase-9 cleavage. J Biol Chem. 275:31733–31738.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Park SY, Lee JH, Ha M, Nam JW and Kim VN:
miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat
Struct Mol Biol. 16:23–29. 2009. View Article : Google Scholar
|
|
43
|
Cui F, Li X, Zhu X, Huang L, Huang Y, Mao
C, Yan Q, Zhu J, Zhao W and Shi H: MiR-125b inhibits tumor growth
and promotes apoptosis of cervical cancer cells by targeting
phosphoinositide 3-kinase catalytic subunit delta. Cell Physiol
Biochem. 30:1310–1318. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu L, Yu X, Guo X, Tian Z, Su M, Long Y,
Huang C, Zhou F, Liu M, Wu X, et al: miR-143 is downregulated in
cervical cancer and promotes apoptosis and inhibits tumor formation
by targeting Bcl-2. Mol Med Rep. 5:753–760. 2012.
|