1. Introduction
To date, it has been considerably appreciated that
extracellular vesicles (EVs) are crucial for intercellular
communication, although they have previously been viewed as
mediating these functions through soluble factors (1). EVs are majorly classified into three
classes: ectosomes or shedding microvesicles (MVs), exosomes, and
apoptotic bodies (ABs), based on their sizes and origins. MVs are
released from the plasma membrane instead of internal membranes (as
is the case for exosomes) and contain different protein components.
In addition, MVs are larger and more heterogeneous in size than
exosomes, ranging from 100 nm to 1 µm, while exosomes are 40-100 nm
(2). Although MVs are not exactly
identical to exosomes, they share similar biological functions
(3). This review majorly focuses
on cargo sorting in EVs, in particular in MVs and exosomes. With
small dimensions and indistinguishable structures, these shedding
vesicles conveniently function as bioactive cargo in order to
facilitate intercellular communication (4). Indeed, the biological functions of
EVs are not restricted to cell communication, and may have several
applications. In order to comprehensively understand EV functions,
we identified the role of each EV protein cargo (reported data to
date from the Vesiclepedia database; http://microvesicles.org/) in general biological
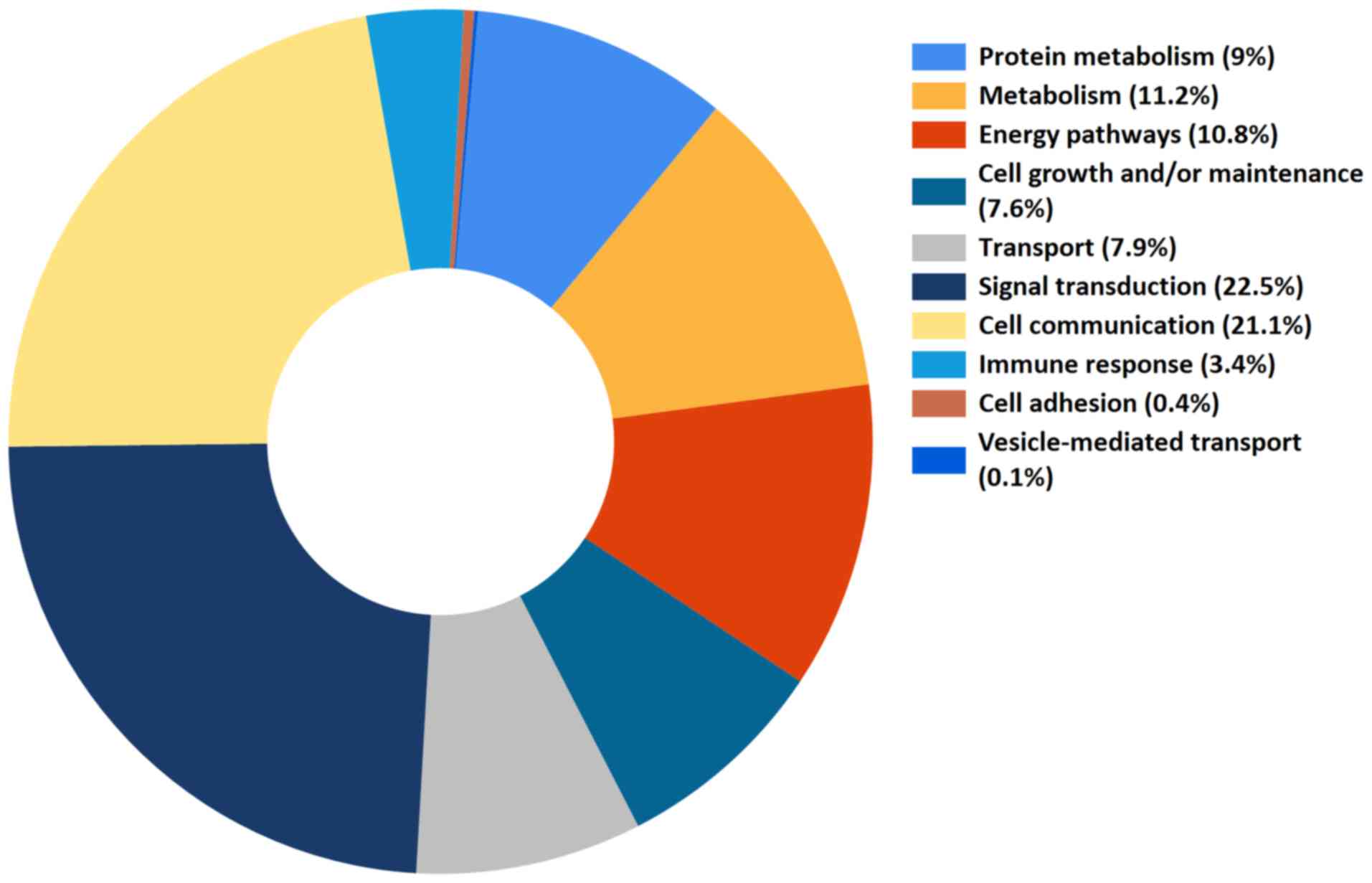
processes, and the 10 primary biological activities were noted, as
presented in Fig. 1 (5). Notably, the two major biological
roles of EV protein contents were signal transduction and cell
communication (22.5 and 21.1%, respectively), accounting for ~50%
of the total EV proteins. The importance of EVs in cell-to-cell
communication and signal transduction was reported recently
(6,7). These membrane-bound sacs are
assembled, transported across lipid bilayer membranes, and
eventually shed from the surface of their parent cells. Once
formed, EVs are paracellularly or distantly transported to the
target release sites and are internalized into the recipient cells.
Their cargo content is further released and mediates various
processes, including signal transduction, cell communication, and
transport (8). Considering the
heterogeneity of membrane-bound sacs, they may also contribute to
other biological activities, such as metabolism, cell growth and
adhesion (9,10).
EVs’ abilities of mass transport and information
exchange between the cells is generally owed to their deposited
complex molecules, such as multiple types of DNA, mRNA, small
noncoding RNA, and proteins (11).
Membrane-bound sacs secreted from different cell sources vary
greatly in the quantity, dimension, their deposition, and even
surface markers; thus, heterogeneity is observed among these
membranous structures (12). A
high-resolution proteomic and lipidomic analysis of exosomes and
MVs from various cell sources revealed that U87 exosomes were
enriched in sphingomyelins, whereas Huh7 and MSC exosomes were
specifically enriched in cardiolipins (13). In the present review article, four
gene sets of EV cargo content from different cell sources (data
from Vesiclepedia database to date; http://microvesicles.org/) were compared and the
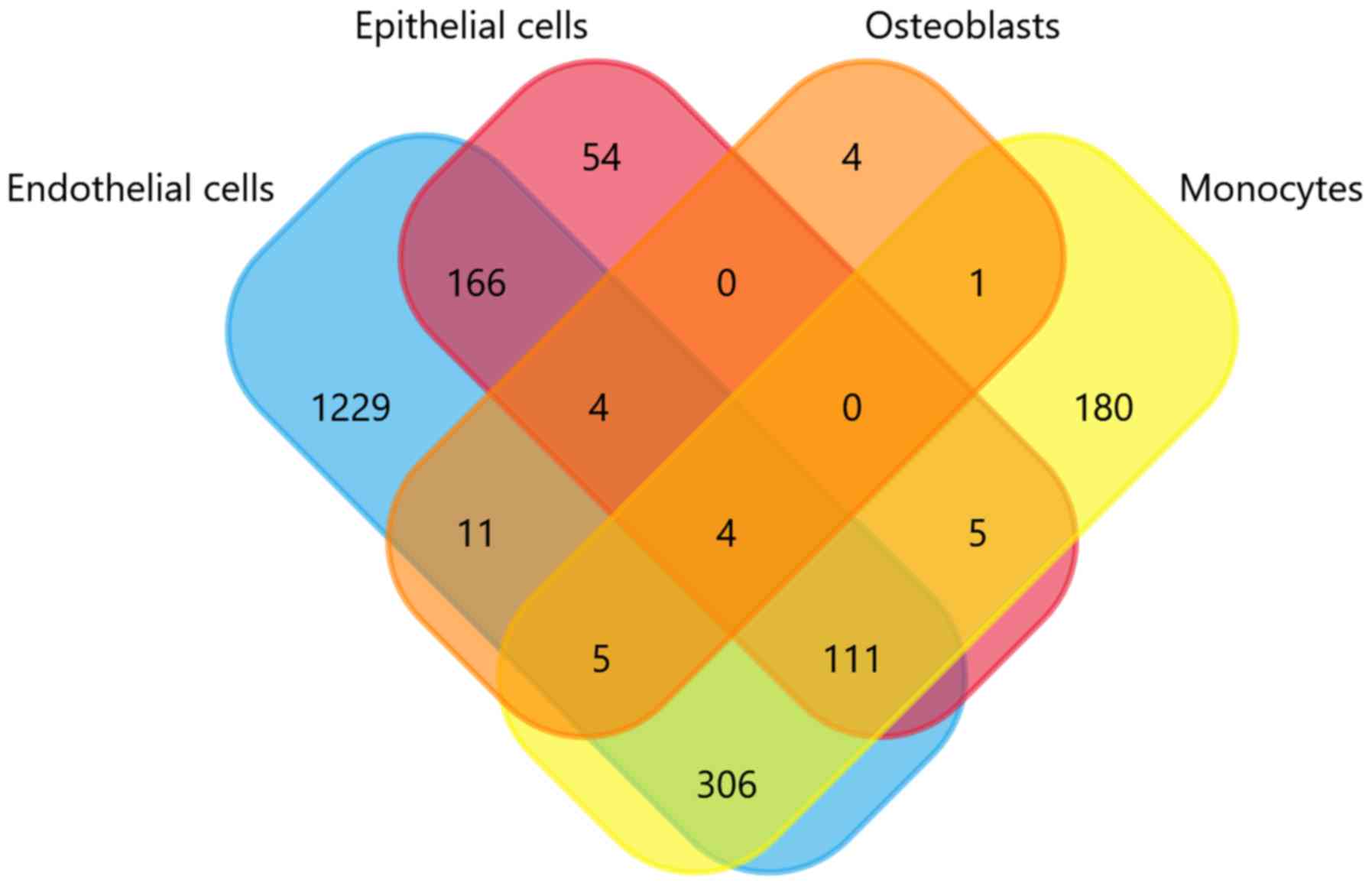
results are depicted in the Venn diagram of Fig. 2. As presented in Fig. 2, the gene expression profiles of
the endothelial cells, epithelial cells, osteoblasts, and monocytes
derived from extracellular vesicles (EN-EVs, EP-EVs, OS-EVs, and
MO-EVs, respectively) differ markedly, despite some small numbers
of overlapping genes. The larger portion of the EV cargo gene
profile in each cell source completely differs from the other three
sources, and the proportion of EN-EVs, EP-EVs, MO-EVs, and OS-EVs
is 67.6, 16.1, 29.8 and 13.8%, respectively. In specific, the
noncoincident regions of the four cargo gene sets contain
respective marker genes that represent their original tissue
characteristics. For example, in the present analysis there are
four genes that exist only in the osteoblasts, namely four and half
LIM domains 2 (FHL2), alkaline phosphatase biomineralization
associated (ALPL), acid phosphatase 1 (ACP1), and calcium
voltage-gated channel auxiliary subunit α2δ1 (CACNA2D1). Notably,
both ALPL and ACP1 phosphatases are specific markers of osteogenic
differentiation, and are crucial in the formation, development, and
metabolism of bone tissues (14,15).
This indicates that EVs tend to load tissue-specific cargo content,
which expresses similar characteristics with their parent
cells.
Indeed, EVs generally carry a series of
tissue-specific characteristics or ‘labels’ that reveal the
secretory cells from which they have originated (16,17).
For example, exosomes derived from cardiomyocytes can be denoted as
‘cardiosomes’ and facilitate a series of metabolic processes in
target cells (18). This is
crucial for distinguishing between numerous types of EVs and
further investigating their specific roles. To date, studies aimed
at discovering such labels have majorly focused on the proteome or
lipidome within EVs. A recent study revealed that the content of
exosomes and MVs derived from various cell sources considerably
differed from each other at the proteomic level, which might be
explored as an additional ‘vesiculome’ biomarker. By contrast, it
has been demonstrated that protein enrichment in the exosomes
distinguished cancer cells from stem cells, suggesting that they
might essentially become a useful general cancer marker (19). Hence, it can be concluded that
there is a difference between the applications of exosomes and MVs:
the biomarker value of exosomes might lie in indicating sorting
dysregulation in their source cells, whereas that of MVs might lie
in reflecting the content of their source cells.
In vivo experiments have also been performed
in recent years in an effort to identify the different MV origins
from certain cell type sources. For instance, labeling MVs/exosomes
with fluorescent lipophilic dyes (DiI, DiO, DiD and DiR) or
lipophilic radiotracer (99mTc-HMPAO) is an important way to track
or monitor the movement of MV in vivo (20,21).
However, these methods are mainly based on the non-specific lipid
compositions of the membrane structure of MV, and thus these dyes
are currently unable to identify the specific origin of MVs from
different cell types. In particular, a novel method integrated with
ultrasmall Mn-magnetofunctionalized Ag2Se quantum dots and
excellent near-infrared fluorescence or magnetic resonance imaging
capabilities has been developed for instant efficient labeling of
MVs for their in vivo high-resolution dual-mode tracking
(22). However, even this
effective and sensitive tracking method is unable to recognize the
derivation of MVs, since it is non-specific and labeling of MVs
occurs in vitro. From a review of the recent literature, it
appears that no effective means exist to date to determine which
MVs come from which cell type in in vivo experiments, and
further studies will be required to address this important
issue.
In addition to cell sources, physiological and
disease conditions can also affect the content of vesicle cargo
(23). The ratio of
specific/non-specific proteins in EVs changes under pathological
state. For example, brain-specific proteins in MVs, such as myelin
basic protein, proteins of coagulation cascade and focal adhesion,
were upregulated, while non-specific proteins, such as albumin,
were downregulated with adverse outcomes in lacunar infarction
(24). In particular, the
malignant transformation of normal cells generally affects EV
secretion, leading to aberrant shedding and oncogenic nucleic acid
and protein content enrichment (25). The selection of EVs is strictly
regulated; nevertheless, this highly regulated process is
frequently perturbed in tumors (26,27).
The following sections of the present review will
focus on recent reports on tumor derived EVs (TD-EVs) and consider
their specific and complex characteristics. In addition, the recent
literature on genetically modified MVs and engineered exosomes
associated with the tumor-targeted gene therapy will be
discussed.
2. General characteristics of EVs and
TD-EVs
Collectively, EVs can be isolated and separated from
culture supernatants and various body fluids, such as plasma,
urine, lymph, tissue, and cerebrospinal fluid (28). Ultracentrifugation and
immunomagnetic separation techniques are common methods for EV
separation, and via high resolution microscopy, the secretion
process of EVs as well as their size, morphology, and
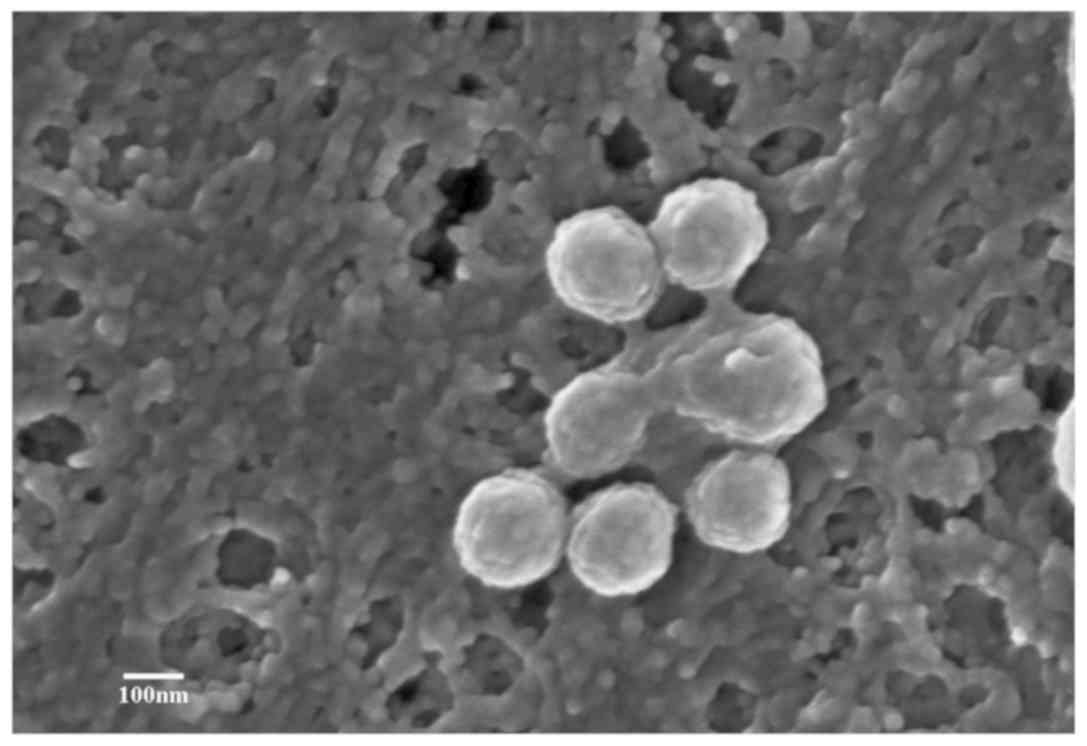
ultrastructures can be visually observed. Scanning electron
microscopy was used by our group to observe the secretion and
structure of human bone marrow mesenchymal stem cell
(hBMSC)-derived MVs (29). The
hBMSCs were isolated from three male patients with acute myeloid
leukemia aged 31, 35 and 36, that were admitted to Traditional
Chinese Medicine Hospital of Xintai (Xintai, China) from June 2018
to September 2018, with written informed consent. To observe the
release process of MVs from the cell surface, hBMSC-MVs were imaged
under a scanning electron microscope. As presented in Fig. 3, several membrane-bound sacs were
observed being shed from the membrane of hBMSCs. These were small,
spheroidal, membranous structures ranging from 200 to 1,000 nm in
size (exosomes range from 30 to 150 nm).
In coordination with morphological identification,
the molecular phenotype of EVs is generally detected via flow
cytometric analysis; for example, CD9, CD81, CD82, CD122 and CD163,
which are related to lipid microdomains, are relatively specific
molecular markers expressed on the membrane surface of MVs. Two of
these proteins, CD9 and CD63, are among the top 12 most commonly
identified proteins in MVs (30).
Therefore, they are commonly used for detection and
immunopurification of MVs following isolation (31). In general, the protein cargo
carried by EVs belongs to two categories: specific and nonspecific
proteins. The latter majorly comprises proteins associated with
biogenesis and common biological functions of EVs such as HSPs,
TM4SF, metabolic enzymes, and cytoplasmic and signal transduction
proteins (32). Specific proteins
exist only in EVs derived from certain distinctive cell sources,
which always carry their own ‘labels’, as aforementioned. These
label proteins are closely associated with the function of
releasing EVs, which also reflects the biological effect of their
parent cell sources (19). In
addition to protein cargo, nucleic acids, in particular noncoding
RNA, have been reported to be crucial in biological functions of
EVs (33). Collectively, all cargo
content within EVs (proteins, nucleic acids or lipids) coordinate
with each other to fulfill their own responsibilities.
Of note, TD-EVs have their own specific ‘labels,’
which distinguish them from the normal cell-derived EVs. Several
studies have reported that TD-EVs can carry oncogenic membrane
proteins or nucleic acids to facilitate tumor progression and a
significant difference was observed in the proportion of oncogenic
proteins and nucleic acids between normal and malignant cell
derived EVs (34). For example,
chromosome segregation 1 like (CSE1L), an important membrane
protein carried by MVs, has been reported to mediate Rastriggered
MV generation and metastasis of B16F10 melanoma cells (35). Another study reported that
endothelial cells developed chemoresistance upon uptake of
transient receptor potential cation channel subfamily C member 5
(TrpC5) carried by adriamycin-resistant breast cancer cell-derived
MVs (36). Thus, tumor cells are
skilled in employing TD-EVs to spread oncogenic information
throughout the body of an organism, facilitating the progression of
cancer. We reviewed the recent literature regarding the roles of
TD-EVs, and discovered that TD-EVs have been demonstrated to
accelerate various aspects of cancer progression.
3. TD-EVs associated with cancer
Massive release of TD-EVs consecutive to
cancer treatment
It has been reported that cancer therapies, such as
photodynamic treatment, radiotherapy, or chemotherapy, trigger
massive EV production, which is followed by release of oncogenes
and oncoproteins (37). To evade
chemotherapeutic agents, cancer cells can increase active drug
efflux via EV shedding, and the enhanced level of TD-EVs has been
identified as one of the most important mechanisms contributing to
cancer chemotherapy resistance (38). Remarkably, it has been demonstrated
that TD-EVs can cause the development of tumor cells with an
acquired drug resistance phenotype and contribute to multidrug
resistance. The release of multidrug resistance proteins, such as
Pgp-1 and lipid ceramide derived from TD-MVs, was reported to
mediate drug resistance (39). In
addition, TD-EVs could also mediate chemoresistance by taking up
chemotherapeutic drugs, and thus, limiting their bioavailability
for treating cancer cells (40).
Considering its multidrug resistant role, recent studies have been
devoted to inhibiting the release of TD-EVs from tumor cells in
order to sensitize them to chemotherapy. For instance, a study by
Kholia et al (41) reported
that inhibiting the release of TD-EVs from prostate cancer cells
reduced the drug resistance of these cells upon methotrexate
treatment. In addition to mediating drug resistance, the elevated
levels of EVs in tumor patients have also been reported to
influence multiple tumorigenic processes, including cell
proliferation, epithelial-mesenchymal transition (EMT),
angiogenesis, and premetastatic niche formation. Thus, the
transport of pathological growth factor receptors, various soluble
proteins, and miRNAs by TD-EVs aids tumor survival and spread.
TD-EVs transfer oncogenic cargo content
to promote tumor progression
Recent studies emphasized on the role of TD-EVs in
tumor colonization and progression by regulating tumor invasion,
angiogenesis, and suppressing immunity (42). An important method of TD-EVs to
facilitate tumor development and invasion is via transferring the
oncogenic cargo content. It is undeniable that several other
alternatives exist for tumor cell communication: direct
cell-to-cell contact; paracrine soluble cytokines signaling to
local cells; and cell-matrix interactions (43). Furthermore, another important way
of cell communication occurs through the TD-EVs transferring their
cargo to distant loci, a process that had been demonstrated to
facilitate the premetastatic niche formation and tumor metastasis.
The present review focuses on the role of TD-EVs in tumor
proliferation, EMT, extracellular matrix remodeling, angiogenesis,
and immunosuppression. Notably, TD-EVs were abundant in the
noncoding and specific coding RNA; the elevated levels of
amplified, missing, or mutated oncogene sequences and transposable
elements, might confer oncogenic information in the recipient cells
(44). It has been reported that
gastric cancer-derived exosomes could promote tumor proliferation
via activating the PI3K/AKT and MAPK/ERK signaling pathways
(45). It is well-known that EMT,
one of the hallmarks of aggressive cancer, confers tumors with a
more malignant and dedifferentiated phenotype, because of a
conversion from motionless epithelial cells into a more active and
motile cell type, mesenchymal cells (46). Previous studies have reported that
TD-EVs carried the full-length tissue factor (flTF) III or CD142
and induced the mesenchymal phenotype expression in tumors
(47). Transforming growth
factor-β is a key regulator responsible for EMT, which contributes
to the migration and spread of the tumor. Kim et al
(48) reported that TD-MVs’
content cargo (enriched in miR-23a) induced EMT in human lung
adenocarcinoma A549 cells via TGF-β1 signaling, suggesting that the
tumor could facilitate its invasion and malignant progression in an
autocrine way by releasing EVs.
The famous hypothesis ‘seed and soil’ put forward by
Stephen Paget in 1889 was a milestone for the study of tumor
metastasis and remains current even today (49). This hypothesis signifies the
importance of the microenvironment at the distant site (soil), not
simply focusing on the tumor itself (seed). Tumor microenvironment
(TME) is the foundation of tumor development and is necessary for
malignant cell survival (50). It
comprises of cellular [such as inflammatory cells, immunocytes,
endotheliocytes, mesenchymal stem cells (MSCs), and
cancer-associated fibroblasts (CAFs)] and noncellular components
(such as cytokines, chemokines, and matrix proteins). These two
components coordinate together to form the whole complex TME and to
support the survival and growth of tumors (51). Extracellular matrix (ECM),
predominantly comprised of glycoproteins, is the scaffold including
all the materials surrounding the cells, except from lymph and
blood, and is an essential component of the TME (52). TD-EVs shuttle across this scaffold
and construct a bridge between the tumor cells and surrounding
stromal cells to promote the spread of tumors. The degradation of
ECM further releases growth factors, enhancing the migration and
invasion capacity of the malignant cells (53). In addition, it has been reported
that the TME facilitates the differentiation of fibroblasts to
myofibroblasts or CAFs, which subsequently secrete matrix
metalloproteinases, further degrading the matrix proteins and
remodeling the ECM (54). For
example, Cho et al (55)
reported that breast cancer-derived exosomes could convert the
adipose derived mesenchymal stem cells into tumor-associated
myofibroblast-like cells, which contributed to the progression and
malignancy of tumors (55).
Collectively, TD-EVs appear to be crucial in ECM remodeling and as
a result in tumor migration and invasion (Fig. 4).
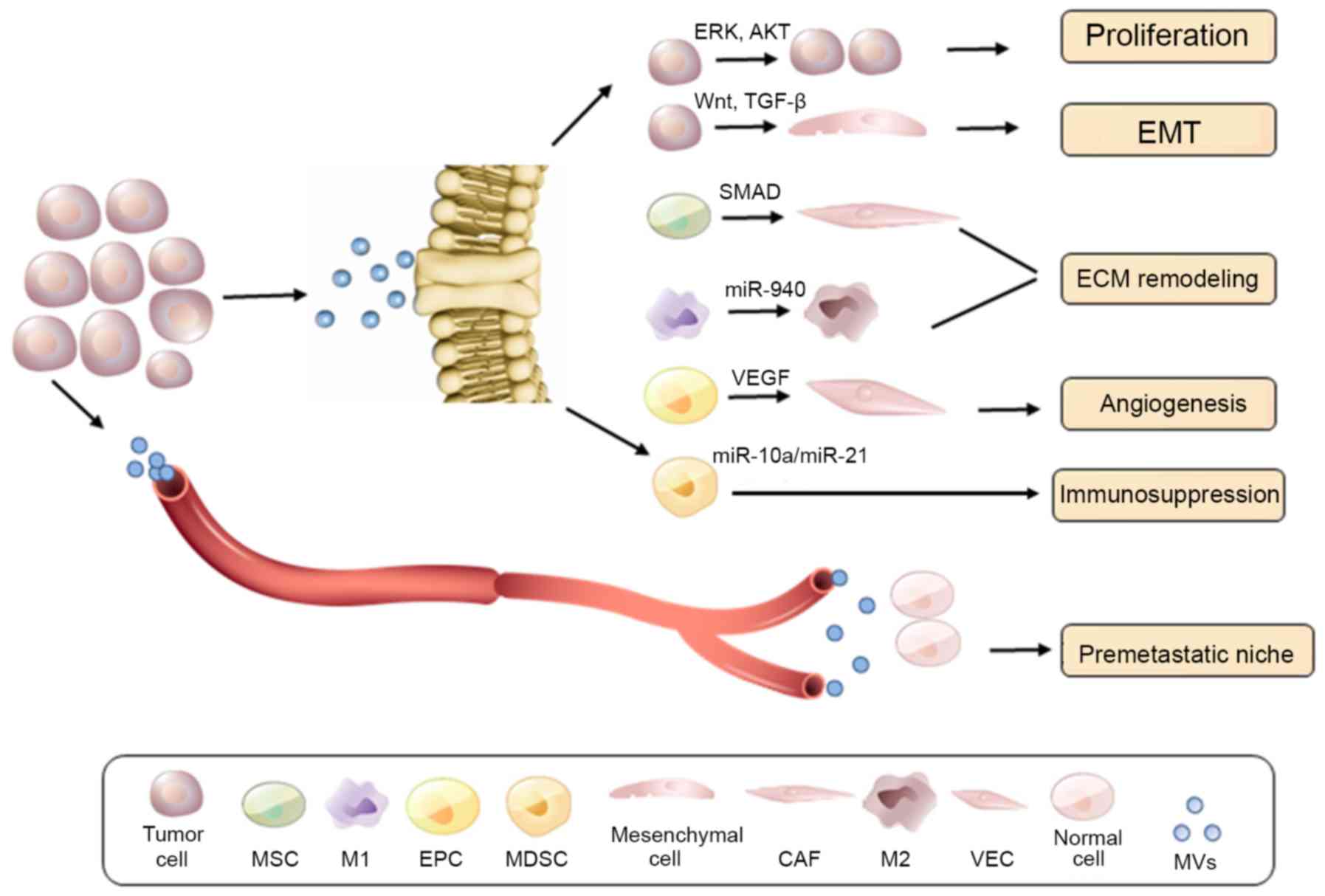 | Figure 4TD-EV mediated tumor progression and
premetastatic niche education. At the primary tumor site, shed
TD-EVs can promote tumor proliferation, EMT, ECM remodeling,
angiogenesis, and potentially immunosuppression, via its oncogenic
cargo content release. Furthermore, TD-EVs contribute to the
distant early metastatic niche formation, which facilitates cancer
metastasis. TD-EV, tumor-derived extracellular vesicle; EMT,
epithelial-mesenchymal transition; ECM, extracellular matrix; MSC,
mesenchymal stem cell; EPC, epithelial progenitor cell; MDSC,
myeloid-derived suppressor cell; CAF, cancer-associated fibroblast;
VEC, vascular endothelial cell; MVs, microvesicles; ERK,
extracellular signal-regulated kinase; AKT, AKT serine/threonine
kinase; TGF, transforming growth factor; VEGF, vascular endothelial
growth factor. |
Furthermore, increasing evidence suggests that
TD-EVs promote tumor angiogenesis and repress the antitumor immune
response, in order to protect the tumor cells from a hostile
environment (56). The exact
mechanisms for these effects of TD-EVs, however, remain unclear.
Tumor-associated macrophages (TAMs) generally adopt an M2-like
phenotype in tumors and the M2 polarization of TAMs has been
regarded as one of most important hallmarks of cancer progression
and metastasis (57). It has been
reported that TD-EVs could deliver cargo content to facilitate the
polarization of TAMs (58). For
example, Ying et al (59)
reported that epithelial ovarian cancer-derived exosomes induce
polarization of TAMs via transport of miR222-3p, which activates
the suppressor of cytokine signaling 3 (SOCS3)/signal transducer
and activator of transcription 3 (STAT3) signaling pathway. Despite
this, the delivery of miR-21 could also promote M2-like
polarization of TAMs in snail-overexpressing cancer cells (60). Essentially, TAMs further secrete
various angiogenic growth factors, such as vascular endothelial
growth factor (VEGF), interleukin (IL)-6, granulocyte-colony
stimulating factor (G-CSF), and tumor necrosis factor α (TNF-α), to
induce formation of new vessels within the tumor tissues, which is
essential for tumor cell survival under hypoxia conditions
(61). Furthermore, TD-EVs were
reported to repress the antitumor immune response by activating
regulatory T cells and myeloid-derived suppressor cells (MDSCs),
which further inhibit the targeted immune effect mediated by
CD8+ T cells (62).
Finally, the expression of Fas ligand (FasL) and TNF-related
apoptosis-inducing ligand (TRAIL) on the surface of TD-EVs could
also induce apoptosis of the cytotoxic CD8+ T cells,
potentially enhancing tumor immune evasion.
TD-EVs facilitate premetastatic niche
formation and tumor microenvironment remodeling
Tumor cells can communicate with the surrounding
tumor and stromal cells via the transport of cargo through TD-EVs;
this facilitates tumor invasion, angiogenesis, and immunity
suppression (63). Alternatively,
TD-EVs also contribute to the formation of a premetastatic niche
for the distant metastasis of cancer (64). Although the exact mechanism is not
clear, TD-EVs can induce vascular leakage and interact with cells
residing in remote organs. This interaction is selective and
highly-specific, because TD-EVs have been demonstrated to be
recruited to particular cell types located in specific organs,
depending on their membrane protein composition (65). For instance, tumor exosome
integrins have been reported to be involved in organotropic
metastasis: integrin avb5 is associated with hepatic metastases,
whereas α6b4 and α6b1 are related to lung metastases (66). Upon endocytosis by recipient cells,
TD-EVs induce the expression of multiple inflammatory factors, such
as S100, IL-8, IL-6, TGF-β and TNF-α, which contribute to the
activation of stromal cells and remodeling of the ECM (67).
Stromal cells, ECM, inflammatory immune cells, and
TD-EVs collectively form a permissive and attractive environment
for the metastatic cells, also called the premetastatic niche
(PMN). Presumably, this early metastatic niche has been engineered
and educated by TD-EVs to be prepared for metastatic tumor cell
implantation (68). Hood et
al (69) reported that
melanoma cells preferred migrating to places with abundant TD-MVs.
Melo et al (70)
demonstrated that glypican-1 on the membrane of MVs released in
body fluids of patients with cancer was considerably overexpressed
and promoted proliferation and metastasis in cancer cells (70). Based on these evidences, TD-EVs are
thought to be released to the perivascular space and easily get
across into vessels or lymph capillaries (several nm in size) and
the release of their cargo content (always abundant in oncogenic
nucleic acids and proteins) at a distant site then contributes to
the engineering of the PMN. This hypothesis might provide an
explanation for the potential mechanism of tumor metastasis.
Education of PMN by TD-EVs might involve various
aspects. For example, hypoxic TME notably contributes to an
aggressive phenotype and drug resistance that leads to tumor
progression, recurrence, and metastasis; however, the mechanisms
underlying these processes remain unclear (71). Recently, it was reported that
TD-EVs could be recruited and forced to arrive at the metastatic
locus, which was conducive to the improvement of tumor oxygen
levels, angiogenesis and metastasis (72). This might be one of the major
causes of hypoxia in the tumor microenvironment. Furthermore,
another important method through which TD-EV mediates the
engineering of early metastatic niches is by participating in the
formation of new blood vessels (73). Tumors commonly release a myriad of
growth factors to induce blood vessel formation (angiogenesis), for
providing oxygen and other essential nutrients through a dedicated
blood supply to the tumor. It has been reported that chronic
myeloid leukemia-derived exosomes induce angiogenic activity in
HUVEC cells and promote angiogenesis in a Src-dependent manner
(74). Collectively, through the
education or engineering of the PMN by TD-EVs, tumor cells that
have invaded and disseminated from an established primary tumor,
are led to a distant locus via blood vessels to implant and grow
into a secondary tumor (Fig.
4).
4. EVs applied for diagnosis and therapy of
cancer
Diagnostic role of EVs in cancer
Several studies have reported the significance of
TD-EVs in tumor progression. Nevertheless, research on the
application of TD-MVs for cancer diagnosis and therapy is relative
scarce, and yet not well-rounded. The present article reviewed the
recent literature regarding the TD-EV application for tumor
diagnosis and therapy. To seek personalized precision healthcare,
recent studies have emphasized the diagnostic role of EVs in cancer
(75). With the rise of liquid
biopsy tests and circulating tumor DNA analysis, scientists have
been focusing on EV diagnostic technologies comprising total
nucleic acid co-isolation [exosomal RNA (exoRNA) and circulating
tumor DNA (ctDNA)], target capture, and bioinformatics (76). As MVs or exosomes can be detected
in various body fluids, including saliva, urine and blood, EV
diagnostics have been one of the most important components in
liquid biopsy tests in addition to ctDNA and circulating tumor
cells (CTCs). In addition, tests on circulating EVs might be a more
precise and sensitive method for diagnosing cancer compared with
CTCs, due to the high variability in the number of CTCs among
different cancer patients (77).
It has been reported that an apparent increase of MV concentration
in the peripheral circulation of ovarian, breast, and pancreatic
cancer is observed (78).
Therefore, the concentrations of EVs could be measured in the body
fluids of patients for an early cancer diagnosis. Another important
part of EV diagnostics includes testing the cargo content, such as
proteins and nucleic acids. Considering the common characteristics
of EVs due to similar biogenesis and the overlap in its cargo
content, they can be isolated and identified via general biomarkers
regardless of their tissue of origin (79). Nevertheless, as aforementioned, EVs
commonly carry a series of tissue-specific characteristics or
‘labels’ similar to their secretory cells in order to reveal their
origin. Therefore, this property provides an identifiable and
unique biosignature for individual cancer patients (80).
In particular, TD-EVs considerably differ from the
common EVs in terms of cargo content, owing to their cancer
promoting properties (81). For
example, the expression of miR-21 in MVs derived from patients with
esophagus squamous cell carcinoma was considerably higher compared
with normal individuals, and this was closely associated with the
progression and malignancy of esophageal carcinoma (82). Additionally, miR-141 derived from
the serum of prostate cancer patients was overexpressed in the
primary and secondary tumors (83). Whole-genome sequencing analysis
revealed that MVs in sera from patients with pancreatic cancer
covered the genomic dsDNA in all 24 human chromosomes (84). In addition, the genetic mutations
associated with pancreatic ductal adenocarcinoma could be detected
in these TD-MVs (85). These
evidences indicate that the results of whole-genome sequencing
analysis in TD-EVs match the gene profile of primary and secondary
tumors. Proteins in TD-EVs are also associated with tumor
progression and have been previously discussed in detail.
Therapeutic role of EVs in cancer
Considering the facilitating role of TD-EVs in PMN
formation and tumor microenvironment remodeling, they may serve in
the future as a target for the clinical treatment of cancer
(86). For example, inhibition of
EV release prevents invasion of the tumor cells to surrounding
stromal cells, by inhibiting the interactions between them, thereby
hindering cancer angiogenesis and metastasis (87). In addition, it has been reported in
several studies that TD-EVs promote drug-resistance of tumor cells
in chemotherapy in several ways (88). Reports indicated that TD-MVs
mediated the transition of the tumor cells from sensitive to
drug-resistant, and thus elimination of TD-MVs might contribute in
improving drug sensitivity and effect of chemotherapy (89). In addition, Her-2 positive MVs
could repress the antiproliferation effect of trastuzumab on breast
cancer cells; therefore, it may be possible to improve response to
trastuzumab in patients by removing Her-2 positive MVs from their
tumors (90).
Recently, nanotechnology has been widely applied for
EV detection, characterization, and especially engineering
(91). The use of EVs as a drug
delivery platform for nanomedicine applications has been previously
emphasized (92). Tian et
al (93) used modified
exosomes as doxorubicin delivery vehicles to target
αv-integrin-positive tumor cells. Furthermore, Mizrak et al
(94) reported that genetically
modified MVs that carried suicide nucleic acids or proteins could
induce the death of schwannoma tumor cells. Liposoluble
chemotherapeutic drugs, such as paclitaxel and lomustine, are able
to penetrate the blood brain barrier, thus leading to increased
side effects. Application of exosome engineering for targeted
therapy has a great advantage in reducing drug side effects,
compared with conventional methods, owing to its lipid bilayer
structure, which can enwrap the therapeutic drugs to form a
hydrophobic structure (95).
Collectively, genetically engineered EVs may prove to be extremely
useful novel strategies for targeted therapy in cancer.
5. Conclusions and future direction
EVs are heterogeneous membrane-bound sacs with
multiple biological functions, such as signal transduction and
intracellular communication. The release of their cargo content,
including nucleic acids, proteins and lipids, contributes to
several physiological and pathological processes. EVs commonly
carry a series of tissue-specific characteristics or ‘labels’ that
match their secretory cells to reveal their origin, a process that
provides an identifiable and unique biosignature for individual
cancer patients (96). TD-EVs are
involved in various processes of tumor progression, such as
malignant proliferation, EMT, ECM remodeling, and angiogenesis. In
addition, they are crucial for premetastatic niche formation and
tumor microenvironment remodeling, processes that contribute to
cancer metastasis. Tests employing circulating EVs might be a more
accurate and sensitive way for diagnosis of cancer compared with
CTCs, and they may provide an easily identifiable and unique
biosignature for individual cancer patients. Finally, thanks to the
development of nanotechnology, EVs have been used as a drug
delivery vehicle for targeted tumor therapy.
Taken together, this review article provides a
comprehensive understanding of the roles of EVs in cancer
progression, and reveals the characteristics of EVs originated from
different sources, especially from different cancer cells, which
has not been reported to date. Furthermore, the latest progress in
the potential clinical applications of EVs were discussed. Our
knowledge of the basic structures, cargo content and biological
roles of EVs have tremendously progressed; however, more
achievement is expected. For example, little is known as to how
TD-EVs are recruited to distant loci and connect with the
surrounding stromal cells (97).
In addition, although it is established that the signatures among
EVs differ based on their tissue of origin, their biological
functions still cannot be distinguished according to these
signatures. Further research will fully elucidate the roles and
functions of TD-EVs, with the hope that EV-based strategies will be
beneficial in the future for personalized precision healthcare in
patients with cancer.
Funding
This study was supported by the Shandong Medical and
Health Science and Technology Development Plan (grant no.
2018WS047).
Availability of data and materials
Not applicable.
Authors’ contributions
FHQ, PP, YBM, YJ and RM were involved in the
conception of the study subject. JPY, JS, YZ, ZYW, XQL and NC were
involved in the study design and the acquisition of data. PP, FHQ
and RM were involved in the writing of the article and revised this
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests
Acknowledgments
Not applicable.
References
|
1
|
Lee TH, D’Asti E, Magnus N, Al-Nedawi K,
Meehan B and Rak J: Microvesicles as mediators of intercellular
communication in cancer–the emerging science of cellular ‘debris’.
Semin Immunopathol. 33:455–467. 2011.
|
|
2
|
Meckes DG Jr and Raab-Traub N:
Microvesicles and viral infection. J Virol. 85:12844–12854.
2011.
|
|
3
|
Raposo G and Stoorvogel W: Extracellular
vesicles: Exosomes, microvesicles, and friends. J Cell Biol.
200:373–383. 2013.
|
|
4
|
Barteneva NS, Maltsev N and Vorobjev IA:
Microvesicles and intercellular communication in the context of
parasitism. Front Cell Infect Microbiol. 3:492013.
|
|
5
|
Kalra H, Simpson RJ, Ji H, Aikawa E,
Altevogt P, Askenase P, Bond VC, Borràs FE, Breakefield X, Budnik
V, et al: Vesiclepedia: A compendium for extracellular vesicles
with continuous community annotation. PLoS Biol.
10:e10014502012.
|
|
6
|
Ratajczak J, Wysoczynski M, Hayek F,
Janowska-Wieczorek A and Ratajczak MZ: Membrane-derived
microvesicles: Important and underappreciated mediators of
cell-to-cell communication. Leukemia. 20:1487–1495. 2006.
|
|
7
|
Martínez MC, Larbret F, Zobairi F,
Coulombe J, Debili N, Vainchenker W, Ruat M and Freyssinet JM:
Transfer of differentiation signal by membrane microvesicles
harboring hedgehog morphogens. Blood. 108:3012–3020. 2006.
|
|
8
|
Akers JC, Gonda D, Kim R, Carter BS and
Chen CC: Biogenesis of extracellular vesicles (EV): Exosomes,
microvesicles, retrovirus-like vesicles, and apoptotic bodies. J
Neurooncol. 113:1–11. 2013.
|
|
9
|
Lawson C, Vicencio JM, Yellon DM and
Davidson SM: Microvesicles and exosomes: New players in metabolic
and cardiovascular disease. J Endocrinol. 228:R57–R71. 2016.
|
|
10
|
Jansa R, Sustar V, Frank M, Susanj P,
Bester J, Mancek-Keber M, Krzan M and Iglic A: Number of
microvesicles in peripheral blood and ability of plasma to induce
adhesion between phos-pholipid membranes in 19 patients with
gastrointestinal diseases. Blood Cells Mol Dis. 41:124–132.
2008.
|
|
11
|
Loyer X, Vion AC, Tedgui A and Boulanger
CM: Microvesicles as cell-cell messengers in cardiovascular
diseases. Circ Res. 114:345–353. 2014.
|
|
12
|
Sullivan R: Epididymosomes: A
heterogeneous population of microvesicles with multiple functions
in sperm maturation and storage. Asian J Androl. 17:726–729.
2015.
|
|
13
|
Didiot MC, Hall LM, Coles AH, Haraszti RA,
Godinho BM, Chase K, Sapp E, Ly S, Alterman JF, Hassler MR, et al:
Exosome-mediated Delivery of Hydrophobically Modified siRNA for
Huntingtin mRNA Silencing. Mol Ther. 24:1836–1847. 2016.
|
|
14
|
Cao FY, Fan JX, Long Y, Zeng X and Zhang
XZ: A smart fluorescence nanoprobe for the detection of cellular
alkaline phosphatase activity and early osteogenic differentiation.
Nanomedicine (Lond). 12:1313–1322. 2016.
|
|
15
|
Lau KH and Baylink DJ: Osteoblastic
tartrate-resistant acid phosphatase: Its potential role in the
molecular mechanism of osteogenic action of fluoride. J Bone Miner
Res. 18:1897–1900. 1900.
|
|
16
|
Aliotta JM, Pereira M, Johnson KW, de Paz
N, Dooner MS, Puente N, Ayala C, Brilliant K, Berz D and Lee D:
Microvesicle entry into marrow cells mediates tissue-specific
changes in mRNA by direct delivery of mRNA and induction of
transcription. Exp Hematol. 38:233–245. 2010.
|
|
17
|
Collino F, Deregibus MC, Bruno S, Sterpone
L, Aghemo G, Viltono L, Tetta C and Camussi G: Microvesicles
derived from adult human bone marrow and tissue specific
mesenchymal stem cells shuttle selected pattern of miRNAs. PLoS
One. 5:e118032010.
|
|
18
|
Waldenström A, Gennebäck N, Hellman U and
Ronquist G: Cardiomyocyte microvesicles contain DNA/RNA and convey
biological messages to target cells. PLoS One. 7:e346532012.
|
|
19
|
Haraszti RA, Didiot MC, Sapp E, Leszyk J,
Shaffer SA, Rockwell HE, Gao F, Narain NR, DiFiglia M, Kiebish MA,
et al: High-resolution proteomic and lipidomic analysis of exosomes
and microvesicles from different cell sources. J Extracell
Vesicles. 5:325702016.
|
|
20
|
Zheng T, Pu J, Chen Y, Mao Y, Guo Z, Pan
H, Zhang L, Zhang H, Sun B and Zhang B: Plasma Exosomes Spread and
Cluster Around β-Amyloid Plaques in an Animal Model of Alzheimer’s
Disease. Front Aging Neurosci. 9:122017.
|
|
21
|
Hwang DW, Choi H, Jang SC, Yoo MY, Park
JY, Choi NE, Oh HJ, Ha S, Lee YS, Jeong JM, et al: Noninvasive
imaging of radio-labeled exosome-mimetic nanovesicle using
(99m)Tc-HMPAO. Sci Rep. 5:156362015.
|
|
22
|
Zhao JY, Chen G, Gu YP, Cui R, Zhang ZL,
Yu ZL, Tang B, Zhao YF and Pang DW: Ultrasmall Magnetically
Engineered Ag2Se Quantum Dots for Instant Efficient Labeling and
Whole-Body High-Resolution Multimodal Real-Time Tracking of
Cell-Derived Microvesicles. J Am Chem Soc. 138:1893–1903. 2016.
|
|
23
|
Abels ER and Breakefield XO: Introduction
to Extracellular Vesicles: Biogenesis, RNA Cargo Selection,
Content, Release, and Uptake. Cell Mol Neurobiol. 36:301–312.
2016.
|
|
24
|
Datta A, Chen CP and Sze SK: Discovery of
prognostic biomarker candidates of lacunar infarction by
quantitative proteomics of microvesicles enriched plasma. PLoS One.
9:e946632014.
|
|
25
|
Tricarico C, Clancy J and D’Souza-Schorey
C: Biology and biogenesis of shed microvesicles. Small GTPases.
8:220–232. 2017.
|
|
26
|
Al-Nedawi K, Meehan B, Micallef J, Lhotak
V, May L, Guha A and Rak J: Intercellular transfer of the oncogenic
receptor EGFRvIII by microvesicles derived from tumour cells. Nat
Cell Biol. 10:619–624. 2008.
|
|
27
|
Principe S, Hui AB, Bruce J, Sinha A, Liu
FF and Kislinger T: Tumor-derived exosomes and microvesicles in
head and neck cancer: Implications for tumor biology and biomarker
discovery. Proteomics. 13:1608–1623. 2013.
|
|
28
|
Lee K, Shao H, Weissleder R and Lee H:
Acoustic purification of extracellular microvesicles. ACS Nano.
9:2321–2327. 2015.
|
|
29
|
Tickoo SK, Lee MW, Eble JN, Amin M,
Christopherson T, Zarbo RJ and Amin MB: Ultrastructural
observations on mitochondria and microvesicles in renal oncocytoma,
chromophobe renal cell carcinoma, and eosinophilic variant of
conventional (clear cell) renal cell carcinoma. Am J Surg Pathol.
24:1247–1256. 2000.
|
|
30
|
Silverman JM and Reiner NE: Exosomes and
other microvesicles in infection biology: Organelles with
unanticipated phenotypes. Cell Microbiol. 13:1–9. 2011.
|
|
31
|
Mathivanan S, Lim JW, Tauro BJ, Ji H,
Moritz RL and Simpson RJ: Proteomics analysis of A33
immunoaffinity-purified exosomes released from the human colon
tumor cell line LIM1215 reveals a tissue-specific protein
signature. Mol Cell Proteomics. 9:197–208. 2010.
|
|
32
|
Wenzel D, Schauermann G, von Lüpke A and
Hinz G: The cargo in vacuolar storage protein transport vesicles is
stratified. Traffic. 6:45–55. 2005.
|
|
33
|
Miranda KC, Bond DT, Levin JZ, Adiconis X,
Sivachenko A, Russ C, Brown D, Nusbaum C and Russo LM: Massively
parallel sequencing of human urinary exosome/microvesicle RNA
reveals a predominance of non-coding RNA. PLoS One.
9:e960942014.
|
|
34
|
Arendt BK, Walters DK, Wu X, Tschumper RC
and Jelinek DF: Multiple myeloma dell-derived microvesicles are
enriched in CD147 expression and enhance tumor cell proliferation.
Oncotarget. 5:5686–5699. 2014.
|
|
35
|
Liao CF, Lin SH, Chen HC, Tai CJ, Chang
CC, Li LT, Yeh CM, Yeh KT, Chen YC, Hsu TH, et al: CSE1L, a novel
microvesicle membrane protein, mediates Ras-triggered microvesicle
generation and metastasis of tumor cells. Mol Med. 18:1269–1280.
2012.
|
|
36
|
Dong Y, Pan Q, Jiang L, Chen Z, Zhang F,
Liu Y, Xing H, Shi M, Li J, Li X, et al: Tumor endothelial
expression of P-glycoprotein upon microvesicular transfer of TrpC5
derived from adriamycin-resistant breast cancer cells. Biochem
Biophys Res Commun. 446:85–90. 2014.
|
|
37
|
Aubertin K, Silva AKA, Luciani N, Espinosa
A, Djemat A, Charue D, Gallet F, Blanc-Brude O and Wilhelm C:
Massive release of extracellular vesicles from cancer cells after
photo-dynamic treatment or chemotherapy. Sci Rep. 6:353762016.
|
|
38
|
Qiu J, Yang G, Feng M, Zheng S, Cao Z, You
L, Zheng L, Zhang T and Zhao Y: Extracellular vesicles as mediators
of the progression and chemoresistance of pancreatic cancer and
their potential clinical applications. Mol Cancer. 17:22018.
|
|
39
|
Muralidharan-Chari V, Clancy JW, Sedgwick
A and D’Souza-Schorey C: Microvesicles: Mediators of extracellular
communication during cancer progression. J Cell Sci. 123:1603–1611.
2010.
|
|
40
|
Wang X, Xu C, Hua Y, Sun L, Cheng K, Jia
Z, Han Y, Dong J, Cui Y and Yang Z: Exosomes play an important role
in the process of psoralen reverse multidrug resistance of breast
cancer. J Exp Clin Cancer Res. 35:1862016.
|
|
41
|
Kholia S, Jorfi S, Thompson PR, Causey CP,
Nicholas AP, Inal JM and Lange S: A novel role for peptidylarginine
deiminases in microvesicle release reveals therapeutic potential of
PAD inhibition in sensitizing prostate cancer cells to
chemotherapy. J Extracell Vesicles. 4:261922015.
|
|
42
|
Al-Nedawi K, Meehan B and Rak J:
Microvesicles: Messengers and mediators of tumor progression. Cell
Cycle. 8:2014–2018. 2009.
|
|
43
|
Wendler F, Favicchio R, Simon T,
Alifrangis C, Stebbing J and Giamas G: Extracellular vesicles swarm
the cancer micro-environment: From tumor-stroma communication to
drug intervention. Oncogene. 36:877–884. 2017.
|
|
44
|
Balaj L, Lessard R, Dai L, Cho YJ, Pomeroy
SL, Breakefield XO and Skog J: Tumour microvesicles contain
retrotransposon elements and amplified oncogene sequences. Nat
Commun. 2:1802011.
|
|
45
|
Qu JL, Qu XJ, Zhao MF, Teng YE, Zhang Y,
Hou KZ, Jiang YH, Yang XH and Liu YP: Gastric cancer exosomes
promote tumour cell proliferation through PI3K/Akt and MAPK/ERK
activation. Dig Liver Dis. 41:875–880. 2009.
|
|
46
|
Salnikov AV, Liu L, Platen M, Gladkich J,
Salnikova O, Ryschich E, Mattern J, Moldenhauer G, Werner J,
Schemmer P, et al: Hypoxia induces EMT in low and highly aggressive
pancreatic tumor cells but only cells with cancer stem cell
characteristics acquire pronounced migratory potential. PLoS One.
7:e463912012.
|
|
47
|
Zhou Y, Xiong M, Fang L, Jiang L, Wen P,
Dai C, Zhang CY and Yang J: miR-21-containing microvesicles from
injured tubular epithelial cells promote tubular phenotype
transition by targeting PTEN protein. Am J Pathol. 183:1183–1196.
2013.
|
|
48
|
Kim J, Kim TY, Lee MS, Mun JY, Ihm C and
Kim SA: Exosome cargo reflects TGF-β1-mediated
epithelial-to-mesenchymal transition (EMT) status in A549 human
lung adenocarcinoma cells. Biochem Biophys Res Commun. 478:643–648.
2016.
|
|
49
|
Ribatti D, Mangialardi G and Vacca A:
Stephen Paget and the ‘seed and soil’ theory of metastatic
dissemination. Clin Exp Med. 6:145–149. 2006.
|
|
50
|
Witz IP: The tumor microenvironment: The
making of a paradigm. Cancer Microenviron. 2(Suppl 1): 9–17.
2009.
|
|
51
|
Wiig H, Tenstad O, Iversen PO, Kalluri R
and Bjerkvig R: Interstitial fluid: The overlooked component of the
tumor micro-environment? Fibrogenesis Tissue Repair. 3:122010.
|
|
52
|
Chouaib S, Kieda C, Benlalam H, Noman MZ,
Mami-Chouaib F and Rüegg C: Endothelial cells as key determinants
of the tumor microenvironment: Interaction with tumor cells,
extracellular matrix and immune killer cells. Crit Rev Immunol.
30:529–545. 2010.
|
|
53
|
Vlodavsky I, Korner G, Ishai-Michaeli R,
Bashkin P, Bar-Shavit R and Fuks Z: Extracellular matrix-resident
growth factors and enzymes: Possible involvement in tumor
metastasis and angio-genesis. Cancer Metastasis Rev. 9:203–226.
1990.
|
|
54
|
Micke P and Ostman A: Exploring the tumour
environment: Cancer-associated fibroblasts as targets in cancer
therapy. Expert Opin Ther Targets. 9:1217–1233. 2005.
|
|
55
|
Cho JA, Park H, Lim EH and Lee KW:
Exosomes from breast cancer cells can convert adipose
tissue-derived mesenchymal stem cells into myofibroblast-like
cells. Int J Oncol. 40:130–138. 2012.
|
|
56
|
Dings R, Vang K, Griffioen A, Farrar M and
Mayo K: Angiogenesis inhibitors promote T-cell mediated anti-tumor
response by diminishing tumor counter immuno-surveillance. Cancer
Res. 68:11192008.
|
|
57
|
Caillou B, Talbot M, Weyemi U,
Pioche-Durieu C, Al Ghuzlan A, Bidart JM, Chouaib S, Schlumberger M
and Dupuy C: Tumor-associated macrophages (TAMs) form an
interconnected cellular supportive network in anaplastic thyroid
carcinoma. PLoS One. 6:e225672011.
|
|
58
|
Yang M, Chen J, Su F, Yu B, Su F, Lin L,
Liu Y, Huang JD and Song E: Microvesicles secreted by macrophages
shuttle invasion-potentiating microRNAs into breast cancer cells.
Mol Cancer. 10:1172011.
|
|
59
|
Ying X, Wu Q, Wu X, Zhu Q and Wang X,
Jiang L, Chen X and Wang X: Epithelial ovarian cancer-secreted
exosomal miR-222-3p induces polarization of tumor-associated
macrophages. Oncotarget. 7:43076–43087. 2016.
|
|
60
|
Hsieh CH, Tai SK and Yang MH:
Snail-overexpressing Cancer Cells Promote M2-Like Polarization of
Tumor-Associated Macrophages by Delivering MiR-21-Abundant
Exosomes. Neoplasia. 20:775–788. 2018.
|
|
61
|
Huang Q, Duan L, Qian X, Fan J, Lv Z,
Zhang X, Han J, Wu F, Guo M, Hu G, et al: IL-17 Promotes Angiogenic
Factors IL-6, IL-8, and Vegf Production via Stat1 in Lung
Adenocarcinoma. Sci Rep. 6:365512016.
|
|
62
|
Szajnik M, Czystowska M, Szczepanski MJ,
Mandapathil M and Whiteside TL: Tumor-derived microvesicles induce,
expand and up-regulate biological activities of human regulatory T
cells (Treg). PLoS One. 5:e114692010.
|
|
63
|
Blonska M, Agarwal NK and Vega F: Shaping
of the Tumor Microenvironment: Stromal Cells and Vessels. Semin
Cancer Biol. 34:3–13. 2015.
|
|
64
|
Atala A: Re: Microvesicles released from
human renal cancer stem cells stimulate angiogenesis and formation
of lung premeta-static niche. J Urol. 187:1506–1507. 2012.
|
|
65
|
Martins VR, Dias MS and Hainaut P:
Tumor-cell-derived microvesicles as carriers of molecular
information in cancer. Curr Opin Oncol. 25:66–75. 2013.
|
|
66
|
Alipoor SD, Mortaz E, Varahram M,
Movassaghi M, Kraneveld AD, Garssen J and Adcock IM: The Potential
Biomarkers and Immunological Effects of Tumor-Derived Exosomes in
Lung Cancer. Front Immunol. 9:8192018.
|
|
67
|
Heinrich LF, Andersen DK, Cleasby ME and
Lawson C: Long-term high fat feeding of rats results in increased
numbers of circulating microvesicles with pro-inflammatory effects
on endothelial cells. Br J Nutr. 113:1704–1711. 2015.
|
|
68
|
Aguado BA, Bushnell GG, Rao SS, Jeruss JS
and Shea LD: Engineering the pre-metastatic niche. Nat Biomed Eng.
1:1–28. 2017.
|
|
69
|
Hood JL, San RS and Wickline SA: Exosomes
released by melanoma cells prepare sentinel lymph nodes for tumor
metastasis. Cancer Res. 71:3792–3801. 2011.
|
|
70
|
Melo SA, Luecke LB, Kahlert C, Fernandez
AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari
N, et al: Glypican-1 identifies cancer exosomes and detects early
pancreatic cancer. Nature. 523:177–182. 2015.
|
|
71
|
Semenza GL: The hypoxic tumor
microenvironment: A driving force for breast cancer progression.
Biochim Biophys Acta. 1863:382–391. 2016.
|
|
72
|
Svensson K: Mechanistic Studies On the
Role of Polyamines and Microvesicles in Tumor Growth and
Hypoxia-mediated Angiogenesis. Scand J Dent Res. 80:139–154.
2012.
|
|
73
|
Lima LG, Chammas R, Monteiro RQ, Moreira
ME and Barcinski MA: Tumor-derived microvesicles modulate the
establishment of metastatic melanoma in a
phosphatidyl-serine-dependent manner. Cancer Lett. 283:168–175.
2009.
|
|
74
|
Mineo M, Garfield SH, Taverna S, Flugy A,
De Leo G, Alessandro R and Kohn EC: Exosomes released by K562
chronic myeloid leukemia cells promote angiogenesis in a
Src-dependent fashion. Angiogenesis. 15:33–45. 2012.
|
|
75
|
Rahbari M, Rahbari N, Reissfelder C, Weitz
J and Kahlert C: Exosomes: Novel implications in diagnosis and
treatment of gastrointestinal cancer. Langenbecks Arch Surg.
401:1097–1110. 2016.
|
|
76
|
Diaz LA Jr and Bardelli A: Liquid
biopsies: Genotyping circulating tumor DNA. J Clin Oncol.
32:579–586. 2014.
|
|
77
|
Armstrong D and Wildman DE: Extracellular
Vesicles and the Promise of Continuous Liquid Biopsies. J Pathol
Transl Med. 52:1–8. 2018.
|
|
78
|
Wieckowski EU, Kim JW, Stanson JD, Taylor
DD, Reichert TE and Whiteside TL: FasL+ and FasL-microvesicles in
the circulation of patients with cancer induce apoptosis of
activated T lymphocytes. Cancer Res. 64:Issue 72004.
|
|
79
|
Hosseinibeheshti E, Pham S, Adomat H, Li N
and Tomlinson Guns ES: Exosomes as biomarker enriched
microvesicles: characterization of exosomal proteins derived from a
panel of prostate cell lines with distinct AR phenotypes. Mol Cell
Proteomics. 11:863–885. 2012.
|
|
80
|
Baran J, Baj-Krzyworzeka M, Weglarczyk K,
Szatanek R and Zembala M, Barbasz J, Czupryna A, Szczepanik A and
Zembala M: Circulating tumour-derived microvesicles in plasma of
gastric cancer patients. Cancer Immunol Immunother. 59:841–850.
2010.
|
|
81
|
Valenti R, Huber V, Filipazzi P, Pilla L,
Sovena G, Villa A, Corbelli A, Fais S, Parmiani G and Rivoltini L:
Human tumor-released microvesicles promote the differentiation of
myeloid cells with transforming growth factor-beta-mediated
suppressive activity on T lymphocytes. Cancer Res. 66:9290–9298.
2006.
|
|
82
|
Kimura S, Naganuma S, Susuki D, Hirono Y,
Yamaguchi A, Fujieda S, Sano K and Itoh H: Expression of microRNAs
in squamous cell carcinoma of human head and neck and the
esophagus: miR-205 and miR-21 are specific markers for HNSCC and
ESCC. Oncol Rep. 23:1625–1633. 2010.
|
|
83
|
Kachakova D, Mitkova A, Popov E, Popov I,
Vlahova A, Dikov T, Christova S, Mitev V, Slavov C and Kaneva R:
Combinations of serum prostate-specific antigen and plasma
expression levels of let-7c, miR-30c, miR-141, and miR-375 as
potential better diagnostic biomarkers for prostate cancer. DNA
Cell Biol. 34:189–200. 2015.
|
|
84
|
Kahlert C, Melo SA, Protopopov A, Tang J,
Seth S, Koch M, Zhang J, Weitz J, Chin L, Futreal A, et al:
Identification of double-stranded genomic DNA spanning all
chromosomes with mutated KRAS and p53 DNA in the serum exosomes of
patients with pancreatic cancer. J Biol Chem. 289:3869–3875.
2014.
|
|
85
|
Borecka M, Zemankova P, Vocka M, Soucek P,
Soukupova J, Kleiblova P, Sevcik J, Kleibl Z and Janatova M:
Mutation analysis of the PALB2 gene in unselected pancreatic cancer
patients in the Czech Republic. Cancer Genet. 209:199–204.
2016.
|
|
86
|
Vader P, Breakefield XO and Wood MJ:
Extracellular vesicles: Emerging targets for cancer therapy. Trends
Mol Med. 20:385–393. 2014.
|
|
87
|
Sidhu SS, Mengistab AT, Tauscher AN,
LaVail J and Basbaum C: The microvesicle as a vehicle for EMMPRIN
in tumor-stromal interactions. Oncogene. 23:956–963. 2004.
|
|
88
|
Jorfi S and Inal JM: The role of
microvesicles in cancer progression and drug resistance. Biochem
Soc Trans. 41:293–298. 2013.
|
|
89
|
Chen WX, Zhong SL, Ji MH, Pan M, Hu Q, Lv
MM, Luo Z, Zhao JH and Tang JH: MicroRNAs delivered by
extracellular vesicles: An emerging resistance mechanism for breast
cancer. Tumour Biol. 35:2883–2892. 2014.
|
|
90
|
Esteva FJ: Novel strategies for
HER-2-positive metastatic disease: Mechanisms and therapeutic
options to overcome trastuzumab resistance. Breast Cancer Res.
9(Suppl 1): 1–2. 2007.
|
|
91
|
Silva AK, Luciani N, Gazeau F, Aubertin K,
Bonneau S, Chauvierre C, Letourneur D and Wilhelm C: Combining
magnetic nanoparticles with cell derived microvesicles for drug
loading and targeting. Nanomedicine. 11:645–655. 2015.
|
|
92
|
Vader P, Mol EA, Pasterkamp G and
Schiffelers RM: Extracellular vesicles for drug delivery. Adv Drug
Deliv Rev. 106(Pt A): 148–156. 2016.
|
|
93
|
Tian Y, Li S, Song J, Ji T, Zhu M,
Anderson GJ, Wei J and Nie G: A doxorubicin delivery platform using
engineered natural membrane vesicle exosomes for targeted tumor
therapy. Biomaterials. 35:2383–2390. 2014.
|
|
94
|
Mizrak A, Bolukbasi MF, Ozdener GB,
Brenner GJ, Madlener S, Erkan EP, Ströbel T, Breakefield XO and
Saydam O: Genetically engineered microvesicles carrying suicide
mRNA/protein inhibit schwannoma tumor growth. Mol Ther. 21:101–108.
2013.
|
|
95
|
Kim HO, Choi SM and Kim HS: Mesenchymal
stem cell-derived secretome and microvesicles as a cell-free
therapeutics for neuro-degenerative disorders. Tissue Eng Regen
Med. 10:93–101. 2013.
|
|
96
|
Miller VM, Lahr BD, Bailey KR, Hodis HN,
Mulvagh SL and Jayachandran M: Specific cell-derived microvesicles:
Linking endothelial function to carotid artery intima-media
thickness in low cardiovascular risk menopausal women.
Atherosclerosis. 246:21–28. 2016.
|
|
97
|
Kahlert C and Kalluri R: Exosomes in tumor
microenvironment influence cancer progression and metastasis. J Mol
Med (Berl). 91:431–437. 2013.
|