Introduction
Breast cancer is a deadly form of cancer and the
main cause of cancer-associated mortality among women worldwide
(1). Surgical resection,
radiotherapy, chemotherapy and molecular-targeted therapy are the
main methods of clinical breast cancer treatment (2). At present, improvements in early
diagnostic methods and treatment strategies have led to notable
decreases in the mortality rate; however, a considerable portion of
patients eventually succumb with tumor recurrence and drug
resistance (3,4). Therefore, it is crucial to develop
novel and alternative therapeutic agents for the treatment of
breast cancer.
Near infrared (NIR) has a high potential in the
diagnosis and treatment of various diseases, including colorectal
cancer, gastric cancer and kidney cancer (5-7),
owing to its well-visualized and notable tissue permeability
properties (8). Of all types of
NIR agents, IR-783 has received increasing attention for its
excellent imaging and tumor targeting properties (9,10).
Previously, several studies have revealed that IR-783 can be
specifically taken up and enriched by cancer cells, targeting
tumors of the brain, prostate and colon with low-toxicity for
normal tissues (10-12). In recent years, researchers have
reported that IR-783 possessed the ability to reduce the viability
of cancer cells via pH switchable photothermal therapy and
photodynamic therapy (13,14). In addition, our group previously
reported that IR-783 decreased MDA-MB-231 cell viability and
promoted mitochondrial apoptosis of human breast cancer cells
(15,16).
Mitochondria are important double-membrane cellular
organelles, which are essential for cell homeostasis and survival,
as well as generating the majority of cellular energy in the form
of adenosine triphosphate (ATP) (17-19).
Furthermore, mitochondria are dynamic organelles that undergo
continuous fission and fusion; the structure and function of which
are highly regulated to meet the physiological needs of the cell
(20,21). The imbalance of fission and fusion,
of mitochondria leads to structural changes and dysfunction of the
mitochondria, and serves an important role in cell-cycle
progression, apoptosis, mitophagy and ATP production (15,21-23).
Importantly, mitochondrial dynamics are essential for cell
proliferation, development, death and diseases (24). Additionally, previous studies have
demonstrated that IR-783 promoted breast cancer cells apoptosis by
inducing mitochondrial fission (15). Therefore, we hypothesized that
mitochondria may also contribute to IR-783-induced inhibition of
breast cancer cell proliferation and migration.
In the present study, we aimed to investigate the
inhibitory effects of IR-783 on breast cancer cell proliferation
and migration. Our results indicated that IR-783 inhibited
MDA-MB-231 and MCF-7 cell proliferation by inducing cell cycle
arrest at the G0/G1 phase. Additionally, IR-783 inhibited
MDA-MB-231 and MCF-7 cell migration, which was accompanied with
inhibiting filopodia formation with reductions in filamentous actin
(F-actin) polymerization. Furthermore, mechanistic analysis
revealed that IR-783 treatment induced mitochondrial fission
through inhibiting the expression of mitochondrial fusion proteins
optic atrophy 1 (OPA1), mitofusin 1 (Mfn1) and mitofusin 2 (Mfn2),
but promoting that of the fission proteins, mitochondrial fission
factor (MFF), mitochondrial fission 1 protein (Fis1) and
dynamin-related protein 1 (Drp1). Our data indicated the underlying
mechanism of IR-783 on inhibiting breast cancer cell proliferation
and migration, and provide a mechanistic basis for the potential
application of IR-783 in the treatment of breast cancer.
Materials and methods
Chemicals and antibodies
The NIR dye IR-783 was obtained from Sigma-Aldrich
(Merck KGaA) and dissolved in sterile distilled water as a stock
solution. The antibodies used in the present study were as follows:
Anti-Cyclin D1 (cat. no. sc-8396), anti-Cyclin E (cat. no. sc-481),
anti-cyclin-dependent kinase 2 (CDK2; cat. no. sc-6248), anti-OPA1
(cat. no. sc-393296), anti-Mfn1 (cat. no. sc-1666447), anti-Mfn2
(cat. no. sc-515647), anti-MFF (cat. no. sc-398617), anti-Fis1
(cat. no. sc-376447) and anti-Drp1 (cat. no. sc-271583) were
obtained from Santa Cruz Biotechnology, Inc. Anti-matrix
metalloproteinase-2 (MMP-2; cat. no. 40994) and anti-MMP-9 (cat.
no. 13667) were obtained from Cell Signaling Technology, Inc.
Anti-GAPDH (cat. no. AG019-1) was obtained from Beyotime Institute
of Biotechnology.
Cell culture
The human breast cancer cell lines MDA-MB-231 and
MCF-7 were purchased from the American Type Culture Collection. The
cells were cultured in Dulbecco's modified Eagle's medium (DMEM)
containing 10% fetal bovine serum (FBS), all from Gibco (Thermo
Fisher Scientific, Inc.). Cells were cultured at 37°C with 5%
CO2 and digested every 2 days by 0.25% trypsin (Gibco;
Thermo Fisher Scientific, Inc.).
MTT assay
Cells (2×104 cells/well) were seeded onto
96-well plates and allowed to attach. After treatment with various
concentrations of IR-783 (0, 20, 40, 60, 80, 100, 120, 140 and 160
μM) for 24 h or with 80 μM IR-783 for different time
intervals (0, 3, 6, 12, 24, 36, 48 and 72 h) in a 5% CO2
incubator at 37°C, cells were incubated with 20 μl MTT
solution (5 mg/ml; Sigma-Aldrich; Merck KGaA), cells were treated
at 37°C for 4 h. Then, the medium was removed slowly and 150
μl dimethyl sulfoxide (Eastman Kodak) was added to dissolve
the formazan crystals. The proliferation of cells was determined
using a microplate reader (Varioskan Flash; Thermo Fisher
Scientific, Inc.) at 490 nm. All results were repeated in three
independent experiments.
Clone formation assay
Cells were digested by 0.25% trypsin and seeded in a
5-cm sterile petri-dish at a density of 100 cells per dish
overnight. Then, cells were treated with different concentrations
of IR-783 (0, 80 and 160 μM) the next day and incubated at
37°C. After 24 h, the supernatant was discarded carefully and fresh
DMEM with 10% FBS was added. Cells were cultured for 2-3 weeks
until clones were visible. After removing culture medium, clones
were washed for three times with PBS, fixed with 4%
paraformaldehyde for 15 min, then stained with 0.1% crystal violet
for 10 min at room temperature. Clones were counted using Photoshop
CS6 software (version 13.0; Adobe Systems, Inc.). All results were
performed for three independent times.
5′-Ethynyl-2′-deoxyuridine (EdU)
assay
Cell proliferation was assessed with a BeyoClick™
EDU-488 imaging Kit (cat. no. C0071S; Beyotime Institute of
Biotechnology). Cells were inoculated onto slides of 24-well plates
at a density of 1×104 cells/well and incubated overnight
at 37°C. Then, IR-783 (0, 80 and 160 μM) was added to cells
and cultured for 24 h. Subsequently, cells were exposed to 20
μM EdU for 2 h at 37°C. Cells were then fixed with 4%
paraformaldehyde for 15 min, followed by the addition of 200
μl 0.5% Triton X-100 (AppliChem GmbH) for 10 min to increase
cell permeability at room temperature. After washing with PBS for
three times, 200 μl of click reaction solution was added
according to the instructions and cells were incubated for another
30 min at room temperature in the dark. Next, cells were washed
again and stained with 100 μl of Hoechst 33342 for 30 min at
room temperature. The samples were analyzed and imaged with a
fluorescence microscope (BX63; Olympus Corporation) at x20
magnification. EdU-positive cells were analyzed in three randomly
selected field by using Photoshop CS6 software (version 13.0; Adobe
Systems, Inc.).
Cell cycle analysis
Cell cycle analysis was performed by flow cytometry.
Cells were seeded in a 6-well plate at a density of 1×106
cells/well and cultured overnight. IR-783 (0, 80 and 160 μM)
was added and incubated for 24 h. Cells were collected and washed
twice with cold PBS, then fixed in cold 75% ethanol at 4°C
overnight. Subsequently, the cells were washed with cold PBS and
incubated with 50 μg/ml RNase and 100 μg/ml propidium
iodide (cat. no. 556547; BD Biosciences) for 30 min at 37°C in the
dark. The samples were analyzed with a flow cytometer (FACScan)
using CellQuest software (version 5.1) (both from BD
Biosciences).
Wound healing assay
The migration ability of MDA-MB-231 cells was
assessed using a wound healing assay. Cells (5×105
cells/well) were cultured in 6-well plates and allowed to grow to
80-90% confluence. Scratches of a predetermined length were created
with a 200 μl pipette tip. After washing the scratched cells
with PBS, IR-783 (0, 80 and 160 μM) were added and incubated
for 24 or 48 h at 37°C. The images of wounds were imaged using a
Cell Imaging System (Carl Zeiss AG). The wound width was measured
using ImageJ software (version 1.52a; National Institute of
Health); wound healing rate (%) = 100% × (0 h width-24/48 h
width)/0 h width.
Transwell assay
Cells (2×104 cells/well) were plated in
the upper chamber of a Transwell chamber with 200 μl
serum-free DMEM, while 600 μl media containing 30% FBS
(Gibco; Thermo Fisher Scientific, Inc.) was added in the lower
chamber. IR-783 (0, 80 and 160 μM) was applied to both
chambers. After 24 h of treatment in a 37°C incubator, non-invasive
cells on the upper surface of the membrane were gently wiped away
with a cotton swab, while the invasive cells on the low surface of
the membrane were fixed with 4% paraformaldehyde for 15 min and
stained with 0.1% crystal violet for 10 min at room temperature.
Three independent fields in each well were imaged under a light
microscope (DP26; Olympus Corporation) at x20 magnification, and
the number of cells in each field was counted.
ATP luminescence assay
The cellular ATP levels were measured with an ATP
Assay Kit (cat. no. S0026; Beyotime Institute of Biotechnology). A
standard curve of ATP concentration (0.01, 0.03, 0.1, 0.3, 1.0,
3.0, 10 μM) was created from a known amount of ATP and then
used to determine the ATP content of the samples. Briefly, after
exposure to different concentrations of IR-783 (0, 40, 80, 120 and
160 μM) for 24 h, cells were harvested and washed twice with
PBS. Then, cells were lysed and centrifuged at 12,000 × g for 10
min at 4°C, and the supernatant was collected and mixed with 100
μl ATP detection working solution. The luminescence values
were determined using a microplate reader at 420 nm (Varioskan
Flash; Thermo Fisher Scientific, Inc.). The levels of ATP were
expressed as a percentage of the control, which was set at
100%.
Transmission electron microscopy
For electron microscopy, after 24 h incubation with
80 μM IR-783, MDA-MB-231 cells were collected and washed
twice with PBS, and fixed in fresh 2.5% glutaraldehyde at 4°C
overnight. Samples were fixed for 2 h in 2% osmium tetroxide at
4°C, dehydrated using a graded series of ethanol and embedded in
Epon. Then, sections (70 nm) were made using an ultra-Microtome
(Leica EM UC7; Leica Microsystems, Inc.), stained with 3% uranyl
acetate and 3% lead citrate for 15 min at room temperature. The
morphology of mitochondria was studied using a Tecnai 10
transmission electron microscope (Philips Healthcare).
Fluorescence microscopy
Cells were inoculated into 24-well plates with
coverslips overnight. After treatment with 80 μM IR-783,
cells were stained with 100 nM MitoTracker Red CMXRos (Beyotime
Institute of Biotechnology) for 30 min at 37°C in the dark, then
washed three times with culture medium for 5 min each time.
Subsequently, cells were fixed with 4% paraformaldehyde for 10 min
at room temperature, washed with PBS for three times, and observed
using a laser-scanning confocal microscope (LSM780NLO; Carl Zeiss
AG) at x63 magnification, the representative images were randomly
selected from five fields of view. For staining of the actin
cytoskeleton, cells were treated with 80 μM IR-783 for 24 h
at 37°C, then cells were fixed with 4% paraformaldehyde for 15 min
and permeabilized with 0.1% Triton X-100 for 5 min at room
temperature. F-actin was incubated with Alexa Fluor® 488 Phalloidin
(CST, 8878S) for 30 min at 37°C in the dark; cells were
counterstained with DAPI for 5 min at room temperature (cat. no.
C1002; Beyotime Institute of Biotechnology). Cell images were
collected using a laser-scanning confocal microscope (LSM780NLO;
Carl Zeiss AG) at x63 magnification, the representative images were
randomly selected from five fields of view.
Western blotting
MDA-MB-231 cells (1×106 cells/ml) were
treated with IR-783 for 24 h, then harvested and lysed in cell
lysis solution (cat. no. P0013; Beyotime Institute of
Biotechnology) with PMSF. A BCA protein assay kit (cat. no. P0010,
Beyotime Institute of Biotechnology) was used to quantify protein
concentration. Proteins were quantified via a BCA assay. The
proteins lysate samples (15, 30 or 60 μg) were separated via
10-12% SDS-PAGE and then transferred to polyvinylidene difluoride
membranes (EMD Millipore). The membranes were blocked with 5% skim
milk for 2 h at room temperature and incubated with specific
primary antibodies overnight at 4°C. The following antibodies were
used: Anti-Cyclin D1 (diluted 1:500), anti-Cyclin E (diluted
1:500), anti-CDK2 (diluted 1:500), anti-OPA1 (diluted 1:500),
anti-Mfn1 (diluted 1:500), anti-Mfn2 (diluted 1:500), anti-MFF
(diluted 1:500), anti-Fis1 (diluted 1:500), anti-Drp1 (diluted
1:500), anti-MMP-2 (diluted 1:1000), anti-MMP-9 (diluted 1:1,000)
and anti-GAPDH (diluted 1:2,000). Membranes were washed three times
with TBST and then incubated with anti-rabbit (diluted 1:50,000,
cat. no. 150204) or anti-mouse (diluted 1:50,000, cat, no. 130639)
horseradish peroxidase secondary antibodies (Kirkegaard & Perry
Laboratories Inc.) for 2 h at room temperature. The immunoreactive
bands were visualized using an enhanced chemiluminescence kit
(Amersham ECL or ECL Plus; GE Healthcare Life Sciences); protein
expression was quantified using Quantity One software (version 4.6;
Bio-Rad Laboratories, Inc.).
Statistical analysis
All data were presented as the mean ± standard
deviation using GraphPad prism 6.0 software (GraphPad Software,
Inc.). Statistical analysis between different groups were
determined using one-way analysis of variance with a Dunnett's or
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
IR-783 suppresses MDA-MB-231 and MCF-7
cell proliferation
The chemical structure of IR-783 is presented in
Fig. 1A. First, we evaluated the
inhibitory effects of IR-783 on MDA-MB-231 and MCF-7 cells using an
MTT assay. As shown in Fig. 1B and
C, we reported that IR-783 treatment significantly decreased
cell proliferation in a dose- and time-dependent manner compared
with the control. To further confirm the effects of IR-783 on cell
proliferation, a colony formation assay was performed. As presented
in Fig. 1D, our results indicated
that the number of colonies formed in IR-783-treated group was
significantly reduced in MDA-MB-231 and MCF-7 breast cancer cells
compared with the control. Furthermore, the effects of IR-783 on
cell proliferation in MDA-MB-231 and MCF-7 cells was examined with
an EdU assay. The results revealed significant fewer EdU-positive
cells following treatment with IR-783 compared with the control
(Fig. 1E). Taken together, these
findings demonstrated that IR-783 suppresses MDA-MB-231 and MCF-7
cell proliferation.
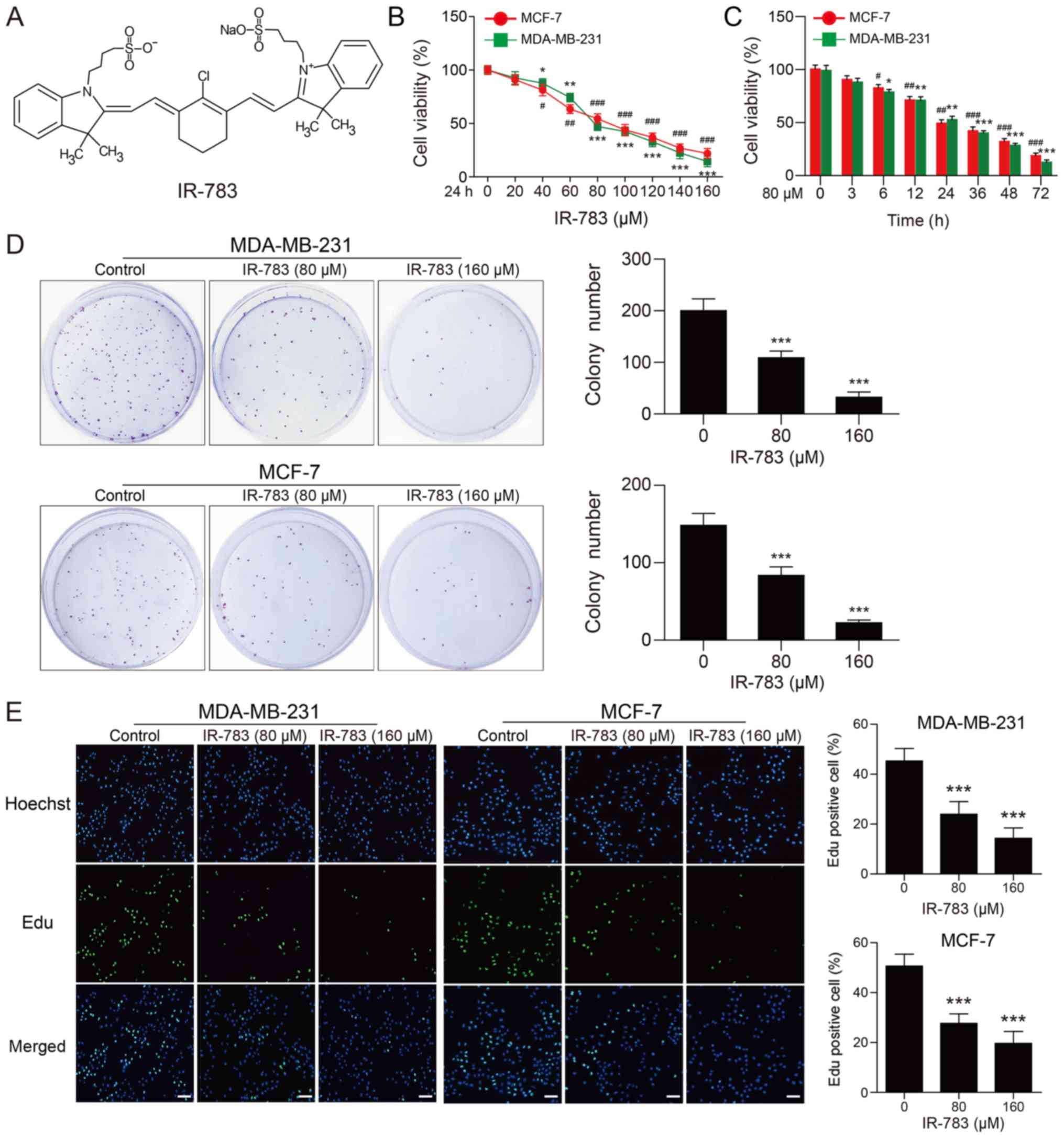 | Figure 1IR-783 suppresses MDA-MB-231 and
MCF-7 cell proliferation. (A) Chemical structure of IR-783. (B and
C) MDA-MB-231 and MCF-7 cell proliferation as determined by an MTT
assay; cells were treated with different concentrations of IR-783
(0, 20, 40, 60, 80, 100, 120, 140 and 160 μM) for 24 h or
with 80 μM IR-783 for different time intervals (0, 3, 6, 12,
24, 36, 48 and 72 h). All data are expressed as the mean ± standard
error of the mean, from three independent experiments.
#P<0.05, ##P<0.01,
###P<0.001 vs. the MCF-7 control group,
*P<0.05, **P<0.01,
***P<0.001 vs. the MDA-MB-231 control group. (D) In
the colony formation assay, MDA-MB-231 and MCF-7 cells were treated
with IR-783 (0, 80 and 160 μM) for 24 h, then fixed and
stained with crystal violet after cultured for 2-3 weeks. All data
are expressed as the mean ± standard error of the mean, from three
independent experiments. ***P<0.001 vs. the control
group. (E) MDA-MB-231 and MCF-7 cells were treated with various
concentrations of IR-783 (0, 80 and 160 μM) for 24 h. An EdU
staining assay was performed to determine the effects of IR-783 on
the proliferation of MDA-MB-231 and MCF-7 cells.
***P<0.001 vs. the control group. Scale bar, 200
μm. All data are expressed as the mean ± standard error of
the mean, from three independent experiments. |
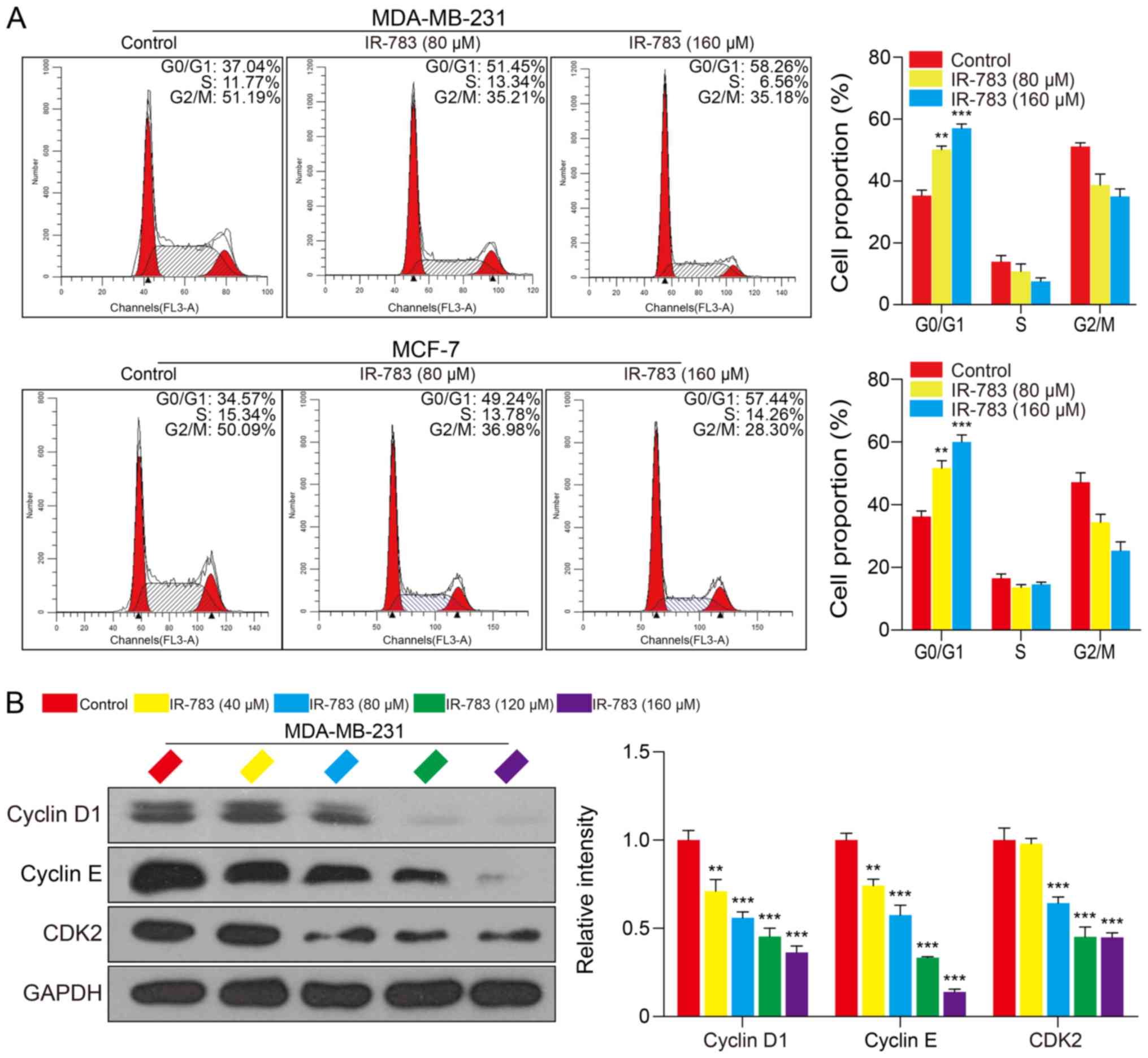
IR-783 induces MDA-MB-231 and MCF-7 cell
cycle arrest at the G0/G1 phase
To further determine the underlying mechanisms of
IR-783-induced inhibition of the growth of MDA-MB-231 and MCF-7
cells, we examined the effects of IR-783 on MDA-MB-231 and MCF-7
cell cycle distribution. After treatment with IR-783 (0, 80 and 160
μM) for 24 h, the proportion of MCF-7 cells in G0/G1 phase
significantly increased from 34.57% (without IR-783 treatment) to
57.45% (cells treated with 160 μM IR-783); the proportion of
MDA-MB-231 cells in G0/G1 phase significantly increased from 37.04%
(without IR-783 treatment) to 58.26% (cells treated with 160
μM IR -783) (Fig. 2A). No
significant differences in the number of cells in S and G2/M phase
were observed following treatment with IR-783. These results
suggested that IR-783 induced MDA-MB-231 and MCF-7 cell cycle
arrest at the G0/G1 phase. Furthermore, we detected alterations in
the expression of G0/G1 phase-related proteins Cyclin D1, Cyclin E
and CDK2 of MDA-MB-231 cells using western blotting. The results
demonstrated that IR-783 significantly reduced the expression of
Cyclin D1, Cyclin E and CDK2 compared with the control (Fig. 2B). These results suggested that
IR-783 induces cell cycle arrest at G0/G1 phase.
IR-783 inhibits MDA-MB-231 and MCF-7 cell
migration
In addition, a Transwell assay was conducted to
examine the effects of IR-783 on MDA-MB-231 and MCF-7 cell
migration; a wound healing assay was performed to determine the
effects of IR-783 on MDA-MB-231 cell migration. The Transwell assay
revealed that IR-783 significantly inhibited the migration of
MDA-MB-231 and MCF-7 cells compared with the control (Fig. 3A). Furthermore, the wound healing
assay indicated that IR-783 significantly decreased the migration
of MDA-MB-231 cells in a dose- and time-dependent manner (Fig. 3B). MMPs, especially MMP-2 and
MMP-9, have been reported to be critical regulators involved tumor
migration (25). Thus, western
blotting was conducted to investigate whether IR-783 affected the
expression of MMP-2 and MMP-9 in MDA-MB-231 cells. The western blot
assay demonstrated that IR-783 treatment significantly decreased
the expression of MMP-2 and MMP-9 in a concentration-dependent
manner compared with the control group (Fig. 3C). Collectively, these results
indicated that IR-783 inhibits MDA-MB-231 and MCF-7 cell
migration.
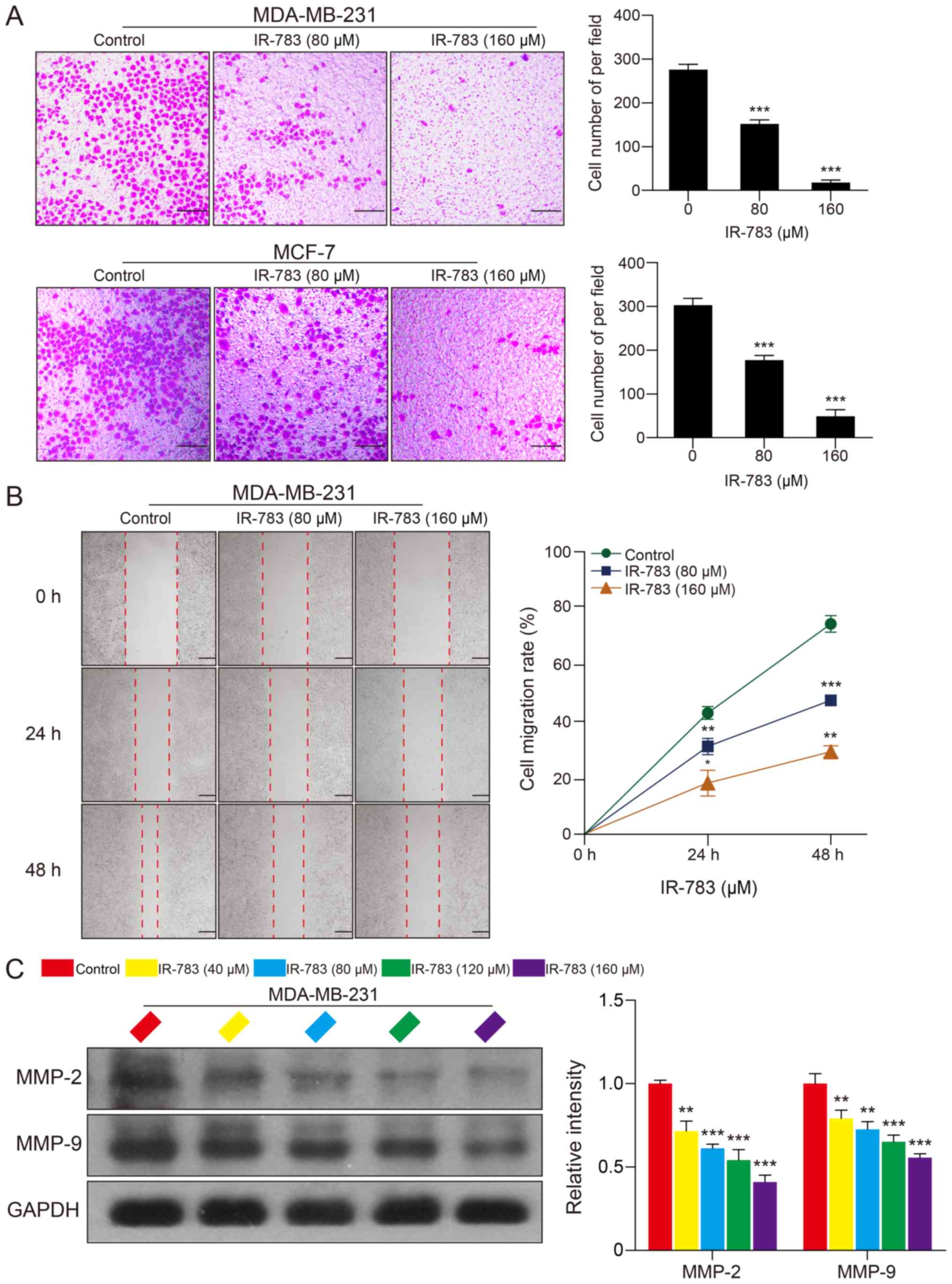 | Figure 3IR-783 inhibits MDA-MB-231 and MCF-7
cell migration. (A) Cells were exposed to various concentrations of
IR-783 (0, 80 and 160 μM) for 24 h. The effects of IR-783 on
MDA-MB-231 and MCF-7 cell migration were analyzed using a Transwell
assay. Scale bar, 200 μm. ***P<0.001 vs. the
control group. (B) Cells were seeded onto a 6-well plate and
treated with different concentrations of IR-783 (0, 80 and 160
μM) for 24 or 48 h. The effects of IR-783 on MDA-MB-231 cell
migration were analyzed by a wound healing assay. Scale bar, 100
μm. *P<0.05, **P<0.01,
***P<0.001 vs. 0 h. (C) After treatment with IR-783
(0, 40, 80, 120 and 160 μM) for 24 h, western blotting was
conducted to analyze the expression of the migration-associated
proteins MMP-2 and MMP-9 in MDA-MB-231 cell. Quantity One software
was used to analyze the relative quantification of detected
proteins; GAPDH was employed as loading control. All data are
expressed as the mean ± standard deviation. (n=3).
**P<0.01, ***P<0.001 vs. the control
group. MMP, matrix metalloproteinase. |
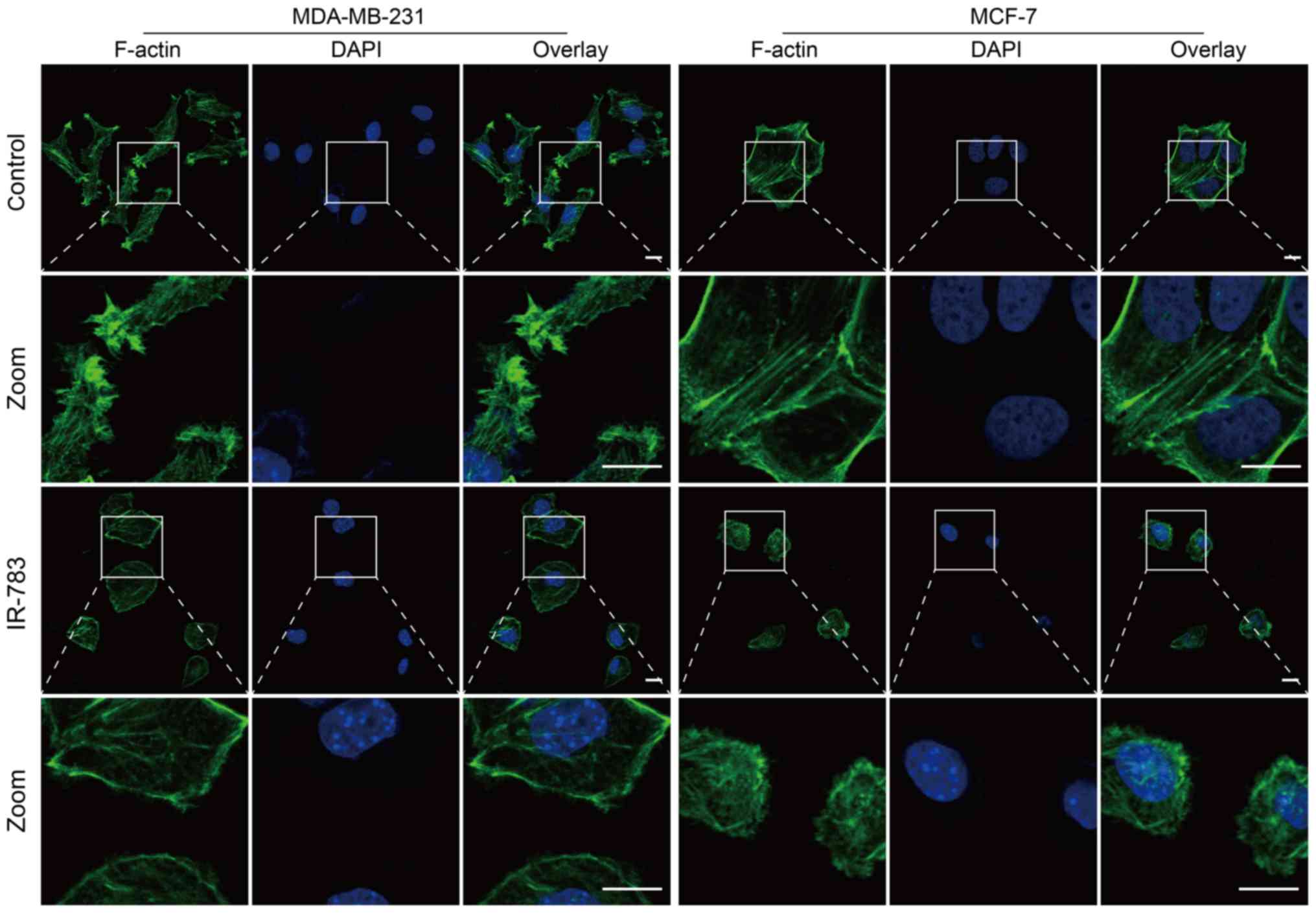
IR-783 inhibits filopodia formation by
reducing F-actin formation
Filopodia are critical actin cytoskeletal protrusion
structures, and are key factors involved in tumor metastasis
(26). Metastatic tumor cells
contain numerous filopodia, and the numbers of filopodia are
correlated with the invasiveness and migration (27). F-actin is the fundamental component
of the cytoskeleton that supports the structure of filopodia,
stabilizing them in migrating cancer cells, and it is involved
infilopodium formation via the polymerization of actin into bundles
under the cell membrane (28). The
present study reported that the control cell group exhibited a
large number of F-actin filaments in the marginal zone and
cytoplasm. Incubation with IR-783 induced MDA-MB-231 and MCF-7 cell
shrinkage and a marked decrease in the abundance of F-actin
(Fig. 4). Collectively, these data
suggested that IR-783 inhibits filopodia formation by reducing
F-actin formation.
IR-783 induces ATP depletion and
mitochondrial fission in MDA-MB-231 and MCF-7 cells
ATP, which provides energy for cells, serves an
important role in cell life and is central to the fate of cancer
cells (29). It has been reported
that cancer cells have higher ATP levels than normal cells, and
decreases in ATP levels of cancer cells indicates alterations in
cancer cell viability (30). As
presented in Fig. 5A, the
MDA-MB-231 and MCF-7 cellular ATP levels decreased in a
dose-dependent manner in response to the IR-783. Mitochondria are
the main organelle in cells involved in the synthesis of ATP. ATP
depletion has been reported as an indicator of mitochondrial
dysfunction (30). Our
transmission electron microscopy results showed that mitochondria
of MDA-MB-231 cells swelled; the cristae of mitochondria
disintegrated partly as demonstrated by the transparent electronic
density area in IR-783 treated cells. On the contrary, the
mitochondria presented a slender flat structure in control cells,
suggesting that IR-783 induced mitochondrial injury in MDA-MB-231
cells (Fig. 5B). The morphology of
mitochondria is dynamically controlled by fission and fusion
(20). When alterations in
mitochondrial morphology occurred, the balance between fission and
fusion is disrupted (20). Next,
MDA-MB-231 and MCF-7 cells were stained with MitoTracker Red CMXRos
to assess the influence of IR-783 on mitochondrial dynamics.
Confocal laser scanning microscopy results revealed that IR-783
treatment promoted mitochondrial fission, as observed by a marked
increase in number of small, punctate mitochondria in IR-783
treated cells compared with control cells, which exhibited stable
and elongated structures (Fig.
5C). To further confirm these results, western blotting was
used to evaluate the expression of mitochondrial fusion regulators
OPA1, Mfn1 and Mfn2, and the expression of mitochondrial fission
regulators MFF, Fis1 and Drp1 which affect mitochondrial dynamics
at the outer mitochondrial membrane. We found that exposure of
MDA-MB-231 cells to IR-783 resulted in a significant decrease in
the expression of OPA1, Mfn1 and Mfn2, and increased the levels of
MFF, Fis1 and Drp1 compared with the control (Fig. 5D and E). These results suggested
that IR-783 induces mitochondrial fission and subsequent ATP
depletion in MDA-MB-231 and MCF-7 cells.
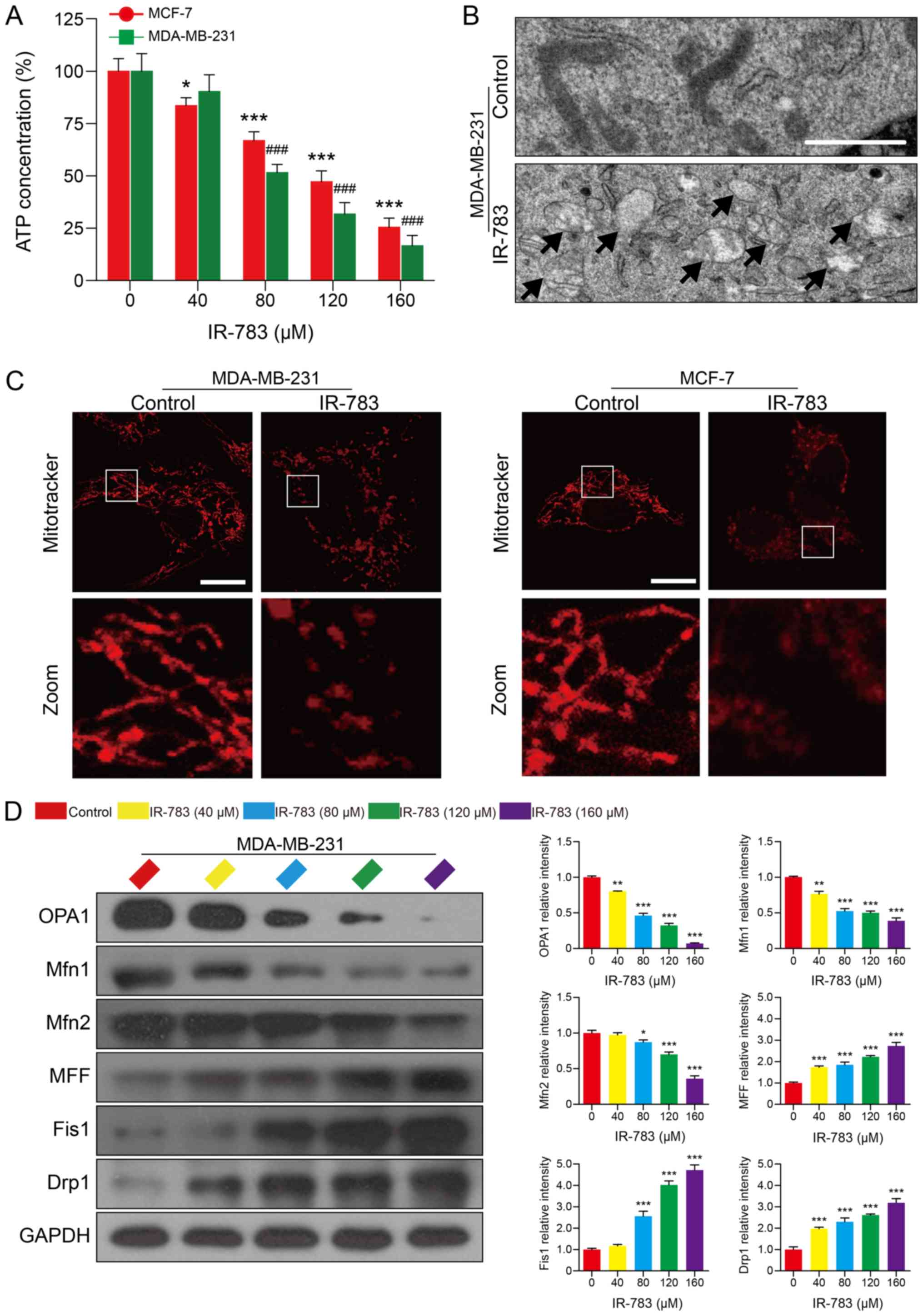 | Figure 5IR-783 induces ATP depletion and
mitochondrial fission in MDA-MB-231 and MCF-7 cells. (A) MDA-MB-231
and MCF-7 cells were treated with IR-783 (0, 40, 80, 120 and 160
μM) for 24 h and the intracellular ATP content was measured
by a Luminometer microplate reader. All data are expressed as the
mean ± standard error of the mean, from three independent
experiments. *P<0.05, ***P<0.001 vs.
the MCF-7 control group, ###P<0.001 vs. the
MDA-MB-231 control group. (B) MDA-MB-231 cells were exposed to 80
μM IR-783 for 24 h and mitochondria were imaged with a
transmission electron microscope. Scale bar, 2 μm. (C)
MDA-MB-231 and MCF-7 cells were treated with 80 μM IR-783
for 24 h, then stained with MitoTracker Red CMXRos (red) and
analyzed by a confocal microscope. Scale bar, 20 μm. (D)
Western blotting was performed to assess the expression of OPA1,
Mfn1, Mfn2, MFF, Fis1 and Drp1 of MDA-MB-231 cells, and GAPDH was
used as the loading control. The relative expression of proteins
compared with GAPDH was presented, and was determined with Quantity
One software. All data are expressed as the mean ± standard
deviation (n=3). *P<0.05, **P<0.01,
***P<0.001 vs. the control group. Drp1,
dynamin-related protein 1; Fis1, mitochondrial fission 1 protein;
Mfn, mitofusin; MFF, mitochondrial fission factor; OPA1, optic
atrophy 1. |
Discussion
In recent years, NIR imaging has received increasing
attention in the diagnosis and treatment of cancer. IR-783 is a
promising imaging NIR dye, and it possesses anti- cancer activity,
specific towards prostate, bladder and breast cancers (31,32).
IR-783 was determined to induce breast cancer cell apoptosis in
vitro and in vivo (15); however, the effects of IR-783 on
breast cancer cell proliferation and migration requires further
investigation. In the present study, we found that IR-783
significantly suppressed the proliferation and migration of
MDA-MB-231 and MCF-7 cells through inhibition of cell cycle
progression and filopodia formation.
Cancer cells have greater proliferation ability
compared with normal cells. Proliferation of cancer cells is the
essential steps of tumor incidence and development (33). In the present study, IR-783
treatment effectively inhibited the proliferation and colony
formation ability of MDA-MB-231 and MCF-7 cells. Additionally,
IR-783 was reported to induce cell cycle arrest at the G0/G1 phase
in a dose-dependent manner in the present study. The cell cycle is
a physiological process comprising G0/G1, S and G2/M phases.
Cyclins bind to corresponding CDKs and activate them to regulate
the transition of cell cycle phases, which serves a key role in
regulation of the cell cycle (34). In the present study, Cyclin D1,
Cyclin E and CDK2 were significantly downregulated in MDA-MB-231
cells following IR-783 treatment. These findings suggested that
IR-783 induced cell cycle arrest at the G0/G1 phase by
downregulating Cyclin D1, Cyclin E and CDK2. Direct analysis of DNA
synthesis is one of the most accurate ways of assessing cell
proliferation. Thus, we performed an EdU assay, an immunochemical
method for the detection of the nucleotide analog which is
incorporated into replicated DNA (35). We observed that the number of
EdU-positive cells was significantly lower in IR-783-treated cells
compared with control group cells; These findings indicated that
IR-783 inhibited MDA-MB-231 and MCF-7 cell proliferation.
Metastasis remains the cause of 90% of mortalities
from solid tumors (36). The
migration and invasive abilities of tumor cells are essential for
tumor metastasis. Members of the MMP family are commonly considered
as biomakers for cancers. In particular, MMP-2 and MMP-9 are
typical members among the MMPs, and are able to degrade type IV
collagen, which induces cancer cell metastasis by promoting the
primary tumor migration (37,38).
In the present study, IR-783 treatment markedly inhibited
wound-closure ability of MDA-MB-231 in a wound healing assay and
the migration ability of MDA-MB-231 and MCF-7 cells was also
suppressed as determined via a Transwell assay. In addition, the
expression of MMP-2 and MMP-9 in MDA-MB-231 cells were decreased
after IR-783 treatment. On the contrary, previous researchers have
revealed that filopodia are associated with cancer cell migration
and invasion (39). Furthermore,
cancer cell migration can be suppressed by inhibiting filopodia
formation ability in vitro (40). F-actin has been reported as a key
factor in filopodia, and participated in filopodia extension and
retraction at the cell surface. In the present study, IR-783
inhibited MDA-MB-231 and MCF-7 cell filopodia formation, while
decreases in polymerized F-actin were detected, indicating that the
metastatic ability of MDA-MB-231 and MCF-7 cell was attenuated by
IR-783 through inhibiting filopodia formation.
It has been reported that blocking ATP production
can inhibit filopodia formation and cell migration (41). We observed that IR-783 treatment
inhibited MDA-MB-231 and MCF-7 cell migration and filopodia
formation. In addition, we assessed the content of ATP using a
microplate reader. The results revealed that IR-783 treatment
decreased the levels of ATP in MDA-MB-231 and MCF-7 cells in a
dose-dependent manner. ATP depletion has also been reported as an
indicator of mitochondrial dysfunction (30). Our results of transmission electron
microscopy and immunofluorescence analysis showed that IR-783
treatment led to mitochondrial injury and a notable increase in
mitochondrial fission, evidenced by more small, punctate, swelled,
cristae disintegrating and vacant mitochondria. Abnormal
mitochondrial morphology is attributed to imbalances in
mitochondrial fission and fusion (21,42).
Numerous proteins and pathways are involved in mitochondrial fusion
and fission, while the initial fusion of mitochondria requires the
presence of OPA1, Mfn1 and Mfn2 at the inner or outer mitochondrial
membrane (21,43). Whereas fission is primarily
regulated by the proteins: Drp1, Fis1 and MFF (43). After cells are stimulated under
fission-promoting conditions, outer mitochondrial membrane proteins
Fis1 and MFF can recruit Drp1 translocation from the cytosol to the
mitochondria, and initiate mitochondrial fission (44). Our findings of western blotting
demonstrated that IR-783 treatment significantly decreased the
expression of the mitochondrial fusion proteins OPA1, Mfn1 and
Mfn2, whereas that of the mitochondrial fission proteins Drp1, Fis1
and MFF had increased in MDA-MB-231 cells. Collectively, these
results demonstrated that IR-783 inhibited ATP production by
inducing mitochondrial fission.
In summary, these data demonstrated that IR-783
inhibited MDA-MB-231 and MCF-7 cell proliferation and migration by
inducing mitochondrial fission and subsequently decreasing ATP
levels, resulted in cell cycle arrest and filopodia formation
inhibition. Our findings could provide a novel mechanistic basis
for the application of IR-783 in the treatment of breast
cancer.
Acknowledgments
Not applicable.
Funding
This work was supported by the National Natural
Science Foundation of China (grant nos. 81874357, 81703481 and
81801273), Natural Science Foundation of Chongqing (grant nos.
cstc2018jcyjAX0183 and cstc2015jcyjA10011).
Availability of data and materials
The datasets generated and analyzed during the
current study are available from the corresponding author upon
reasonable request.
Authors' contributions
PL, YZ and RZ conceived and designed the study. PL,
FS, YL, WL, MZ and QT performed the experiments. FL, JH, HZ, CH and
WL analyzed the data. PL, YL and YZ wrote the manuscript. GL and QZ
reviewed and revised the manuscript. All authors read and approved
the manuscript and agreed to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the report work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dai X, Li T, Bai Z, Yang Y, Liu X, Zhan J
and Shi B: Breast cancer intrinsic subtype classification, clinical
use and future trends. Am J Cancer Res. 5:2929–2943.
2015.PubMed/NCBI
|
|
2
|
Wang B, Xing Z, Wang F, Yuan X and Zhang
Y: Fangchinoline inhibits migration and causes apoptosis of human
breast cancer MDA-MB-231 cells. Oncol Lett. 14:5307–5312.
2017.PubMed/NCBI
|
|
3
|
He MY, Rancoule C, Rehailia-Blanchard A,
Espenel S, Trone JC, Bernichon E, Guillaume E, Vallard A and Magné
N: Radiotherapy in triple-negative breast cancer: Current situation
and upcoming strategies. Crit Rev Oncol Hematol. 131:96–101. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mathe A, Wong-Brown M, Morten B, Forbes
JF, Braye SG, Avery-Kiejda KA and Scott RJ: Novel genes associated
with lymph node metastasis in triple negative breast cancer. Sci
Rep. 5:158322015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jones JE, Busi SB, Mitchem JB,
Amos-Landgraf JM and Lewis MR: Evaluation of a tumor-targeting,
near-infrared fluorescent peptide for early detection and
endoscopic resection of polyps in a rat model of colorectal cancer.
Mol Imaging. 17:15360121187900652018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagaya T, Okuyama S, Ogata F, Maruoka Y,
Choyke PL and Kobayashi H: Endoscopic near infrared
photoimmunotherapy using a fiber optic diffuser for peritoneal
dissemination of gastric cancer. Cancer Sci. 109:1902–1908. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang X, Shao C, Wang R, Chu CY, Hu P,
Master V, Osunkoya AO, Kim HL, Zhau HE and Chung LWK: Optical
imaging of kidney cancer with novel near infrared heptamethine
carbocyanine fluorescent dyes. J Urol. 189:702–710. 2013.
View Article : Google Scholar
|
|
8
|
Holt D, Okusanya O, Judy R, Venegas O,
Jiang J, DeJesus E, Eruslanov E, Quatromoni J, Bhojnagarwala P,
Deshpande C, et al: Intraoperative near-infrared imaging can
distinguish cancer from normal tissue but not inflammation. PLoS
One. 9:e1033422014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Deng G, Li S, Sun Z, Li W, Zhou L, Zhang
J, Gong P and Cai L: Near-infrared fluorescence imaging in the
largely unexplored window of 900-1,000 nm. Theranostics.
8:4116–4128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu JB, Shi C, Chu GC, Xu Q, Zhang Y, Li Q,
Yu JS, Zhau HE and Chung LW: Near-infrared fluorescence
heptamethine carbo-cyanine dyes mediate imaging and targeted drug
delivery for human brain tumor. Biomaterials. 67:1–10. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Shao C, Liao CP, Hu P, Chu CY, Zhang L,
Bui MH, Ng CS, Josephson DY, Knudsen B, Tighiouart M, et al:
Detection of live circulating tumor cells by a class of
near-infrared heptamethine carbocyanine dyes in patients with
localized and metastatic prostate cancer. PLoS One. 9:e889672014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen S and Margel S; S. C S M:
Engineering of near IR fluorescent albumin nanoparticles for in
vivo detection of colon cancer. J Nanobiotechnology. 10:362012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Liu Z, Lian P, Qian J, Li X, Wang
L, Fu W, Chen L, Wei X and Li C: Selective imaging and cancer cell
death via pH switchable nearinfrared fluorescence and photothermal
effects. Chem Sci. 7:5995–6005. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Thomas RG and Jeong YY: NIRF heptamethine
cyanine dye nanocomplexes for multi modal theranosis of tumors.
Chonnam Med J. 53:83–94. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tang Q, Liu W, Zhang Q, Huang J, Hu C, Liu
Y, Wang Q, Zhou M, Lai W, Sheng F, et al: Dynamin-related protein
1-mediated mitochondrial fission contributes to IR-783-induced
apoptosis in human breast cancer cells. J Cell Mol Med.
22:4474–4485. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hou L, Yang X, Ren J, Wang Y, Zhang H,
Feng Q, Shi Y, Shan X, Yuan Y and Zhang Z: A novel redox-sensitive
system based on single-walled carbon nanotubes for
chemo-photothermal therapy and magnetic resonance imaging. Int J
Nanomedicine. 11:607–624. 2016.PubMed/NCBI
|
|
17
|
Tokarz P and Blasiak J: Role of
mitochondria in carcinogenesis. Acta Biochim Pol. 61:671–678. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eirin A, Lerman A and Lerman LO:
Mitochondria: A pathogenic paradigm in hypertensive renal disease.
Hypertension. 65:264–270. 2015. View Article : Google Scholar :
|
|
19
|
Ruan S, Zhang Z, Tian X, Huang D, Liu W,
Yang B, Shen M and Tao F: Compound fuling granule suppresses
ovarian cancer development and progression by disrupting
mitochondrial function, galactose and fatty acid metabolism. J
Cancer. 9:3382–3393. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Scott I and Youle RJ: Mitochondrial
fission and fusion. Essays Biochem. 47:85–98. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Han XJ, Yang ZJ, Jiang LP, Wei YF, Liao
MF, Qian Y, Li Y, Huang X, Wang JB, Xin HB, et al: Mitochondrial
dynamics regulates hypoxia-induced migration and antineoplastic
activity of cisplatin in breast cancer cells. Int J Oncol.
46:691–700. 2015. View Article : Google Scholar
|
|
22
|
Matsuishi YI, Kato H, Masuda K, Yamaza H,
Hirofuji Y, Sato H, Wada H, Kiyoshima T and Nonaka K: Accelerated
dentinogenesis by inhibiting the mitochondrial fission factor,
dynamin related protein 1. Biochem Biophys Res Commun.
495:1655–1660. 2018. View Article : Google Scholar
|
|
23
|
Archer SL: Mitochondrial dynamics -
mitochondrial fission and fusion in human diseases. N Engl J Med.
369:2236–2251. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Westermann B: Mitochondrial fusion and
fission in cell life and death. Nat Rev Mol Cell Biol. 11:872–884.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eatemadi A, Aiyelabegan HT, Negahdari B,
Mazlomi MA, Daraee H, Daraee N, Eatemadi R and Sadroddiny E: Role
of protease and protease inhibitors in cancer pathogenesis and
treatment. Biomed Pharmacother. 86:221–231. 2017. View Article : Google Scholar
|
|
26
|
Han S, Huang J, Liu B, Xing B, Bordeleau
F, Reinhart-King CA, Li W, Zhang JJ and Huang XY: Improving fascin
inhibitors to block tumor cell migration and metastasis. Mol Oncol.
10:966–980. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shibue T, Brooks MW, Inan MF, Reinhardt F
and Weinberg RA: The outgrowth of micrometastases is enabled by the
formation of filopodium-like protrusions. Cancer Discov. 2:706–721.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Atilgan E, Wirtz D and Sun SX: Mechanics
and dynamics of actin-driven thin membrane protrusions. Biophys J.
90:65–76. 2006. View Article : Google Scholar
|
|
29
|
Ke R, Xu Q, Li C, Luo L and Huang D:
Mechanisms of AMPK in the maintenance of ATP balance during energy
metabolism. Cell Biol Int. 42:384–392. 2018. View Article : Google Scholar
|
|
30
|
Wang X, Li Y, Qian Y, Cao Y, Shriwas P,
Zhang H and Chen X: Extracellular ATP, as an energy and
phosphorylating molecule, induces different types of drug
resistances in cancer cells through ATP internalization and
intracellular ATP level increase. Oncotarget. 8:87860–87877.
2017.PubMed/NCBI
|
|
31
|
Yang X, Shi C, Tong R, Qian W, Zhau HE,
Wang R, Zhu G, Cheng J, Yang VW, Cheng T, et al: Near IR
heptamethine cyanine dye-mediated cancer imaging. Clin Cancer Res.
16:2833–2844. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan J, Yi X, Yan F, Wang F, Qin W, Wu G,
Yang X, Shao C and Chung LW: Near infrared fluorescence imaging of
prostate cancer using heptamethine carbocyanine dyes. Mol Med Rep.
11:821–828. 2015. View Article : Google Scholar
|
|
33
|
Papaccio F, Paino F, Regad T, Papaccio G,
Desiderio V and Tirino V: Concise Review: Cancer cells, cancer stem
cells, and mesenchymal stem cells: Influence in cancer development.
Stem Cells Transl Med. 6:2115–2125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun L, Huang Y, Wei Q, Tong X, Cai R,
Nalepa G and Ye X: Cyclin E-CDK2 protein phosphorylates plant
homeodomain finger protein 8 (PHF8) and regulates its function in
the cell cycle. J Biol Chem. 290:4075–4085. 2015. View Article : Google Scholar :
|
|
35
|
Zhang R, Wei YH, Zhao CY, Song HY, Shen N,
Cui X, Gao X, Qi ZT, Zhong M and Shen W: EDIL3 depletion suppress
epithelial-mesenchymal transition of lens epithelial cells via
transforming growth factor β pathway. Int J Ophthalmol. 11:18–24.
2018.
|
|
36
|
Gupta GP and Massagué J: Cancer
metastasis: Building a framework. Cell. 127:679–695. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Benson CS, Babu SD, Radhakrishna S,
Selvamurugan N and Ravi Sankar B: Expression of matrix
metalloproteinases in human breast cancer tissues. Dis Markers.
34:395–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mendes O, Kim HT and Stoica G: Expression
of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat
model. Clin Exp Metastasis. 22:237–246. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Flamini MI, Fu X-D, Sanchez AM, Giretti
MS, Garibaldi S, Goglia L, Pisaneschi S, Tosi V, Genazzani AR and
Simoncini T: Effects of raloxifene on breast cancer cell migration
and invasion through the actin cytoskeleton. J Cell Mol Med.
13:2396–2407. 2009. View Article : Google Scholar
|
|
40
|
Li Y, Zhang Z, Zhou X, Li L, Liu Q, Wang
Z, Bai X, Zhao Y, Shi H, Zhang X, et al: The oncoprotein HBXIP
enhances migration of breast cancer cells through increasing
filopodia formation involving MEKK2/ERK1/2/Capn4 signaling. Cancer
Lett. 355:288–296. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ozawa S, Ueda S, Imamura H, Mori K,
Asanuma K, Yanagita M and Nakagawa T: Glycolysis, but not
Mitochondria, responsible for intracellular ATP distribution in
cortical area of podocytes. Sci Rep. 5:185752015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tang D, Kang R, Livesey KM, Kroemer G,
Billiar TR, Van Houten B, Zeh HJ III and Lotze MT: High-mobility
group box 1 is essential for mitochondrial quality control. Cell
Metab. 13:701–711. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Newell C, Shutt TE, Ahn Y, Hittel DS, Khan
A, Rho JM and Shearer J: Tissue specific impacts of a ketogenic
diet on mitochondrial dynamics in the BTBRT+tf/j mouse. Front
Physiol. 7:6542016. View Article : Google Scholar
|
|
44
|
Kar R, Mishra N, Singha PK, Venkatachalam
MA and Saikumar P: Mitochondrial remodeling following fission
inhibition by 15d-PGJ2 involves molecular changes in mitochondrial
fusion protein OPA1. Biochem Biophys Res Commun. 399:548–554. 2010.
View Article : Google Scholar : PubMed/NCBI
|