Introduction
Glutamate, a fast excitatory neurotransmitter in the
central nervous system (1), is
also responsible for neurotoxicity via excitotoxicity and oxidative
pathways (2,3). As reported previously, excessive
release of glutamate leads to acute and chronic brain diseases
including brain ischemia, traumatic brain injury, and
neurodegenerative disorders (4,5).
Several signaling pathways participate in glutamate-induced
neurotoxicity, one of which is associated with the mitochondria
(6). B-cell lymphoma 2 (Bcl-2) and
Bax, located in the mitochondria, are considered to be associated
with mitochondrial function (7).
As reported by a previous study, reactive oxygen species (ROS), a
byproduct of cellular oxidative processes, is responsible for
mitochondrial depolarization (8).
In addition, enhanced mitochondrial permeabilization causes
intracellular ROS accumulation, which further results in
mitochondria dysfunction (9,10).
Experiments demonstrate that the reduction of mitochondrial
membrane potential (MMP, Δψ m) leads to the release of cytochrome
c (Cyto C), whose cytoplasmic localization initiates
caspase-dependent apoptotic cell death.
Due to the various pharmacological functions of
polysaccharides, the natural purified polysaccharides are widely
used in the food and pharmaceutical industries. It was confirmed
that the chemical composition, glycosidic linkages, conformation,
molecular weight, and degree of branching of polysaccharides were
associated with their bioactivities (11). As potent macrofungi-derived
substances, polysaccharides separated from Cordyceps
militaris (12), Ganoderma
lucidum (13), and
Tricholoma matsutake (14)
have been extensively investigated. However, limited work has been
conducted on the purification and bioactivities of polysaccharides
separated from Tremella fuciformis.
Tremella fuciformis, known as a nutritious
mushroom, is popular in China as a medicinal remedy with tonic
actions for treating debility and exhaustion. As one of the primary
active components, polysaccharides obtained from the Tremella
fuciformis are reported to possess immunomodulatory (15), anticancer (16), and anti-inflammatory activities
(17). Tremella fuciformis
successfully enhances the neurite outgrowth of PC12 cells and
restores trimethyltin-induced impairment of memory in rats
(18). Polysaccharides isolated
from Tremella fuciformis exhibits a protective effect
against radiation-induced damage in mice (19). It is also reported that the
purified Tremella fuciformis polysaccharides possess
anti-oxidant activity (20). All
these data indicate that polysaccharides isolated from Tremella
fuciformis may exhibit a neuroprotective function.
In the present study, polysaccharides extracted from
Tremella fuctiformis were purified and characterized. The
neuroprotective effect of purified polysaccharides on glutamate
induced differentiated PC12 (DPC12) cell damage and the underlying
mechanisms were investigated. The data revealed that the purified
polysaccharides improved cell viability and mitochondrial function,
and restored the abnormal expression of apoptosis-related proteins.
All the findings demonstrated that mitochondria-related pathways
are essential for neuroprotection against glutamate-induced
toxicity in DPC12 cells.
Materials and methods
Crude extract preparation and preliminary
identification
As demonstrated in a previous study (21), 100 g Tremella fuciformis
powder was extracted twice using 90°C water for 2.5 h. After
centrifuging at 2667 x g for 10 min, the supernatant was
sequentially concentrated and freeze-dried for further
experiments.
Purification of Tremella fuciformis
polysaccharides
The protein in Tremella fuciformis water
extract was removed using Sevag reagent [V (n-butanol): V
(chloroform)=1:4, 50 ml] (22).
After adding 4X ethanol, the precipitation was dissolved in double
distilled (dd) H2O and subjected to the DEAE-52
cellulose anion exchange column (2.6 × 35 cm; Whatman; GE
Healthcare Life Sciences, Chalfont, UK). The column was eluted with
ddH2O followed by 0.1 mol/l and 0.3 mol/l NaCl at a flow
rate of 1 ml/min. Collected polysaccharides were further purified
using a gel permeation chromatography system Sepharose G-100
(Pfizer, New York City, NY, USA). The column was eluted with
ddH2O at a flow rate of 0.4 ml/min. The fractions (20
ml) were collected.
Cellular morphology analysis
PC12 cells (1×105), obtained from the
American Type Culture Collection, were seeded into each well in a
six-well plate. After differentiation, cells were pre-treated with
5 and 20 mg TL04 for 3 h, followed with 12 h co-incubation with 20
mM L-Glu (Beijing Dingguo Tech Changseng Biotechnology Co., Ltd.,
Beijing, China). Then, cells were incubated with Hoechst 33342 (5
mg/mL; Sigma-Aldrich, St. Louis, MO, USA) for 15 min at 37°C in
darkness. The fluorescence intensity in the nucleus was
photographed using a fluorescent microscope (x20 objective; CCD
camera, Nikon Corporation, Tokyo, Japan) after being washed with
phosphate buffered saline 3 times.
Fourier transform infrared spectroscopy
(FTIR) determination
In total, a 4 mg sample was ground thoroughly with
150 mg KBr into a smooth mortar. The average transmission spectra
(n=100) were recorded via an IRPrestige-21 FTIR spectrometer
(Shimadzu, Tokyo, Japan) at a wavelength ranging from 400 to 4,000
cm−1.
Homogeneity and molecular weight
determination
The homogeneity and molecular weight were analyzed
by LC-10ATvp high performance liquid chromatography (HPLC) system
(Shimadzu, Tokyo, Japan) equipped with a TSK-GEL G4000PWXL column
(Tosoh Co., Tokyo, Japan) and an Alltech 2000ES ELSD (Shimadzu,
Tokyo, Japan). Similar to a previous study (23), ddH2O served as the
mobile phase, the flow rate was 0.45 ml/min, aerosol level was 60%,
drift tube temperature was 120°C and nebulising nitrogen pressure
was 25 psi. The dextran standards were used to create a calibration
curve.
Periodate oxidation-smith degradation
reaction of polysaccharides
Similar to a previous study (23), 20 mg polysaccharide was dissolved
in 15 mM NaIO4 (25 ml, pH 4) in darkness at 4°C.
HIO4 consumption was calculated and the formic acid
production was determined by titration. After 48-h dialyzing
against ddH2O, the dialysate was concentrated and
reduced with potassium borohydride (70 mg) overnight at room
temperature. Adjusting to pH 7.0 by addition of acetic acid, the
solution was dialyzed against ddH2O for another 24 h.
Then, 3 ml of sample was detected by the HPLC/ELSD system. The rest
of the product was hydrolyzed with 1 M H2SO4
at 25°C for 40 h, and adjusted to pH 7.0 by BaCO3. The
solution was centrifuged at 2134 x g for 10 min to separate the
hydrolysates, which were further analyzed by HPLC/ELSD.
Cell culture
PC12 cells, were grown in Dulbecco's modified
Eagle's medium (DMEM) supplemented with 10% horse serum (HS), 5%
fetal bovine serum (FBS), penicillin (100 U/ml) and streptomycin
(100 µg/ml) (all from Thermo Fisher Scientific, Inc.), under
a humidified atmosphere containing 5/95% CO2/air at
37°C. The culture medium was changed every 3 days. PC12 cells were
differentiated for 48 h with 20 ng/ml nerve growth factor dissolved
in DMEM medium containing 1% FBS and 1% HS.
Cell viability analysis
As reported previously, a quantitative colorimetric
assay with 3-(4,5-cimethylthiazol-2-yl)-2,5-diphenyl tetrazolium
bromide (MTT; Sigma-Aldrich) was applied to measure cell viability
(24). Differentiated PC12 cells
were seeded into 96-well plates at 1×104 cells/well.
Cells were treated with TL04 (5 and 20 µg) alone for 24 h,
or pretreated with TL04 (5 and 20 µg) for 3 h and exposed to
20 mM glutamate for another 24 h. In another separated experiment,
cells were pretreated with 10 µM Ac-DEVD-CHO for 30 min, and
exposed to 5 µg and 20 µg TL04 for 3 h, followed by
another 24 h co-incubation with 20 mM glutamate. After incubation
with 0.5 mg/ml MTT solution for 4 h at 37°C in darkness, 100
µl dimethyl sulfoxide was added to dissolve crystals. A
microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
was used to detect the absorbance at 540 nm. Viability values of
treated cells were expressed as a percentage of that from
corresponding control cells.
Mitochondrial membrane potential (MMP)
analysis
PC12 cells (1×105) were seeded into each
well of a 6-well plate. After differentiation, cells were
pre-treated with 5 µg and 20 µg TL04 for 3 h,
followed by 12-h co-incubation with 20 mM glutamate. The changes in
MMP were measured via 5,5′, 6,6′-tetrachloro-1,1′,3,3′
tetraethylbenzimidazolylcarbocyanine iodide (JC-1; Sigma-Aldrich)
staining. Treated cells were incubated with 2 µM JC-1 at
37°C for 10 min. Fluorescent microscope (20x objective; Axio
Observer Z1, CCD camera; Carl Zeiss, Germany) was applied to record
the fluorescent color in each group. Average ratio of red (590 nm;
healthy cells) to green (540 nm; apoptotic or unhealthy cells)
fluorescence intensity of each cell was calculated by ImageJ
software (n=50; National Institutes of Health, Bethseda, MA, USA).
Values of treated cells were expressed as a percentage of that from
corresponding control cells.
Intracellular lactate dehydrogenase (LDH)
release, ROS accumulation and caspase-3 activity determination
PC12 cells were seeded into a 6-well plate at a
density of 1×105 cells/well. After differentiation,
cells were pre-treated with 5 µg and 20 µg TL04 for 3
h, followed by 12-h co-incubation with 20 mM glutamate. Cultured
medium was collected, and the intracellular LDH release was
detected via in vitro Toxicology Assay kit (Sigma-Aldrich).
Treated cells were lysed with radioimmunoprecipitation assay (RIPA)
buffer (Sigma-Aldrich), intracellular ROS accumulation was measured
by a ROS detection kit (Nanjing Biotechnology Co. Ltd., Nanjing,
China), and caspase-3 activity was analyzed by a caspase-3
colorimetric detection kit (Enzo Life Sciences International, Inc.)
according to the manufacturer's protocol.
Western blot analysis
DPC12 cells were pre-treated with 5 µg and 20
µg TL04 for 3 h, followed by 24-h co-incubation with 20 mM
glutamate. Cells were lysed by RIPA buffer (Sigma-Aldrich)
containing with 2% phenylmethanesulfonyl fluoride (Sigma-Aldrich)
and 1% protease inhibitor cocktail (Sigma-Aldrich). According to a
previous study, cytoplasmic extracts were prepared for Cyto C
release detection (25). After
determination of protein concentration via the Bradford method
using Coomassie Brilliant Blue G 250 (EMD Millipore), proteins were
separated via 12% sodium dodecyl sulfate-polyacrylamide gel
electrophoresis gel and transferred electrophoretically onto
nitrocellulose membranes (Bio Basic, Int., Amherst, NY, USA). The
transferred membranes were then washed with Tris-buffered saline
with 0.1% Tween-20 5 times every 5 min, and blocked with 5% bovine
serum albumin (Genview Scientific, Inc., El Monte, CA USA) for 3 h
at room temperature. Then, the membranes were blotted with the
following primary antibodies: Monoclonal rabbit Bcl-2 (ab32124),
monoclonal rabbit Bax (ab32503), monoclonal mouse Cyto C
(ab110325), polyclonal rabbit cleaved caspase-9 (ab2325) (all
purchased from Abcam, Cambridge, UK), polyclonal rabbit cleaved
caspase-8 (AB1879), polyclonal rabbit cleaved caspase-3 (AB3623)
(both purchased from EMD Millipore, Billerica, MA, USA) and
polyclonal rabbit glyceraldehyde-3-phosphate dehydrogenase (GAPDH;
sc-25778; Santa Cruz Biotechnology, Inc., Danvers, MA, USA) (all
1:1,000) at 4°C overnight, followed by incubation with horseradish
peroxidase-conjugated mouse anti-rabbit IgG (sc-2357) and goat
anti-mouse IgG (sc-2005) secondary antibodies (both 1:2,000; both
purchased from Santa Cruz Biotechnology Inc.). Chemiluminescence
was detected using enhanced chemiluminescence detection kits (GE
Healthcare). The intensity of the bands was quantified by scanning
densitometry using software Image J.
Statistical analysis
All data are presented as the mean ± standard
deviation. SPSS version 16.0 was used to perform all statistical
analyses (IBM SPSS, Amronk, NY, USA). Data were evaluated by
one-way analysis of variance to detect statistical significance,
followed by post hoc multiple comparisons (Dunn's test). P<0.05
was considered to indicate a statistically significant
difference.
Results
Purification and characterization of
polysaccharides purified from Tremella fuciformis
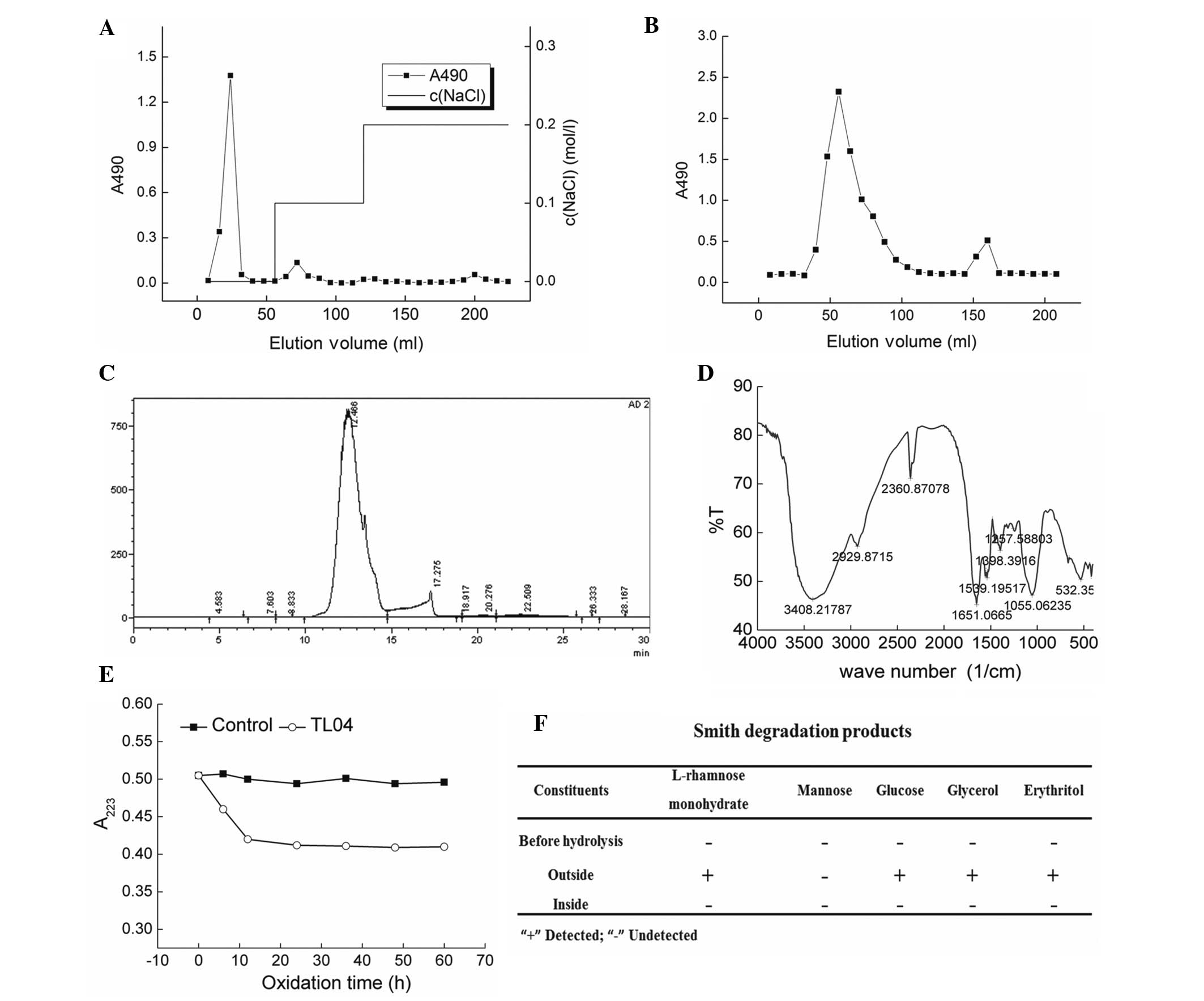
Anion exchange chromatography (DEAE-cellulose
column) was performed to separate the crude polysaccharides
obtained via water extraction (Fig.
1A). The samples were further purified by a gel permeation
chromatography system Sepharose G-100, and an elution peak that
appeared at 56 min was collected and named TL04 (Fig. 1B). Via calibration using the
molecular weight curve, the molecular weight of TL04 was determined
to be 2,033 kDa (Fig. 1C).
According the HPLC finger print, the percentage of rhamnose,
mannose and glucose contained in TL04 was 1:5.04:1.87. The
characteristic structures of TL04 were elucidated by FTIR spectra.
A strong hydroxyl absorption peak (3,400 cm−1), a weak
C–H absorption peak (2,930 cm−1) (26), and a C=O characteristic absorption
peak (1,650 cm−1) were observed (Fig. 1D). The absorption between 950–1,200
cm−1 indicated the existence a pyran ring skeleton
structure within TL04 (27). The
linkage mode of glucose present in TL04 was detected via Periodate
oxidation-Smith degradation (Fig. 1E
and F). After hydrolysis of TL04, L-rhamnose monohydrate,
glucose, glycerol and erythritol were observed (Fig. 1F).
Effect of TL04 against glutamate-induced
DPC12 cell damage
TL04 alone was not identified to affect DPC12 cell
proliferation. Exposure to 20 mM glutamate for 24 h resulted in
32.7% cell death (P<0.001); however, pretreatment with TL04 at 5
and 20 µg strongly prevented the cell viability loss
(P<0.05 and P<0.01, respectively; Fig. 2A). Pretreatment with 20 µg
TL04 for 3 h following incubation with 20 mM glutamate for another
24 h, significantly suppressed glutamate-enhanced LDH release was
(135.2±6.4 vs. 111.3±3.6%; P<0.01; Fig. 2B). Furthermore, TL04 (5 and 20
µg) normalized the glutamate-induced over-accumulation of
intracellular ROS (165.3±4.4 vs. 130.7±7.8 and 111.3±3.6%;
P<0.01 and P<0.001, respectively; Fig. 2C) and hyper-activity of caspase-3
(155.3±13.4 vs. 120.7±10.8 and 91.3±9.6%; P<0.001; Fig. 2D). In addition, results from
Hoechst 33342 staining confirmed that TL04 markedly attenuated
glutamate-induced apoptotic nuclei in DPC12 cells indicated by the
reduction in the intensity of blue fluorescence (Fig. 2E).
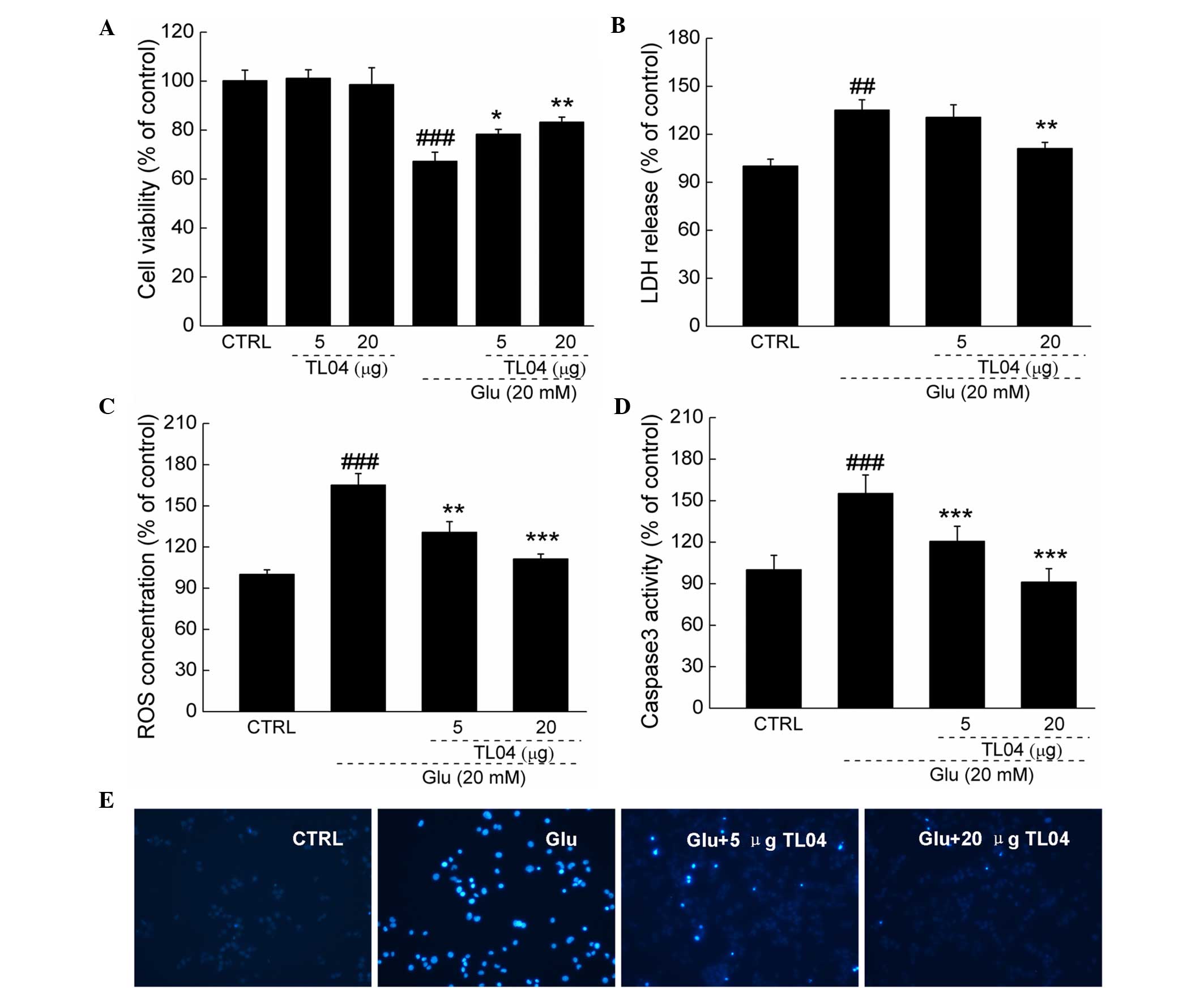 | Figure 2Effects of TL04 against Glu-induced
neurotoxicity in DPC12 cells. Cells were pretreated with 5 and 20
µg TL04 for 3 h, followed by exposure to 20 mM glutamate for
24 h. Compared with Glu-treated cells, TL04 pretreatment (A)
enhanced cell viability, reduced (B) LDH release, (C) intracellular
ROS accumulation, (D) caspase-3 activity and (E) apoptosis rate.
Data are expressed as a percentage of corresponding control cells
and the mean ± standard deviation (n=6). ##P<0.01,
###P<0.001 vs. the untreated cells;
*P<0.05, **P<0.01 and
***P<0.001 vs. the Glu-exposed cells. Glu, glutamate;
LDH, lactose dehydrogenase; ROS, reactive oxygen species; CTRL,
control. |
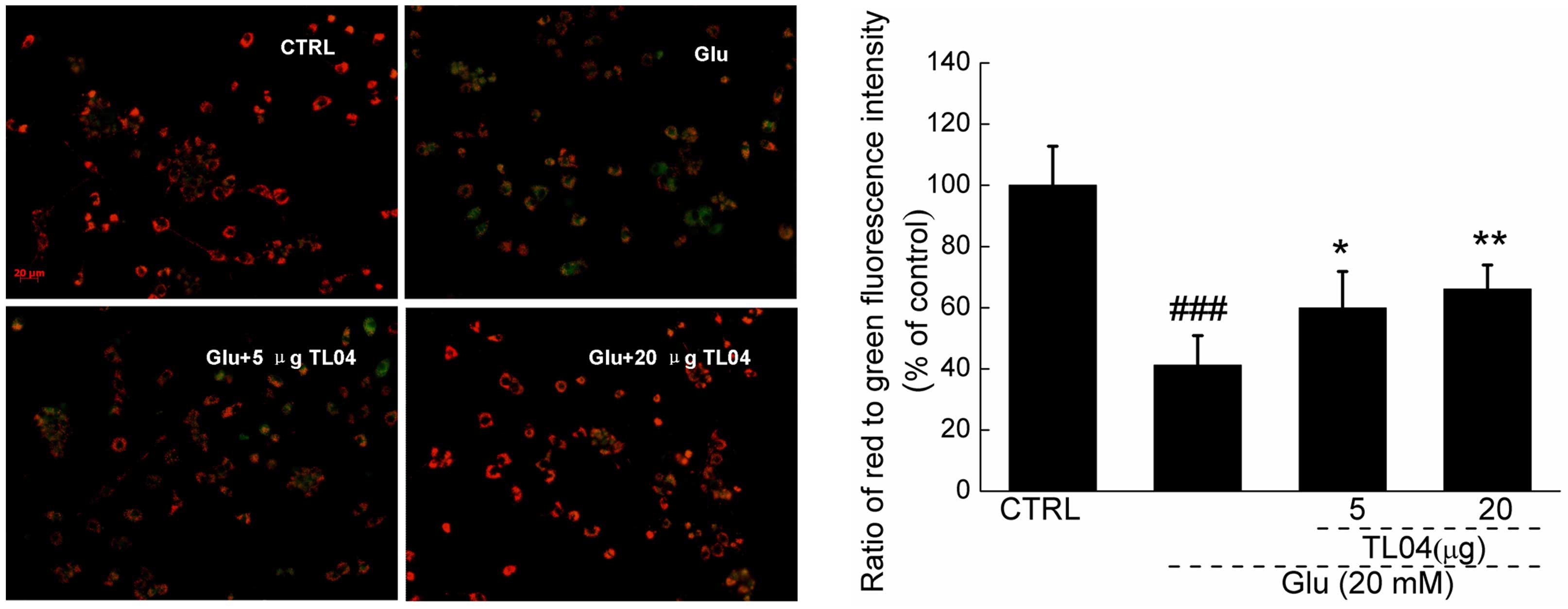
TL04 restores the dissipation of MMP
caused by glutamate
Results from JC-1 staining showed that TL04
significantly restored the dissipation of MMP indicated by the
increment of red fluorescence emission (Fig. 3). Compared with the
glutamate-treated cells, TL04 pretreatment at doses of 5 and 20
µg significantly restored MMP up to 18.7±9.7 and 24.9±7.9%
(P<0.05 and P<0.01, respectively; Fig. 3).
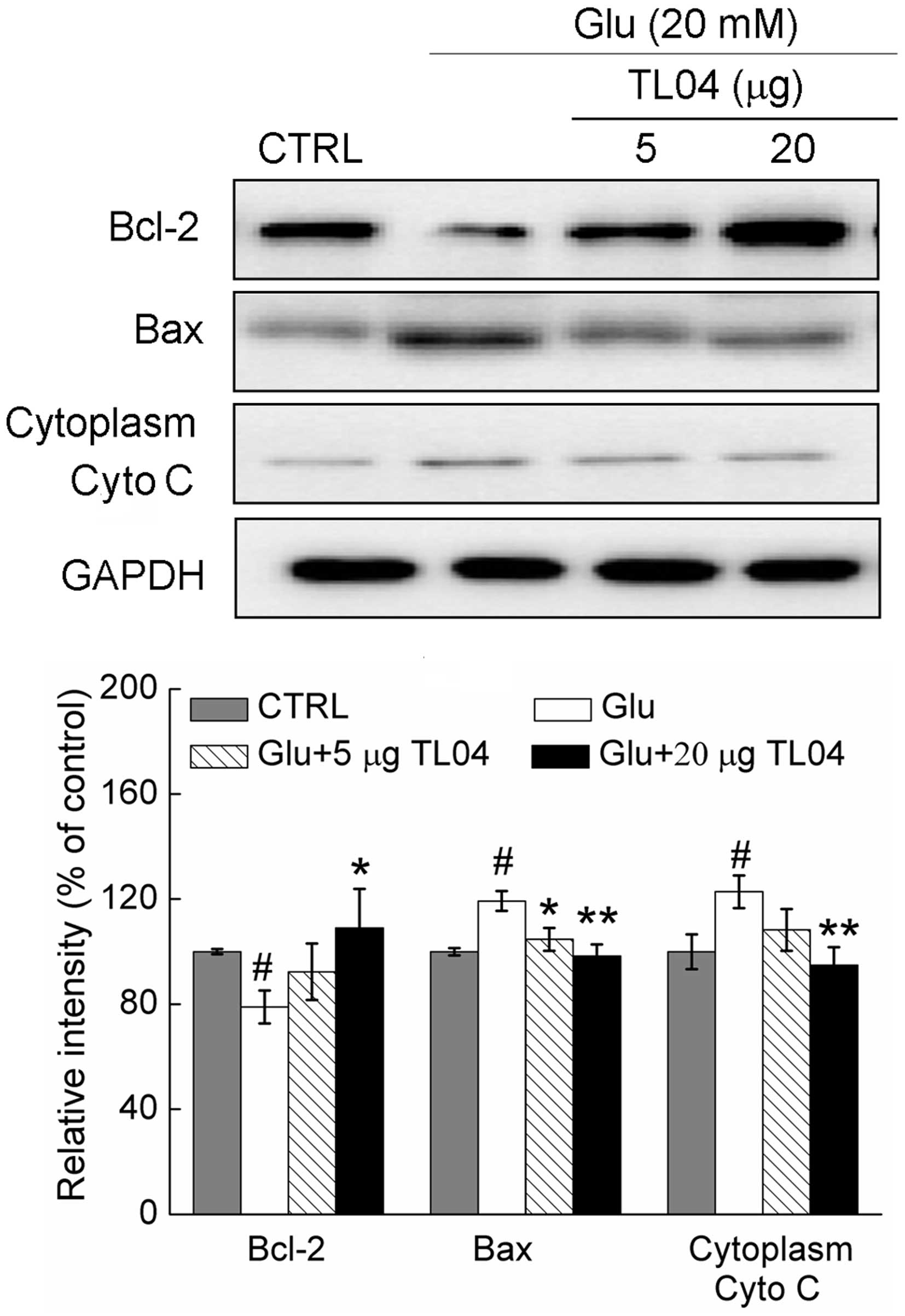
Effect of TL04 on the expression of
Bcl-2, Bax and Cyto C
Treatment with glutamate (20 mM) resulted in a
21.1±6.3% reduction in the expression of Bcl-2, a 19.3±3.8%
increase in the expression of Bax, and a 22.8±6.2% reduction in the
level of cytoplasm Cyto C (P<0.05; Fig. 4). Pretreatment with 20 µg
TL04 restored the glutamate-reduced Bcl-2 level to 109.1±14.9%
(P<0.05), normalized glutamate-increased Bax expression to
98.3±4.4% (P<0.01) and suppressed Cyto C release to 94.8±6.7%
(P<0.01; Fig. 4).
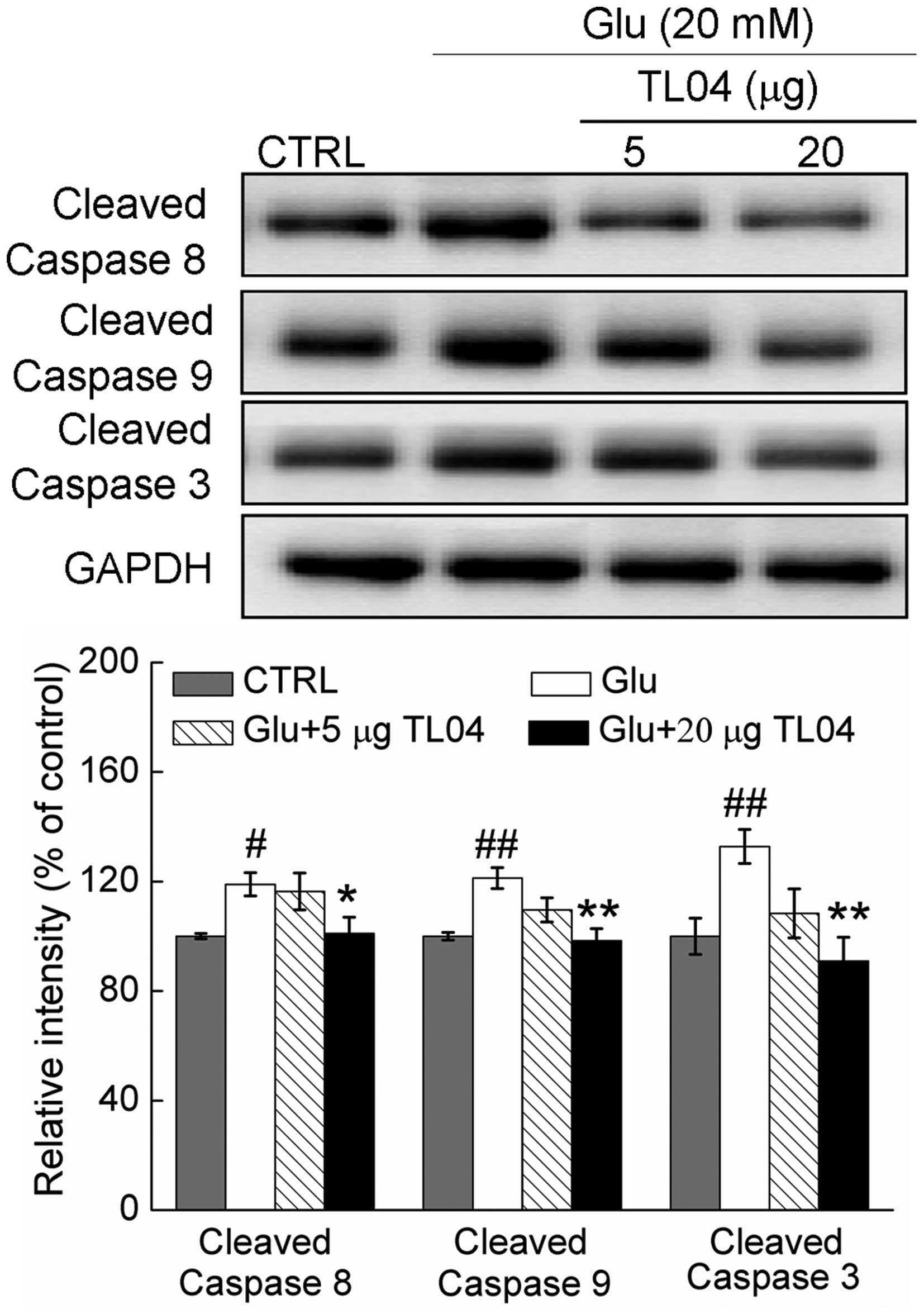
Caspase-dependent pathway contributes to
TL04-mediated neuroprotection
Compared with control cells, the activity of
caspase-8, caspase-9, and caspase-3 were enhanced to 118.9±4.4
(P<0.05), 121.3±3.8 (P<0.01) and 132.8±3.2% (P<0.01) in
DPC12 cells exposed to 20 mM glutamate for 24 h (Fig. 5). Pretreatment with 20 µg
TL04 decreased the levels of cleaved caspase-8, caspase-9 and
caspase-3 to 101.1±5.4 (P<0.05), 98.3±4.3 (P<0.01) and
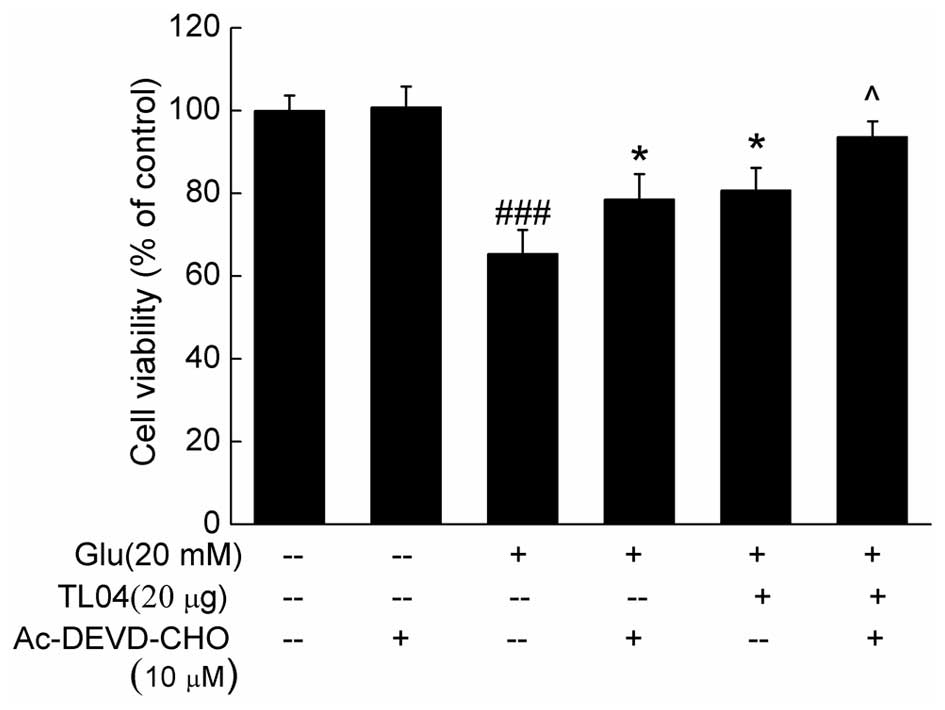
90.9±8.8% (P<0.01), respectively (Fig. 5). Furthermore, pretreatment with 10
µM Ac-DEVD-CHO (a caspase-3 inhibitor) for 30 min, and then
co-incubation with or without TL04 for another 24 h significantly
enhanced cell viability compared with glutamate-treated cells
(P<0.05; Fig. 6).
Discussion
Recently, research on the structure and
pharmacological activity of polysaccharides from Tremella
fuciformis has received extensive attention (20,28).
The aim of the present study was to investigate the neuroprotective
effect of the purified polysaccharide separated from Tremella
fuciformis (TL04) against glutamate-induced DPC12 cell damage
and the molecular mechanisms underlying these effects. The present
results support the hypothesis that TL04 mediates neuroprotection
of DPC12 cells indicated by the activity against glutamate-induced
neurotoxicity on cell viability, ROS accumulation, LDH release,
nuclear morphology, mitochondrial apoptotic alternation, and the
expression of apoptotic proteins in DPC12 cells. Further data
revealed that the caspase-dependent pathway is the predominant
attribution for the neuroprotective activity of TL04.
Polysaccharides possess effective biological
activities which are associated with their chemical structures.
TL04, purified from Tremella fuciformis water extract, was
characterized in the present study. The preliminary data from the
ultraviolet spectrum suggested the absence of contamination of
nucleic acid and proteins indicated by the lack of the absorbance
peak at wavelengths of 260 nm and 280 nm. The existence of C-H,
C=O, C-O-C and C-O-H within TL04 was confirmed via FTIR spectra.
Periodate oxidation-Smith degradation was applied to analyze the
possible linkage of monosaccharides within TL04. During 48-h
oxidation, little formic acid was produced suggesting inexistence
of 1→ and/or (1→6) linkage within TL04. The consumption of <1
mol periodate (0.14 mol for TL04) indicated the existence of (1→3)
linkage, and no glycerinum and erythritol were observed in the
hydrolysate of TL04. Combining the data from permanganate
oxidation, the backbone of TL04 is composed of (1→2)- and (1→4)
linked-mannose and (1→3) -linked-glucans.
Although a previous study indicated that Tremella
fuciformis significantly promotes neurite outgrowth of PC12
cells (18), the present study
focuses on the neuroprotective effect of TL04 against
glutamate-induced toxicity in differentiated PC12 cells related to
mitochondrial function. A series of experiments performed in
differentiated PC12 cells supported the neuroprotection of TL04
against glutamate-induced toxicity in the present study.
Mitochondrial function is considered to be key in cell apoptosis
(29). The expression of Bcl-2 and
Bax, located in the mitochondria, served as a hallmark to determine
the levels of cell apoptosis and mitochondrial function (30). TL04 restored the dissipation of
MMP, suppressed the expression of Bax, enhanced the level of Bcl-2
and inhibited Cyto C release compared with glutamate-treated cells.
It was previously demonstrated that when MMP dissipation was
observed, the release of Cyto C from the mitochondria to the
cytoplasm was enhanced (31).
Moreover, TL04 successfully suppressed the glutamate-enhanced
intracellular ROS level suggesting that ROS accumulation is
responsible for mitochondrial membrane permeability (6,32).
At physiologically low levels, ROS function as redox messengers in
intracellular signaling and regulation, whereas excess ROS induce
oxidative modification of cellular macromolecules, cause disruption
of intracellular redox homeostasis, and is related to the opening
of mitochondrial permeability transition pores (32). Thus the mitochondria-dependent
apoptotic pathway was shown to be involved in the protective
activity of TL04 against glutamate-associated neurotoxicity.
Caspases, a family of cysteine proteases, have been
investigated as a mediator during neuronal apoptosis and
neurodegeneration (33). Initiator
caspases (caspase-8, -9 and -10) catalyze the proteolytic
maturation of effector caspases (such as caspase-3, -6, -7), which
result in cell death (34). The
auto-catalytic activation of procasapase-8 in the extrinsic
apoptotic pathway further leads to the increase in mitochondrial
membrane permeability (31,35).
Consequently, apoptotic stimuli trigger the release of
mitochondrial intermembrane space proteins particularly Cyto C
(36), which promotes caspase
activation via forming a protein complex composed of Cyto C, Apaf-1
and caspase-9 (37). Activated
caspase-9 in turn activates executioner caspase-3, which executes
apoptosis (38). In the present
study, TL04 suppressed the activation of caspase-8, caspase-9 and
caspase-3 in glutamate-treated DPC12 cells. Moreover, Ac-DEVD-CHO
(a caspase-3 inhibitor) and TL04 co-treatment strongly enhanced
cell viability compared with TL04-treated cells. These data suggest
that TL04-mediated neuroprotection is associated with the
caspase-dependent mitochondrial pathway.
In conclusion, the present study purified and
characterized polysaccharides from Tremella fuciformis and
confirmed the protective effect of TL04 against glutamate-induced
neurotoxicity in DPC12 cells. In addition, the underlying mechanism
was demonstrated to be associated with the caspase-dependent
mitochondrial pathway. These findings raise the potential
therapeutic application of Tremella fuciformis and purified
polysaccharides TL04 against neurodegenerative diseases.
Acknowledgments
This study was supported by the National Science and
Technology Support Program of P.R. China (grant no.
2012BAL29B05).
References
|
1
|
Traynelis SF, Wollmuth LP, McBain CJ,
Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ and
Dingledine R: Glutamate receptor ion channels: Structure,
regulation and function. Pharmacol Rev. 62:405–496. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Reynolds IJ and Hastings TG: Glutamate
induces the production of reactive oxygen species in cultured
forebrain neurons following NMDA receptor activation. J Neurosci.
15:3318–3327. 1995.PubMed/NCBI
|
|
3
|
Akaishi T, Nakazawa K, Sato K, Saito H,
Ohno Y and Ito Y: Hydrogen peroxide modulates whole cell
Ca2+ currents through L-type channels in cultured rat
dentate granule cells. Neurosci Lett. 356:25–28. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chamoun R, Suki D, Gopinath SP, Goodman JC
and Robertson C: Role of extracellular glutamate measured by
cerebral microdialysis in severe traumatic brain injury. J
Neurosurg. 113:564–570. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Coyle JT and Puttfarcken P: Oxidative
stress, glutamate and neurodegenerative disorders. Science.
262:689–695. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
El-Najjar N, Chatila M, Moukadem H,
Vuorela H, Ocker M, Gandesiri M, Schneider-Stock R and
Gali-Muhtasib H: Reactive oxygen species mediate
thymoquinone-induced apoptosis and activate ERK and JNK signaling.
Apoptosis. 15:183–195. 2010. View Article : Google Scholar
|
|
7
|
Adams JM and Cory S: The Bcl-2 protein
family: Arbiters of cell survival. Science. 281:1322–1326. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tsujimoto Y and Shimizu S: Role of the
mitochondrial membrane permeability transition in cell death.
Apoptosis. 12:835–840. 2007. View Article : Google Scholar
|
|
9
|
Simon HU, Haj-Yehia A and Levi-Schaffer F:
Role of reactive oxygen species (ROS) in apoptosis induction.
Apoptosis. 5:415–418. 2000. View Article : Google Scholar
|
|
10
|
Ricci JE, Gottlieb RA and Green DR:
Caspase-mediated loss of mitochondrial function and generation of
reactive oxygen species during apoptosis. J Cell Biol. 160:65–75.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Methacanon P, Madla S, Kirtikara K and
Prasitsil M: Structural elucidation of bioactive fungi-derived
polymers. Carbohydrate Polymers. 60:199–203. 2005. View Article : Google Scholar
|
|
12
|
Zeng Y, Han Z, Qiu P, Zhou Z, Tang Y, Zhao
Y, Zheng S, Xu C, Zhang X, Yin P, et al: Salinity-induced
anti-angiogenesis activities and structural changes of the
polysaccharides from cultured cordyceps militaris. PLoS One.
9:e1038802014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Habijanic J, Berovic M, Boh B, Plankl M
and Wraber B: Submerged cultivation of Ganoderma lucidum and the
effects of its polysaccharides on the production of human cytokines
TNF-α, IL-12, IFN-γ, IL-2, IL-4, IL-10 and IL-17. N Biotechnol.
32:85–95. 2015. View Article : Google Scholar
|
|
14
|
Ren M, Ye L, Hao X, Ren Z, Ren S, Xu K and
Li J: Polysaccharides from Tricholoma matsutake and Lentinus edodes
enhance 5-fluorouracil-mediated H22 cell growth inhibition. J
Tradit Chin Med. 34:309–316. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Q, Seljelid R, Chen H and Jiang R:
Characterisation of acidic heteroglycans from Tremella fuciformis
Berk with cytokine stimulating activity. Carbohydr Res.
288:135–142. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ukai S, Kiriki H, Nagai K and Kiho T:
Synthesis and antitumor activities of conjugates of mitomycin
C-polysaccharide from Tremella fuciformis. Yakugaku Zasshi.
112:663–668. 1992.In Japanese. PubMed/NCBI
|
|
17
|
Ukai S, Kiho T, Hara C, Kuruma I and
Tanaka Y: Polysaccharides in fungi. XIV. Anti-inflammatory effect
of the polysaccharides from the fruit bodies of several fungi. J
Pharmacobiodyn. 6:983–990. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park HJ, Shim HS, Ahn YH, Kim KS, Park KJ,
Choi WK, Ha HC, Kang JI, Kim TS, Yeo IH, et al: Tremella fuciformis
enhances the neurite outgrowth of PC12 cells and restores
trimethyltin-induced impairment of memory in rats via activation of
CREB transcription and cholinergic systems. Behav Brain Res.
229:82–90. 2012. View Article : Google Scholar
|
|
19
|
Xu W, Shen X, Yang F, Han Y, Li R, Xue D
and Jiang C: Protective effect of polysaccharides isolated from
Tremella fuciformis against radiation-induced damage in mice. J
Radiat Res. 53:353–360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Z, Wang X, Zhao M and Qi H:
Free-radical degradation by
Fe2+/Vc/H2O2 and antioxidant
activity of polysaccharide from Tremella fuciformis. Carbohydr
Polym. 112:578–582. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du L, Song J, Wang H, Li P, Yang Z, Meng
L, Meng F, Lu J and Teng L: Optimization of the fermentation medium
for Paecilomyces tenuipes N45 using statistical approach. African
Journal of Microbiology Research. 6:6130–6141. 2012. View Article : Google Scholar
|
|
22
|
Yan H, Zhu D, Xu D, Wu J and Bian X: A
study on Cordyceps militaris polysaccharide purification,
composition and activity analysis. African Journal of
Biotechnology. 7:4004–4009. 2008.
|
|
23
|
Dong Y, Hu S, Liu C, Meng Q, Song J, Lu J,
Cheng Y, Gao C, Liu Y, Wang D and Teng L: Purification of
polysaccharides from Cordyceps militaris and their anti-hypoxic
effect. Mol Med Rep. 11:1312–1317. 2015.
|
|
24
|
Mosmann T: Rapid colorimetric assay for
cellular growth and survival: Application to proliferation and
cytotoxicity assays. J Immunol Methods. 65:55–63. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang CL, Chik SC, Li JC, Cheung BK and Lau
AS: Identification of the bioactive constituent and its mechanisms
of action in mediating the anti-inflammatory effects of black
cohosh and related Cimicifuga species on human primary blood
macrophages. J Med Chem. 52:6707–6715. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Santhiya D, Subramanian S and Natarajan K:
Surface chemical studies on sphalerite and galena using
extracellular polysaccharides isolated from Bacillus polymyxa. J
Colloid Interface Sci. 256:237–248. 2002. View Article : Google Scholar
|
|
27
|
Pielesz A: Vibrational spectroscopy and
electrophoresis as a 'golden means' in monitoring of
polysaccharides in medical plant and gels. Spectrochim Acta A Mol
Biomol Spectrosc. 93:63–69. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Du XJ, Zhang JS, Yang Y, Tang QJ, Jia W
and Pan YJ: Purification, chemical modification and
immunostimulating activity of polysaccharides from Tremella
aurantialba fruit bodies. J Zhejiang Univ Sci B. 11:437–442. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lee CS, Kim YJ, Lee MS, Han ES and Lee SJ:
18beta-Glycyrrhetinic acid induces apoptotic cell death in SiHa
cells and exhibits a synergistic effect against antibiotic
anti-cancer drug toxicity. Life Sci. 83:481–489. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Raisova M, Hossini AM, Eberle J, Riebeling
C, Wieder T, Sturm I, Daniel PT, Orfanos CE and Geilen CC: The
Bax/Bcl-2 ratio determines the susceptibility of human melanoma
cells to CD95/Fas-mediated apoptosis. J Invest Dermatol.
117:333–340. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kroemer G, Dallaporta B and Resche-Rigon
M: The mitochondrial death/life regulator in apoptosis and
necrosis. Annu Rev Physiol. 60:619–642. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Circu ML and Aw TY: Reactive oxygen
species, cellular redox systems and apoptosis. Free Radic Biol Med.
48:749–762. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang L and Zhang S: Modulating Bcl-2
family proteins and caspase-3 in induction of apoptosis by
paeoniflorin in human cervical cancer cells. Phytother Res.
25:1551–1557. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu Q, Wu D, Chen W, Yan Z and Shi Y:
Proteolytic processing of the caspase-9 zymogen is required for
apoptosome-mediated activation of caspase-9. J Biol Chem.
288:15142–15147. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Boatright KM, Renatus M, Scott FL,
Sperandio S, Shin H, Pedersen IM, Ricci JE, Edris WA, Sutherlin DP,
Green DR and Salvesen GS: A unified model for apical caspase
activation. Mol Cell. 11:529–541. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fulda S and Vucic D: Targeting IAP
proteins for therapeutic intervention in cancer. Nat Rev Drug
Discov. 11:109–124. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yuan S and Akey CW: Apoptosome structure,
assembly and procaspase activation. Structure. 21:501–515. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Jin Z and El-Deiry WS: Overview of cell
death signaling pathways. Cancer Biol Ther. 4:139–163. 2005.
View Article : Google Scholar : PubMed/NCBI
|