Introduction
Low back pain (LBP) is a widely spread disease,
which generally requires medical care and can lead to chronic
disabilities (1). It is reported
that ~84% of the general population suffer from LBP in their
lifetime (2). Degenerative disc
disease (DDD) is a commonly observed reason for LBP (3). However, it is very challenging to
investigate the pathogenesis of DDD on the account of the vague DDD
definitions and multiple interdependent factors involved, including
changed mechanical loading (4),
hampered nutrition supply (5),
hereditary factors (6) and altered
extracellular matrix (ECM) composition (7). The removal of protruding disc tissues
is the prevalent therapeutic method for DDD, which relieves painful
symptoms of patients; however, this overlooks the underlying
biological changes of discs. In terms of drawbacks of current
treatments, advanced novel therapies are urgently required, which
directly deal with the underlying biochemical causes of DDD to both
relieve symptoms and reverse disc degeneration. In recent years,
cell-based therapies regenerating disc structure and function have
aroused people's interests (8). In
particular, mesenchymal stem cells (MSCs) are recognized as an
eligible cell source for disc targeting tissue engineering. A great
number of previous studies have confirmed the ability of MSCs to
self-renew, expand and of multilineage differentiation (9–12).
However, the microenvironment of degenerated discs does not suit
exogenous MSCs due to the pathological changes occurring in
degenerated discs. Hence, it may be a better choice to concentrate
on stem cells in situ in degenerated discs. Numerous
previous studies have reported evidence for existence of stem cells
in degenerated intervertebral discs (IVDs) (11,13,14).
Notably, our research group has previous isolated cartilage
endplate-derived stem cells (CESCs) and confirmed their
multilineage differentiation capacity (13). The cartilage endplate (CEP) refers
to a thin layer of hyaline cartilage existing between the vertebral
body and the disc, and it prevents nucleus pulposus (NP) from
protruding out to the adjacent vertebrae. It is speculated that CEP
degeneration may be involved in the initiation and development of
DDD (15,16), since CEP is the predominant path
through which nutrients get to IVDs and supply them. CEP
degeneration has several manifestations, including CEP
calcification (17), proteoglycan
loss (18) and hampered ECM
synthesis (19). It is reported
that calcification or sclerosis of CEP inhibited nutrient diffusion
into adjacent IVDs, resulting in DDD (20). Therefore, it may be helpful to
modify the differentiation ability of CESCs to relieve CEP
calcification and restore CEP structure. However, the mechanism of
CESC differentiation remains to be fully understood.
Alternative splicing (AS) is a sophisticated
regulatory process in which a single pre-mRNA generates different
RNA isoforms, potentially leading to structurally and functionally
diverse proteins (21,22). It is estimated that AS occurs to
~95% of multi-exonic genes in eukaryotic organisms (23,24).
Generally speaking, AS is divided into the following types: Exon
skip/inclusion, mutually exclusive exons, alternative 5′/3′ splice
sites, intron retention, alternative promoters and polyadenylation
sites (22). In addition,
regulation of AS is elaborately achieved through cell type-,
development- and extracellular signal-related pathways (25). Aberrated AS of genes is reported to
serve roles in numerous human diseases, including autoimmune
diseases, neurodegenerative diseases and cancer (26–28).
Lately, the AS mechanism underlying stem cell differentiation has
piqued people's interest. Kazantseva et al (29) reported that depletion of hTAF4-TAFH
domain from TAF4 isoforms caused enhanced chondrogenic
differentiation of human MSCs (29). In addition, a novel alternative
trasncript-quantitative polymerase chain reaction method was
created by McAlinden et al (30) to quantify isoforms of alternatively
spliced Col2a1 gene, and the results indicated that the
majority of ATDC5 cells were the chondroprogenitor cells induced by
the standard chondrogenic differentiation method. Furthermore,
Longo et al (31) found
that in osteogenic differentiation of human MSCs, PTHrP
isoforms became increasingly selective and were considered as novel
molecular markers of stem cell state. Therefore, it is very
meaningful and rewarding to elucidate AS mechanism during stem cell
differentiation.
As mentioned above, it may be helpful to promote
chondrogenic differentiation and inhibit osteogenic differentiation
of CESCs to alleviate CEP calcification and rebuild nutrition
provision, repairing and regenerating disc degeneration. The
present study aimed to investigate the mechanism of AS underlying
osteogenic differentiation of CESCs. The isolated CESCs were
induced to undergo osteogenic differentiation and a genome-wide
analysis was performed on both the undifferentiated and
differentiated samples using Affymetrix Human Transcriptome Array
(HTA) 2.0 system. Following data extraction and pre-treatment, a
comparative analysis of alternative splicing events (ASE) was
performed between the controlled CESCs (undifferentiated) and
osteogenically differentiated CESCs. Gene Ontology (GO) and Kyoto
Encyclopedia of Genes and Genomes (KEGG) pathway analyses were
performed to add functional annotation of genes of interest to
exemplify AS mechanisms. The present study is the first, to the
best of our knowledge, to elucidate the AS mechanisms in osteogenic
differentiation of stem cells on a genome-wide scale.
Materials and methods
Ethics statement
The CEP tissues used in the present study was
obtained from seven patients who underwent discectomy and fusion
surgeries as a result of lumbar degenerative diseases at the
Department of Orthopedics, Xinqiao Hospital, Third Military Medical
University (Chongqing, China) (Table
I). The present study was approved by the Ethics Committee of
Xinqiao Hospital, Third Military Medical University. All procedures
described below were in accordance with the Helsinki Declaration.
Written informed consent was obtained from each patient and we
extensive precautions were made to protect the privacy of each
donor.
 | Table IPatient information. |
Table I
Patient information.
| Case no. | Gender | Age (years) | Diagnosis | Degenerated disc
level | Surgery type |
|---|
| 1 | Female | 56 | Disc
herniation | L4-L5 | MEDa |
| 2 | Female | 67 | Disc
herniation | L4-L5 | MED |
| 3 | Male | 68 | Disc
herniation | L5-S1 | TLIFb |
| 4 | Female | 50 |
Spondylolisthesis | L4-L5 | Quadrant assisted
TLIF |
| 5 | Male | 52 | Disc
herniation | L5-S1 | MED |
| 6 | Male | 58 | Disc
herniation | L5-S1 | TLIF |
| 7 | Male | 60 | Disc
herniation | L4-L5 | TLIF |
Agarose culture to select CESCs
The agarose culture method was used, according to a
previous study (32). Briefly, a
60 mm-diameter culture dish was coated with 1% low-melting point
agarose containing an equal volume of DMEM/F12 (37°C) and 2%
low-melting point agarose. Then, 0.75 ml DMEM/F12, 0.75 ml 2%
low-melting point agarose and 1.5 ml DMEM/F12 (20% FBS) containing
5×104 P1 CEP-derived cells were mixed and transferred to
the coated culture dishes. The final concentration of FBS was 10%.
The culture dishes were maintained at 4°C for 15 min until the gel
solidified. The culture dishes were subsequently incubated in a
humidified atmosphere containing 5% CO2 at 37°C. The
culture medium was changed twice every week. After 6 weeks, cell
clusters with a diameter >50 µm were isolated by sterile
Pasteur pipette and were sub-cultured in 6-well plates (Corning
Inc., Corning, NY, USA). Passage 3 CESCs were used in the present
study.
Osteogenic differentiation assay
The CESCs were seeded at 3×104
cells/cm2 in 6-well culture plates pre-coated with
gelatin. The complete osteogenic differentiation medium (cat. no.
HUXMA-90021; Cyagen Biosciences, Inc., Guangzhou, China) consisted
of 175 ml basal medium, 20 ml FBS, 2 ml penicillin-streptomycin, 2
ml glutamine, 400 µl ascorbate, 2 ml β-Glycerophosphate and
20 µl dexamethasone. After CESCs reached 50–70% confluence,
the culture medium was replaced with 2 ml complete medium. The
complete medium was replaced every 3 days and the cells were
cultured for 21 days.
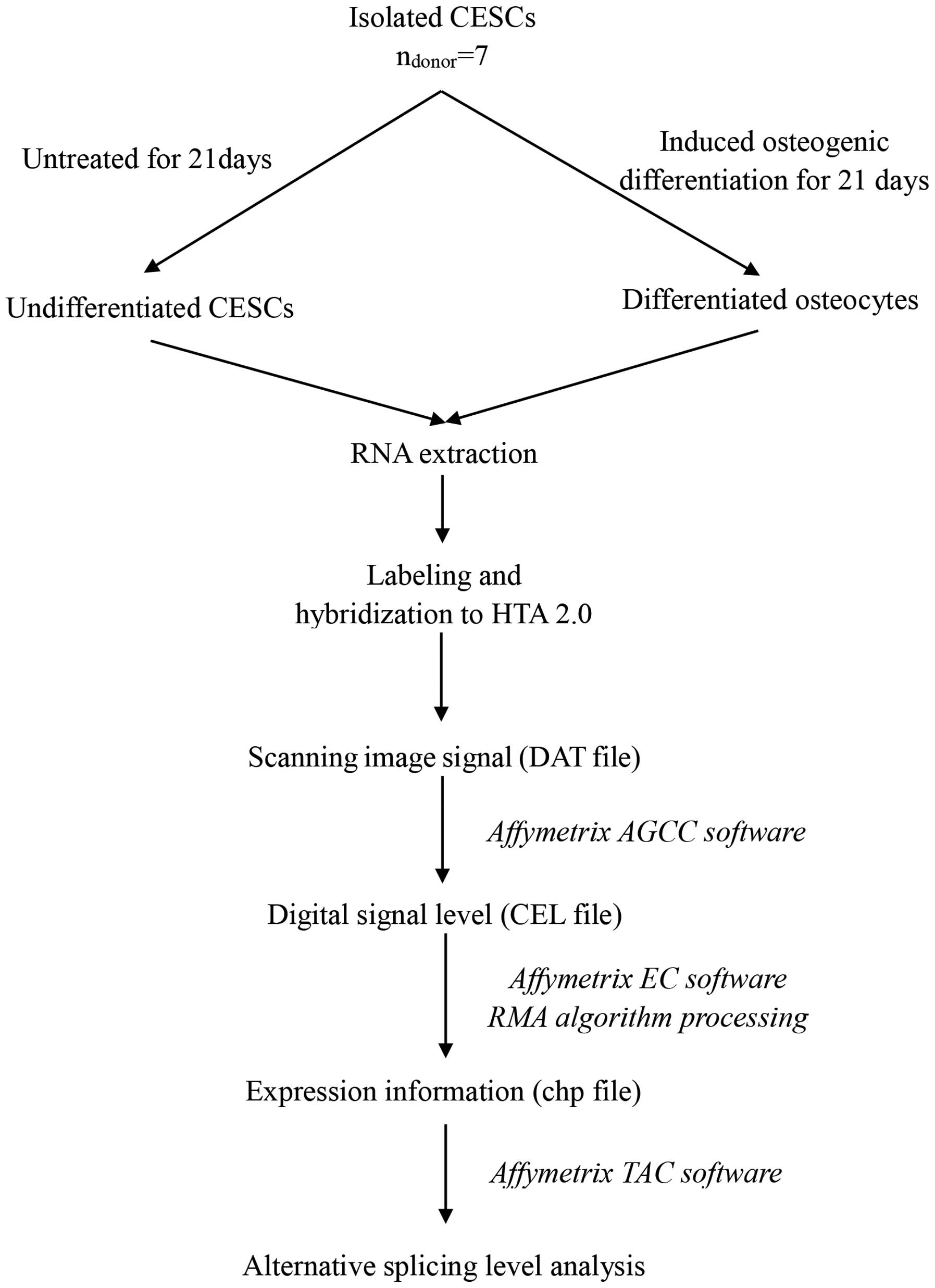
Affymetrix HTA 2.0
CESCs were induced to undergo osteogenic
differentiation or left untreated in the undifferentiated state.
Differentiated and undifferentiated samples were treated with
TRIzol reagent and sent to Bioassay Laboratory of CapitalBio
Corporation (Beijing, China). The alternative splicing events were
analyzed using HTA 2.0, purchased from Affymetrix (Santa Clara, CA,
USA). The Affymetrix HTA 2.0 contained ~339,000 probe sets (10
probes/exon and 4 probes/junction), covering ~67,000 transcript
clusters and 573,000 probe selection regions (PSRs). Transcript
clusters were referred to as genes in the present study for
simplicity. The HTA 2.0 allowed probes to target exons and
junctions within genes and provided AS information. The labeling,
hybridization, scanning and data extraction of microarray were
performed by Bioassay Laboratory of CapitalBio Corporation
(Beijing, China), according to the recommended Affymetrix
protocols. Briefly, the fluorescence signals of the microarray were
scanned and saved as DAT image files. The Affymetrix
GeneChip® Command Console software transformed DAT files
into CEL files, to change image signals into digital signals, which
recorded the fluorescence density of probes. Next, the Affymetrix
Expression Console software to pre-treat CEL files through Robust
Multichip Analysis algorithm (33), including background correction,
probeset signal integration and quantile normalization. Following
pre-treatment, the obtained chp files were analyzed by Affymetrix
Transcriptome Analysis Console software to detect alternatively
spliced genes (ASGs). The Expression Console and Transcriptome
Analysis Console software were provided by Affymetrix. To identify
significantly enriched GO terms and functional pathways, both the
publicly available web-tools KEGG (http://www.genome.jp/kegg/) and DAVID (http://david.abcc.ncifcrf.gov/tools.jsp), and the
commercial database Molecule Annotation System (MAS; CapitalBio
Corporation) were used. The microarray data were submitted to
NCBI's Gene Expression Omnibus (accession number, GSE63897). The
overall workflow of the HTA data analysis is presented in Fig. 1.
Criteria for detecting ASGs
The Spicing Index (SI) model (34,35)
was used to identify ASGs. The SI represented the ratio of the exon
signal intensities normalized against the gene signal intensities
between two experimental conditions, and was used to detect the
exon exclusion/inclusion level. The SI value was calculated in
following ways:
The NI (i,j)A represented the signal
intensity of i-th exon normalized against the j-th gene in
condition A. The subscript U indicated undifferentiated condition
and the subscript D indicated the differentiated condition. The
default filter criteria was set as SI (linear) ≤−2 or ≥2.
ASG validation by semi-quantitative
reverse transcription (RT)-PCR
RT-PCR was performed to identify the ASGs. The total
RNA was extracted using TRIzol reagent and was used to generate
cDNA using the the relevant kits (Takara Bio, Inc., Otsu, Japan),
according to the manufacturer's protocol. The quality of the total
RNA was examined using a spectrophotometer (Nanodrop 2000; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 260 and 280 nm. An
oligo (dT) primer was used to reverse transcribe 1 µg total
RNA into cDNA using the PrimeScrip RT reagent kit with gDNA Eraser
(cat. no. RR047A; Takara Bio, Inc.) and 1 µl cDNA template
was added for each reaction. The primers of genes of interest were
designed in expressed constitutive exons flanking the target exon,
using the Primer Premier 6.0 software (Premier Biosoft
International, Palo Alto, CA, USA). GAPDH was used as the internal
control. All primers are listed in Table II. The candidate genes for ASG
validation were selected according to following criteria: i) Higher
absolute value of SI was firstly considered; ii) whole exon
gain/skip was privileged; iii) first and last alternative exons
were excluded because of primer design difficulties.
 | Table IIPrimer sequences for alternatively
spliced gene confirmation by RT-PCR. |
Table II
Primer sequences for alternatively
spliced gene confirmation by RT-PCR.
| Gene symbol | Transcript ID | Primer sequences
(5′-3′) |
|---|
| ADH1C | NM_000669 | F:
CGTTCAGATGAGCATGTGGTT |
| | R:
AAGGTGCTGACGCCGAC |
| KDM5D | NM_004653 | F:
TTAAGGCCCGACATGGAACC |
| | R:
CCGCTCCACATTGGGAATCT |
| PDE4B | NM_002600 | F:
GCAGGAGTGTGATGACGGTG |
| | R:
GATCCAGTGGACTCCGACCT |
| USP9Y | NM_004654 | F:
ACTGTGCGTTCTTCTCCGTCA |
| | R:
AAGACACAAGCATAAAGGTAGCAG |
| ADAMTSL3 | NM_207517 | F:
TGTCCTGGACGTTGCATGGG |
| | R:
GCAGCACCTTTGTTTGTAGCG |
| NTRK2 | NM_006180 | F:
ACAATGCACGCAAGGACTTC |
| | R:
AAATCTCCCACAACACGACCC |
| C7 | NM_000587 | F:
CCTCAGGTTGGCATTTTGTCG |
| | R:
GCAATGGCACAGACAATGGG |
| PKP2 | NM_004572 | F:
TGTGTGGGGCCTTGAGAAAC |
| | R:
CTCCGTCAGCGTAAGCAATG |
| RXFP1 | NM_001253732 | F:
TGTAACGGTGTGGACGACTG |
| | R:
ACCGATGGAACAGCTCGTAA |
| MLPH | NM_001042467 | F:
TGCTTGCCCCCATTATCCAG |
| | R:
CTCGTTCAGATGGGCAGTGT |
| GAPDH | NM_002046.5 | F:
CTCTGCTCCTCCTGTTCGAC |
| | R:
GCGCCCAATACGACCAAATC |
Statistical analysis
Student's t-test was used to determine the
significance between groups. The data were expressed as the mean ±
standard deviation. P<0.05 was considered to indicate a
statistically significant difference.
Results
ASG detection and validation during
osteogenic differentiation of CESCs
Analysis of HTA 2.0 data was performed using
rigorous statistical methods to detect ASGs in the osteogenic
differentiation of CESCs. According to the criteria and the SI
algorithm mentioned above, this analysis of genome-wide AS
identified 11,040 alternatively spliced exons, which belonged to
3,802 ASGs during osteogenic differentiation of CESCs. In addition,
6,149 (55%) alternatively spliced exons with ≥2 SI value were
considered as 'general exon inclusion' events, while the remaining
4,891 (45%) exons were referred to as 'general exon exclusion'
events. Furthermore, it was found that 52% (1,990/3,802) of the
ASGs contained 83% (9,228/11,040) of the alternatively spliced
exons, thus confirming that multiple AS events can occur to the
same gene. During osteogenic differentiation of CESCs, each ASG had
4.6 (9,228/1,990) alternatively spliced exons on average. The
ADH1C gene was a typical example, which had 9 alternatively
spliced exons detected and suggested complicated splicing
regulation. Based on these results, 10 ASGs were selected for
RT-PCR validation (Table III).
Fig. 2 showed that 6/10 the
selected ASGs were validated successfully.
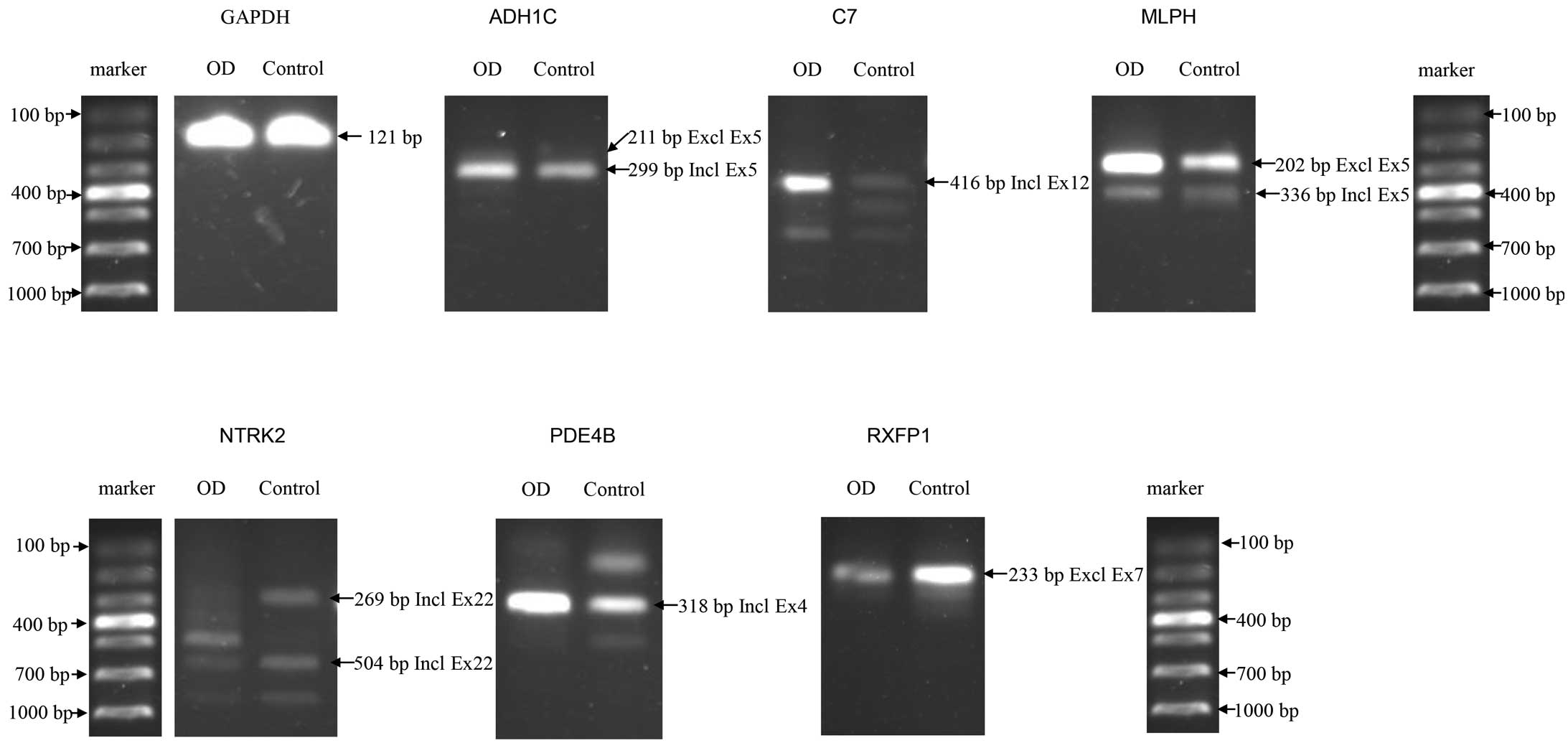 | Figure 2ASGs confirmed in osteogenic
differentiation of CESCs by RT-PCR. A total of 6/10 ASGs were
confirmed successfully by RT-PCR. The control sample refers to
undifferentiated CESCs and GAPDH was used as internal control. ASG,
Alternatively spliced gene; CESCs, cartilage endplate-derived stem
cells; RT-PCR, reverse transcription-polymerase chain reaction; OD,
osteogenically differentiated; ADH1C, alcohol dehyrogenase 1C; C7,
complement component 7; MLPH, melanophilin; NTRK2, neurotrophic
tyrosine kinase, receptor, type 2; PDE4B, phosphodiesterase 4B;
RXFP1, relaxin/insulin-like family peptide receptor 1; GAPDH,
glyceraldehyde 3-phosphate dehydrogenase; Incl, including; Excl,
excluding; Ex, exon; bp, base pairs. |
 | Table IIISummary of alternatively spliced
genes selected for validation by RT-PCR. |
Table III
Summary of alternatively spliced
genes selected for validation by RT-PCR.
| Gene symbol | AS exon | PSR ID | Splicing index | Microarray
results | RT-PCR results |
|---|
| ADH1C | 5 |
PSR04019704.hg.1 | 8.1 | Exon inclusion | Exon inclusion |
| C7 | 12 |
PSR05002182.hg.1 | 5.02 | Exon inclusion | Exon inclusion |
| MLPH | 5 |
PSR02023043.hg.1 | 5.33 | Exon inclusion | Exon inclusion |
| NTRK2 | 22 |
PSR09004159.hg.1 | 4.94 | Exon inclusion | Exon inclusion |
| PDE4B | 4 |
PSR01011724.hg.1 | 4.47 | Exon inclusion | Exon inclusion |
| RXFP1 | 7 |
PSR04011118.hg.1 | −5.4 | Exon exclusion | Exon exclusion |
| ADAMTSL3 | 29 |
PSR15007327.hg.1 | −3.94 | Exon exclusion | Exon inclusion |
| KDM5D | 3 |
PSR0Y001860.hg.1 | 7.87 | Exon inclusion | Exon exclusion |
| PKP2 | 7 |
PSR12017926.hg.1 | −5.17 | Exon exclusion | Exon inclusion |
| USP9Y | 39 |
PSR0Y000610.hg.1 | 10.43 | Exon inclusion | Exon exclusion |
Molecular function analysis of ASGs
during osteogenic differentiation of CESCs
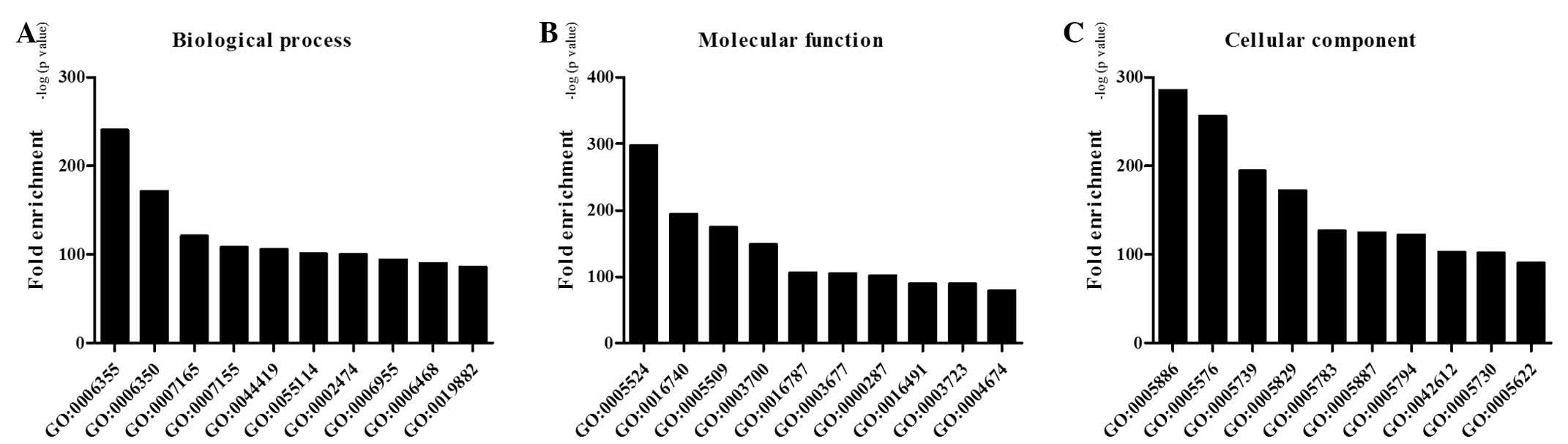
GO enrichment analysis was performed on the ASGs
during osteogenic differentiation of CESCs. The results suggested
that numerous important GO terms were regulated by AS in osteogenic
differentiation of CESCs, including regulation of transcription,
metal ion binding, signal transduction and cell adhesion. Fig. 3 highlighted the top 10 GO functions
regulated in the biological process, molecular function and
cellular component categories during osteogenic differentiation of
CESCs at the level of alternative splicing.
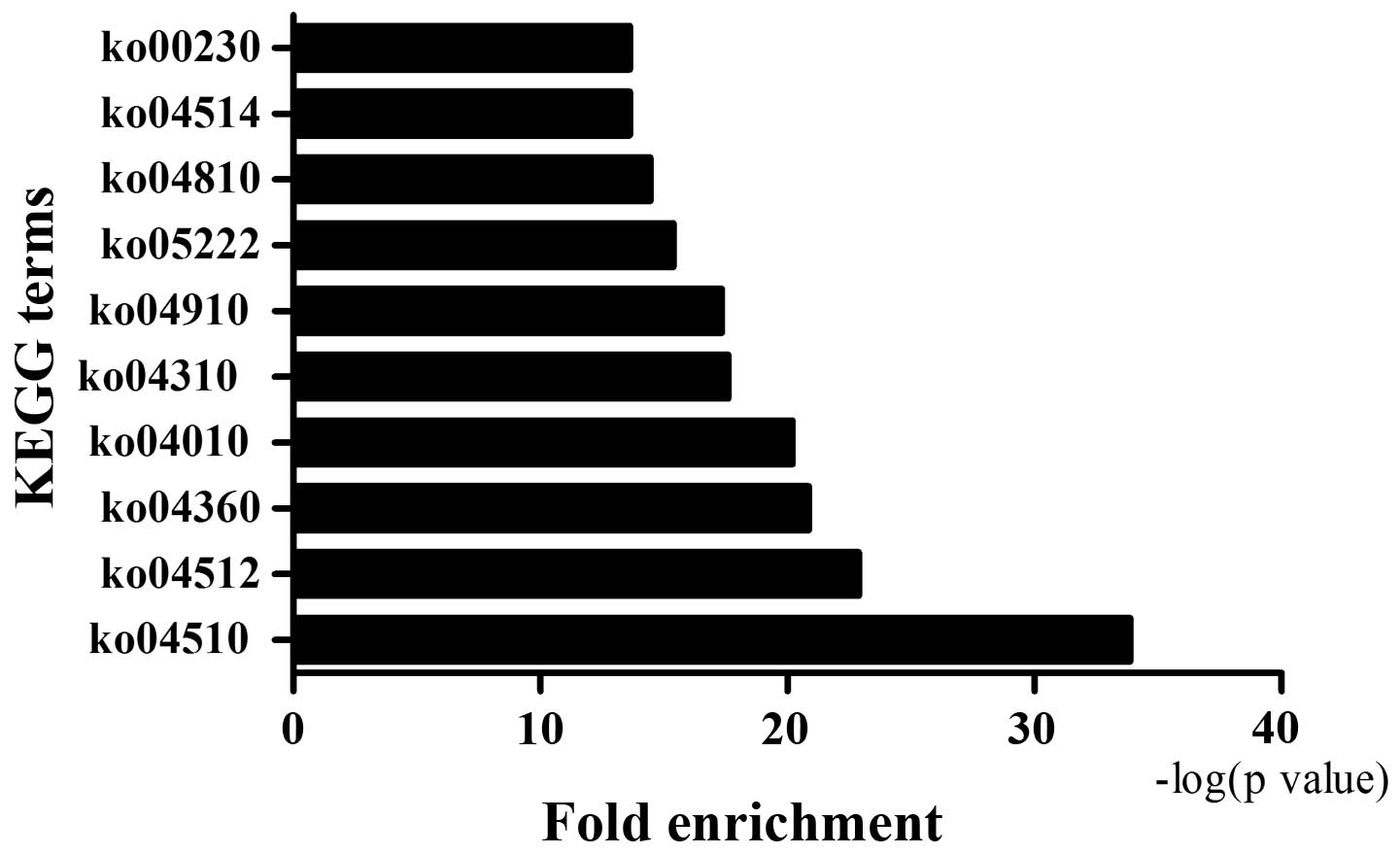
The 3,802 ASGs were analyzed by KEGG pathway
analysis in order to determine functional cellular pathways
regulated during osteogenic differentiation of CESCs. Based on the
results, several cellular pathways were affected, including the
MAPK signaling pathway, insulin signaling pathway, cell adhesion
molecules and calcium signaling pathway. Fig. 4 demonstrated the top 10 KEGG
pathways regulated in osteogenic differentiation of CESCs at the
level of AS.
Discussion
Tissue engineering is a bioengineering method to
combine seed cells, biomaterials and biological factors to repair
and regenerate damaged tissues and organs (36). The differentiation capacity is of
great importance to seed cells, usually stem cells, to accomplish
the repair and regeneration tasks. In recent years, the molecular
mechanism of osteogenic differentiation of stem cells has been
extensively studied (37–39). In particular, it is a very powerful
approach to analyze gene transcription and translation on a
genome-wide scale to fully elucidate the mechanisms of osteogenic
differentiation of stem cells. This approach has been applied to
several previous studies to investigate the global gene expression,
and post-transcriptional and epigenetic changes in the
differentiation process (40–43).
However, little contribution has been made to obtain a
comprehensive and coherent view of AS mechanisms of stem cell
osteogenic differentiation in a genome-wide scale. The present
study detected and verified ASEs in osteogenic differentiation of
CESCs, and analyzed molecular functions and pathways using
bioinformatics methods. To the best of our knowledge, the present
study is the first to determine the ASEs in osteogenic
differentiation of stem cells on the whole genome level.
The HTA 2.0 platform was used in the present study
to cover the entire genome to both identify evidence-based
sequences and discover novel ASEs. Previously, researchers tended
to analyzed expression sequence tag (EST) data for the purpose of
discovering novel ASEs (44).
However, it should be noted that the number of sequenced ESTs is
really small and the available EST data leave more ASEs
undetectable. The present study detected 3,802 ASGs with 11,040
ASEs in osteogenic differentiation of CESCs. Additionally, 6 ASGs
were validated successfully by RT-PCR. These novel validated
isoforms of ASGs have not been recorded in the NCBI Reference
Sequences database previously, hence further studies are required
to be carried out to determine cellular and molecular functions of
these isoforms. In terms of the molecular functions and pathways of
these detected ASGs, GO analysis presented enrichment of numerous
GO terms, including signal transduction, cell differentiation and
cell cycle arrest. The enriched signal transduction process
suggested that AS may modulate signaling pathways to exert its
influence on cellular function networks. Besides, during osteogenic
differentiation process, the state of CESCs shifted from
proliferation to differentiation. Therefore, cell cycle arrest
occurred and cell growth stopped slowly. Furthermore, the present
study also performed KEGG pathway analysis and revealed several
enriched signaling pathways, including focal adhesion, ECM-receptor
interaction and the MAPK signaling pathway. The focal adhesion
signaling pathway is critical for cell-matrix adhesion and serves
important roles in numerous biological processes, including cell
proliferation, cell differentiation and gene expression regulation
(45–47). The significantly enriched focal
adhesion pathway suggested an CESC and ECM interaction in
osteogenic differentiation. Additionally, the MAPK signaling
cascade is a highly conserved module, which is involved in diverse
cellular processes, including cell differentiation, migration and
proliferation (48–50). Therefore, the results of pathway
analysis indicate that AS interacts with signaling pathways to
regulate the osteogenic differentiation process.
The present stusdy used the HTA 2.0 platform to
investigate AS events in osteogenic differentiation of CESCs. The
results revealed various ASGs, and associated molecular functions
and pathways. Further research is required to illuminate downstream
mechanisms of AS modulation. The structure and function of novel
isoforms may be potential targets in future studies.
Acknowledgments
The present study was financially supported by the
National Natural Science Foundation of China (nos. 81401801 and
81301944).
References
|
1
|
Andersson GB: Epidemiological features of
chronic low-back pain. Lancet. 354:581–585. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Walker BF: The prevalence of low back
pain: A systematic review of the literature from 1966 to 1998. J
Spinal Disord. 13:205–217. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Freemont AJ: The cellular pathobiology of
the degenerate intervertebral disc and discogenic back pain.
Rheumatology (Oxford). 48:5–10. 2009. View Article : Google Scholar
|
|
4
|
Stokes IA and Iatridis JC: Mechanical
conditions that accelerate intervertebral disc degeneration:
Overload versus immobilization. Spine (Phila Pa 1976).
29:2724–2732. 2004. View Article : Google Scholar
|
|
5
|
Urban JP, Smith S and Fairbank JC:
Nutrition of the intervertebral disc. Spine (Phila Pa 1976).
29:2700–2709. 2004. View Article : Google Scholar
|
|
6
|
Battié MC and Videman T: Lumbar disc
degeneration: Epidemiology and genetics. J Bone Joint Surg Am.
88(Suppl 2): S3–S9. 2006. View Article : Google Scholar
|
|
7
|
Zhao CQ, Wang LM, Jiang LS and Dai LY: The
cell biology of intervertebral disc aging and degeneration. Ageing
Res Rev. 6:247–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Smith LJ, Nerurkar NL, Choi KS, Harfe BD
and Elliott DM: Degeneration and regeneration of the intervertebral
disc: Lessons from development. Dis Model Mech. 4:31–41. 2011.
View Article : Google Scholar :
|
|
9
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bajada S, Mazakova I, Richardson JB and
Ashammakhi N: Updates on stem cells and their applications in
regenerative medicine. J Tissue Eng Regen Med. 2:169–183. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Risbud MV, Guttapalli A, Tsai TT, Lee JY,
Danielson KG, Vaccaro AR, Albert TJ, Gazit Z, Gazit D and Shapiro
IM: Evidence for skeletal progenitor cells in the degenerate human
intervertebral disc. Spine (Phila Pa 1976). 32:2537–2544. 2007.
View Article : Google Scholar
|
|
12
|
Sakaguchi Y, Sekiya I, Yagishita K and
Muneta T: Comparison of human stem cells derived from various
mesenchymal tissues: Superiority of synovium as a cell source.
Arthritis Rheum. 52:2521–2529. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu LT, Huang B, Li CQ, Zhuang Y, Wang J
and Zhou Y: Characteristics of stem cells derived from the
degenerated human intervertebral disc cartilage endplate. PLoS One.
6:e262852011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blanco JF, Graciani IF, Sanchez-Guijo FM,
Muntión S, Hernandez-Campo P, Santamaria C, Carrancio S, Barbado
MV, Cruz G, Gutierrez-Cosío S, et al: Isolation and
characterization of mesenchymal stromal cells from human
degenerated nucleus pulposus: Comparison with bone marrow
mesenchymal stromal cells from the same subjects. Spine (Phila Pa
1976). 35:2259–2265. 2010. View Article : Google Scholar
|
|
15
|
Boyd LM and Carter AJ: Injectable
biomaterials and vertebral endplate treatment for repair and
regeneration of the intervertebral disc. Eur Spine J. 15(Suppl 3):
S414–S421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rajasekaran S, Venkatadass K, Naresh Babu
J, Ganesh K and Shetty AP: Pharmacological enhancement of disc
diffusion and differentiation of healthy, ageing and degenerated
discs: Results from in-vivo serial post-contrast MRI studies in 365
human lumbar discs. Eur Spine J. 17:626–643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng B, Hou S, Shi Q and Jia L: The
relationship between cartilage end-plate calcification and disc
degeneration: An experimental study. Chin Med J (Engl).
114:308–312. 2001.
|
|
18
|
Roberts S, Urban JP, Evans H and
Eisenstein SM: Transport properties of the human cartilage endplate
in relation to its composition and calcification. Spine (Phila Pa
1976). 21:415–420. 1996. View Article : Google Scholar
|
|
19
|
Antoniou J, Goudsouzian NM, Heathfield TF,
Winterbottom N, Steffen T, Poole AR, Aebi M and Alini M: The human
lumbar endplate. Evidence of changes in biosynthesis and
denaturation of the extracellular matrix with growth, maturation,
aging, and degeneration. Spine (Phila Pa 1976). 21:1153–1161. 1996.
View Article : Google Scholar
|
|
20
|
Benneker LM, Heini PF, Alini M, Anderson
SE and Ito K: 2004 Young Investigator Award Winner: Vertebral
endplate marrow contact channel occlusions and intervertebral disc
degeneration. Spine (Phila Pa 1976). 30:167–173. 2005. View Article : Google Scholar
|
|
21
|
Black DL: Mechanisms of alternative
pre-messenger RNA splicing. Annu Rev Biochem. 72:291–336. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Blencowe BJ: Alternative splicing: New
insights from global analyses. Cell. 126:37–47. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pan Q, Shai O, Lee LJ, Frey BJ and
Blencowe BJ: Deep surveying of alternative splicing complexity in
the human transcriptome by high-throughput sequencing. Nat Genet.
40:1413–1415. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang ET, Sandberg R, Luo S, Khrebtukova I,
Zhang L, Mayr C, Kingsmore SF, Schroth GP and Burge CB: Alternative
isoform regulation in human tissue transcriptomes. Nature.
456:470–476. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
David CJ and Manley JL: The search for
alternative splicing regulators: New approaches offer a path to a
splicing code. Genes Dev. 22:279–285. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lukong KE, Chang KW, Khandjian EW and
Richard S: RNA-binding proteins in human genetic disease. Trends
Genet. 24:416–425. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Martinez-Contreras R, Cloutier P, Shkreta
L, Fisette JF, Revil T and Chabot B: hnRNP proteins and splicing
control. Adv Exp Med Biol. 623:123–147. 2007. View Article : Google Scholar
|
|
28
|
Ng B, Yang F, Huston DP, Yan Y, Yang Y,
Xiong Z, Peterson LE, Wang H and Yang XF: Increased noncanonical
splicing of auto-antigen transcripts provides the structural basis
for expression of untolerized epitopes. J Allergy Clin Immunol.
114:1463–1470. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kazantseva J, Kivil A, Tints K, Kazantseva
A, Neuman T and Palm K: Alternative splicing targeting the
hTAF4-TAFH domain of TAF4 represses proliferation and accelerates
chondrogenic differentiation of human mesenchymal stem cells. PLoS
One. 8:e747992013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McAlinden A, Shim KH, Wirthlin L,
Ravindran S and Hering TM: Quantification of type II procollagen
splice forms using alternative transcript-qPCR (AT-qPCR). Matrix
Biol. 31:412–420. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Longo A, Librizzi M, Naselli F, Caradonna
F, Tobiasch E and Luparello C: PTHrP in differentiating human
mesenchymal stem cells: Transcript isoform expression, promoter
methylation, and protein accumulation. Biochimie. 95:1888–1896.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Thornemo M, Tallheden T, Sjogren Jansson
E, Larsson A, Lovstedt K, Nannmark U, Brittberg M and Lindahl A:
Clonal populations of chondrocytes with progenitor properties
identified within human articular cartilage. Cells Tissues Organs.
180:141–150. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Purdom E, Simpson KM, Robinson MD, Conboy
JG, Lapuk AV and Speed TP: FIRMA: A method for detection of
alternative splicing from exon array data. Bioinformatics.
24:1707–1714. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Srinivasan K, Shiue L, Hayes JD, Centers
R, Fitzwater S, Loewen R, Edmondson LR, Bryant J, Smith M,
Rommelfanger C, et al: Detection and measurement of alternative
splicing using splicing-sensitive microarrays. Methods. 37:345–359.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Clark TA, Sugnet CW and Ares M Jr:
Genomewide analysis of mRNA processing in yeast using
splicing-specific microarrays. Science. 296:907–910. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ahmed TA and Hincke MT: Strategies for
articular cartilage lesion repair and functional restoration.
Tissue Eng Part B Rev. 16:305–329. 2010. View Article : Google Scholar
|
|
37
|
Caldwell KL and Wang J: Cell-based
articular cartilage repair: The link between development and
regeneration. Osteoarthritis Cartilage. 23:351–362. 2015.
View Article : Google Scholar :
|
|
38
|
Veronesi F, Giavaresi G, Tschon M, Borsari
V, Nicoli Aldini N and Fini M: Clinical use of bone marrow, bone
marrow concentrate, and expanded bone marrow mesenchymal stem cells
in cartilage disease. Stem Cells Dev. 22:181–192. 2013. View Article : Google Scholar
|
|
39
|
Locke M, Feisst V and Dunbar PR: Concise
review: Human adipose-derived stem cells: Separating promise from
clinical need. Stem Cells. 29:404–411. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Babadagli ME, Tezcan B, Yilmaz ST and
Tufan AC: Matrilin-3 as a putative effector of C-type natriuretic
peptide signaling during TGF-β induced chondrogenic differentiation
of mesenchymal stem cells. Mol Biol Rep. 41:5549–5555. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fernandes AM, Herlofsen SR, Karlsen TA,
Kuchler AM, Fløisand Y and Brinchmann JE: Similar properties of
chondrocytes from osteoarthritis joints and mesenchymal stem cells
from healthy donors for tissue engineering of articular cartilage.
PLoS One. 8:e629942013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Herlofsen SR, Bryne JC, Høiby T, Wang L,
Issner R, Zhang X, Coyne MJ, Boyle P, Gu H, Meza-Zepeda LA, et al:
Genome-wide map of quantified epigenetic changes during in vitro
chon-drogenic differentiation of primary human mesenchymal stem
cells. BMC Genomics. 14:1052013. View Article : Google Scholar
|
|
43
|
Weber M, Sotoca AM, Kupfer P, Guthke R and
van Zoelen EJ: Dynamic modelling of microRNA regulation during
mesenchymal stem cell differentiation. BMC Syst Biol. 7:1242013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kan Z, Rouchka EC, Gish WR and States DJ:
Gene structure prediction and alternative splicing analysis using
genomically aligned ESTs. Genome Res. 11:889–900. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Petit V and Thiery JP: Focal adhesions:
Structure and dynamics. Biol Cell. 92:477–494. 2000. View Article : Google Scholar
|
|
46
|
Mitra SK, Hanson DA and Schlaepfer DD:
Focal adhesion kinase: In command and control of cell motility. Nat
Rev Mol Cell Biol. 6:56–68. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
47
|
Comoglio PM, Boccaccio C and Trusolino L:
Interactions between growth factor receptors and adhesion
molecules: Breaking the rules. Curr Opin Cell Biol. 15:565–571.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Chen Z, Gibson TB, Robinson F, Silvestro
L, Pearson G, Xu B, Wright A, Vanderbilt C and Cobb MH: MAP
kinases. Chem Rev. 101:2449–2476. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yang SH, Sharrocks AD and Whitmarsh AJ:
Transcriptional regulation by the MAP kinase signaling cascades.
Gene. 320:3–21. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tanoue T and Nishida E: Docking
interactions in the mitogen-activated protein kinase cascades.
Pharmacol Ther. 93:193–202. 2002. View Article : Google Scholar : PubMed/NCBI
|