Introduction
Cryptorchidism and hypospadias in newborn males, and
infertility and testicular germ cell cancer in adult males, are
common male reproductive system disorders worldwide (1,2).
These disorders have been hypothesized to be associated with
testicular dysgenesis syndrome (TDS), which originates in male
fetal life (3). An important
factor contributing to TDS is androgen dysfunction during the
masculinization programming window. Masculinization is a pivotal
event during reproductive tract development, driven by androgen
produced by the fetal testes (4).
Specific factors influencing this process, including exposure to
certain environmental pollutants, may cause inadequate production
of androgen, and ultimately lead to abnormal reproductive
development (5–7).
Acrolein, a cyclophosphamide metabolite, is a common
environmental and dietary pollutant arising from the combustion of
fuels, plastic and fried food, and is additionally a primary
component of tobacco (8). Acrolein
may be generated endogenously during cellular metabolism by lipid
peroxidation and degradation of threonine and polyamines. It has
been identified as one of the most harmful non-biological air
pollutants in residences in the United States (9,10).
Increased acrolein exposure has been reported to be associated with
various diseases, including diabetes mellitus, hepatotoxicity,
cardiovascular disease and Alzheimer's disease (11–13).
Acrolein has been demonstrated to induce embryo
lethality and teratogenicity in cultured rat embryos, and causes
reproductive toxicity in a yeast gametogenesis model (14,15).
Cyclophosphamide (CP), the precursor of acrolein, is used as a
therapeutic agent for the treatment of childhood cancers; however,
it may be associated with a high risk of infertility and long-term
gonadal toxicity in male survivors (16). Additionally, cytochrome P450 3A 4
and 5, the two key enzymes that metabolize CP into acrolein, are
highly expressed in mammalian testes, which may lead to an
increased concentration of acrolein in the testes of patients
treated with CP (17,18). As acrolein efficiently reaches the
testes, it may interfere with steroidogenesis during fetal
development, when testosterone production by Leydig cells is
critical for normal sexual development. However, little is known
regarding the effects of acrolein exposure in maternal rats on the
steroidogenic function and sexual development of male offspring.
The present study aimed to investigate the dose-dependent effects
of acrolein on prenatal testosterone production, and the expression
levels of the factors involved in testosterone biosynthesis in the
fetal rat testes.
Materials and methods
Ethical approval
The present study was performed in compliance with
the regulations of the Medical Ethics Committee of Peking
University Third Hospital (Beijing, China; ethical approval no.
LA2015205).
Chemicals and reagents
Acrolein (10 mg/ml; CAS107-02-8) was purchased from
AccuStandard, Inc. (New Haven, CT, USA). Anti-3β-hydroxysteroid
dehydrogenase (3β-HSD; catalog no. ab150384), anti-steroidogenic
acute regulatory protein (StAR; catalog no. ab203193),
anti-4-hydroxynonenal (4-HNE; catalog no. ab46545) and
anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; catalog no.
ab181602) rabbit primary antibodies were obtained from Abcam
(Cambridge, MA, USA). All other reagents were purchased from
Sigma-Aldrich; Merck Millipore (Darmstadt, Germany) or as otherwise
specified.
Animals and treatments
Pregnant Sprague-Dawley rats (n=32, with n=8 used
for preliminary studies and 24 for formal studies; Vital River
Laboratories, Co., Ltd., Beijing, China) were individually housed
and maintained under a 12-h light/dark cycle at a controlled
temperature (20–25°C) and humidity (50±5%) for one week prior to
the experiments. The study was performed according to the Guide for
the Care and Use of Laboratory Animals published by the National
Institutes of Health (Bethesda, MD, USA). Acrolein was administered
intraperitoneally (i.p.), in accordance with previous studies of
maternal exposure (19). Our
preliminary study revealed that rats died 4 days following
injection of 10 mg/kg acrolein, and as testosterone levels in the
fetal testes decreased significantly in the 5 mg/kg group, a dose
of 5 mg/kg was used in the present study. A total of 24 pregnant
rats at gestational days (GD) 14–20 were divided into four groups
(n=6) and injected i.p. with 1, 2 or 5 mg/kg acrolein, with an
equal volume of saline serving as the control according to previous
studies (20,21). Pregnant rats were anesthetized with
an i.p. injection of 50 mg/kg sodium pentobarbital (Sinopharm
Chemical Reagent, Co., Ltd., Beijing, China) at GD 21. The fetal
rats were harvested by cesarean section, weighed and dissected
under a stereomicroscope. Gender was determined by morphology and
the gonads. All male fetuses were sacrificed by decapitation and
whole blood was collected in a tube with heparin for testosterone
analysis. Fetal testes were aseptically removed and stored at −80°C
for analysis of testosterone levels, reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
histology.
RT-qPCR
Total RNA was extracted using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA). The quantity and quality of the purified RNA was evaluated by
spectroscopy. cDNA was synthesized using a RevertAid First Strand
cDNA Synthesis Kit (catalog no. K1621; Thermo Fisher Scientific,
Inc.), according to the manufacturer's protocol. qPCR was performed
using the SYBR® Green Master mix (Fermentas; Thermo
Fisher Scientific, Inc.) and the Applied Biosystems 7500 Real-Time
PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.).
The cycling conditions were as follows: 1 cycle at 95°C for 5 min,
and 40 cycles of amplification at 95°C for 15 sec and 60°C for 1
min. All the samples were run in duplicate using the threshold
suggested by the software for the instrument to calculate the
quantitation cycle (Cq). Following RT-qPCR, a melting curve
analysis was performed to demonstrate the specificity of the PCR
products, which revealed that the melting curve for the PCR product
of each gene transcript had a single peak (data not shown). To
normalize the readings, Cq values from GAPDH served as internal
controls for each run, obtaining a ΔCq value for each gene.
Relative alterations in the gene expression data were analyzed
using the 2−ΔΔCq method (22). Primer sequences are presented in
Table I.
 | Table I.Sequences of the specific
oligonucleotide primers used for polymerase chain reaction
amplification. |
Table I.
Sequences of the specific
oligonucleotide primers used for polymerase chain reaction
amplification.
| Target gene | Genbank no. | Product length
(bp) | Primer sequence
(5′-3′) |
|---|
| Scavenger receptor
class B | NM_031541 | 134 |
F:ctcctgactttctccgtctttc |
|
|
|
|
R:caggatctggaactgcttgt |
| Steroidogenic acute
regulatory protein | NM_031558 | 125 |
F:tcaactggaagcaacactctac |
|
|
|
|
R:cctgctggctttccttctt |
| Cytochrome P-450
side chain cleavage | NM_017286 | 156 |
F:ctggtgacaatggttggataaac |
|
|
|
|
R:ccttagggtccaggatgtaaac |
| 3β-hydroxysteroid
dehydrogenase | M38178 | 141 |
F:tgttggtgcaggagaaagaa |
|
|
|
|
R:ggtactgggcatccagaatatc |
| Cytochrome P-450,
family 17 | NM_012753 | 170 |
F:gcctttgcagatgctggta |
|
|
|
|
R:ggcgtggacaggtctat |
| 17β-hydroxysteroid
dehydrogenase | NM_012851 | 180 |
F:aggctttaccagggtctttc |
|
|
|
|
R:cagtggtcctctcaatctcttc |
| Insulin-like factor
3 | NM_053680 | 130 |
F:gcacccagcaagaccttt |
|
|
|
|
R:tagggatcctccaaggcaat |
| GAPDH | NM_017008 | 155 |
F:actcccattcttccacctttg |
|
|
|
|
R:gtccagggtttcttactccttg |
Western blot analysis
Fetal testes were washed twice with ice-cold
phosphate buffered saline (PBS) and homogenized with RIPA lysis
buffer containing protease inhibitors (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). The protein
concentration was quantified using the Bicinchoninic Acid Protein
assay kit (Beijing CoWin Biotech, Co., Ltd., Beijing, China).
Proteins (30 µg) in lysates were separated by 10% SDS-PAGE,
transferred to nitrocellulose membranes (Applygen Technologies,
Inc., Beijing, China), and blocked with 5% bovine serum albumin
(BSA; Sigma-Aldrich; Merck Millipore) at room temperature for 1 h.
Membranes were subsequently incubated at 4°C overnight with the
following rabbit primary antibodies, all at a 1:1,000 dilution:
Anti-StAR, anti-4-HNE or anti-GAPDH, following which corresponding
IRDye-conjugated goat anti-rabbit IgG secondary fluorescent
antibodies (cat. no. 925-32211; 1:10,000 dilution; LI-COR
Biosciences, Lincoln, NE, USA) were added and incubated for 1 h at
room temperature in a dark place. Membranes were scanned using the
Odyssey® CLx Imaging system (LI-COR Biosciences) and the
protein expression was quantified using Image Studio™ Software
(LI-COR Biosciences).
Radioimmunoassay (RIA) for
testosterone analysis
Serum was separated from blood collected from male
offspring by centrifugation at 3000 × g for 10 min at room
temperature and stored at −80°C until required for the testosterone
assay. Fetal testes were rinsed with 0.01 M PBS and homogenized in
150 µl 0.01 M PBS. The homogenate was centrifuged for 10 min at
5000 × g at 4°C, and the supernatant was collected and
stored at −80°C until required. 125I-based RIA kits were
purchased from the Beijing North Institute of Biological Technology
(Beijing, China). Testosterone levels were measured according to
the manufacturer's protocol, and expressed as ng/ml.
Histopathology and
immunohistochemistry
The testes were immersed in 4% paraformaldehyde for
fixation, dehydrated via a graded series of ethanol washes followed
by xylene, and embedded in paraffin. Paraffin-embedded tissues were
serially sectioned (5-µm thick), mounted onto glass slides coated
with poly-L-lysine, deparaffinized with xylene and rehydrated with
graded ethanol. At least two non-serial sections were stained with
hematoxylin and eosin (H&E) using standard procedures for
morphological analyses. For histological evaluation of apoptosis,
DNA fragmentation was examined using the Terminal Deoxynucleotidyl
Transferase dUTP Nick-End Labeling assay (TUNEL) Detection kit
(cat. no. 11684817910; Roche Diagnostics, Basel, Switzerland)
according to the manufacturer's protocol. Slides were incubated
with 20 µg/ml proteinase K for 15 min at room temperature and
washed with PBS three times. The slides were subsequently incubated
with TUNEL reaction buffer for 60 min at 37°C in a humidified
atmosphere in the dark. Following a further wash with PBS, the
slides were incubated with an anti-converter-peroxidase secondary
antibody, which was part of the TUNEL kit (Roche Diagnostics) for
30 min at 37°C, and the signal was visualized with diaminobenzidine
(DAB; OriGene Technologies, Inc., Beijing, China). The number of
positive cells was calculated for analysis under a light
microscope.
Testicular Leydig cells were identified in 5-µm
thick paraffin sections by immunohistochemistry for 3β-HSD. Antigen
retrieval was performed by microwave oven heating for 5 min in 0.01
M citrate buffer (pH 6.0). The slides were incubated for 10 min in
3% (v/v) hydrogen peroxide in PBS to block endogenous peroxidase
activity and subsequently washed with PBS. Following blocking with
normal goat serum (Beijing Zhongshan Golden Bridge Biotechnology;
OriGene Technologies, Inc., Rockville, MD, USA) diluted 1:5 in PBS
containing 5% BSA, the slides were incubated overnight at 4°C with
a rabbit polyclonal anti-3β-HSD antibody (diluted 1:100 in antibody
dilutions liquid (cat. no. ZLI-9028; Beijing Zhongshan Golden
Bridge Biotechnology; OriGene Technologies, Inc.). Following this,
the slides were washed with PBS and incubated with the horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(provided at working dilution; cat. no. PV-6001; Beijing Zhongshan
Golden Bridge Biotechnology; OriGene Technologies, Inc.) at 37°C
for 30 min. The slides were subsequently stained using a DAB kit,
washed with water, then stained with hematoxylin, dehydrated using
sequential concentrations ethanol, being washed for 2 min in each
starting with 80%, followed by 95% and finishing with 100% ethanol
twice, and placed under cover slips. The density of
3β-HSD-immunoreactivity was detected as described previously
(23). Briefly, photomicrographic
digital images were obtained from 3β-HSD-immunostained sections of
the fetal testes, and regions of these photomicrographs were
analyzed to measure the density of 3β-HSD immunoreactivity in the
interstitial region of the testes. The surface area of the total
interstitial region and the 3β-HSD-immunoreactive area were
subsequently measured using ImageJ software version 1.46 (National
Institute of Health). Areas darker than 100 of 256 pixels were
determined to be 3β-HSD-immunoreactive. The positive
3β-HSD-immunoreactive area was normalized by dividing by the total
area of the interstitial region of interest, and was expressed as
the 3β-HSD-immunoreactive area/1-mm2 area of the
interstitial region of the fetal testes.
Statistical analysis
Statistical analysis was performed using SPSS
software version 12.0 (SPSS, Inc., Chicago, IL, USA). The data were
examined for normal distribution and homogeneity of variance.
Normally distributed and variance homogeneous data were analyzed by
one-way analysis of variance. Dunnett's post hoc test was used to
compare the values from acrolein-treated animals with the control
group. Data are presented as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
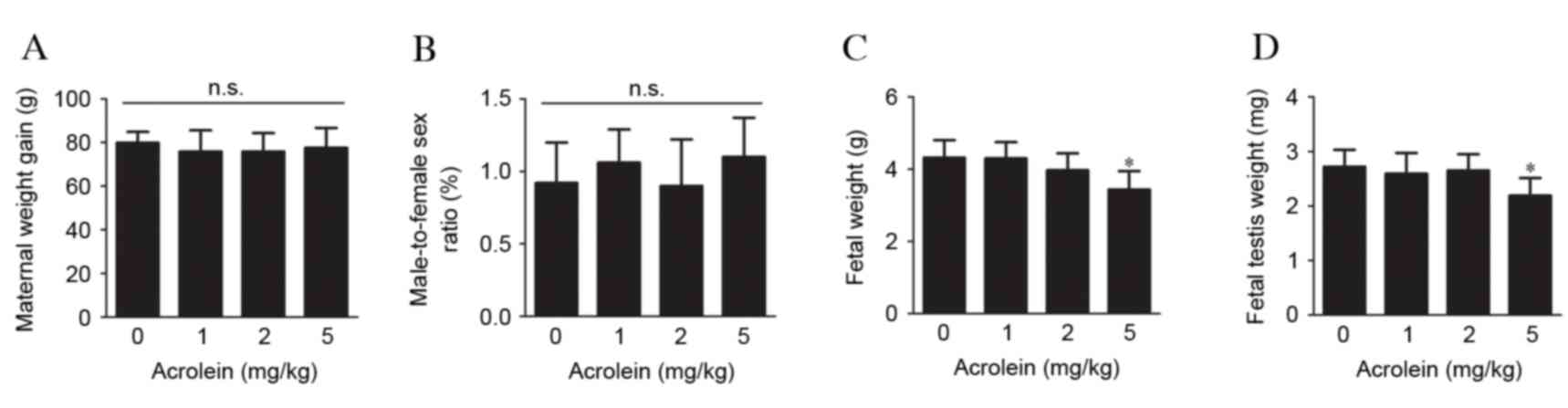
Effects of maternal acrolein exposure
on pregnant rats and fetus development
To investigate the effects of maternal acrolein
exposure during pregnancy on maternal weight gain and fetus
development, pregnant rats were injected i.p. with 0, 1, 2 or 5
mg/kg acrolein daily from GD 14–20. No significant differences in
body weight gain in the pregnant rats were observed between the
acrolein-treated and control groups (Fig. 1A). Although no significant
differences were observed in the male-to-female sex ratio (Fig. 1B), the weight of the pups was
markedly reduced when pregnant rats received acrolein at 5 mg/kg
compared with the control (P=0.003, Fig. 1C). However, no significant effect
on fetal weight was observed in groups administered with 1 or 2
mg/kg acrolein. In addition, the weight of the fetal testes in the
group treated with 5 mg/kg acrolein was reduced compared with the
control group (P=0.001), while no significant alterations were
observed in the other groups (Fig.
1D).
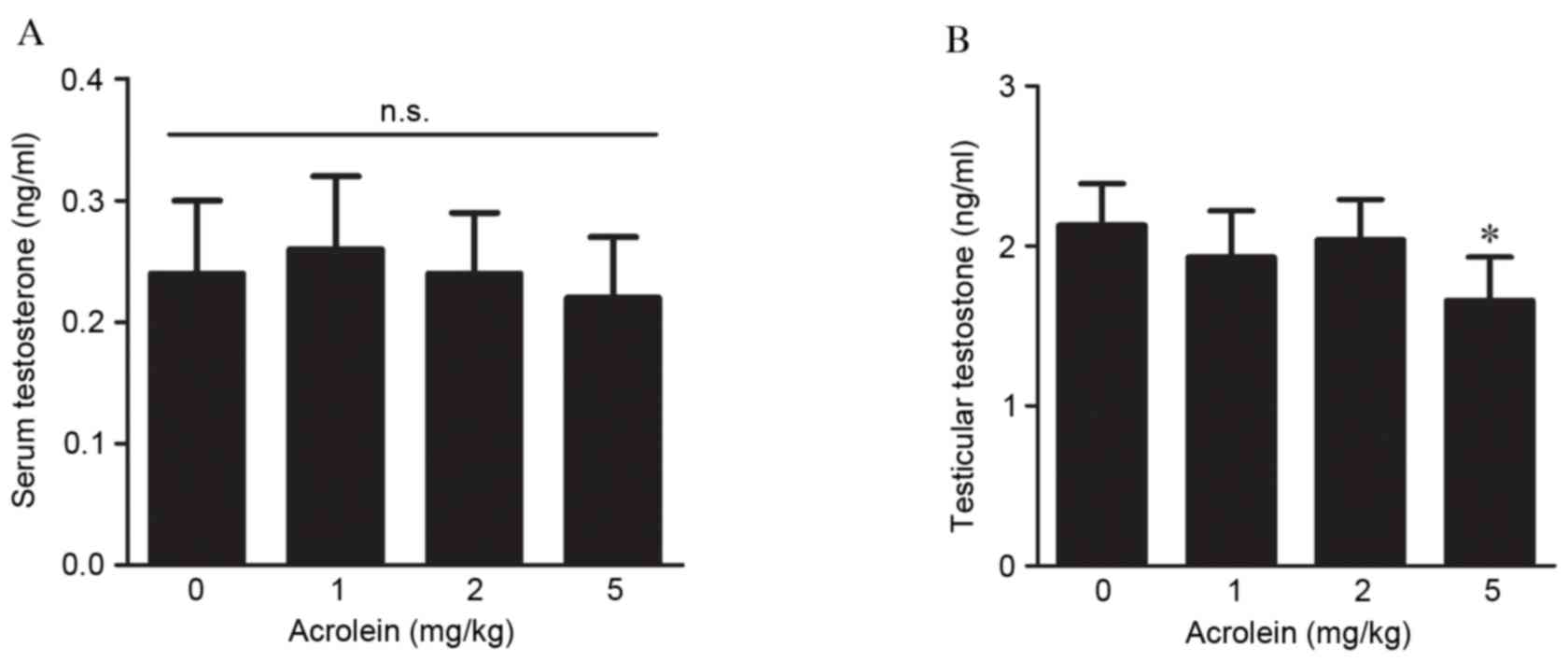
Effects of maternal acrolein exposure
on testosterone production in fetal rats
The effects of maternal acrolein exposure during
pregnancy on testosterone production in the serum and testes were
analyzed. As presented in Fig. 2A,
no significant effects of acrolein on serum testosterone were
observed in fetal rats. However, compared with the control, the
intratesticular testosterone concentration was markedly reduced
following treatment with 5 mg/kg acrolein (P<0.001, Fig. 2B). Although testosterone in the
groups treated with 1 and 2 mg/kg acrolein decreased compared with
the control group, no statistically significant differences were
observed.
Effects of maternal acrolein exposure
on the expression levels of steroidogenic genes and insulin-like
factor 3 (Insl3)
A panel of genetic markers associated with
testosterone production was used to assess the steroidogenic
function in the fetal testes following prenatal exposure to
acrolein. No significant differences were observed in the mRNA
expression levels of the scavenger receptor class B member 1
(SR-B1; Fig. 3A). However, there
was a slight increase in 17β-HSD mRNA expression levels in the 1
mg/kg group (P=0.032, Fig. 3B). No
significant differences in mRNA expression levels were observed in
the other steroidogenic enzymes assessed, cholesterol side-chain
cleavage enzyme (P450scc; Fig. 3C)
and steroid 17 alpha-hydroxylase/17,20 lyase (P450c17; Fig. 3D). The mRNA expression levels of
3β-HSD were only reduced in the 5 mg/kg group (P=0.020, Fig. 3E). These alterations were
consistent with reduced intratesticular testosterone levels. No
significant alterations in the mRNA expression levels of Insl-3, a
critical gene involved in testicular descent, were observed in any
of the groups treated with acrolein, compared with the control
group (Fig. 3F). The mRNA
expression levels of the cholesterol transporter StAR in fetal
testes were reduced following exposure to 2 and 5 mg/kg acrolein
(P=0.009 and P=0.038, respectively), compared with the control
group, while no significant differences were observed in the 1
mg/kg group (Fig. 3G). Alterations
in protein expression levels of StAR were in accordance with mRNA
expression levels (Fig. 3H).
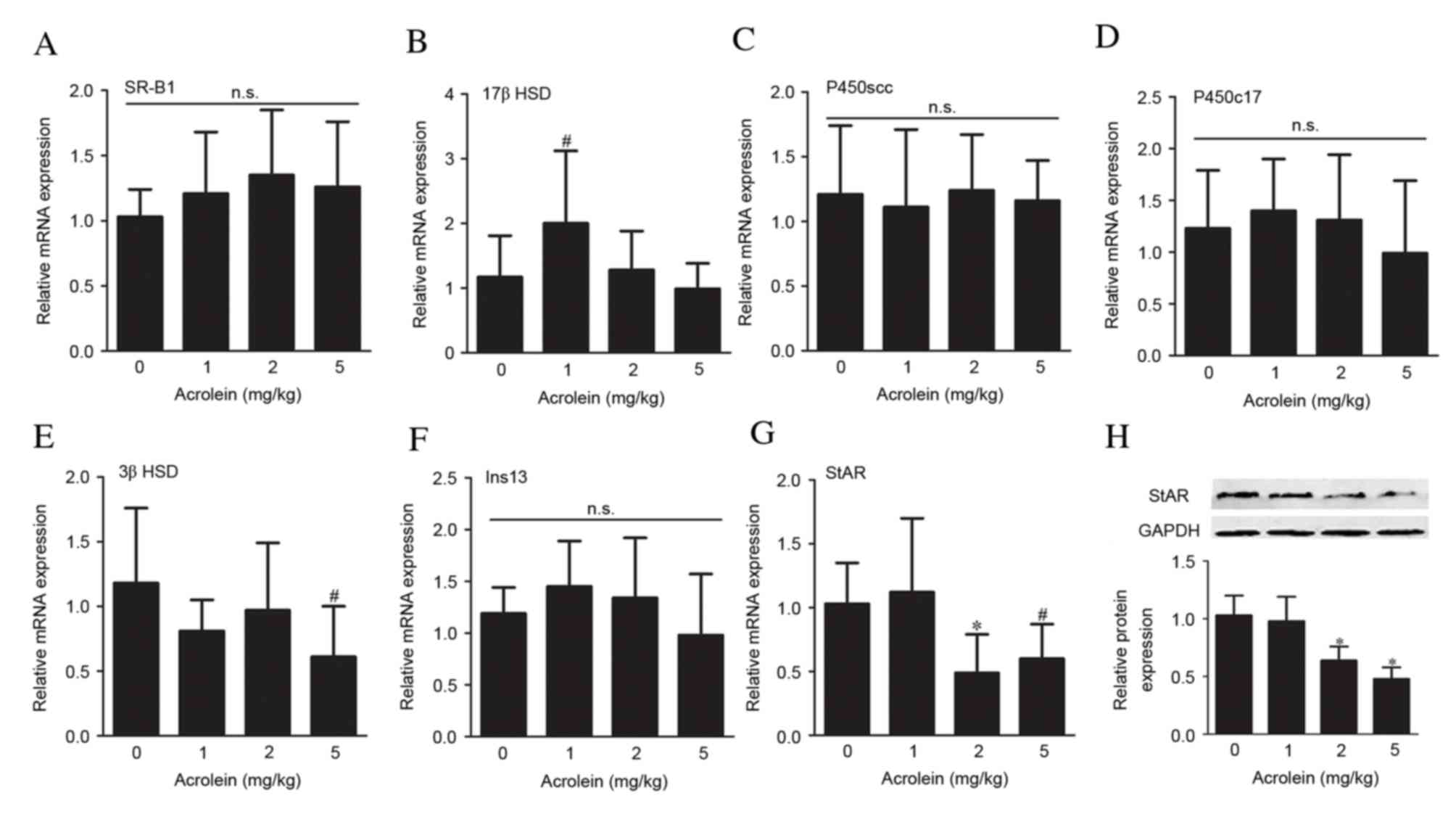 | Figure 3.Gene expression analysis. Reverse
transcription-quantitative polymerase chain reaction analysis of
the expression of (A) SR-B1, (B) 17β HSD, (C) P450scc, (D) P450c17,
(E) 3β HSD, (F) Ins13 and (G) StAR. (H) Representative western blot
images and analysis of StAR protein expression levels in fetal
testes following maternal acrolein exposure. Data are presented as
the mean ± standard deviation (n=6–8). #P<0.05 and
*P<0.01 vs. 0 mg/kg acrolein. SR-B1, scavenger receptor class
B;StAR, steroidogenic acute regulatory protein; P450scc,
cholesterol side-chain cleavage enzyme; P450c17, steroid 17
alpha-hydroxylase/17,20 lyase; HSD, hydroxysteroid dehydrogenase;
Ins13, insulin-like factor 13; n.s., non-significant. |
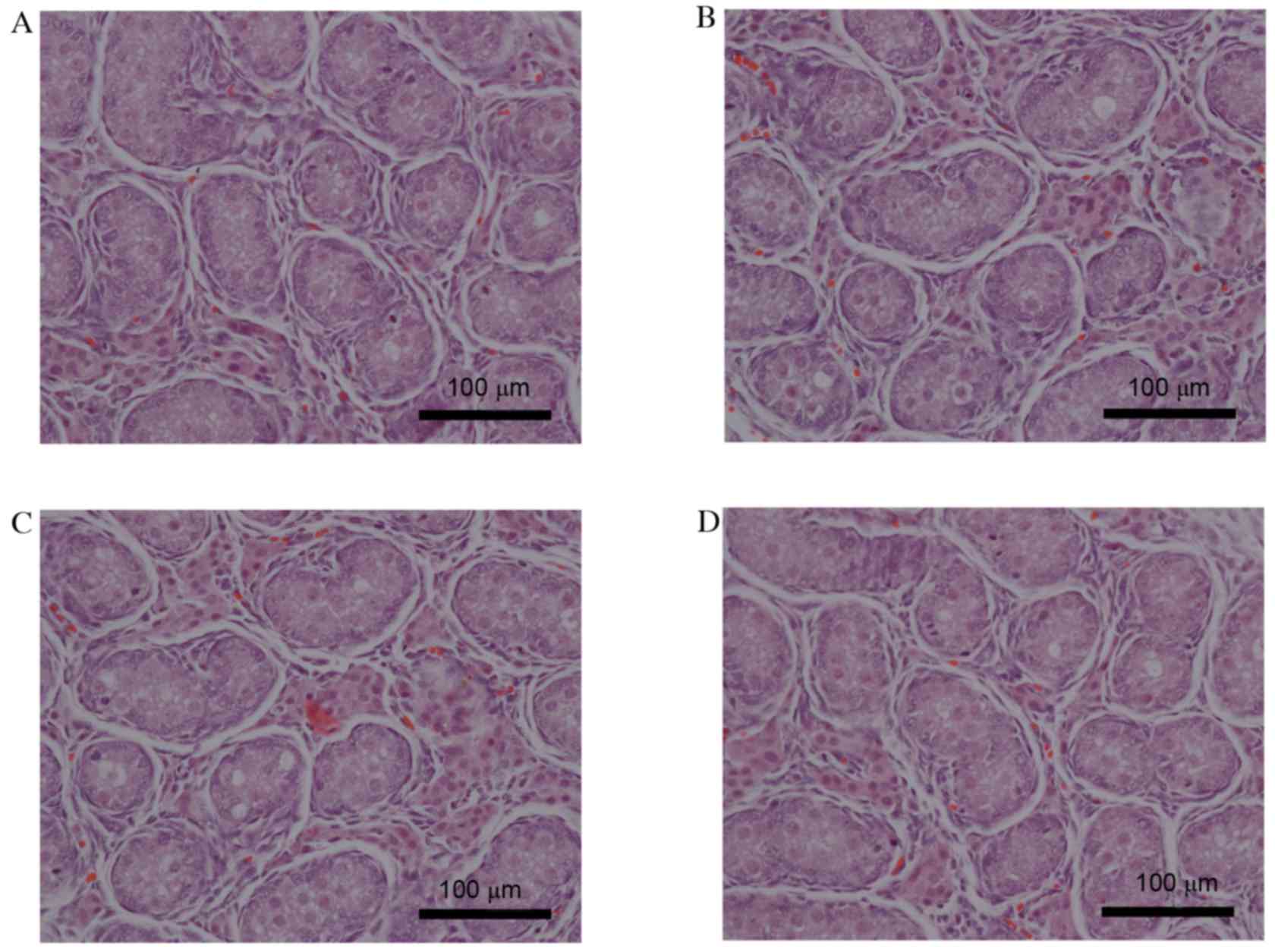
Effects of maternal acrolein exposure
on fetal testes histopathology
The effects of maternal acrolein exposure during
pregnancy on fetal testes histology were determined by H&E
staining. As presented in Fig. 4,
no abnormal morphology was observed in the fetal testes of the
acrolein-treated rats compared with the control group. The
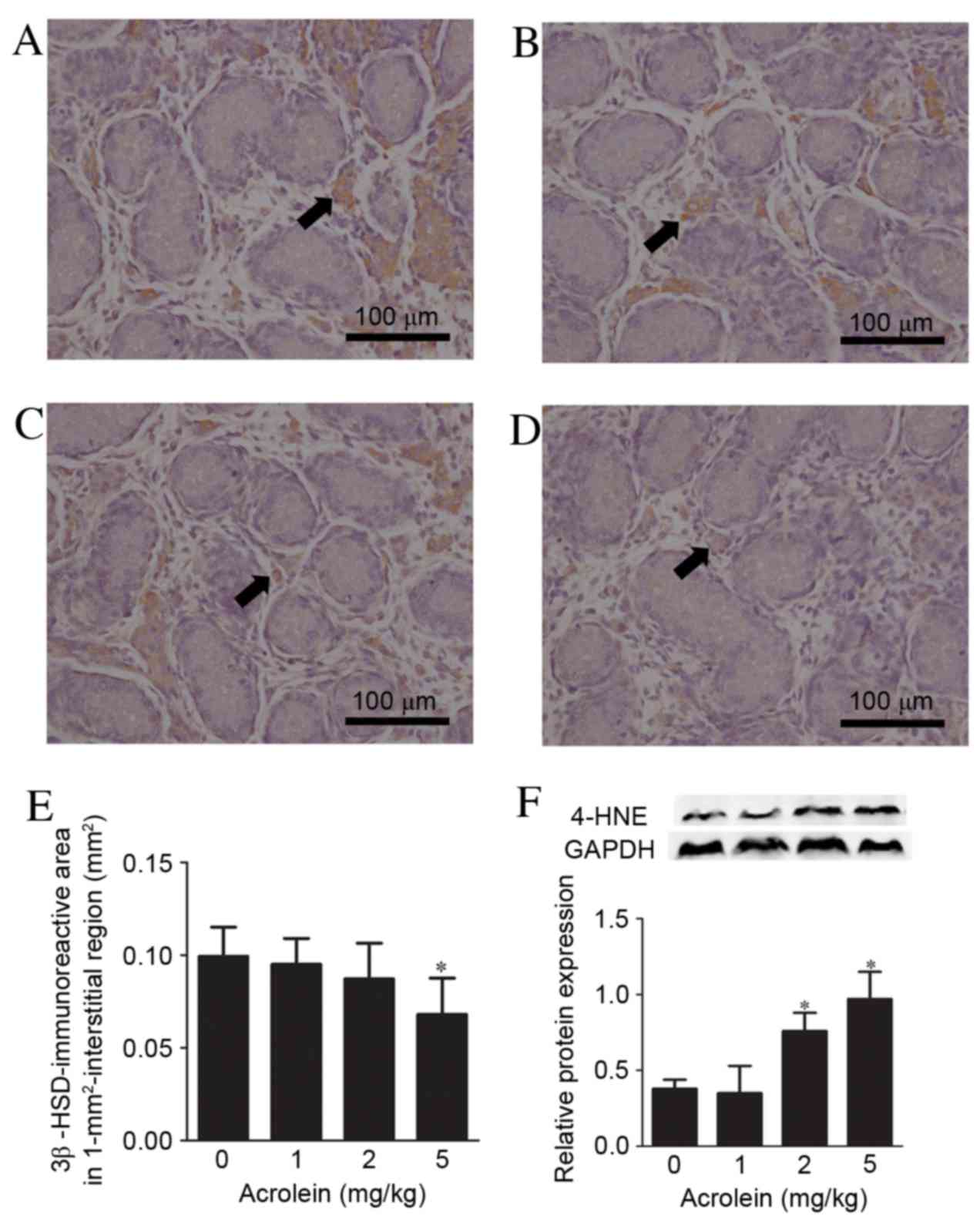
distribution of Leydig cells was identified by immunohistochemical
staining for the Leydig cell type-specific marker, 3β-HSD (Fig. 5A-D). The 3β-HSD immunoreactive area
in the interstitial region of the fetal testes was reduced
following exposure to 5 mg/kg acrolein during pregnancy (P=0.004),
whereas the immunoreactive areas in the 1 and 2 mg/kg groups were
unaltered compared with controls (Fig.
5E). ATUNEL assay revealed that there were no significant
differences in cell death in fetal testes between the acrolein
exposure and control groups (data not shown). The protein
expression levels of 4-HNE, an indicator of oxidative stress, were
subsequently examined in fetal testes. As presented in Fig. 5F, the protein expression levels of
4-HNE increased following fetal exposure to acrolein in a
dose-dependent manner (P<0.001).
Discussion
Acrolein has been reported to have diverse toxic
effects in numerous organs, including the reproductive system
(11). Various studies have
demonstrated that acrolein exposure impairs male germ cells,
sertoli cells and spermatogenesis (14,24,25).
In the present study, the dose-associated effects of acrolein
exposure during pregnancy on fetal testosterone production and gene
expression were investigated. The results demonstrated that the
weight of pups and fetal testes were significantly decreased when
prenatally exposed to high doses of acrolein (5 mg/kg).
Furthermore, maternal acrolein exposure during pregnancy resulted
in a reduction of intratesticular testosterone production and the
expression of steroidogenic genes, including StAR and 3β-HSD, and
impaired function of Leydig cells. These results indicated that
high doses of acrolein exposure in utero impair
steroidogenic capacity during the fetal period.
Testosterone is primarily synthesized by testicular
Leydig cells. Fetal Leydig cells are a distinct population that
exhibit a specific origin, structure and capacity for testosterone
production, and are regulated by hormones and growth factors
(26,27). Leydig cells begin to appear in the
interstitial tissue of the developing testes following the
formation of testicular cords, reach peak numbers around birth and
gradually disappear following postnatal day 7 (28). Previous studies have demonstrated
that various environmental toxicants, including phthalate and
perfluorooctane sulfonate, may cause impairment of rat fetal Leydig
cells and reduction of testosterone production (29,30).
Although no direct evidence has indicated that acrolein impairs
steroidogenic capacity, males treated with high doses of CP, the
precursor of acrolein, for sarcoma during childhood had abnormally
elevated gonadotrophin-releasing hormone-stimulated luteinizing
hormone levels, suggesting a degree of Leydig cell insufficiency
(16). In the present study, a
high dose of acrolein (5 mg/kg) significantly decreased fetal
testicular testosterone concentration following prenatal exposure,
whereas fetal testosterone was unaffected following treatment with
low doses of acrolein (1 and 2 mg/kg). Exposure to pollutants,
including toluene, during pregnancy is associated with an abnormal
3β-HSD immunoreactive area in fetal testes (23); the present study additionally
demonstrated that prenatal acrolein treatment had similar effects.
Furthermore, high doses of acrolein exposure (5 mg/kg) during
pregnancy were revealed to lead to weight reduction in the male
fetus and fetal testes.
The synthesis of testosterone in Leydig cells
requires a series of steroidogenic enzymes, including SR-B1, StAR,
P450scc, 3β-HSD, P450c17 and 17β-HSD (31). The mechanisms underlying reduced
fetal testosterone levels following exposure to acrolein were
investigated by assessing the mRNA expression levels of these
steroidogenic enzymes. SR-B1 is responsible for the transport of
high-density lipoprotein (HDL) cholesteryl esters into the cell;
the present study demonstrated that the expression levels of SR-B1
were unaffected following exposure to acrolein. StAR is important
for the delivery of cholesterol from the outer to the inner
mitochondrial membrane in fetal testes (32). In the present study, the mRNA and
protein expression levels of this cholesterol transport molecule
were markedly decreased following treatment with 2 and 5 mg/kg
acrolein. Additionally, previous studies have demonstrated that
acrolein impairs cholesterol transport by modification of
apolipoprotein A-I and HDL, and that cholesterol is essential for
testosterone biosynthesis (33).
P450scc is involved in catalyzing the conversion of cholesterol to
pregnenolone, which is subsequently transported to the smooth
endoplasmic reticulum in the cytoplasm, where 3β-HSD converts it to
progesterone, following conversion to testosterone by P450c17 and
17β-HSD (34). In the present
study, the mRNA expression levels of 3β-HSD were reduced by
exposure to 5 mg/kg acrolein, while the mRNA expression levels of
P450scc, P450c17 and 17β-HSD were not significantly altered, with
the exception of a slight increase in 17β-HSD in the 2 mg/kg group.
These results suggested that high doses of acrolein exposure
impairs the steroidogenic capacity in testosterone synthesis by
reducing StAR and 3β-HSD expression levels in fetal testes, and
that the inhibitory effect on steroidogenesis appears to result
from selective alterations of gene expression.
Insl3 is another important factor secreted by fetal
Leydig cells, and specifically binds to the leucine-rich
repeat-containing G protein-coupled receptor 8 in the gubernaculums
to induce scrotal descent of the testes (35). Therefore, interference with the
expression of Insl3 may lead to cryptorchidism. The present data
suggested that prenatal acrolein exposure does not affect the mRNA
expression levels of Insl3. In addition, fetal testes were not
histologically damaged following maternal acrolein exposure.
Previous studies have demonstrated that acrolein impairs the
sertoli cytoskeleton and induces germ cell apoptosis by induction
of oxidative stress in vitro (24,25),
and that it negatively regulates meiosis in a yeast gametogenesis
model by inhibiting premeiotic DNA synthesis (14). The present results revealed that
prenatal acrolein in vivo does not lead to an evident
morphological alteration, which is consistent with a study that
demonstrated that the mating pattern and fertility of rats were
unaffected following acrolein treatment (36). Additionally, Kuwada et al
(37) revealed that neonatal
endocrine disruptors exposure decreases weight and steroidogenesis
of juvenile testes, whereas spermatogenesis was restored during
puberty. However, the effects of prenatal acrolein exposure on the
function of sertoli and germ cells remain to be fully elucidated.
Taken together, these studies indicated that maternal acrolein
exposure during pregnancy does not cause a distinct pathological
impairment in fetal testes, with the exception of reduced
steroidogenic capacity at a dose of 5 mg/kg acrolein.
Exposure to environmental pollutants often generates
excessive levels of reactive oxygen species (ROS) and induces
oxidative stress responses in cells (38). ROS, including oxygen radicals, have
been demonstrated to disrupt the balance of the endocrine system
and inhibit testicular steroidogenesis (39,40).
Acrolein, an unsaturatedα, β-aldehyde, reacts with and depletes
cell antioxidants, including glutathione, and affects the cellular
redox balance (25). 4-HNE is an
aldehydic product of lipid peroxidation and has been considered an
indicator of oxidative stress-induced cell death (41). Thus, the protein expression levels
of 4-HNE in fetal testes were examined following maternal acrolein
exposure. It was demonstrated that the protein expression levels of
4-HNE were increased in testes following fetal exposure to acrolein
in a dose-dependent manner, indicating that acrolein-induced
oxidative stress damage may be implicated in the etiology of fetal
testicular pathological alterations.
In conclusion, the results of the present study
indicated that prenatal exposure to high doses of acrolein
significantly reduced fetal and testes weight and most notably,
testicular testosterone production capacity. The abnormal
expression of StAR and 3β-HSD, and oxidative stress damage, may
contribute to the impairment of steroidogenesis. Consequently,
these alterations may result in the maldevelopment of the testes
and affect masculinization.
Acknowledgements
The present study was supported by the Peking
University 985 Clinical Hospital Cooperation Program (grant no.
B67463). The authors would like to thank all staff of the Key
Laboratory of Assisted Reproduction (Peking University Third
Hospital, Beijing, China) for their assistance.
References
|
1
|
Skakkebaek NE, Rajpert-De Meyts E and Main
KM: Testicular dysgenesis syndrome: An increasingly common
developmental disorder with environmental aspects. Hum Reprod.
16:972–978. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schnack TH, Poulsen G, Myrup C, Wohlfahrt
J and Melbye M: Familial coaggregation of cryptorchidism,
hypospadias, and testicular germ cell cancer: A nationwide cohort
study. J Natl Cancer Inst. 102:187–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sharpe RM and Skakkebaek NE: Testicular
dysgenesis syndrome: Mechanistic insights and potential new
downstream effects. Fertil Steril. 89:(2 Suppl). e33–e38. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Macleod DJ, Sharpe RM, Welsh M, Fisken M,
Scott HM, Hutchison GR, Drake AJ and van den Driesche S: Androgen
action in the masculinization programming window and development of
male reproductive organs. Int J Androl. 33:279–287. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu D, Shen L, Tao Y, Kuang Y, Cai L, Wang
D, He M, Tong X, Zhou S, Sun J, et al: Alterations in gene
expression during sexual differentiation in androgen receptor
knockout mice induced by environmental endocrine disruptors. Int J
Mol Med. 35:399–404. 2015.PubMed/NCBI
|
|
6
|
Araki A, Mitsui T, Miyashita C, Nakajima
T, Naito H, Ito S, Sasaki S, Cho K, Ikeno T, Nonomura K and Kishi
R: Association between maternal exposure to di (2-ethylhexyl)
phthalate and reproductive hormone levels in fetal blood: The
Hokkaido study on environment and children's health. PLoS One.
9:e1090392014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wilson VS, Lambright CR, Furr JR,
Howdeshell KL and Gray Earl L Jr: The herbicide linuron reduces
testosterone production from the fetal rat testis during both in
utero and in vitro exposures. Toxicol Lett. 186:73–77. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Stevens JF and Maier CS: Acrolein:
Sources, metabolism, and biomolecular interactions relevant to
human health and disease. Mol Nutr Food Res. 52:7–25. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cahill TM: Ambient acrolein concentrations
in coastal, remote, and urban regions in California. Environ Sci
Technol. 48:8507–8513. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Logue JM, Price PN, Sherman MH and Singer
BC: A method to estimate the chronic health impact of air
pollutants in U.S. residences. Environ Health Perspect.
120:216–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moghe A, Ghare S, Lamoreau B, Mohammad M,
Barve S, McClain C and Joshi-Barve S: Molecular mechanisms of
acrolein toxicity: Relevance to human disease. Toxicol Sci.
143:242–255. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
DeJarnett N, Conklin DJ, Riggs DW, Myers
JA, O'Toole TE, Hamzeh I, Wagner S, Chugh A, Ramos KS, Srivastava
S, et al: Acrolein exposure is associated with increased
cardiovascular disease risk. J Am Heart Assoc. 3:pii: e000934.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lovell MA, Xie C and Markesbery WR:
Acrolein is increased in Alzheimer's disease brain and is toxic to
primary hippocampal cultures. Neurobiol Aging. 22:187–194. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Golla U, Bandi G and Tomar RS: Molecular
cytotoxicity mechanisms of allyl alcohol (acrolein) in budding
yeast. Chem Res Toxicol. 28:1246–1264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Slott VL and Hales BF: The embryolethality
and teratogenicity of acrolein in cultured rat embryos. Teratology.
34:155–163. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kenney LB, Laufer MR, Grant FD, Grier H
and Diller L: High risk of infertility and long term gonadal damage
in males treated with high dose cyclophosphamide for sarcoma during
childhood. Cancer. 91:613–621. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Westlind A, Malmebo S, Johansson I, Otter
C, Andersson TB, Ingelman-Sundberg M and Oscarson M: Cloning and
tissue distribution of a novel human cytochrome p450 of the CYP3A
subfamily, CYP3A43. Biochem Biophys Res Commun. 281:1349–1355.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ekhart C, Doodeman VD, Rodenhuis S, Smits
PH, Beijnen JH and Huitema AD: Influence of polymorphisms of drug
metabolizing enzymes (CYP2B6, CYP2C9, CYP2C19, CYP3A4, CYP3A5,
GSTA1, GSTP1, ALDH1A1 and ALDH3A1) on the pharmacokinetics of
cyclophosphamide and 4-hydroxycyclophosphamide. Pharmacogenet
Genomics. 18:515–523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ji YL, Wang H, Liu P, Zhao XF, Zhang Y,
Wang Q, Zhang H, Zhang C, Duan ZH, Meng C and Xu DX: Effects of
maternal cadmium exposure during late pregnant period on testicular
steroidogenesis in male offspring. Toxicol Lett. 205:69–78. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Auerbach SS, Mahler J, Travlos GS and
Irwin RD: A comparative 90-day toxicity study of allyl acetate,
allyl alcohol and acrolein. Toxicology. 253:79–88. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rashedinia M, Lari P, Abnous K and
Hosseinzadeh H: Proteomic analysis of rat cerebral cortex following
subchronic acrolein toxicity. Toxicol Appl Pharmacol. 272:199–207.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsukahara S, Nakajima D, Kuroda Y, Hojo R,
Kageyama S and Fujimaki H: Effects of maternal toluene exposure on
testosterone levels in fetal rats. Toxicol Lett. 185:79–84. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu F, Li XL, Lin T, He DW, Wei GH, Liu JH
and Li LS: The cyclophosphamide metabolite, acrolein, induces
cytoskeletal changes and oxidative stress in Sertoli cells. Mol
Biol Rep. 39:493–500. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
He X, Song W, Liu C, Chen S and Hua J:
Rapamycin inhibits acrolein-induced apoptosis by alleviating
ROS-driven mitochondrial dysfunction in male germ cells. Cell
Prolif. 47:161–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Griswold SL and Behringer RR: Fetal Leydig
cell origin and development. Sex Dev. 3:1–15. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
O'Shaughnessy PJ, Baker PJ and Johnston H:
The foetal Leydig cell-differentiation, function and regulation.
Int J Androl. 29:90–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Habert R, Lejeune H and Saez JM: Origin,
differentiation and regulation of fetal and adult Leydig cells. Mol
Cell Endocrinol. 179:47–74. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhao B, Li L, Liu J, Li H, Zhang C1, Han
P, Zhang Y, Yuan X, Ge RS and Chu Y: Exposure to perfluorooctane
sulfonate in utero reduces testosterone production in rat fetal
Leydig cells. PLoS One. 9:e788882014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin H, Ge RS, Chen GR, Hu GX, Dong L, Lian
QQ, Hardy DO, Sottas CM, Li XK and Hardy MP: Involvement of
testicular growth factors in fetal Leydig cell aggregation after
exposure to phthalate in utero. Proc Natl Acad Sci USA.
105:7218–7222. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Payne AH and Hales DB: Overview of
steroidogenic enzymes in the pathway from cholesterol to active
steroid hormones. Endocr Rev. 25:947–970. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tyczewska M, Rucinski M, Ziolkowska A,
Trejter M, Szyszka M and Malendowicz LK: Expression of selected
genes involved in steroidogenesis in the course of
enucleation-induced rat adrenal regeneration. Int J Mol Med.
33:613–623. 2014.PubMed/NCBI
|
|
33
|
Chadwick AC, Holme RL, Chen Y, Thomas MJ,
Sorci-Thomas MG, Silverstein RL, Pritchard KA Jr and Sahoo D:
Acrolein impairs the cholesterol transport functions of high
density lipoproteins. PLoS One. 10:e01231382015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Luu-The V: Assessment of steroidogenesis
and steroidogenic enzyme functions. J Steroid Biochem Mol Biol.
137:176–182. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ivell R and Bathgate RA: Reproductive
biology of the relaxin-like factor (RLF/INSL3). Biol Reprod.
67:699–705. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Parent RA, Caravello HE and Hoberman AM:
Reproductive study of acrolein on two generations of rats. Fundam
Appl Toxicol. 19:228–237. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kuwada M, Kawashima R, Nakamura K, Kojima
H, Hasumi H, Maki J and Sugano S: Neonatal exposure to endocrine
disruptors suppresses juvenile testis weight and steroidogenesis
but spermatogenesis is considerably restored during puberty.
Biochem Biophys Res Commun. 295:193–197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Monroe RK and Halvorsen SW: Environmental
toxicants inhibit neuronal Jak tyrosine kinase by mitochondrial
disruption. Neurotoxicology. 30:589–598. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi Z, Feng Y, Wang J, Zhang H, Ding L and
Dai J: Perfluorododecanoic acid-induced steroidogenic inhibition is
associated with steroidogenic acute regulatory protein and reactive
oxygen species in cAMP-stimulated Leydig cells. Toxicol Sci.
114:285–294. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhou L, Beattie MC, Lin CY, Liu J, Traore
K, Papadopoulos V, Zirkin BR and Chen H: Oxidative stress and
phthalate-induced down-regulation of steroidogenesis in MA-10
Leydig cells. Reprod Toxicol. 42:95–101. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ayala A, Muñoz MF and Argüelles S: Lipid
peroxidation: Production, metabolism, and signaling mechanisms of
malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev.
2014:3604382014. View Article : Google Scholar : PubMed/NCBI
|