Introduction
Forkhead box L2 (FOXL2) is a gene encoding a
fork head transcription factor, which belongs to the large family
of forkhead/winged-helix transcription factors. It is a single-exon
gene encoding a protein of 376 amino acids, and the protein
contains a forkhead DNA-binding domain and a polyalanine tract of
14 residues (1). In humans, as
well as mice and goats, the expression level of FOXL2 has been
consistently observed in the developing eyelids, ovaries and the
pituitary glands (2–4). Increasing evidence indicates that
FOXL2 is an important transcription factor that is involved
in various key life processes in humans, and its mutation may lead
to diseases, such as blepharophimosis, ptosis, and epicanthus in
versus syndrome (BPES) and premature ovarian failure.
FOXL2 is considered to be a possible inductor
of female sex determination, actively repressing male genes, such
as SRY-box 9 (SOX9) during development and more prominently
later in adult life (5,6). In addition, mutations of FOXL2 are
responsible for BPES (7,8), which includes eyelid and mild
craniofacial defects that are either associated with primary
ovarian insufficiency (type I) or not (type II) (9). Furthermore, a previous study
demonstrated the involvement of FOXL2 in virtually all
stages of ovarian development and function, as well as in granulose
cell (GC)-associated pathologies (10). In the ovaries, FOXL2 is involved in
the regulation of cholesterol and steroid metabolism, apoptosis,
reactive oxygen species detoxification and cell proliferation
(11). In a previous study in
mice, FOXL2 was identified as the earliest ovarian maker, its
expression being detectable from 12.0 d.p.c. (12).
However, the regulation of FOXL2 is less well
understood and there are only a few studies regarding this. Dai
et al (13) identified that
microRNA-133b (miR-133b) bound to the 3′-untranslated region
(3′-UTR) of FOXL2 mRNA, and miR-133b overexpression reduced
the FOXL2 expression at the mRNA and protein levels. In addition,
Luo et al (14)
demonstrated that the 3′-UTR of mouse FOXL2 mRNA has one
putative miR-133a binding site, and the expression level of FOXL2
was demonstrated to be downregulated by subsequent western blot
analysis.
Signal transducer and activator of transcription 3
(STAT3) is a clinically significant latent transcription factor
that is activated by multiple extracellular and intracellular
signals, including the Janus kinase (JAK) family and the receptor
tyrosine kinase (15). The
phosphorylation of STAT3 results in its activation and nuclear
translocation (16), and the
activated STAT3 regulates gene expression in various biological
processes, including proliferation, cell survival and inflammation
(17). In addition, STAT3 is
persistently activated in various human cancer cell lines,
including human myeloma, breast and prostate cancer cells, and is
involved in cancer development and progression, and contributes to
cancer cell survival (18).
The present study predominantly focuses on the
upstream transcriptional regulation of FOXL2. Following the
successful cloning of the FOXL2 promoter region, it was
analyzed using bioinformatics software and the results indicated
that it may be regulated by STAT3. Subsequently, the the HeLa cells
were exposed to the STAT3 inhibitors, and the dual luciferase
reporter (DLR) analysis and western blot results demonstrated that
FOXL2 was transcriptionally regulated by STAT3. Furthermore,
using the xCELLigence real-time cellular analysis (RTCA) system,
the growth and viability of the HeLa cells were demonstrated to be
markedly suppressed compared with the control cells.
Materials and methods
Cell lines and treatments
The HeLa cell line, which was obtained from the
Provincial Key Laboratory of Plastic and Microscopic Reconstructive
Surgery Techniques of Shandong (Shandong, China), was cultured in
high-glucose Dulbeccos modified Eagles medium (DMEM; Hyclone; GE
Healthcare Life Sciences, Logan, UT, USA) supplemented with 10%
fetal bovine serum (Hyclone; GE Healthcare Life Sciences) at 37°C
in a humidified environment with 5% CO2.
STAT3 inhibitors
The STAT3 inhibitors, C188–9 and WP1066, were
synthesized and supplied by Dr. Shaopeng Yuan (Institute of Materia
Medica, Chinese Academy of Medical Sciences, Beijing, China), and
dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) as stock solution. This was serially diluted to
the desired concentration using DMEM. The final concentration of
DMSO in the culture systems was <0.05%.
Cloning and analysis of the promoter
region of FOXL2
Based on the human mRNA sequence of FOXL2
[GenBank (https://www.ncbi.nlm.nih.gov/genbank/) accession no.
NM023067], the human genome sequence was analyzed using the Basic
Local Alignment Search Tool (BLAST) and the ~2 kb region upstream
of the predicted transcription start site was selected. Forward and
reverse primers (pro-forward, 5-CAG GGA TTC TGC GGA AGT GC-3 and
pro-reverse, 5-GTT TCC CGA AGC ACG ACC C-3) were designed using
Primer Premier software version 5.0 (Premier Biosoft International,
Palo Alto, CA, USA) according to the sequences in NCBI (https://www.ncbi.nlm.nih.gov/). The promoter region of
human FOXL2 (~1.9 kb) was amplified by polymerase chain
reaction (PCR) from human blood genomic DNA. PCR was carried out in
a 25 µl reaction containing 0.3 µl EasyTaq DNA Polymerase (Beijing
Transgen Biotech Co., Ltd., Beijing, China), with thermocycling
conditions as follows: Initial denaturation at 94°C for 2 min,
followed by 30 cycles at 94°C for 30 sec, at 61°C for 30 sec and at
72°C for 2 min, with a final extension at 72°C for 20 min. The PCR
products were cloned into the pMD19-T simple vector (Takara Bio,
Inc., Otsu, Japan) and sequenced by Sangon Biotech Co., Ltd.
(Shanghai, China) to confirm the resulting sequence. The cloned
sequence was registered in GenBank (GenBank accession no.
KR030055). The cis-regulatory elements in the promoter region of
FOXL2 were predicted using TFSERACH (http://www.cbrc.jp/research/db/TFSEARCH.html) and
Nsite (http://www.softberry.com/berry.phtml?topic=nsite&group=programs&subgroup=promoter).
Plasmid construction
The promoter sequence of FOXL2 was isolated
from the recombinant plasmid pMD19-T by PCR, as aforementioned,
with the following primers synthesized using Primer Premier
software version 5.0 (Premier Biosoft International): Pro-FE, 5-ATG
AGC TCC AGG GAT TCT GCG GAA GTG C-3 and Pro-RE, 5-ATA CTC GAG GTT
TCC CGA AGC ACG ACC C-3, containing restriction enzyme cutting
sites (underlined), and was confirmed by 10% agarose gel
electrophoresis (Beijing Transgen Biotech Co., Ltd., Beijing,
China) and sequencing by Sangon Biotech Co., Ltd. Following
digestion with SacI and XhoI (Takara Bio, Inc.), the
fragments were ligated into pGL3-Basic and pGL3-Enhancer vectors
(Promega Corporation, Madison, WI, USA). Binary vectors containing
ProFOXL2::luc were transformed into Escherichia coli DH5α
(Beijing Transgen Biotech Co., Ltd.) using the freeze-thaw method.
The recombinant plasmid was extracted using a Mini Plasmid kit
(Tiangen Biotech Co., Ltd., Beijing, China) according to the
manufacturers protocol.
Transient transfection and luciferase
reporter assays
For transient transfection using Invitrogen
Lipofectamine® 3000 (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), the Lipofectamine® 3000 Reagent was
diluted in Opti-MEM medium (Thermo Fisher Scientific, Inc.), and
the master plasmid was prepared by diluting the recombinant
pGL3-ProFOXL2 plasmid in Opti-MEM medium and adding P3000 Reagent
(Thermo Fisher Scientific, Inc.). Subsequently, diluted plasmid was
added to each tube of diluted Lipofectamine® 3000
Reagent (1:1 ratio) and incubated for 5 min at room temperature.
Different plasmid-lipid complexes (0.25 µg/well) were then added to
HeLa cells plated in 12-well plates (70–90% confluent) at 37°C
separately. All cells were co-transfected with the Renilla
luciferase reporter plasmid (5 ng/well, pRL-TK; Promega
Corporation) as a control for transfection efficiency. Luciferase
(LUC) activity was assayed 48 h after transfection using the
Dual-Luciferase Reporter Assay system (Promega Corporation) and
measured using a Lumat 3 LB9508 luminescence counter (Berthold
Technologies GmbH & Co. KG, Bad Wildbad, Germany). A minimum of
three transfection assays was performed to obtain statistically
significant data.
Western blot analysis
HeLa cells (1.5×106 cells/well) in 6-well
plates, which had been grown under normal conditions, were treated
with different concentrations (1, 2.5, 5 or 10 µM) WP1066 for 48 h;
cells treated with DMSO to a final concentration at 1‰ served as
the control group. Proteins were collected using 150 µl/well
radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc.), frozen for 30 min and stored at −20°C.
For western blot analysis, proteins that had been
extracted from HeLa cells were boiled in 1X SDS-PAGE loading buffer
(Takara Bio, Inc.). Protein concentrations were measured using a
bicinchoninic acid protein assay and equal amounts of extracted
protein samples (30 µl lysate/lane) were separated on 10% SDS-PAGE,
as previously described (19) and
transferred onto polyvinylidene difluoride membrane (EMD Millipore,
Billerica, MA, USA). The membrane was blocked using skim milk for
2.5 h at room temperature. Immunoblotting was performed using the
following primary antibodies for 3 h at room temperature:
Anti-FOXL2 (cat no. ab188584; 1:5,000; Abcam, Cambridge, MA, USA),
anti-GAPDH (cat no. ab181602; 1:10,000; Abcam). Membranes were then
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-rabbit secondary antibodies (cat no. BA1054; 1:3,000; Wuhan
Boster Biological Technology, Ltd., Wuhan, China) for 2.5 h at room
temperature. An enhanced HRP-diaminobenzidine chromogenic substrate
kit (Tiangen Biotech Co., Ltd., Beijing, China) was used for
immunodetection, according to the manufacturers instructions. Blots
were semi-quantified by densitometry using the FluroChem R system
and AlphaView software (ProteinSimple; Bio-Techne, Minneapolis, MN,
USA).
Real-time cell viability assay by
RTCA
The cytotoxic effect of C188-8 or WP1066 was
determined using the xCELLigence system (Roche Diagnostics, Basel,
Switzerland), which measures cell status (provided as cell index)
utilizing an electric current to determine cellular attachment to
an electrode-containing plate. Cells (5×103 cells/well;
100 µl) were seeded in the electrode-containing plate (e-plate) and
incubated for 24 h at 37°C in a humidified environment with 5%
CO2. The inhibitors of STAT3, C188-9 or WP1066, were
added (at a concentration of 5 or 10 µM) and the electrical
impedance, caused by cell adhesion to the plate (which is directly
proportional to cellular viability) was numerically reported. Cells
treated with DMSO served as a negative control. Data from the
xCELLigence system was collected using the software provided with
the system, and values were normalized at the point of treatment
with compounds for all cell lines.
Statistical analysis
Statistical analysis was performed using the Data
Processing System software version 7.0 developed by Zhejiang
University (Hangzhou, China). Data are expressed as the mean ±
standard error of the mean of at least 3 independent experiments.
The statistical significance of the differences between groups was
evaluated using one-way analysis of variance followed by a post hoc
Duncans test for multiple comparisons. P<0.05 was considered to
indicate a statistically significant difference.
Results
Cloning and BLASTing of the 5 flanking
region of FOXL2
In order to characterize the transcriptional
regulation mechanism of FOXL2, the promoter region of FOXL2
was cloned. Using the genomic DNA of normal human blood samples
(Fig. 1A) as templates, the
1,931-bp 5 region upstream of the transcription start site was
cloned (Fig. 1B) and registered in
Gen Bank (Gen Bank accession no. KR030055), and the BLASTing result
indicated that the 5 flanking region of FOXL2 was
successfully cloned (data not shown).
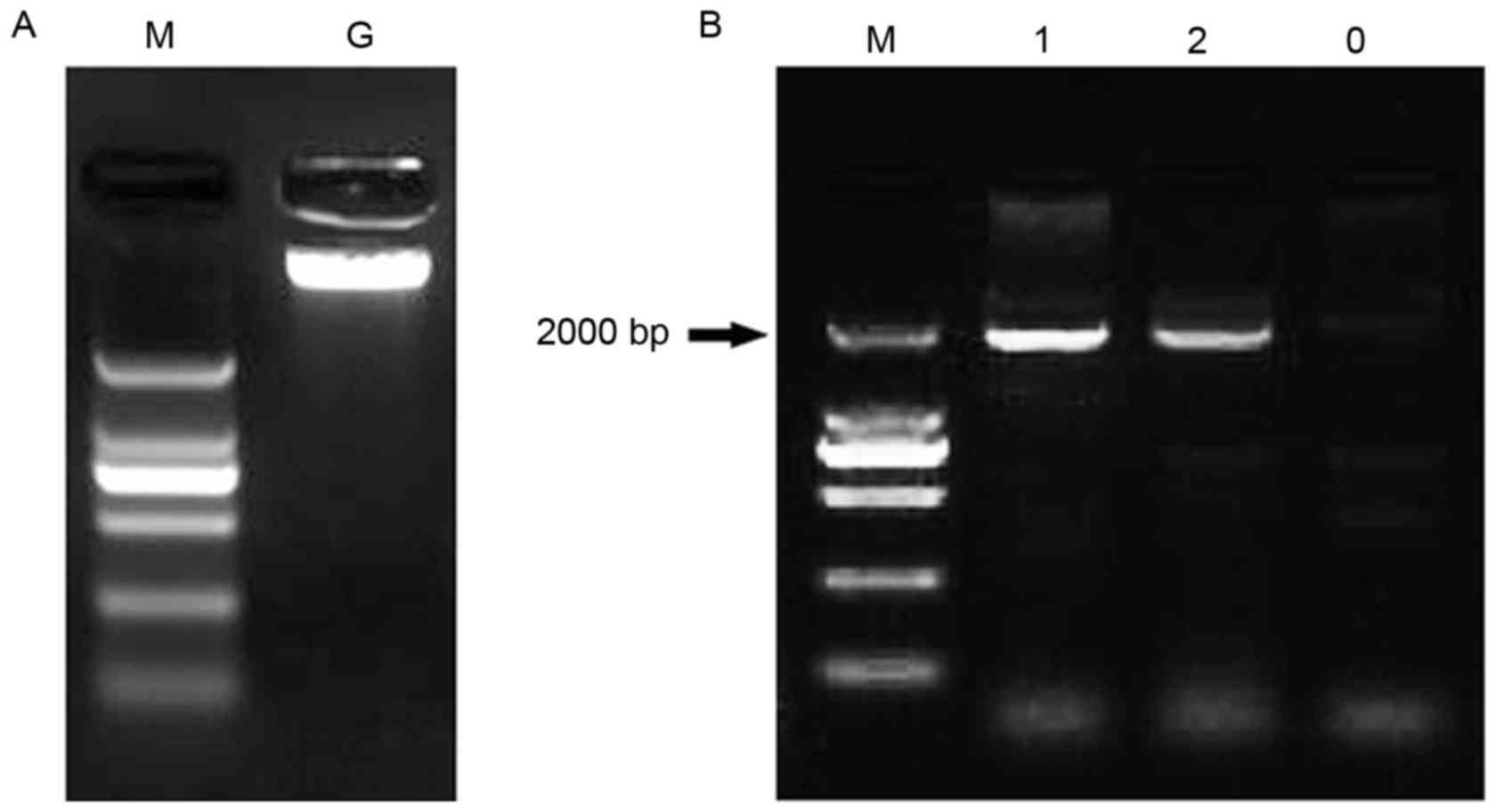 | Figure 1.Cloning of FOXL2 promoter from
healthy human blood. (A) Extraction of genomic DNA from normal
human blood. Lane M, DL2000 DNA marker (2,000, 1,000, 750, 500, 250
and 100 bp); lane G, normal genomic DNA. (B) Cloning of the
promoter of FOXL2 using normal human genomic DNA. Lanes 1 and 2,
the 1,931-bp upstream region of FOXL2; lane 0, negative control;
FOXL2, forkhead box L2. |
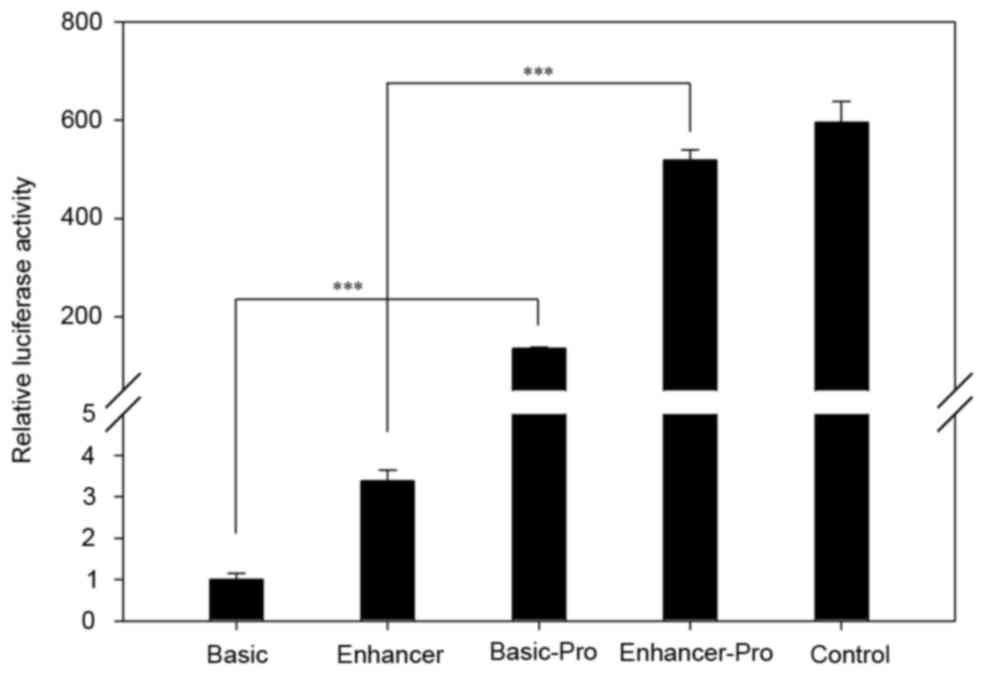
LUC activity was significantly induced
by the cloned 5 flanking region
To assess whether the cloned 5 flanking region was
actually the promoter of FOXL2, the region was fused to pGL3
(pGL3-Basic and pGL3-Enhancer) vectors, which contain luciferase
reporter genes (Fig. 2A). The
digested result of double restriction enzymes is presented in
Fig. 2B, which demonstrates that
the recombinant vectors containing the 1,931-bp 5 flanking region
of FOXL2 were successfully constructed (Fig. 2B).
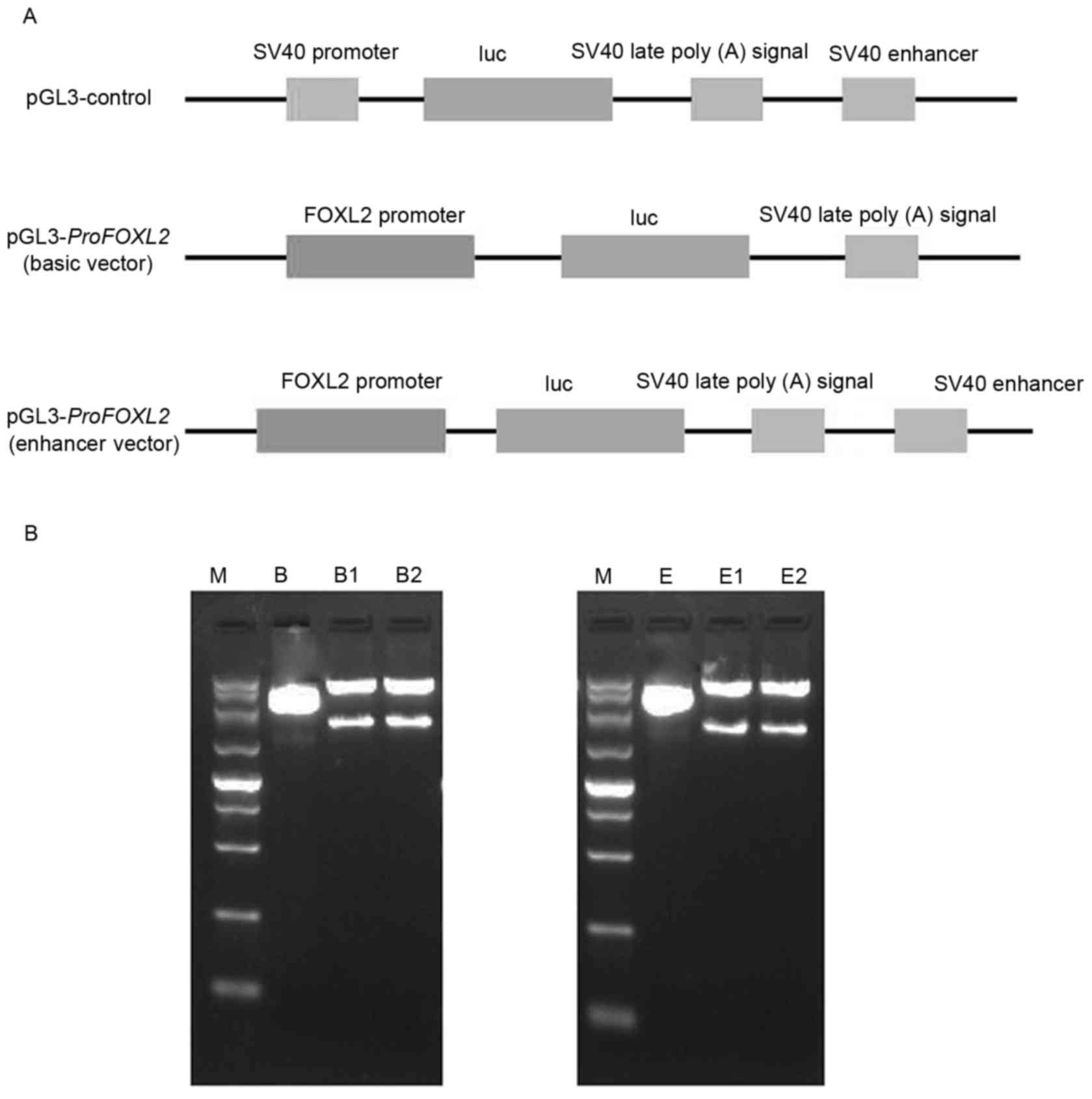 | Figure 2.Construction of vectors containing
the promoter of FOXL2 and the luciferase reporter gene. (A)
Schematic representation of the pGL3-ProFOXL2 basic and
enhancer fusion constructs, and the pGL3-Control construct. (B)
Electrophoresis results of pGL3-ProFOXL2 basic vector (left)
and pGL3-ProFOXL2 enhancer vector (right) following
digestion by double restriction enzymes (SacI and
XhoI); the basic and enhancer vectors without the fusion
promoter served as the negative control. Lane M, DL5000 (5,000,
3,000, 2,000, 1,500, 1,000, 750, 500, 250 and 100 bp); lane B, pGL3
basic vector; lanes B1 and B2, pGL3-ProFOXL2 basic vector;
lane E, pGL3 enhancer vector; lanes E1 and E2, pGL3-ProFOXL2
enhancer vector. FOXL2, forkhead box L2; luc, luciferase. |
Then the constructed vectors were transient
transfected into HeLa cells, and the LUC activity was measured
using a DLR assay system. As shown in Fig. 3, the relative LUC activities in
pGL3-Basic Pro and pGL3-Enhancer Pro were significantly higher than
the negative control (empty vector of pGL3-Basic and
pGL3-Enhancer). Thus, the results in Figs. 2 and 3 indicate that the 1,931-bp fragment was
the promoter of FOXL2 and it significantly induces the
expression of the luciferase gene.
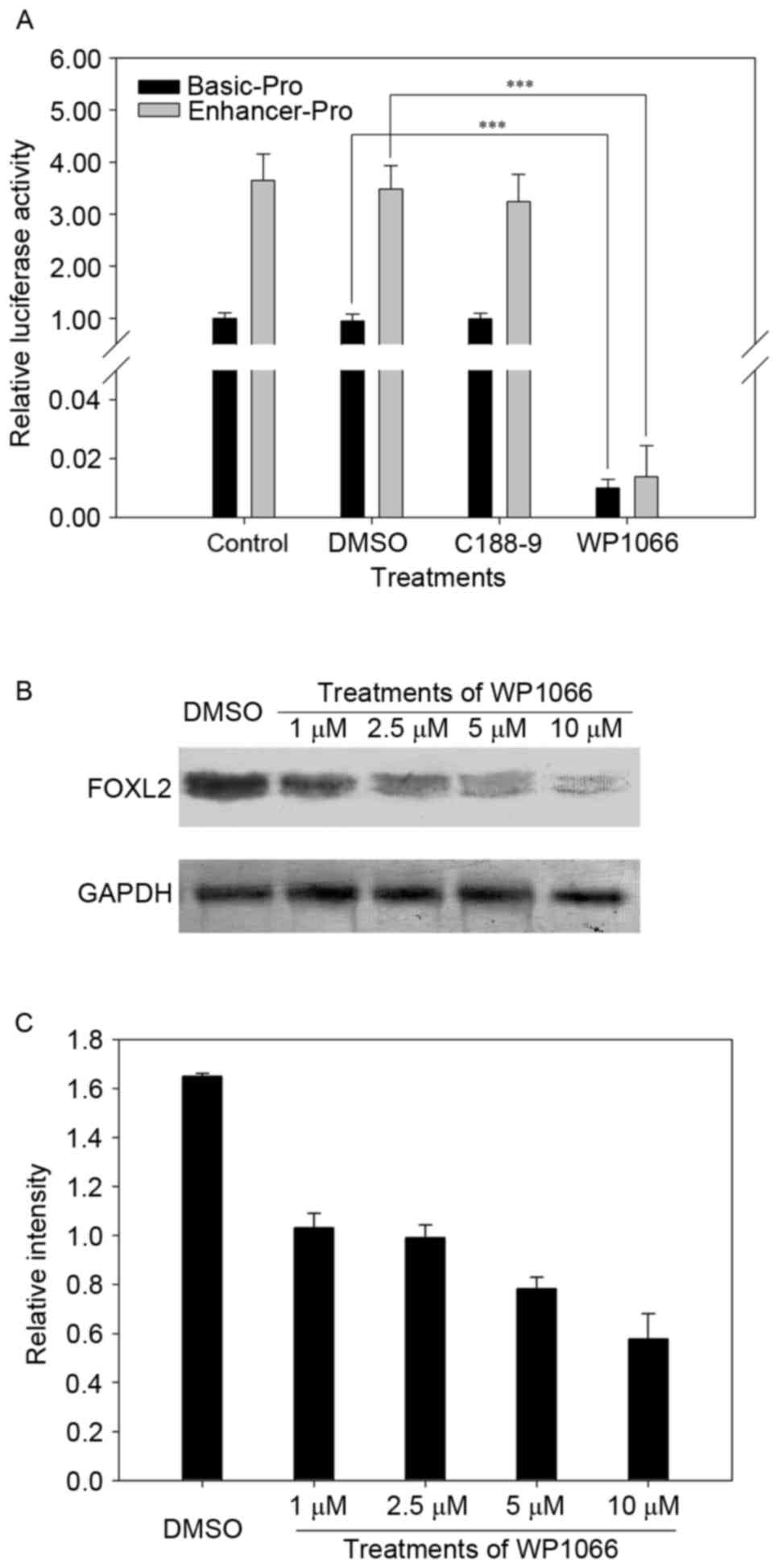
Promoter of FOXL2 has STAT3 binding
sites, and the expression level of FOXL2 was down regulated by
STAT3 inhibitor
Using TFSERACH and Nsite, the Cis-regulatory
elements in the promoter of FOXL2 were predicted, and the
result demonstrated STAT3 binding sites (TGTCTTCCCAGTCTGT) in the
promoter region (data not shown). To verify this prediction, two
STAT3 inhibitors (C188-9 and WP1066) were used to treat the cells
that were transfected with pGL3-Basic Pro and pGL3-Enhancer Pro
vectors, and then the LUC activities were measured (Fig. 4A). As shown in Fig. 4A, the LUC activity with WP1066
treatment was significant lower than that without WP1066, while the
cell treatment with C188-9 did not show any obvious changes.
Subsequently, further detection was performed using
western blotting to verify the regulation of FOXL2 by STAT3.
The expression level of FOXL2 protein decreased markedly following
treatment with different concentrations of STAT3 inhibitor when
compared with the control (Fig.
4B). Thus, the results confirmed that the promoter region of
FOXL2 contains STAT3 binding sites, and the expression level
of FOXL2 was down-regulated by the inhibitor of STAT3 in protein
level (Fig. 4).
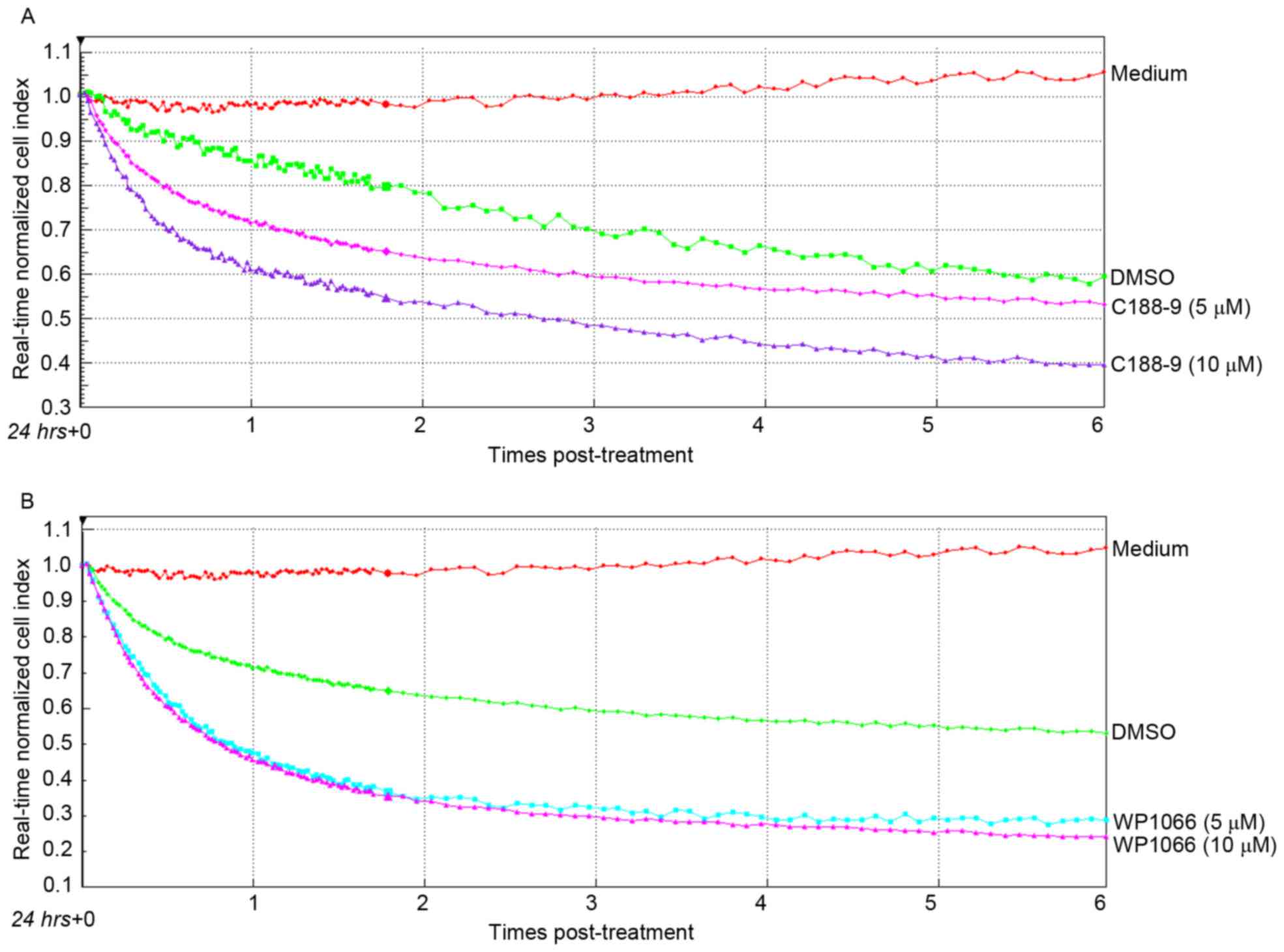
Cell growth was repressed by the
inhibitor of STAT3
For further analysis of the influence of the STAT3
inhibitor, HeLa cells, which were plated onto an e-plate, were
treated with different concentration inhibitors of STAT3, and the
real-time cell viabilities (cell index) were detected (Fig. 5). As predicted, cell indexes
demonstrated a marked decline after the normal HeLa cells were
exposed to the inhibitors, C188-9 and WP1066, compared with cells
that were treated with the same volume of medium or DMSO. WP1066
markedly reduced cell growth (0.3–0.4 cell index) when compared
with C188-9 (0.4–0.55 cell index) at 6 h after treatment (Fig. 5A and B). However, cells treated
with DMSO also exhibited a decreased normalized cell index compared
with control cells.
Discussion
As a transcription factor, FOXL2 is highly
conserved among vertebrates, and has emerged as a key factor in
ovarian biology, for example during early ovarian development and
female sex determination, as well as in postnatal somatic cell
differentiation and follicle maintenance (20). In addition, mutations of
FOXL2 are responsible for the BPES (13), and previous studies have
demonstrated that FOXL2 has various potential direct and
indirect transcriptional targets, including SOX9 (21), cytochrome P450 family 19 subfamily
A member 1 (CYP19A1) (22),
SMAD family member 3 (SMAD3) (6), follistatin (FST) (21), steroidogenic acute regulatory
protein (STAR) (23),
follicle stimulating hormone beta subunit (FSHB) (24), gonadotropin releasing hormone
receptor (GNRHR) (25),
superoxide dismutase 2 (SOD2) and sirtuin 1 (SIRT1)
(26). However, studies regarding
the underlying mechanisms of FOXL2 regulation are
limited.
In the present study, the promoter region of
FOXL2 was cloned (Fig. 1)
and the binary vector containing the luciferase gene was
successfully constructed (Fig. 2).
DLR analysis indicated that LUC activity was significantly induced
when compared with the negative control vector (Fig. 3). The bioinformatics analysis then
indicated that FOXL2 may be regulated by STAT3.
As a transcription factor, STAT3 is constitutively
expressed in a wide variety of tissue types, and is activated by
various cytokines and growth factors, as well as proto-oncogenes
and oncogenes (27,28). The present results confirmed that
the activity of luciferase, which fused to the promoter of
FOXL2, is significantly suppressed by the STAT3 inhibitor
WP1066 (Fig. 4A). This result
implied that FOXL2 may be transcriptionally regulated by STAT3,
which was further demonstrated by the western blot result (Fig. 4B). To the best of our knowledge,
this is a novel finding on the upstream regulation of FOXL2.
However, the binding sequence of STAT3 (data not shown) in the
promoter region of FOXL2 was only predicted using the
bioinformatics software, thus further evidence is required to
confirm the results in Fig, 4, and
further experiments have been conducted in our next paper.
Although the expression level of the FOXL2
promoter was suppressed significantly by WP1066, another inhibitor
of STAT3, C188-9, did not significantly suppress LUC activity
(Fig. 4A). As a transcription
factor, STAT3 is activated by phosphorylation of tyrosine residue,
after which it dimerizes and translocates into the nucleus. WP1066
is a novel inhibitor of STAT3 that is structurally associated with
AG490, which is a kinase inhibitor and originally selected from a
group of tyrphostins screened for their ability to block JAK-2
activity, but is significantly more potent and active (29,30).
While C188-9 (a second-generation compound of C188), which was
identified to be active in inhibiting granulocyte colony
stimulating factor, induced STAT3 phosphorylation (31). Thus, the current results indicate
that FOXL2 was transcriptionally regulated by STAT3, via the
JAK2-STAT3 signaling pathway.
DMSO is an important aprotic solvent that
solubilizes a wide variety of otherwise poorly soluble polar and
nonpolar molecules (32). In the
present study, inhibitors of STAT3 (WP1066 and C188-9) were
dissolved in DMSO as stock solution. The RTCA using the xCELLigence
system indicated that although the concentration of DMSO was low
(<0.05%), it may suppress the viability of HeLa cells (Fig. 5). In a previous study, similar
toxicity was observed in a retinal neuronal cell line (33). Thus, caution is required when DMSO
is used as a control treatment in future studies using cell
lines.
A previous study demonstrated that FOXL2 regulates
certain genes, which are directly or indirectly involved in cell
proliferation and apoptosis, such as tumor necrosis factor receptor
1 (TNF-R1), FAS or TNF-related apoptosis-inducing
ligand receptor (TRAIL-R) (34), which was similar to the results
presented in Figs. 4 and 5, where cell proliferation was markedly
inhibited subsequent to HeLa cells being exposed to STAT3
inhibitors. Similarly, Cheng et al (25) identified that FOXL2 activates the
expression of the GNRH receptor in human and mouse GCs, which may
exert a pro-apoptotic role.
In conclusion, the promoter of FOXL2 was
successfully cloned and registered in Genbank (GenBank accession
no. KR030055), and LUC activity was observed to be significantly
induced by the promoter. Notably, the current results demonstrated
for the first time that FOXL2 was regulated by STAT3, according to
the DLR and western blot analysis. In addition, cell viability was
revealed to be suppressed by DMSO even at a low concentration.
Acknowledgements
The authors would like to thank Dr Shaopeng Yuan for
kindly providing the inhibitors of STAT3. The present study was
supported by the National Natural Foundation of China (grant nos.
81501683 and 81471880), Natural Science Foundation of Shandong
Province (grant no. ZR2015HL057) and the Doctoral Foundation of
Weifang Medical University (Weifang, China).
Glossary
Abbreviations
Abbreviations:
|
BPES
|
blepharophimosis, ptosis, and
epicanthus inversus syndrome
|
|
DLR
|
dual luciferase reporter
|
|
FOXL2
|
forkhead box L2
|
|
GC
|
granulose cell
|
|
LUC
|
luciferase
|
|
RTCA
|
real-time cellular analysis
|
|
STAT3
|
signal transducer and activator of
transcription 3
|
References
|
1
|
Verdin H and De Baere E: FOXL2 impairment
in human disease. Horm Res Paediatr. 77:2–11. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rosario R, Cohen PA and Shelling AN: The
role of FOXL2 in the pathogenesis of adult ovarian granulosa cell
tumours. Gynecol Oncol. 133:382–387. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Georges A, Auguste A, Bessière L, Vanet A,
Todeschini AL and Veitia RA: FOXL2: A central transcription factor
of the ovary. J Mol Endocrinol. 52:R17–R33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cocquet J, Pailhoux E, Jaubert F, Servel
N, Xia X, Pannetier M, De Baere E, Messiaen L, Cotinot C, Fellous M
and Veitia RA: Evolution and expression of FOXL2. J Med Genet.
39:916–921. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Uhlenhaut NH, Jakob S, Anlag K,
Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C,
Holter NI, et al: Somatic sex reprogramming of adult ovaries to
testes by FOXL2 ablation. Cell. 139:1130–1142. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Garcia-Ortiz JE, Pelosi E, Omari S,
Nedorezov T, Piao Y, Karmazin J, Uda M, Cao A, Cole SW, Forabosco
A, et al: Foxl2 functions in sex determination and histogenesis
throughout mouse ovary development. BMC Dev Biol. 9:362009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qian X, Shu A, Qin W, Xing Q, Gao J, Yang
J, Feng G and He L: A novel insertion mutation in the FOXL2 gene is
detected in a big Chinese family with
blepharophimosis-ptosis-epicanthus inversus. Mutat Res. 554:19–22.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li WX, Wang XK, Sun Y, Wang YL, Lin LX and
Tang SJ: A novel mutation in the FOXL2 gene in a Chinese family
with blepharophimosis, ptosis, and epicanthus inversus syndrome.
Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 22:372–375. 2005.PubMed/NCBI
|
|
9
|
Yu HC, Geiger EA, Medne L, Zackai EH and
Shaikh TH: An individual with blepharophimosis-ptosis-epicanthus
inversus syndrome (BPES) and additional features expands the
phenotype associated with mutations in KAT6B. Am J Med Genet A.
164A:950–957. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duffin K, Bayne RA, Childs AJ, Collins C
and Anderson RA: The forkhead transcription factor FOXL2 is
expressed in somatic cells of the human ovary prior to follicle
formation. Mol Hum Reprod. 15:771–777. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Caburet S, Georges A, LHôte D, Todeschini
AL, Benayoun BA and Veitia RA: The transcription factor FOXL2: At
the crossroads of ovarian physiology and pathology. Mol Cell
Endocrinol. 356:55–64. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Auguste A, Chassot AA, Grégoire EP,
Renault L, Pannetier M, Treier M, Pailhoux E and Chaboissier MC:
Loss of R-spondin1 and Foxl2 amplifies female-to-male sex reversal
in XX mice. Sex Dev. 5:304–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dai A, Sun H, Fang T, Zhang Q, Wu S, Jiang
Y, Ding L, Yan G and Hu Y: MicroRNA-133b stimulates ovarian
estradiol synthesis by targeting Foxl2. FEBS Lett. 587:2474–2482.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo Y, Wu X, Ling Z, Yuan L, Cheng Y, Chen
J and Xiang C: microRNA133a targets Foxl2 and promotes
differentiation of C2C12 into myogenic progenitor cells. DNA Cell
Biol. 34:29–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Eiring AM, Page BD, Kraft IL, Mason CC,
Vellore NA, Resetca D, Zabriskie MS, Zhang TY, Khorashad JS, Engar
AJ, et al: Combined STAT3 and BCR-ABL1 inhibition induces synthetic
lethality in therapy-resistant chronic myeloid leukemia. Leukemia.
29:586–597. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Takeda K, Kaisho T, Yoshida N, Takeda J,
Kishimoto T and Akira S: Correction: Stat3 activation is
responsible for IL-6-dependent T cell proliferation through
preventing apoptosis: Generation and characterization of T
cell-specific Stat3-deficient mice. J Immunol. 194:35262015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ and
Settleman J: Drug resistance via feedback activation of Stat3 in
oncogene-addicted cancer cells. Cancer Cell. 26:207–221. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Furqan M, Akinleye A, Mukhi N, Mittal V,
Chen Y and Liu D: STAT inhibitors for cancer therapy. J Hematol
Oncol. 6:902013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang T, Li F and Tang S: MiR-30a
upregulates BCL2A1, IER3 and cyclin D2 expression by targeting
FOXL2. Oncol Lett. 9:967–971. 2015.PubMed/NCBI
|
|
20
|
Pisarska MD, Barlow G and Kuo FT:
Minireview: Roles of the forkhead transcription factor FOXL2 in
granulosa cell biology and pathology. Endocrinology. 152:1199–1208.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kashimada K, Pelosi E, Chen H,
Schlessinger D, Wilhelm D and Koopman P: FOXL2 and BMP2 act
cooperatively to regulate follistatin gene expression during
ovarian development. Endocrinology. 152:272–280. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rosario R, Araki H, Print CG and Shelling
AN: The transcriptional targets of mutant FOXL2 in granulosa cell
tumours. PLoS One. 7:e462702012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosario R, Blenkiron C and Shelling AN:
Comparative study of microRNA regulation on FOXL2 between
adult-type and juvenile-type granulosa cell tumours in vitro.
Gynecol Oncol. 129:209–215. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tran S, Lamba P, Wang Y and Bernard DJ:
SMADs and FOXL2 synergistically regulate murine FSHbeta
transcription via a conserved proximal promoter element. Mol
Endocrinol. 25:1170–1183. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheng JC, Klausen C and Leung PC:
Overexpression of wild-type but not C134W mutant FOXL2 enhances
GnRH-induced cell apoptosis by increasing GnRH receptor expression
in human granulosa cell tumors. PLoS One. 8:e550992013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Benayoun BA, Batista F, Auer J,
Dipietromaria A, LHôte D, De Baere E and Veitia RA: Positive and
negative feedback regulates the transcription factor FOXL2 in
response to cell stress: Evidence for a regulatory imbalance
induced by disease-causing mutations. Hum Mol Genet. 18:632–644.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Staniszewska AD, Pensa S, Caffarel MM,
Anderson LH, Poli V and Watson CJ: Stat3 is required to maintain
the full differentiation potential of mammary stem cells and the
proliferative potential of mammary luminal progenitors. PLoS One.
7:e526082012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rokavec M, Öner MG, Li H, Jackstadt R,
Jiang L, Lodygin D, Kaller M, Horst D, Ziegler PK, Schwitalla S, et
al: IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated
colorectal cancer invasion and metastasis. J Clin Invest.
124:1853–1867. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Iwamaru A, Szymanski S, Iwado E, Aoki H,
Yokoyama T, Fokt I, Hess K, Conrad C, Madden T, Sawaya R, et al: A
novel inhibitor of the STAT3 pathway induces apoptosis in malignant
glioma cells both in vitro and in vivo. Oncogene. 26:2435–2444.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Daniel JM, Dutzmann J, Bielenberg W,
Widmer-Teske R, Gündüz D, Hamm CW and Sedding DG: Inhibition of
STAT3 signaling prevents vascular smooth muscle cell proliferation
and neointima formation. Basic Res Cardiol. 107:2612012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Redell MS, Ruiz MJ, Alonzo TA, Gerbing RB
and Tweardy DJ: Stat3 signaling in acute myeloid leukemia:
Ligand-dependent and -independent activation and induction of
apoptosis by a novel small-molecule Stat3 inhibitor. Blood.
117:5701–5709. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gad SE and Sullivan DW: Dimethyl Sulfoxide
(DMSO)Encyclopedia Toxicol. Wexler P: 3rd. Academic Press/Elsevier;
London: pp. 166–168. 2014, View Article : Google Scholar
|
|
33
|
Galvao J, Davis B, Tilley M, Normando E,
Duchen MR and Cordeiro MF: Unexpected low-dose toxicity of the
universal solvent DMSO. FASEB J. 28:1317–1330. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim JH, Yoon S, Park M, Park HO, Ko JJ,
Lee K and Bae J: Differential apoptotic activities of wild-type
FOXL2 and the adult-type granulosa cell tumor-associated mutant
FOXL2 (C134W). Oncogene. 30:1653–1663. 2011. View Article : Google Scholar : PubMed/NCBI
|