Introduction
With the rapid development and widespread
application of nanomaterials, the health impact of nanomaterial
exposure has attracted increasing attention. Copper nanoparticles
(CuNPs) have a number of desirable properties, including a large
surface area, ductility, and excellent optical, electrical,
catalytic and antimicrobial properties. Therefore, they have been
widely used in lithium ion batteries (1), lubricant oil, ceramics (2), polymers/plastics, inks, metallics,
coatings, osteoporosis treatment drugs, drug delivery, intrauterine
contraceptive devices, and additives to livestock and poultry feed
(3–6). However, numerous studies have
indicated that the gastrointestinal system, liver and kidney are
sensitive targets of copper toxicity following oral exposure beyond
the range of biological tolerance (7). The symptoms of copper poisoning are
drowsiness and anorexia in the early stages, followed by disruption
of the epithelial lining of the liver, gastrointestinal distress,
hepatocellular necrosis, hemolysis, jaundice and kidney damage
(8,9).
Cytochrome P450 (CYP450) enzymes metabolize a number
of exogenous and endogenous compounds, such as antidepressants,
opiates, steroids, arachidonic acid, dopamine and serotonin
(10–12). These enzymes are abundant in the
brain, liver and other organs (10–15).
Brain CYP450 content is low compared with that in the liver
(0.5–2%), which makes it unlikely to affect systemic drug and
peripheral metabolite levels (12). However, local brain levels of
centrally acting compounds and the resulting therapeutic effects
may be regulated by brain CYP450-mediated metabolism independently
of peripheral metabolism and systemic drug levels. Variable brain
CYP450 activity has substantial potential impact; effects have been
demonstrated on behavior, neurotoxicity and drug response (16,17).
The expression of a specific CYP450 enzyme within an organ can
increase or decrease substantially in response to certain inducers
and inhibitors (18,19). A number of CYP450 enzymes have
tissue- and cell type-specific expression levels and regulators,
and brain tissue expresses a unique set of these enzymes (20). For instance, CYP450 2D (CYP2D) has
been identified in the liver and brain, and is involved in the
metabolism of numerous centrally acting drugs, but is essentially
uninducible in the liver. Brain CYP2D, however, can be induced by
nicotine and clozapine (21,22).
Most studies of CuNPs explore their hepatotoxicity
and nephrotoxicity (23,24); whether CuNPs affect the expression
of brain CYP450 enzymes remains unknown. The present study
investigated the effect of CuNPs on CYP450 enzymes in the rat brain
by measuring the protein and gene expression of CYP450 isoenzymes
in brain tissue. To identify the changes of CYP450s in the
neurotoxicity of CuNPs, the effects of CuNPs on the levels of
oxidative stress and nuclear receptors in the rat brain were
investigated.
Materials and methods
Materials
The tested CuNPs (cat. no. H1605061), copper
microparticles (cat. no. A1711069) and copper ions
(CuCl2·2H2O, cat. no. F1620012) were obtained
from Aladdin Industrial Co., Ltd. The sizes of the CuNPs and copper
microparticles were 80 nm and 1 µm, respectively. Western blotting
and SDS-PAGE preparation kits were purchased from Chengdu Baihe
Technology Co., Ltd. Other molecular biology reagents were
purchased from Bio-Rad Laboratories, Inc. Antibodies were purchased
from Abcam. All analytical commercial kits were purchased from
Nanjing Jiancheng Bioengineering Institute.
Particle characterization
The sizes of the CuNPs and copper microparticles
were confirmed using a Phenom ProX scanning electron microscope
(Phenom Scientific Instruments Co., Ltd.). The CuNPs were dispersed
in purified water, shaken and sonicated in an ice bath to avoid
aggregation. The distribution of particle sizes in the suspension
was characterized by dynamic light scattering studies performed
using a Zetasizer Nano ZS (Malvern Panalytical, Ltd.) immediately
following sonication.
Animals and treatments
A total of 60 specific pathogen-free (SPF) male rats
(100–120 g, 6 weeks old) were purchased from Chengdu Dossy
Biological Technology Co., Ltd. Male rats were chosen as the
subjects of the study due to differences in the expression of
CYP450 between female and male rats (25,26).
Rats were housed in plastic cages under SPF conditions at 25±2°C
and 70±10% relative humidity, under a 12-h light/dark cycle. Water
and food were provided ad libitum. Copper particles were
suspended in 1% hydroxypropyl methylcellulose (HPMC) solution (w/v)
(Shanghai Ryon Biological Technology Co., Ltd.) every day prior to
use. Following 7 days of acclimatization, rats were randomly
divided into a control group, which was administered with 1% HPMC,
and five test groups that were administered with different
concentrations of copper by gavage for 28 days: i) 200 mg/kg 1 µm
copper; ii) 200 mg/kg CuCl2·2H2O
(Cu2+); iii) 50 mg/kg CuNPs (low dose); iv) 100 mg/kg
CuNPs (medium dose); v) 200 mg/kg CuNPs (high dose) (n=10 per
group). The study was approved by Sichuan Agricultural University
(Chengdu, China), and the protocols for animal care and treatment
were in accordance with their guidelines for animal experiments
(approval no. 20170314). All possible efforts were made to relieve
unnecessary suffering of the experimental animals.
Sample collection
On day 28 of the experiment, following an overnight
fast, the rats were anesthetized by gas anesthesia with diethyl
ether at the rate of 0.2 l/min. Anesthesia was confirmed by
righting reflex, and the animals were rapidly taken out of the
anesthesia machine and sacrificed by cervical dislocation. Brain
tissues were snap-frozen and stored at −80°C for oxidative stress
and reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) analyses.
Brain microsomes were used to analyze the protein
expression of the nuclear receptors pregnane X receptor (PXR) and
constitutive androstane receptor (CAR) and CYP450 enzymes, which
were prepared by differential centrifugation as previously
described (27). The tissue was
homogenized in a 0.05 mM Tris/KCl buffer (pH 7.4; Boster Biological
Technology), centrifuged at 10,000 × g at 4°C for 30 min, and the
supernatant was centrifuged at 105,000 × g at 4°C for 60 min.
Subsequently, the brain protein settlement was re-suspended with
0.05 mM Tris/KCl buffer (pH 7.4) and stored at −80°C until western
blot analyses were performed. The protein content in the brain
microsomes was determined using the Bicinchoninic Acid Protein
Assay kit (Beyotime Biological Technology Co., Ltd.) with the
Thermo Scientific™ Multiskan™ GO Microplate reader (Thermo Fisher
Scientific, Inc.).
Oxidative stress
The levels of total superoxide dismutase (T-SOD),
glutathione (GSH), hydroxyl radicals (·OH) and malondialdehyde
(MDA) in the rat brain were determined to evaluate oxidative stress
and damage. This was performed using commercial assay kits from
Nanjing Jiancheng Bioengineering Institute, which were: Total
Superoxide Dismutase (T-SOD) assay kit (Hydroxylamine method);
Malondialdehyde (MDA) assay kit (TBA method); Reduced glutathione
(GSH) assay kit (Spectrophotometric method); and Hydroxyl Free
Radical assay kit. All assays were performed according to the
manufacturer's instructions.
Gene expression
The expression levels of CYP450 1A2, 2D22,
2E1 and 3A11 in the brain were analyzed using RT-qPCR as
previously described (28). Total
RNA was extracted using an OMGA total RNA kit II (Omega Bio-Tek,
Inc.) and cDNA was synthesized using PrimeScript™ RT reagent kit
with gDNA Eraser (Takara Bio, Inc.). The qPCR was performed using
iQ SYBR® Premix Ex Taq™ II (Tli RNaseH Plus; cat. no.
RR820A; Takara Bio, Inc.). The qPCR was performed under the
following conditions: 45 cycles, each involving 5 sec of
denaturation at 95°C, and 40 sec of amplification at 60°C. The
housekeeping gene GAPDH was used as an internal control. All
primers were designed with Primer premier v 5.0 software (Premier
Biosoft International) and commercially produced (BGI Tech
Solutions Co., Ltd.; Table I)
based on the target gene. Melting curves and PCR efficiency were
used as standard quality criteria for each qPCR run. The target
gene mRNA expression was normalized to GAPDH expression, and were
analyzed using the 2−ΔΔCq method (29).
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primers. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primers.
| Target | Primer sequence
(5′-3′) |
|---|
| CYP450 1A1 | F: |
GGGAGGTTACTGGTTCTGG |
|
| R: |
ATGAGGCTGTCTGTGATGTC |
| CYP450 2C11 | F: |
AATCCGCAGTCTGAGTTTACCC |
|
| R: |
GGTTTCTGCCAATTACACGTTCT |
| CYP450 2D6 | F: |
AGCTTCAACACCGCTATGGT |
|
| R: |
CAGCAGTGTCCTCTCCATGA |
| CYP450 3A1 | F: |
TGCCATCACGGACACAGA |
|
| R: |
ATCTCTTCCACTCCTCATCCTTAG |
| CAR | F: |
CCACGGGCTATCATTTCCAT |
|
| R: |
CCCAGCAAACGGACAGATG |
| PXR | F: |
TGGACAAACTCTCCGTTCTAAGG |
|
| R: |
GATTTTAATGCAACATCAAAGAA GCT |
| GAPDH | F: |
GATGGTGAAGGTCGGTGTG |
|
| R: |
ATGAAGGGGTCGTTGATGG |
Western blot analysis
The protein levels of CYP450 1A1, CYP450 2C11,
CYP450 2D6, CYP450 3A1, CAR and PXR in the brain microsome of rats
were estimated using western blot analysis as previously described
(30,31). Microsomal proteins (10 µg) were
separated by 10% SDS-PAGE and transferred to PVDF membranes (Pall
Corporation). The membranes were blocked with skimmed milk (Beijing
Solarbio Science & Technology Co., Ltd.) and incubated for 12 h
at 4°C with primary antibodies against CYP450 1A1 (cat. no.
ab22717; 1:1,000; Abcam), CYP450 2C11 (cat. no. ab3571; 1:1,000;
Abcam), CYP450 2D6 (cat. no. 73867S; 1:1,000; Cell Signaling
Technology, Inc.), CYP450 3A1 (cat. no. ab22724; 1:1,500; Abcam),
PXR (cat. no. ab118336; 1:500; Abcam), CAR (cat. no. ab62590;
1:1,500; Abcam), and β-actin (cat. no. bs-0061R; 1:10,000; Beijing
Biosynthesis Biotechnology Co., Ltd.). Following incubation with
primary antibody, the blots were incubated with a horseradish
peroxidase-labeled secondary antibody for 1 h at room temperature
(cat. no. bs-0295G; 1:10,000; Beijing Biosynthesis Biotechnology
Co., Ltd.). β-actin was used as an internal loading control. The
bands were visualized using enhanced chemiluminescence (ECL Western
Blotting Substrate; Beijing Solarbio Science & Technology Co.,
Ltd.) and densitometric analysis was performed using ImageJ
software version 1.48u (National Institutes of Health).
Statistical analysis
The assays were performed in triplicate. All data
were expressed as the mean ± standard deviation. Statistical
analysis was performed by one-way ANOVA in SPSS version 19.0 (IBM
Corp.), and the least significant difference test was used
following comparison of the mean values with the control group.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Physiochemical characterization of
CuNPs and copper microparticles
Physiochemical characteristics of CuNPs and copper
microparticles were evaluated using scanning electron microscopy
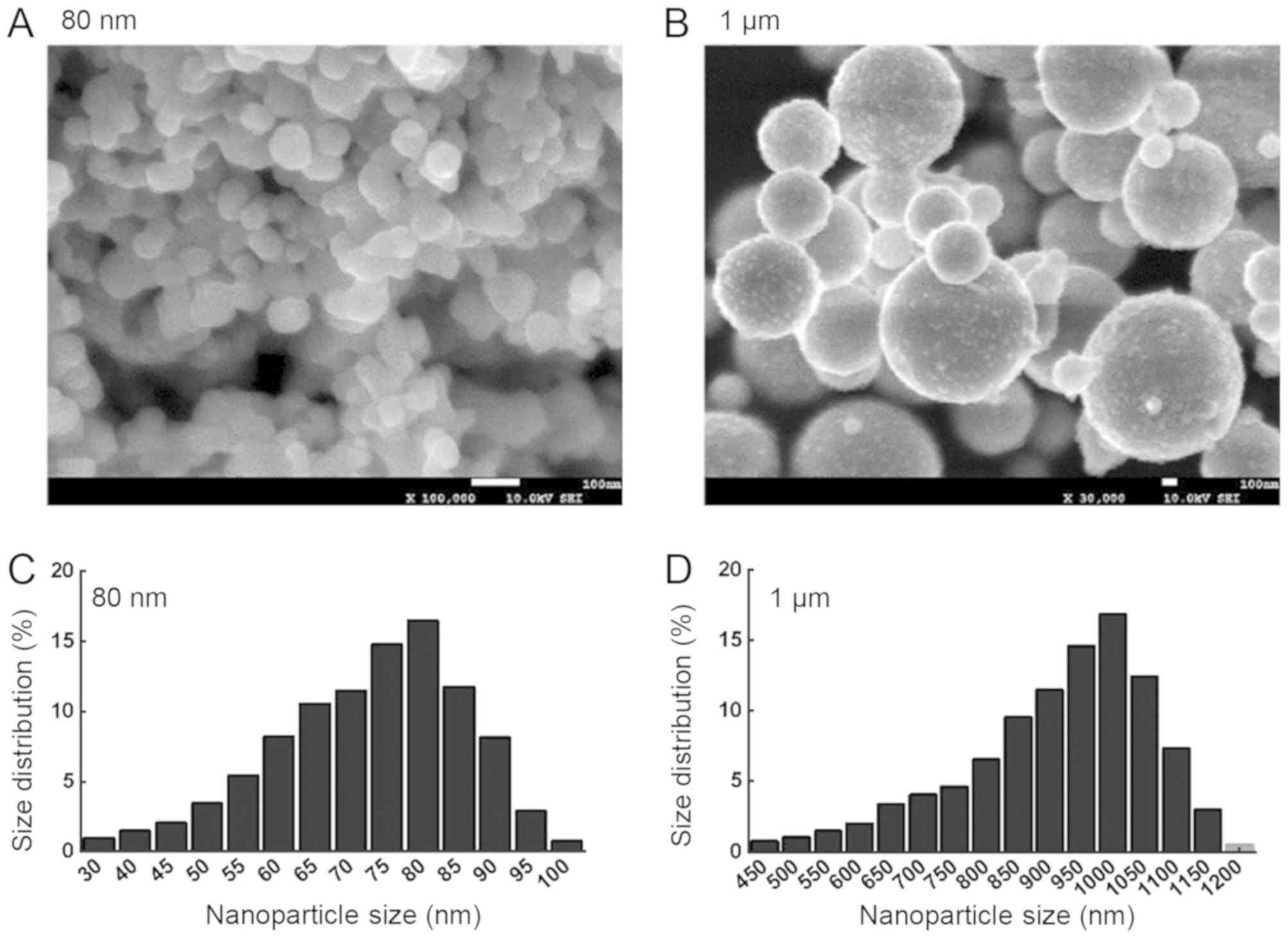
and a laser particle size analyzer (Fig. 1). CuNPs and copper microparticles
exhibited spherical morphology (Fig.
1A and B), and the size distribution is presented in Fig. 1C and D. The most common sizes of
the CuNPs and copper microparticles were 80 nm (average size:
82.5±33.4 nm) and 1 µm (average size: 987.4±436.7 nm),
respectively.
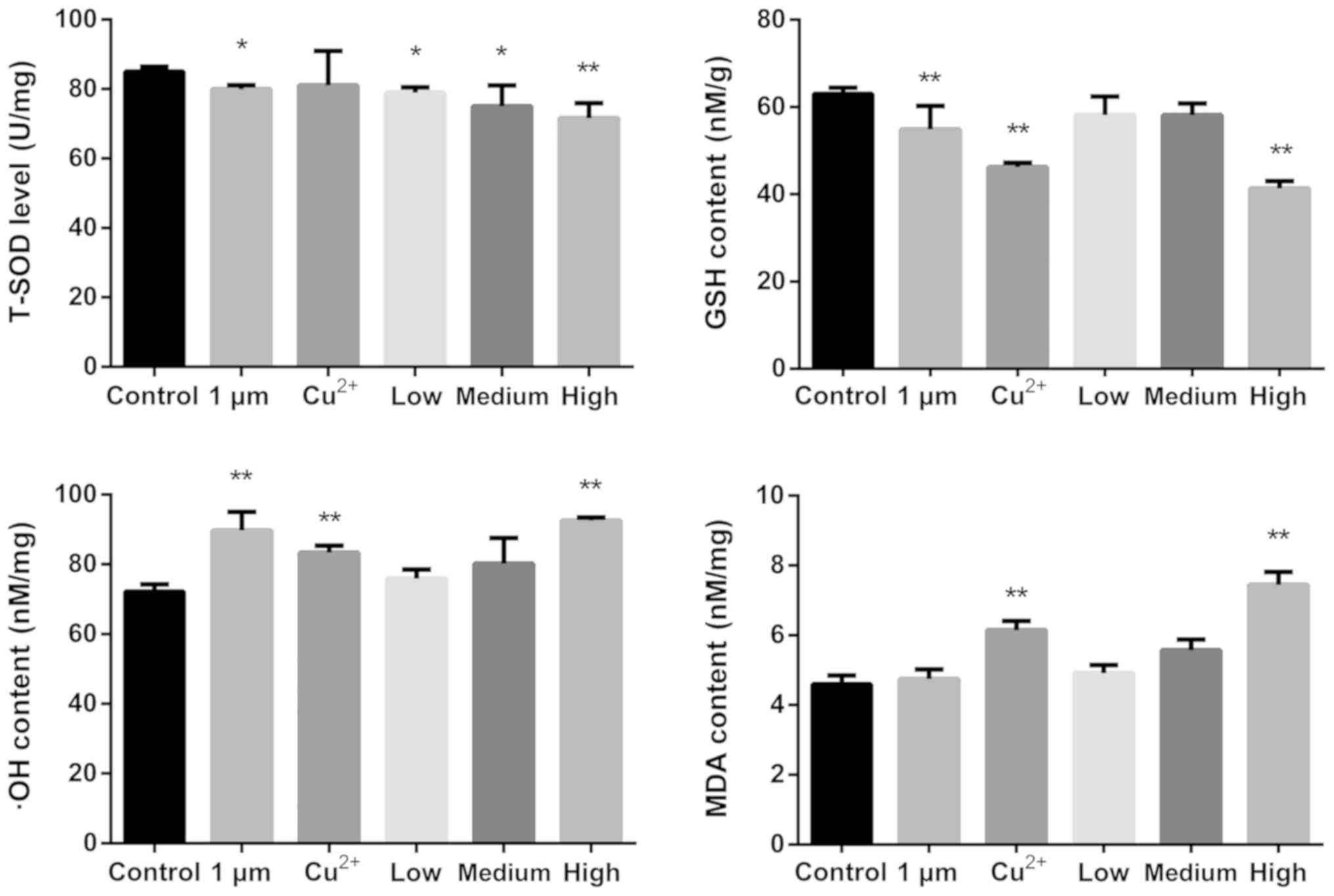
Oxidative stress
The levels of oxidative stress markers in the rat
brains were determined using commercial assay kits (Fig. 2). The levels of T-SOD were
significantly decreased compared with those of the control
following all treatments, with the exception of Cu2+.
GSH content was decreased significantly in the 1 µm,
Cu2+ and high-dose CuNPs groups compared with that in
the control group. The levels of ·OH were increased in the 1 µm,
Cu2+ and high-dose CuNPs groups compared with that in
the control group. The level of MDA was increased in the
Cu2+ and high-dose CuNPs groups compared with that in
the control.
mRNA expression of nuclear receptors
and CYP450s
RT-qPCR was performed to determine the mRNA
expression levels of different CYP450s (Fig. 3). CYP450 1A1 mRNA expression levels
were significantly decreased in the 1 µm and Cu2+ groups
and significantly increased in the low-dose CuNPs group compared
with that in the control group. The mRNA expression levels of CYP
2C11 were significantly decreased in rats treated with high-dose
CuNPs compared with that in the control. The mRNA expression levels
of CYP450 2D6 were significantly decreased in the Cu2+
group, but significantly increased in the low- and medium-dose
CuNPs groups compared with that in the control group. The mRNA
expression levels of CYP450 3A1 were increased in the 1 µm,
Cu2+ and low-dose groups, but significantly suppressed
in the high-dose CuNPs group compared with that in the control. The
mRNA expression levels of CAR were reduced in the low and medium
CuNPs groups, and significantly reduced in the high CuNPs groups,
whereas PXR mRNA expression levels of were reduced significantly in
the medium and high dose CuNPs groups, and significantly increased
in the 1 µm, Cu2+ and low-dose CuNPs groups compared
with that in the control group.
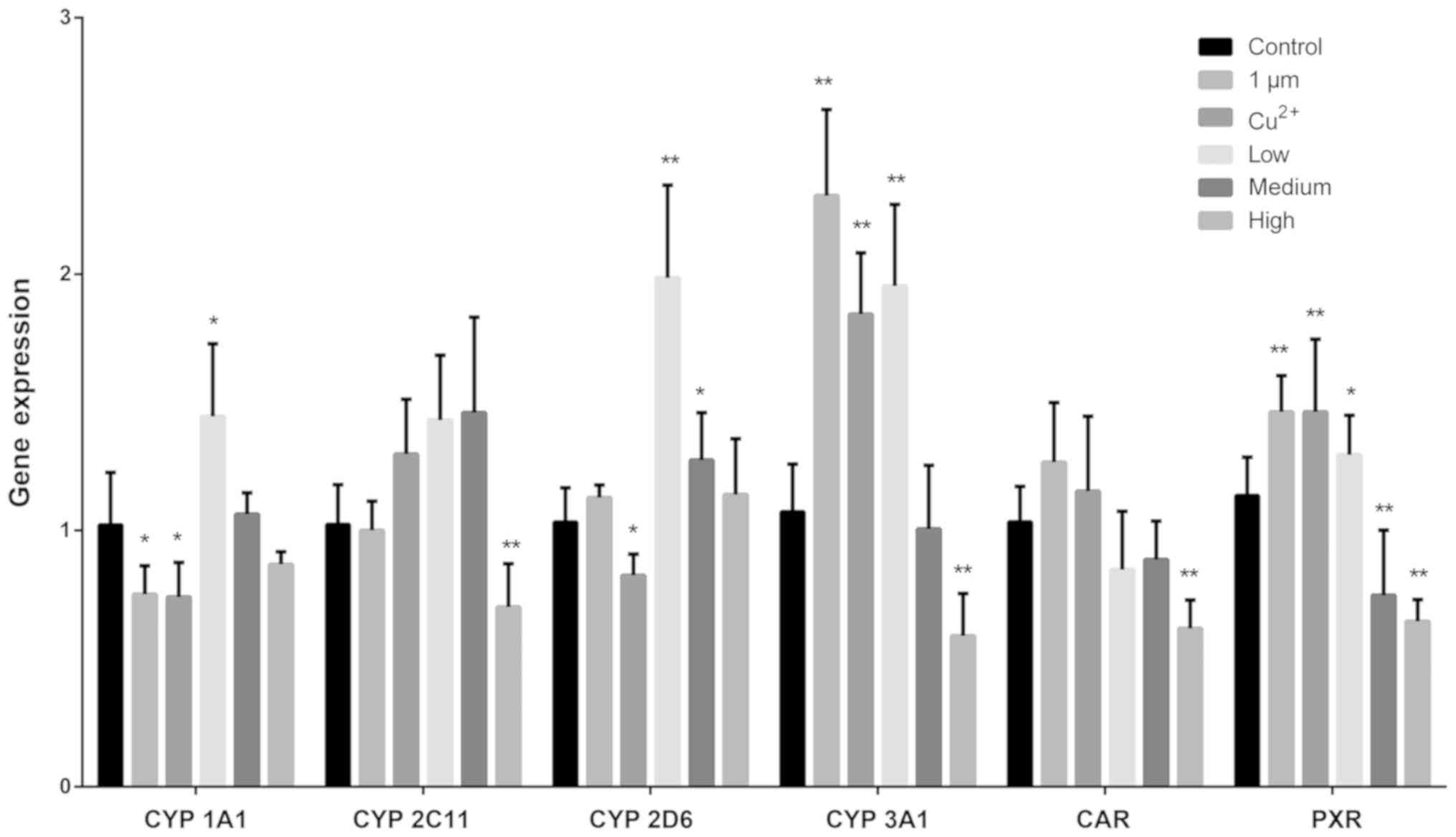 | Figure 3.mRNA expression levels of CYP450 1A1,
2C11, 2D6, 3A1, CAR and PXR in the rat brain. Different sources of
copper had a different impact on the mRNA expression of brain
CYP450s. A high dose of CuNPs decreased the expression levels of
CAR, PXR, CYP450 2C11 and CYP450 3A1. A low-dose of CuNPs increased
the expression of CYP 450 enzymes (except for CYP450 2C11) and
PXR. *P<0.05 and **P<0.01 vs. control. CYP450,
cytochrome P450; CAR, constitutive receptor; PXR, pregnane X
receptor; CuNPs, copper nanoparticles; Low, low-dose CuNPs; Medium,
medium-dose CuNPs; High, high-dose CuNPs. |
Protein expression of nuclear
receptors and CYP450 enzymes
Western blot analysis was performed to determine the
protein expression levels of CYP450 enzymes (Fig. 4). Protein expression levels of
CYP450 1A1 were decreased significantly in the high-dose CuNPs
group compared with that in the control. The levels of CYP450 2C11
were significantly decreased in the medium- and high-dose CuNPs
groups, but increased in the 1 µm group compared with that in the
control group. The protein expression levels of CYP450 2D6 were
suppressed in the medium- and high-dose CuNPs groups compared with
that in the control. The activity of CYP450 3A1 was suppressed in
all treatment groups compared with that in the control group. The
CAR protein expression levels did not change under any treatment,
whereas the protein levels of PXR were decreased in the
Cu2+, and the low-, medium- and high-dose CuNPs groups
compared with that in the control.
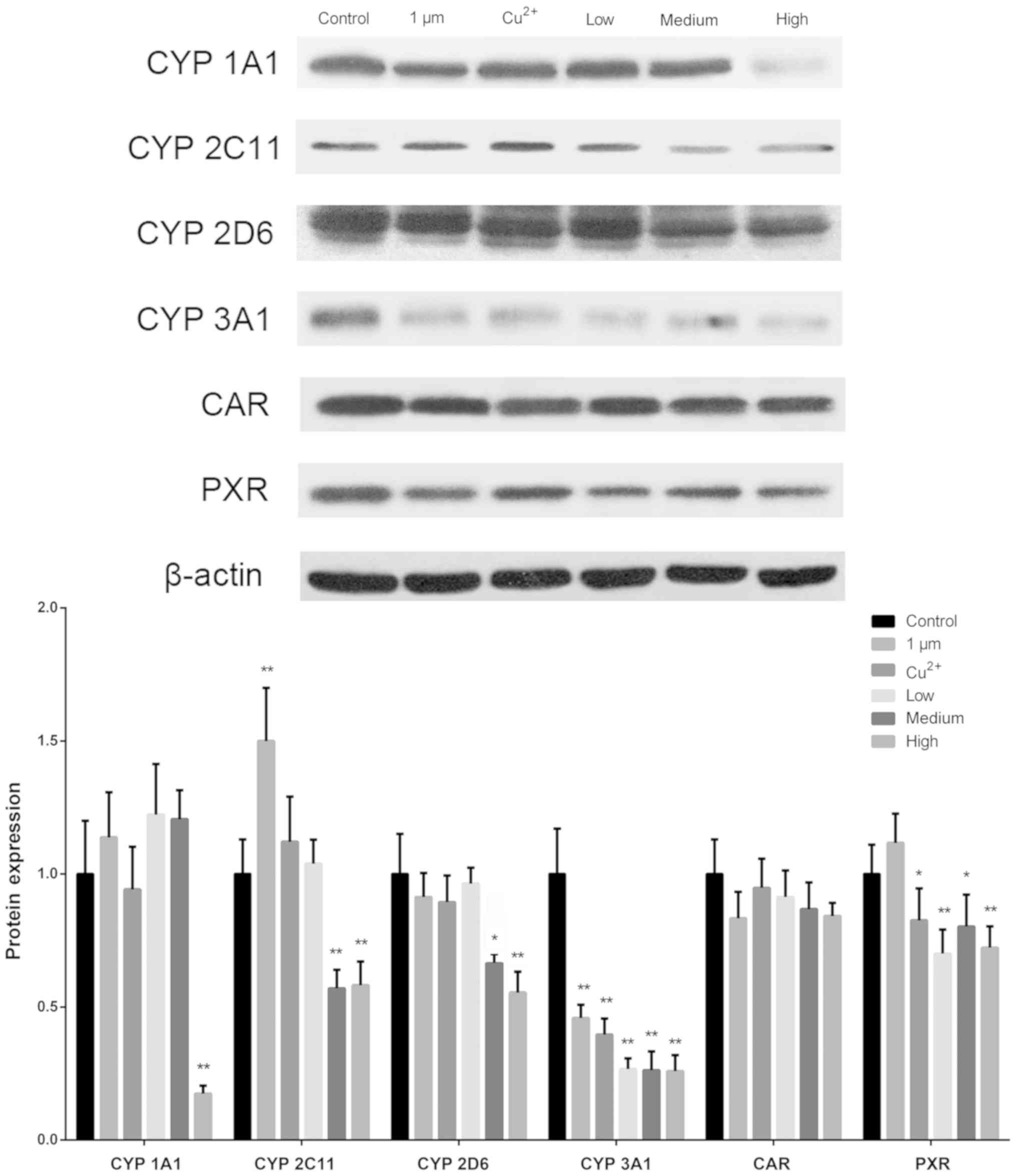 | Figure 4.Protein expression levels of CYP450
1A1, 2C11, 2D6, 3A1, CAR and PXR in the rat brain. CYP450 3A1 was
the most strongly affected of the tested CYP450s when analyzing all
sources of copper. A high dose of CuNPs decreased the protein
expression of all CYP450 enzymes and PXR. *P<0.05 and
**P<0.01 vs. control. CYP450, cytochrome P450; CAR, constitutive
receptor; PXR, pregnane X receptor; CuNPs, copper nanoparticles;
Low, low-dose CuNPs; Medium, medium-dose CuNPs; High, high-dose
CuNPs. |
Discussion
Brain CYP450 is expressed in glial cells in the
barrier regions and in neurons throughout the brain, and certain
endogenous compounds, as well as central nervous system drugs, are
metabolized by CYP450s in the brain (32). The function of brain CYP450 and the
associated changes may be important for the development of drugs
that act and are metabolized locally in the brain, as well as
therapeutics that directly target brain CYPs (33). CuNPs not only cause lesions and
blood-brain barrier breakdown where copper accumulates, but also
affect neurotransmitter levels in the brain; it is not clear
whether these changes depend on CYP450s (34).
The underlying molecular mechanism of brain CYP450
regulation remains poorly understood, but a large body of data has
demonstrated that CYP450 expression can be regulated by oxidative
stress via the activation of nuclear receptor signaling pathways
(35–37). Oxidative stress is a state of redox
disequilibrium, which occurs when reactive oxygen species (ROS)
production exceeds the antioxidant defense capacity of a cell
(38). Previous studies have
suggested that exposure to CuNPs leads to oxidative stress, as
indicated by elevated ROS levels and decreased antioxidant enzyme
activity (39,40). ROS, including superoxide anions,
hydrogen peroxide and hydroxyl radicals, exhibit higher reactivity
than molecular oxygen (41).
Exposure to CuNPs increases the production of ROS, which may result
in damage to nuclear DNA and alterations of proteins, lipids and
carbohydrates when present at a high level (42,43).
In the present study, the levels of antioxidants (T-SOD and GSH)
were decreased, whereas the levels of lipid peroxidation products
(·OH and MDA) were increased upon exposure of the rat brain to
CuNPs, when compared with results in untreated rats.
Cu2+ and high-dose CuNPs induced severe oxidative
stress. These results suggested that CuNPs may induce redox
disequilibrium and exert negative effects on CYP450 in the rat
brain.
Brian CYP450s regulate cellular mechanisms
transcriptionally, post-transcriptionally and post-translationally
(44–47). Brain CYP450s are sensitive to
xenobiotic inducers, which may differ from the induction of liver
CYPs. The regulation of brain CYP450s is highly dependent on the
isoform and inducer of CYP (19).
CuNPs are small (1- to 100-nm) particles that can cross the
blood-brain barrier and damage the brain (48). The results of the present study
demonstrated that the mRNA expression levels of CYP450s and nuclear
receptors were increased or suppressed by different copper
treatments compared with the control group, but CYP450 protein
expression levels were decreased in the CuNP-treated groups
compared with that in the control. The expression of CYP450 3A1 and
PXR exhibited similarly trends following treatment with different
levels of copper nano-particles, which is in line with a previous
study that posited that PXR is a regulator of CYP450 3A enzymes
(49). CAR and PXR are thought to
be activated in response to exogenous stimuli, and are involved in
CYP450 regulation (49–51). The results of the present study
indicate that oxidative stress may suppress the expression of PXR
expression through CuNPs. Therefore, the toxicity of CuNPs may
decrease the expression of CYP450 in the brain, and their main
target is CYP450 3A1. The mRNA expression level of CYP450s were
either unchanged or reduced following a high dose of CuNPs, but
overall higher doses were shown to reduce the protein level of
CYP450s. Although an increase in mRNA expression was observed for
both CYP450 2D6 and CYP450 3A1, the western blotting analyses
showed a reduction in protein levels following CuNP treatment in a
seemingly dose dependent manner. This demonstrated that CuNPs may
affect CYP450 enzymes differently depending on whether they act at
the post-transcriptional and/or post-translational levels.
CYP450 enzymes of the brain serve an important role
in maintaining brain homeostasis, therefore it is of interest to
continue researching the role of CYP450s in the metabolism of
endogenous neurochemicals, some of which have already been
described (52,53). The results of the present study
provide evidence that CuNPs may have an impact on rat brain CYP450
enzymes, and unnecessary neurotoxicity and nervous system disorders
should be avoided in practical applications.
In conclusion, the present study demonstrates that
CuNPs may have an impact on brain CYP450 enzymes through ROS
accumulation. The understanding of the roles of CuNPs in the
regulation of brain CYP450s may be useful for better prediction,
prevention and treatment of the toxicity of copper therapeutics in
the brain.
Acknowledgements
The authors would like to thank Akram M. Salam (PhD,
Emory University, Atlanta, GA, USA) for language editing.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW, HT and YL made substantial contributions to
conception, design, acquisition of data, analysis and
interpretation of data, and were major contributors in writing the
manuscript. MX and JL helped in experimental operation and data
analysis. LZ, FS, GY, and CL contributed to the experimental
design. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by Sichuan Agricultural
University, and the protocols for animal care and treatment were in
accordance with their guidelines for animal experiments (approval
no. 20170314).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CYP450
|
cytochrome P450 enzyme
|
|
CAR
|
constitutive androstane receptor
|
|
PXR
|
pregnane X receptor
|
|
CuNPs
|
copper nanoparticles
|
|
GSH
|
glutathione
|
|
·OH
|
hydroxyl radicals
|
|
MDA
|
malondialdehyde
|
|
HPMC
|
hydroxypropyl methylcellulose
|
|
RT-qPCR
|
reverse-transcriptase polymerase chain
reaction
|
References
|
1
|
Guo K, Pan Q, Wang L and Fang S:
Nano-scale copper-coated graphite as anode material for lithium-ion
batteries. J Appl Electrochem. 32:679–685. 2002. View Article : Google Scholar
|
|
2
|
Liu G, Li X, Qin B, Xing D, Guo Y and Fan
R: Investigation of the mending effect and mechanism of copper
nano-particles on a tribologically stressed surface. Tribol Lett.
17:961–966. 2004. View Article : Google Scholar
|
|
3
|
Cioffi N, Ditaranto N, Torsi L, Picca RA,
Sabbatini L, Valentini A, Novello L, Tantillo G, Bleve-Zacheo T and
Zambonin PG: Analytical characterization of bioactive fluoropolymer
ultra-thin coatings modified by copper nanoparticles. Anal Bioanal
Chem. 381:607–616. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lei R, Wu C, Yang B, Ma H, Shi C, Wang Q,
Wang Q, Yuan Y and Liao M: Integrated metabolomic analysis of the
nano-sized copper particle-induced hepatotoxicity and
nephrotoxicity in rats: A rapid in vivo screening method for
nanotoxicity. Toxicol Appl Pharmacol. 232:292–301. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Magdassi S, Grouchko M and Kamyshny A:
Copper nanoparticles for printed electronics: Routes towards
achieving oxidation stability. Materials (Basel). 3:4626–4638.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoon KY, Hoon ByeonJ, Park JH and Hwang J:
Susceptibility constants of Escherichia coli and Bacillus
subtilis to silver and copper nanoparticles. Sci Total Environ.
373:572–575. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee IC, Ko JW, Park SH, Lim JO, Shin IS,
Moon C, Kim SH, Heo JD and Kim JC: Comparative toxicity and
biodistribution of copper nanoparticles and cupric ions in rats.
Int J Nanomedicine. 11:2883–2900. 2016.PubMed/NCBI
|
|
8
|
Chen Z, Meng H, Xing G, Chen C, Zhao Y,
Jia G, Wang T, Yuan H, Ye C, Zhao F, et al: Acute toxicological
effects of copper nanoparticles in vivo. Toxicol Lett. 163:109–120.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sarkar A, Das J, Manna P and Sil PC:
Nano-copper induces oxidative stress and apoptosis in kidney via
both extrinsic and intrinsic pathways. Toxicology. 290:208–217.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guengerich FP: Human Cytochrome P450
Enzymes. Cytochrome P450: Structure, Mechanism, and Biochemistry.
Ortiz de Montellano PR: Springer International Publishing; Cham:
pp. 523–785. 2015
|
|
11
|
Backman JT, Filppula AM, Niemi M and
Neuvonen PJ: Role of cytochrome P450 2C8 in drug metabolism and
interactions. Pharmacol Rev. 68:168–241. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hedlund E, Gustafsson JA and Warner M:
Cytochrome P450 in the brain; a review. Curr Drug Metab. 2:245–263.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Miksys S and Tyndale RF: Cytochrome
P450-mediated drug metabolism in the brain. J Psychiatry Neurosci.
38:152–163. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toselli F, Dodd PR and Gillam EM: Emerging
roles for brain drug-metabolizing cytochrome P450 enzymes in
neuropsychiatric conditions and responses to drugs. Drug Metab Rev.
48:379–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang X, Li J, Dong G and Yue J: The
endogenous substrates of brain CYP2D. Eur J Pharmacol. 724:211–218.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schilter B, Andersen MR, Acharya C and
Omiecinski CJ: Activation of cytochrome P450 gene expression in the
rat brain by phenobarbital-like inducers. J Pharmacol Exp Ther.
294:916–922. 2000.PubMed/NCBI
|
|
17
|
Huang P, Rannug A, Ahlbom E, Håkansson H
and Ceccatelli S: Effect of 2,3,7,8-tetrachlorodibenzo-p-dioxin on
the expression of cytochrome P450 1A1, the aryl hydrocarbon
receptor, and the aryl hydrocarbon receptor nuclear translocator in
rat brain and pituitary. Toxicol Appl Pharmacol. 169:159–167. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sánchez-Catalán MJ, Hipólito L, Guerri C,
Granero L and Polache A: Distribution and differential induction of
CYP2E1 by ethanol and acetone in the mesocorticolimbic system of
rat. Alcohol Alcohol. 43:401–407. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Miksys SL and Tyndale RF:
Drug-metabolizing cytochrome P450s in the brain. J Psychiatry
Neurosci. 27:406–415. 2002.PubMed/NCBI
|
|
20
|
Hedlund E, Wyss A, Kainu T, Backlund M,
Köhler C, Pelto-Huikko M, Gustafsson JA and Warner M: Cytochrome
P4502D4 in the brain: Specific neuronal regulation by clozapine and
toluene. Mol Pharmacol. 50:342–350. 1996.PubMed/NCBI
|
|
21
|
Mann A, Miksys S, Lee A, Mash DC and
Tyndale RF: Induction of the drug metabolizing enzyme CYP2D in
monkey brain by chronic nicotine treatment. Neuropharmacology.
55:1147–1155. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Strobel HW, Thompson CM and Antonovic L:
Cytochromes P450 in brain: Function and significance. Curr Drug
Metab. 2:199–214. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Funae Y, Kishimoto W, Cho T, Niwa T and
Hiroi T: CYP2D in the brain. Drug Metab Pharmacokinet. 18:337–349.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferguson CS and Tyndale RF: Cytochrome
P450 enzymes in the brain: Emerging evidence of biological
significance. Trends Pharmacol Sci. 32:708–714. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bai R, Zhang L, Liu Y, Li B, Wang L, Wang
P, Autrup H, Beer C and Chen C: Integrated analytical techniques
with high sensitivity for studying brain translocation and
potential impairment induced by intranasally instilled copper
nanoparticles. Toxicol Lett. 226:70–80. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liao M and Liu H: Gene expression
profiling of nephrotoxicity from copper nanoparticles in rats after
repeated oral administration. Environ Toxicol Pharmacol. 34:67–80.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sarkar P, Narayanan J and Harder DR:
Differential effect of amyloid β on the cytochrome P450 epoxygenase
activity in rat brain. Neuroscience. 194:241–249. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Auyeung DJ, Kessler FK and Ritter JK: An
alternative promoter contributes to tissue-and inducer-specific
expression of the rat UDP-glucuronosyltransferase 1A6 gene. Toxicol
Appl Pharmacol. 174:60–68. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Daniel WA, Haduch A, Syrek M and Boksa J:
Direct and indirect interactions between antidepressant drugs and
CYP2C6 in the rat liver during long-term treatment. Eur
Neuropsychopharmacol. 16:580–587. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haduch A, Wójcikowski J and Daniel WA: The
effect of tricyclic antidepressants, selective serotonin reuptake
inhibitors (SSRIs) and newer antidepressant drugs on the activity
and level of rat CYP3A. Eur Neuropsychopharmacol. 16:178–186. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meyer RP, Gehlhaus M, Knoth R and Volk B:
Expression and function of cytochrome p450 in brain drug
metabolism. Curr Drug Metab. 8:297–306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Navarro-Mabarak C, Camacho-Carranza R and
Espinosa-Aguirre JJ: Cytochrome P450 in the central nervous system
as a therapeutic target in neurodegenerative diseases. Drug Metab
Rev. 50:95–108. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang L, Bai R, Liu Y, Meng L, Li B, Wang
L, Xu L, Le Guyader L and Chen C: The dose-dependent toxicological
effects and potential perturbation on the neurotransmitter
secretion in brain following intranasal instillation of copper
nanoparticles. Nanotoxicology. 6:562–575. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kakehashi A, Hagiwara A, Imai N, Nagano K,
Nishimaki F, Banton M, Wei M, Fukushima S and Wanibuchi H: Mode of
action of ethyl tertiary-butyl ether hepatotumorigenicity in the
rat: Evidence for a role of oxidative stress via activation of CAR,
PXR and PPAR signaling pathways. Toxicol Appl Pharmacol.
273:390–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tolson AH and Wang H: Regulation of
drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv
Drug Deliv Rev. 62:1238–1249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Waxman DJ: P450 gene induction by
structurally diverse xenochemicals: Central role of nuclear
receptors CAR, PXR, and PPAR. Arch Biochem Biophys. 369:11–23.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Deres P, Halmosi R, Toth A, Kovacs K,
Palfi A, Habon T, Czopf L, Kalai T, Hideg K, Sumegi B and Toth K:
Prevention of doxorubicin-induced acute cardiotoxicity by an
experimental antioxidant compound. J Cardiovasc Pharmacol.
45:36–43. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu P, Xu J, Liu S, Ren G and Yang Z: In
vitro toxicity of nanosized copper particles in PC12 cells induced
by oxidative stress. J Nanopart Res. 14:9062012. View Article : Google Scholar
|
|
40
|
Xu P, Xu J, Liu S and Yang Z: Nano copper
induced apoptosis in podocytes via increasing oxidative stress. J
Hazard Mater. 241-242:279–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Thannickal VJ and Fanburg BL: Reactive
oxygen species in cell signaling. Am J Physiol Lung Cell Mol
Physiol. 279:L1005–L1028. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Martindale JL and Holbrook NJ: Cellular
response to oxidative stress: Signaling for suicide and survival. J
Cell Physiol. 192:1–15. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yoshida Y, Itoh N, Saito Y, Hayakawa M and
Niki E: Application of water-soluble radical initiator, 2,2′-azobis
[2-(2-imidazolin-2-yl) propane] dihydrochloride, to a study of
oxidative stress. Free Radic Res. 38:375–384. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yadav S, Dhawan A, Singh RL, Seth PK and
Parmar D: Expression of constitutive and inducible cytochrome P450
2E1 in rat brain. Mol Cell Biochem. 286:171–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Roberts BJ, Shoaf SE, Jeong KS and Song
BJ: Induction of CYP2E1 in liver, kidney, brain and intestine
during chronic ethanol administration and withdrawal: Evidence that
CYP2E1 possesses a rapid phase half-life of 6 hours or less.
Biochem Biophys Res Commun. 205:1064–1071. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Joshi M and Tyndale RF: Induction and
recovery time course of rat brain CYP2E1 after nicotine treatment.
Drug Metab Dispos. 34:647–652. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Miksys S, Wadji FB, Tolledo EC, Remington
G, Nobrega JN and Tyndale RF: Rat brain CYP2D enzymatic metabolism
alters acute and chronic haloperidol side-effects by different
mechanisms. Prog Neuropsychopharmacol Biol Psychiatry. 78:140–148.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ramsden CS, Smith TJ, Shaw BJ and Handy
RD: Dietary exposure to titanium dioxide nanoparticles in rainbow
trout, (Oncorhynchus mykiss): No effect on growth, but subtle
biochemical disturbances in the brain. Ecotoxicology. 18:939–951.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Aleksunes LM and Klaassen CD: Coordinated
regulation of hepatic phase I and II drug-metabolizing genes and
transporters using AhR-, CAR-, PXR-, PPARα-, and Nrf2-null mice.
Drug Metab Dispos. 40:1366–1379. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Thompson EE, Kuttab-Boulos H, Krasowski MD
and Di Rienzo A: Functional constraints on the constitutive
androstane receptor inferred from human sequence variation and
cross-species comparisons. Hum Genomics. 2:168–178. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tien ES and Negishi M: Nuclear receptors
CAR and PXR in the regulation of hepatic metabolism. Xenobiotica.
36:1152–1163. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Fradette C, Yamaguchi N and Du Souich P:
5-Hydroxytryptamine is biotransformed by CYP2C9, 2C19 and 2B6 to
hydroxylamine, which is converted into nitric oxide. Br J
Pharmacol. 141:407–414. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bromek E, Haduch A, Gołembiowska K and
Daniel WA: Cytochrome P450 mediates dopamine formation in the brain
in vivo. J Neurochem. 118:806–815. 2011. View Article : Google Scholar : PubMed/NCBI
|