Introduction
Endometrial cancer is the most common gynecologic
malignancy, and its incidence is increasing worldwide (1). A strong association exists between
endometrial cancer and metabolism. Individuals with diabetes
mellitus or obesity have 1.8 or 1.5-fold higher relative risks for
developing endometrial cancer, respectively (2,3). In
addition, metabolic modifiers, including metformin (an oral
antidiabetic drug for type-II diabetes mellitus), have been
reported to induce antitumor effects in endometrial cancer
(4,5).
Resveratrol (RSV) is a natural polyphenol found in a
variety of plant-based foods and beverages, such as red wine
(6). RSV is able to regulate various
physiological functions, such as blocking inflammation and
protecting against cardiovascular dysfunctions and obesity
(6–8).
These activities suggest that RSV may serve as a promising
metabolic modifier in endometrial cancer. Indeed, an antitumor role
of RSV has been reported in endocrine-associated cancers, including
endometrial cancer (9–11). However, the mechanism underlying its
antiproliferative effect is debated. The effects of RSV have been
suggested to be dependent on estrogen, epidermal growth factor
downregulation, protein kinase B (AKT) inactivation, and adenosine
monophosphate-activated protein kinase (AMPK) activation (11–14). Loss
of AMPK activity can promote oncogenesis (15). Metformin is known to activate AMPK
through liver kinase B1 (LKB1) phosphorylation, and this activation
is suggested to be involved in its antitumor effect (16). RSV was previously revealed to activate
sirtuin 1 (SIRT1) (17). SIRT1 is
able to deacetylate certain proteins that regulate longevity and
cellular stress, such as tumor protein p53 (TP53) (18,19). Thus,
various factors are associated with the antitumor effects of RSV.
In addition, cytostatic and cytotoxic effects have been observed
following RSV treatment in cancer cells (20).
By contrast, RSV may also induce oncogenesis.
Notably, RSV is associated with autophagy induction (21–24) and
activation of the Raf/MEK/ERK signal transduction cascade (25). Autophagy, which literally means
‘self-eating’ is a major degradation system that promotes the
lysosomal digestion of organelles and cytoplasmic components
(26). Autophagic activity is
commonly assessed through measuring the expression levels of
microtubule-associated protein 1 light chain 3 (LC3). LC3-II is a
standard marker of autophagic flux and localizes to autophagosomes.
Autophagy-related (ATG) genes 5 (ATG5) and 7 (ATG7)
directly regulate autophagic processes (26). Autophagy has been suggested to promote
cancer progression through driving cell metabolism (27). Activation of AMPK and/or extracellular
signal-regulated kinase (ERK) signaling was demonstrated to induce
autophagy in human cancers (28,29), which
may induce the antitumor effect of RSV on cancer cells.
Chloroquine (CQ) is an autophagy inhibitor with an
antimalarial effect (30). In
addition, CQ and its derivative, hydroxychloroquine, have been used
to treat connective tissue diseases, including rheumatoid
arthritis, systemic lupus erythematosus and Sjögren's syndrome
(31–33). CQ exhibits antitumor effects in
vitro and in vivo by inhibiting autophagy, and various
clinical trials have been conducted using CQ in certain types of
cancer (34,35). We recently reported that autophagy
inhibition by CQ suppressed endometrial cancer cell proliferation,
and improved cisplatin sensitivity (36). Therefore, autophagy inhibition may
potentiate the antitumorigenic effects of RSV in endometrial cancer
cells.
The purpose of the present study was to investigate
the effects of RSV on endometrial cancer cell proliferation and
autophagy. In addition, the study also addressed whether autophagy
inhibition enhances the effect of RSV, which would suggest a
potential new treatment strategy for endometrial cancer.
Materials and methods
Chemicals and antibodies
RSV and CQ were obtained from Sigma-Aldrich (St.
Louis, MO, USA). Mouse monoclonal antibodies against LC3 (#M152-3)
and β-actin (#M177-3) were obtained from MBL International
Corporation (Woburn, MA, USA) and Sigma-Aldrich, respectively.
Rabbit monoclonal antibodies against SIRT1 (#ab32441) were
purchased from Abcam (Cambridge, UK). Antibodies against
phospho-AMPKα (p-AMPKα) at Thr172 (#2535), phospho-AKT at Ser473
(#9271P), phospho-Erk1/2 (p44/42 MAPK; #9101), phospho S6 ribosomal
protein at Ser240/244 (#2215), LC3β (#2775), and cleaved poly
(ADP-ribose) polymerase (PARP) (#9544) were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA). An Alexa Fluor
488-conjugated goat anti-mouse immunoglobulin (Ig)G secondary
antibody (#A-11001) was obtained from Invitrogen, Thermo Fisher
Scientific, Inc. (Waltham, MA, USA).
Cell culture
The Ishikawa endometrial cancer cell line was
provided by Dr Masato Nishida (National Hospital Organization
Kasumigaura Medical Center, Tsuchiara, Japan). Ishikawa cells were
grown at 37°C in Dulbecco's modified Eagle's medium (DMEM)
supplemented with 10% fetal bovine serum (FBS; both obtained from
Thermo Fisher Scientific, Inc.) in a humidified 5% CO2
incubator.
MTT assays
Ishikawa cells (3,000 cells/well) were seeded 24 h
prior to RSV treatment. Subsequently, the cells were grown for 72 h
in DMEM, which contained increasing doses of RSV (0.1–200 µM). At
the endpoint, 10 µl of the Cell Counting kit-8 reagent containing
the tetrazolium salt WST-8 was added to the wells, according to the
protocol of the manufacturer (Dojindo, Molecular Technologies,
Inc., Kumamoto, Japan), and absorbance (450 nm) was measured in a
microplate reader (BioTek Instruments, Inc., Winooski, VT, USA).
Proliferation was normalized to absorbance measurements observed in
control cells treated with dimethyl sulfoxide alone.
Cell cycle analysis
Ishikawa cells (5×105 cells/60-mm dish)
were grown in the presence of RSV (25 µM) for 72 h. Cell cycle
analysis was performed as previously described (36) in three independent experiments.
Apoptosis measurements by double
staining with annexin V and propidium iodide (PI)
Ishikawa cells were plated in 60-mm dishes for 24 h
prior to 24 h incubations at 37°C with the indicated drugs and/or
small interfering RNAs (siRNAs), at the indicated doses. As
described previously (36), the cells
were trypsinized, washed two times with phosphate-buffered saline
(PBS), and stained with PI and fluorescein isothiocyanate
(FITC)-conjugated annexin V, using the FITC Annexin-V Apoptosis
Detection kit I (BD Biosciences, San Jose, CA, USA), as directed by
the manufacturer. Apoptotic cells were measured as double-positive
cells in three independent experiments using a BD FACSCalibur flow
cytometer, and expressed on a percentage basis.
Western blot analysis
Soluble proteins from Ishikawa cell lysates were
extracted as described previously (36), followed by western blot analysis with
the aforementioned primary antibodies (1:1,000) at 4°C overnight.
Bands were detected using the BioRad Blotting system (BioRad
Laboratories, Inc., Hercules, CA, USA) with the ECL Select
Detection Reagent (GE Healthcare, Little Chalfont, UK).
Immunofluorescence
Ishikawa cells were cultured in DMEM in 6-well
plates, on glass coverslips coated with PBS containing 0.1%
gelatin. After 24-h incubation at 37°C, the medium was replaced
with DMEM alone (control cells) or DMEM supplemented with 25 µM
RSV. The cells were then incubated for an additional 48-h.
Subsequently, the cells were washed in PBS, fixed with 4%
paraformaldehyde, and permeabilized with 0.2% Triton X-100 prior to
blocking in 6% bovine serum albumin (Thermo Fisher Scientific,
Inc.). The cells were then incubated overnight at 4°C with a
primary anti-LC3 antibody (diluted 1:200). On the following day,
the cells were incubated for 1 h at room temperature with a
secondary Alexa Fluor 488-conjugated goat, anti-mouse IgG antibody
(1:200). Nuclei were counterstained with Hoechst 33342 dye at a
1:1,000 dilution. The slides were analyzed by confocal fluorescence
microscopy (BX50; Olympus Corporation, Tokyo, Japan).
Gene silencing
Ishikawa cells were grown in culture for 24 h prior
to gene-silencing experiments conducted with Stealth RNAi siRNAs
against ATG5 or ATG7 (Invitrogen; Thermo Fisher
Scientific, Inc.), using Lipofectamine RNAiMAX (Invitrogen; Thermo
Fisher Scientific, Inc.). A negative control siRNA was used as a
control (Invitrogen; Thermo Fisher Scientific, Inc.). siRNA
transfections were performed as described previously (36).
Statistical analysis
The data were presented as the mean ± standard error
from at least three independent determinations. The significance of
differences between ≥3 samples were analyzed by one-way analysis of
variance and post-hoc testing, whereas the significance
between two samples were analyzed by a Mann-Whitney U test, using
GraphPad Prism, version 6.0 (GraphPad Software, San Diego, CA,
USA). P<0.05 was considered to indicate a statistically
significant result.
Results
RSV suppresses the proliferation of
Ishikawa cells by apoptosis induction
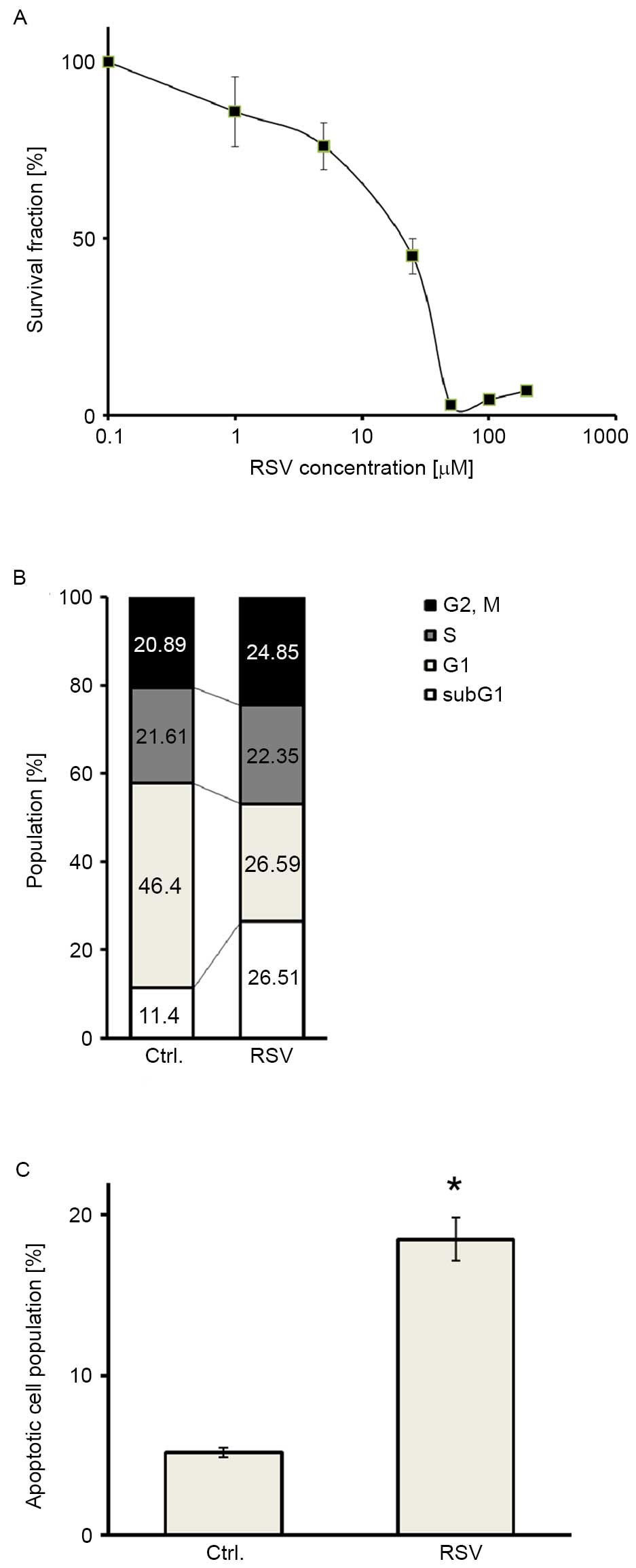
MTT assays were performed in Ishikawa endometrial
cancer cells to assess the antitumor activity of RSV. RSV inhibited
the proliferation of Ishikawa cells in a dose-dependent manner
(Fig. 1A). The half-maximal (50%)
inhibitory concentration IC50 value was 20 µM. Cell
cycle analysis was also performed to elucidate whether growth
inhibition by RSV was attributable to cell cycle arrest or cell
death. Cell cycle analysis demonstrated that RSV caused a
significant increase in the abundance of the sub-G1 population of
Ishikawa cells (Fig. 1B). In
addition, annexin V-PI double staining showed a significant
accumulation of double-positive cells following RSV treatment in
Ishikawa cells (Fig. 1C), indicating
that RSV induced apoptosis in Ishikawa cells. These results
suggested that RSV inhibits the growth of Ishikawa cells, mainly
via its cytotoxic effect.
RSV induces autophagy in Ishikawa
cells
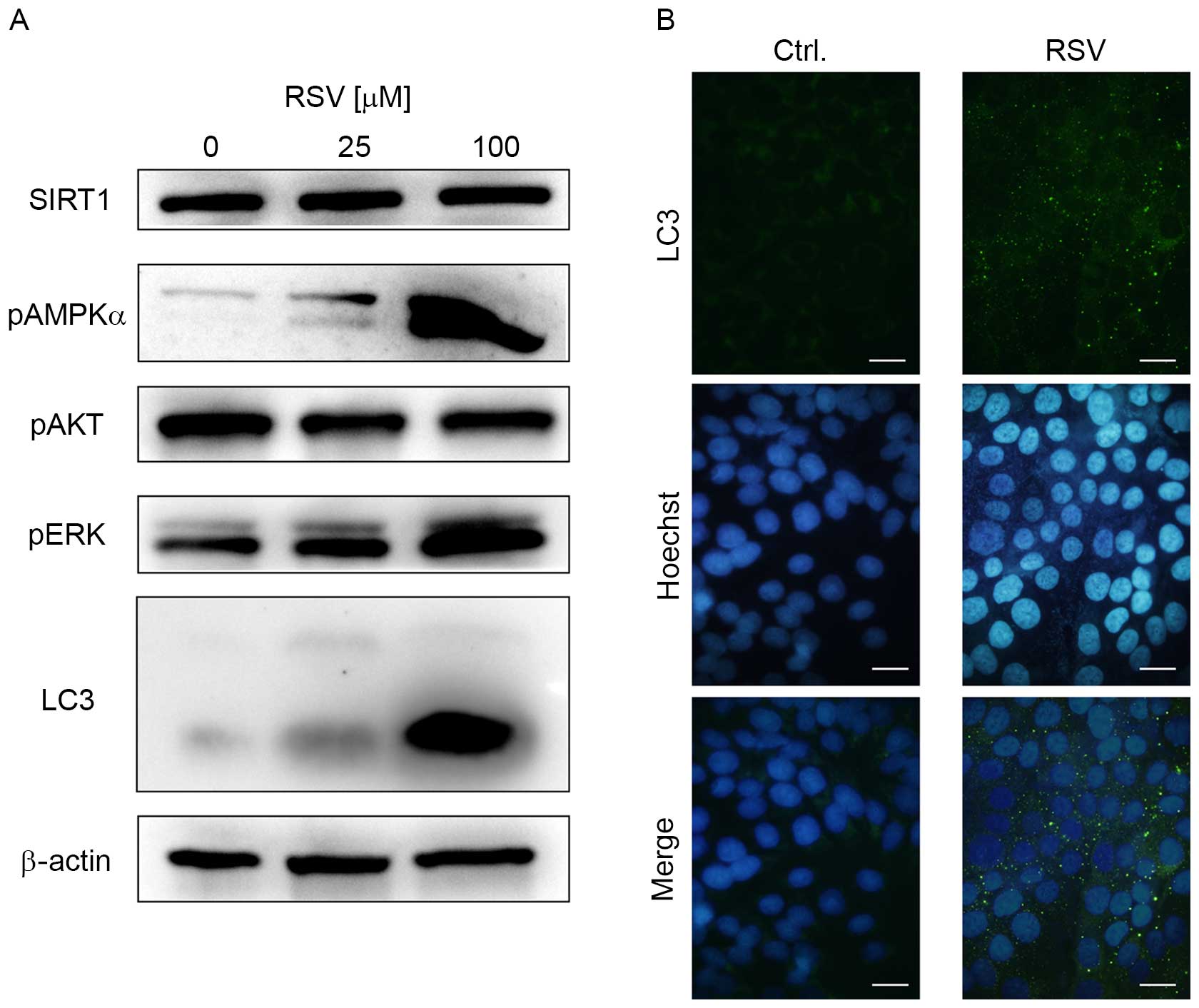
To elucidate which proteins are associated with
growth inhibition by RSV, immunoblotting was performed against cell
growth-associated proteins expressed in Ishikawa cells. RSV
markedly increased the expression of p-AMPKα and p-ERK (Fig. 2A). However, RSV did not increase SIRT1
expression, or decrease the expression of p-AKT (Fig. 2A). RSV induced LC3-II expression, and
LC3-immunofluorescence experiments revealed autophagosome
accumulation in the cytosol of Ishikawa cells following 20 µM RSV
treatment (Fig. 2A and B). These data
strongly suggest that RSV activates AMPK and ERK signaling in
Ishikawa cells, with an induction of autophagy.
Pharmacologic autophagy inhibition by
CQ augments RSV-inducible apoptosis in Ishikawa cells
Next, we addressed whether RSV-mediated autophagy
affects the RSV antitumor effect in Ishikawa cells, by adding CQ in
combination with RSV. Cell viability was significantly suppressed
by combination treatment (25 µM RSV and 5 µM CQ), compared with RSV
treatment alone at 25 µM (Fig. 3A).
Combination treatment induced significant cleaved PARP
accumulation, compared with RSV treatment alone, as determined by
western blot analysis (Fig. 3B). In
addition, combination treatment showed a trend towards an increased
population of double-positive (apoptotic) cells in the annexin V-PI
double staining assays (Fig. 3C).
These data indicated that combination treatment with RSV and CQ may
induce greater cytotoxicity in Ishikawa cells, as compared with RSV
treatment alone.
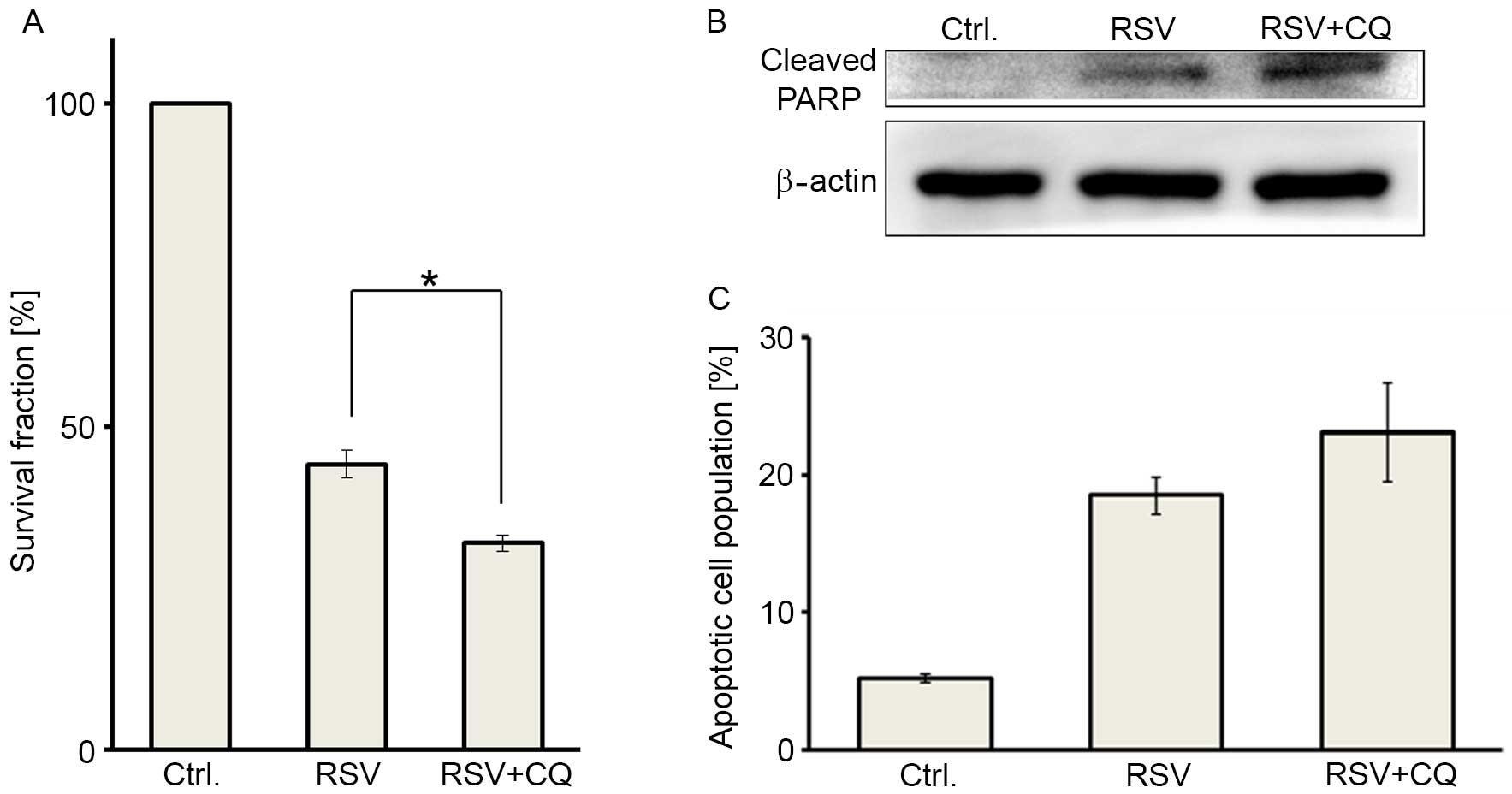 | Figure 3.Pharmacologic autophagy inhibition by
chloroquine augments RSV-induced apoptosis in Ishikawa cells. (A)
Cell viabilities were assessed by performing MTT assays in three
groups, including: Untreated control cells (left), cells treated
with 25 µM RSV (middle), and cells administered a combination
treatment with 25 µM RSV and 5 µM CQ (right). Treated cell survival
fraction (%) was compared with the non-treated group (set as 100%).
The results are presented as the mean ± SE of three independent
experiments. *P<0.05. (B) Immunoblotting of cleaved PARP
following each treatment, as described above. β-actin was used as a
loading control. (C) Apoptosis was measured by annexin V-PI double
staining following each treatment, using the aforementioned RSV and
CQ concentrations. The results are presented as the mean ± SE of
three independent experiments. RSV, resveratrol; CQ, chloroquine;
SE, standard error; PARP, poly ADP ribose polymerase; PI, propidium
iodide. |
Autophagy inhibition by ATG5 and ATG7
siRNAs augments RSV-induced apoptosis in Ishikawa cells
To elucidate whether RSV-inducible autophagy renders
the antiproliferative effect of RSV, the core ATGs, ATG5 or
ATG7, were knocked down in Ishikawa cells using two
independent siRNAs for each gene. The efficacy of gene silencing
and autophagy inhibition by these siRNAs was already confirmed in
our previous report (36). MTT assay
revealed that the cells were more sensitive to RSV when either
ATG5 or ATG7 was knocked down (Fig. 4A). Moreover, annexin V-PI double
staining revealed that RSV-induced apoptosis was enhanced by
silencing ATG5 or ATG7, whereas the knockdown of
ATG5, or ATG7, alone did not affect apoptosis in
cells without RSV treatment (Fig.
4B).
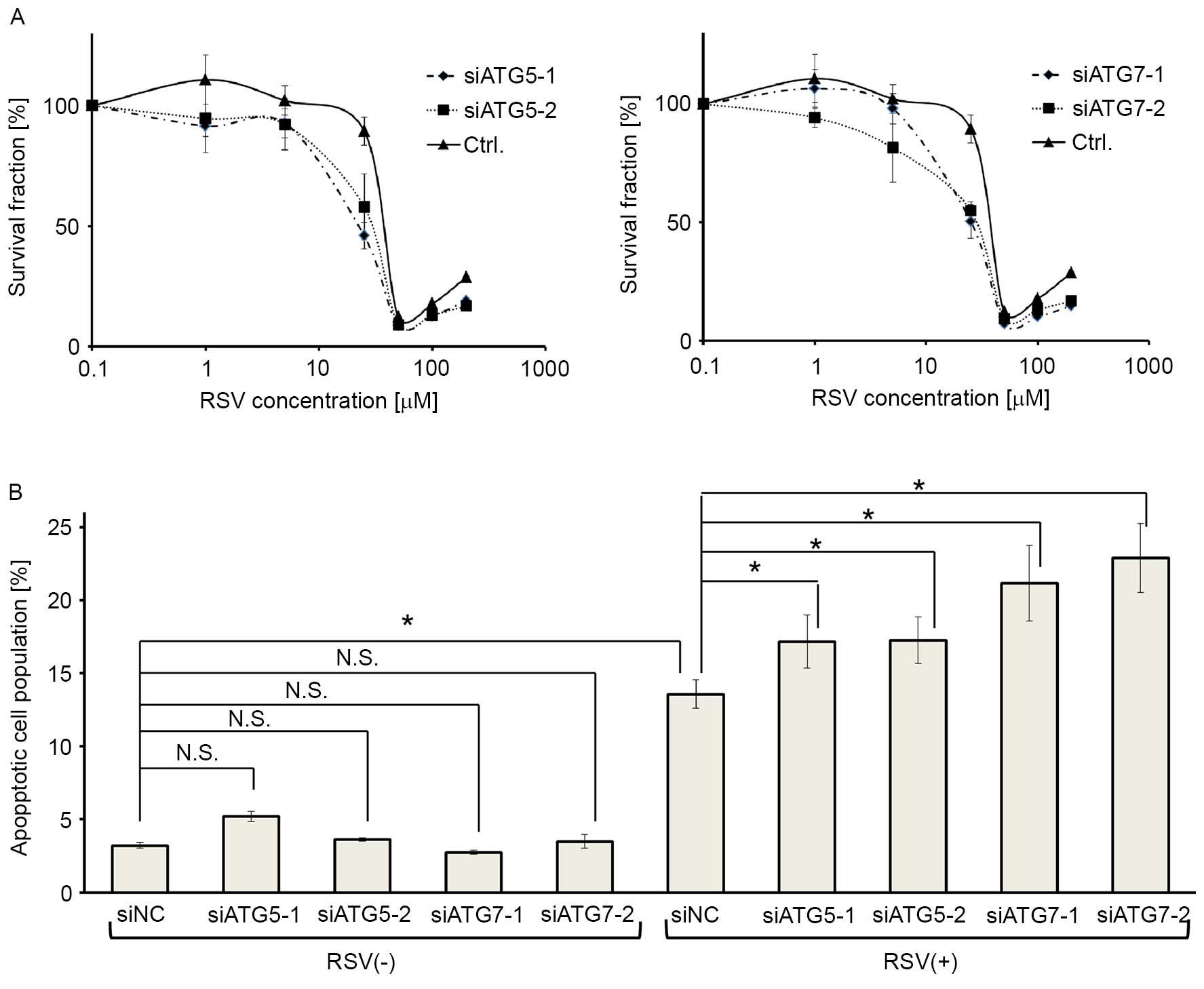 | Figure 4.Autophagy inhibition by ATG5
and ATG7 siRNA augments RSV-induced apoptosis in Ishikawa
cells. (A) MTT assays following RSV treatment (0.1–200 µM)
following gene knockdown in Ishikawa cells. Two siRNAs targeting
ATG5 (siATG5-1, siATG5-2; left panel) or ATG7 mRNA
(siATG7-1, siATG7-2; right panel), and a negative control siRNA
were used for this assay. The results are presented as the mean ±
SE of quadruplicate samples. (B) Annexin V-PI double staining
following ATG5 or ATG7 knockdown, with and without 25
µM RSV treatment in Ishikawa cells. Four siRNAs (siATG5-1,
siATG5-2, siATG7-1, and siATG7-2) and a negative control siRNA
(siNC) were used, as described above. Three independent experiments
were performed. These results show the percentage of
double-positive cells following each treatment. The results are
presented as the mean ± SE of three independent experiments.
*P<0.05. ATG, autophagy related gene; RSV, resveratrol; si,
small interfering; PI, propidium iodide; SE, standard error; CQ,
chloroquine. |
Discussion
RSV is an active compound in foods that can prevent
cell proliferation of various types of cancer cells. However, RSV
also induces autophagy, which can promote stress tolerance and cell
survival by maintaining energy production. Therefore,
RSV-associated autophagy may hamper its antitumor effect. In this
study, we focused on i) antitumor activity and apoptosis induction
by RSV, ii) autophagy induction by RSV, and iii) the efficacy of
combined autophagy inhibition and RSV treatment in Ishikawa
endometrial cancer cells.
Initially, the results demonstrated that RSV
suppressed the proliferation of Ishikawa cells. The IC50
value of 20 µM for RSV in the Ishikawa endometrial cancer cells was
lower than those of cervical, bladder, breast and liver cancer
cells (37–39). This result implies that at least
certain endometrial cancer cells may be more sensitive to RSV
treatment than other types of cancer cells. The antiproliferative
effect of RSV on the tumor cells was revealed to be primarily
cytotoxic, not cytostatic. Although the mechanism underlying RSV
induction of apoptosis remains unclear, AMPK-dependent signaling
pathways may be associated with its ability to induce apoptosis
(40). Indeed, RSV markedly increased
the expression of p-AMPKα in this study. Although a previous report
indicated that RSV attenuated cancer cell proliferation in a
SIRT1-dependent manner (41), SIRT1
did not accumulate following RSV treatment in Ishikawa cells.
Therefore, RSV-induced apoptosis may be independent from SIRT1.
Further investigation is warranted to elucidate the mechanism
underlying apoptosis induction by RSV.
In addition, autophagy was induced by RSV treatment
in Ishikawa cells, results which were concordant with previous
findings in ovarian and cervical cancer cells (21,23). To
our knowledge, this is the first report of RSV-mediated autophagy
in endometrial cancer cells. Activation of either AMPK or ERK has
also been reported to induce autophagy (29,42). AMPK
Activation inhibits the mammalian target of the rapamycin (mTOR)
signaling pathway, which is frequently activated via phosphatase
and tensin homolog mutations in endometrial cancers, including
Ishikawa cells (43,44). As activation of mTOR signaling is
associated with autophagy inhibition (45), AMPK activation by RSV may counteract
mTOR-dependent autophagy inhibition (thereby promoting autophagy)
in Ishikawa cells. ERK activation is also associated with autophagy
induction, as well as cell proliferation (29). Although the effect of RSV-mediated
autophagy on cancer cells is thought to be cancer-type specific
(i.e., tumor suppressive in glioma and esophageal cancer (46–48), or
tumor-promoting in ovarian and cervical cancer cells (21,23), the
results of the present study suggest that RSV-mediated autophagy
may serve a protective role against apoptosis in endometrial cancer
cells.
Finally, autophagy inhibition by CQ augmented
RSV-induced apoptosis in Ishikawa cells. Moreover, specific
autophagy inhibition by siRNAs against either ATG5 or
ATG7 significantly enhanced apoptotic cell death by RSV. We
previously reported that CQ treatment alone caused apoptosis in
endometrial cancer cells (36). The
results indicate that combined RSV and CQ treatment may be a
promising therapeutic strategy through autophagy inhibition and
apoptosis induction.
This study has several limitations. The precise
mechanism underlying RSV-induced apoptosis and autophagy remains
unclear. Autophagy induction may also be mediated by other factors
that are independent of AMPK and ERK signaling. Biomarkers for
predicting sensitivity to RSV or combined treatment (RSV+CQ) should
be identified for clinical applications. In addition, the safety
and efficacy of combination RSV and CQ therapy should be examined
in in vivo studies.
In conclusion, the results of the present study
revealed that RSV increased apoptosis, and that RSV-mediated
autophagy rendered its apoptotic function in Ishikawa cells.
Combined autophagy inhibition with RSV treatment significantly
augmented apoptosis. Considering that CQ is widely used in clinical
settings, combination RSV/CQ therapy may be a viable option for
treating endometrial cancer.
Acknowledgements
We thank Dr. Chinami Makii and Ms. Otoe Hagiwara for
their support and assistance. We also thank Dr. Masato Nishida for
generously providing the Ishikawa cells. This work was financially
supported by a Grant-in-Aid for Scientific Research (grant no.
26462515); by Grants-in-Aid for Young Scientific Research (grant
no. 25893229 and 25861471) from the Ministry of Education, Culture,
Sports, Science, and Technology of Japan; and by a research program
of the Project for Development of Innovative Research on Cancer
Therapeutics (P-Direct) from the Ministry of Education, Culture,
Sports, Science, and Technology of Japan (grant no. 11114014). We
would also like to thank Editage (www.editage.com) for English language editing.
Glossary
Abbreviations
Abbreviations:
|
AMPK
|
AMP-activated protein kinase
|
|
ATG
|
autophagy-related gene
|
|
CQ
|
chloroquine
|
|
DMEM
|
Dulbecco's modified Eagle's medium
|
|
FITC
|
fluorescein isothiocyanate
|
|
IC50
|
half-maximal (50%) inhibitory
concentration
|
|
LC3
|
light chain 3
|
|
MTT
|
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
|
|
PBS
|
phosphate-buffered saline
|
|
RSV
|
resveratrol
|
|
siRNA
|
small interfering RNA
|
References
|
1
|
SGO Clinical Practice Endometrial Cancer
Working Group, . Burke WM, Orr J, Leitao M, Salom E, Gehrig P,
Olawaiye AB, Brewer M, Boruta D, Villella J, et al: Endometrial
cancer: A review and current management strategies: Part I. Gynecol
Oncol. 134:385–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liao C, Zhang D, Mungo C, Tompkins DA and
Zeidan AM: Is diabetes mellitus associated with increased incidence
and disease-specific mortality in endometrial cancer? A systematic
review and meta-analysis of cohort studies. Gynecol Oncol.
135:163–171. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Renehan AG, Tyson M, Egger M, Heller RF
and Zwahlen M: Body-mass index and incidence of cancer: A
systematic review and meta-analysis of prospective observational
studies. Lancet. 371:569–578. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sivalingam VN, Myers J, Nicholas S, Balen
AH and Crosbie EJ: Metformin in reproductive health, pregnancy and
gynaecological cancer: Established and emerging indications. Hum
Reprod Update. 20:853–868. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Febbraro T, Lengyel E and Romero IL: Old
drug, new trick: Repurposing metformin for gynecologic cancers?
Gynecol Oncol. 135:614–621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jang M, Cai L, Udeani GO, Slowing KV,
Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta
RG, et al: Cancer chemopreventive activity of resveratrol, a
natural product derived from grapes. Science. 275:218–220. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pendurthi UR, Williams JT and Rao LV:
Resveratrol, a polyphenolic compound found in wine, inhibits tissue
factor expression in vascular cells: A possible mechanism for the
cardiovascular benefits associated with moderate consumption of
wine. Arterioscler Thromb Vasc Biol. 19:419–426. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Baur JA, Pearson KJ, Price NL, Jamieson
HA, Lerin C, Kalra A, Prabhu VV, Allard JS, Lopez-Lluch G, Lewis K,
et al: Resveratrol improves health and survival of mice on a
high-calorie diet. Nature. 444:337–342. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Singh CK, Ndiaye MA and Ahmad N:
Resveratrol and cancer: Challenges for clinical translation.
Biochim Biophys Acta. 1852:1178–1185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Carter LG, D'Orazio JA and Pearson KJ:
Resveratrol and cancer: Focus on in vivo evidence. Endocr Relat
Cancer. 21:R209–R225. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bhat KP and Pezzuto JM: Resveratrol
exhibits cytostatic and antiestrogenic properties with human
endometrial adenocarcinoma (Ishikawa) cells. Cancer Res.
61:6137–6144. 2001.PubMed/NCBI
|
|
12
|
Kaneuchi M, Sasaki M, Tanaka Y, Yamamoto
R, Sakuragi N and Dahiya R: Resveratrol suppresses growth of
Ishikawa cells through down-regulation of EGF. Int J Oncol.
23:1167–1172. 2003.PubMed/NCBI
|
|
13
|
Sexton E, Van Themsche C, LeBlanc K,
Parent S, Lemoine P and Asselin E: Resveratrol interferes with AKT
activity and triggers apoptosis in human uterine cancer cells. Mol
Cancer. 5:452006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gasparrini M, Giampieri F, Alvarez M,
Suarez J, Mazzoni L, Forbes Y, Hernandez T, Quiles LJ, Bullon P and
Battino M: AMPK as a new attractive therapeutic target for disease
prevention: The role of dietary compounds AMPK and Disease
Prevention. Curr Drug Targets. 17:865–889. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Faubert B, Vincent EE, Poffenberger MC and
Jones RG: The AMP-activated protein kinase (AMPK) and cancer: Many
faces of a metabolic regulator. Cancer Lett. 356:165–170. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Umene K, Banno K, Kisu I, Yanokura M,
Nogami Y, Tsuji K, Masuda K, Ueki A, Kobayashi Y, Yamagami W, et
al: New candidate therapeutic agents for endometrial cancer:
Potential for clinical practice (review). Oncol Rep. 29:855–860.
2013.PubMed/NCBI
|
|
17
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend Saccharomyces
cerevisiae lifespan. Nature. 425:191–196. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Luo J, Nikolaev AY, Imai S, Chen D, Su F,
Shiloh A, Guarente L and Gu W: Negative control of p53 by Sir2alpha
promotes cell survival under stress. Cell. 107:137–148. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Roth M and Chen WY: Sorting out functions
of sirtuins in cancer. Oncogene. 33:1609–1620. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Borriello A, Bencivenga D, Caldarelli I,
Tramontano A, Borgia A, Pirozzi AV, Oliva A and Ragione F Della:
Resveratrol and cancer treatment: Is hormesis a yet unsolved
matter? Curr Pharm Des. 19:5384–5393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Opipari AW Jr, Tan L, Boitano AE, Sorenson
DR, Aurora A and Liu JR: Resveratrol-induced autophagocytosis in
ovarian cancer cells. Cancer Res. 64:696–703. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Trincheri NF, Follo C, Nicotra G,
Peracchio C, Castino R and Isidoro C: Resveratrol-induced apoptosis
depends on the lipid kinase activity of Vps34 and on the formation
of autophagolysosomes. Carcinogenesis. 29:381–389. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hsu KF, Wu CL, Huang SC, Wu CM, Hsiao JR,
Yo YT, Chen YH, Shiau AL and Chou CY: Cathepsin L mediates
resveratrol-induced autophagy and apoptotic cell death in cervical
cancer cells. Autophagy. 5:451–460. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Puissant A, Robert G, Fenouille N, Luciano
F, Cassuto JP, Raynaud S and Auberger P: Resveratrol promotes
autophagic cell death in chronic myelogenous leukemia cells via
JNK-mediated p62/SQSTM1 expression and AMPK activation. Cancer Res.
70:1042–1052. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
In K, Park J and Park H: Resveratrol at
high doses acts as an apoptotic inducer in endothelial cells.
Cancer Res Treat. 38:48–53. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mizushima N and Komatsu M: Autophagy:
Renovation of cells and tissues. Cell. 147:728–741. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo JY, Xia B and White E:
Autophagy-mediated tumor promotion. Cell. 155:1216–1219. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pineda CT, Ramanathan S, Tacer K Fon, Weon
JL, Potts MB, Ou YH, White MA and Potts PR: Degradation of AMPK by
a cancer-specific ubiquitin ligase. Cell. 160:715–728. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Corcelle E, Djerbi N, Mari M, Nebout M,
Fiorini C, Fénichel P, Hofman P, Poujeol P and Mograbi B: Control
of the autophagy maturation step by the MAPK ERK and p38: Lessons
from environmental carcinogens. Autophagy. 3:57–59. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Solomon VR and Lee H: Chloroquine and its
analogs: A new promise of an old drug for effective and safe cancer
therapies. Eur J Pharmacol. 625:220–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
van der Heijden JW, Dijkmans BA, Scheper
RJ and Jansen G: Drug insight: Resistance to methotrexate and other
disease-modifying antirheumatic drugs-from bench to bedside. Nat
Clin Pract Rheumatol. 3:26–34. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lee SJ, Silverman E and Bargman JM: The
role of antimalarial agents in the treatment of SLE and lupus
nephritis. Nat Rev Nephrol. 7:718–729. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Brito-Zeron P, Sisó-Almirall A, Bové A,
Kostov BA and Ramos-Casals M: Primary Sjögren syndrome: An update
on current pharmacotherapy options and future directions. Expert
Opin Pharmacother. 14:279–289. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang ZJ, Chee CE, Huang S and Sinicrope
FA: The role of autophagy in cancer: Therapeutic implications. Mol
Cancer Ther. 10:1533–1541. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Amaravadi RK, Lippincott-Schwartz J, Yin
XM, Weiss WA, Takebe N, Timmer W, DiPaola RS, Lotze MT and White E:
Principles and current strategies for targeting autophagy for
cancer treatment. Clin Cancer Res. 17:654–666. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fukuda T, Oda K, Wada-Hiraike O, Sone K,
Inaba K, Ikeda Y, Miyasaka A, Kashiyama T, Tanikawa M, Arimoto T,
et al: The anti-malarial chloroquine suppresses proliferation and
overcomes cisplatin resistance of endometrial cancer cells via
autophagy inhibition. Gynecol Oncol. 137:538–545. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Su D, Cheng Y, Liu M, Liu D, Cui H, Zhang
B, Zhou S, Yang T and Mei Q: Comparison of piceid and resveratrol
in antioxidation and antiproliferation activities in vitro. PLoS
One. 8:e545052013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wu ML, Li H, Yu LJ, Chen XY, Kong QY, Song
X, Shu XH and Liu J: Short-term resveratrol exposure causes in
vitro and in vivo growth inhibition and apoptosis of bladder cancer
cells. PLoS One. 9:e898062014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zhao XY, Yang S, Chen YR, Li PC, Dou MM
and Zhang J: Resveratrol and arsenic trioxide act synergistically
to kill tumor cells in vitro and in vivo. PLoS One. 9:e989252014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen S, Zhou N, Zhang Z, Li W and Zhu W:
Resveratrol induces cell apoptosis in adipocytes via AMPK
activation. Biochem Biophys Res Commun. 457:608–613. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Yang Q, Wang B, Zang W, Wang X, Liu Z, Li
W and Jia J: Resveratrol inhibits the growth of gastric cancer by
inducing G1 phase arrest and senescence in a Sirt1-dependent
manner. PLoS One. 8:e706272013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Samari HR and Seglen PO: Inhibition of
hepatocytic autophagy by adenosine, aminoimidazole-4-carboxamide
riboside, and N6-mercaptopurine riboside. Evidence for involvement
of amp-activated protein kinase. J Biol Chem. 273:23758–23763.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Miyasaka A, Oda K, Ikeda Y, Wada-Hiraike
O, Kashiyama T, Enomoto A, Hosoya N, Koso T, Fukuda T, Inaba K, et
al: Anti-tumor activity of olaparib, a poly (ADP-ribose) polymerase
(PARP) inhibitor, in cultured endometrial carcinoma cells. BMC
Cancer. 14:1792014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oda K, Ikeda Y, Kawana K, Osuga Y and
Fujii T: mTOR signaling in endometrial cancer: From a molecular and
therapeutic point of view. Curr Obstet Gynecol Rep. 4:1–10. 2015.
View Article : Google Scholar
|
|
45
|
Hung CM, Garcia-Haro L, Sparks CA and
Guertin DA: mTOR-dependent cell survival mechanisms. Cold Spring
Harb Perspect Biol. 4(pii): a0087712012.PubMed/NCBI
|
|
46
|
Li J, Qin Z and Liang Z: The prosurvival
role of autophagy in resveratrol-induced cytotoxicity in human U251
glioma cells. BMC Cancer. 9:2152009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Filippi-Chiela EC, Villodre ES, Zamin LL
and Lenz G: Autophagy interplay with apoptosis and cell cycle
regulation in the growth inhibiting effect of resveratrol in glioma
cells. PLoS One. 6:e208492011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Tang Q, Li G, Wei X, Zhang J, Chiu JF,
Hasenmayer D, Zhang D and Zhang H: Resveratrol-induced apoptosis is
enhanced by inhibition of autophagy in esophageal squamous cell
carcinoma. Cancer Lett. 336:325–337. 2013. View Article : Google Scholar : PubMed/NCBI
|