Introduction
Prostate cancer is the second most prevalent cancer
in the United States of America, and also the second leading cause
of mortality in the western world (1). Approximately 25% of all diagnoses of
cancer in the male population of the United States of America are
prostate cancer (2). Due to the
increasing risk of prostate cancer over the previous decade,
studies associating lifestyle with prostate cancer risk have become
increasingly prevalent (1). However,
the primary etiology of prostate cancer remains obscure, as no
specific carcinogen is known to be responsible for this disease
(2–4).
Epidemiological studies suggest that certain risk factors,
including aging, Afro-American ethnicity and positive family
history are associated with the likelihood of developing prostate
cancer (5). However, according to
several studies, genetic factors are not the sole etiology of
prostate cancer; it is also associated with lifestyle, dietary and
environmental factors (5–7).
The majority of prostate cancer-associated
mortalities are due to the acquisition of the metastatic phenotype
of the disease, and the epithelial to mesenchymal transition (EMT)
is known to serve a pivotal role in tumor metastasis (8). EMT is a series of coordinated events,
during which cancer cells acquire enhanced migratory and invasive
properties, may invade through the basement membrane and survive in
systemic circulation due to the resistance to apoptosis (8–10). This
EMT is accompanied by the extravasation at distant organ sites,
followed by the adhesion of cancer cells to extracellular matrix
proteins, including fibronectin, which finally leads to tumor
metastasis (11,12). Downregulation of the gap junction
protein Epithelial (E)-cadherin, which serves an important role in
cell-to-cell adhesion, and the upregulation of the mesenchymal
proteins Neural (N)-cadherin, vimentin, Twist-related protein 1 are
known to be the hallmark events in the prostate cancer-associated
EMT process (8,10,13).
Previous studies suggested that EMT serves a crucial role in the
development and maintenance of stemness in prostate carcinoma
(14–16), and is also responsible for the
resistance to therapeutic drugs (17).
Flavonoids are naturally occurring polyphenols,
which constitute a major part of the human diet, and are abundantly
present in fruits, grains, vegetables and traditional medicinal
herbs (18,19). Almost all of the chemically
synthesized drugs currently used in cancer therapy exhibit high
toxicity to normal cells (20), but
the naturally occurring flavonoids have demonstrated selective
cytotoxicity to human cancer cells, with minimum toxicity to the
normal cells (19). In an attempt to
identify improved chemopreventive or chemotherapeutic agents,
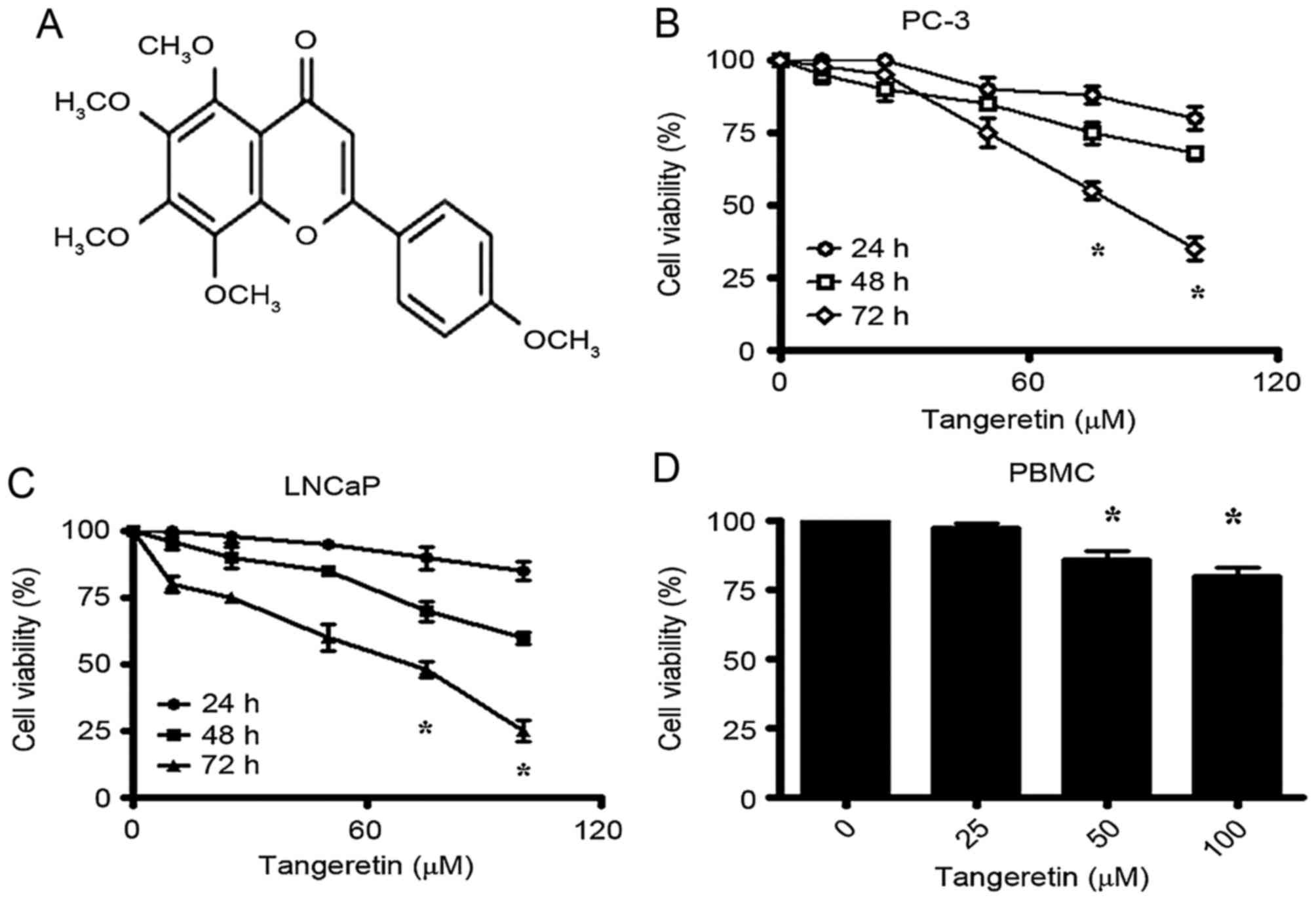
tangeretin, a 4′,5,6,7,8-pentamethoxyflavone (Fig. 1A) that is abundant in the peel of
citrus fruits (21), was selected.
The tumor suppressive role of tangeretin is well documented, and it
has been suggested to inhibit the growth and progression of several
types of cancer cells such as ovarian cancer, colorectal, gastric
and breast cancer (21–25). However; there are no studies
investigating tangeretin in prostate cancer, to the best of our
knowledge. Taking into consideration the potent anticancer property
of tangeretin, the present study investigated the effect of this
dietary flavonoid on prostate cancer PC-3 cells.
Materials and methods
Materials
Tangeretin was purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Dulbecco's modified Eagle's medium
(DMEM; supplemented with 1 mM L-glutamine), fetal bovine serum,
penicillin-streptomycin and 0.25% Trypsin-EDTA were purchased from
Invitrogen (Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Primary antibodies including rabbit polyclonal anti-B-cell lymphoma
2 (Bcl-2)-associated X protein (Bax) antibody (cat. no., sc-493),
and mouse monoclonal anti- Bcl-2 antibody (cat. no., sc-7382), were
purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA),
and rabbit polyclonal anti-cleaved caspase-3 (cat. no., 9661),
rabbit monoclonal cleaved anti-caspase-9 (cat. no., 7237), rabbit
monoclonal anti-phosphorylated (p)-protein kinase B (pAkt; cat.
no., 4060), rabbit monoclonal anti-Akt (cat. no., 4691), rabbit
monoclonal anti-p-mammalian target of rapamycin (pmTOR; cat. no.,
5536) and rabbit monoclonal anti-mTOR (cat. no., 2983) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The single-stranded DNA (ssDNA) Apoptosis ELISA Kit (cat. no.,
APT225) was purchased from EMD Millipore (Billerica, MA, USA).
Cell culture and maintenance
The prostate cancer PC-3 and LNCaP cell lines were
purchased from American Type Culture Collection (Manassas, VA, USA)
and routinely maintained in DMEM supplemented with 10% FBS and 100
U/ml penicillin and 100 µg/ml streptomycin, at 37°C in a humidified
chamber.
Isolation and maintenance of human
peripheral blood mononuclear cells (PBMC)
Human PBMC were isolated from the whole blood of
adult healthy donors using the density gradient Ficoll-Hypaque
(Histopaque 1077, Sigma Aldrich; Merck KGaA) method. Whole blood
collected from the donor was carefully mixed with equal volume of
Ficoll-Hypaque and centrifuged at 400 × g for 30 min at room
temperature. The PBMC were collected from the plasma/Ficoll-Hypaque
interphase, washed in PBS (twice for 30 min) and resuspended in
RPMI-1640 complete medium (Invitrogen; Thermo Fisher Scientific,
Inc.) supplemented with 10% FBS. The present study was approved by
the Ethical Committee Board of Linyi People's Hospital (Linyi,
China). Donors provided written informed consent to the inclusion
of their samples in the present study.
Cell viability assay (MTT assay)
Cultured prostate cancer cells (1×104
cells/ml) were treated with different concentrations of tangeretin
(0, 25, 50 µM) for 24, 48 and 72 h. Following treatment, MTT
solution (0.5 mg/ml; Thermo Fisher Scientific, Inc.) was added to
each well followed by 100 µl of isopropanol (10%)/PBS and the
resultant purple-blue formazan complex was measured using a Varian
Cary 50MPR microplate reader (Akribis Scientific, Knutsford, UK) at
an absorbance 570 nm, as described previously (26).
Apoptosis assay
Induction of apoptosis by tangeretin in prostate
cancer cells was determined by ELISA-based apoptosis detection kit
(EMD Millipore) following previously described protocol (27). Cultured prostate cancer cells
(1×104 cells/ml) were treated with different
concentrations of tangeretin (0, 50 and 75 µM) for 72 h, and
apoptosis was determined according to the manufacturer's protocol.
Results were indicative to the absorbance recorded at 405 nm using
s Varian Cary 50MPR microplate reader.
Hoechst 33258 staining of the
apoptotic nuclei
Cultured prostate cancer cells (1×104
cells/ml) were treated with tangeretin (0, 50 and 75 µM) for 72 h
and following treatment, cells were fixed in 4% paraformaldehyde
and stained with 20 µM Hoechst 33258 for 20 min. Cells were then
observed and images (NIS imaging system software 4.5 ver) were
captured at a magnification of ×100 using an inverted fluorescence
microscope.
Anchorage-dependent and -independent
colony formation assay
The anchorage-dependent growth properties of PC-3
cells were evaluated by their ability to form viable colonies.
Cultured PC-3 cells (1×104 cells/ml) were treated with
tangeretin (0, 50 and 75 µM) for 72 h. Following treatment, single
cell suspensions were prepared from the control and treated groups,
and cells were finally seeded at a density of ~500 cells/ml. Cells
were cultured for 7 days and the viable colonies were stained for
10 h with 0.5 mg/ml crystal violet at 37°C. Colony forming
efficiency (CFE) was determined by re-suspending the crystal violet
stained cells in 10% acetic acid solution and measuring the
absorbance at 600 nm.
Anchorage-independent growth was assessed by seeding
the control and tangeretin-treated cells on soft agar (0.4% top
layer, 0.8% bottom layer). Cultured PC-3 cells (1×104
cells/ml) were treated with tangeretin (0, 50 and 75 µM) for 72 h.
Following treatment, single cell suspension was prepared from the
control and treated groups, and finally seeded on soft agar-coated
96-well plates, following previously described protocol (28). The colonies were counted after 14 days
using an inverted microscope (magnification, ×200).
Wound healing assay
The migratory activities of PC-3 cells were
determined by a wound-healing assay. Cultured PC-3 cells were grown
to ~80% confluency, and a wound was created with a sterile plastic
pipette tip. Cells were then allowed to migrate for 48 h in the
absence and presence of 75 µM tangeretin, and images were captured
using a phase contrast microscope (magnification, ×100).
Determination of invasion by Boyden
Chamber Assay
The invasive properties of PC-3 cells were
determined by a Boyden chamber assay, using
Matrigel®-coated invasion chambers (BD Biosciences,
Franklin Lakes, NJ, USA) (29). PC-3
cells were treated with 75 µM tangeretin for 72 h, and
1×103 cells were loaded in the upper part of the Boyden
chamber. Cells that invaded into the lower surface of the membrane
were stained with 0.5 mg/ml crystal violet for 6 h and the
resultant crystal violet complex was then dissolved in 10% acetic
acid. Finally, the absorbance was measured at 600 nm, using the
Varian Cary 50MPR microplate reader to determine the extent of
invasion.
Quantitative reverse transcription
polymerase chain reaction (RT-qPCR)
Total RNA from the control and tangeretin-treated
PC-3 cells were isolated using TRIzol reagent (Invitrogen; Thermo
Fisher Scientific, Inc.) according to the manufacturers' protocol.
Reverse transcription of the extracted RNA to corresponding
complementary DNA was performed using a commercially available kit
(Takara Bio, Inc., Otsu, Japan). RT-qPCR was performed with
QuantiTech SYBR® Green PCR Kit (Qiagen GmbH, Hilden,
Germany) according to the manufacturer's protocol, as previously
described (30). A list of the used
primers for all genes are in Table I.
The reaction parameters selected were: 95°C for 5 min, and the 40
cycles of 95°C for 10 sec and 60°C for 30 sec. The GAPDH
gene was used as the endogenous control. Relative quantification
values for each sample were determined using the
2−(ΔΔCq) method (30).
 | Table I.List of genes, with their primer
sequences, used for quantitative reverse transcription polymerase
chain reaction. |
Table I.
List of genes, with their primer
sequences, used for quantitative reverse transcription polymerase
chain reaction.
| Gene | Primer sequence
(5′-3′) |
|---|
| Vimentin |
|
|
Forward |
AACTTAGGGGCGCTCTTGTC |
|
Reverse | CCTGCTGTCCCGCCG |
| CD44 |
|
|
Forward |
CCCAGATGGAGAAAGCTCTG |
|
Reverse |
GTTGTTTGCTGCACAGATGG |
| N-cadherin |
|
|
Forward |
CCTTTCACTGCGGATACGTG |
|
Reverse |
GATCCAGGGGCTTTGTCACC |
| E-cadherin |
|
|
Forward |
TGAGTGTCCCCCGGTATCTT |
|
Reverse |
GAATCATAAGGCGGGGCTGT |
| Cytokeratin |
|
|
Forward |
CGGGGCCTCACTCTGCGATATAA |
|
Reverse |
GCGAGTGGTGAAGCTCATGC |
Western blot analysis
Cultured PC-3 cells were treated with tangeretin (0,
50 and 75 µM) for 72 h, washed with PBS and incubated with RIPA
lysis buffer (Sigma-Aldrich; Merck KGaA) for 2 h at 37°C. The
resultant cell lysates were centrifuged at 3,000 × g for 10 min at
37°C and total cellular protein was calculated using a BCA Protein
Assay Reagent kit (BioVision, Inc., Milpitas, CA, USA). Equal
quantities of protein (50 µg/lane) was separated by 10% SDS-PAGE,
and then electrotransferred onto a nitrocellulose membrane by a
semi-dry blotting system (GE Healthcare, Little Chalfont, UK). The
membrane was blocked with Tris-buffered saline (TBS) containing
Tween-20 and 5% skimmed milk and probed with the following primary
antibodies: Rabbit polyclonal anti-B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax) (cat. no., sc493; 1:1,200)
antibody, mouse monoclonal anti- Bcl-2 antibody (cat. no., sc-7382;
1:1,000) (Santa Cruz Biotechnology, Inc., Dallas, TX, USA) and
rabbit polyclonal anti-cleaved caspase-3, rabbit monoclonal cleaved
anti-caspase-9, rabbit monoclonal anti-phosphorylated (p)-protein
kinase B (pAkt) (cat. no., 4060; 1:1,000), rabbit monoclonal
anti-Akt (cat. no., 4691; 1:800), rabbit monoclonal
anti-p-mammalian target of rapamycin (pmTOR) (cat. no., 5536;
1:1,000) and rabbit monoclonal anti-mTOR (cat. no., 2983; 1:1,200)
as well as mouse monoclonal anti-rat β-actin antibody (cat. no.,
5723; 1:1,500) (Cell Signaling Technology, Inc., Danvers, MA, USA)
at 4°C for 10 h. Subsequently, samples were incubated with goat
anti-rabbit (cat. no., sc-2979; dilution, 1:10,000) and anti-mouse
(cat. no., sc-358914; dilution, 1:10,000) secondary antibodies
conjugated to horseradish peroxidase-conjugated (Santa Cruz
Biotechnology, Inc.) secondary antibody in TBS at room temperature
for 1 h and washed with TBS. The bound antibodies were visualized
using an Enhanced Chemiluminescence system (Bio-Rad Laboratories,
Inc., Hercules, CA, USA) and densitometry analysis was then
performed (ChemiDoc-17001401; Image Lab-5.2.1; Bio-Rad
Laboratories, Inc.).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Statistically significant differences between groups
were determined by using the paired Student's two-tailed t-test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Tangeretin induces loss of cell
viability in prostate cancer cells, with negligible toxicity
towards normal cells
The effect of tangeretin treatment on the viability
of androgen-insensitive PC-3 and androgen-sensitive LNCaP cells was
evaluated by MTT assay. Tangeretin induces a significant reduction
of cell viability in PC-3 cells in a time- and dose-dependent
manner (Fig. 1B). The IC50
for PC-3 cells was obtained following 72 h of incubation at 75 µM
dose of tangeretin. The androgen-sensitive LNCaP cells were
identified to exhibit a slightly higher sensitivity to tangeretin
treatment, with the IC50 obtained around 65 µM following
72 h of treatment (Fig. 1C).
To evaluate the toxicity of tangeretin on normal
cells, the viability of human PBMC in the presence of tangeretin
was determined. Following treatment for 72 h, it was observed that
tangeretin exhibited negligible cytotoxicity on PBMC compared with
the cancer cells, and in the presence of 100 µM tangeretin, the
cell viability was decreased by only 20% (Fig. 1D).
Tangeretin induces apoptosis in
prostate cancer cells with the modulation of pro- and
anti-apoptotic markers
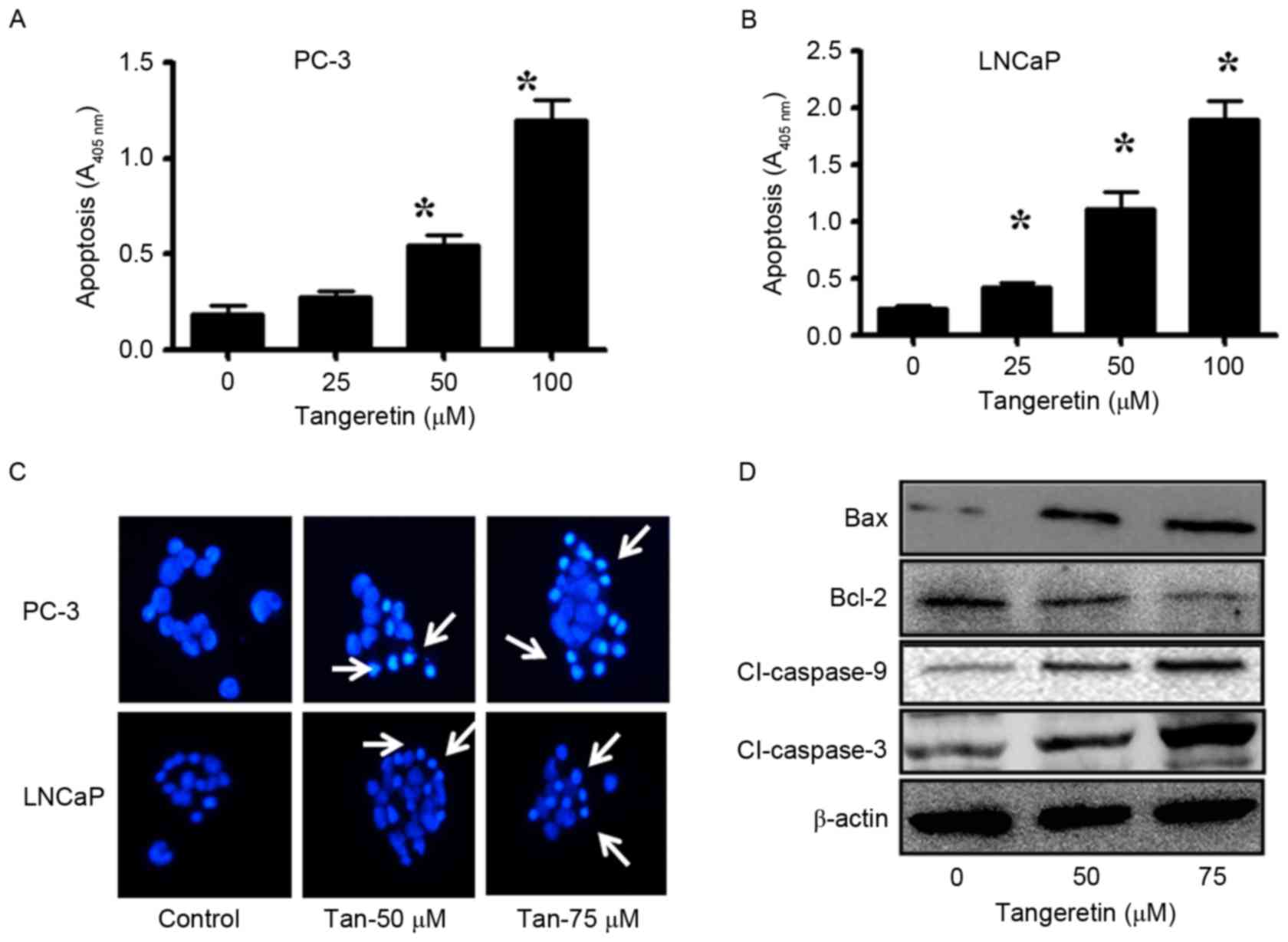
To determine the mode of cell death induced by
tangeretin, the apoptosis assay was performed with
tangeretin-treated PC-3 and LNCaP cells. The induction of apoptosis
was determined using the ssDNA Apoptosis ELISA kit. Tangeretin
treatment resulted in a dose-dependent induction of apoptosis in
PC-3 cells (Fig. 2A). At a 50 µM dose
of tangeretin, apoptosis was increased ~3-fold (P<0.05), while
in the presence of 75 µM tangeretin, apoptosis was increased
6.4-fold (P<0.05) compared with the control. Similarly, in LNCaP
cells, treatment with 50 µM tangeretin resulted in a 5.5-fold
increase in apoptosis (P<0.05), while in the presence of 75 µM
tangeretin, apoptosis was increased by 7.5-fold compared with the
control (P<0.05; Fig. 2B).
Furthermore, in PC-3 and LNCaP cells, tangeretin treatment resulted
in nuclear shrinkage, chromatin condensation and the formation of
apoptotic bodies, as observed by Hoechst 33258 staining (Fig. 2C).
Subsequent to confirmation of the involvement of
apoptosis in tangeretin-mediated cell death, the status of several
anti- and pro-apoptotic markers was also investigated. The
pro-apoptotic markers such as Bax, caspase-9 and caspase-3 were
upregulated, and the anti-apoptotic Bcl-2 was downregulated in
tangeretin-treated PC-3 cells (Fig.
2D).
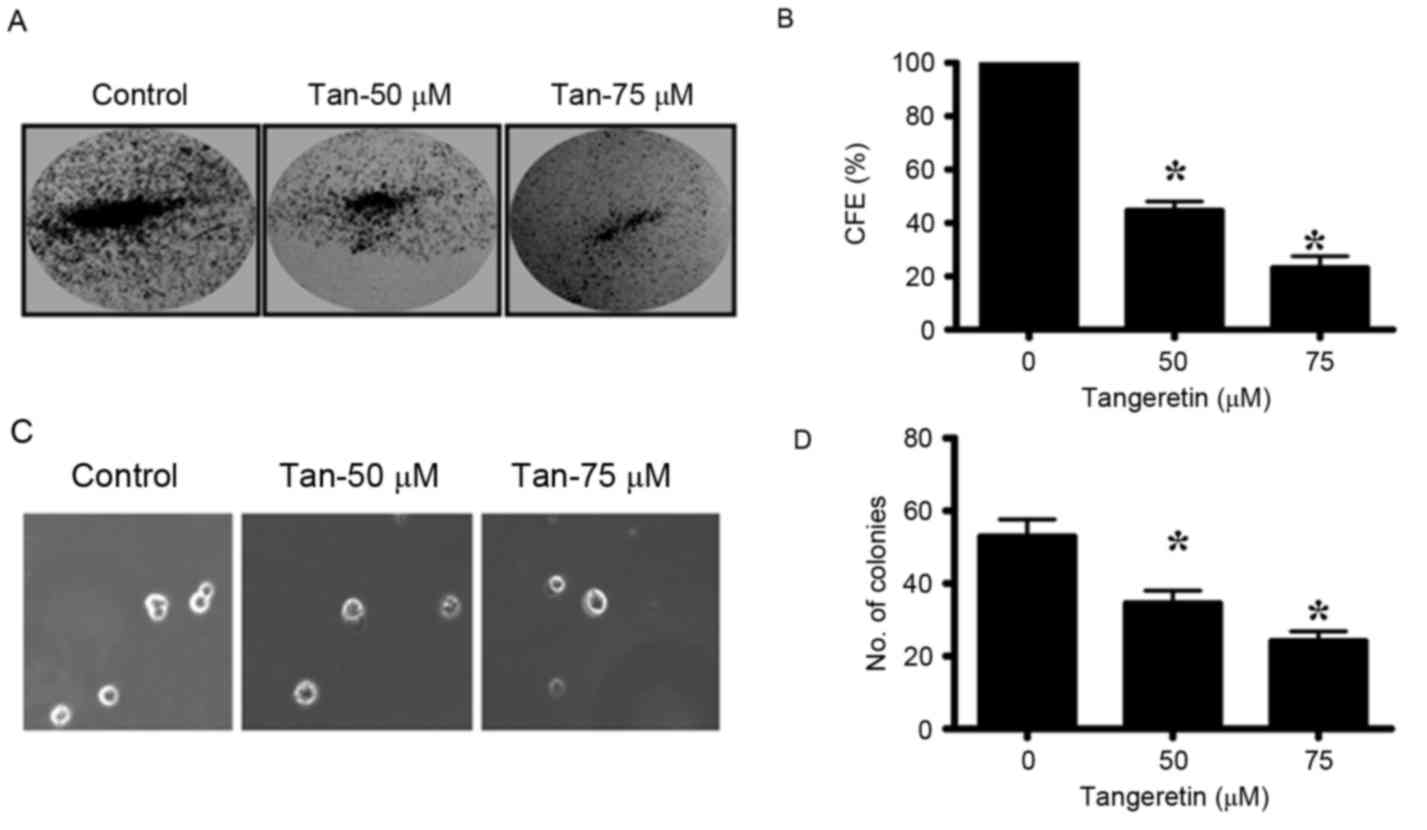
Tangeretin inhibits colony-forming
ability and anchorage-independent growth properties of PC-3
cells
The colony-forming ability of PC-3 cells was
markedly inhibited by tangeretin in a dose-dependent manner. PC-3
cells were treated with different doses of tangeretin (0–75 µM) for
72 h and the residual cells were collected. Equal numbers of cells
from control and treatment groups were then seeded to observe the
colony formation and anchorage independent growth. The CFE was
determined by crystal violet staining of the viable colonies. It
was observed that tangeretin significantly inhibited the
colony-forming ability of PC-3 cells in a dose-dependent manner
(P<0.05; Fig. 3A and B). In the
presence of 50 µM tangeretin, the CFE was reduced by 45%, while at
75 µM tangeretin the CFE is reduced by 23%. Therefore, these
results indicate that tangeretin inhibits the clonal growth in PC-3
cells.
The ability of the cancer cells to form colonies on
soft agar due to their ability of anchorage-independent growth, is
considered to be the hallmark of tumorigenesis. The untreated PC-3
cells, when seeded in the soft agar, formed several viable
colonies. However, in the tangeretin-treated groups, the number of
viable colonies was revealed to be significantly reduced
(P<0.05; Fig. 3C and D).
Therefore, it may be concluded that tangeretin significantly
inhibits the anchorage-independent growth potential of PC-3
cells.
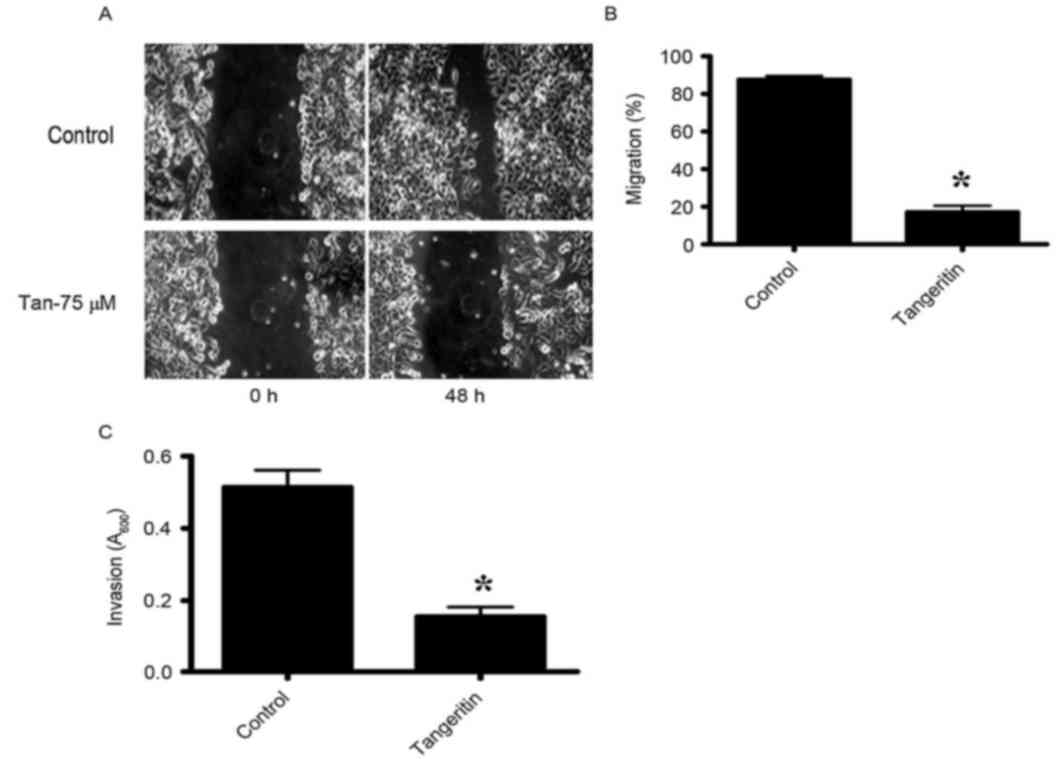
Tangeretin inhibits the motility of
PC-3 cells
The migratory activities of PC-3 cells were
identified to be markedly inhibited by tangeretin as determined by
the wound-healing assay (Fig. 4A and
B). The control PC-3 cells covered ~80% of the wound following
48 h of incubation. However, in the presence of 75 µM tangeretin,
PC-3 cells completely failed to migrate. To additionally determine
whether tangeretin may perturb the invasive properties of PC-3
cells, a matrigel invasion assay was performed using a Boyden
Chamber assay. As hypothesized, the invasive properties of PC-3
cells were identified to be markedly inhibited in the presence of
75 µM tangeretin (Fig. 4C).
Therefore, the results clearly indicated that tangeretin reduced
the migratory or invasive phenotype of PC-3 cells.
Tangeretin induces reprogramming of
epithelial to mesenchymal transition in PC-3 cells
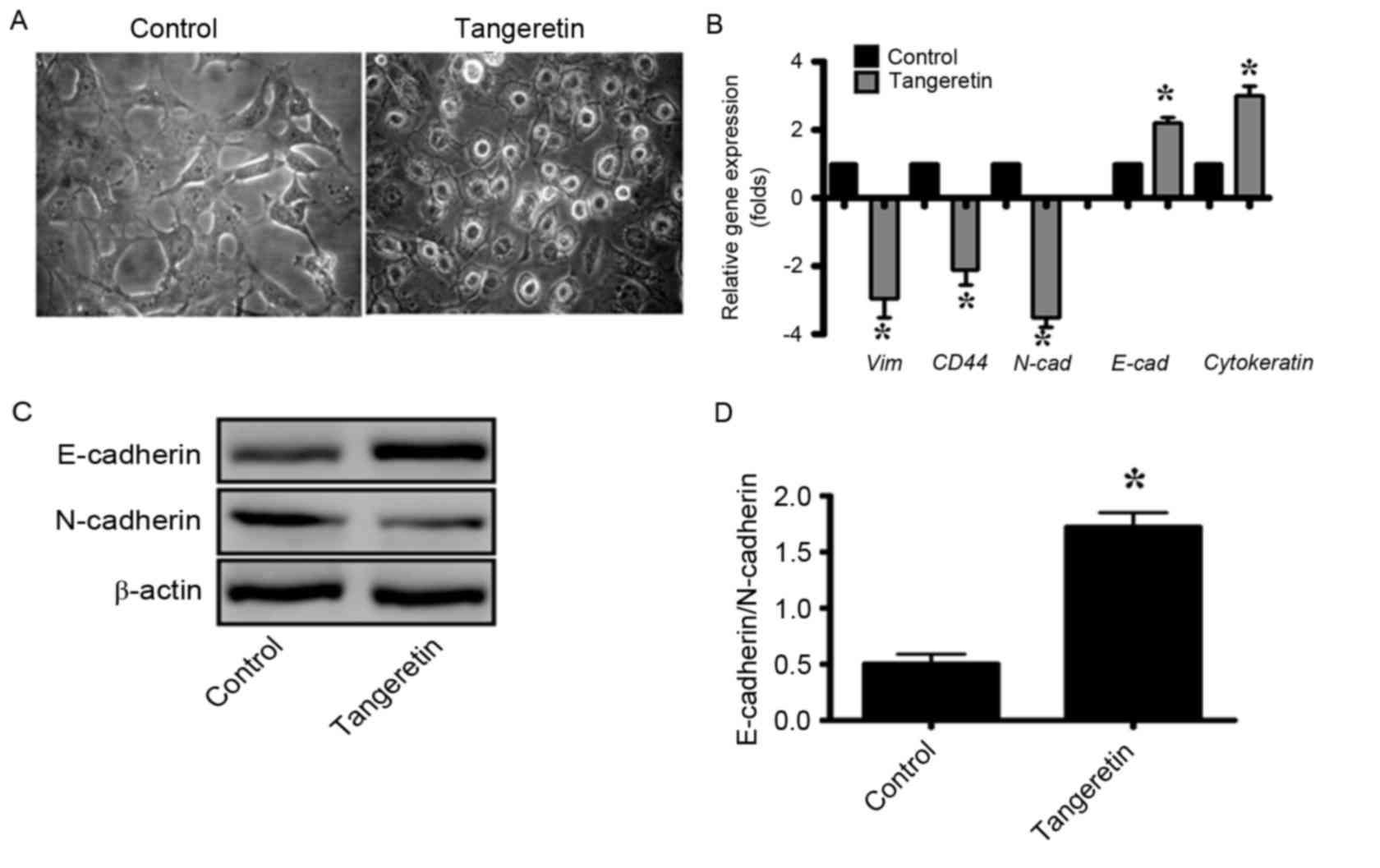
It was observed that subsequent to treatment with
tangeretin, the morphology of PC-3 cells was significantly altered,
and the treated cells exhibited an increased epithelial-like
morphology compared with the mesenchymal morphology of the control
cells (Fig. 5A). As EMT serves an
important role in the progression and metastasis of prostate
cancer, the gene expression of several EMT markers was determined
by RT-qPCR analysis (Fig. 5B). It was
observed that the expression levels of the genes encoding the
mesenchymal proteins vimentin, cluster of differentiation (CD)44
and N-cadherin were decreased by 3-, 2- and 3.5-fold, respectively
whereas that of the epithelial markers such as E-cadherin and
cytokeratin-19 were increased by 2.2- and 3-fold, respectively, in
tangeretin-treated cells (as compared with the control; Fig. 5B). In addition, the western blot
analysis revealed that the E-cadherin/N-cadherin ratio was
significantly increased in tangeretin-treated cells compared with
the control (P<0.05; Fig. 5C and
D).
Tangeretin targets Akt/mTOR pathway in
PC-3 cells
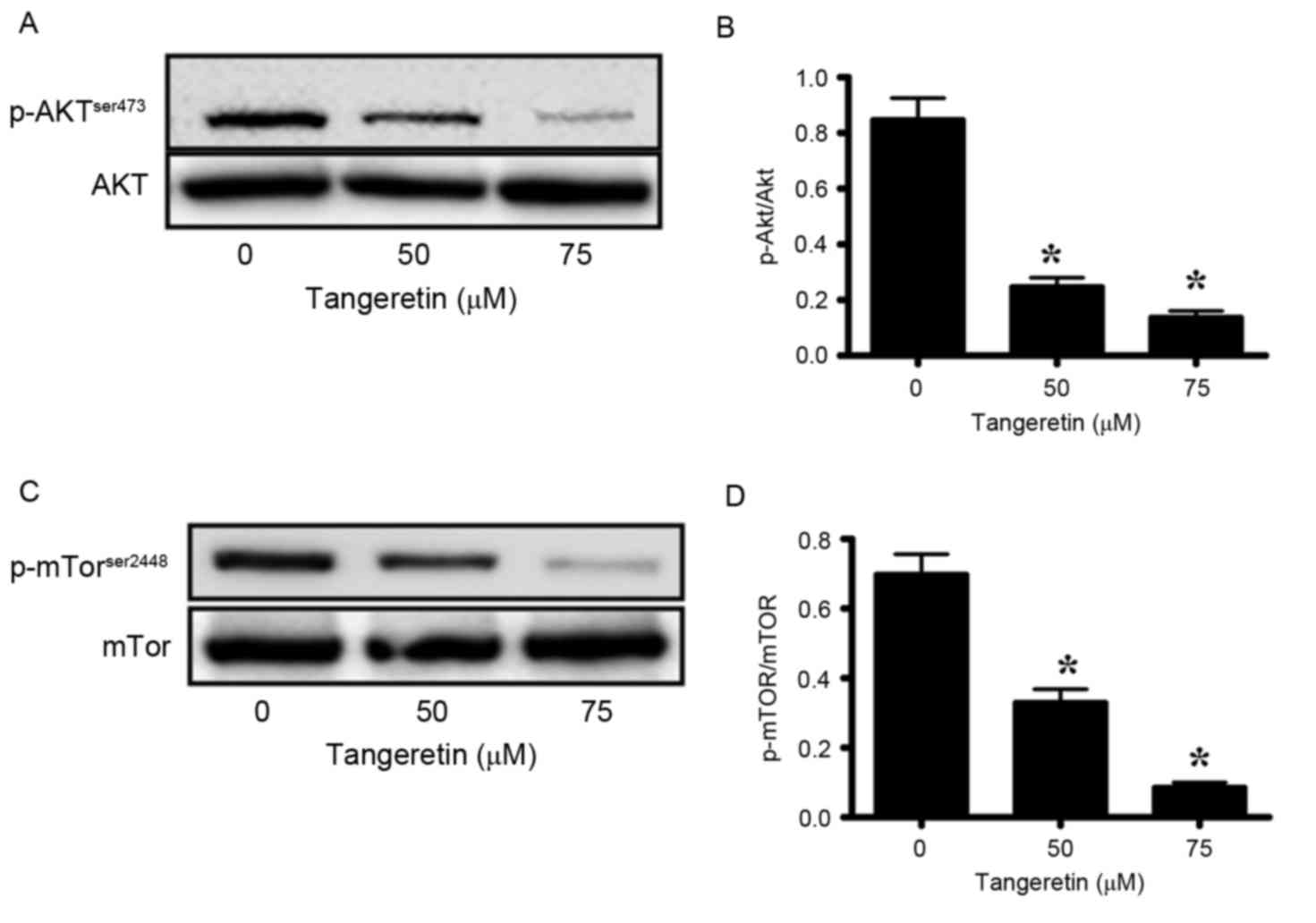
Akt-signaling serves an important role in the
maintenance of the tumor phenotype in prostate cancer (31,32).
Therefore, the present study investigated the phosphorylation of
Akt, and its downstream modulator mTOR, in tangeretin-treated PC-3
cells. Tangeretin significantly decreased the phosphorylation
levels of Akt in a dose-dependent manner (P<0.05; Fig. 6A and B). In the presence of 50 and 75
µM tangeretin, pAkt levels were reduced by 64 and 82%, respectively
when compared with the control (Fig. 6A
and B). Similarly, the expression levels of p-mTOR were
significantly reduced by 49 and 85%, respectively (P<0.05;
Fig. 6C and D). These results
suggested that tangeretin efficiently inhibited Akt signaling in
PC-3 cells.
Discussion
A number of epidemiological studies have indicated
that, instead of a particular specific carcinogen, several factors
associated with lifestyle, dietary, and environmental factors may
serve as the etiology of prostate cancer (2,4–7). Due to the minimum toxicity towards
normal cells, and selective cytotoxicity against cancer cells,
naturally-occurring dietary flavonoids have gained importance as
anticancer therapeutics (18–20). In the present study, the anticancer
potential of tangeretin, a 4′,5,6,7,8-pentamethoxyflavone, against
prostate carcinoma was investigated. Although tangeretin has been
suggested to be effective against several types of cancer (22–25), its
role against prostate cancer has not been determined and requires
additional investigation.
The present study observed that treatment of the
prostate cancer PC-3 and LNCaP cell lines with tangeretin resulted
in dose-and time-dependent loss of cell viability, with negligible
cytotoxicity in PBMC. In addition, it was also observed that
tangeretin induces caspase-3-mediated apoptosis in prostate cancer
cells. The ability to form colonies by PC-3 cells under
anchorage-dependent and -independent conditions was also inhibited
by tangeretin in a dose-dependent manner. As hypothesized, it was
also observed that tangeretin treatment also inhibited the motility
of PC-3 cells, as revealed by the migration and invasion
assays.
EMT is an important pathophysiological process which
serves an important role in the metastasis of prostate cancer to
distant organs, and also in the maintenance of stemness (8,13,15,16). As
tangeretin induces a marked alteration to the morphology of PC-3
cells, the statuses of EMT markers in tangeretin-treated PC-3 cells
were investigated. The expression levels of the genes encoding the
mesenchymal proteins vimentin, CD-44 and N-cadherin were
significantly downregulated by tangeretin, whereas the epithelial
markers such as E-cadherin and cytokeratin-19 were significantly
upregulated. Additionally, the E-cadherin/N-cadherin ratio was
significantly upregulated by tangeretin treatment, indicating the
reversal of EMT in tangeretin-treated PC-3 cells.
Akt, a serine/threonine protein kinase, is a key
regulator of apoptosis, regulating the downstream signaling pathway
of apoptosis, whereas mTOR acts as a downstream effector for Akt
and regulates key processes such as cell growth and proliferation
and cell cycle progression (31,32).
Deregulation of this pathway in prostate cancer is well documented,
and it has been demonstrated that the phosphoinositide 3-kinase
(PI3K)/Akt/mTOR pathway is deregulated in 42% of localized disease
and 100% of advanced-stage carcinoma (33). Therefore, this signaling pathway may
be a potential drug target in the treatment of prostate cancer. In
the present study, it was observed that tangeretin treatment of
PC-3 cells resulted in a marked reduction of p-Akt levels, and also
p-mTOR levels were also decreased. However, the total Akt or mTOR
levels remained unaltered.
Therefore, the present study presented a novel
therapeutic approach to prostate cancer. The dietary flavonoid
tangeretin was identified to be effective against PC-3 cells.
Reprogramming of the EMT process, via downregulation of
PI3K/Akt/mTOR pathway serves as the primary mechanism of
tangeretin-induced cytotoxicity in PC-3 cells.
References
|
1
|
Shelke AR and Mohile SG: Treating prostate
cancer in elderly men: How does aging affect the outcome? Curr
Treat Options Oncol. 12:263–275. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Crawford ED: Understanding the
epidemiology, natural history, and key pathways involved in
prostate cancer. Urology. 73(5 Suppl): S4–S10. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bosland MC: The role of steroid hormones
in prostate carcinogenesis. J Natl Cancer Inst Monogr. 1–66.
2000.PubMed/NCBI
|
|
4
|
Crawford ED: Prostate cancer.
Introduction. Urology. 62(6 Suppl 1): S1–S2. 2003. View Article : Google Scholar
|
|
5
|
Grönberg H: Prostate cancer epidemiology.
Lancet. 361:859–864. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Crawford ED: Epidemiology of prostate
cancer. Urology. 62(6 Suppl 1): S3–S12. 2003. View Article : Google Scholar
|
|
7
|
Whittemore AS, Kolonel LN, Wu AH, John EM,
Gallagher RP, Howe GR, Burch JD, Hankin J, Dreon DM, West DW, et
al: Prostate cancer in relation to diet, physical activity, and
body size in blacks, whites, and Asians in the United States and
Canada. J Natl Cancer Inst. 87:652–661. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gravdal K, Halvorsen OJ, Haukaas SA and
Akslen LA: A switch from E-cadherin to N-cadherin expression
indicates epithelial to mesenchymal transition and is of strong and
independent importance for the progress of prostate cancer. Clin
Cancer Res. 13:7003–7011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tomita K, van Bokhoven A, van Leenders GJ,
Ruijter ET, Jansen CF, Bussemakers MJ and Schalken JA: Cadherin
switching in human prostate cancer progression. Cancer Res.
60:3650–3654. 2000.PubMed/NCBI
|
|
11
|
Shiozawa Y, Pedersen EA, Havens AM, Jung
Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, et al:
Human prostate cancer metastases target the hematopoietic stem cell
niche to establish footholds in mouse bone marrow. J Clin Invest.
121:1298–1312. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Steeg PS: Cancer biology: Emissaries set
up new sites. Nature. 438:750–751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Alexander NR, Tran NL, Rekapally H,
Summers CE, Glackin C and Heimark RL: N-cadherin gene expression in
prostate carcinoma is modulated by integrin-dependent nuclear
translocation of Twist1. Cancer Res. 66:3365–3369. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kong D, Banerjee S, Ahmad A, Li Y, Wang Z,
Sethi S and Sarkar FH: Epithelial to mesenchymal transition is
mechanistically linked with stem cell signatures in prostate cancer
cells. PLoS One. 5:e124452010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Maitland NJ and Collins A: A tumour stem
cell hypothesis for the origins of prostate cancer. BJU Int.
96:1219–1223. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pavese JM and Bergan RC: Circulating tumor
cells exhibit a biologically aggressive cancer phenotype
accompanied by selective resistance to chemotherapy. Cancer Lett.
352:179–186. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sak K: Site-specific anticancer effects of
dietary flavonoid quercetin. Nutr Cancer. 66:177–193. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sak K: Cytotoxicity of dietary flavonoids
on different human cancer types. Pharmacogn Rev. 8:122–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta S, Afaq F and Mukhtar H: Selective
growth-inhibitory, cell-cycle deregulatory and apoptotic response
of tangeretin in normal versus human prostate carcinoma cells.
Biochem Biophys Res Commun. 287:914–920. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Pan MH, Chen WJ, Lin-Shiau SY, Ho CT and
Lin JK: Tangeretin induces cell-cycle G1 arrest through inhibiting
cyclin-dependent kinases 2 and 4 activities as well as elevating
Cdk inhibitors p21 and p27 in human colorectal carcinoma cells.
Carcinogenesis. 23:1677–1684. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
el SA Arafa, Zhu Q, Barakat BM, Wani G,
Zhao Q, El-Mahdy MA and Wani AA: Tangeretin sensitizes
cisplatin-resistant human ovarian cancer cells through
downregulation of phosphoinositide 3-kinase/Akt signaling pathway.
Cancer Res. 69:8910–8917. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dong Y, Cao A, Shi J, Yin P, Wang L, Ji G,
Xie J and Wu D: Tangeretin, a citrus polymethoxyflavonoid, induces
apoptosis of human gastric cancer AGS cells through extrinsic and
intrinsic signaling pathways. Oncol Rep. 31:1788–1794. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lakshmi A and Subramanian S:
Chemotherapeutic effect of tangeretin, a polymethoxylated flavone
studied in 7, 12-dimethylbenz(a)anthracene induced mammary
carcinoma in experimental rats. Biochimie. 99:96–109. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Periyasamy K, Baskaran K, Ilakkia A,
Vanitha K, Selvaraj S and Sakthisekaran D: Antitumor efficacy of
tangeretin by targeting the oxidative stress mediated on
7,12-dimethylbenz(a) anthracene-induced proliferative breast cancer
in Sprague-Dawley rats. Cancer Chemother Pharmacol. 75:263–272.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Das A, Bhattacharya A, Chakrabarty S,
Ganguli A and Chakrabarti G: Smokeless tobacco extract
(STE)-induced toxicity in mammalian cells is mediated by the
disruption of cellular microtubule network: A key mechanism of
cytotoxicity. PLoS One. 8:e682242013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balint K, Xiao M, Pinnix CC, Soma A, Veres
I, Juhasz I, Brown EJ, Capobianco AJ, Herlyn M and Liu ZJ:
Activation of Notch1 signaling is required for
beta-catenin-mediated human primary melanoma progression. J Clin
Invest. 115:3166–3176. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Di Cello F, Flowers VL, Li H,
Vecchio-Pagán B, Gordon B, Harbom K, Shin J, Beaty R, Wang W,
Brayton C, et al: Cigarette smoke induces epithelial to mesenchymal
transition and increases the metastatic ability of breast cancer
cells. Mol Cancer. 12:902013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tanno B, Sesti F, Cesi V, Bossi G,
Ferrari-Amorotti G, Bussolari R, Tirindelli D, Calabretta B and
Raschellà G: Expression of Slug is regulated by c-Myb and is
required for invasion and bone marrow homing of cancer cells of
different origin. J Biol Chem. 285:29434–29445. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chang L, Graham PH, Hao J, Ni J, Bucci J,
Cozzi PJ, Kearsley JH and Li Y: Acquisition of
epithelial-mesenchymal transition and cancer stem cell phenotypes
is associated with activation of the PI3K/Akt/mTOR pathway in
prostate cancer radioresistance. Cell Death Dis. 4:e8752013.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ni J, Cozzi P, Hao J, Beretov J, Chang L,
Duan W, Shigdar S, Delprado W, Graham P, Bucci J, et al: Epithelial
cell adhesion molecule (EpCAM) is associated with prostate cancer
metastasis and chemo/radioresistance via the PI3K/Akt/mTOR
signaling pathway. Int J Biochem Cell Biol. 45:2736–2748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Taylor BS, Schultz N, Hieronymus H,
Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva
B, et al: Integrative genomic profiling of human prostate cancer.
Cancer Cell. 18:11–22. 2010. View Article : Google Scholar : PubMed/NCBI
|