Introduction
Approximately 250,000 people worldwide are diagnosed
with brain cancer annually. Unfortunately, only ~20% of all brain
tumors are of primary origin at the time of diagnosis, while ~80%
are metastatic (1). Gliomas are the
most common primary brain tumor in adults and more than half are
malignant (2). The current therapy
for gliomas is multimodal, including surgical resection,
radiotherapy and chemotherapy. However, due to the invasive
properties of the cells, these treatment options are not always
effective. Furthermore, the prognosis of patients with glioblastoma
is poor and of invariable recurrence, with a median survival time
of slightly >1 year (3,4). In the search for alternative treatment
modalities, oncolytic viruses have recently received increasingly
widespread attention for their potential in novel cancer therapy
(5,6).
Oncolytic viruses are effective for tumor therapy as they have the
capacity to selectively replicate in tumor cells, to spread viral
progeny in tumor tissues and to eventually induce tumor cell
apoptosis (7). In this respect,
conditionally replicative adenoviruses (CRAds) appear to be
attractive anticancer agents and are currently being evaluated in
clinical trials (8). The replication
of CRAds occurs only in tumor cells and not in normal cells, which
reflects the safety of this agent.
Conditionally replicative adenoviruses are designed
to exert intrinsic anticancer activity through selective
replication in cancer cells and to induce lysis in order to destroy
these cells (9). Oncolytic viruses
have been developed by adding specific promoter elements and
therapeutic genes, including the human telomerase reverse
transcriptase (hTERT) gene to control gene expression essential for
adenoviral replication, and the p53 gene to promote the dissolution
of tumor cells (7,10). In addition, the release of viral
progeny from infected tumor cells offers a potential to amplify the
oncolytic effects of CRAds by lateral spread in a solid tumor
(6,9).
Self-replicated adenoviruses have previously been
demonstrated to affect the progression of cancer cells in
vitro by expressing p53. Promoters have been used previously to
increase adenoviral replication or p53 expression, but the use of
two promoters at same time is rare (6,10). In the
present study, to enhance the oncolysis of CRAd on tumor cells, a
P74-Tp-Gp53 plasmid, containing the p53 gene, the early region 1A
(E1A) gene and two tumor-specific promoters hTERT and glial
fibrillary acidic protein (GFAP), was constructed. The plasmid
p74-Tp-Gp53 was restructured with the plasmid of an adenovirus
skeleton, PPE3, into the recombinant oncolytic adenovirus,
Ad-Tp-E1A-Gp-p53, to selectively replicate in tumor cells and to
restrain cell growth by expressing the p53 gene. Therefore, this
approach may serve as a promising therapeutic agent for the
treatment of numerous types of cancer.
Materials and methods
Cell culture
The human glioma U251 and T98G cell lines, and the
human embryonic lung MRC-5 cell line as well as the 293 cell line
were cultured in Dulbecco's modified Eagle's medium (DMEM) plus
GlutaMAX™ (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA), supplemented with 10% heat-inactivated fetal
bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml
penicillin and 100 g/ml streptomycin at 37°C in 5%
CO2.
Plasmid construction
hTERT and GFAP were designed according to the
sequences published in GenBank (https://www.ncbi.nlm.nih.gov/genbank/; accession
numbers, NM_198253 and M67446, respectively), and were synthesized
by Generay Biotech Co., Ltd. (Shanghai, China). Next, restriction
sites, BgIII (5′-AGATCT-3′) and HindIII
(5′-AAGCTT-3′), were added upstream and downstream of hTERT.
Subsequently, hTERT and GFAP were cloned into the
Dual-Luciferase® Reporter vector, pGL3-Basic (empty
vector; Promega Corporation, Madison, WI, USA), resulting in the
plasmids pGL3-hTERTp and pGL3-GFAP, respectively. Using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), plasmids were transfected into the MRC-5
(11), U251 and T98 G cells for 2 h.
A total of 24 h after transfection, the activity of the promoters
was detected using the Dual-Luciferase reporter gene detection
system (Invitrogen; Thermo Fisher Scientific, Inc.). Cells in the
normal control group remained intact and pRL-TK (Promega
Corporation) was used as a control reporter vector, and was used in
combination with any experimental reporter vector to co-transfect
mammalian cells. Renilla promoters (Promega Corporation) were
co-transfected as an internal control. Firefly luciferase activity
was normalized to renilla luciferase activity for individual
analysis.
To enhance the activity of pGL3-GFAP, four optimized
HIF-binding site optHBS enhancer sites
(5′-TACGTGCAGTACGTGCAGTACGTGCAGTACGTGCAG-3′) were cloned
(Invitrogen; Thermo Fisher Scientific, Inc.) into
MluI/XhoI sites of pGL3-GFAP, resulting in the
plasmid pGL3-ENGFAP. pGL3-ENGFAP was then identified by excising
with two different double enzyme systems (MluI/XhoI
and PvuI/PvuII) and the excision product was detected
by 2% agarose gel electrophoresis.
A 1,836 bp fragment was obtained from the double
digestion of pGL3-ENGFAP with MluI/HindIII followed
by EcoRI/XbaI and cloned into the
EcoRI/XbaI sites of PCA19 to generate the plasmid
PCA19-ENGFAP. Subsequently, two different enzyme systems
(EcoRI/XbaI and PstI) and 2% agarose gel
electrophoresis were used to verify the generation of PCA19-ENGFAP.
Next, the early viral1A (E1A) gene was cloned into
NcoI/SalI sites of pGL3-hTERT, and the plasmid
pGL3-hTERT-E1A (P74-Tp) was generated. The p53 gene was excised by
double digestion of PENTER15-p53 (Invitrogen; Thermo Fisher
Scientific, Inc.) with SalI and EcoRI and was cloned
into the SalI and EcoRI sites in PCA19-ENGFAP,
resulting in the plasmid PCA19-Gp53. The plasmid PCA19-Gp53 was
excised by digestion with EcoRI, SalI and
PVUII, and the excised product was detected by 2% agarose
gel electrophoresis. Additionally, the 3,765 bp fragment excised
from PCA19-Gp53 using BglII was cloned into the 8,465 bp
fragment excised from pGL3-hTERT-E1A (P74-TP) using BglII,
to yield the plasmid p74-Tp-Gp53. A different enzyme system
(BamHI and HindIII; Invitrogen; Thermo Fisher
Scientific, Inc.) was used to identify the p74-Tp-Gp53 plasmid
prior to detection of the excised fragments by 2% agarose gel
electrophoresis.
Recombination of oncolytic
adenovirus
The shuttle plasmid p74-Tp-Gp53 and the adenovirus
vector PPE3 (both Invitrogen; Thermo Fisher Scientific, Inc.) were
co-transfected into well-cultured 293 cells using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) to complete the virus recombination. Following 9
days of incubation at 37°C, virus plaques were observable and were
purified three times. Subsequently, 293 cells were alternately
frozen and thawed to collect the restructured oncolytic adenovirus,
Ad-Tp-E1A-Gp-p53. Polymerase chain reaction was then performed to
determine the recombination of the Ad-Tp-E1A-Gp-p53 oncolytic
adenovirus.
DNA extraction and PCR
Genomic DNA of the oncolytic adenovirus,
Ad-Tp-E1A-Gp-p53, was extracted using a Blood Genome DNA Extraction
kit (Takara Biotechnology Co., Inc., Dalian, China) according to
the manufacturer's protocol and was stored at −80°C. To determine
the recombination sites of the oncolytic adenovirus, PCR was
performed with repeated three times using the following primers:
GT154+GT156 forward, 5′-CCCACCGGTCACAGACGCCCAGGAC-3′ and reverse,
5′-GTGGCCGGGGCCAGGGCTTCCC-3′; W267+W268 forward,
5′-CCGGACGATATTGAACAATGGTTC-3′ and reverse,
5′-GTGAAATATTCTCCATCCAGTGG-3′; and W331+W332 forward,
5′-CGACGCGTCCCTCTAGATACGTGCAGTACGTGCAGTACGTGCAGTACGTGCAGAA-3′ and
reverse, 5′-CCGCTCGAGTTCCCACACATCAGCCTGGAGAGAT-3′ to amplify hTERT,
p53 and GFAP, respectively. The PCR reaction was set at an initial
denaturation step of 3 min at 95°C followed by 35 cycles of 95°C
for 40 sec, 58°C for 40 sec, 72°C for 90 sec and, finally, 72°C for
10 min. The PCR products were subsequently detected by 2% agarose
gel electrophoresis.
Detection of viral titer by 50% cell
culture infection dose (TCID50)
293 cells (permissive to viral infection) were
seeded onto 96-well plates (104 cells/well). After 24 h,
eight serial 10-fold dilutions of freeze/thaw lysates from infected
cells were seed into a 96-well plate (100 µl/well). After 10 days
of incubation at 37°C, the observable cytopathic effect (CPE) per
dilution was counted, and the ratio between infected and unaffected
wells was determined. The viral titer was then calculated using the
following equation: TCID50=10L+d (s-0.5), where L is the
log of the lowest dilution (e.g., If 10−1 is the minimum
dilution degree, L=1), d is the dilution coefficient (e.g., If
there is a 10-fold dilution, d=1), and s is the sum of the CPE rate
observed at each dilution.
Western blot analysis
U251 cells were infected with Ad-Tp-E1A-Gp-p53 at
different multiplicities of infection (MOIs; 0, 1, 10, 100 and
1,000). A bicinchoninic acid assay was used to determine protein
concentrations. After 48 h of incubation, infected U251 cells were
lysed using lysis buffer [0.125 M TRIS-HCl (pH 6.8), 2% SDS and
proteinase inhibitor; Abcam, Cambridge, UK]. Total protein (20
µg/lane) was then separated on a SDS polyacrylamide gel (12.5%) and
blotted onto HyBond N membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with a 5% skimmed milk solution in TBS
with 0.1% Tween-20 for 2 h at room temperature, adenoviral p53
expression was detected using the following primary antibodies:
Anti-p53 [DO-1] (cat. no. ab1101; dilution, 1:1,000; Abcam) and
anti-GAPDH [6C5] (cat. no. ab8245; dilution, 1:2,000; Abcam), and
incubated for 12 h at 4°C. GAPDH (cat. no. ab8245; dilution,
1:2,000; Abcam) served as a loading control. Membranes were then
incubated with a horseradish peroxidase-conjugated goat anti-mouse
immunoglobulin G secondary antibody (cat. no. ab6789; dilution,
1:5,000; Abcam) for 2 h at room temperature. The antigen-antibody
complexes were visualized using an enhanced chemiluminescence
detection plus kit (cat. no. PE0010; EMD Millipore), according to
the manufacturer's protocol.
Detection of the growth inhibiting
effect of Ad-Tp-E1A-Gp-p53 on U251 cells by MTT
Tumor U251 cells were seeded onto 96-well plates
(104 cells/well) and were infected with freeze/thaw
lysates of infected cells at different MOIs (0, 1, 10, 100 and
1,000) following incubation for 24 h at 37°C (Control, 0 MOI; Group
1, 1 MOI; group 2, 10 MOI; group 3, 100 MOI; group 4, 1,000 MOI).
After 2 h, the culture solution was discarded and 5% FBS/DMEM was
added. After incubation for 72 h at 37°C, MTT (5 mg/ml) was added
to the 96-well plates (20 µl/well). After 4 h, 200 µl dimethyl
sulfoxide was added to the 96-well plates, and then the 96-well
plates were agitated to dissolve the purple formazan. Subsequently,
optical density (OD) values were measured at 490 nm. The cell
growth inhibition rate was calculated using the following equation:
Inhibition rate (%)=(control group OD value-experimental group OD
value)/control group OD value.
Statistical analysis
All analyses were performed using SPSS software
package (version 19.0; IBM Corp., Armonk, NY, USA). Statistical
differences between all groups (experimental groups and the control
group) were compared using one-way analysis of variance, followed
by the Student-Newman-Keuls post hoc test. Data are expressed as
the mean ± standard deviation of three independent experiments.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Activity of pGL3-hTERTp and
pGL3-GFAP
The plasmids, pGL3-hTERTp and pGL3-GFAP, were
transfected into the MRC-5, U251 and T98 G cells with pRL-TK
(Invitrogen; Thermo Fisher Scientific, Inc.) at a ratio of 50:1,
respectively. After 24 h of incubation, the activity was detected
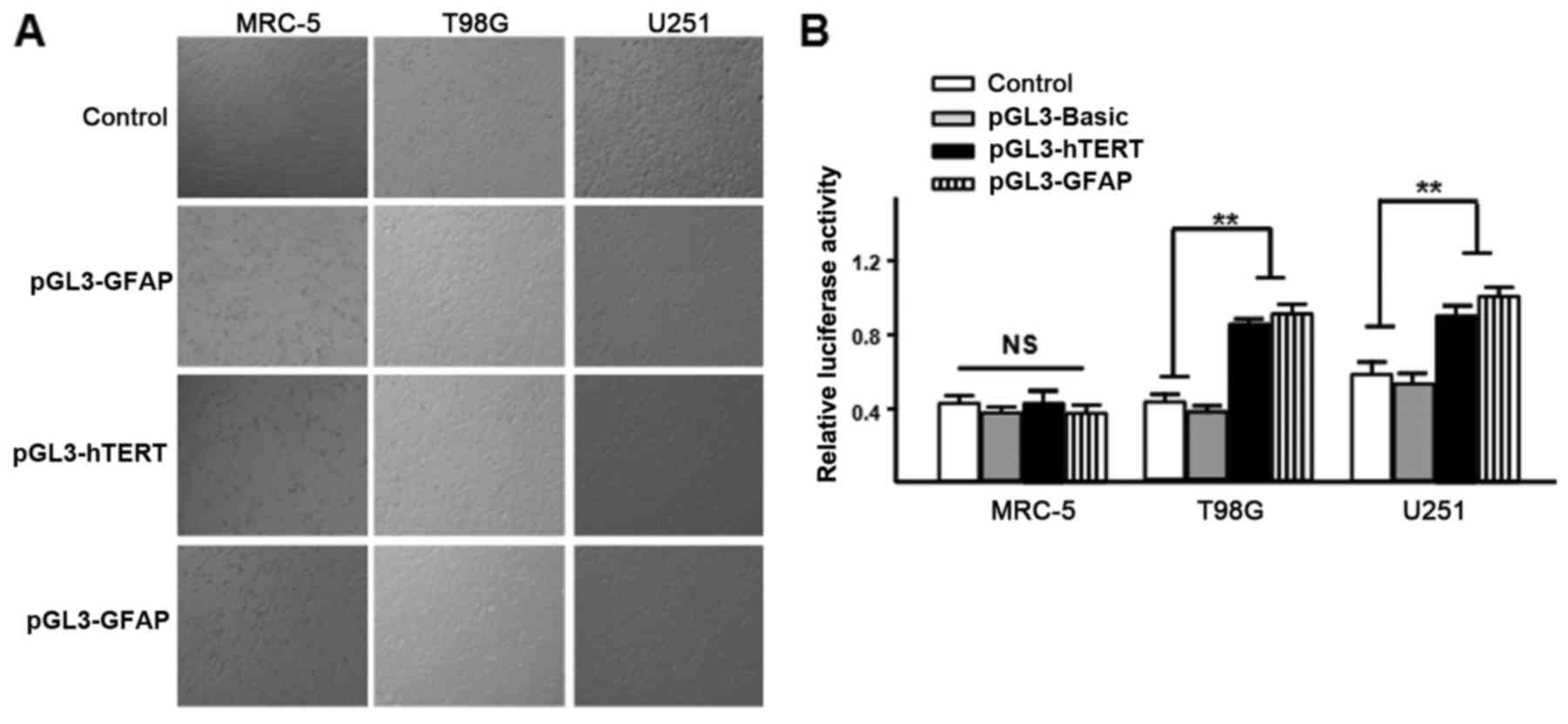
using a dual-luciferase reporter assay. It was observed that the
hTERT promoter is active in U251 and T98G glioma cells, but it is
not active in human MRC-5 cells, and the same finding was obtained
for the GFAP promoter (Fig. 1). These
results indicate that there is a good level of specificity of the
hTERT and GFAP promoters to glioma cells.
Verification of pGL3-ENGFAP,
PCA19-ENGFAP, PCA19-Gp53, P74-Tp-Gp53 plasmids and the recombinant
adenovirus Ad-Tp-E1A-Gp-p53
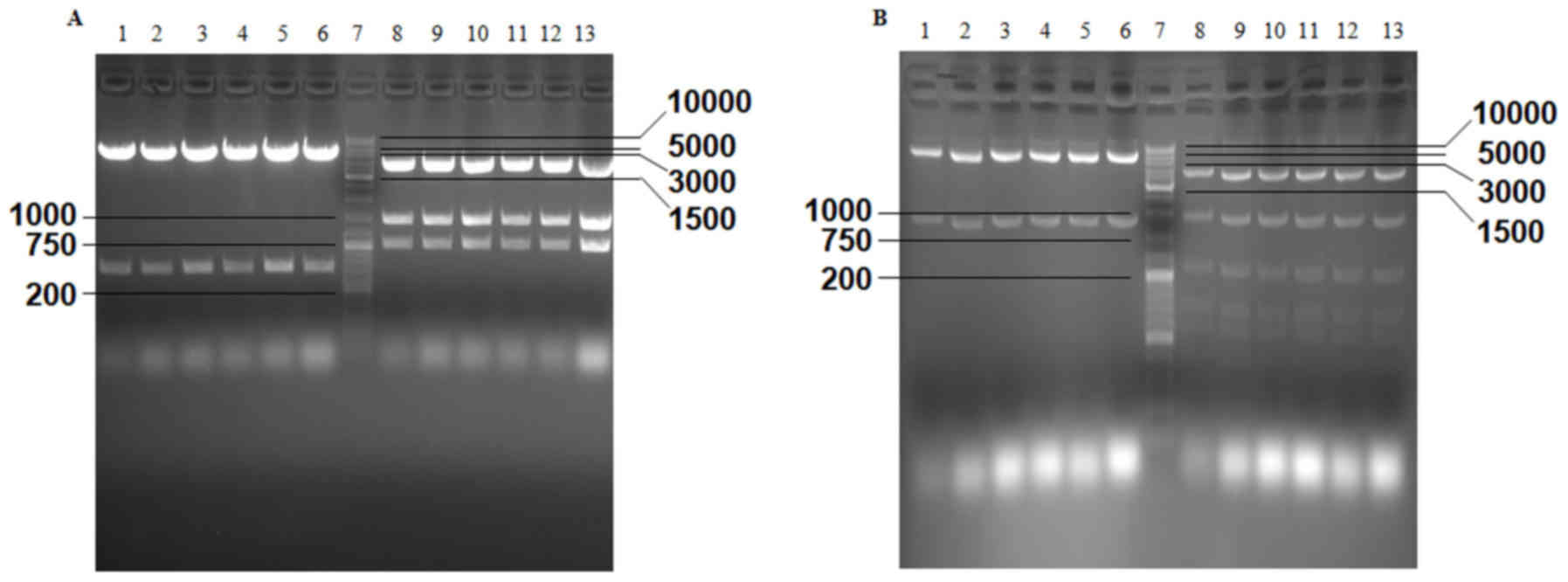
The results of agarose gel electrophoresis revealed
that two predicted fragments were obtained from pGL3-ENGFAP, which
was double digested with MluI and XhoI, and three
fragments were obtained from the double digestion of pGL3-ENGFAP
with PvuI and PvuII (Fig.
2A). Following double digestion of PCA19-ENGFAP with
EcoRI and XbaI and digestion with PstI, two
and seven predicted fragments were obtained (Fig. 2B). EcoRI+SalI and
PvuII were used to identify PCA19-Gp53, and two and seven
predicted fragments were generated, respectively (Fig. 3A). As a result of the excision with
BglII, SacI+XbaI, NcoI+SalI,
BamHI, PstI and NcoI, different numbers of
predicted fragments (2, 5, 6, 4, 7 and 5, respectively) were
obtained from p74-Tp-Gp53 (Fig. 3B).
Additionally, the results demonstrated that partial fragments of
TERTp (size, 266 bp), p53 (size, 847 bp) and GFAP (size, 744 bp)
were successfully amplified from Ad-Tp-E1A-Gp-p53 using PCR
(Fig. 3C).
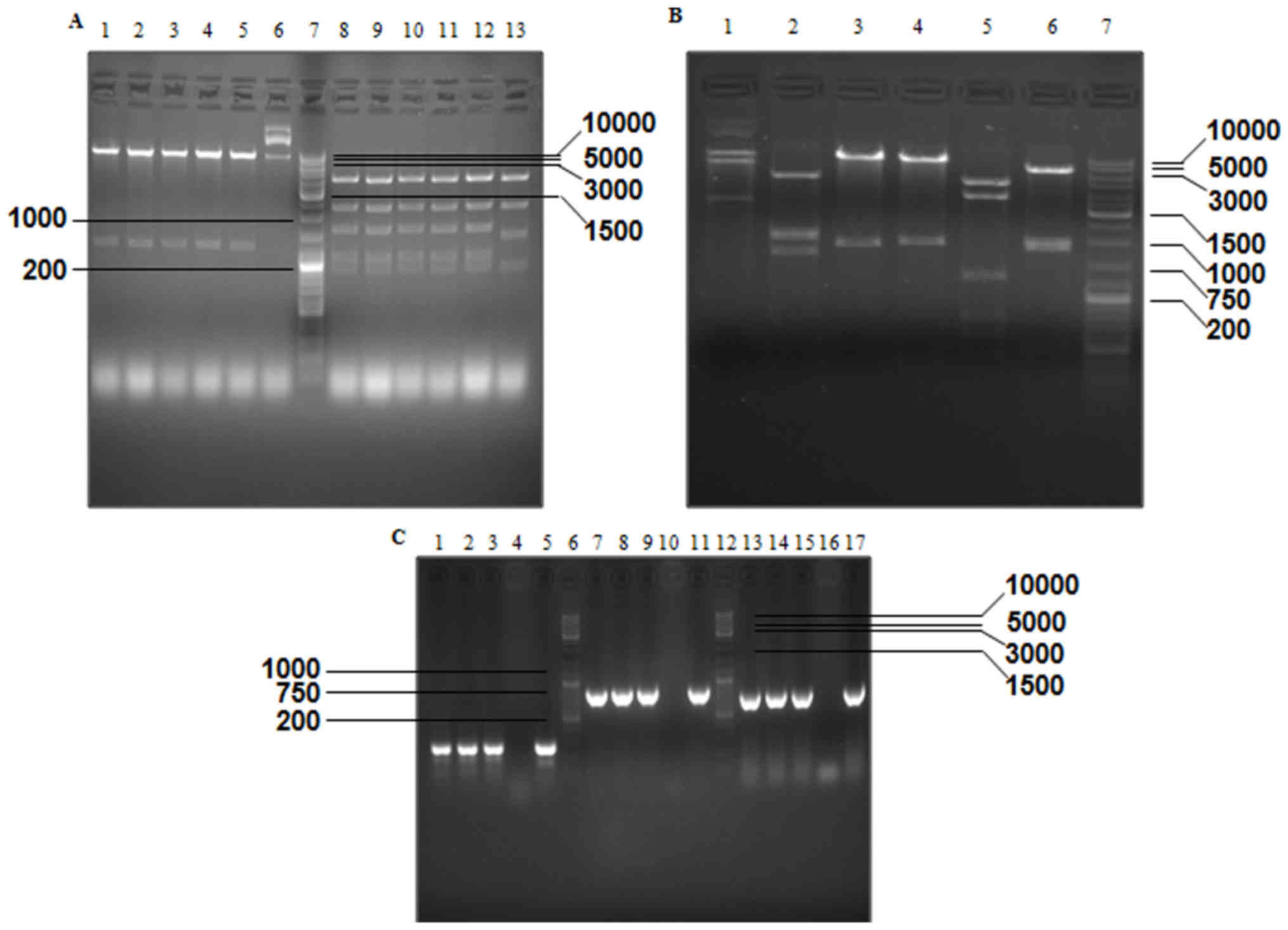 | Figure 3.Verification of PCA19-Gp53 and
p74-Tp-Gp53 plasmids by gel electrophoresis, according to the size
(bp) of excised fragments. (A) Excised fragments of PCA19-Gp53
detected by gel electrophoresis. Lanes 1–6, double digestion with
EcoRI and SalI; lane 7, GeneRuler™ DNA Ladder mix
(10,000 bp); lanes 8–13, digestion with PvuII. (B) Excised
fragments of P74-Tp-Gp53 detected by gel electrophoresis. Lanes
1–6, digestion of PCA19-Gp53 by BglII, SacI and
XbaI, NcoI and SalI, BamHI, PstI
and NcoI, respectively; and lane 7, GeneRuler™ DNA Ladder
Mix (10,000 bp). (C) DNA fragments of Ad-Tp-E1A-Gp-p53 were
detected by gel electrophoresis. Lanes 1–3, amplification of
Ad-Tp-E1A-Gp-p53 using the GT154+GT156 primer; lanes 4, 10 and 16,
negative control; lane 5, amplification of plasmid P74-TP using the
GT154+GT156 primer; lanes 6 and 12, GeneRuler™ DNA Ladder Mix;
lanes 7–9, amplification of Ad-Tp-E1A-Gp-p53 using the W267+W268
primer; lane 11, amplification of plasmid PENTER-p53 using the
W267+W268 primer; lanes 13–15, amplification of Ad-Tp-E1A-Gp-p53
using the W331+W332 primer; and lane 17, amplification of
plasmidpGL3-GFAP by using the W331+W332 primer. E1A, early viral
1A; GFAP, glial fibrillary acidic protein. |
Viral titer of p74-Tp-Gp53
Using the aforementioned equation for
TCID50=10L+d (s-0.5), a viral titer of
4.15×1011 PFU/ml Ad-Tp-E1A-Gp-p53 was calculated.
Expression of functional p53 in cancer
cells infected with adenovirus
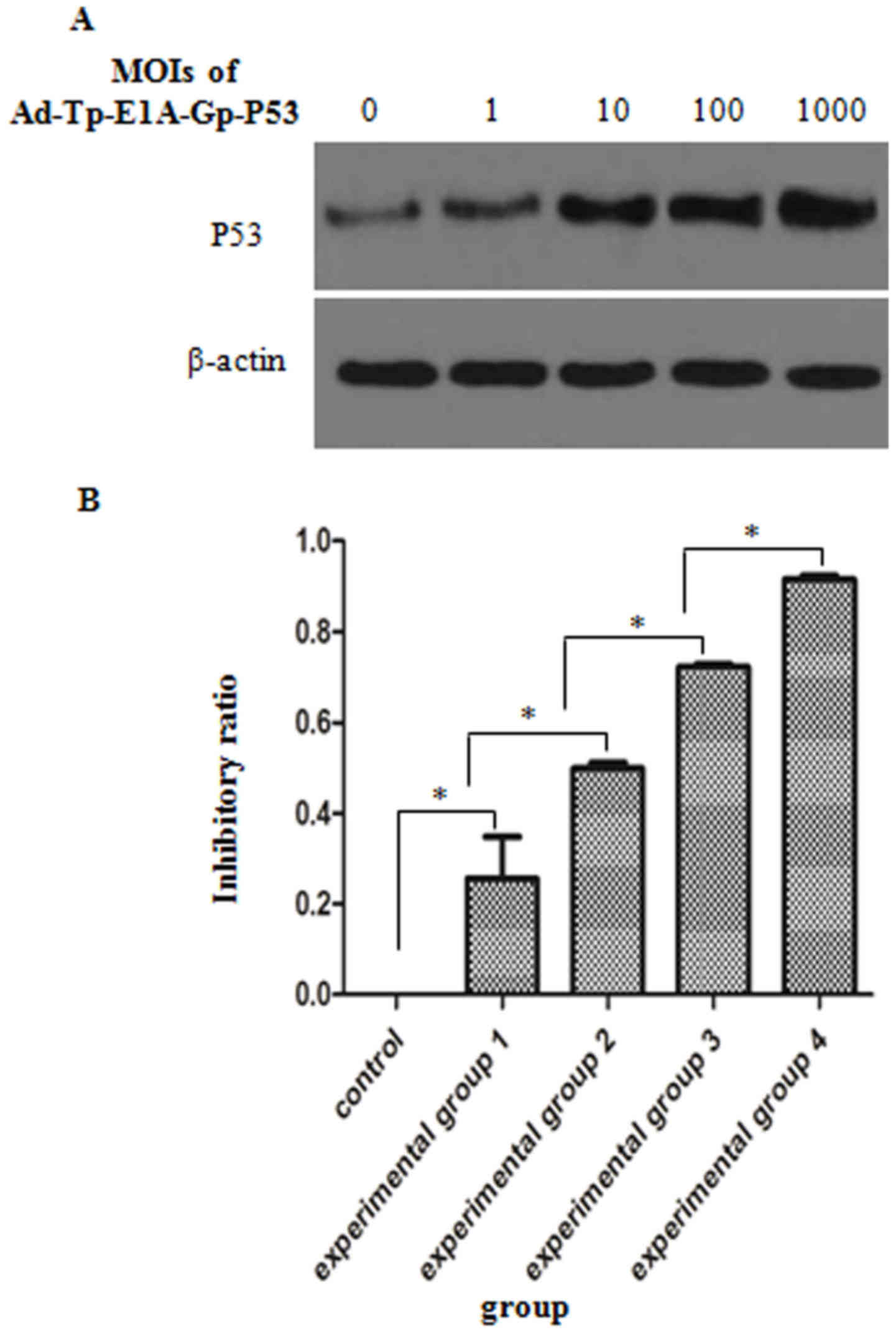
To investigate whether Ad-Tp-E1A-Gp-p53 affects the
expression of p53, western blot analysis was performed and the
levels of p53 expression in the Ad-Tp-E1A-Gp-p53-infected U251
cells at different MOIs were compared. As indicated in Fig. 4A, infection with Ad-Tp-E1A-Gp-p53 was
able to upregulate p53 expression in U251 cells, and the level of
p53 expression gradually increased with an increase in MOI.
Inhibitory effect of Ad-Tp-E1A-Gp-p53
on U251 cell growth
To evaluate the efficacy of viral transmission and
the therapeutic potential of Ad-Tp-E1A-Gp-p53 in vitro, U251
cells derived from human brain gliomas were infected with
Ad-Tp-E1A-Gp-p53 at different MOIs and the inhibition rate was
calculated (Fig. 4B). The inhibitory
effect of different MOIs of Ad-Tp-E1A-Gp-p53 was significantly
different (P<0.05), in which the inhibition ratios of the
experimental groups 1–4 were significantly higher compared with the
control group (P<0.05). Additionally, the inhibition ratio of
the four experimental groups exhibited an increasing trend as the
MOI of Ad-Tp-E1A-Gp-p53 increased.
Discussion
In the present study, pGL3-hTERTp and pGL3-GFAP
plasmids were successfully constructed and due to low activity,
four optHBS enhancer sites were cloned into pGL3-GFAP.
Additionally, a recombinant p74-Tp-Gp53 plasmid was constructed to
specifically express E1A and p53 in tumor cells. Furthermore,
Ad-Tp-E1A-Gp-p53 was demonstrated to suppress the growth of U251
cells through the expression of functional p53.
With the development of molecular biology and
in-depth knowledge of viral gene function, it has become possible
to genetically re-engineer viruses, and these may be used to
selectively target tumor cells through the use of adenoviruses and
herpesviruses (12,13). Conditionally replicative adenoviruses
(CRAds) have recently presented as novel agents for cancer therapy
and the use of these CRAds has been evaluated in preclinical trials
(14). These evaluations have already
demonstrated the potential of these adenoviruses in the therapy of
malignant brain tumors. For example, the conditionally replicative
adenovirus, ONYX-015, has entered into clinical trials for
malignant glioma (6,15,16). The
release and spread of conditionally replicative adenoviruses
progeny depend on the replication efficiency of the adenovirus in
cancer cells and their oncolytic capacity to induce death in cancer
cells at the late stages of infection (9).
Since the E1A protein has been identified and widely
recognized to be essential for adenoviral replication and the
production of progeny virions in human cells, a number of
telomerase promoter-regulated adenoviral vectors retain E1A genes
(17). Additionally, the catalytic
component of hTERT is not expressed in the majority of primary
somatic human cells, whereas major cancer cells are able to
reactivate telomerase by transcriptional upregulation of hTERT
(18). Furthermore, the hTERT gene,
as a promoter, controls the gene expression that is essential for
adenoviral replication (19).
Therefore, hTERT is usually used to induce E1A expression and
adenovirus replication in tumor cells. In the present study, hTERT
was cloned together with the E1A gene into pGL3-Basic to induce E1A
expression in tumor cells. GFAP, which was used in the present
study as a promoter, has been previously demonstrated to be able to
promote p53 expression and is downregulated in glioblastoma cells
(20,21). GFAP and hTERT have been demonstrated
to be glioma specific and are not expressed in MRC-5 cells
(11), and these findings were
confirmed in the present study.
The p53 gene, as a tumor suppressor, regulates
diverse cellular processes, including cell cycle arrest, cell
autophagy, senescence and apoptosis (22). In normal cells, the interaction
between cellular proteins and encoded proteins, including cellular
p53 proteins with viral E1A proteins, is necessary to complete the
viral life cycle. However, when this interaction is dysregulated,
normal cells gain the ability to develop into tumor cells (9,23).
The p53 suppressor protein is one component of the
cell apoptosis pathway that is exploited by adenoviruses.
Unfortunately, p53 is frequently inactivated by genetic alterations
in ~50% of all types of human cancer. For example, dysfunction of
the p53 signaling pathway is common in malignant gliomas, which
leads to a non-functional p53 pathway and inhibition of
CRAd-induced cell death (6,24).
It was reported in a previous study that p53
expression was higher in high-grade brain glioma compared with
low-grade brain glioma (P<0.05), and the expression was not
associated with patient sex or age, or the size of the tumor,
demonstrating that p53 serves an important role in the occurrence
and development of brain glioma (25). Therefore, restoring the function of
wild-type p53 in p53-inactivated tumor cells is a potential
treatment for certain types of tumor. In recent years,
adenovirus-mediated p53 gene therapy has been rapidly developed as
a promising antitumor strategy and has been employed in a number of
preclinical experiments and clinical studies (22,25,26). In
this regard, p53 is a common gene inserted in CRAd to control tumor
cell growth and to cause cell apoptosis.
A number of experiments involving the use of p53 in
CRAd have been performed. For example, Chen et al (26) demonstrated that p53-induced
microRNA-107 inhibited brain tumor cell growth, which indicates
that it serves as a tumor suppressor and thus, may be used as a
target for glioma therapy. Additionally, Yang et al
(27) suggested a combination of
recombinant adenovirus-p53 (rAd-p53) with fractionated stereotactic
radiotherapy (fSRT) is an effective and relatively safe method for
the treatment of primary hepatocellular carcinoma (HCC) compared
with fSRT monotherapy, indicating that rAd-p53 combined with fSRT
may be preferred as a local method to treat primary HCC if the
patients are unable to undergo surgery or refuse operation.
In the present study, a conditionally replicating
adenovirus (Ad-Tp-E1A-Gp-p53) was constructed using the two
plasmids pGL3-hTERT-E1A (p74-Tp) and PCA19-Gp53. Next, the
expression level of functional p53 in adenovirus-infected U251
cells was detected by western blot analysis, and the results
revealed that the expression of p53 was upregulated with an
increasing MOI. Additionally, the inhibitory effect of
Ad-Tp-E1A-Gp-p53 on U251 cells was detected by MTT and the results
indicated that the inhibitory effects of Ad-Tp-E1A-Gp-p53 in
different experimental groups (with different MOIs) were
significantly different (P<0.05), with the inhibition ratio of
the experimental groups being higher compared with the control
group (P<0.05). Furthermore, the inhibition ratio increased with
increases in MOI, indicating that the expression of functional p53
enhances the inhibitory effect of Ad-Tp-E1A-Gp-p53 on U251
cells.
In conclusion, in the present study, p74-Tp,
PCA19-Gp53 and Ad-Tp-E1A-Gp-p53 were constructed. It was also
demonstrated that hTERT and GFAP were able to promote the
expression of their downstream genes in vitro. Furthermore,
Ad-Tp-E1A-Gp-p53 was able to suppress the growth of U251 cells and
the inhibitory effect increased with increasing MOIs of
Ad-Tp-E1A-Gp-p53. Therefore, Ad-Tp-E1A-Gp-p53 may contribute to
more effective treatment for different types of human cancer to
enhance the potency of CRAd through the expression of functional
p53. Although the present study demonstrated that the constructed
CRAd represses the growth of glioma cells, there were certain
limitations. The human MRC-5 cell line was used rather than normal
glial cells to verify glioma specificity of the constructed virus
and thus, future experiments should use normal glial cells as a
control to further verify the effectiveness of the constructed
virus.
Acknowledgements
The authors would like to thank Dr Qijun Qian and Dr
Hongping Wu from The Second Military Medical University (Shanghai,
China) for providing technical assistance during the experiments.
The present study was supported by the Science and Technology
Mutual Fund of Guizhou Province (grant no. [2014] 7116) and the
Science and Technology Fund of Guizhou Health and Family Planning
Commission (grant no. gzwjkj 2014-2-144).
Glossary
Abbreviations
Abbreviations:
|
E1A
|
early viral 1A
|
|
CRAd
|
conditionally replicative
adenovirus
|
|
hTERT
|
human telomerase reverse
transcriptase
|
|
GFAP
|
glial fibrillary acidic protein
|
|
CPE
|
cytopathic effect
|
|
SDS
|
sodium dodecyl sulfate
|
References
|
1
|
Ulasov I, Borovjagin AV, Kaverina N,
Schroeder B, Shah N, Lin B, Baryshnikov A and Cobbs C: MT1-MMP
silencing by an shRNA-armed glioma-targeted conditionally
replicative adenovirus (CRAd) improves its anti-glioma efficacy in
vitro and in vivo. Cancer Lett. 365:240–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gilbert MR, Dignam JJ, Armstrong TS, Wefel
JS, Blumenthal DT, Vogelbaum MA, Colman H, Chakravarti A, Pugh S,
Won M, et al: A randomized trial of bevacizumab for newly diagnosed
glioblastoma. N Engl J Med. 370:699–708. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Westphal M, Ylä-Herttuala S, Martin J,
Warnke P, Menei P, Eckland D, Kinley J, Kay R and Ram Z; ASPECT
Study Group, : Adenovirus-mediated gene therapy with sitimagene
ceradenovec followed by intravenous ganciclovir for patients with
operable high-grade glioma (ASPECT): a randomised, open-label,
phase 3 trial. Lancet Oncol. 14:823–833. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Holland EC: Glioblastoma multiforme: The
terminator. Proc Natl Acad Sci USA. 97:pp. 6242–6244. 2000;
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nemunaitis J and Edelman J: Selectively
replicating viral vectors. Cancer Gene Ther. 9:987–1000. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Geoerger B, Vassal G, Opolon P, Dirven CM,
Morizet J, Laudani L, Grill J, Giaccone G, Vandertop WP, Gerritsen
WR and van Beusechem VW: Oncolytic activity of p53-expressing
conditionally replicative adenovirus AdDelta24-p53 against human
malignant glioma. Cancer Res. 64:5753–5759. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei F, Wang H, Chen X, Li C and Huang Q:
Dissecting the roles of E1A and E1B in adenoviral replication and
RCAd-enhanced RDAd transduction efficacy on tumor cells. Cancer
Biol Ther. 15:1358–1366. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Alemany R, Balagué C and Curiel DT:
Replicative adenoviruses for cancer therapy. Nat Biotechnol.
18:723–727. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Beusechem VW, van den Doel PB, Grill
J, Pinedo HM and Gerritsen WR: Conditionally replicative adenovirus
expressing p53 exhibits enhanced oncolytic potency. Cancer Res.
62:6165–6171. 2002.PubMed/NCBI
|
|
10
|
Fukuda K, Abei M, Ugai H, Kawashima R, Seo
E, Wakayama M, Murata T, Endo S, Hamada H, Hyodo I and Yokoyama KK:
E1A, E1B double-restricted replicative adenovirus at low dose
greatly augments tumor-specific suicide gene therapy for
gallbladder cancer. Cancer Gene Ther. 16:126–136. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu J, Mo Y, Wang X, Liu J, Zhang X, Wang
J, Hu L, Yang C, Chen L and Wang Y: Conditionally replicative
adenovirus-based mda-7/IL-24 expression enhances sensitivity of
colon cancer cells to 5-fluorouracil and doxorubicin. J
Gastroenterol. 48:203–213. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chu RL, Post DE, Khuri FR and Van Meir EG:
Use of replicating oncolytic adenoviruses in combination therapy
for cancer. Clin Cancer Res. 10:5299–5312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chiocca EA: Oncolytic viruses. Nature Rev
Cancer. 2:938–950. 2002. View
Article : Google Scholar
|
|
14
|
Fueyo J, Alemany R, Gomez-Manzano C,
Fuller GN, Khan A, Conrad CA, Liu TJ, Jiang H, Lemoine MG, Suzuki
K, et al: Preclinical characterization of the antiglioma activity
of a tropism-enhanced adenovirus targeted to the retinoblastoma
pathway. J Natl Cancer Inst. 95:652–660. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Geoerger B, Grill J, Opolon P, Morizet J,
Aubert G, Terrier-Lacombe MJ, Bressac De-Paillerets B, Barrois M,
Feunteun J, Kirn DH and Vassal G: Oncolytic activity of the E1B-55
kDa-deleted adenovirus ONYX-015 is independent of cellular p53
status in human malignant glioma xenografts. Cancer Res.
62:764–772. 2002.PubMed/NCBI
|
|
16
|
Lamfers ML, Grill J, Dirven CM, Van
Beusechem VW, Geoerger B, van den Berg J, Alemany R, Fueyo J,
Curiel DT, Vassal G, et al: Potential of the conditionally
replicative adenovirus Ad5-Delta24RGD in the treatment of malignant
gliomas and its enhanced effect with radiotherapy. Cancer Res.
62:5736–5742. 2002.PubMed/NCBI
|
|
17
|
Wirth T, Kuhnel F and Kubicka S:
Telomerase-dependent gene therapy. Curr Mol Med. 5:243–251. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wirth T, Zender L, Schulte B, Mundt B,
Plentz R, Rudolph KL, Manns M, Kubicka S and Kühnel F: A
telomerase-dependent conditionally replicating adenovirus for
selective treatment of cancer. Cancer Res. 63:3181–3188.
2003.PubMed/NCBI
|
|
19
|
Alemany R: Conditionally replicating
adenoviruses for cancer treatment. Cancer Gene Ther. 1–248.
2005.PubMed/NCBI
|
|
20
|
Wilhelmsson U, Eliasson C, Bjerkvig R and
Pekny M: Loss of GFAP expression in high-grade astrocytomas does
not contribute to tumor development or progression. Oncogene.
22:3407–3411. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee K, Jeon K, Kim JM, et al:
Downregulation of GFAP, TSP-1 and p53 in human glioblastoma cell
line, U373MG, by IE1 protein from human cytomegalovirus. Glia.
51:1–12. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tazawa H, Kagawa S and Fujiwara T:
Advances in adenovirus-mediated p53 cancer gene therapy. Expert
Opin Biol Ther. 13:1569–1583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heise C, Hermiston T, Johnson L, Brooks G,
Sampson-Johannes A, Williams A, Hawkins L and Kirn D: An adenovirus
E1A mutant that demonstrates potent and selective systemic
anti-tumoral efficacy. Nat Med. 6:1134–1139. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Collins V: Gene amplification in human
gliomas. Glia. 15:289–296. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin T, Wang M, Liang HS and Liu EZ: The
expression of p53, mgmt and egfr in brain glioma and clinical
significance. J Biol Regul Homeost Agents. 29:143–149.
2015.PubMed/NCBI
|
|
26
|
Chen L, Zhang R, Li P, Liu Y, Qin K, Fa
ZQ, Liu YJ, Ke YQ and Jiang XD: p53-induced microRNA-107 inhibits
proliferation of glioma cells and down-regulates the expression of
CDK6 and Notch-2. Neurosci Lett. 534:327–332. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang ZX, Wang D, Wang G, Zhang QH, Liu JM,
Peng P and Liu XH: Clinical study of recombinant adenovirus-p53
combined with fractionated stereotactic radiotherapy for
hepatocellular carcinoma. J Cancer Res Clin Oncol. 136:625–630.
2010. View Article : Google Scholar : PubMed/NCBI
|