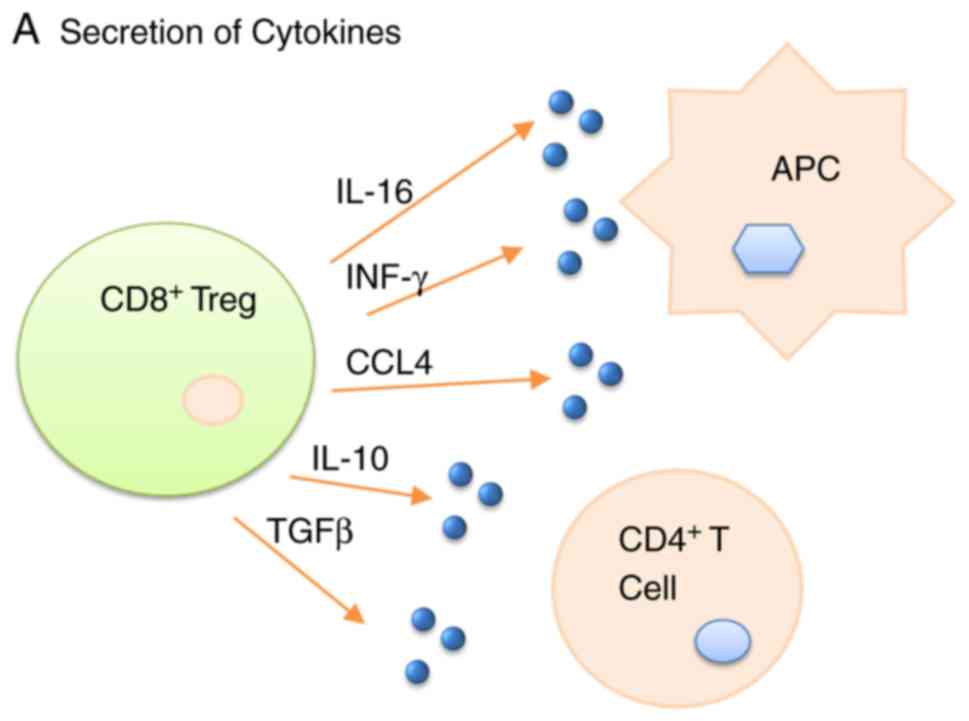
Immunization is critical for the maintenance of
biological homeostasis. It refers to the physiological function by
which a body's immune system fights against the invasion of foreign
substances and distinguishes internal components from external
ones. The immune system is responsible for the body's immune
response and immune function. While it produces robust immune
responses to attack various antigens, it also negatively regulates
or inhibits abnormal immune responses, maintaining a relatively
stable immune reactivity (1–3). The immune system consists of three major
components, namely the immune organs, immune cells and
immunologically active substances. In particular, immune cells are
produced, mature and are concentrated in the immune organs. These
cells can be divided into phagocytic cells and lymphocytes, which
are composed of T and B cells that mature in the thymus and bone
marrow, respectively. Immunologically active substances mainly
refer to antibodies, lymphokines and lysozyme (4–6).
Physiologically, the tolerance of internal
components and the response to ‘non-self’ antigens are under the
strict control of the body's immune regulation mechanism. Immune
regulation is crucial for the maintenance of the physical
environment stability in the human body (7,8).
Therefore, dysfunction of immune regulation will result in serious
pathological consequences. For instance, if the immune system
develops a strong immune attack on the body's own components,
autoimmune diseases occur (9,10). The body may also be harmed if the
immune system cannot respond adequately to an infection caused by
external pathogenic microorganisms. In this case, a weak response
can result in severe infection, whereas an excessively strong
response can result in allergy (11–18).
Therefore, immune regulation determines the occurrence and the
strength of an immune response. This elegant and complicated
regulation functions in multiple steps in an immune response
process.
In the process of thymic negative selection, only T
cell clones with high affinity to autologous antigens are removed
(29,30). Therefore, certain T cells with low
affinity to autologous antigens are leaked to the peripheral immune
system. Under certain conditions, they may be activated and result
in autoimmune diseases. Therefore, this process is monitored by a
series of peripheral immune tolerance mechanisms, including Tregs
with immunosuppressive effects, namely CD4+ and
CD8+ Tregs (31–33).
Common molecules that serve as markers for Tregs
include FoxP3, CD25, CD127, CD39 and CD73. Among them, FoxP3 is a
member of the fork-like transcription factor family and was first
reported in 2001 by Brunkow et al (53). Studies have identified that FoxP3
expression and function are closely correlated with Tregs. FoxP3 is
mainly expressed in lymphoid organs and tissues, including in the
thymus, spleen and lymph nodes (54–56). In
mice, FoxP3 has been reported to be preferentially expressed in
CD4+CD25+ T cells, while its expression in
CD8+ T cells was limited. By contrast, in humans, FoxP3
can be expressed in both CD4+CD25+ T cells
and CD8+ T cells (57,58).
However, its expression in CD4+ T cells is significantly
higher in comparison with that in CD8+ T cells. Thus
far, FoxP3 has been recognized as the most sensitive marker of
Tregs (54–56).
Traditionally, the identification of Tregs mainly
relied on CD25 labeling. However, it was later reported that
identifying Tregs merely based on CD25 positivity was not accurate
(59,60).
The mouse protein Qa-1 (homologous to HLA-E in
humans) is essential for immune protection and immune regulation.
In particular, Qa-1-restricted CD8+ Tregs recognize the
MHC-Ib molecule Qa-1, and therefore inhibit the development and
recurrence of autoimmune diseases (27,85).
Notably, the immune response phenotype of Qa-1-deficient mice
demonstrated two opposite effects: Enhanced CD4-dependent immune
responses revealed the influence of Qa-1 target loss on
CD8+ Treg activity, whereas a weakened CD4-dependent
immune response demonstrated an unimpeded NKG2A-Qa-1/Qdm inhibition
(86,87).
In order to illustrate the two Qa-1-dependent
regulatory pathways, researchers performed experiments with
Qa-1-deficient mice expressing different surface determinants
(88,89). Notably, Qa-1 D227K is a mutant of Qa-1
that interferes with the binding of Qa-1 and CD8 co-receptors to
prevent the expression of CD8+ cell effective molecules.
As expected, Qa-1 D227K-deficient mice generated no active
CD8+ Tregs, accompanied by worsened EAE symptoms. In
addition, Qa-1/Qdm was demonstrated to bind to CD94/NKG2A on
CD8+ Tregs, and may thus suppress the inhibitory
activity of CD8+ Tregs through signaling factors
(90,91). Therefore, the key for preventing
CD8+ T cell autoimmune responses may be the regulation
of Qa-1-restricted auto-activated cells through interaction between
Qa-1-Qdm and CD94/NKG2A. It was also reported that the interaction
of Qa-1-NKG2A with antibodies in EAE mice attenuated the pathogenic
condition to complete remission (89,92). These
findings demonstrated that Qa-1 serves a key role in the
development and mediation of CD8+ Treg activity.
Furthermore, a molecular level inhibitory mechanism emerged based
on these results, suggesting that MHC-TCR interactions relied on
the co-receptors of the CD8 molecule. Identification of
Qa-1-restricted CD8+ Tregs enriched the occurrence and
development mechanism of autoimmune disease, providing a
theoretical basis for its treatment.
The ability of excess tissue and organs to incite a
strong immune response depends on the clearance of autoreactive T
or B lymphocytes. However, immature and mature autoreactive T and B
cells are not thoroughly cleared, and may require an effort from
cell reprograming to suppress the immune response. Notably, a
sublineage of CD8+ Tregs is necessary for the
maintenance of self-tolerance and the prevention of autoimmune
diseases in mice (101–103). The interruption of gene functions
associated with the interaction between these CD8+ T
cells and Qa-1+ follicular helper T cells can to lead to
the development of systemic lupus erythematosus, indicating that
these sublineage T cells serve an important role in the regulation
of immune response, as well as in the surveillance of immune
tolerance (104–106).
Currently, several issues remain unaddressed
regarding the functions of Tregs and the corresponding research
methodology (21,23). Firstly, phenotype identification and a
functional/mechanistic study of CD8+ Tregs have to be
further conducted in different experiment systems. In addition, it
remains unclear whether different CD8+ cell
subpopulations originate from common precursor cells. Finally, the
application of CD8+ Tregs can be further explored in the
scenarios of prevention, diagnosis and clinical treatment of
diseases.
Not applicable.
The present study was supported by Project of
Natural Scientific Foundation of Guangxi University of Science and
Technology (grant no. 174516).
Not applicable.
YY was responsible for writing original draft,
editing, analysis and designing of the work, and interpretation of
data for the work. XM performed analysis, editing and validation.
RG provided resources and performed review. JZ is mainly
responsible for editing of references, reviewing and data analysis
for the work. LW provided software asssitance. JY made substantial
contributions to the study design and acquisition and analysis of
data. In addition JY was responsible for providing supervision and
ensuring that questions related to the accuracy or integrity of any
part of the work were appropriately investigated and resolved.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Andersen MH: Immune regulation by
self-recognition: Novel possibilities for anticancer immunotherapy.
J Natl Cancer Inst. 107:pii: djv154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andersen MH: Novel understanding of
self-reactive T cells. Oncoimmunology. 5:e10836722015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ahmad Munir S, Martinenaite E, Hansen M,
Junker N, Borch TH, Met Ö, Donia M, Svane IM and Andersen MH: PD-L1
peptide co-stimulation increases immunogenicity of a dendritic
cell-based cancer vaccine. Oncoimmunology. 5:e12023912016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Markman JL and Shiao SL: Impact of the
immune system and immunotherapy in colorectal cancer. J
Gastrointest Oncol. 6:208–223. 2015.PubMed/NCBI
|
|
5
|
Sun Q, Burton RL and Lucas KG: Cytokine
production and cytolytic mechanism of CD4(+) cytotoxic T
lymphocytes in ex vivo expanded therapeutic Epstein-Barr
virus-specific T-cell cultures. Blood. 99:3302–3309. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Guo H, Cheng Y, Shapiro J and McElwee K:
The role of lymphocytes in the development and treatment of
alopecia areata. Expert Rev Clin Immunol. 11:1335–1351. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lemke H: Antigen receptor-intrinsic
non-self: The key to understanding regulatory lymphocyte-mediated
idiotypic control of adaptive immune responses. Crit Rev Immunol.
36:13–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Plasilova M, Risitano A and Maciejewski
JP: Application of the molecular analysis of the T-cell receptor
repertoire in the study of immune-mediated hematologic diseases.
Hematology. 8:173–181. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lopez-Pedrera C, Perez-Sanchez C,
Ramos-Casals M, Santos-Gonzalez M, Rodriguez-Ariza A and Cuadrado
MJ: Cardiovascular risk in systemic autoimmune diseases: Epigenetic
mechanisms of immune regulatory functions. Clin Dev Immunol.
2012:9746482012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Amaya-Amaya J, Montoya-Sanchez L and
Rojas-Villarraga A: Cardiovascular involvement in autoimmune
diseases. Biomed Res Int. 2014:3673592014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Michelsen SW, Soborg B, Diaz LJ, Hoff ST,
Agger EM, Koch A, Rosenkrands I, Wohlfahrt J and Melbye M: The
dynamics of immune responses to Mycobacterium tuberculosis
during different stages of natural infection: A longitudinal study
among Greenlanders. PLoS One. 12:e01779062017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hunniger K, Lehnert T, Bieber K, Martin R,
Figge MT and Kurzai O: A virtual infection model quantifies innate
effector mechanisms and Candida albicans immune escape in
human blood. PLoS Comput Biol. 10:e10034792014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gounder K, Padayachi N, Mann JK, Radebe M,
Mokgoro M, van der Stok M, Mkhize L, Mncube Z, Jaggernath M, Reddy
T, et al: High frequency of transmitted HIV-1 Gag HLA class
I-driven immune escape variants but minimal immune selection over
the first year of clade C infection. PLoS One. 10:e01198862015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang SS, Banner D, Degousee N, Leon AJ,
Xu L, Paquette SG, Kanagasabai T, Fang Y, Rubino S, Rubin B, et al:
Differential pathological and immune responses in newly weaned
ferrets are associated with a mild clinical outcome of pandemic
2009 H1N1 infection. J Virol. 86:13187–13201. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bourke CD, Maizels RM and Mutapi F:
Acquired immune heterogeneity and its sources in human helminth
infection. Parasitology. 138:139–159. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Furman D and Davis MM: New approaches to
understanding the immune response to vaccination and infection.
Vaccine. 33:5271–5281. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Redgrove KA and McLaughlin EA: The role of
the immune response in chlamydia trachomatis infection of the male
genital tract: A double-edged sword. Front Immunol. 5:5342014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kotzamanis K, Angulo A and Ghazal P:
Infection homeostasis: Implications for therapeutic and immune
programming of metabolism in controlling infection. Med Microbiol
Immunol. 204:395–407. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gershon RK and Kondo K: Cell interactions
in the induction of tolerance: The role of thymic lymphocytes.
Immunology. 18:723–737. 1970.PubMed/NCBI
|
|
20
|
Afonina IS, Zhong Z, Karin M and Beyaert
R: Limiting inflammation-the negative regulation of NF-κB and the
NLRP3 inflammasome. Nat Immunol. 18:861–869. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dinesh RK, Skaggs BJ, La Cava A, Hahn BH
and Singh RP: CD8+ Tregs in lupus, autoimmunity, and
beyond. Autoimmun Rev. 9:560–568. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Emregul E, David A, Balthasar JP and Yang
VC: A GPIIb/IIIa bioreactor for specific treatment of immune
thrombocytopenic purpura, an autoimmune disease. Preparation, in
vitro characterization, and preliminary proof-of-concept animal
studies. J Biomed Mater Res A. 75:648–655. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Smith TR and Kumar V: Revival of
CD8+ Treg-mediated suppression. Trends Immunol.
29:337–342. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu D, Li G, Avella DM, Kimchi ET, Kaifi
JT, Rubinstein MP, Camp ER, Rockey DC, Schell TD and
Staveley-O'Carroll KF: Sunitinib represses regulatory T cells to
overcome immunotolerance in a murine model of hepatocellular
cancer. Oncoimmunology. 7:e13720792017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chakraborty S, Panda AK, Bose S, Roy D,
Kajal K, Guha D and Sa G: Transcriptional regulation of FOXP3
requires integrated activation of both promoter and CNS regions in
tumor-induced CD8+ Treg cells. Sci Rep. 7:16282017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou C, Wu J, Borillo J, Torres L, McMahon
J and Lou YH: Potential roles of a special CD8 alpha alpha+ cell
population and CC chemokine thymus-expressed chemokine in ovulation
related inflammation. J Immunol. 182:596–603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Tang X, Maricic I, Purohit N, Bakamjian B,
Reed-Loisel LM, Beeston T, Jensen P and Kumar V: Regulation of
immunity by a novel population of Qa-1-restricted
CD8alphaalpha+TCRalphabeta+ T cells. J Immunol. 177:7645–7655.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Bian Y, Shang S, Siddiqui S, Zhao J,
Joosten SA, Ottenhoff THM, Cantor H and Wang CR: MHC Ib molecule
Qa-1 presents Mycobacterium tuberculosis peptide antigens to
CD8+ T cells and contributes to protection against infection. PLoS
Pathog. 13:e10063842017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takada K, Kondo K and Takahama Y:
Generation of peptides that promote positive selection in the
thymus. J Immunol. 198:2215–2222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ali Mohammed HH and Drela N: Role of
thymic B cells in the development of thymus-derived regulatory T
cell in vitro. Immunol Lett. 185:56–63. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Talotta R, Atzeni F, Batticciotto A,
Benucci M, Bongiovanni S and Sarzi-Puttini P: Biological agents in
rheumatoid arthritis: A cross-link between immune tolerance and
immune surveillance. Curr Rheumatol Rev. 2016.PubMed/NCBI
|
|
32
|
Huang G, Xu J, Lefever DE, Glenn TC, Nagy
T and Guo TL: Genistein prevention of hyperglycemia and improvement
of glucose tolerance in adult non-obese diabetic mice are
associated with alterations of gut microbiome and immune
homeostasis. Toxicol Appl Pharmacol. 332:138–148. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Capece T and Kim M: The role of lymphatic
niches in T Cell Differentiation. Mol Cells. 39:515–523. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang N, Li Z, Jiao Z, Gu P, Zhou Y, Lu L
and Chou KY: A Trichosanthin-derived peptide suppresses type 1
immune responses by TLR2-dependent activation of CD8(+)CD28(-)
Tregs. Clin Immunol. 153:277–287. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vuddamalay Y and van Meerwijk JP: CD28-
and CD28lowCD8+ regulatory T cells: Of mice and men. Front Immunol.
8:312017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Morimoto C, Takeuchi T and Schlossman SF:
Characterization of the CD8+CD45R+(2H4+) suppressor effector cell.
Clin Exp Rheumatol. 7 Suppl 3:S3–S7. 1989.PubMed/NCBI
|
|
37
|
Takeuchi T, Rudd CE, Tanaka S, Rothstein
DM, Schlossman SF and Morimoto C: Functional characterization of
the CD45R (2H4) molecule on CD8 (T8) cells in the autologous mixed
lymphocyte reaction system. Eur J Immunol. 19:747–755. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Raziuddin S and Elawad ME:
Immunoregulatory CD4+ CD45R+ suppressor/inducer T lymphocyte
subsets and impaired cell-mediated immunity in patients with Down's
syndrome. Clin Exp Immunol. 79:67–71. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wei S, Kryczek I, Zou L, Daniel B, Cheng
P, Mottram P, Curiel T, Lange A and Zou W: Plasmacytoid dendritic
cells induce CD8+ regulatory T cells in human ovarian carcinoma.
Cancer Res. 65:5020–5026. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wu J, Li S, Yang Y, Zhu S, Zhang M, Qiao
Y, Liu YJ and Chen J: TLR-activated plasmacytoid dendritic cells
inhibit breast cancer cell growth in vitro and in vivo. Oncotarget.
8:11708–11718. 2017.PubMed/NCBI
|
|
41
|
Kourtzelis I and Rafail S: The dual role
of complement in cancer and its implication in anti-tumor therapy.
Ann Transl Med. 4:2652016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen L, Hasni MS, Jondal M and Yakimchuk
K: Modification of anti-tumor immunity by tolerogenic dendritic
cells. Autoimmunity. 50:370–376. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang L: Adaptive Treg generation by DCs
and their functional analysis. Methods Mol Biol. 595:403–412. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao JF, McIntyre MS, Juvet SC, Diao J, Li
X, Vanama RB, Mak TW, Cattral MS and Zhang L: Regulation of
antigen-expressing dendritic cells by double negative regulatory T
cells. Eur J Immunol. 41:2699–2708. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Suciu-Foca N, Manavalan JS, Scotto L,
Kim-Schulze S, Galluzzo S, Naiyer AJ, Fan J, Vlad G and Cortesini
R: Molecular characterization of allospecific T suppressor and
tolerogenic dendritic cells: Review. Int Immunopharmacol. 5:7–11.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Song ZY, Yamasaki R, Kawano Y, Sato S,
Masaki K, Yoshimura S, Matsuse D, Murai H, Matsushita T and Kira J:
Peripheral blood T cell dynamics predict relapse in multiple
sclerosis patients on fingolimod. PLoS One. 10:e01249232015.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hendrikx TK, Velthuis JH, Klepper M, van
Gurp E, Geel A, Schoordijk W, Baan CC and Weimar W: Monotherapy
rapamycin allows an increase of CD4 CD25 FoxP3 T cells in renal
recipients. Transpl Int. 22:884–891. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Assadiasl S, Ahmadpoor P, Nafar M,
Pezeshki Lessan M, Pourrezagholi F, Parvin M, Shahlaee A, Sepanjnia
A, Nicknam MH and Amirzargar A: Regulatory T cell subtypes and
TGF-beta1 gene expression in chronic allograft dysfunction. Iran J
Immunol. 11:139–152. 2014.PubMed/NCBI
|
|
49
|
Negrini S, Fenoglio D, Parodi A, Kalli F,
Battaglia F, Nasi G, Curto M, Tardito S, Ferrera F and Filaci G:
Phenotypic alterations involved in CD8+ Treg impairment in systemic
sclerosis. Front Immunol. 8:182017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Velasquez-Lopera MM, Correa LA and Garcia
LF: Human spleen contains different subsets of dendritic cells and
regulatory T lymphocytes. Clin Exp Immunol. 154:107–114. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang B, Jiao Z, Shao X, Lu L, Yang N, Zhou
X, Xin L, Zhou Y and Chou KY: Phenotypic alterations of dendritic
cells are involved in suppressive activity of trichosanthin-induced
CD8+CD28- regulatory T cells. J Immunol. 185:79–88. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Nikoueinejad H, Amirzargar A, Sarrafnejad
A, Einollahi B, Nafar M, Ahmadpour P, Pour-Reze-Gholi F, Sehat O
and Lesanpezeshki M: Dynamic changes of regulatory T cell and
dendritic cell subsets in stable kidney transplant patients: A
prospective analysis. Iran J Kidney Dis. 8:130–138. 2014.PubMed/NCBI
|
|
53
|
Brunkow ME, Jeffery EW, Hjerrild KA,
Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF
and Ramsdell F: Disruption of a new forkhead/winged-helix protein,
scurfin, results in the fatal lymphoproliferative disorder of the
scurfy mouse. Nat Genet. 27:68–73. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Ochs HD, Ziegler SF and Torgerson TR:
FOXP3 acts as a rheostat of the immune response. Immunol Rev.
203:156–164. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Niemz J, Kliche S, Pils MC, Morrison E,
Manns A, Freund C, Crittenden JR, Graybiel AM, Galla M, Jänsch L,
et al: The guanine-nucleotide exchange factor Caldag gefi
fine-tunes functional properties of regulatory T cells. Eur J
Microbiol Immunol (Bp). 7:112–126. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Maranduba CM, De Castro SB, de Souza GT,
Rossato C, da Guia FC, Valente MA, Rettore JV, Maranduba CP, de
Souza CM, do Carmo AM, et al: Intestinal microbiota as modulators
of the immune system and neuroimmune system: Impact on the host
health and homeostasis. J Immunol Res. 2015:9315742015. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Fontenot JD, Gavin MA and Rudensky AY:
FOXP3 programs the development and function of CD4+CD25+ regulatory
T cells. Nat Immunol. 4:330–336. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
58
|
Bin Dhuban K, Kornete M, Mason S E and
Piccirillo CA: Functional dynamics of Foxp3+ regulatory
T cells in mice and humans. Immunol Rev. 259:140–158. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cosmi L, Liotta F, Lazzeri E, Francalanci
M, Angeli R, Mazzinghi B, Santarlasci V, Manetti R, Vanini V,
Romagnani P, et al: Human CD8+CD25+ thymocytes share phenotypic and
functional features with CD4+CD25+ regulatory thymocytes. Blood.
102:4107–4114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Maslanka T, Ziolkowska N, Ziolkowski H and
Malaczewska J: CD25+CD127+Foxp3- cells represent a major
subpopulation of CD8+ T cells in the eye chambers of normal mice.
PLoS One. 12:e01700212017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Jun C, Ke W, Qingshu L, Ping L, Jun D, Jie
L, Bo C and Su M: Protective effect of CD4(+)CD25(high)CD127(low)
regulatory T cells in renal ischemia-reperfusion injury. Cell
Immunol. 289:106–111. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Su H, Longhi MS, Wang P, Vergani D and Ma
Y: Human CD4+CD25(high)CD127 (low/neg) regulatory T cells. Methods
Mol Biol. 806:287–299. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Simonetta F, Chiali A, Cordier C, Urrutia
A, Girault I, Bloquet S, Tanchot C and Bourgeois C: Increased CD127
expression on activated FOXP3+CD4+ regulatory T cells. Eur J
Immunol. 40:2528–2538. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Rodriguez-Perea AL, Arcia ED, Rueda CM and
Velilla PA: Phenotypical characterization of regulatory T cells in
humans and rodents. Clin Exp Immunol. 185:281–291. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang HY, Yan KX, Huang Q, Ma Y, Fang X
and Han L: Target tissue ectoenzyme CD39/CD73-expressing Foxp3+
regulatory T cells in patients with psoriasis. Clin Exp Dermatol.
40:182–191. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Allard B, Longhi MS, Robson SC and Stagg
J: The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor
targets. Immunol Rev. 276:121–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bono MR, Fernandez D, Flores-Santibanez F,
Rosemblatt M and Sauma D: CD73 and CD39 ectonucleotidases in T cell
differentiation: Beyond immunosuppression. FEBS Lett.
589:3454–3460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Matyash M, Zabiegalov O, Wendt S, Matyash
V and Kettenmann H: The adenosine generating enzymes CD39/CD73
control microglial processes ramification in the mouse brain. PLoS
One. 12:e01750122017. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Hasan D, Blankman P and Nieman GF:
Purinergic signalling links mechanical breath profile and alveolar
mechanics with the pro-inflammatory innate immune response causing
ventilation-induced lung injury. Purinergic Signal. 13:363–386.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Drakes ML and Stiff PJ: Harnessing
immunosurveillance: Current developments and future directions in
cancer immunotherapy. Immunotargets Ther. 3:151–165. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Stallone G, Infante B, Di Lorenzo A,
Rascio F, Zaza G and Grandaliano G: mTOR inhibitors effects on
regulatory T cells and on dendritic cells. J Transl Med.
14:1522016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Oberg HH, Juricke M, Kabelitz D and Wesch
D: Regulation of T cell activation by TLR ligands. Eur J Cell Biol.
90:582–592. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Su J, Xie Q, Xu Y, Li XC and Dai Z: Role
of CD8(+) regulatory T cells in organ transplantation. Burns
Trauma. 2:18–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Ferguson AR and Engelhard VH: CD8 T cells
activated in distinct lymphoid organs differentially express
adhesion proteins and coexpress multiple chemokine receptors. J
Immunol. 184:4079–4086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Croft M and Siegel RM: Beyond TNF: TNF
superfamily cytokines as targets for the treatment of rheumatic
diseases. Nat Rev Rheumatol. 13:217–233. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xu Z, Ho S, Chang CC, Zhang QY, Vasilescu
ER, Vlad G and Suciu-Foca N: Molecular and cellular
characterization of human CD8 T suppressor cells. Front Immunol.
7:5492016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Parkes MD, Halloran PF and Hidalgo LG:
Mechanistic sharing between NK cells in ABMR and effector T cells
in TCMR. Am J Transplant. 18:63–73. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Nagy E, Lei Y, Martinez-Martinez E, Body
SC, Schlotter F, Creager M, Assmann A, Khabbaz K, Libby P, Hansson
GK, et al: Interferon-gamma released by activated CD8+ T
lymphocytes impairs the calcium resorption potential of osteoclasts
in calcified human aortic valves. Am J Pathol. 187:1413–1425. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Hasegawa H, Kawahata K, Mizoguchi F,
Okiyama N, Miyasaka N and Kohsaka H: Direct suppression of
autoaggressive CD8+ T cells with CD80/86 blockade in CD8+ T
cell-mediated polymyositis models of mice. Clin Exp Rheumatol.
35:593–597. 2017.PubMed/NCBI
|
|
80
|
Nejad Beyranvand E, van der Sluis TC, van
Duikeren S, Yagita H, Janssen GM, van Veelen PA, Melief CJ, van der
Burg SH and Arens R: Tumor eradication by cisplatin is sustained by
CD80/86-mediated costimulation of CD8+ T cells. Cancer Res.
76:6017–6029. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Pierini A, Schneidawind D, Nishikii H and
Negrin RS: Regulatory T cell immunotherapy in immune-mediated
diseases. Curr Stem Cell Rep. 1:177–186. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Long SA, Thorpe J, DeBerg HA, Gersuk V,
Eddy J, Harris KM, Ehlers M, Herold KC, Nepom GT and Linsley PS:
Partial exhaustion of CD8 T cells and clinical response to
teplizumab in new-onset type 1 diabetes. Sci Immunol. 1:pii:
eaai7793. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Joosten SA, Sullivan LC and Ottenhoff TH:
Characteristics of HLA-E restricted T-cell responses and their role
in infectious diseases. J Immunol Res. 2016:26953962016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Varthaman A, Clement M, Khallou-Laschet J,
Fornasa G, Gaston AT, Dussiot M, Caligiuri G, Cantor H, Kaveri S
and Nicoletti A: Physiological induction of regulatory
Qa-1-restricted CD8+ T cells triggered by endogenous CD4+ T cell
responses. PLoS One. 6:e216282011. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sinha S, Itani FR and Karandikar NJ:
Immune regulation of multiple sclerosis by CD8+ T cells. Immunol
Res. 59:254–265. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Leavenworth JW, Tang X, Kim HJ, Wang X and
Cantor H: Amelioration of arthritis through mobilization of
peptide-specific CD8+ regulatory T cells. J Clin Invest.
123:1382–1389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kambayashi T, Kraft-Leavy JR, Dauner JG,
Sullivan BA, Laur O and Jensen PE: The nonclassical MHC class I
molecule Qa-1 forms unstable peptide complexes. J Immunol.
172:1661–1669. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jiang H: The Qa-1 dependent CD8+ T cell
mediated regulatory pathway. Cell Mol Immunol. 2:161–167.
2005.PubMed/NCBI
|
|
89
|
Chen L, Reyes-Vargas E, Dai H, Escobar H,
Rudd B, Fairbanks J, Ho A, Cusick MF, Kumánovics A, Delgado J, et
al: Expression of the mouse MHC class Ib H2-T11 gene product, a
paralog of H2-T23 (Qa-1) with shared peptide-binding specificity. J
Immunol. 193:1427–1439. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Jensen PE, Sullivan BA, Reed-Loisel LM and
Weber DA: Qa-1, a nonclassical class I histocompatibility molecule
with roles in innate and adaptive immunity. Immunol Res. 29:81–92.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Ramsingh AI, Manley K, Rong Y, Reilly A
and Messer A: Transcriptional dysregulation of inflammatory/immune
pathways after active vaccination against Huntington's disease. Hum
Mol Genet. 24:6186–6197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Shiina T, Blancher A, Inoko H and Kulski
JK: Comparative genomics of the human, macaque and mouse major
histocompatibility complex. Immunology. 150:127–138. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chiswick EL, Mella JR, Bernardo J and
Remick DG: Acute-phase deaths from murine polymicrobial sepsis are
characterized by innate immune suppression rather than exhaustion.
J Immunol. 195:3793–3802. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
O'Leary S, Lloyd ML, Shellam GR and
Robertson SA: Immunization with recombinant murine cytomegalovirus
expressing murine zona pellucida 3 causes permanent infertility in
BALB/c mice due to follicle depletion and ovulation failure. Biol
Reprod. 79:849–860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cheng MH and Nelson LM: Mechanisms and
models of immune tolerance breakdown in the ovary. Semin Reprod
Med. 29:308–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li XL, Menoret S, Bezie S, Caron L,
Chabannes D, Hill M, Halary F, Angin M, Heslan M, Usal C, et al:
Mechanism and localization of CD8 regulatory T cells in a heart
transplant model of tolerance. J Immunol. 185:823–833. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Nambu Y, Hayashi T, Jang KJ, Aoki K, Mano
H, Nakano K, Osato M, Takahashi K, Itoh K, Teramukai S, et al:
In situ differentiation of CD8αα T cells from CD4 T cells in
peripheral lymphoid tissues. Sci Rep. 2:6422012. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Tang X, Maricic I and Kumar V: Anti-TCR
antibody treatment activates a novel population of nonintestinal
CD8 alpha alpha+ TCR alpha beta+ regulatory T cells and prevents
experimental autoimmune encephalomyelitis. J Immunol.
178:6043–6050. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Kumar V and Sercarz E: An integrative
model of regulation centered on recognition of TCR peptide/MHC
complexes. Immunol Rev. 182:113–121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Ge PL, Ma LP, Wang W, Li Y and Zhao WM:
Inhibition of collagen-induced arthritis by DNA vaccines encoding
TCR Vbeta5.2 and TCR Vbeta8.2. Chin Med J (Engl). 122:1039–1048.
2009.PubMed/NCBI
|
|
101
|
Xu H, Wang X, Malam N, Aye PP, Alvarez X,
Lackner AA and Veazey RS: Persistent simian immunodeficiency virus
infection drives differentiation, aberrant accumulation, and latent
infection of germinal center follicular T helper cells. J Virol.
90:1578–1587. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bruno F, Fornara C, Zelini P, Furione M,
Carrara E, Scaramuzzi L, Cane I, Mele F, Sallusto F, Lilleri D, et
al: Follicular helper T-cells and virus-specific antibody response
in primary and reactivated human cytomegalovirus infections of the
immunocompetent and immunocompromised transplant patients. J Gen
Virol. 97:1928–1941. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Muema DM, Macharia GN, Olusola BA, Hassan
AS, Fegan GW, Berkley JA, Urban BC and Nduati EW: Proportions of
circulating follicular helper T cells are reduced and correlate
with memory B cells in HIV-infected children. PLoS One.
12:e01755702017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Sugimoto T and Watanabe T: Follicular
Lymphoma: The role of the tumor microenvironment in prognosis. J
Clin Exp Hematop. 56:1–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Kurita D, Miyoshi H, Yoshida N, Sasaki Y,
Kato S, Niino D, Sugita Y, Hatta Y, Takei M, Makishima M, et al: A
clinicopathologic study of lennert lymphoma and possible prognostic
factors: the importance of follicular helper T-cell markers and the
association with angioimmunoblastic T-cell lymphoma. Am J Surg
Pathol. 40:1249–1260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Miles B, Miller SM, Folkvord JM, Levy DN,
Rakasz EG, Skinner PJ and Connick E: Follicular regulatory CD8 T
cells impair the germinal center response in SIV and ex vivo HIV
infection. PLoS Pathog. 12:e10059242016. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Tsai S, Clemente-Casares X and Santamaria
P: CD8(+) Tregs in autoimmunity: Learning ‘self’-control from
experience. Cell Mol Life Sci. 68:3781–3795. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Krausz LT, Major ZZ, Muresanu DF, Chelaru
E, Nocentini G and Riccardi C: Characterization of CD4+ and CD8+
Tregs in a Hodgkin's lymphoma patient presenting with
myasthenia-like symptoms. Ideggyogy Sz. 66:343–348. 2013.PubMed/NCBI
|
|
109
|
Spadaro M, Montarolo F, Perga S, Martire
S, Brescia F, Malucchi S and Bertolotto A: Biological activity of
glatiramer acetate on Treg and anti-inflammatory monocytes persists
for more than 10 years in responder multiple sclerosis patients.
Clin Immunol. 181:83–88. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
van Nierop GP, van Luijn MM, Michels SS,
Melief MJ, Janssen M, Langerak AW, Ouwendijk WJD, Hintzen RQ and
Verjans GMGM: Phenotypic and functional characterization of T cells
in white matter lesions of multiple sclerosis patients. Acta
Neuropathol. 134:383–401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Rådinger M, Bossios A, Alm AS, Jeurink P,
Lu Y, Malmhäll C, Sjöstrand M and Lötvall J: Regulation of
allergen-induced bone marrow eosinophilopoiesis: Role of CD4+ and
CD8+ T cells. Allergy. 62:1410–1418. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Zhang H, Kong H, Zeng X, Guo L, Sun X and
He S: Subsets of regulatory T cells and their roles in allergy. J
Transl Med. 12:1252014. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Martin-Orozco E, Norte-Munoz M and
Martinez-Garcia J: Regulatory T cells in allergy and asthma. Front
Pediatr. 5:1172017. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Tulunay A, Yavuz S, Direskeneli H and
Eksioglu-Demiralp E: CD8+CD28-, suppressive T cells in systemic
lupus erythematosus. Lupus. 17:630–637. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Zabinska M, Krajewska M,
Koscielska-Kasprzak K and Klinger M: CD3(+)CD8(+)CD28(-) T
lymphocytes in patients with lupus nephritis. J Immunol Res.
2016:10581652016. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Ceeraz S, Hall C, Choy EH, Spencer J and
Corrigall VM: Defective CD8+CD28+ regulatory T cell suppressor
function in rheumatoid arthritis is restored by tumour necrosis
factor inhibitor therapy. Clin Exp Immunol. 174:18–26. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Mikulkova Z, Praksova P, Stourac P,
Bednarik J, Strajtova L, Pacasova R, Belobradkova J, Dite P and
Michalek J: Numerical defects in CD8+CD28- T-suppressor lymphocyte
population in patients with type 1 diabetes mellitus and multiple
sclerosis. Cell Immunol. 262:75–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Arosa FA: CD8+CD28- T cells: Certainties
and uncertainties of a prevalent human T-cell subset. Immunol Cell
Biol. 80:1–13. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Sfanos KS and De Marzo AM: Prostate cancer
and inflammation: The evidence. Histopathology. 60:199–215. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Mhawech-Fauceglia P, Wang D, Ali L, Lele
S, Huba MA, Liu S and Odunsi K: Intraepithelial T cells and
tumor-associated macrophages in ovarian cancer patients. Cancer
Immun. 13:12013.PubMed/NCBI
|
|
121
|
Longoria TC and Tewari KS: Pharmacologic
management of advanced cervical cancer: Antiangiogenesis therapy
and immunotherapeutic considerations. Drugs. 75:1853–1865. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Liu H, Wang Y, Zeng Q, Zeng YQ, Liang CL,
Qiu F, Nie H and Dai Z: Suppression of allograft rejection by
CD8+CD122+PD-1+ Tregs is dictated by their Fas ligand-initiated
killing of effector T cells versus Fas-mediated own apoptosis.
Oncotarget. 8:24187–24195. 2017.PubMed/NCBI
|
|
123
|
Beres AJ, Haribhai D, Chadwick AC, Gonyo
PJ, Williams CB and Drobyski WR: CD8+ Foxp3+ regulatory T cells are
induced during graft-versus-host disease and mitigate disease
severity. J Immunol. 189:464–474. 2012. View Article : Google Scholar : PubMed/NCBI
|