Introduction
Gastric cancer (GC) is an aggressive disease and a
leading cause of cancer-associated mortality in China (1). A study reported 679,100 novel cases of
GC in China, but the diagnostic rate of early GC remained extremely
low at <10% in 2012 (1). Certain
advances in GC therapy, including surgical resection with adjuvant
chemotherapy or chemoradiation, have improved the overall survival
time for patients with GC. However, due to tumor recurrence and
metastasis at advanced stages, the disease continues to exhibit a
poor prognosis (2,3). Therefore, studies investigating novel
and more efficient tumor markers and potential targets for GC
therapy are required.
MicroRNAs (miRNAs/miRs) are a class of small,
regulatory non-coding RNAs involved in post-translational
regulation by targeting the 3′-untranslated region (3′-UTR) of mRNA
(4,5).
miR-140-5p has been reported to function as a tumor suppressor in
certain types of tumor. Jing et al (6) confirmed that microR-140-5p suppresses
tumor cell migration and invasion by targeting the ADAM10-mediated
Notch1 signaling pathway in hypopharyngeal squamous cell carcinoma.
Wei et al (7) reported that
miR-140-5p attenuates chemotherapeutic drug-induced cell death by
regulating autophagy through inositol 1,4,5-trisphosphate kinase 2
in human osteosarcoma cells. In GC development, Fang et al
(8) revealed that miR-140-5p
suppresses the proliferation, migration and invasion of GC by
regulating YES1. However, the underlying mechanisms of miR-140-5p
involved in GC remain largely unknown.
The present study demonstrated that miR-140-5p,
which was downregulated in GC tissues and cells, functioned as a
tumor suppressor. Lower expression of miR-140-5p was revealed to be
associated with a shorter survival time in patients with GC.
Furthermore, it was demonstrated that miR-140-5p suppressed cell
proliferation and invasion by targeting the WNT1-mediated
Wnt/β-catenin signaling pathway. Therefore, the results of the
present study indicated that miR-140-5p may serve as a prognostic
maker for the survival of patients with GC and as a potential
target of GC treatment.
Materials and methods
Patient tissue samples
The present study was approved by Huizhou Municipal
Central Hospital of Guangdong Province (Huizhou, China). Written
informed consent was obtained from all patients. A total of 60
paired GC tissues and adjacent non-tumor tissues were obtained from
42 male and 18 female patients (mean age, 52.5 years; range, 30–82
years), who underwent radical gastrectomy at Huizhou Municipal
Central Hospital of Guangdong Province between March 2011 and April
2015. None of the patients had received any radiotherapy or
chemotherapy prior to surgery. Tissue samples were snap-frozen in
liquid nitrogen following surgery until further analyses. The
clinical data of patients with GC are presented in Table I. All specimens had a confirmed
pathological diagnosis and were staged according to the 2009
UICC-Tumor-Node-Metastasis (TNM) Classification of Malignant Tumors
(9).
 | Table I.Association between miR-140-5p
expression and clinical data in patients. |
Table I.
Association between miR-140-5p
expression and clinical data in patients.
| Clinicopathological
factor | No. patients | High expression
(n=30) | Low expression
(n=30) | P-value |
|---|
| Age, years |
|
|
| 0.630 |
| ≤55 | 37 | 22 | 15 |
|
|
>55 | 23 | 8 | 15 |
|
| Sex |
|
|
| 0.691 |
| Male | 42 | 20 | 22 |
|
|
Female | 18 | 10 | 8 |
|
| Tumor size, cm |
|
|
| 0.292 |
|
<3 | 36 | 20 | 16 |
|
|
>3 | 24 | 10 | 14 |
|
| Histological
grade |
|
|
| 0.390 |
| High and
middle | 43 | 23 | 20 |
|
| Poor | 17 | 7 | 10 |
|
| Lymph node
metastasis |
|
|
| 0.018a |
|
Negative | 35 | 22 | 13 |
|
|
Positive | 25 | 8 | 17 |
|
| TNM stage |
|
|
| 0.038a |
| I–II | 32 | 20 | 12 |
|
|
III–IV | 28 | 10 | 18 |
|
Cell line culture
The human GC MKN-45, BGC-823 and SGC-7901 cell
lines, and the human immortalized normal gastric mucosa GES-1 cell
line were purchased from the Shanghai Institute of Biochemistry and
Cell Biology (Shanghai, China). Cells were cultured in RPMI-1640
medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA),
supplemented with 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.), 1% penicillin and 1% streptomycin, and were cultured at 37°C
in humidified air with 5% CO2.
Cell transfection
The following sequences were constructed by and
purchased from Shanghai GenePharma Co., Ltd. (Shanghai, China):
miR-140-5p mimic,
5′-CAGUGGUUUUACCCUAUGGUAGACCAUAGGGUAAAACCACUGUU-3′; miR-140-5p
inhibitor, 5′-AACCCAUGGAAUUCAGUUCUCA-3′ and the corresponding
negative control, 5′-UUCUCCGAACGUGUCACGUTTACGUGACACGUUCGGAGAATT-3′.
The cells were transfected with miR-140-5p mimic or inhibitor (100
nM) or respective negative control (NC), according to the
manufacturer's protocol. The pcDNA3.1-WNT1 and the negative control
(pcDNA3.1-vector) plasmids were obtained from Shanghai GenePharma
Co., Ltd. Transfection was performed using Lipofectamine 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. The RNA analysis and protein analysis
were performed at 48 h following transfection.
Identification of potential target
genes
TargetScan (www.targetscan.org) and MiRanda (www.microrna.org) databases were used for predicting
the target gene of miR-140-5p.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
TRIzol reagent (Takara, Dalian, China), according to the
manufacturer's protocols. RNA was reverse transcribed to cDNA using
a reverse transcription kit (Takara, Dalian, China) according to
the manufacturer's protocols. RT-qPCR was performed using a
SYBRGreen PCR kit (Takara, Dalian, China), according to the
manufacturer's protocols. The reaction was performed on an Applied
Biosystems Step One Plus Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The relative expression of GAPDH
or U6 was used as an internal control for WNT1 or miR-140-5p
expression, respectively. The primer sequences were as follows:
miR-140-5p forward, 5′-GAGTGTCAGTGGTTTTACCCT-3′ and reverse,
5′-GCAGGGTCCGAGGTATTC-3′; WNT1 forward, 5′-CTGTGCGAGAGTGCAAATGG-3′
and reverse, 5′-GATGAACGCTGTTTCTCGGC-3′; and GAPDH forward,
5′-AGACACCATGGGGAAGGTGAA-3′ and GAPDH reverse,
5′-ATTGCTGATGATCTTGAGGCTG-3′. The thermocycling conditions were as
follows: 50°C for 2 min, followed by 40 cycles of 95°C for 15 sec
and 60°C for 1 min. The mRNA expression was calculated using the
2−ΔΔCq methods (10).
Cell proliferation assay
A cell proliferation assay was performed using a
CCK-8 kit (Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
In brief, 2,000 MKN-45 or BGC-823 cells/well were seeded into
96-well plates. Cells were cultured in RPMI-1640 medium, containing
10% FBS at 37°C in a humidified atmosphere with 5% CO2.
Next, at indicated time points (12, 24, 48 and 72 h), CCK-8
solution (10 µl/well) was added into each well according to the
manufacturer's protocols. Finally, cell proliferation rate was
detected using a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) at an absorbance of 450 nm.
Colony formation assay
For the colony formation assay, 300 cells were
transfected with miR-NC, miR-140-5p mimic or miR-140-5p inhibitor,
and cultured on 12-well plates. After 14 days, cell colonies were
fixed with 100% methanol for 20 min and stained with 0.1% crystal
violet for 20 min at room temperature, and the number of cell
colonies was calculated in randomly selected fields. The cell
colony number was quantified by counting the colonies with >50
cells.
Cell invasion assay
A cell invasion assay was performed using 8-µm pores
coated with Matrigel (BD Biosciences, Franklin Lakes, NJ, USA). A
total of 1×105 MKN-45 or BGC-823 cells were resuspended
in serum-free RPMI-1640 medium and seeded into the top chamber of
the inserts, and 500 µl PRIM-1640 medium, supplemented with 10%
FBS, was added to the lower chamber. Following cell transfection at
48 h, cells in the lower chamber were fixed with 100% methanol for
20 min at room temperature and stained with 0.1% crystal violet for
30 min at room temperature. Cells were counted under a light
microscope (AZ100; Nikon Corporation, Tokyo, Japan) in ten randomly
selected fields, ×200, magnification.
Western blot analysis
Total protein was isolated from transfected GC cells
at 48 h after transfection using radioimmunoprecipitation assay
buffer (Beyotime Institute of Biotechnology, Haimen, China). The
concentration of protein was detected using a bicinchoninic acid
protein assay kit (Beyotime Institute of Biotechnology), according
to the manufacturer's protocols. Protein samples were separated on
10% SDS-PAGE and were transferred onto polyvinylidene difluoride
membranes (Bio-Rad Laboratories, Inc.). Next, the membranes were
incubated with primary antibodies against WNT1 (dilution, 1:3,000;
cat. no. ab15251; Abcam, Cambridge, MA, USA and GAPDH (dilution,
1:3,000; cat. no. ab14247; Abcam) at 4°C overnight. Following
incubation with horseradish peroxidase-conjugated anti-rabbit
immunoglobulin G secondary antibodies (dilution, 1:5,000; cat. no.
ab151318; Abcam) at room temperature for 1 h, the protein bands
were visualized using an enhanced chemiluminescence system. GAPDH
expression was used as an internal control.
Dual-luciferase reporter assay
The wild-type 3′-UTR of WNT1 mRNA that had a
putative miR-140-5p binding site and mutant type 3′-UTR of WNT1
were inserted into pMIRGLO vectors (Promega Corporation, Madison,
WI, USA). MKN-45 cells were co-transfected with miR-140-5p mimic or
miR-negative control and pMIRGLO-wild type 3′UTR of WNT1 vector or
pMIRGLO-mutant type 3′-UTR of WNT1 vector. Following cell
transfection for 48 h, luciferase activity was detected using a
dual-luciferase reporter assay system (E1910; Promega
Corporation).
Statistical analysis
All the experiments in the present study were
analyzed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA).
The results are presented as the mean ± standard deviation.
Differences between two groups was analyzed using Student's t-test.
Differences among groups were analyzed using one-way analysis of
variance, followed by the Student-Newman-Keuls post hoc test. The
χ2 test was used to assess the associations between
miR-140-5p and clinicopathological factors in patients with GC.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of miR-140-5p is
significantly downregulated in GC tissues and is associated with
prognosis
The present study analyzed the miR-140-5p expression
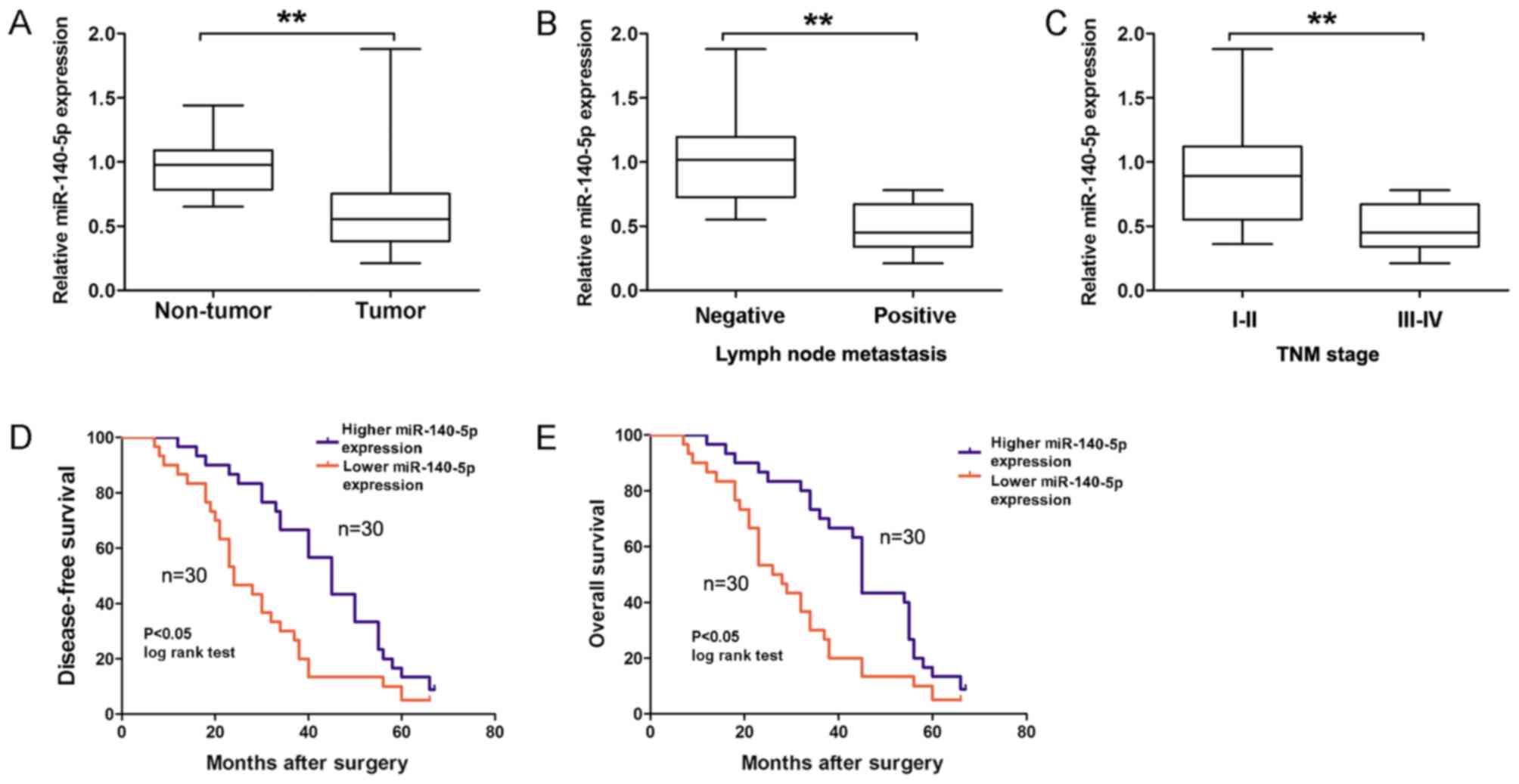
in 60 GC tissues and adjacent non-tumor tissues using RT-qPCR. As
demonstrated in Fig. 1A, the relative
expression of miR-140-5p was significantly downregulated in GC
tissues compared with adjacent non-tumor tissues (P<0.01).
Furthermore, miR-140-5p expression was divided into two (higher or
lower expression) groups according to the median expression of
miR-140-5p in GC tissues. Analysis results revealed that a lower
miR-140-5p expression was significantly associated with lymph node
metastasis (Fig. 1B; Table I; P=0.018) and advanced TNM stage
(Fig. 1C; Table I; P=0.038) in patients with GC.
Kaplan-Meier survival analysis revealed that a lower miR-140-5p
expression predicted a poorer disease-free survival (DFS; Fig. 1D; log-rank=8.55; P<0.05) and
overall survival (OS; Fig. 1E;
log-rank=9.53; P<0.05) time in patients with GC, compared with
the higher miR-140-5p expression group. Therefore, these results
suggested that miR-140-5p expression was downregulated in GC and
associated with GC prognosis.
miR-140-5p suppresses cell
proliferation and invasion of GC in vitro
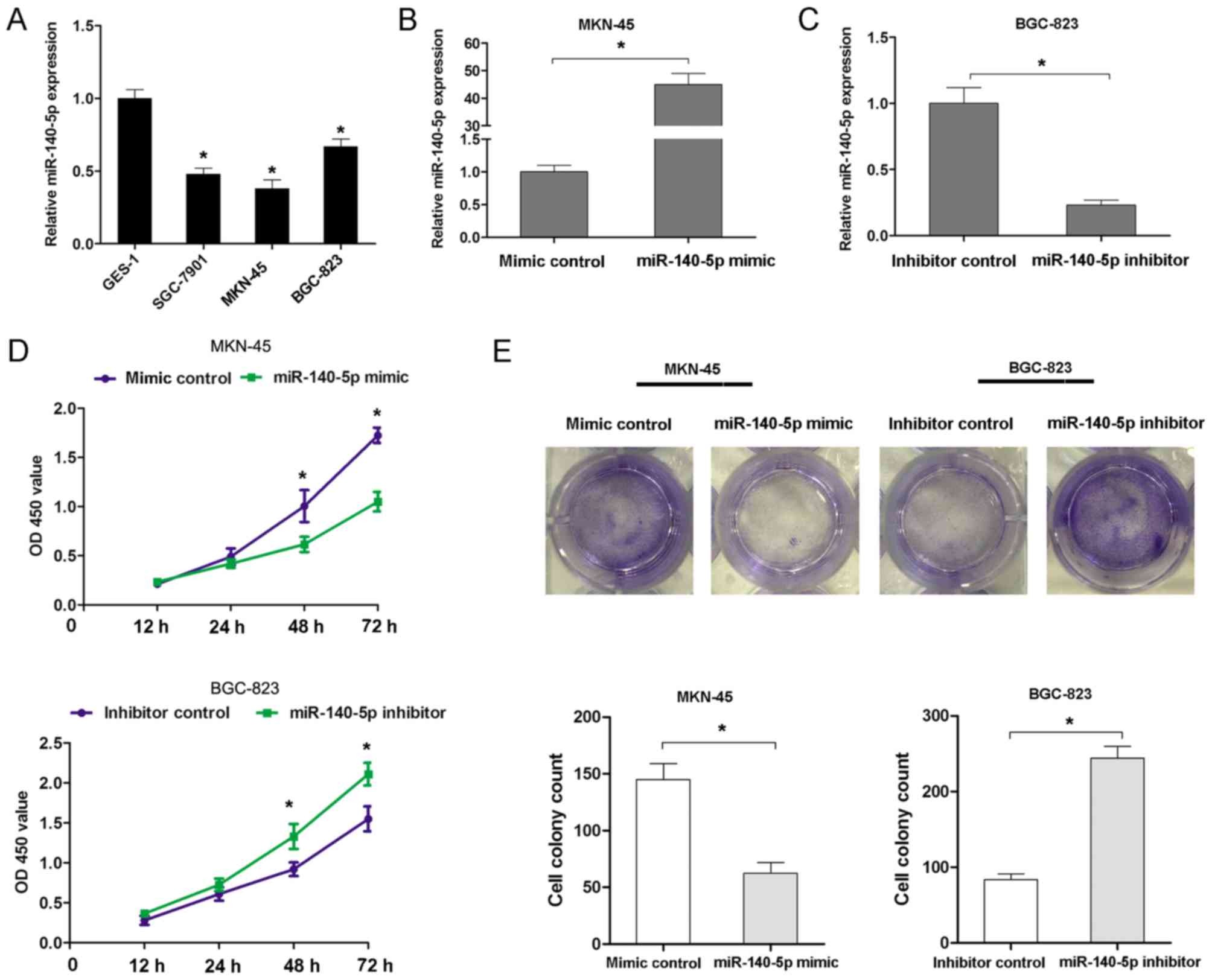
miR-140-5p expression was further investigated in
three GC cell lines (MKN-45, BGC-823 and SGC-7901 cells) and GES-1
cells using RT-qPCR analyses. The results confirmed that miR-140-5p
expression was notably lower in GC cell lines than in GES-1 cells
(Fig. 2A). To assess the effects of
miR-140-5p expression on proliferation and invasion, a
gain-of-function assay was performed in MKN45 cells and a
loss-of-function assay was performed in BGC-823 cells. The assays
were performed according to the expression level of miR-140-5p in
MKN-45 cells (higher expression level) and BGC-823 cells (lower
expression level). The miR-140-5p mimic significantly increased the
expression of miR-140-5p in MKN-45 cells, while the miR-140-5p
inhibitor decreased the expression of miR-140-5p in BGC-823 cells
(Fig. 2B and C). Transfection of
MKN-45 cells with the miR-140 mimic significantly suppressed the
cell proliferation rate at 48 and 72 h, but transfection of BGC-823
cells with the miR-140-5p inhibitor resulted in an increased cell
proliferation rate at 48 and 72 h (Fig.
2D). Furthermore, transfection of MKN45 cells with miR-140
mimic resulted in the formation of fewer cell colonies, compared
with the control group. However, transfection of BGC-823 cells with
the miR-140-5p inhibitor significantly increased the cell colony
number (Fig. 2E). Additionally, the
present study examined whether miR-140-5p expression affected the
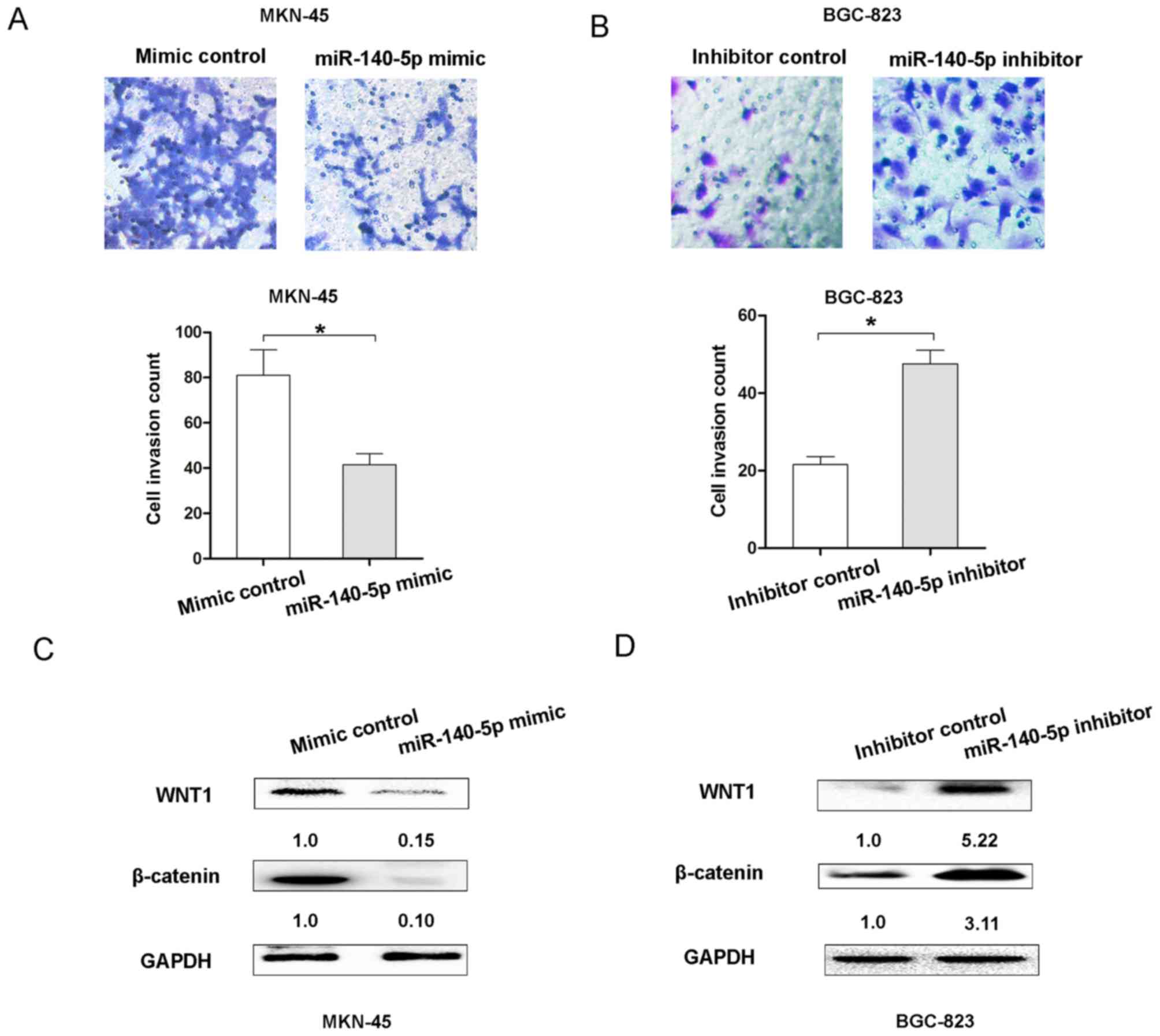
invasion of GC cells. The Transwell cell invasion assay results
demonstrated that transfection of MKN45 cells with the miR-140
mimic resulted in decreasing cell invasion ability, but
transfection of BGC-823 cells with the miR-140-5p inhibitor
enhanced cell invasion ability (Fig. 3A
and B). Therefore, these results indicated that miR-140-5p
could inhibit cell proliferation and invasion of GC in
vitro.
miR-140-5p suppresses the
Wnt/β-catenin signaling pathway by targeting WNT1 in GC cells
The Wnt/β-catenin signaling pathway was revealed to
be associated with the proliferation and invasion of GC cells
(11). To demonstrate whether
miR-140-5p expression affected the Wnt/β-catenin signaling pathway,
the expression of Wnt/β-catenin signaling molecules, WNT1 and
β-catenin, was detected following upregulation or downregulation of
miR-140-5p in GC cells. The results demonstrated that miR-140-5p
overexpression suppressed the expression of WNT1 and β-catenin in
MKN-45 cells, compared with the control group (Fig. 3C). However, decreasing miR-140-5p
expression upregulated the expression levels of WNT1 and β-catenin
in BGC-823 cells (Fig. 3D). These
results indicated that miR-140-5p suppressed the Wnt/β-catenin
signaling pathway in GC.
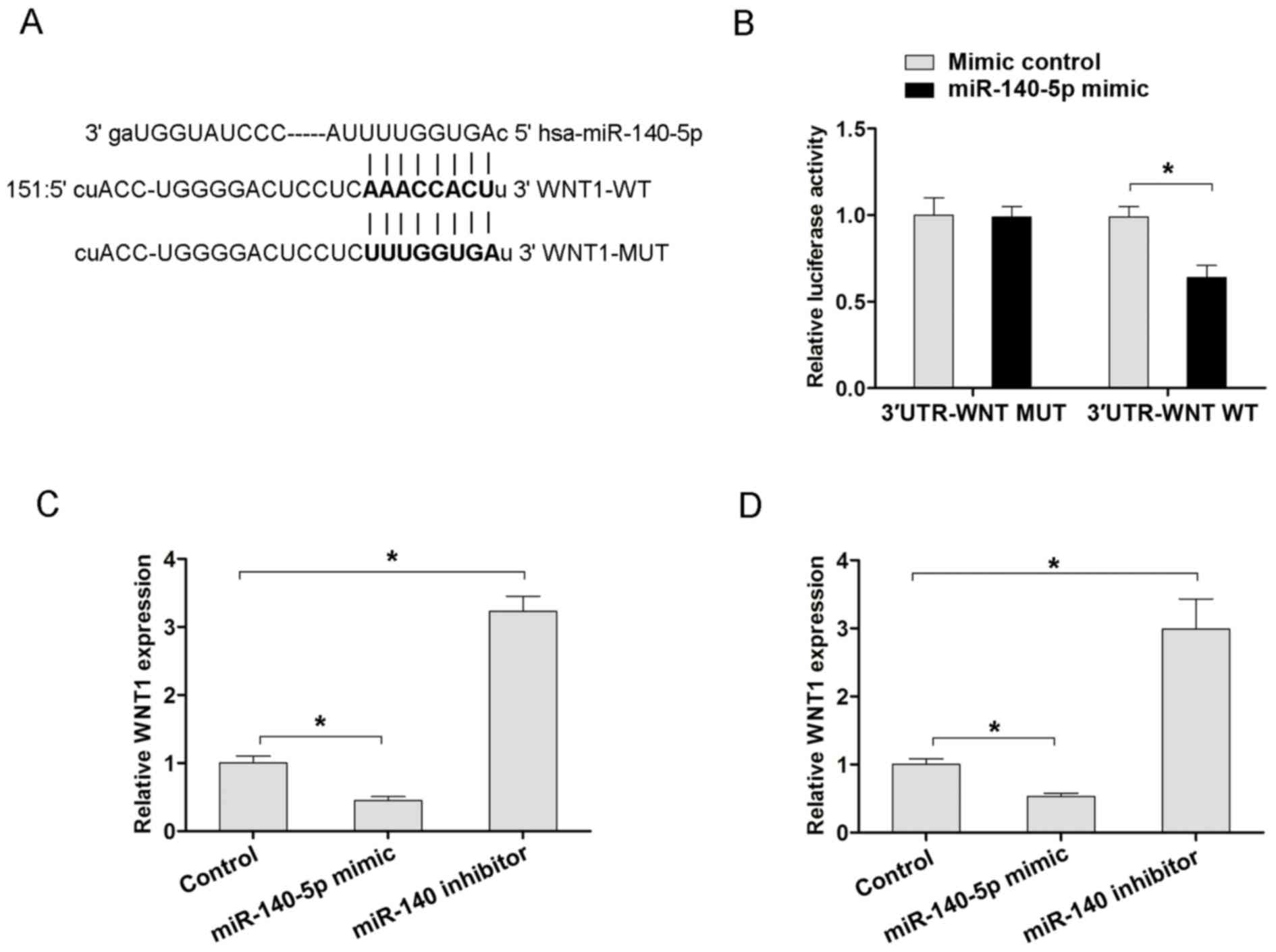
Furthermore, the TargetScan (www.targetscan.org) or MiRanda (www.microrna.org) databases were used to search
potential target genes of miR-140-5p. From the miRanda data, it was
revealed that WNT1 was a potential target of miR-140-5p (Fig. 4A). Furthermore, the WT 3′-UTR of WNT1
mRNA that had a putative miR-140-5p binding site and Mut type
3′-UTR of WNT1 were inserted into the pMIRGLO vectors, prior to
luciferase assays being performed. The results revealed that
miR-140-5p overexpression decreased the luciferase activity of WT
3′-UTR of the WNT1 vector, but not in the Mut 3′-UTR of the WNT1 in
MKN45 cells (Fig. 4B). Furthermore,
the present study also examined the mRNA expression of WNT1
following miR-140-5p overexpression or downregulation in MKN-45 and
BGC-823 cells. Transfection with the miR-140-5p mimic in MKN45 and
BGC-823 cells resulted in decreased WNT1 mRNA, but transfection
with the miR-140-5p inhibitor exhibited increased WNT1 mRNA
expression, compared with the corresponding control groups
(Fig. 4C and D). Therefore, the
aforementioned results indicated that WNT1 is a target of
miR-140-5p, and that miR-140-5p suppressed the Wnt/β-catenin
signaling pathway by targeting WNT1 in GC.
Overexpression of WNT1 reverses the
effects of miR-140-5p on the proliferation and invasion of GC
cells
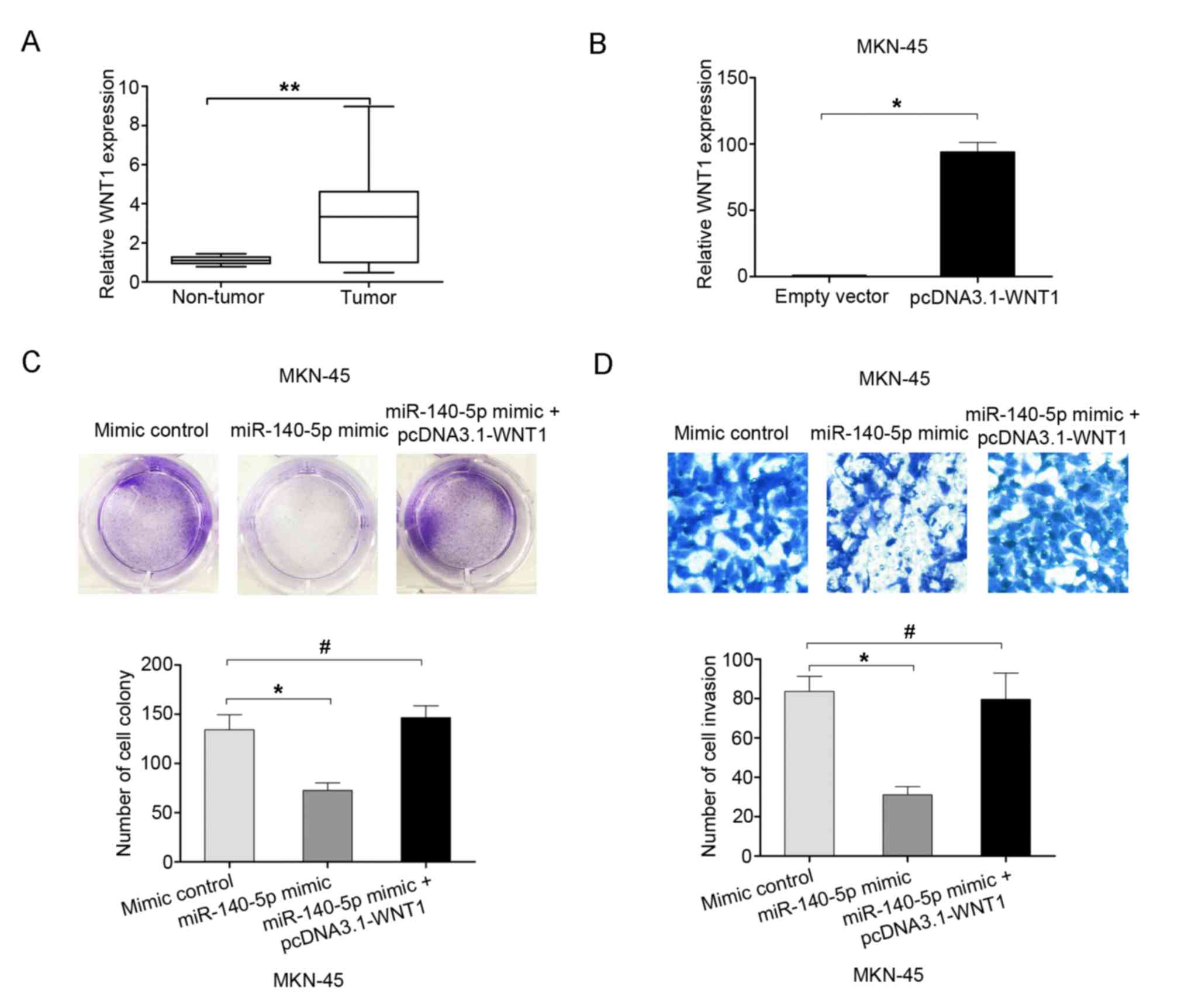
The expression of WNT1 was examined in 60 GC tissues
and adjacent non-tumor tissues using RT-qPCR analyses. The results
indicated that WNT1 mRNA expression was higher in GC tissues than
in adjacent non-tumor tissues (Fig.
5A). To demonstrate whether WNT mediated the effects of
miR-140-5p on the proliferation and invasion of GC cells, rescue
experiments were performed by overexpressing WNT1 using the
pcDNA3.1-WNT1 plasmid in MKN-45 cells (Fig. 5B). The cell colony formation assay
results demonstrated that the cell proliferation ability was
inhibited following miR-140-5p overexpression in MKN-45 cells, but
this effect was reversed by co-transfection with miR-140-5p mimic
and pcDNA3.1-WNT1 (Fig. 5C). The cell
invasion assay results demonstrated that cell invasion ability was
inhibited following miR-140-5p overexpression in MKN-45 cells, but
the effect was reversed by co-transfection with miR-140-5p mimic
and pcDNA3.1-WNT1 (Fig. 5D).
Therefore, the results of the present study indicated that
overexpression of WNT1 may reverse the effects of miR-140-5p on
cell proliferation and invasion.
Discussion
Recent studies have indicated that deregulation of
miRNAs contributes toward the tumorigenesis and progression of GC
(12,13). miR-140-5p acts as tumor suppressor to
serve important regulatory roles in cancer development. miR-140-5p
suppresses tumor growth and metastasis by targeting transforming
growth factor β receptor 1 and fibroblast growth factor 9 in
hepatocellular carcinoma (14).
miR-140-5p could suppress the proliferation of lung cancer cells by
regulating Erk signaling (15).
Overexpression of hsa-miR-140-5p in colorectal cancer (CRC) cell
lines decreases Smad2 expression levels, leading to decreased cell
invasion and proliferation, and increased cell cycle arrest
(16). miR-140-5p was significantly
downregulated in CRC and inhibits the progression of CRC by
targeting VEGFA (17). The present
study demonstrated that miR-140-5p was significantly downregulated
in GC. Lower miR-140-5p expression was significantly associated
with lymph node metastasis and advanced clinical stages in patients
with GC. A recent study also demonstrated that miR-140-5p
suppresses the proliferation, migration and invasion of GC by
regulating YES1 (8). Additionally,
the present study demonstrated that miR-140-5p overexpression
inhibited cell proliferation and invasion ability.
Furthermore, it was demonstrated that miR-140-5p
suppressed the Wnt/β-catenin signaling pathway. Additionally, the
Wnt/β-catenin signaling pathway has been identified as a key
signaling pathway in the proliferation, invasion and metastatic
cascade of GC cells (18,19). A previous study undertaken by Yue
et al (20) reported that
miR-519d suppresses the GC epithelial-mesenchymal transition via
Twist1 and inhibits the Wnt/β-catenin signaling pathway. Wu et
al (21) demonstrated that
miRNA-27a promotes the proliferation and invasion of human GC
MGC803 cells by targeting SFRP1 via the Wnt/β-catenin signaling
pathway. miR-194 activates the Wnt/β-catenin signaling pathway in
GC by targeting the negative Wnt regulator, SUFU (22). Another study revealed that LINC00052
promotes GC cell proliferation and metastasis via activating the
Wnt/β-Catenin signaling pathway (23). These studies have identified the
importance of the Wnt/β-Catenin signaling pathway in GC
progression. The present study demonstrated that miR-140-5p
suppressed the Wnt/β-Catenin signaling pathway by targeting the
3′-UTR region of WNT1 (a key molecule of the Wnt/β-Catenin
signaling pathway), and regulated its mRNA and protein expression.
In addition, the present study demonstrated that overexpression of
WNT1 could reverse the effects of miR-140-5p on cell proliferation
and invasion. Therefore, these results indicated that miR-140-5p
suppressed cell proliferation and invasion by regulating the
WNT1-mediated Wnt/β-Catenin signaling pathway in GC.
In conclusion, the results of the present study
indicated that miR-140-5p was downregulated in GC and that a lower
expression of miR-140-5p predicted a poorer prognosis in patients
with GC. Furthermore, it was revealed that miR-140-5p suppressed
cell proliferation and invasion. Additionally, the present study
demonstrated that miR-140-5p inhibited the Wnt/β-Catenin signaling
pathway by targeting WNT1. Therefore, these results indicated that
miR-140-5p may serve as a prognostic biomarker of GC and a
potential target for GC treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
YC, YH and KO performed the experiments in the
present study. HX, JL and XY performed the data analysis and YC and
XY designed the present study and wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by Huizhou Municipal
Central Hospital of Guangdong Province (Huizhou, China). Written
informed consent was obtained from all patients.
Patient consent for publication
All participants agreed to the publication of the
present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leja M, You W, Camargo MC and Saito H:
Implementation of gastric cancer screening-the global experience.
Best Pract Res Clin Gastroenterol. 28:1093–1106. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Carcas LP: Gastric cancer review. J
Carcinog. 13:142014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rao M, Zhu Y, Cong X and Li Q: Knockdown
of CREB1 inhibits tumor growth of human gastric cancer in vitro and
in vivo. Oncol Rep. 37:3361–3368. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xie M, Dart DA, Guo T, Xing XF, Cheng XJ,
Du H, Jiang WG, Wen XZ and Ji JF: MicroRNA-1 acts as a tumor
suppressor microRNA by inhibiting angiogenesis-related growth
factors in human gastric cancer. Gastric Cancer. 21:41–54. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jing P, Sa N, Liu X, Liu X and Xu W:
MicroR-140-5p suppresses tumor cell migration and invasion by
targeting ADAM10-mediated Notch1 signaling pathway in
hypopharyngeal squamous cell carcinoma. Exp Mol Pathol.
100:132–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wei R, Cao G, Deng Z, Su J and Cai L:
miR-140-5p attenuates chemotherapeutic drug-induced cell death by
regulating autophagy through inositol 1,4,5-trisphosphate kinase 2
(IP3k2) in human osteosarcoma cells. Biosci Rep. 36:e003922016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang Z, Yin S, Sun R, Zhang S, Fu M, Wu Y,
Zhang T, Khaliq J and Li Y: miR-140-5p suppresses the
proliferation, migration and invasion of gastric cancer by
regulating YES1. Mol Cancer. 16:1392017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Novák J and Fabian P: Comments on the TNM
classification of malignant tumours-7th edition. Klin Onkol.
24:149–150. 2011.(In Czech). PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang T, Wu Y, Fang Z, Yan Q, Zhang S, Sun
R, Khaliq J and Li Y: Low expression of RBMS3 and SFRP1 are
associated with poor prognosis in patients with gastric cancer. Am
J Cancer Res. 6:2679–2689. 2016.PubMed/NCBI
|
|
12
|
Wang J, Chen X, Su L, Li P, Cai Q, Liu B,
Wu W and Zhu Z: MicroRNA-126 inhibits cell proliferation in gastric
cancer by targeting LAT-1. Biomed Pharmacother. 72:66–73. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiang XJ, Deng J, Liu YW, Wan LY, Feng M,
Chen J and Xiong JP: MiR-1271 inhibits cell proliferation, invasion
and EMT in gastric cancer by targeting FOXQ1. Cell Physiol Biochem.
36:1382–1394. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang H, Fang F, Chang R and Yang L:
MicroRNA-140-5p suppresses tumor growth and metastasis by targeting
transforming growth factor β receptor 1 and fibroblast growth
factor 9 in hepatocellular carcinoma. Hepatology. 58:205–217. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li W and He F: Monocyte to macrophage
differentiation-associated (MMD) targeted by miR-140-5p regulates
tumor growth in non-small cell lung cancer. Biochem Biophys Res
Commun. 450:844–850. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhai H, Fesler A, Ba Y, Wu S and Ju J:
Inhibition of colorectal cancer stem cell survival and invasive
potential by hsa-miR-140-5p mediated suppression of Smad2 and
autophagy. Oncotarget. 6:19735–19746. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang W, Zou C, Pan L, Xu Y, Qi W, Ma G,
Hou Y and Jiang P: MicroRNA-140-5p inhibits the progression of
colorectal cancer by targeting VEGFA. Cell Physiol Biochem.
37:1123–1133. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Y, Yan W, Liu X, Jia Y, Cao B, Yu Y, Lv
Y, Brock MV, Herman JG, Licchesi J, et al: DACT2 is frequently
methylated in human gastric cancer and methylation of DACT2
activated Wnt signaling. Am J Cancer Res. 4:710–724.
2014.PubMed/NCBI
|
|
19
|
Nunez F, Bravo S, Cruzat F, Montecino M
and De Ferrari GV: Wnt/β-catenin signaling enhances
cyclooxygenase-2 (COX2) transcriptional activity in gastric cancer
cells. PLoS One. 6:e185622011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yue H, Tang B, Zhao Y, Niu Y, Yin P, Yang
W, Zhang Z and Yu P: MIR-519d suppresses the gastric cancer
epithelial-mesenchymal transition via Twist1 and inhibits
Wnt/β-catenin signaling pathway. Am J Transl Res. 9:3654–3664.
2017.PubMed/NCBI
|
|
21
|
Wu F, Li J, Guo N, Wang XH and Liao YQ:
MiRNA-27a promotes the proliferation and invasion of human gastric
cancer MGC803 cells by targeting SFRP1 via Wnt/β-catenin signaling
pathway. Am J Cancer Res. 7:405–416. 2017.PubMed/NCBI
|
|
22
|
Peng Y, Zhang X, Ma Q, Yan R, Qin Y, Zhao
Y, Cheng Y, Yang M, Wang Q, Feng X, et al: MiRNA-194 activates the
Wnt/β-catenin signaling pathway in gastric cancer by targeting the
negative Wnt regulator, SUFU. Cancer Lett. 385:117–127. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shan Y, Ying R, Jia Z, Kong W, Wu Y, Zheng
S and Jin H: LINC00052 promotes gastric cancer cell proliferation
and metastasis via activating Wnt/β-catenin signaling pathway.
Oncol Res. 25:1589–1599. 2017. View Article : Google Scholar : PubMed/NCBI
|