Introduction
Osteosarcoma (OS) is one of the most commonly
diagnosed types of malignant bone cancer in adolescents and young
adults (1). The long-term
disease-free survival of patients with OS remains low (~65%) and
disease-free survival of patients exhibiting metastasis have a
lower survival rate (2). Due to the
improvement of chemotherapy drugs, the 5-year survival rate of OS
patients has increased in recent decades (3). However, chemoresistance remains a major
problem, and contributes to the low 5-year survival rate (4). Previous studies have indicated that
various oncogenes and tumor suppressors are involved in the
chemoresistance of OS cells (5,6). However,
the existing understanding of the mechanism of chemoresistance in
OS is limited and further investigation is urgently required.
MicroRNAs (miRNAs/miRs), a group of small non-coding
RNAs that regulate gene expression by binding to the
3′-untranslated region (3′-UTR) of mRNAs, serve important roles in
various cellular processes, including proliferation, apoptosis,
invasion and differentiation (7,8). miRNAs
are dysregulated in various types of cancer, acting as oncogenes or
tumor suppressors during tumorigenesis (9). Evidence demonstrated that miRNA serves a
regulatory role in the chemosensitivity of various types of cancer
cells. miRNA-107 enhanced chemosensitivity to paclitaxel in
non-small cell lung cancer by targeting the antiapoptotic factor
Bcl-2 (10). miRNA-625 has been
demonstrated to increase chemosensitivity in glioma by directly
targeting AKT serine/threonine kinase 2 AKT2 (11). The aim of the present study was to
elucidate the regulatory role of miR-19a-3p in the chemosensitivity
of OS cells. miR-19a-3p was reported to enhance proliferation and
insulin secretion, while inhibiting the apoptosis of pancreatic
β-cells through the inhibition of suppressor of cytokine signaling
3 (SOCS3) (12). However, the role of
miR-19a-3p in the regulation of chemosensitivity is unclear and
requires further examination.
Down-regulation of phosphatase and tensin homolog
(PTEN), a tumor suppressor gene, was reported in various human
primary tumors (13). Previous
studies have also demonstrated that PTEN was associated with
chemosensitivity of cancer cells. The study of Shen et al
(14) indicated that downregulation
of miRNA-147 expression increased the chemosensitivity of gastric
cancer cells to 5-fluorouracil by directly targeting PTEN. Using
bioinformatics analysis, it was revealed in the present study that
PTEN was a putative target gene of miR-19a-3p. Accordingly, the
hypothesis was that miR-19a-3p may be involved in the regulation of
chemosensitivity through targeting PTEN in OS cells.
In the present study, overexpression of miR-19a-3p
was identified in OS cells and that silencing of miR-19a-3p
enhanced the chemosensitivity of OS cells by elevating the
expression of PTEN. These results will assist in the understanding
of the underlying mechanism of involvement of miR-19a-3p in
regulating chemosensitivity of OS cells.
Materials and methods
Cell culture and induction of
cisplatin resistant cells
Bone marrow-derived stroma cells (BMSCs) were
purchased from BeNa Culture Collection (Bejing, China) and the OS
cell lines, MNNG/HOS, U-2 OS, MG63, Saos-2, were purchased from the
American Type Culture Collection (Manassas, VA, USA). Cells were
cultured in DMEM (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Gibco;
Thermo Fisher Scientific, Inc.) at 37°C in 5% CO2.
Cisplatin was purchased from Sigma-Aldrich (Merck KGaA, Darmstadt,
Germany) and dissolved in PBS. For the induction of
cisplatin-resistant cells, MG63 cells were treated by gradually
increasing doses of cisplatin in the cell culture medium throughout
the passages for 6~8 months. The cells were maintained in the
presence of 5 µM cisplatin in the culture medium for 48 h in every
alternate passage. Bpv(HOpic) (Merck KGaA, Darmstadt, Germany), a
PTEN inhibitor, was used to treat cells at a concentration of 1
µM.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The expression level of miR-19a-3p in the cell lines
was measured via RT-qPCR. Total RNA was extracted from the cells
using the TRIzol (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol. cDNA was synthesized
using a miScript reverse transcription kit (Qiagen, GmbH, Hilden,
Germany) and the PCR reaction was performed using the SYBR Premix
Ex Taq™ II kit (Takara Bio, Inc., Otsu, Japan), according to the
manufacturer's instructions. The primers used were as following,
miR-19a-3p forward, 5′-GGGGGGGTGTGCAAATCT-3′, and reverse,
5′-GTGCGTGTCGTGGAGTCG-3′; U6, forward,
5′-GCTTCGGCAGCACATATACTAAAAT-3′, and reverse,
5′-CGCTTCACGAATTTGCGTGTCAT-3′. The amplification protocol included
an initial denaturation step at 95°C for 10 min, followed by 40
cycles of 95°C for 15 sec and 60°C for 60 sec. The expression
levels were calculated using the 2−ΔΔCq method with U6
used for normalization (15).
Transfection
Cells were seeded into 96-well plates to reach 60%
confluence for transfection. miR-19a-3p mimics (cat. no.
MIMAT0000073) and inhibitor (cat. no. MIMAT0021837) were purchased
from Invitrogen; (Thermo Fisher Scientific, Inc.). According to
manufacturer's protocol, transfections were performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufactuerer's protocol. For
overexpression of PTEN, the full-length PTEN sequence
(5′-UUCACAUCCUACCCCUUUGCACU-3′) obtained from Invitrogen (Thermo
Fisher Scientific, Inc.) was cloned into a pcDNA3.0 vector
(Invitrogen; Thermo Fisher Scientific, Inc). Cells were transfected
with pcDNA3.0-PTEN using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufactuerer's protocol. After transfection for 48 h, cells were
collected for subsequent experimentation.
Cell Counting Kit-8 (CCK-8) assay
Cells were seeded at 5×103 per well in
96-well plates and incubated for 0–5 days with and without
cisplatin treatment (3 µM). A total of 10 µl of CCK-8 solution
(Beyotime Institute of Biotechnology, Shanghai, China) was
incubated at 37°C for 2 h. The absorbance was measured at 450 nm
using a microplate spectrophotometer (Molecular Devices, LLC,
Sunnyvale, CA, USA). Triplicate wells were used in each group.
Western blotting
According to the manufacturer's protocol, proteins
were extracted from cells using RIPA lysis buffer (Beyotime
Institute of Biotechnology). Homogenized samples were washed with
ice-cold PBS and centrifuged at 10,000 × g for 15 min at 4°C. The
supernatant was collected and the protein concentration was
determined using a BCA protein assay kit (Beijing Solarbio Science
& Technology Co., Ltd., Beijing, China). A total of 20 µg
protein were loaded per lane and separated by 10% SDS-PAGE and then
transferred into PVDF membranes (Merck KGaA). The membrane was
blocked with 5% bovine serum albumin for 1 h at room temperature
and then incubated with the following primary monoclonal antibodies
(at a 1:1,000 dilution): Anti-Ki67 (cat. no. ab92742; Abcam,
Cambridge, UK), anti-PCNA (cat. no. 2586; Cell Signaling
Technology, Inc., Danvers, MA, USA), anti-Bax (cat. no. ab32503;
Abcam), anti-Bcl-2 (cat. no. ab32124; Abcam), anti-PTEN (cat. no.
ab79156; Abcam) and GAPDH (cat. no. 5174; Cell Signaling
Technology, Inc.) and gently agitated at 4°C overnight. Then the
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (Anti-rabbit IgG, HRP-linked Antibody, cat.
no. 7074; Cell Signaling Technology, Inc.; dilution 1:3,000) for 1
h at room temperature. An enhanced chemiluminescence system
(Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used for the
detection of antibody-bound proteins, according to manufacturer's
instructions. Analysis was performed using ImageJ software (version
1.48, National Institutes of Health, Bethesda, MD, USA).
Cell apoptosis assay
An Annexin V-FITC/propidium iodide (PI) apoptosis
detection kit (Multisciences, Shanghai, China, http://liankebio.biomart.cn/) was used for the cell
apoptosis assay, according to manufacturer's protocol. A total of
3×105 cells were washed with PBS, resuspended and
incubated with 5 µl Annexin V-FIFC and 10 µl PI. Cell apoptosis was
analyzed using a flow cytometer (BD Biosciences, Franklin Lakes,
NJ, USA). FACS data was processed using FlowJo (version 7.5.5; Tree
Star Inc., Ashland, OR, USA).
MiRNA target prediction
Genes containing the binding sites of the miR-19a-3p
3′-UTR were obtained using TargetScan (http://www.targetscan.org/vert_71/), MiRanda
(http://www.microrna.org/microrna/home.do), PICTAR
(http://www.pictar.org/) and miRDB (http://mirdb.org/miRDB/custom.html) target
prediction algorithms.
Luciferase reporter assay
The fragment of PTEN containing the target sequence
5′-UUUGCAC-3′, Invitrogen, Thermo Fisher Scientific, Inc.) of
miR-19a-3p was cloned into pGL3 Luciferase Reporter Vectors
(Promega Corporation, Madison, WI, USA) to form the reporter vector
PTEN-wild-type (PTEN WT), while the PTEN-mutated-type (PTEN MUT)
contained the mutated binding site. The cells were co-transfected
with PTEN WT or PTEN MUT and miR-19a-3p mimics using
Lipofectamine® 2000, according to manufacturer's
protocol. Following transfection for 48 h, luciferase activity was
measured using the Dual-Luciferase® Reporter assay
system kit (Promega Corporation), according to the manufacturer's
instructions. Renilla luciferase activity was used for
normalization.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed by Student's t-test
or one-way analysis of variance with Student-Newman-Keuls post hoc
test, using SPSS 19.0 (IBM Corp., Armonk, NY, USA). P>0.05 was
considered to indicate a statistically significant difference.
Results
miR-19a-3p is overexpressed in OS
cells
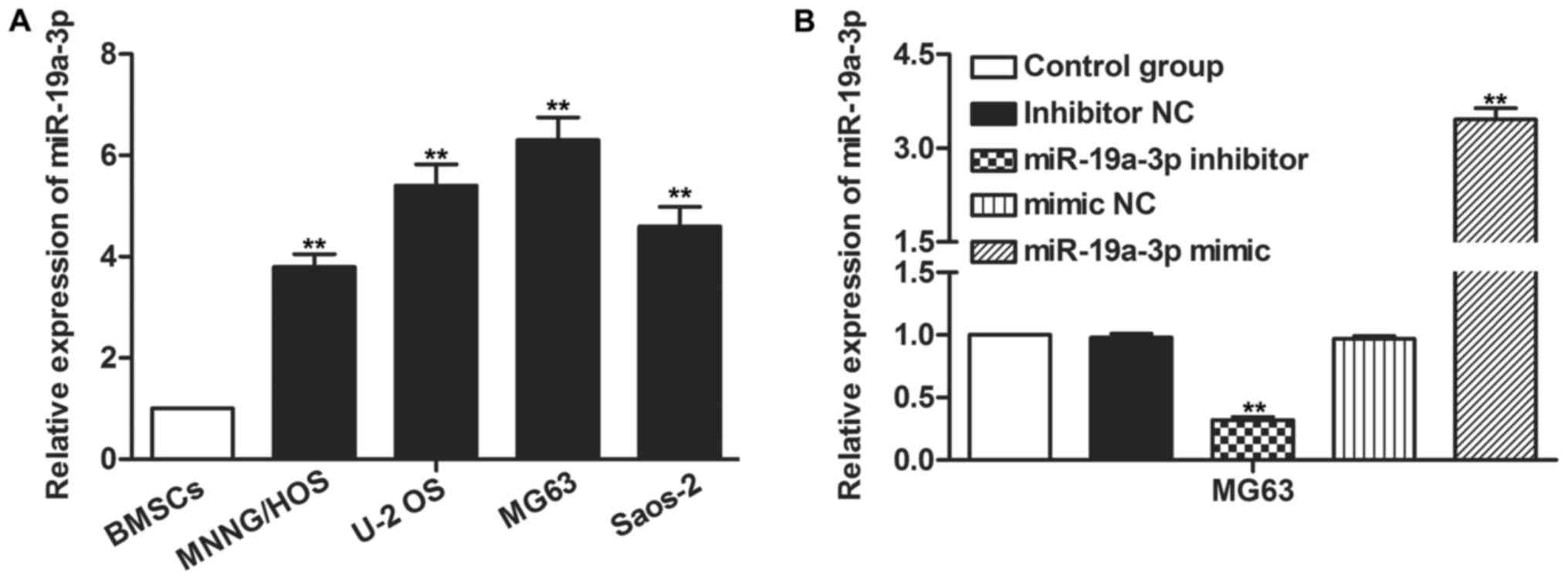
To investigate the role of miR-19a-3p in OS, its
expression level in OS cells was detected using RT-qPCR. The
expression of miR-19a-3p that was revealed in OS cells, MNNG/HOS,
U-2 OS, MG63 and Saos-2, was higher than that in normal BMSCs
(P<0.01; Fig. 1A). Transfection
with miR-19a-3p mimics or inhibitor increased and decreased the
expression of miR-19a-3p significantly in U-2 OS and MG63 cells,
respectively (P<0.01; Fig. 1B).
The expression of miR-19a-3p was highest in MG63 cells, thus, MG63
cells were selected for the following experiments.
Inhibition of miR-19a-3p enhances
chemosensitivity of OS cells
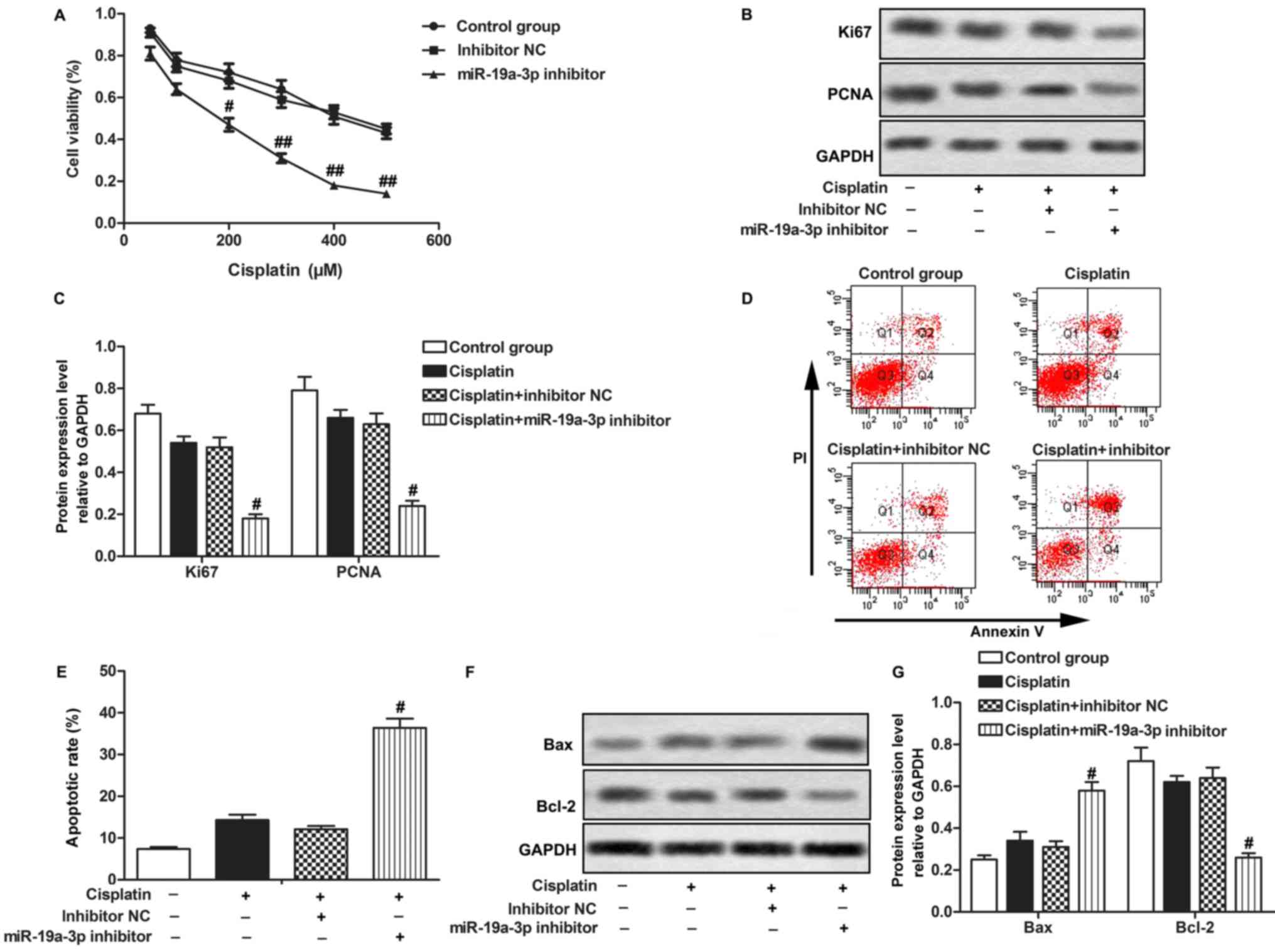
A Cisplatin-resistant MG-63 cell line was
constructed, as described in the Materials and methods section.
Cisplatin treatment did not significantly affect cell proliferation
or apoptosis of cisplatin-resistant MG-63 cells. However,
transfection with the miR-19a-3p inhibitor downregulated the rate
of cell proliferation and decreased the expression of the
proliferation-associated proteins, Ki67 and PCNA. Transfection with
miR-19a-3p inhibitor NC did not have a significant effect compared
with the Cisplatin group (P>0.05; Fig.
2A-C), suggesting that downregulation of miR-19a-3p suppressed
the proliferation of Cisplatin-resistant OS cells under Cisplatin
treatment. Transfection with miR-19a-3p inhibitor increased cell
apoptotic rate, while miR-19a-3p mimics did not have a significant
effect compared with the Cisplatin group (P>0.05; Fig. 2D and E). Transfection with miR-19a-3p
inhibitor increased the expression of Bax and decreased the
expression of Bcl-2 compared with the Cisplatin group (P<0.05;
Fig. 2F and G), suggesting that
downregulation of miR-19a-3p expression promoted apoptosis of
Cisplatin-resistant OS cells under Cisplatin treatment. The data
indicates that miR-19a-3p inhibitor increases the chemosensitivity
of OS cells to Cisplatin.
PTEN is a target of miR-19a-3p in OS
cells
To explore the chemosensitivity regulatory mechanism
of miR-19a-3p in OS cells, the public database, TargetScan was used
to search for potential targets of miR-19a-3p. It was revealed that
the PTEN mRNA 3′-UTR contained highly conserved sequences, which
were targeted by miR-19a-3p (Fig.
3A). Expression of endogenous PTEN protein was detected in OS
cells. PTEN expression was revealed to be significantly
downregulated in MNNG/HOS, U-2 OS, MG63 and Saos-2 cells compared
with BMSCs cells (P<0.05, P<0.01; Fig. 3B and C). Expression of PTEN was
downregulated in the miR-19a-3p mimics group and upregulated in the
miR-19a-3p inhibitor group, compared with the control group
(P<0.05; Fig. 3D and E). To
confirm the association between PTEN and miR-19a-3p in OS cells, a
luciferase reporter assay was conducted. It was revealed that
co-transfection with miR-19a-3p mimics and PTEN WT led to a
significant decrease in luciferase activity. However,
co-transfection with miR-19a-3p mimics and PTEN MUT did not have a
significant effect on luciferase activity (P>0.01; Fig. 3F). In summary, these results indicated
that PTEN was a target of miR-19a-3p and that the expression of
PTEN was negatively regulated by miR-19a-3p in OS cells.
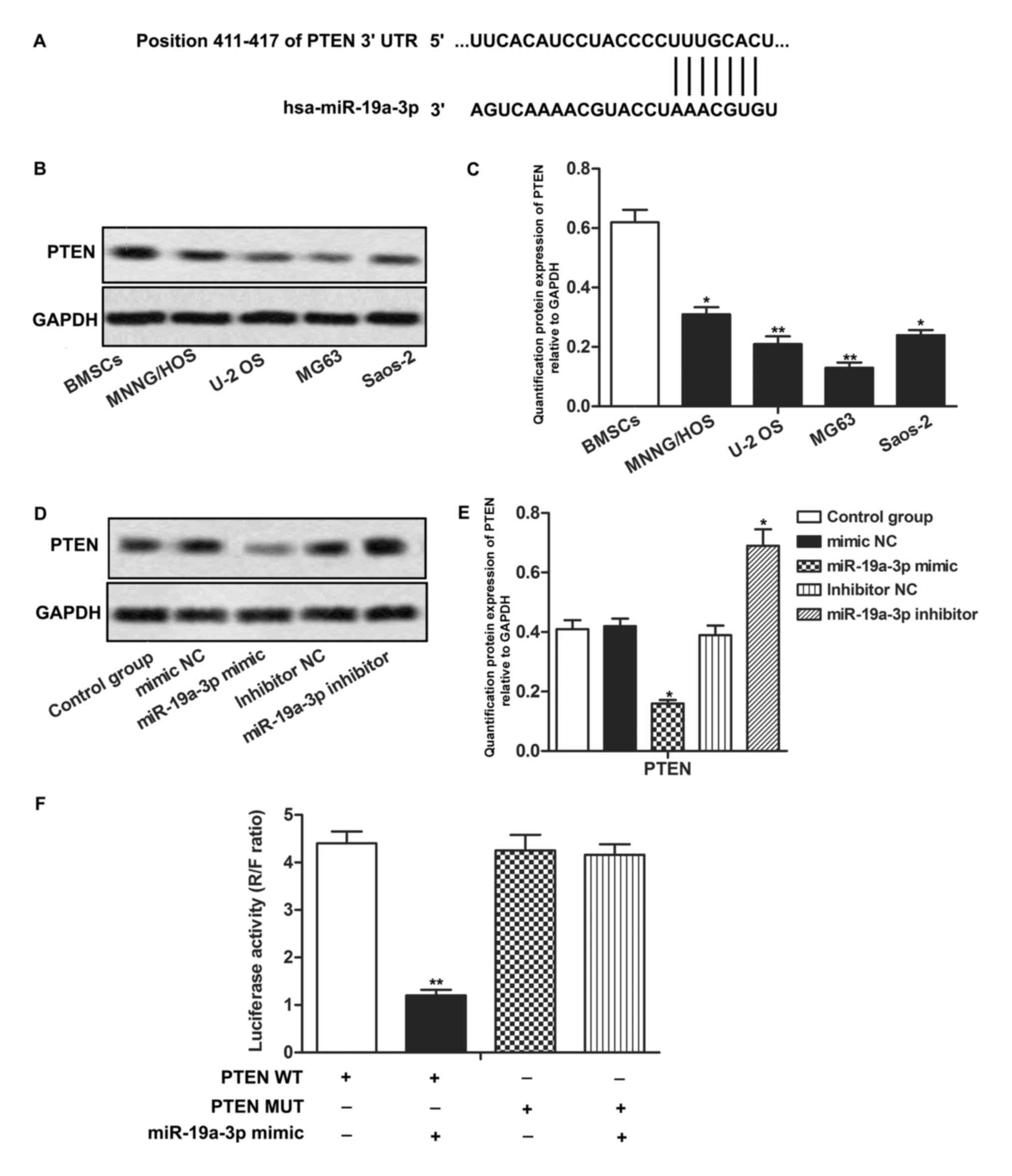 | Figure 3.PTEN is a target of miR-19a-3p in OS
cells. (A) The putative binding site of miR-19a-3p in 3′-UTR of
PTEN. (B) Protein expression of PTEN in OS cell lines and bone
marrow-deived stromal cells was measured through western blotting.
(C) Quantification of the protein expression of PTEN in MNNG/HOS,
U-2 OS, MG63, Saos-2. *P<0.05, **P<0.01 compared with BMSCs
group. (D) Relative expression of PTEN in MG63 cells transfected
with miR-19a-3p mimics or inhibitor was measured through western
blotting. (E) Quantification of the protein expression level of
PTEN relative to GAPDH. *P<0.05, compared with control group.
(F) Results of the luciferase reporter assay. **P<0.01 compared
with PTEN WT group. PTEN, phosphatase and tensin homolog; miR,
microRNA; OS, osteosarcoma; 3′-UTR, 3′-untranslated region; BMSC,
bone marrow derived stroma cell; WT, wild type; MUT, mutated; NC,
negative control; R, Renilla; F, Firefly. |
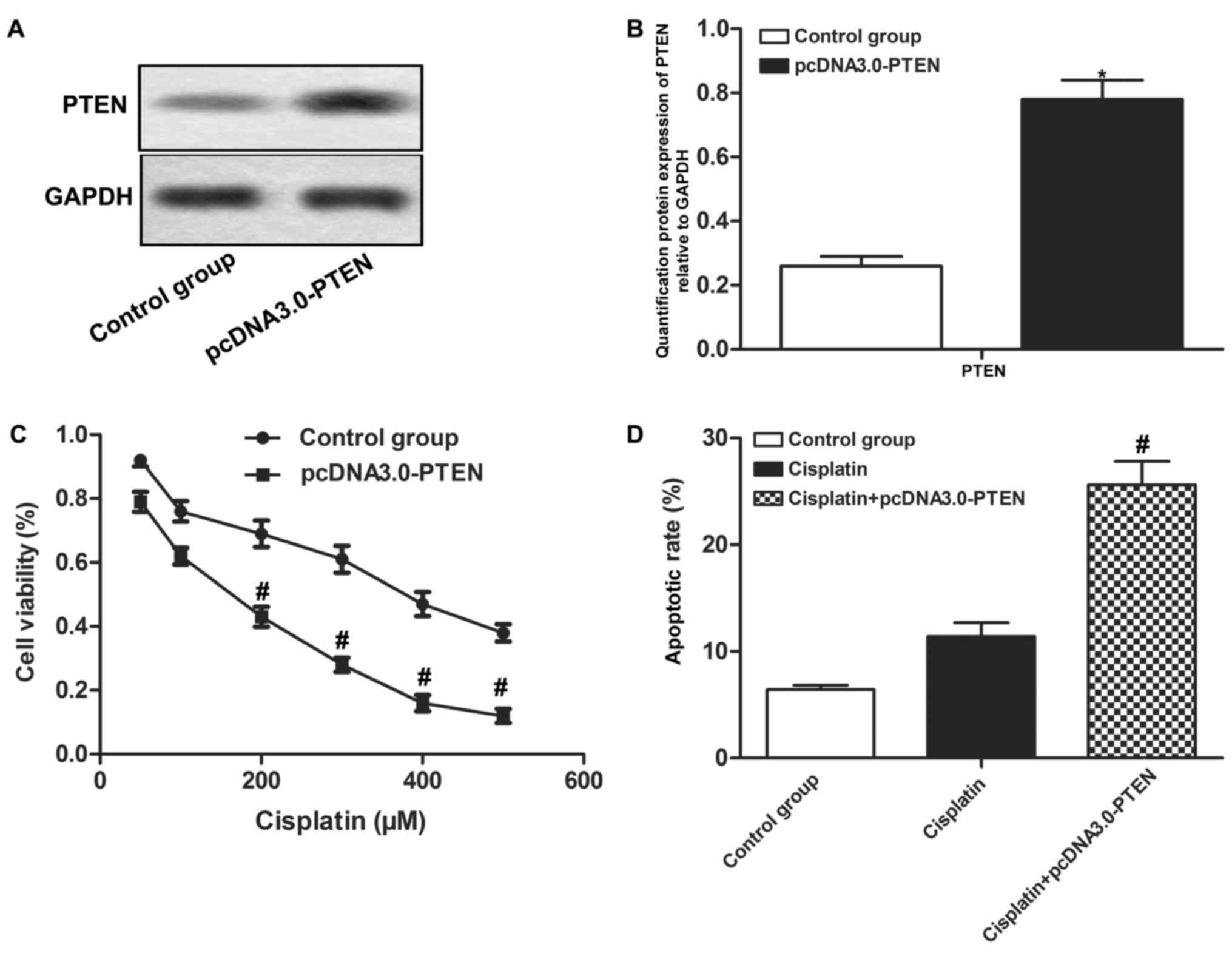
Overexpressed PTEN enhances the
chemosensitivity of OS cells
MG-63 cells were transfected with pcDNA3.0-PTEN to
increase the protein expression level of PTEN (P<0.05; Fig. 4A and B). It was demonstrated that
overexpressed PTEN decreased cell proliferation and increased
apoptotic rate compared with the Cisplatin group (P<0.05;
Fig. 4C and D). The results indicate
that overexpressed PTEN enhances the chemosensitivity of OS
cells.
PTEN inhibitor BpV(HOpic) counteracts the effect of
miR-19a-3p inhibitor in the regulation of chemosensitivity. To
investigate the interaction between PTEN and miR-19a-3p, the PTEN
inhibitor, BpV(HOpic), was used in the present study. It was
observed that BpV(HOpic) treatment increased cell proliferation and
decreased apoptotic rate compared with the Cisplatin-treated
miR-19a-3p inhibitor group (P<0.05Fig. 5A and B). These results
indicate that inhibition of PTEN counteracted the effect of the
miR-19a-3p inhibitor on the regulation of chemosensitivity in OS
cells.
Discussion
In the treatment of OS, the combination of
chemotherapy and surgery has improved the overall 5-year survival
rate of patients with OS (16).
Cisplatin is one of the most commonly used platinum-based
anticancer drugs for the treatment of OS, interacting with
nucleophilic N7-sites of purine bases to induce DNA
damage that leads to cell death (17). However, Cisplatin resistance can
develop and limit the effectiveness of chemotherapeutics.
Therefore, understanding the molecular mechanism of chemoresistance
to Cisplatin is essential to develop a more effective treatment for
OS. In the present study, it was demonstrated that silencing of
miR-19a-3p enhanced chemosensitivity to Cisplatin in OS cells by
up-regulating the expression of PTEN. These results can assist in
finding a better therapeutic strategy against Cisplatin resistance
in OS.
miRNAs, a class of non-coding regulatory RNAs, have
been reported to be involved in tumor progression (18,19). As
miRNAs act as oncogenes or tumor suppressor genes, they constitute
a large gene network for the regulation of various cellular
processes during tumorigenesis (9).
Evidence suggests that numerous miRNAs are associated with
chemoresistance in various types of cancers. For example, miR-491
has been demonstrated to inhibit chemoresistance of OS cells by
targeting αB-crystallin (20).
miR-608 has been demonstrated to inhibit gemcitabine
chemoresistance by regulating resistance genes in pancreatic cancer
cells (21). Previous studies have
reported that miR-19a-3p expression was correlated with cell
proliferation and autophagy (12,22). In
addition, the serum level of miR-19a-3p has been reported to be
significantly higher in patients with colorectal cancer compared
with healthy patients (23).
Similarly, it was demonstrated that the expression of miR-19a-3p
was upregulated in OS cells compared with normal BMSCs. In the
present study a cispaltin-resistant MG-63 cell line was constructed
to investigate the role of miR-19a-3p in the chemosensitivity of OS
cells. It was observed that Cisplatin treatment alone did not
significantly affect proliferation or apoptosis of
cispaltin-resistant MG-63 cells. However, Cisplatin treatment
combined with miR-19a-3p inhibitor transfection suppressed cell
proliferation and decreased the expression of
proliferation-associated proteins, including Ki67 and PCNA,
compared with the Cisplatin treatment group. Cisplatin treatment
combined with miR-19a-3p inhibitor transfection increased the
apoptotic rate, increased the expression of Bax, and decreased the
expression of Bcl-2 compared with the Cisplatin treatment group. In
summary, these results suggest that the silencing of miR-19a-3p
enhances chemosensitivity to Cisplatin in OS cells by suppressing
cell proliferation and promoting cell apoptosis in treatment with
Cisplatin.
PTEN is a tumor suppressor and its expression is
often downregulated in various types of tumors (24,25).
Similarly, downregulation of the expression of PTEN in OS cells was
observed in the present study. Silencing of PTEN has been reported
to induce activation of the PI3K/AKT pathway, which leads to cell
survival and apoptosis resistance (26,27).
Previous studies have reported that PTEN is regulated by miRNAs in
many types of cancers. Liu et al (26) indicated that miR-206 inhibited head
and neck squamous cell carcinoma (HNSCC) cell progression by
targeting HDAC6 via the PTEN/AKT/mTOR pathway. miR-130a increased
cell proliferation and tumor growth through repression of PTEN
(28). miRNA-21 enhanced
chemoresistance to Cisplatin by negatively regulating PTEN in
epithelial ovarian cancer (29). The
miRNA/PTEN axis may serve an important role in the regulation of
tumorigenesis. In the present study, bioinformatics tools
demonstrated that the 3′-UTR of the PTEN gene harbored a putative
binding site for miR-19a-3p. Expression of PTEN was significantly
downregulated in the miR-19a-3p mimics group and significantly
upregulated in the miR-19a-3p inhibitor group, indicating that the
expression of PTEN was negatively regulated by miR-19a-3p. To
further examine the interaction between PTEN and miR-19a-3p, a
luciferase reporter assay was performed and the results suggested
that miR-19a-3p directly targeted PTEN in OS cells. In order to
examine the effect of PTEN in the chemosensitivity of OS cells,
cispaltin-resistant MG-63 cells were transfected with pcDNA3.0-PTEN
to up-regulate the expression of PTEN. The present study revealed
that Cisplatin treatment combined with pcDNA3.0-PTEN transfection
decreased cell proliferation and increased apoptotic rate compared
with Cisplatin treatment alone, suggesting that overexpression of
PTEN enhanced chemosensitivity of OS cells to Cisplatin. Therefore,
silencing of miR-19a-3p may result in upregulation of the
expression of PTEN in OS cells. To verify this result, Bpv(HOpic),
a PTEN inhibitor, was used to treat miR-19a-3p
inhibitor-transfected cells. It was observed that inhibition of
PTEN upregulated cell proliferation and downregulated apoptotic
rate compared with the Cisplatin-treated miR-19a-3p inhibitor
group, indicating that inhibition of PTEN counteracted the effect
of the miR-19a-3p inhibitor on the chemosensitivity of OS
cells.
Taken together, the results of the present study
indicate a role of miR-19a-3p in the chemosensitivity of OS cells.
miR-19a-3p was overexpressed in OS cell lines and downregulation of
miR-19a-3p enhanced chemosensitivity to Cisplatin by elevating the
expression of tumor suppressor PTEN in OS cells. Therefore, an
miR-19a-3p/PTEN axis may be a potential target for the treatment of
OS.
Acknowledgements
The authors would like to thank members of The First
People's Hospital of Wenling, for providing helpful discussion
regarding the present study.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BZ interpreted the main data regarding the cell
survival and apoptosis analysis. JNZ was responsible for western
blot and statistical analysis. YL was responsible for study design
and drafting of the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
OS
|
osteosarcoma
|
|
miRNAs
|
microRNAs
|
|
Bax
|
Bcl-2 associated X, apoptosis
regulator
|
|
3′-UTR
|
3′-untranslated region
|
|
PTEN
|
phosphatase and tensin homolog
|
|
BMSCs
|
bone marrow derived stroma cells
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
|
CCK-8
|
Cell Counting Kit-8
|
References
|
1
|
Arndt CA, Rose PS, Folpe AL and Laack NN:
Common musculoskeletal tumors of childhood and adolescence. Mayo
Clin Proc. 87:475–487. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ta HT, Dass CR, Choong PF and Dunstan DE:
Osteosarcoma treatment: State of the art. Cancer Metastasis Rev.
28:247–263. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kempf-Bielack B, Bielack SS, Jurgens H,
Branscheid D, Berdel WE, Exner GU, Göbel U, Helmke K, Jundt G,
Kabisch H, et al: Osteosarcoma relapse after combined modality
therapy: An analysis of unselected patients in the Cooperative
Osteosarcoma Study Group (COSS). J Clin Oncol. 23:559–568. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thompson LD: Osteosarcoma. Ear, Nose
Throat J. 92:288–290. 2013.
|
|
5
|
Chang Z, Huo L, Li K, Wu Y and Hu Z:
Blocked autophagy by miR-101 enhances osteosarcoma cell
chemosensitivity in vitro. ScientificWorldJournal.
2014:e7947562014. View Article : Google Scholar
|
|
6
|
Zhou Y, Huang Z, Wu S, Zang X, Liu M and
Shi J: miR-33a is up-regulated in chemoresistant osteosarcoma and
promotes osteosarcoma cell resistance to cisplatin by
down-regulating TWIST. J Exp Clin Cancer Res. 33:122014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang Y and Lee CG: MicroRNA and
cancer-focus on apoptosis. J Cell Mol Med. 13:12–23. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Iorio MV and Croce CM: MicroRNAs in
cancer: Small molecules with a huge impact. J Clin Oncol.
27:5848–5856. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lu C, Xie Z and Peng Q: MiRNA-107 enhances
chemosensitivity to paclitaxel by targeting antiapoptotic factor
Bcl-w in non small cell lung cancer. Am J Cancer Res. 7:1863–1873.
2017.PubMed/NCBI
|
|
11
|
Zhang J, Qiu W, Xu S, Yu Q, Liu C, Wang Y,
Lu A, Zhang J and Lu X: 1MicroRNA-625 inhibits the
proliferation and increases the chemosensitivity of glioma by
directly targeting AKT2. Am J Cancer Res. 7:1835–1849.
2017.PubMed/NCBI
|
|
12
|
Li Y, Luo T, Wang L, Wu J and Guo S:
MicroRNA-19a-3p enhances the proliferation and insulin secretion,
while it inhibits the apoptosis of pancreatic β cells via the
inhibition of SOCS3. Int J Mol Med. 38:1515–1524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Soria JC, Lee HY, Lee JI, Wang L, Issa JP,
Kemp BL, Liu DD, Kurie JM, Mao L and Khuri FR: Lack of PTEN
expression in non-small cell lung cancer could be related to
promoter methylation. Clin Cancer Res. 8:1178–1184. 2002.PubMed/NCBI
|
|
14
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shen J, Niu W and Zhang H, Jun M and Zhang
H: Downregulation of MicroRNA-147 Inhibits Cell Proliferation and
Increases the Chemosensitivity of Gastric Cancer Cells to
5-Fluorouracil by Directly Targeting PTEN. Oncol Res. doi:
10.3727/096504017X15061902533715. 2017.
|
|
16
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lei W, Yan C, Ya J, Yong D, Yujun B and
Kai L: MiR-199a-3p affects the multi-chemoresistance of
osteosarcoma through targeting AK4. BMC cancer. 18:6312018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xie B, Li Y, Zhao R, Xu Y, Wu Y, Wang J,
Xia D, Han W and Chen D: Identification of key genes and miRNAs in
osteosarcoma patients with chemoresistance by bioinformatics
analysis. Biomed Res Int. 2018:47610642018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang SN, Luo S, Liu C, Piao Z, Gou W, Wang
Y, Guan W, Li Q, Zou H, Yang ZZ, et al: miR-491 inhibits
osteosarcoma lung metastasis and chemoresistance by targeting
alphaB-crystallin. Mol Ther. 25:2140–2149. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rajabpour A, Afgar A, Mahmoodzadeh H,
Radfar JE, Rajaei F and Teimoori-Toolabi L: MiR-608 regulating the
expression of ribonucleotide reductase M1 and cytidine deaminase is
repressed through induced gemcitabine chemoresistance in pancreatic
cancer cells. Cancer Chemother Pharmacol. 80:765–775. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zou M, Wang F, Gao R, Wu J, Ou Y, Chen X,
Wang T, Zhou X, Zhu W, Li P, et al: Autophagy inhibition of
hsa-miR-19a-3p/19b-3p by targeting TGF-β R II during TGF-β1-induced
fibrogenesis in human cardiac fibroblasts. Sci Rep. 6:247472016.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhu M, Huang Z, Zhu D, Zhou X, Shan X, Qi
LW, Wu L, Cheng W, Zhu J, Zhang L, et al: A panel of microRNA
signature in serum for colorectal cancer diagnosis. Oncotarget.
8:17081–17091. 2017.PubMed/NCBI
|
|
24
|
Lotan TL, Heumann A, Rico SD, Hicks J,
Lecksell K, Koop C, Sauter G, Schlomm T and Simon R: PTEN loss
detection in prostate cancer: Comparison of PTEN
immunohistochemistry and PTEN FISH in a large retrospective
prostatectomy cohort. Oncotarget. 8:65566–65576. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma J, Guo X, Zhang J, Wu D, Hu X, Li J,
Lan Q, Liu Y and Dong W: PTEN gene induces cell invasion and
migration via regulating AKT/GSK-3β/β-Catenin signaling pathway in
human gastric cancer. Dig Dis Sci. 62:3415–3425. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu F, Zhao X, Qian Y, Zhang J, Zhang Y
and Yin R: MiR-206 inhibits Head and neck squamous cell carcinoma
cell progression by targeting HDAC6 via PTEN/AKT/mTOR pathway.
Biomed Pharmacother. 96:229–237. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma Y, Zhang P, Gao Y, Fan H, Zhang M and
Wu J: Evaluation of AKT phosphorylation and PTEN loss and their
correlation with the resistance of rituximab in DLBCL. Int J Clin
Exp Pathol. 8:14875–14884. 2015.PubMed/NCBI
|
|
28
|
Wei H, Cui R, Bahr J, Bahr J, Zanesi N,
Luo Z, Meng W, Liang G and and Croce CM: miR-130a deregulates PTEN
and stimulates tumor growth. Cancer Res. 77:6168–6178. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yu X, Chen Y, Tian R, Li J, Li H, Lv T and
Yao Q: miRNA-21 enhances chemoresistance to cisplatin in epithelial
ovarian cancer by negatively regulating PTEN. Oncol Lett.
14:1807–1810. 2017. View Article : Google Scholar : PubMed/NCBI
|