Introduction
Colorectal cancer (CRC) is a common malignancy and
the fifth leading cause of cancer-associated cases of mortality for
males and females in China (1). The
morbidity of CRC has increased rapidly in recent years and distant
metastasis accounts for the majority of cancer-associated cases of
mortality in patients with CRC (2).
Evidence has suggested that the overall five-year survival rate for
patients with metastatic CRC is approximately 10–15%, showing an
unsatisfactory prognosis (3,4). Therefore, improved understanding of the
molecular interactions that occur in the initiation and progression
of CRC may be helpful in identifying therapeutic targets and
providing new prognostic treatments.
MicroRNAs (miRNAs or miRs) are a type of non-coding
single-strand RNA molecule with a length of 20–24 nucleotides
(5). These molecules are endogenously
synthesized and negatively regulate the expression of genes by
binding to their 3′-untranslated-region (3′-UTR) (6). Numerous studies have reported that
miRNAs are involved in a wide range of biological processes and
aberrant expression of miRNAs is associated with tumorigenesis and
progression. For example, miR-200a regulates the proliferation and
metastasis of pancreatic cancer through modulating the DEK gene
(7), miR-543 promotes metastasis of
prostate cancer by binding to RKIP (8), and miR-33 is downregulated in breast
cancer tissues and acts as a tumor suppressor by targeting HMGA2
(9).
It has also been reported that a number of miRNAs
are involved in CRC progression and prognosis. Among them, miR-410
has been verified to be aberrantly expressed in several human
malignant cancer types and may function as a tumor suppressor in
endometrial cancer, myeloma, lung cancer and breast cancer
(10–13). Certain studies have reported that
miR-410 regulates biological functions of CRC cells by targeting
FHL1, ITPKB and Bak1 (14–16). In the current study, it was confirmed
that miR-410 functions as a tumor suppressor in CRC cells by
suppressing cell proliferation, migration and invasion.
Furthermore, the current study reported that miR-410 targets
dickkopf-related protein 1 (DKK1) and elucidated the underlying
mechanism of the miR-410/DKK1 axis in CRC. To the best of our
knowledge, the current study is the first to identify this finding
and therefore may shed new light on the therapeutic strategies for
CRC.
Materials and methods
Cell culture and transfection
The CRC cell lines SW-480, SW-620, HT-29, HCT-116
and normal colon epithelial cell line FHC were purchased from
American Type Culture Collection (Manassas, VA, USA) and cultured
in RPMI-1640 medium (Pan Biotech GmbH, Aidenbach, Germany)
supplemented with 10% fetal bovine serum (FBS) (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C in humidified
atmospheric conditions of 5% CO2. For transfection,
miR-410 mimics (AUCAUGAUGGGCUCCUCGGUGUACACCGAGGAGCCCAUCAUGAU),
miR-410 inhibitors (UAGUACUACCCGAGGAGCCACAUGUGGCUCCUCGGGUAGUACUA)
and a negative control inhibitor (NC inhibitor) were constructed by
Biossci Biotechnology Co. (Wuhan, China, http://www.biossci.com/). Transfections were performed
using Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The concentration
of miR-410 mimics was 50 nmol/l and of miR-410 inhibitors was 100
nmol/l, following instructions from Biossci Biotechnology Co. Cells
were harvested after 48 h for further analyses.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from SW-480 and HT-116 cells
using TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. The RNA purity was
determined by a DU800 UV/Vis Spectrophotometer (Beckman Coulter,
Inc., Brea, CA, USA) and 100 ng RNA was used for complementary DNA
(cDNA) synthesis using the ReverTra Ace-α-kit (Toboyo Life Science,
Osaka, Japan) following the manufacturer's protocol. qPCR was
performed using SYBR Green Real-Time PCR Master mix (Toyobo Life
Science). The expression of miR-410 and DKK1 mRNA was normalized to
U6 and β-actin, respectively. PCR was performed with the following
thermocycling conditions: Initial denaturation at 94°C for 4 min,
40 cycles of 94°C for 30 sec, 56°C for 30 sec and 72°C for 25 sec,
using the ABI 7900 Real-Time PCR system (Applied Biosystems; Thermo
Fisher Scientific Inc.), according to the manufacturer's protocols.
The relative amount of miRNA or mRNA was calculated via the
2−∆∆Cq method (17) and
the primer sequences used are shown in Table I.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primer sequences. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primer sequences.
| Gene | Primer sequences
(5′-3′) |
|---|
| DKK1 | F:
AGTACTGCGCTAGTCCCACC |
|
| R:
TCCTCAATTTCTCCTCGGAA |
| miR-410 | F:
AAUAUAACACAGAUGGCCUGU |
|
| R:
CCGUGCUCGACUUUCCGGCG |
| U6 snRNA | F:
CTCGCTTCGGCAGCACATATACT |
|
| R:
ACGCTTCACGAATTTGCGTGTC |
| GAPDH | F:
TGAAGGTCGGTGTGAACGGATTTGGTC |
|
| R:
CATGTAGGCCATGAGGTCCACCAC |
Cell viability assay
The viability of SW-480 and HT-116 cells was
measured by a cell viability test, using Cell Counting Kit-8
(CCK-8; Beyotime Institute of Biotechnology, Jiangsu, China). Cells
were inoculated into 96-well plates at a density of
2×103 cells/well for 24 h and then transfected with
miR-410 inhibitor or NC inhibitor according to the aforementioned
manufacturer's protocol. SW-480 and HT-116 cells were stained with
20 µl of CCK-8 reagent for 4 h before detecting the absorbance at
450 nm using a Multiskan FC spectrophotometer (Thermo Fisher
Scientific, Inc.).
Cell apoptosis assay
Cell apoptosis was examined using the Annexin
V-FITC/Propidium Iodide (PI) staining kit (Dojindo Molecular
Technologies, Inc., Kumamoto, Japan). SW-480 and HT-116 cells were
seeded in 6-well plates at a density of 106 cells/ml. At
24 h after transfection, the cells were labeled with Annexin V-FITC
for 15 min in the dark. A total of 50 µg/ml of PI was added to each
sample for 30 min. Cell apoptosis distribution was analyzed to
evaluate the percentage of apoptotic cells by flow cytometry using
a BD LSR II flow cytometer (BD Biosciences, San Jose, CA, USA).
Cell invasion and migration
assays
Cell invasion and migration assays were performed
using Transwell plates (Corning Life Sciences, NY, USA) with
8-µm-pore size membranes with Matrigel (for invasion assay) or
without Matrigel (for migration assay). SW-480 and HT-116 cells
were used at 48 h post-transfection with miR-410 inhibitor or NC
inhibitor. Briefly, 3×104 cells were seeded in the upper
chamber while medium containing 10% FBS was placed in the lower
chamber. After incubation at 37°C for 24 h, cells on the upper
chamber membrane were wiped away. Then, cells on the lower chamber
membrane were stained with 0.2% crystal violet for 30 min. Five
predetermined fields were counted under a Olympus BX50 light
microscope (magnification, ×100; Olympus Corporation, Tokyo,
Japan). All assays were performed in triplicate.
Database prediction
To explore the association between miR-410 and DKK1,
an in silico prediction was performed using open access
software (TargetScan, http://www.targetscan.org; PicTarget, https://pictar.mdc-berlin.de/ and miRanda http://microrna.org). A putative binding site for
miR-410 was identified within the 3′-UTR of DKK1.
Plasmid construction and luciferase
reporter assays
The putative and mutated miR-410 target binding
sequences in DKK1 were synthesized and cloned into luciferase
reporters to generate the wild-type (DKK1-WT) or mutated-type
(DKK1-MUT) reporter plasmids. The mutant 3′-UTR sequence of DKK1
was obtained using an overlap-extension PCR method (18). Then, the sequences including the
predicted wild and mutant target sites were subcloned into a
psiCHECK-2 vector (Promega Corporation, Madison, WI, USA), and
validated by sequencing by Sangon Biotech Co., Ltd. (Shanghai,
China). For the luciferase reporter assay, SW-480 cells were seeded
at 1×105 cells/well on a 24-well plate. The cells were
then co-transfected with miR-410 inhibitor or NC inhibitor using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.)
following the manufacturer's protocol. Cells were harvested at 48 h
post-transfection and luciferase activities were compared with
Renilla luciferase activity using a Dual-Luciferase Reporter
Assay system (Promega Corporation).
Western blot analysis
Total cellular proteins were lysed using RIPA Buffer
(Beyotime Institute of Biotechnology), followed by centrifugation
at 15,000 × g for 20 min at 4°C. A bicinchoninic acid assay
(Beyotime Institute of Biotechnology) was performed to quantify
protein concentrations. Briefly, equivalent amounts of protein of
30 µg per lane were resolved by 10% SDS-PAGE gel electrophoresis
and subsequently blotted onto polyvinylidene difluoride membranes
followed by blocking at 4°C for 1 h with TBS containing 0.05%
Tween-20 (TBST) buffer with 5% non-fat milk and incubation with the
following primary antibodies: Anti-DKK1(dilution, 1:1,000; cat. no.
ab109416); anti-β-catenin (dilution, 1:5,000; cat. no. ab32572);
anti-GSK-3β (dilution, 1:5,000; cat. no. ab32391) (all from Abcam,
Cambridge UK); and anti-phosphorylated glycogen synthase kinase-3β
(p-GSK-3β) (dilution, 1:1,000; cat. no. D85E12; CST Biological
Reagents Co., Ltd., Shanghai, China) at 4°C overnight. GAPDH was
used as a loading control. After washing with TBST buffer,
membranes were incubated with goat anti-rabbit IgG antibody
(dilution, 1:100; cat. no. LK2003; Sungene Biotech, Co., Ltd,
Tianjin, China) at room temperature for 1 h. All bands were
visualized with an ECL system kit [MultiSciences (Lianke) Biotech
Co., Ltd, Hangzhou, China]. Densitometry was performed by ImageJ
1.48 software (National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All statistical analyses were performed using SPSS
19.0 (SPSS, Inc., Chicago, IL, USA) and GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Data are presented as
the mean ± standard deviation. Differences were assessed by
two-tailed Student's t-test, analysis of variance and a
Student-Newman-Keuls post hoc test as appropriate. All experiments
were performed at least three times. P<0.05 was considered to
indicate a statistically significant difference.
Results
Expression of miR-410 in CRC cell
lines
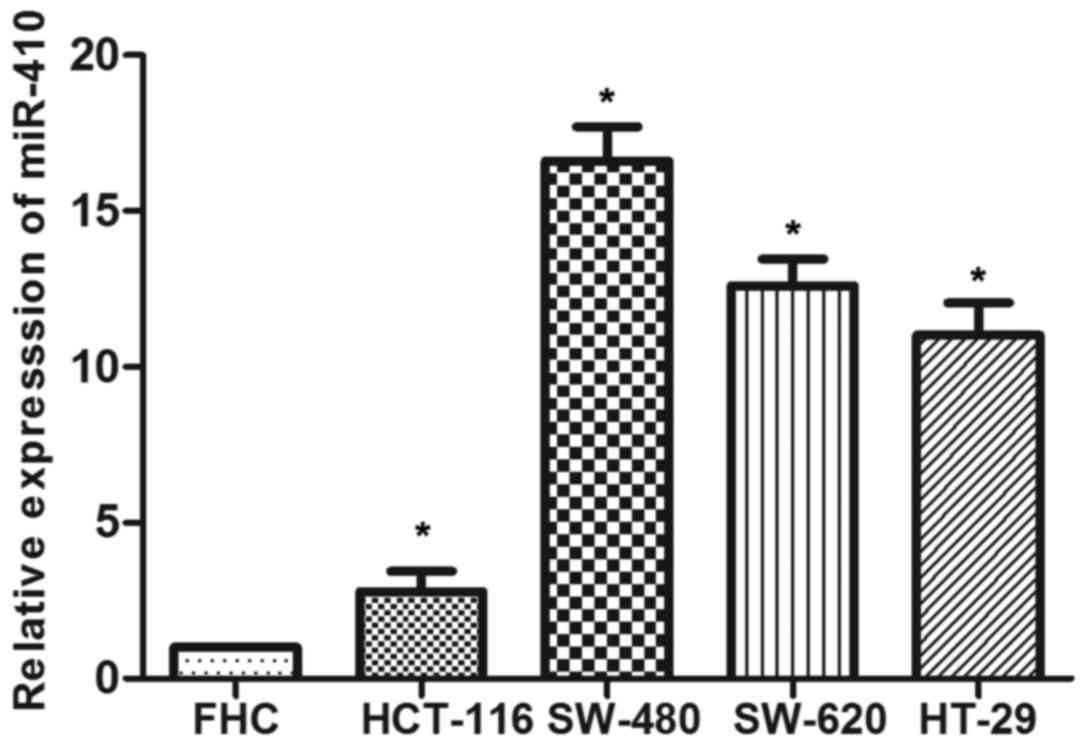
To investigate whether miR-410 is involved in CRC
development, miR-410 levels in four CRC cell lines (SW-480, SW-620,
HT-29 and HCT-116) were examined by RT-qPCR. As shown in Fig. 1, the data demonstrated that miR-410
expression was significantly upregulated in CRC cell lines compared
with the control. SW-480 and HT-116 cell lines were employed in
subsequent experiments.
miR-410 induces CRC cell proliferation
and inhibits cell apoptosis
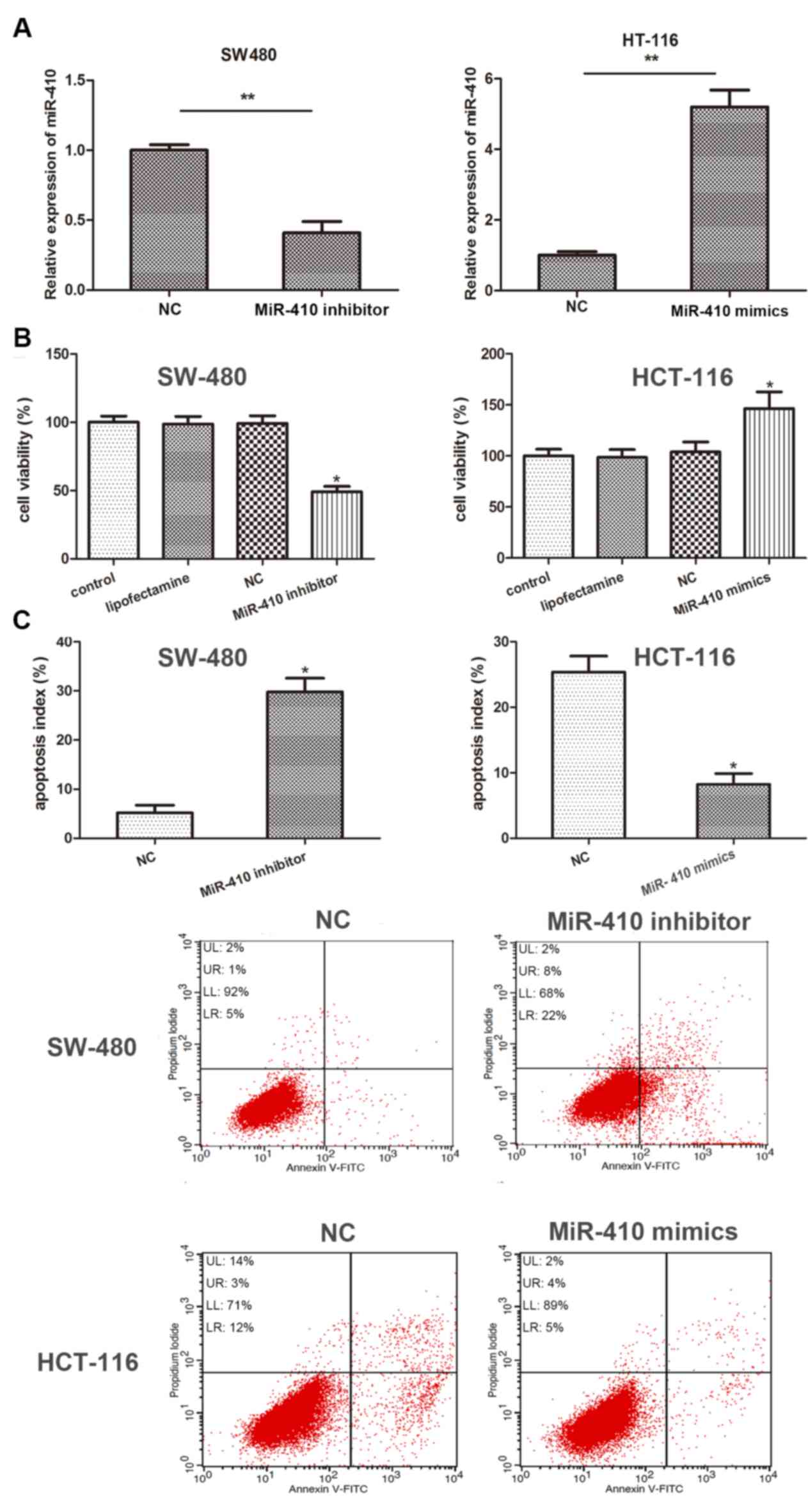
To investigate the effect of miR-410 in CRC
development, miR-410 inhibitor and NC inhibitor were used to
evaluate the biological properties of miR-410 in SW-480 and HT-116
cells. Transfection efficiency was determined using RT-qPCR. As the
results demonstrate, miR-410 expression was significantly inhibited
in SW-480 cells and significantly upregulated in HT-116 cells
following transfection with miR-410 inhibitor and miR-410 mimics,
respectively (Fig. 2A). The results
of the CCK-8 assay revealed that knockdown of miR-410 significantly
inhibited cellular viability compared with NC group, while miR-410
overexpression markedly promoted cellular viability (Fig. 2B). In addition, flow cytometry data
demonstrated that apoptotic rate of SW-480 was significantly
increased by miR-410 inhibitor, while the apoptotic rate of HT-116
cells was suppressed by miR-410 mimics compared with NC group
(Fig. 2C). The current data
demonstrated that miR-410 is closely associated with cell
proliferation and apoptosis in CRC cells.
miR-410 promotes cell migration and
invasion of CRC cells
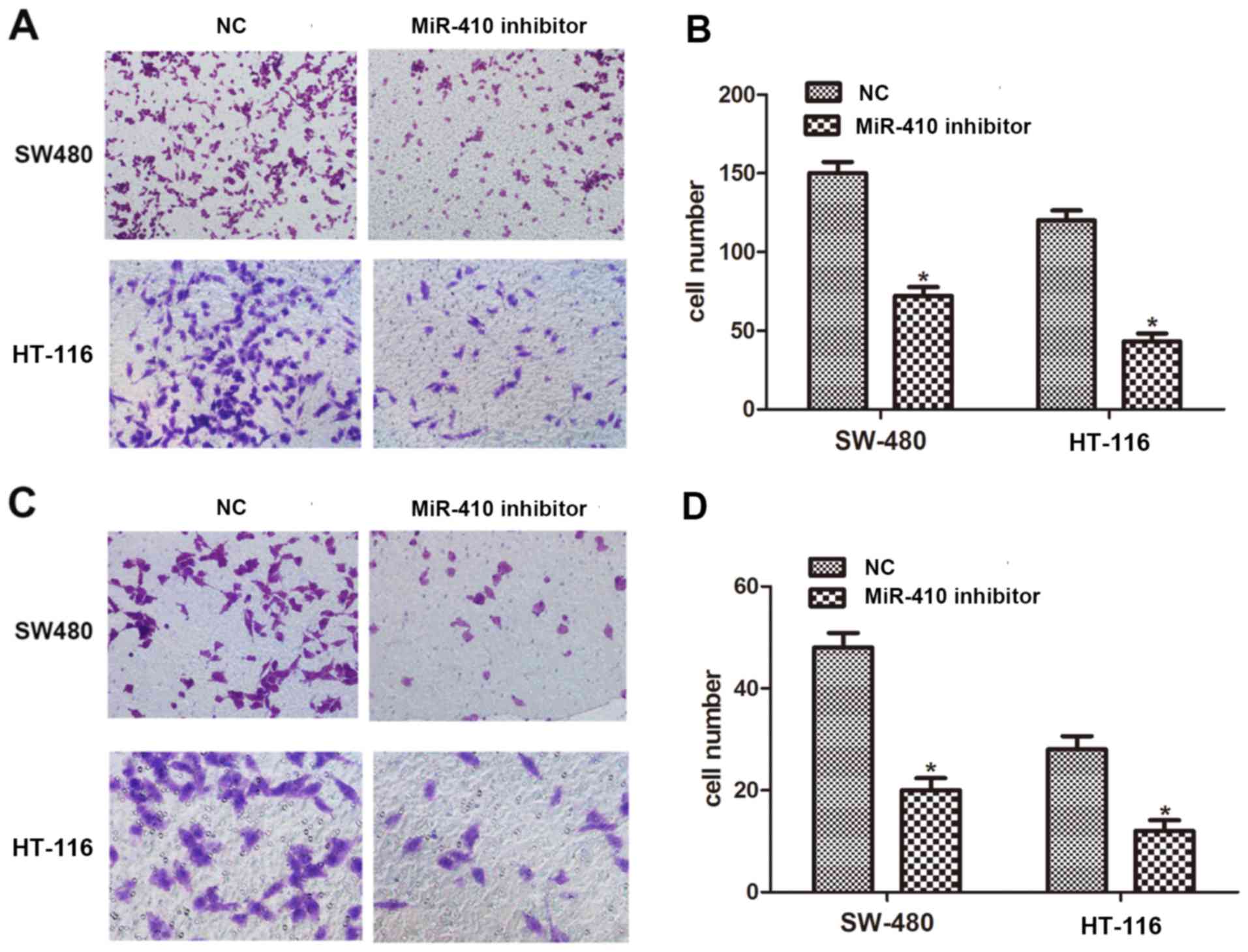
To delineate the role of miR-410 in the metastasis
of CRC, a Transwell assay was employed to evaluate the migration
and invasion capacity of CRC cells. It was identified that
downregulation of miR-410 significantly inhibited cell migration in
SW-480 and HT-116 cells (Fig. 3A and
B). Compared with NC inhibitor, the number of invaded cells was
dramatically reduced when cells were treated with miR-410 inhibitor
(Fig. 3C and D). These results
suggest that miR-410 promotes the migration and invasion capacity
of CRC cells.
miR-410 directly targets DKK1 and
negatively regulates DKK1 expression
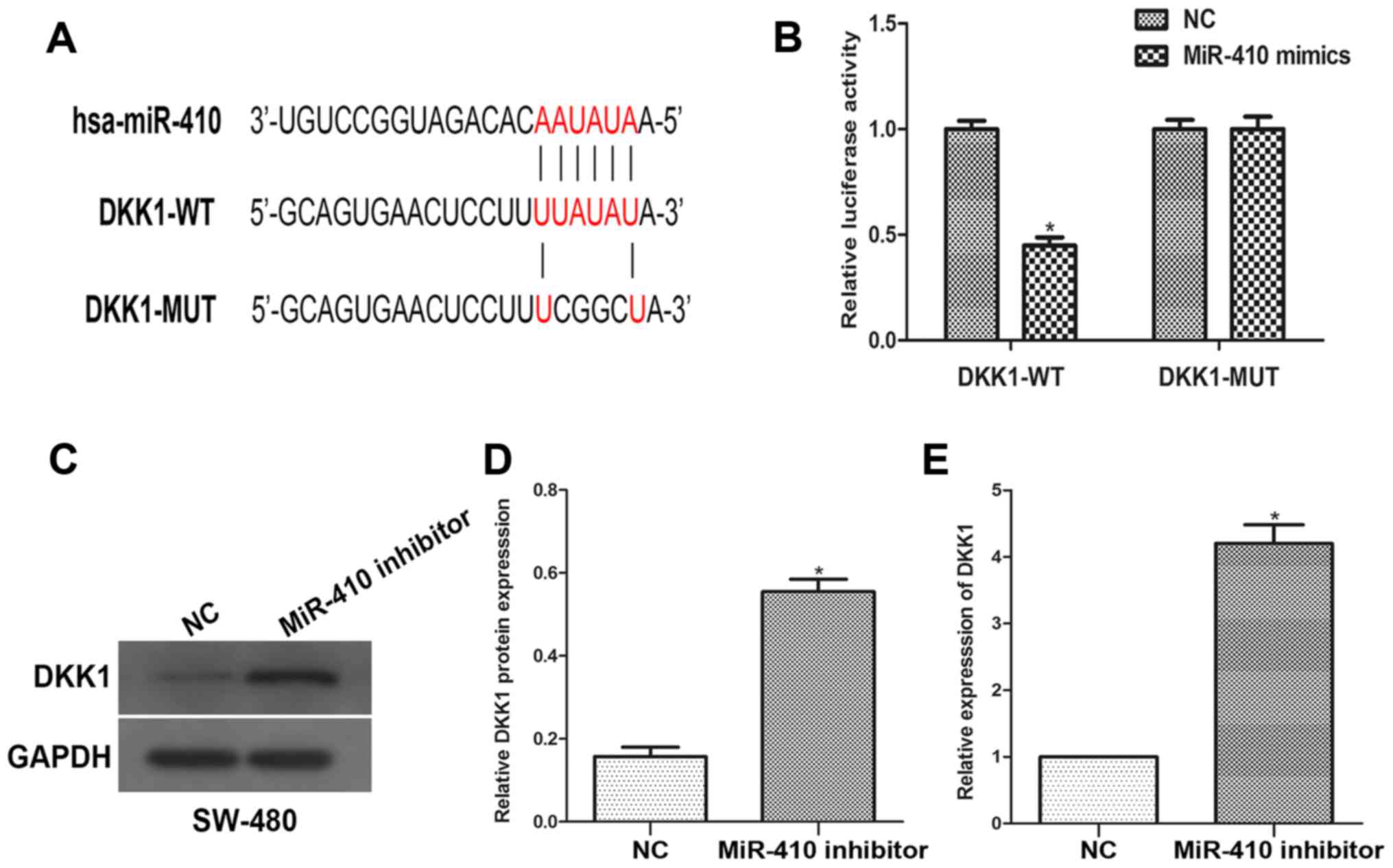
An open access database was employed to predict the
putative binding sequences between miR-410 and DKK1. To further
confirm whether DKK1 is a direct target of miR-410, the luciferase
reporter vectors of the DKK1 3′-UTR containing miR-410 binding
sites (DKK-1 WT), or a mutated version (DKK-MUT), were constructed.
Co-transfection was performed with miR-410 inhibitor (or NC
inhibitor) and DKK-1 WT (or DKK-1 MUT) plasmids into SW480 cells. A
dual-luciferase reporter assay was used to measure the reporter
activities of the different constructs. The results revealed that
knockdown of miR-410 significantly increased reporter vector
activity of DKK-1 3′-UTR in SW-480 cells but had no effect on the
mutated reporter vector (Fig. 4A and
B). To further explore the modulation of DKK1 expression by
miR-410, western blot and RT-qPCR analyses were performed. It was
demonstrated that miR-410 silencing led to an increase in the DKK1
expression level (Fig. 4C-E). The
current results indicate that miR-410 directly targets DKK1 and
negatively regulates DKK1 expression.
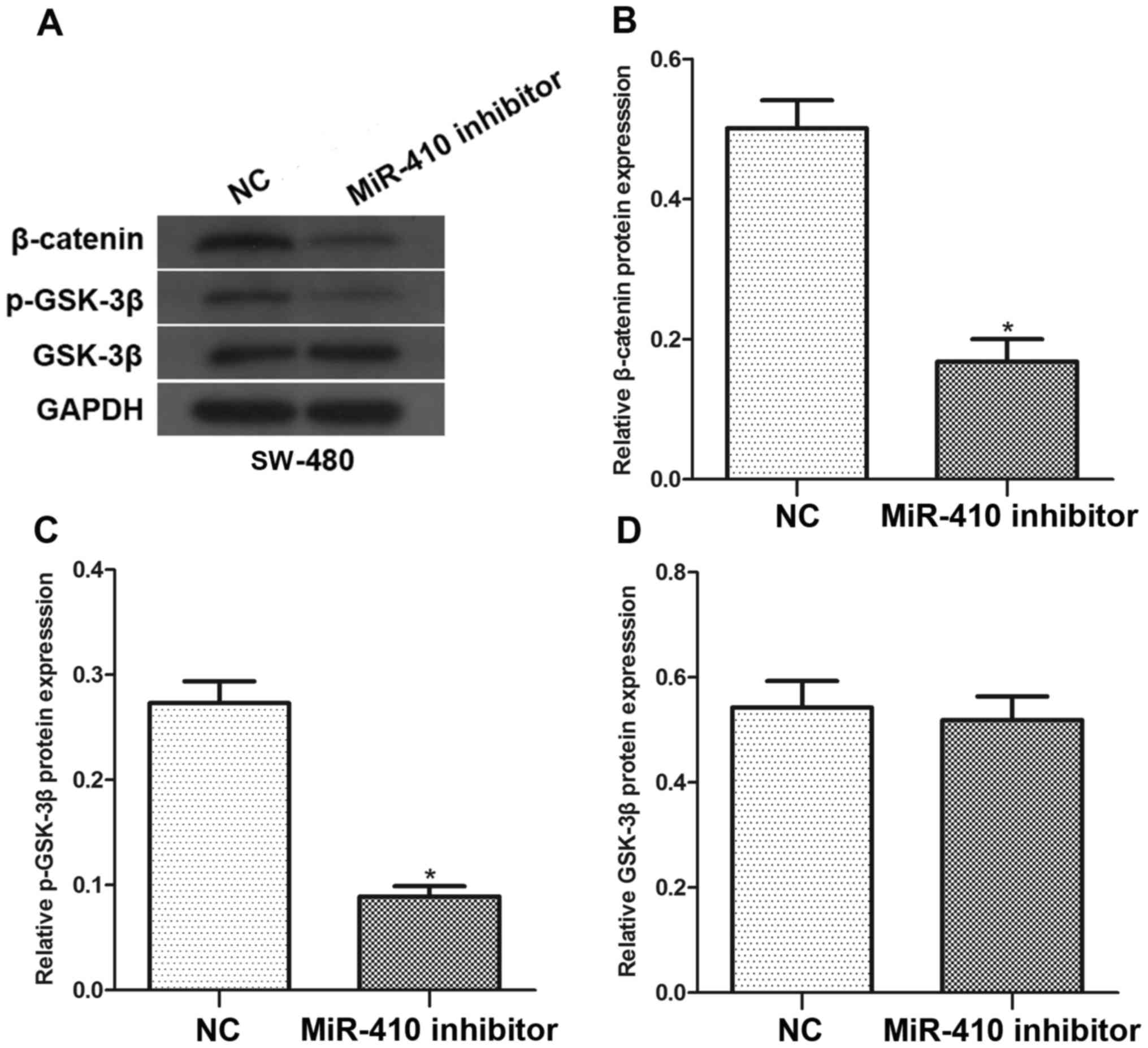
Effects of miR-410 on Wnt/β-catenin
signaling pathways
To further investigate whether miR-410 affected
Wnt/β-catenin signaling pathways, SW-480 cells were transfected
with miR-410 inhibitor or NC inhibitor. As shown in Fig. 5, western blot analysis revealed that
downregulation of miR-410 significantly decreased β-catenin and
p-GSK-3β protein levels in SW-480 cells. However, there was no
difference in the level of GSK-3β with regard to miR-410
downregulation. Collectively, these results demonstrate that
miR-410 may be an important regulator in the Wnt/β-catenin
signaling pathways.
Discussion
Despite advances in diagnosis and treatment for CRC
patients, it remains difficult to eradicate tumors and prevent
recurrence. Evidence has demonstrated that 20–45% of patients who
undergo radical resection experience relapse or metastasis after a
short period of remission (19,20).
Therefore, it is important to uncover the underlying molecular
mechanisms in the progression of CRC and identify more effective
therapeutic targets. Previous studies have revealed that miRNAs
closely associate with tumor proliferation and apoptosis in cancer
cells by targeting specific genetic targets (6,21). The
current study aimed to identify miRNAs that regulate the
progression of CRC.
A growing volume of literature has demonstrated that
miRNA regulates gene expression post-transcriptionally and acts as
an oncogene or tumor suppressor in different cancer types (22,23).
miR-410 has been confirmed to inhibit tumor invasion and metastasis
in several malignancies (24,25). It has also been reported that miR-410
suppresses CRC cell growth, migration and invasion through binding
to certain targets (10,16). In the current study, it was confirmed
that endogenous expression of miR-410 was upregulated in CRC cell
lines, implying that miR-410 may be involved in the tumorigenesis
of CRC. Additionally, the current data demonstrated that miR-410
knockdown inhibited cell proliferation and induced apoptosis in CRC
cell lines. Using a cell Transwell assay, it was identified that
knockdown of miR-410 mitigated migration and invasion capability of
CRC cells in vitro. The aforementioned results are in
accordance with a previous report (14), suggesting that miR-410 functions like
an oncogene in CRC cells and has potential to be used as a
biomarker for diagnosis and prognosis of CRC.
The Wnt/β-catenin signaling pathway is well known
for its critical role in the early progression of metastasis and
tumor growth (26,27). The triggering of Wnt signaling could
prevent GSK-3β from activation and maintain β-catenin
stabilization, characteristics that are strongly associated with
tumor metastasis (28,29). DKK-1 is one of four members of the
extracellular Wnt inhibitors family, which can block signaling by
binding to plasma membrane Wnt-receptor complexes (30,31). It
has been reported that DKK-1 inhibits colorectal cell proliferation
both in vitro and in vivo (32), supporting a tumor suppressor role for
this protein. Additionally, several studies have demonstrated that
the expression of DKK-1 is downregulated in colorectal adenoma
carcinoma at late CRC stages (33).
DKK-1 has also been revealed to be downregulated in chronic
lymphocytic leukemia and papillary thyroid cancer (34,35). The
current study predicted binding between miR-410 and DKK-1 using
bioinformatics methods and then confirmed this interaction by
luciferase reporter assay. Subsequently, the effect of miR-410
knockdown on the expression of DKK-1 and Wnt/β-catenin signaling
was investigated. As the current results revealed, knockdown of
miR-410 significantly promoted the expression of DKK-1 while it
decreased the expression of β-catenin and p-GSK-3β. This suggests
that the biological function of miR-410 in CRC may be associated
with the negative regulation of DKK-1 via the Wnt/β-catenin
signaling pathway.
In conclusion, the current study indicated that
miR-410 acts as an oncogene in CRC cells by promoting cell
proliferation, migration and invasion capacity, and this biological
function may, at least in part, be ascribed to the negative
regulation of DKK1 through the Wnt/β-catenin signaling pathway.
These results implied that miR-410 could potentially be used as a
biomarker for the diagnosis and prognosis of CRC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed data sets generated during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
WW was responsible for conception and design of the
study, data collection and analysis, and manuscript writing. YH
designed the study, performed critical revision and supervised all
phases of the study. JR and MX, data collection and analysis.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen W, Zheng R, Zeng H, Zhang S and He J:
Annual report on status of cancer in China, 2011. Chin J Cancer
Res. 27:2–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ,
Meester RGS, Barzi A and Jemal A: Colorectal cancer statistics,
2017. CA Cancer J Clin. 67:177–193. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan C, Lin Y, Mao Y, Huang Z, Liu AY, Ma
H, Yu D, Maitikabili A, Xiao H, Zhang C, et al: MicroRNA-543
suppresses colorectal cancer growth and metastasis by targeting
KRAS, MTA1 and HMGA2: Oncotarget. 7:21825–21839. 2016.
|
|
4
|
Lan YT, Yang, Chang SC, Liang WY, Li AF,
Wang HS, Jiang JK, Chen WS, Lin TC and Lin JK: Analysis of the
seventh edition of American Joint Committee on colon cancer
staging. Int J Colorectal Dis. 27:657–663. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kala R, Peek GW, Hardy TM and Tollefsbol
TO: MicroRNAs: An emerging science in cancer epigenetics. J Clin
Bioinforma. 3:62013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bushati N and Cohen SM: microRNA
functions. Annu Rev Cell Dev Biol. 23:175–205. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wu X, Wu G, Wu Z, Yao X and Li G: miR-200a
suppresses the proliferation and metastasis in pancreatic ductal
adenocarcinoma through downregulation of DEK gene. Transl Oncol.
9:25–31. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Du Y, Liu XH, Zhu HC, Wang L, Ning JZ and
Xiao CC: miR-543 Promotes proliferation and epithelial-mesenchymal
transition in prostate cancer via targeting RKIP. Cell Physiol
Biochem. 41:1135–1146. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang H, Sun Z, Wang Y, Hu Z, Zhou H, Zhang
L, Hong B, Zhang S and Cao X: miR-33-5p, a novel mechano-sensitive
microRNA promotes osteoblast differentiation by targeting Hmga2.
Sci Rep. 6:231702016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rak B, Mehlich D, Garbicz F, Domosud Z,
Paskal W, Marczewska JM and Włodarski PK: Post-transcriptional
regulation of MMP16 and TIMP2 expression via miR-382, miR-410 and
miR-200b in endometrial cancer. Cancer Genomics Proteomics.
14:389–401. 2017.PubMed/NCBI
|
|
11
|
Yang N, Chen J, Zhang H, Wang X, Yao H,
Peng Y and Zhang W: LncRNA OIP5-AS1 loss-induced microRNA-410
accumulation regulates cell proliferation and apoptosis by
targeting KLF10 via activating PTEN/PI3K/AKT pathway in multiple
myeloma. Cell Death Dis. 8:e29752017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ke X, Yuan Y, Guo C, Yang Y, Pu Q, Hu X,
Tang K, Luo X, Jiang Q, Su X, et al: miR-410 induces stemness by
inhibiting Gsk3β but upregulating β-catenin in non-small cells lung
cancer. Oncotarget. 8:11356–11371. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang YF, Yu Y and Song WZ: miR-410-3p
suppresses breast cancer progression by targeting Snail. Oncol Rep.
36:480–486. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang Y, Fu J, Jiang M, Zhang X, Cheng L,
Xu X, Fan Z, Zhang J, Ye Q and Song H: miR-410 is overexpressed in
liver and colorectal tumors and enhances tumor cell growth by
silencing FHL1 via a direct/indirect mechanism. PLoS One.
9:e1087082014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Shi C, Zhao J and Li B: Long
noncoding RNA CCAT1 functions as a ceRNA to antagonize the effect
of miR-410 on the down-regulation of ITPKB in human HCT-116 and
HCT-8 cells. Oncotarget. 8:92855–92863. 2017.PubMed/NCBI
|
|
16
|
Liu C, Zhang A, Cheng L and Gao Y: miR-410
regulates apoptosis by targeting Bak1 in human colorectal cancer
cells. Mol Med Rep. 14:467–473. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2ˆ(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
18
|
Hussain H and Chong NF: Combined overlap
extension PCR method for improved site directed mutagenesis. Biomed
Res Int. 2016:80415322016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Winawer S, Fletcher R, Rex D, Bond J, Burt
R, Ferrucci J, Ganiats T, Levin T, Woolf S, Johnson D, et al:
Colorectal cancer screening and surveillance: Clinical guidelines
and rationale-Update based on new evidence. Gastroenterology.
124:544–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu W, Sun M, Zou GM and Chen J: MicroRNA
and cancer: Current status and prospective. Int J Cancer.
120:953–960. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Okato A, Goto Y, Kurozumi A, Kato M,
Kojima S, Matsushita R, Yonemori M, Miyamoto K, Ichikawa T and Seki
N: Direct regulation of LAMP1 by tumor-suppressive microRNA-320a in
prostate cancer. Int J Oncol. 49:111–122. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin Y, Liu AY, Fan C, Zheng H, Li Y, Zhang
C, Wu S, Yu D, Huang Z, Liu F, et al: MicroRNA-33b inhibits breast
cancer metastasis by targeting HMGA2, SALL4 and Twist1. Sci Rep.
5:99952015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wen R, Umeano AC, Essegian DJ,
Sabitaliyevich UY, Wang K and Farooqi AA: Role of microRNA-410 in
molecular oncology: A double edged sword. J Cell Biochem. Aug
7–2018.(Epub ahead of print). View Article : Google Scholar
|
|
25
|
Wu H, Li J, Guo E, Luo S and Wang G:
miR-410 acts as a tumor suppressor in estrogen receptor-positive
breast cancer cells by directly targeting ERLIN2 via the ERS
pathway. Cell Physiol Biochem. 48:461–474. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ke Z, He W, Lai Y, Guo X, Chen S, Li S,
Wang Y and Wang L: Overexpression of collagen triple helix repeat
containing 1 (CTHRC1) is associated with tumour aggressiveness and
poor prognosis in human non-small cell lung cancer. Oncotarget.
5:9410–9424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sethi JK and Vidal-Puig A: Wnt signalling
and the control of cellular metabolism. Biochem J. 427:1–17. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Fukumoto S, Hsieh CM, Maemura K, Layne MD,
Yet SF, Lee KH, Matsui T, Rosenzweig A, Taylor WG, Rubin JS, et al:
Akt participation in the Wnt signaling pathway through Dishevelled.
J Biol Chem. 276:17479–17483. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou BP, Deng J, Xia W, Xu J, Li YM,
Gunduz M and Hung MC: Dual regulation of Snail by
GSK-3beta-mediated phosphorylation in control of
epithelial-mesenchymal transition. Nat Cell Biol. 6:931–940. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Glinka A, Wu W, Delius H, Monaghan AP,
Blumenstock C and Niehrs C: Dickkopf-1 is a member of a new family
of secreted proteins and functions in head induction. Nature.
391:357–362. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Aguilera O, Fraga MF, Ballestar E, Paz MF,
Herranz M, Espada J, García JM, Muñoz A, Esteller M and
González-Sancho JM: Epigenetic inactivation of the Wnt antagonist
DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene.
25:4116–4121. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu Z, Sun B, Qi L, Li Y, Zhao X, Zhang D
and Zhang Y: Dickkopf-1 expression is down-regulated during the
colorectal adenoma-carcinoma sequence and correlates with reduced
microvessel density and VEGF expression. Histopathology.
67:158–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pamuk GE, Uyanik MS, Pamuk ON, Maden M and
Tapan U: Decreased dickkopf-1 levels in chronic lymphocytic
leukemia and increased osteopontin levels in non-Hodgkin's lymphoma
at initial diagnosis: Could they be playing roles in pathogenesis?
Hematology. 20:267–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho SW, Kim YA, Sun HJ, Ahn HY, Lee EK, Yi
KH, Oh BC, Park DJ, Cho BY and Park YJ: Therapeutic potential of
Dickkopf-1 in wild-type BRAF papillary thyroid cancer via
regulation of β-catenin/E-cadherin signaling. J Clin Endocrinol
Metab. 99:E1641–E1649. 2014. View Article : Google Scholar : PubMed/NCBI
|