Introduction
Medulloblastoma (MB) is the most common solid tumor
in children, comprising 15–20% of pediatric central nervous system
tumors (1,2). MB may occur at all ages, however its
peak incidence is between 4 and 7 years (3). In 2016, the World Health Organization
classified MB into four subtypes, including WNT-activated,
SHH-activated, group 3 and group 4, by combining molecular
profiling with histology (4). In
addition, as these tumors occur in the posterior fossa, clinical
symptoms are often too vague for accurate and prompt diagnosis
(5). The therapeutic options include
maximal safe surgical resection, radiation and chemotherapy
(3). However, cerebellar mutism may
occur in >25% of the cases following maximal surgical resection
in patients with high-risk MB; recovering patients may still
experience dysarthria and neurocognitive dysfunction (6). In addition, adjuvant chemotherapy and
radiotherapy may lead to hearing loss and the development of
secondary tumors (6). The main cause
of mortality in MB is metastatic disease, which is unresectable
(7). Although multimodal therapy
significantly improves the prognosis of MB, approximately one-third
of the patients eventually succumb to the disease (3). Therefore, further research on the
underlying molecular mechanisms is imperative, in order to design
more efficient and precise treatment strategies to improve patient
survival.
With the completion of the Human Genome Project,
molecular diagnosis and therapy have become available in clinical
practice, which is helpful for improving the accuracy and efficacy
of diagnosis and treatment (4).
Using bioinformatics and microarray analysis, it is possible to
further examine the underlying gene characteristics and molecular
mechanisms involved in the proliferation, invasion and metastasis
of MB (8,9). For example, mitotic kinases and WEE1 G2
checkpoint kinase were identified as rational therapeutic targets
for MB by performing an integrated genomic analysis using
structural and functional methods (10).
In the present study, 4 databases with 115 samples
of MB and normal tissues were downloaded from the Gene Expression
Omnibus (GEO) database. Following screening of differentially
expressed genes (DEGs) though package Limma in R, Gene Ontology
(GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) analyses
were performed to analyze the potential functional and pathway
enrichment, and a protein-protein interaction (PPI) network was
subsequently constructed using the Search Tool for Retrieval of
Interacting Genes (STRING) database and visualized with Cytoscape
software, in order to identify biomarkers and examine the potential
underlying molecular mechanisms in MB. Therefore, the results of
the present study may improve our understanding of MB, identify
potential biomarkers and indicate methods of diagnosis and
treatment for future research.
Materials and methods
Microarray data
In the present study, the gene expression profiles
of GSE35493 (11), GSE50161
(12), GSE74195 (13) and GSE86574 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE86574)
were downloaded from the GEO database (http://www.ncbi.nlm.nih.gov/geo/). A total of 115
samples, including 78 MB and 37 normal samples, had been hybridized
on the Affymetrix Human Genome U133 Plus 2.0 Array (HG-U133_Plus_2)
on the GPL570 platform. GSE35493 included 17 MB and 9 normal
samples, GSE50161 included 22 MB and 13 normal samples, GSE74195
included 23 MB and 5 normal samples, and GSE86574 included 16 MB
and 10 normal samples. GSE85217 from the GPL22286 platform included
613 MB samples.
Identification of DEGs
The raw data in CEL files were transformed into gene
symbols based on the downloaded platform annotation files. The data
were preprocessed, including background correction, normalization
and summarization, via R 3.4.1 software (https://www.r-project.org/) (14). Following robust multiarray average
normalization, Limma in R package (version 3.26.9) was used to
screen DEGs (15). The genes meeting
the cut-off criteria of adjusted P<0.05 and
|log2fold-change (FC)|>1 were selected as the
DEGs.
Functional and pathway enrichment
analysis
After acquiring the DEGs, GO enrichment analysis and
KEGG pathway enrichment analysis were performed through DAVID
(https://david.ncifcrf.gov/) online tool
to identify functional categories of DEGs (16). GO analysis of DEGs included
biological process (BP), molecular function (MF) and cell component
(CC). In addition, the terms with P<0.05 were considered to
indicate a statistically significant difference.
Construction of the PPI network and
selection of modules
To identify hub genes and screen modules, the DEGs
were uploaded to STRING (version 10.5; http://www.string-db.org/) to analyze and set up the
PPI network (17). Subsequently, the
network was visualized in Cytoscape (version 3.5.1; www.cytoscape.org) (18). The Cytoscape software was applied to
search for hub genes with CytoHubba, a plugin to hub an object from
complex networks, and modules with Molecular Complex Detection
(MCODE) (19,20). Furthermore, hub genes in selected
modules were analyzed via DAVID to examine pathway enrichment.
Survival analysis
To assess the association between hub genes and
survival, 613 MB samples with clinical data from GSE85217 were
selected and survival curves were drawn by recruiting the survival
package in R.
Results
Identification of DEGs
Basing on the cut-off criteria of P<0.05 and
|log2fold-change (FC)|>1, a total of 6,305, 4,185,
2,506 and 4,253 DEGs between MB and normal samples were screened
from GSE35493, GSE50161, GSE74195 and GSE86574, respectively. DEGs
from GSE35493 included 5,242 upregulated and 1,063 downregulated
genes. DEGs from GSE50161 included 3,025 upregulated and 1,160
downregulated genes. DEGs from GSE74195 included 1,315 upregulated
and 1,191 downregulated genes. DEGs from GSE86574 included 2,772
upregulated and 1,481 downregulated genes. In addition, a total of
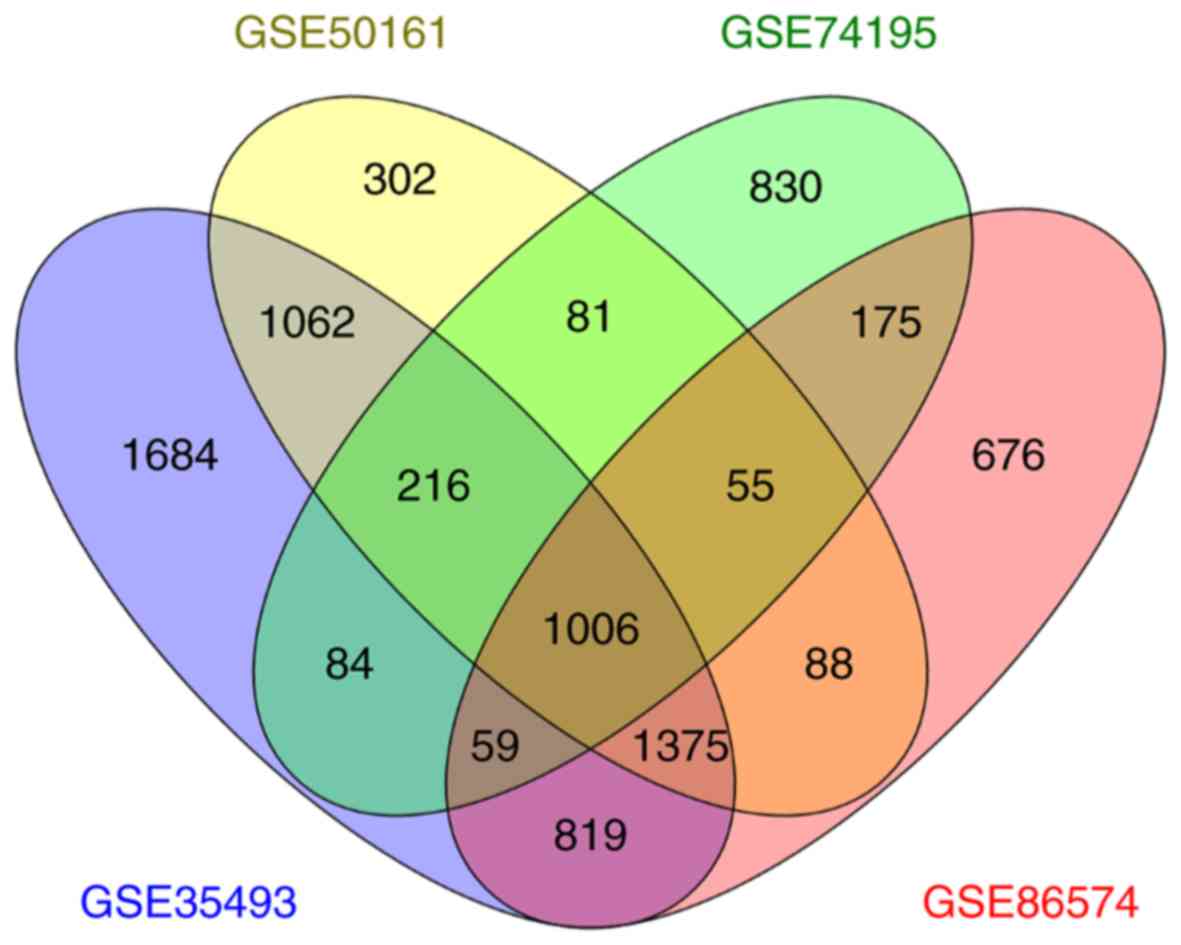
1,006 mutual DEGs were screened, among those 4 datasets, by
performing a Venn diagram analysis (Fig.
1), where 540 were upregulated and 466 were downregulated.
Functional and pathway enrichment
analysis
Submitting 1,006 mutual DEGs to DAVID provided
further insight into the function of these DEGs and the molecular
mechanisms implicated in MB. The top 10 significant terms of each
GO category (Table I) and the top 10
terms of KEGG category (Table II)
were selected. The GO analysis results demonstrated that overlapped
DEGs were significantly associated with cell division, mitotic
nuclear division and chemical synaptic transmission in the BP
category; cell junction, condensed chromosome kinetochore and
postsynaptic membrane in the CC category; and protein binding,
microtubule binding and chromatin binding in the MF category
(P<0.05) (Table I). In addition,
the enriched KEGG pathway analysis results primarily included cell
cycle, DNA replication and retrograde endocannabinoid signaling
(Table II).
 | Table I.Top 10 significant GO terms of BP, MF
and CC. |
Table I.
Top 10 significant GO terms of BP, MF
and CC.
| Category | Term | Description | Count | P-value |
|---|
| BP | GO:0051301 | Cell division | 77 |
1.54×10−27 |
| BP | GO:0007067 | Mitotic nuclear
division | 55 |
6.14×10−20 |
| BP | GO:0007268 | Chemical synaptic
transmission | 45 |
1.47×10−13 |
| BP | GO:0000082 | G1/S transition of
mitotic cell cycle | 28 |
1.02×10−12 |
| BP | GO:0007062 | Sister chromatid
cohesion | 27 |
8.76×10−12 |
| BP | GO:0006260 | DNA
replication | 33 |
1.23×10−11 |
| BP | GO:0000070 | Mitotic sister
chromatid segregation | 12 |
1.48×10−08 |
| BP | GO:0007059 | Chromosome
segregation | 18 |
4.14×10−08 |
| BP | GO:0000086 | G2/M transition of
mitotic cell cycle | 24 |
5.11×10−07 |
| BP | GO:0007076 | Mitotic chromosome
condensation | 8 |
4.23×10−06 |
| CC | GO:0030054 | Cell junction | 65 |
8.14×10−14 |
| CC | GO:0000777 | Condensed
chromosome kinetochore | 24 |
2.74×10−11 |
| CC | GO:0045211 | Postsynaptic
membrane | 37 |
8.92×10−11 |
| CC | GO:0014069 | Postsynaptic
density | 34 |
1.40×10−10 |
| CC | GO:0005654 | Nucleoplasm | 206 |
2.20×10−09 |
| CC | GO:0030425 | Dendrite | 43 |
3.92×10−08 |
| CC | GO:0000775 | Chromosome,
centromeric region | 16 |
8.32×10−08 |
| CC | GO:0030496 | Midbody | 24 |
9.65×10−08 |
| CC | GO:0043025 | Neuronal cell
body | 40 |
1.67×10−07 |
| CC | GO:0005874 | Microtubule | 39 |
3.78×10−07 |
| MF | GO:0005515 | Protein
binding | 531 |
3.92×10−09 |
| MF | GO:0008017 | Microtubule
binding | 32 |
7.31×10−08 |
| MF | GO:0003682 | Chromatin
binding | 40 |
4.88×10−05 |
| MF | GO:0005524 | ATP binding | 110 |
7.45×10−05 |
| MF | GO:0019901 | Protein kinase
binding | 38 |
1.00×10−04 |
| MF | GO:0005201 | Extracellular
matrix structural constituent | 13 |
1.27×10−04 |
| MF | GO:0005509 | Calcium ion
binding | 60 |
1.87×10−04 |
| MF | GO:0017075 | Syntaxin-1
binding | 7 |
1.88×10−04 |
| MF | GO:0004890 | GABA-A receptor
activity | 7 |
2.63×10−04 |
| MF | GO:0005219 | Ryanodine-sensitive
calcium-release channel activity | 4 |
5.07×10−04 |
 | Table II.Top 10 significant Kyoto Encyclopedia
of Genes and Genomes pathways. |
Table II.
Top 10 significant Kyoto Encyclopedia
of Genes and Genomes pathways.
| Term | Description | Count | P-value | Genes |
|---|
| hsa04110 | Cell cycle | 28 |
3.95×10−11 | E2F5, DBF4, TTK,
CHEK1, PTTG1, CHEK2, CCNE2, CDC45, MCM7, CDKN2C, CDK1, ESPL1, CDK6,
CDC20, MCM2, CDK4, MCM3, WEE1, CDC25A, MCM5, CCNB1, MAD2L1, CCNB2,
CCND2, PLK1, PCNA, BUB1B, ABL1 |
| hsa03030 | DNA
replication | 14 |
7.26×10−09 | MCM2, RNASEH2A,
MCM3, MCM5, PRIM1, RFC3, RFC4, MCM7, POLE2, RFC2, POLD1, PRIM2,
PCNA, FEN1 |
| hsa04723 | Retrograde
endocannabinoid signaling | 22 |
1.50×10−08 | GABRD, GABRG1,
GABRA2, GABRA1, GNAI3, GABRA4, GABRB2, GABRB1, GNG13, MAPK10,
GRIA4, RIMS1, KCNJ3, ITPR1, SLC17A7, SLC32A1, KCNJ6, KCNJ9, GRIA1,
MGLL, GNG3, GNG4 |
| hsa04727 | GABAergic
synapse | 20 |
2.14×10−08 | GABRD, GABRG1,
GABRA2, GABARAPL1, GABRA1, GNAI3, SLC6A1, GABRA4, GABRB2, GABRB1,
GABBR1, GNG13, GABBR2, GLS2, SLC32A1, KCNJ6, ABAT, GNG3, GNG4,
GAD1 |
| hsa05032 | Morphine
addiction | 19 |
3.66×10−07 | GABRD, GABRG1,
GABRA2, GNAI3, GABRA1, GABRA4, GABRB2, GABRB1, GABBR1, GNG13,
GABBR2, KCNJ3, ADORA1, SLC32A1, KCNJ6, KCNJ9, PDE1A, GNG3,
GNG4 |
| hsa05033 | Nicotine
addiction | 12 |
2.42×10−06 | SLC17A7, GABRD,
SLC32A1, GABRG1, GABRA2, GABRA1, GABRA4, GRIA1, GABRB2, GABRB1,
GRIN2A, GRIA4 |
| hsa04728 | Dopaminergic
synapse | 20 |
1.55×10−05 | SCN1A, CALY,
PPP2R3A, GNAI3, KIF5A, GRIN2A, GNG13, MAPK10, GRIA4, KCNJ3, ITPR1,
KCNJ6, KCNJ9, PPP1R1B, GRIA1, CREB3L4, CAMK2B, GNG3, PPP3CA,
GNG4 |
| hsa04713 | Circadian
entrainment | 16 |
5.91×10−05 | GNAI3, GRIN2A,
GNG13, GRIA4, KCNJ3, ITPR1, KCNJ6, KCNJ9, GRIA1, RYR3, RYR1, RYR2,
CAMK2B, GUCY1B3, GNG3, GNG4 |
| hsa03430 | Mismatch
repair | 8 |
8.58×10−05 | EXO1, MSH6, RFC3,
RFC4, RFC2, MSH2, POLD1, PCNA |
| hsa04115 | p53 signaling
pathway | 13 |
9.34×10−05 | CCNB1, CCNE2, CDK1,
TP53I3, CCNB2, CCND2, RRM2, SIAH1, CHEK1, CDK6, CHEK2, CDK4,
GTSE1 |
Module screening from the PPI
network
Among the 4 datasets, the overlapped 1,006 DEGs were
analyzed with the PPI network via STRING, and interaction with a
score >0.9 was subsequently obtained in the following analysis.
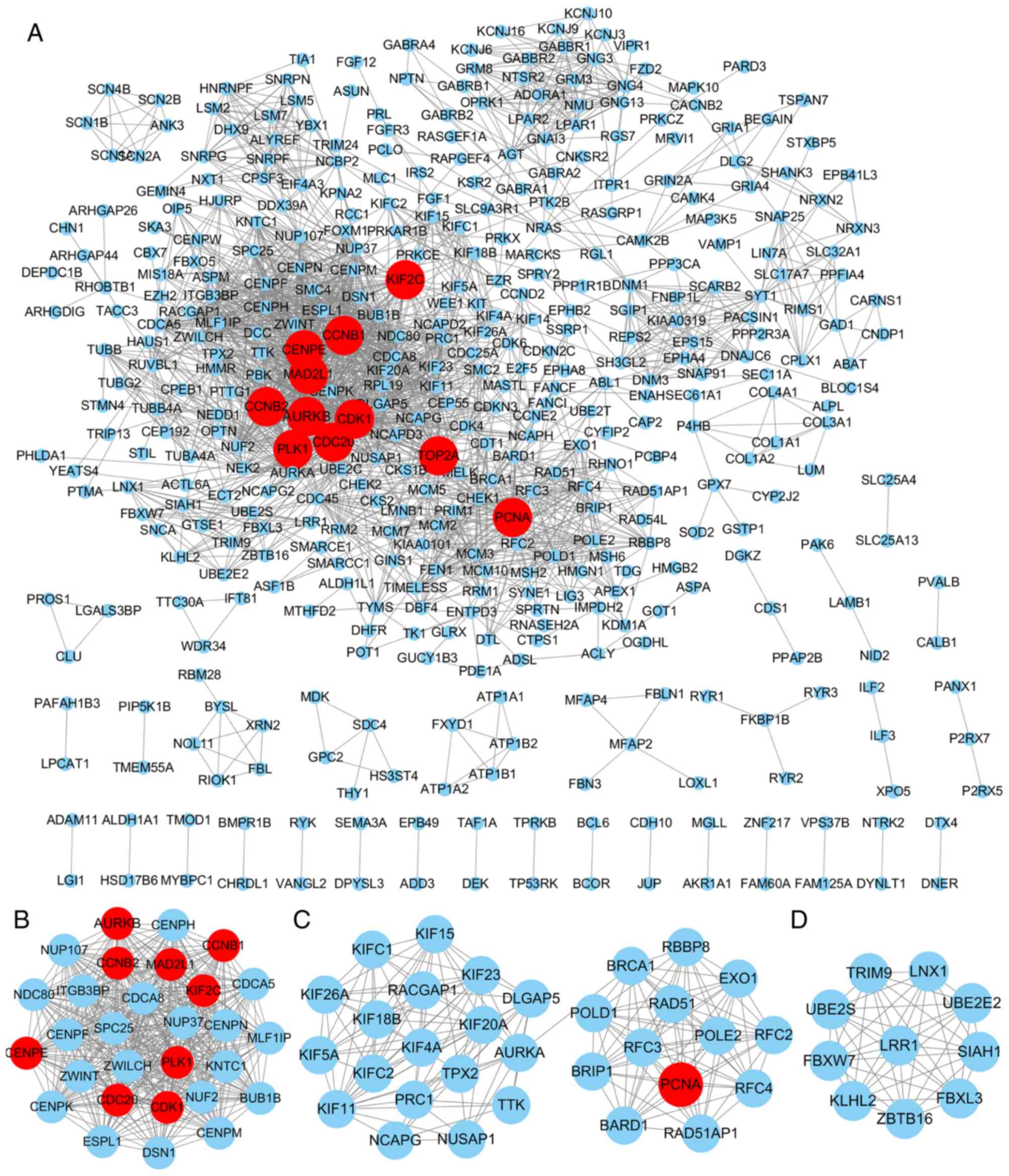
Furthermore, the hub genes with degrees >44 were screened out
based on CytoHubba. In total, 11 nodes were screened out as hub
genes, including CDK1 (degree=90), CCNB1 (degree=68), CCNB2
(degree=60), PLK1 (degree=60), CDC20 (degree=57), MAD2L1
(degree=53), AURKB (degree=50), CENPE (degree=46), TOP2A
(degree=45), KIF2C (degree=45) and PCNA (degree=45; Fig. 2A). Among the 11 hub genes, the node
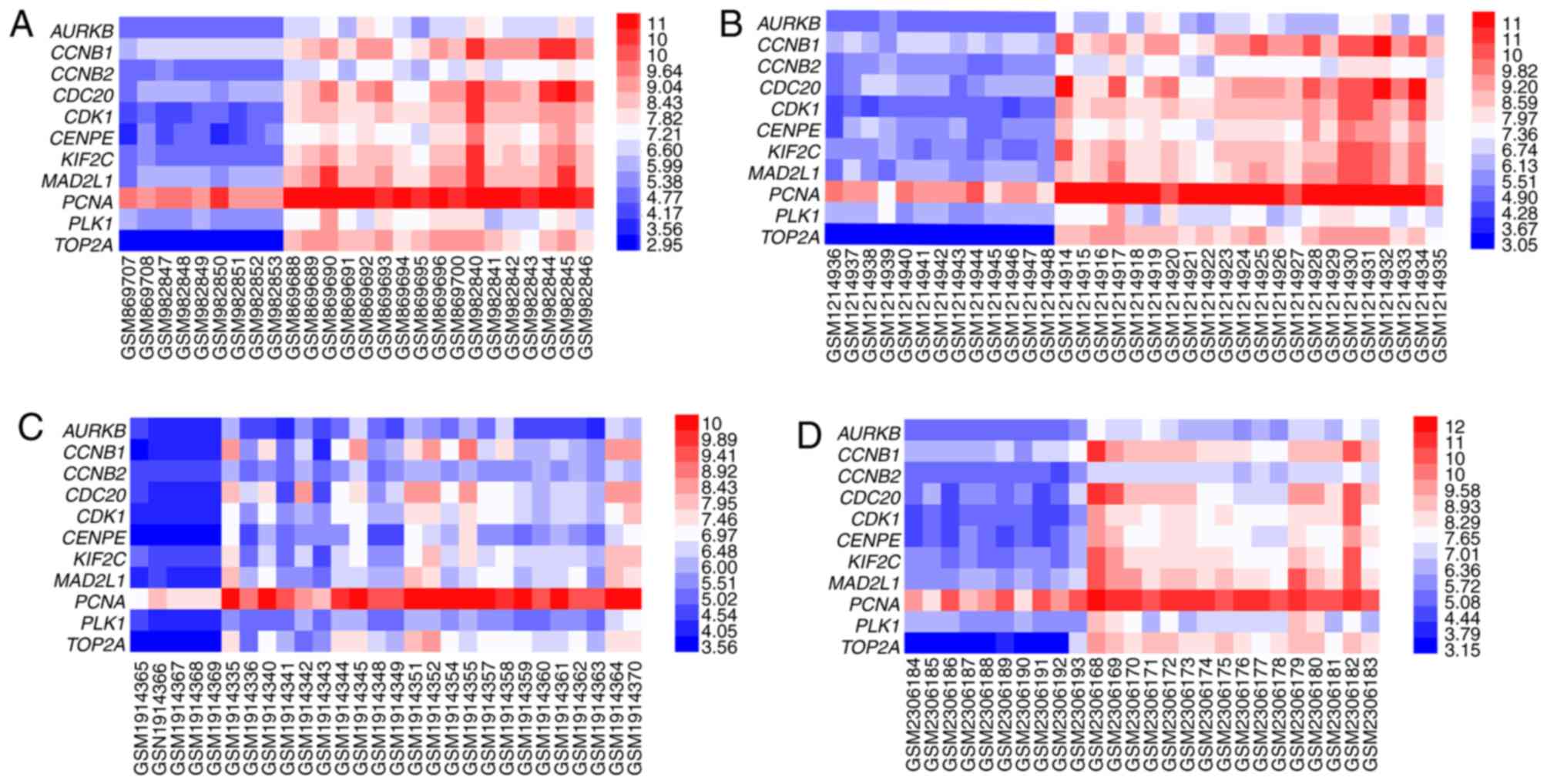
with the highest degree (90) was CDK1. Additionally, four heat maps
of the expression of 11 hub genes in GSE35493, GSE50161, GSE74195
and GSE86574 are presented in Fig.
3.
Furthermore, following MCODE analysis, 7 modules
were identified to be available and the top 3 significant modules
are presented in Fig. 2B-D.
Furthermore, the top 3 pathways in each module are listed in
Table III. Module 1 had 29 nodes,
404 edges and the highest score (score=28.857). In this module, the
top 3 enriched KEGG pathways were cell cycle, progesterone-mediated
oocyte maturation, and oocyte meiosis. In addition, module 2
(score=11.6) had 31 nodes and 174 edges, and the pathways were
primarily associated with mismatch repair, DNA replication and
nucleotide excision repair. Module 3 (score=10) had 10 nodes and 45
edges, which were significantly enriched in ubiquitin-mediated
proteolysis (P<0.05; Table
III).
 | Table III.Top 10 significant KEGG pathways of
the DEGs in top 3 modules. |
Table III.
Top 10 significant KEGG pathways of
the DEGs in top 3 modules.
| Module | Term | KEGG names | Count | P-value | Genes |
|---|
| Module 1 | hsa04110 | Cell cycle | 8 |
5.80×10−11 | CCNB1, CDK1, CDC20,
CCNB2, PLK1, BUB1B, MAD2L1, ESPL1 |
|
| hsa04914 |
Progesterone-mediated oocyte
maturation | 5 |
4.65×10−06 | CCNB1, CDK1, PLK1,
MAD2L1, CCNB2, |
|
| hsa04114 | Oocyte meiosis | 5 |
1.14×10−05 | CDK1, MAD2L1, PLK1,
CDC20, ESPL1 |
| Module 2 | hsa03430 | Mismatch
repair | 6 |
3.25×10−10 | EXO1, RFC3, RFC4,
RFC2, POLD1, PCNA |
|
| hsa03030 | DNA
replication | 6 |
3.59×10−09 | RFC3, RFC4, POLE2,
RFC2, POLD1, PCNA |
|
| hsa03420 | Nucleotide excision
repair | 6 |
1.45×10−08 | RFC3, RFC4, POLE2,
RFC2, POLD1, PCNA |
| Module 3 | hsa04120 | Ubiquitin mediated
proteolysis | 4 |
7.41×10−05 | FBXW7, SIAH1,
UBE2S, UBE2E2 |
Survival analysis
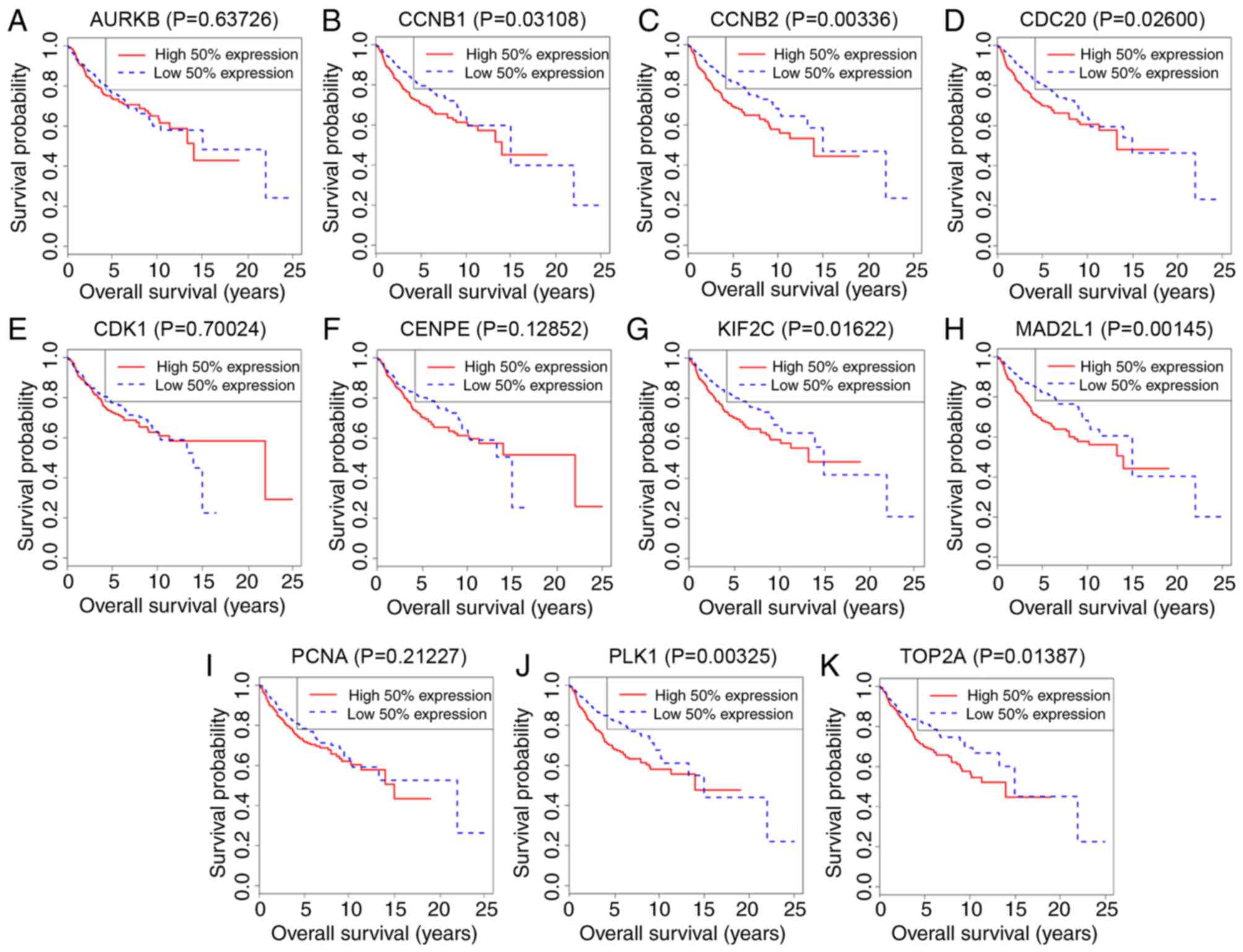
The survival curves for hub genes in the dataset
GSE85217 are presented in Fig. 4.
The expressions of CCNB1 (P=0.03108), CCNB2 (P=0.00336), CDC20
(P=0.026), KIF2C (P=0.01622), MAD2L1 (P=0.00145), PLK1 (P=0.00325)
and TOP2A (P=0.01387) were negatively associated with patient
survival time.
Discussion
Identifying the molecular mechanisms of MB, which
has unique gene expression signatures, is of critical importance
for targeted diagnosis and treatment (21). In the present study, 78 MB and 37
normal samples were collected from the GEO database for
bioinformatics analysis, aiming to identify hidden biomarkers and
elucidate the molecular mechanisms in MB. A total of 1,006 mutual
DEGs were screened from the four microarray datasets GSE35493,
GSE50161, GSE74195 and GSE86574 using the Limma in R package.
The GO analysis results of the DEGs revealed that
the overlapped DEGs were primarily associated with mitosis,
including cell division, mitotic nuclear division, G1/S
transition of the mitotic cell cycle, sister chromatid cohesion,
sister chromatid segregation, chromosome segregation,
G2/M transition of the mitotic cell cycle, and mitotic
chromosome condensation in the BP category. In addition, Aurora
kinase B regulates multiple stages of mitosis, and its inhibitors
may inhibit the growth of Group 3 MBs and prolong survival
(22). These results suggested that
it may be possible to treat MB by regulating the key biomarkers of
mitosis (23,24). In addition, it was also observed that
these genes were enriched in cell junction, condensed chromosome
kinetochore, protein binding, microtubule binding and chromatin
binding. Certain RNA binding proteins, including MSI1, DDX3X and
CCAR1, were reported to play important roles in the growth and/or
maintenance of MB (25).
Following KEGG pathway analysis, the genes were
found to be significantly associated with cell cycle, DNA
replication, retrograde endocannabinoid signaling, GABAergic
synapse, morphine addiction, nicotine addiction, dopaminergic
synapse, circadian entrainment, mismatch repair, and p53 signaling
pathway. A previous study reported that the defect of NEO1, which
was necessary for cell cycle progression, arrests cells at the
G2/M phase in MB (26).
These results indicated that cannabinoids, morphine and nicotine,
consistent with previous studies, were likely associated with the
progression of brain tumors (27–29).
Therefore, these pathway analysis results may enable the prediction
of novel therapeutic targets.
Furthermore, the top 11 hub genes, including CDK1,
CCNB1, CCNB2, PLK1, CDC20, MAD2L1, AURKB, CENPE, TOP2A, KIF2C and
PCNA, were identified using the PPI network. It was reported that
inhibiting the catalytic activity of CDK1 using VMY-1-103 may
severely disrupt the mitotic cycle of MB cells (24). It was previously reported that the
combined expression of MYC, LDHB and CCNB1 as potential prognostic
biomarkers may predict survival and provide a more accurate basis
for the targeted therapy of patients with MB (30). The inhibitors of PLK1, an oncogenic
kinase that controls cell cycle and proliferation, inhibited
mitosis in MB cells, and patients expressing high levels of PLK1
were considered as high-risk. All findings indicated that PLK1 is a
possible therapeutic target for patients with MB (31,32). In
another study, it was reported that the expression levels of TOP2A
may be a potential biomarker for sensitivity to etoposide in
patients with MB (33). PCNA, which
may be used to demonstrate the proliferative phase of the cell
cycle, was significantly associated with MB grade, suggesting that
PCNA may be a biomarker for assessing grade and the possibility of
recurrence in MB (34).
However, 6 hub genes, including CCNB2, CDC20,
MAD2L1, AURKB, CENPE and KIF2C, have yet to be verified, to the
best of our knowledge, in MB by systematic searches through PubMed,
there were a number of studies on other tumors, particularly brain
tumors, including glioma (33–44). It
was reported that CDC20 was a critical regulator of
tumor-initiating cell proliferation and survival of glioblastoma
cells (35). Combining the findings
of other studies, CDC20 was found to play a role in cell cycle
progression, apoptosis and brain development, and these findings
indicated that it may be a potential novel target for therapeutic
intervention in brain tumors, particularly MB (36,37). In
addition, the RNA levels of CDC20 and MAD2L1 were associated with
glioma grade, which suggested a clinical benefit as a biomarker
(38). Other studies demonstrated
that MAD2L1 was of diagnostic value in several tumors, including
salivary duct carcinomas, breast cancer and acute lymphoblastic
leukemia (39–41). Furthermore, CCNB2 was predicted as a
tumor-associated factor similar to CCNB1 (42). AURKA was reported to be the target of
some molecule inhibitors, such as BMS-754807 and SIX3, aiming to
inhibit diffuse intrinsic pontine glioma or astrocytoma (43,44). The
knockdown of CENPE, which is highly expressed in pediatric glioma,
combined with temozolomide treatment, was found to lead to
inhibition of glioma cell proliferation (45). KIF2C was reported to be associated
with histopathological glioma grades, which indicated this gene may
be a potential biomarker for prognosis in patients with glioma
(46). Taken together, although
their significance has yet to been confirmed, to the best of our
knowledge, these findings suggest that the 6 hub genes may be
potential biomarkers in the diagnosis, treatment and prognosis of
MB.
In conclusion, the findings of the present study
provided an integrated bioinformatics analysis of 1,006 overlapped
DEGs that may be involved in the growth, recurrence and metastasis
of MB. A total of 11 hub genes, including CDK1, CCNB1, CCNB2, PLK1,
CDC20, MAD2L1, AURKB, CENPE, TOP2A, KIF2C and PCNA, were identified
as novel potential biomarkers. These findings may provide further
insight into the underlying molecular mechanisms and identify novel
biomarkers for evaluating the diagnosis and prognosis, and advance
the treatment of MB. However, further molecular biological research
is required to confirm the clinical value of our findings.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Scientific
Research Foundation for the Returned Overseas Chinese Scholars
(082003), the Research Foundation of Shanghai Municipal Health and
Family Planning Commission (201540266), Shanghai Jiao Tong
University Medicine-Engineering Cross Research Foundation
(YG2015MS25) and the Research Foundation of Shanghai No. 3 People's
Hospital Affiliated to Shanghai Jiao Tong University School of
Medicine (syz2015-015).
Availability of data and materials
The datasets used and/or analyzed during the present
study were obtained from the GEO, DAVID and STRING databases.
Authors' contributions
SHC and BY were involved in the conception and
design of the research and drafting the manuscript. YBP
participated in the acquisition of data. BY and JXD performed the
analysis and interpretation of data. YBM was involved in the
statistical analysis. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MB
|
medulloblastoma
|
|
GEO
|
Gene Expression Omnibus
|
|
DEGs
|
differentially expressed genes
|
|
DAVID
|
Database for Annotation, Visualization
and Integrated Discovery
|
|
GO
|
Gene Ontology
|
|
KEGG
|
Kyoto Encyclopedia of Genes and
Genomes
|
|
PPI
|
protein-protein interaction
|
|
STRING
|
Search Tool for the Retrieval of
Interacting Genes
|
|
MCODE
|
Molecular Complex Detection
|
|
BP
|
biological process
|
|
MF
|
molecular function
|
|
CC
|
cell component
|
References
|
1
|
Gilbertson RJ: Medulloblastoma: Signalling
a change in treatment. Lancet Oncol. 5:209–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bartlett F, Kortmann R and Saran F:
Medulloblastoma. Clin Oncol (R Coll Radiol). 25:36–45. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gerber NU, Mynarek M, von Hoff K,
Friedrich C, Resch A and Rutkowski S: Recent developments and
current concepts in medulloblastoma. Cancer Treat Rev. 40:356–365.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World health organization
classification of tumors of the central nervous system: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Quinlan A and Rizzolo D: Understanding
medulloblastoma. JAAPA. 30:30–36. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sengupta S, Pomeranz Krummel D and Pomeroy
S: The evolution of medulloblastoma therapy to personalized
medicine. F1000 Res. 6:4902017. View Article : Google Scholar
|
|
7
|
Archer TC, Mahoney EL and Pomeroy SL:
Medulloblastoma: Molecular classification-based personal
therapeutics. Neurotherapeutics. 14:265–273. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shou Y, Robinson DM, Amakye DD, Rose KL,
Cho YJ, Ligon KL, Sharp T, Haider AS, Bandaru R, Ando Y, et al: A
five-gene hedgehog signature developed as a patient preselection
tool for hedgehog inhibitor therapy in medulloblastoma. Clin Cancer
Res. 21:585–593. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Khatua S and Zaky W: The biologic era of
childhood medulloblastoma and clues to novel therapies. Future
Oncol. 10:637–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Harris PS, Venkataraman S, Alimova I,
Birks DK, Balakrishnan I, Cristiano B, Donson AM, Dubuc AM, Taylor
MD, Foreman NK, et al: Integrated genomic analysis identifies the
mitotic checkpoint kinase WEE1 as a novel therapeutic target in
medulloblastoma. Mol Cancer. 13:722014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Birks DK, Donson AM, Patel PR, Sufit A,
Algar EM, Dunham C, Kleinschmidt-DeMasters BK, Handler MH, Vibhakar
R and Foreman NK: Pediatric rhabdoid tumors of kidney and brain
show many differences in gene expression but share dysregulation of
cell cycle and epigenetic effector genes. Pediatr Blood Cancer.
60:1095–1102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Griesinger AM, Birks DK, Donson AM, Amani
V, Hoffman LM, Waziri A, Wang M, Handler MH and Foreman NK:
Characterization of distinct immunophenotypes across pediatric
brain tumor types. J Immunol. 191:4880–4888. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Bont JM, Kros JM, Passier MM,
Reddingius RE, Sillevis Smitt PA, Luider TM, den Boer ML and
Pieters R: Differential expression and prognostic significance of
SOX genes in pediatric medulloblastoma and ependymoma identified by
microarray analysis. Neuro Oncol. 10:648–660. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gautier L, Cope L, Bolstad BM and Irizarry
RA: Affy-analysis of Affymetrix GeneChip data at the probe level.
Bioinformatics. 20:307–315. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ritchie ME, Phipson B, Wu D, Hu Y, Law CW,
Shi W and Smyth GK: limma powers differential expression analyses
for RNA-sequencing and microarray studies. Nucleic Acids Res.
43:e472015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang da W, Sherman BT and Lempicki RA:
Systematic and integrative analysis of large gene lists using DAVID
bioinformatics resources. Nat Protoc. 4:44–57. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jensen LJ, Kuhn M, Stark M, Chaffron S,
Creevey C, Muller J, Doerks T, Julien P, Roth A, Simonovic M, et
al: STRING 8-a global view on proteins and their functional
interactions in 630 organisms. Nucleic Acids Res. 37:D412–416.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Smoot ME, Ono K, Ruscheinski J, Wang PL
and Ideker T: Cytoscape 2.8: New features for data integration and
network visualization. Bioinformatics. 27:431–432. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chin CH, Chen SH, Wu HH, Ho CW, Ko MT and
Lin CY: cytoHubba: Identifying hub objects and sub-networks from
complex interactome. BMC Syst Biol. 4 (Suppl 8):S112014. View Article : Google Scholar
|
|
20
|
Bader GD and Hogue CW: An automated method
for finding molecular complexes in large protein interaction
networks. BMC Bioinformatics. 4:22003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brandes AA, Bartolotti M, Marucci G,
Ghimenton C, Agati R, Fioravanti A, Mascarin M, Volpin L, Ammannati
F, Masotto B, et al: New perspectives in the treatment of adult
medulloblastoma in the era of molecular oncology. Crit Rev Oncol
Hematol. 94:348–359. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Diaz RJ, Golbourn B, Faria C, Picard D,
Shih D, Raynaud D, Leadly M, MacKenzie D, Bryant M, Bebenek M, et
al: Mechanism of action and therapeutic efficacy of Aurora kinase B
inhibition in MYC overexpressing medulloblastoma. Oncotarget.
6:3359–3374. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alimova I, Ng J, Harris P, Birks D, Donson
A, Taylor MD, Foreman NK, Venkataraman S and Vibhakar R: MPS1
kinase as a potential therapeutic target in medulloblastoma. Oncol
Rep. 36:2633–2640. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ringer L, Sirajuddin P, Heckler M, Ghosh
A, Suprynowicz F, Yenugonda VM, Brown ML, Toretsky JA, Uren A, Lee
Y, et al: VMY-1-103 is a novel CDK inhibitor that disrupts
chromosome organization and delays metaphase progression in
medulloblastoma cells. Cancer Biol Ther. 12:818–826. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bish R and Vogel C: RNA binding
protein-mediated post-transcriptional gene regulation in
medulloblastoma. Mol Cells. 37:357–364. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Milla LA, Arros A, Espinoza N, Remke M,
Kool M, Taylor MD, Pfister SM, Wainwright BJ and Palma V: Neogenin1
is a sonic hedgehog target in medulloblastoma and is necessary for
cell cycle progression. Int J Cancer. 134:21–31. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ellert-Miklaszewska A, Grajkowska W,
Gabrusiewicz K, Kaminska B and Konarska L: Distinctive pattern of
cannabinoid receptor type II (CB2) expression in adult and
pediatric brain tumors. Brain Res. 1137:161–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sepsova V, Krusek J, Zdarova Karasova J,
Zemek F, Musilek K, Kuca K and Soukup O: The interaction of
quaternary reversible acetylcholinesterase inhibitors with the
nicotinic receptor. Physiol Res. 63:771–777. 2014.PubMed/NCBI
|
|
29
|
Sardi I, la Marca G, Giovannini MG,
Malvagia S, Guerrini R, Genitori L, Massimino M and Arico M:
Detection of doxorubicin hydrochloride accumulation in the rat
brain after morphine treatment by mass spectrometry. Cancer
Chemother Pharmacol. 67:1333–1340. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
de Haas T, Hasselt N, Troost D, Caron H,
Popovic M, Zadravec-Zaletel L, Grajkowska W, Perek M, Osterheld MC,
Ellison D, et al: Molecular risk stratification of medulloblastoma
patients based on immunohistochemical analysis of MYC, LDHB, and
CCNB1 expression. Clin Cancer Res. 14:4154–4160. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pezuk JA, Brassesco MS, Ramos PMM,
Scrideli CA and Tone LG: Polo-like kinase 1 pharmacological
inhibition as monotherapy or in combination: Comparative effects of
polo-like kinase 1 inhibition in medulloblastoma cells. Anticancer
Agents Med Chem. 17:1278–1291. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Triscott J, Lee C, Foster C, Manoranjan B,
Pambid MR, Berns R, Fotovati A, Venugopal C, O'Halloran K,
Narendran A, et al: Personalizing the treatment of pediatric
medulloblastoma: Polo-like kinase 1 as a molecular target in
high-risk children. Cancer Res. 73:6734–6744. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Uesaka T, Shono T, Kuga D, Suzuki SO,
Niiro H, Miyamoto K, Matsumoto K, Mizoguchi M, Ohta M, Iwaki T and
Sasaki T: Enhanced expression of DNA topoisomerase II genes in
human medulloblastoma and its possible association with etoposide
sensitivity. J Neurooncol. 84:119–129. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kayaselcuk F, Zorludemir S, Gumurduhu D,
Zeren H and Erman T: PCNA and Ki-67 in central nervous system
tumors: Correlation with the histological type and grade. J
Neurooncol. 57:115–121. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xie Q, Wu Q, Mack SC, Yang K, Kim L,
Hubert CG, Flavahan WA, Chu C, Bao S and Rich JN: CDC20 maintains
tumor initiating cells. Oncotarget. 6:13241–13254. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang L, Zhang J, Wan L, Zhou X, Wang Z and
Wei W: Targeting Cdc20 as a novel cancer therapeutic strategy.
Pharmacol Ther. 151:141–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ji P, Zhou X, Liu Q, Fuller GN, Phillips
LM and Zhang W: Driver or passenger effects of augmented c-Myc and
Cdc20 in gliomagenesis. Oncotarget. 7:23521–23529. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bie L, Zhao G, Cheng P, Rondeau G,
Porwollik S, Ju Y, Xia XQ and McClelland M: The accuracy of
survival time prediction for patients with glioma is improved by
measuring mitotic spindle checkpoint gene expression. PLoS One.
6:e256312011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ko YH, Roh JH, Son YI, Chung MK, Jang JY,
Byun H, Baek CH and Jeong HS: Expression of mitotic checkpoint
proteins BUB1B and MAD2L1 in salivary duct carcinomas. J Oral
Pathol Med. 39:349–355. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Krapf G, Kaindl U, Kilbey A, Fuka G,
Inthal A, Joas R, Mann G, Neil JC, Haas OA and Panzer-Grumayer ER:
ETV6/RUNX1 abrogates mitotic checkpoint function and targets its
key player MAD2L1. Oncogene. 29:3307–3312. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Z, Katsaros D, Shen Y, Fu Y, Canuto
EM, Benedetto C, Lu L, Chu WM, Risch HA and Yu H: Biological and
clinical significance of MAD2L1 and BUB1, genes frequently
appearing in expression signatures for breast cancer prognosis.
PLoS One. 10:e01362462015. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Manni I, Mazzaro G, Gurtner A, Mantovani
R, Haugwitz U, Krause K, Engeland K, Sacchi A, Soddu S and Piaggio
G: NF-Y mediates the transcriptional inhibition of the cyclin B1,
cyclin B2, and cdc25C promoters upon induced G2 arrest. J Biol
Chem. 276:5570–5576. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Halvorson KG, Barton KL, Schroeder K,
Misuraca KL, Hoeman C, Chung A, Crabtree DM, Cordero FJ, Singh R,
Spasojevic I, et al: A high-throughput in vitro drug screen in a
genetically engineered mouse model of diffuse intrinsic pontine
glioma identifies BMS-754807 as a promising therapeutic agent. PLoS
One. 10:e01189262015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yu Z, Sun Y, She X, Wang Z, Chen S, Deng
Z, Zhang Y, Liu Q, Liu Q, Zhao C, et al: SIX3, a tumor suppressor,
inhibits astrocytoma tumorigenesis by transcriptional repression of
AURKA/B. J Hematol Oncol. 10:1152017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Liang ML, Hsieh TH, Ng KH, Tsai YN, Tsai
CF, Chao ME, Liu DJ, Chu SS, Chen W, Liu YR, et al: Downregulation
of miR-137 and miR-6500-3p promotes cell proliferation in pediatric
high-grade gliomas. Oncotarget. 7:19723–19737. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Bie L, Zhao G, Wang YP and Zhang B:
Kinesin family member 2C (KIF2C/MCAK) is a novel marker for
prognosis in human gliomas. Clin Neurol Neurosurg. 114:356–360.
2012. View Article : Google Scholar : PubMed/NCBI
|