Introduction
Endometrial cancer (EC) is the most common
malignancy of the female reproductive tract and its incidence is on
the increase (1). Approximately 40%
of all cases can be attributed to obesity (2). Aberration in lipid metabolism
contributes to different aspects of tumorigenesis (3); our research team focused on this
domain of EC in recent years.
NAD-dependent class III histone deacetylase silent
information regulator 1 (SIRT1), that shares the highest degree of
homology with the yeast protein SIR2, can deacetylate both histones
and non-histone proteins (4). Its
deacetylation activity enables it to interact with a variety of
important transcription factors and transcriptional co-regulatory
factors, to regulate gene transcription, chromosome stability and
activity of target proteins, which are involved in tumor metabolism
and development (5,6). The current results demonstrated that
SIRT1 plays a dual role as a tumor promoter as well as a tumor
suppressor (7). Its involvement in
tumorigenesis may be due to its diverse distribution in different
tissues and different upstream and downstream regulatory factors
that regulate its function (8).
Sterol regulatory element binding protein 1 (SREBP1)
belongs to the family of the basic helix-loop-helix leucine zipper
family of DNA binding transcription factors, which can regulate
most enzymes involved in fatty acid biosynthesis, such as
acetyl-CoA carboxylase, fatty acid synthase, Elovl-6 and
stearoyl-CoA desaturase (9).
Lipogenesis is increased in cancer cells (10,11),
and the expression of SREBP1 has been observed to be elevated in
various types of cancer (12–15).
In our previous studies, we demonstrated that SREBP1 is
overexpressed in endometrial and ovarian cancer and the expression
of SREBP1 protein increased with higher FIGO surgical stage and
histological grade of the disease. Furthermore, knockdown of SREBP1
could induce apoptosis, reduce cell proliferation in vitro
and in vivo (16,17). Eberhard et al demonstrated
that inhibition of SREBP1 or its downstream target fatty acid
synthase sensitized resistant cells to death ligands (18). Therefore, SREBP1 plays a role as a
cancer promoter in human cancers.
In the present study, we investigated SIRT1
expression in EC and its effect on tumor cells. We also explored
the correlation between SIRT1 and SREBP1 and we propose to
establish the role of SIRT1 in EC.
Materials and methods
Tissue collection and cell culture
Sixty cases of fresh uterine endometrial
adenocarcinoma and adjacent normal endometrial tissues were
obtained from patients who underwent initial hysterectomy. All
specimens were preserved at −80°C refrigeration. Endorsed informed
consent from all patients was collected prior to the operation.
Human EC cell lines Ishikawa, ECC, RL95-2, KLE were separately
cultured in RPMI-1640, MEM, DMEM/F12, DMEM/F12-medium. The basal
medium was supplemented with 10% FBS. All cells were cultured at
37°C in a humid atmosphere with 5% CO2.
Quantitative real-time PCR analysis
Total RNA was extracted from collected tissues and
cultured cells by TRIzol reagent following the manufacturer’s
protocol (Invitrogen). Complementary DNAs were synthesized from
total RNA using the SuperScript™ II Reverse Transcriptase kit
(Invitrogen). PCR was operated using SYBR-Green Real-Time PCR
Master Mix (Invitrogen). GAPDH was used as a control of
normalization, the primers used for qRT-PCR are listed below. The
gene symbol, forward primer sequence and reverse primer sequence
(amplification size) were: SIRT1, 5′-TGT GAA AGT GAT GAG GAG GAT
AGA and 5′-TAC AGC AAG GCG AGC ATA AAT A (136 bp); SREBP1a, 5′-CGG
CGC TGC TGA CCG ACA TC and 5′-CCC TGC CCC ACT CCC AGC AT (104 bp);
SREBP1c, 5′-GCG CAG ATC GCG GAG CCA T and 5′-CCC TGC CCC ACT CCC
AGC AT (116 bp); GAPDH, 5′-GAG TCA ACG GAT TTG GTC GT and 5′-TTG
AGG TCA ATG AAG GGG TC (103 bp).
Western blot analysis
The collected tissues and cultured cells were lysed
using cell lysis buffer. Protein concentrations were determined by
the BCA protein quantitative analysis kit. The dissolved lysates
were subjected to 10% SDS-PAGE and blotted onto a PVDF membrane.
After blocking for 2 h at room temperature, the membrane was
incubated with primary antibodies against SIRT1 and SREBP1 (Santa
Cruz Biotechnology, Inc.) at 4°C overnight. Horseradish
peroxidase-labeled secondary antibody was used to bind the primary
antibody. Proteins were visualized by ECL plus system. β-actin was
used as a housekeeping protein. Quantitative data were analyzed
using Bio-Rad software.
Cell transduction
Lentiviral vectors containing SIRT1 siRNAs and their
controls were constructed and prepared for virus solutions by
Genesil (Wuhan, China), and these solutions were then utilized to
infect EC cell lines. The efficiency of transduction was determined
by western blotting.
Plate colony formation assay
Logarithmic growth phase cells (2,000) were seeded
in 100-mm culture plates for two weeks. Each group was allotted
three plates. Colonies formed of each plate were stained with
crystal violet, and statistics on the number of colonies were
determined by Gel-Pro Analyzer.
Cell proliferation assays
Stably transfected cells were seeded at a density of
2×105 cells in 60-mm culture plates. Each group had
three plates. The total number of cells was counted for 6 days.
Experiments were repeated three times.
Statistical analysis
Relative quantification of RNA expression was
measured by using the 2−ΔΔCt method. Comparison of the
quantitative data was analyzed by Student’s t-test. The data was
described as means ± SD. The statistical analyses were performed
using SPSS 13.0 software (SPSS Inc.). p-values <0.05 were
considered to indicate a statistically significant difference.
Results
SIRT1 is upregulated in EC
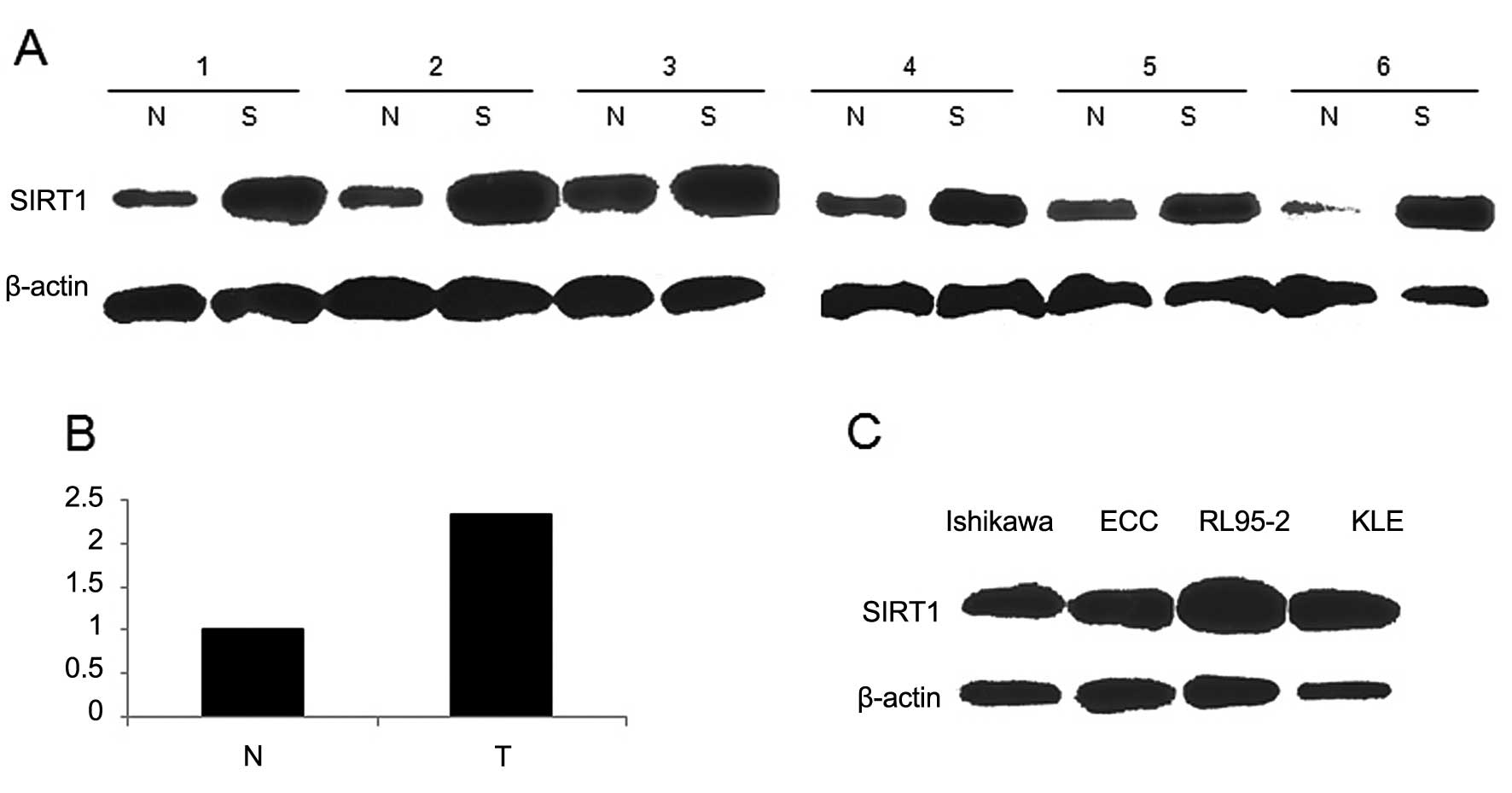
To detect the expression of SIRT1 in EC, we analyzed
the SIRT1 mRNA and protein levels by RT-PCR and western blotting in
40 cases of uterine endometrial adenocarcinoma and adjacent normal
endometrial tissues. Compared to the normal controls, the
expression of SIRT1 mRNA in EC was significantly elevated (Fig. 1A), the expression at protein levels
showed the same trend, consistent with mRNA levels (Fig. 1B). These data demonstrated that
SIRT1 is upregulated in EC. We also detected protein levels in four
EC cell lines (Fig. 1C). RL95-2
cell line that had higher expression of SIRT1 was transduced with
lentiviral vectors in order to knock down the expression of SIRT1
in the following study.
Effect on SREBP1 with the silencing of
SIRT1 in RL95-2 cell line
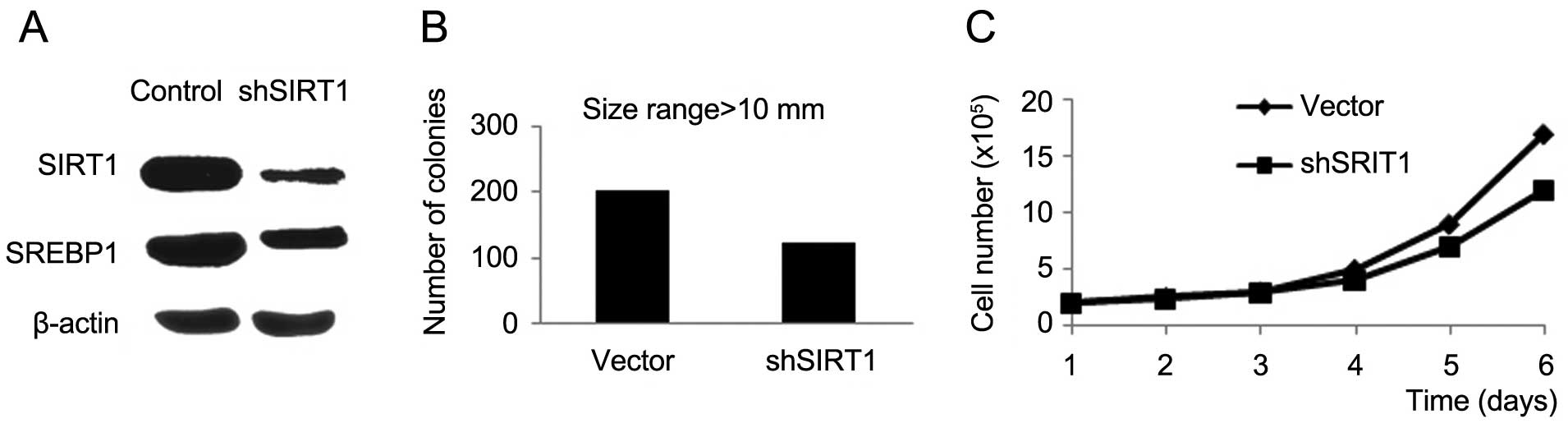
To explore the correlation between SIRT1 and SREBP1
in EC, we used the lentiviral vectors with SIRT1 siRNAs in RL95-2
cell line. Efficiency of transduction was detected by western
blotting (Fig. 2A). The expression
of SREBP1 was clearly reduced at the protein levels (Fig. 2A); lowered expression of SIRT1
downregulated the expression of SREBP1. Accordingly, we established
that SIRT1 positively regulates SREBP1 in EC. Our previous study
showed that overexpression of SREBP1 was found in EC and it
promoted tumor cell proliferation and tumor growth (16), and since the inhibition of SIRT1 can
lower the expression of SREBP1, it can suppress tumor growth and
progression. We further studied the role of SIRT1 in tumor growth
in the following experiment.
Knockdown of SIRT1 inhibits EC cell
growth capacity
To illustrate the effect of SIRT1 knockdown on cell
growth capacity, we carried out direct cell counting and
colony-forming assays. We found that cell number and the number of
colonies were clearly reduced in the RL95-2 cell line with
silencing of SIRT1 compared to the vector control (Fig. 2B and C). These results demonstrate
that SIRT1 plays a significant role in EC cell growth.
Discussion
Accumulating evidence indicates that SIRT1 plays a
dual role in tumorigenesis. Elevated expression of SIRT1 has been
reported in some types of human cancer tissues, such as prostate,
liver, breast and stomach (19–22).
Moreover, Zhao et al discovered that SIRT1 RNAi knockdown
induces apoptosis and senescence, inhibits invasion and enhances
chemosensitivity in pancreatic cancer cells (23). Nakane et al found that
inhibition of cortactin and SIRT1 expression attenuates migration
and invasion of prostate cancer DU145 cells (24). However, other studies demonstrated
that SIRT1 is downregulated in several tumors (25,26).
There is little research exploring the correlation that exists
between SIRT1 and EC.
We showed that SIRT1 is overexpressed in EC compared
to normal endometrium, and its expression varies in a few EC cell
lines. We also discovered that knockdown of SIRT1 clearly
suppressed cell proliferation and cell growth. These results
demonstrate that SIRT1 acts as a tumor promoter in EC. Knockdown of
SIRT1 can affect biological behavior, yet it is insufficient to
inhibit tumor progression. This suggests that SIRT1 alone may not
be adequate in the promotion of carcinogenesis; however,
downregulation of SIRT1 is clearly beneficial for EC treatment.
Many studies have shown an increased degree of
lipogenesis in cancer cells and that the majority of fatty acids in
cancer cells are derived from de novo fatty acid synthesis,
in contrast to normal cells that obtain their fatty acids from the
circulation (3,10,27).
SIRT1 acts as a sensor and a regulator in the metabolism of cancer
(28). SREBP1 is the pivotal
transcription factor in lipogenesis (29), and Bengoechea-Alonso et al
speculated that SREBP1 may provide a link between lipid synthesis,
and cell growth and proliferation (30). Based on these findings, some
researchers have found that SREBP1 plays a role as an oncogene in
human cancer (12–15). It has been reported that SIRT1
deacetylates and inhibits SREBP-1c transactivation by decreasing
its stability and its occupancy at the lipogenic genes.
Furthermore, hepatic overexpression of SIRT1 or treatment with
resveratrol can decrease elevated acetylated SREBP-1c levels in
diet-induced obese mice (31).
Walker et al also demonstrated that SIRT1 negatively
regulates SREBP1 during fasting, and decreased expression of SREBP1
correlates with decreased hepatic lipid and cholesterol levels and
attenuates liver steatosis in diet-induced and genetically obese
mice (32). In contrast, Defour
discovered that knocking out the catalytic domain of SIRT1
decreased SREBP-1c mRNA and protein levels, and SREBP-1c promoter
transactivation was significantly increased in response to SIRT1
overexpression by in vivo genetic electrotransfer in
skeletal muscle (33). The variance
in the contribution of SIRT1 in regulating SREBP1 negatively or
positively depends on genetic background, tissue types and
metabolic environment. The correlation of SIRT1 and SREBP1 in
cancer tissues has not been found.
Our previous studies showed that SREBP1 plays a role
as a tumor promoter in EC; in the present study, we established the
role of SIRT1 as a cancer promoter in EC. The expression of SREBP1
was downregulated by transduction of SIRT1-specific siRNA
lentivirus in RL95-2 cell line. Briefly, SIRT1 affects biological
behaviors by targeting SREBP1 and lipogenesis. Activated FoxO1
inhibits SREBP-1c gene expression by reducing transcriptional
activity of Sp1 and SREBP-1c and disrupting the assembly of
transcriptional initiation complex on the SREBP-1c promoter
(34,35). In cancer, SIRT1 suppresses Fox01
activity by deacetylating (36–38),
while low expression of Fox01 decreases suppression of SREBP1,
consequently resulting in elevated levels of SREBP1. However, the
exact mechanism remains unclear and will be studied further in
subsequent analyses.
Obesity is an established epidemiological risk
factor in EC (2). On the one hand,
obese patients have increased endogenous estrogen levels, which
comes from conversion of androstenedione to estrone and the
aromatization of androgens to estradiol in redundant subcutaneous
adipocytes; on the other hand, abnormal lipometabolism exists in
obese patients and disorder of lipid metabolism contributes to
different aspects of tumorigenesis. In the present study, SIRT1 was
demonstrated to promote endometrial tumor growth by increasing the
levels of SREBP1 and lipogenesis, thus we postulate that we can
attenuate tumor growth by targeting SIRT1 in EC.
Collectively, we confirmed that SIRT1 significantly
upregulates and can promote tumor proliferation, migration and
invasion capacity by targeting SREBP1 and lipogenesis in EC. Thus,
knockdown of SIRT1 or SIRT1 inhibitor may play a substantial role
in suppressing endometrial tumor. Thus, SIRT1 may be regarded as a
therapeutic target in EC.
Acknowledgements
This study was partly funded by the National Natural
Science Foundation of China [81372808 (J.J.)] and [81173614
(Q.T.L)], and was also partly funded by the Science, and the
Science and Technology Developing Planning of Jinan (201303035),
and the Technology Development planning of Shandong [2012G0021823
(J.J)].
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar
|
|
2
|
Modesitt SC, Hsu JY, Chowbina SR, Lawrence
RT and Hoehn KL: Not all fat is equal: differential gene expression
and potential therapeutic targets in subcutaneous adipose, visceral
adipose, and endometrium of obese women with and without
endometrial cancer. Int J Gycecol Cancer. 22:732–741. 2012.
View Article : Google Scholar
|
|
3
|
Santos CR and Schulze A: Lipid metabolism
in cancer. FEBS J. 279:2610–2623. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Imai S: The molecular mechanism of aging
and longevity and the function of Sir2 proteins. Nihon Ronen
Igakkai Zasshi. 38:735–739. 2001.(In Japanese).
|
|
5
|
Liu T, Liu PY and Marshall GM: The
critical role of the class III histone deacetylase SIRT1 in cancer.
Cancer Res. 69:1702–1705. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Katto J, Engel N, Abbas W, Herbein G and
Mahlknecht U: Transcription factor NFκB regulates the expression of
the histone deacetylase SIRT1. Clin Epigenetics. 5:112013.
|
|
7
|
Song NY and Surh YJ: Janus-faced role of
SIRT1 in tumorigenesis. Ann NY Acad Sci. 1271:10–19. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fang Y and Nicholl MB: Sirtuin 1 in
malignant transformation: friend or foe? Cancer Lett. 306:10–14.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shimano H: SREBPs: physiology and
pathophysiology of the SREBP family. FEBS J. 276:616–621. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Swinnen JV, Brusselmans K and Verhoeven G:
Increased lipogenesis in cancer cells: new players, novel targets.
Curr Opin Clin Nutr Metab Care. 9:358–365. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Menendez JA and Lupu R: Fatty acid
synthase and the lipogenic phenotype in cancer pathogenesis. Nat
Rev Cancer. 7:763–777. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li JN, Mahmoud MA, Han WF, Ripple M and
Pizer ES: Sterol regulatory element-binding protein-1 participates
in the regulation of fatty acid synthase expression in colorectal
neoplasia. Exp Cell Res. 261:159–165. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Swinnen JV: Increased lipogenesis in
steroid-responsive cancer cells: mechanisms of regulation, role in
cancer cell biology and perspectives on clinical applications. Verh
K Acad Geneeskd Belg. 63:321–333. 2001.PubMed/NCBI
|
|
14
|
Yang Yu, Morin PJ, Han WF, et al:
Regulation of fatty acid synthase expression in breast cancer by
sterol regulatory element binding protein-1c. Exp Cell Res.
282:132–137. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yahagi N, Shimano H, Hasegawa K, et al:
Co-ordinate activation of lipogenic enzymes in hepatocellular
carcinoma. Eur J Cancer. 41:1316–1322. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li W, Tai Y, Zhou J, et al: Repression of
endometrial tumor growth by targeting SREBP1 and lipogenesis. Cell
Cycle. 11:2348–2358. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Nie LY, Lu QT, Li WH, et al: Sterol
regulatory element-binding protein 1 is required for ovarian tumor
growth. Oncol Rep. 30:1346–1354. 2013.PubMed/NCBI
|
|
18
|
Eberhard Y, Gronda M, Hurren R, et al:
Inhibition of SREBP1 sensitizes cells to death ligands. Oncotarget.
2:186–196. 2011.PubMed/NCBI
|
|
19
|
Huffman DM, Grizzle WE, Bamman MM, et al:
SIRT1 is significantly elevated in mouse and human prostate cancer.
Cancer Res. 67:6612–6618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Choi HN, Bae JS, Jamiyandorj U, et al:
Expression and role of SIRT1 in hepatocellular carcinoma. Oncol
Rep. 26:503–510. 2011.PubMed/NCBI
|
|
21
|
Kalle AM, Mallika A, Badiger J, Alinakhi,
Talukdar P and Sachchidanand: Inhibition of SIRT1 by a small
molecule induces apoptosis in breast cancer cells. Biochem Biophys
Res Commun. 401:13–19. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cha EJ, Noh SJ, Kwon KS, et al: Expression
of DBC1 and SIRT1 is associated with poor prognosis of gastric
carcinoma. Clin Cancer Res. 15:4453–4459. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhao G, Cui J, Zhang JG, et al: SIRT1 RNAi
knockdown induces apoptosis and senescence, inhibits invasion and
enhances chemosensitivity in pancreatic cancer cells. Gene Ther.
18:920–928. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nakane K, Fujita Y, Terazawa R, et al:
Inhibition of cortactin and SIRT1 expression attenuates migration
and invasion of prostate cancer DU145 cells. Int J Urol. 19:71–79.
2012. View Article : Google Scholar
|
|
25
|
Firestein R, Blander G, Michan S, et al:
The SIRT1 deacetylase suppresses intestinal tumorigenesis and colon
cancer growth. PLoS One. 3:e20202008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lai CC, Lin PM, Lin SF, et al: Altered
expression of SIRT gene family in head and neck squamous
cell carcinoma. Tumour Biol. 34:1847–1854. 2013.
|
|
27
|
Medes G, Thomas A and Weinhouse S:
Metabolism of neoplastic tissue. IV A study of lipid synthesis in
neoplastic tissue slices in vitro. Cancer Res. 13:27–29.
1953.PubMed/NCBI
|
|
28
|
Knight JR and Milner J: SIRT1, metabolism
and cancer. Curr Opin Oncol. 24:68–75. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eberlé D, Hegarty B, Bossard P, Ferré P
and Foufelle F: SREBP transcription factors: master regulators of
lipid homeostasis. Biochimie. 86:839–848. 2004.PubMed/NCBI
|
|
30
|
Bengoechea-Alonso MT, Punga T and Ericsson
J: Hyperphos-phorylation regulates the activity of SREBP1 during
mitosis. Proc Natl Acad Sci USA. 102:11681–11686. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ponugoti B, Kim DH, Xiao Z, et al: SIRT1
deacetylates and inhibits SREBP-1C activity in regulation of
hepatic lipid metabolism. J Biol Chem. 285:33959–33970. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Walker AK, Yang F, Jiang K, et al:
Conserved role of SIRT1 orthologs in fasting-dependent inhibition
of the lipid/cholesterol regulator SREBP. Genes Dev. 24:1403–1417.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Defour A, Dessalle K, Castro Perez A, et
al: Sirtuin 1 regulates SREBP-1c expression in a LXR-dependent
manner in skeletal muscle. PLoS One. 7:e434902012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Deng X, Zhang W, O-Sullivan I, et al:
FoxO1 inhibits sterol regulatory element-binding protein-1c
(SREBP-1c) gene expression via transcription factors Sp1 and
SREBP-1c. J Biol Chem. 287:20132–20143. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang W, Patil S, Chauhan B, et al: FoxO1
regulates multiple metabolic pathways in the liver: effects on
gluconeogenic, glycolytic, and lipogenic gene expression. J Biol
Chem. 281:10105–10117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Motta MC, Divecha N, Lemieux M, et al:
Mammalian SIRT1 represses forkhead transcription factors. Cell.
116:551–563. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yang Y, Hou H, Haller EM, Nicosia SV and
Bai W: Suppression of FOXO1 activity by FHL2 through SIRT1-mediated
deacetylation. EMBO J. 24:1021–1032. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Frampton G, Ueno Y, Quinn M, et al: The
novel growth factor, progranulin, stimulates mouse cholangiocyte
proliferation via sirtuin-1-mediated inactivation of FOXO1. Am J
Physiol Gastrointest Liver Physiol. 303:G1202–G1211. 2012.
View Article : Google Scholar
|